Abstract
Objective
To test the effectiveness of a stepped care intervention model targeting posttraumatic stress disorder (PTSD) symptoms after injury.
Background
Few investigations have evaluated interventions for injured patients with PTSD and related impairments that can be feasibly implemented in trauma surgical settings.
Methods
The investigation was a pragmatic effectiveness trial in which 207 acutely injured hospitalized trauma survivors were screened for high PTSD symptom levels and then randomized to a stepped combined, care management, psychopharmacology, and cognitive behavioral psychotherapy intervention (n = 104) or usual care control (n = 103) conditions. The symptoms of PTSD and functional limitations were reassessed at one-, three-, six-, nine-, and twelve-months after the index injury admission.
Results
Regression analyses demonstrated that over the course of the year after injury, intervention patients had significantly reduced PTSD symptoms when compared to controls (group by time effect, CAPS, F(2, 185) = 5.50, P < 0.01; PCL-C, F(4, 185) = 5.45, P < 0.001). Clinically and statistically significant PTSD treatment effects were observed at the six-, nine-, and twelve-month post-injury assessments. Over the course of the year after injury, intervention patients also demonstrated significant improvements in physical function (MOS SF-36 PCS main effect, F(1, 172) = 9.87, P < 0.01).
Conclusion
Stepped care interventions can reduce PTSD symptoms and improve functioning over the course of the year after surgical injury hospitalization. Orchestrated investigative and policy efforts could systematically introduce and evaluate screening and intervention procedures for PTSD at United States trauma centers. (Trial Registration: clinicaltrials.gov identifier: NCT00270959)
INTRODUCTION
Each year between 1.5 – 2.5 million American civilians require surgical hospitalization for the treatment of traumatic physical injury.1–3 The symptoms of posttraumatic stress disorder (PTSD) and related co-morbid conditions, such as depression and alcohol use problems, are common in physically injured patients with and without traumatic brain injury (TBI).4–10
After traumatic injury, PTSD and related co-morbidities are associated with a broad profile of functional and health-related impairments.8, 11–16 A series of investigations have reported an association between high PTSD symptom levels and the development of post-injury impairments in physical functioning.6, 12, 13, 17 In a nationwide U.S. study of injury survivors, PTSD and depression made an independent “dose” related contribution to the inability to return to work after surgical hospitalization; 39% of individuals with no disorder versus 67% of individuals with one disorder and 78% of individuals with both disorders had not returned to work 12 months after injury.13
A body of evidence now suggests that trauma-exposed individuals, including injured trauma survivors, may respond to early cognitive behavioral therapy (CBT) and pharmacologic interventions.18–22 Epidemiologic data, however, suggests that it may take months or years for trauma-exposed individuals with PTSD to enter treatment.23 Effective intervention models that serve to initially engage and then link injured trauma survivors to evidence-based PTSD services are therefore key in the early mental health response to trauma.19, 24
Large-scale randomized trials have established the effectiveness of collaborative care models that integrate care management, evidence-based pharmacotherapy, and CBT in the treatment of primary care medical patients with depressive and anxiety disorders.25–29 Few large-scale investigations have comprehensively targeted PTSD and related functional impairments among injured patients initially treated in inpatient trauma surgical settings.18, 30–32 Finally, no published investigations have assessed the effectiveness of stepped care interventions targeting PTSD for patients with TBI.
This investigation was a pragmatic randomized effectiveness trial designed to assess whether injured patients participating in a stepped collaborative care protocol would demonstrate reductions in the symptoms of PTSD, functional impairments, and co-morbid conditions when compared to patients assigned to a usual care control condition. We hypothesized that patients receiving the collaborative care intervention would demonstrate clinically and statistically significant reductions in the symptoms of PTSD over the course of the year after injury. We also hypothesized that intervention patients would demonstrate improved physical function as well as diminished depressive and alcohol use symptoms. A secondary hypothesis was that the intervention would be equally effective in patients with and without TBI.
METHODS
Design and Setting33
The University of Washington Institutional Review Board approved all study procedures prior to initiating the protocol. Between April 2006 and September 2009, injured trauma survivors admitted to the University of Washington’s Harborview Level I trauma center were approached at bedside for participation. After providing written informed consent, potential participants were screened twice for high PTSD symptom levels with the PTSD Checklist Civilian Version (PCL-C),34 once while surgical inpatients (median hospital days = 4, interquartile range (IQR) = 7), and again in the early days and weeks after hospital discharge (median days after index injury event = 13, IQR = 17), either in the outpatient surgery clinic or over the telephone. It was determined that with 100 patients randomized to each group, loss to follow-up of 20%, and a two-tailed α = 0.05, there would be > 80% power to detect an effect size of ≥0.4 when analyzing PTSD symptoms as a continuous variable.35
Two-hundred seven patients who screened positive at both time points were randomized to the stepped collaborative care intervention (n = 104) or usual care control (n = 103) conditions. All patients received evaluations of PTSD, depression, and alcohol consumption, as well as functional impairments and health service utilization, at baseline in the surgical ward before randomization, and again after randomization at one-, three-, six-, nine-, and twelvemonths post-discharge.
Participants
The investigators screened English-speaking women and men ages 18 and older, who presented to Harborview with injuries severe enough to require inpatient surgical admission. Patients were excluded who required immediate psychiatric intervention (i.e., self-inflicted injury, active psychosis), lived > 100 miles from the trauma center, were currently incarcerated, or who had recent histories of severe violence and were likely to face criminal charges.
Randomization
Randomization occurred in a 1:1 ratio according to a computer-generated random assignment sequence. A series of blocks of either 4 or 6 patients were generated using a random number generator. Research associates conducting all baseline screening assessments and follow-up interviews were blinded to block sizes and intervention or control status.33
Stepped Collaborative Care Intervention
Patients randomized to the intervention condition received care from a trauma center-based mental health team over the course of the 12 months post-injury. The intervention was designed to be feasibly delivered in the surgical inpatient ward, outpatient surgery clinics, and over the telephone, as patients re-engaged in primary care and community rehabilitation. The intervention included care management, as well as evidence-based pharmacotherapy and CBT components.33 The intervention team included masters in social work (MSW) and nurse practitioner (NP) care managers who delivered the intervention in surgical hospital and outpatient settings.
In the early days and weeks post-injury, intervention patients received continuous care management. Care managers coordinated care across surgical inpatient, primary care, and community service delivery settings. To engage injured trauma survivors, care managers first elicited and attempted to problem solve each patient’s unique constellation of post-injury concerns. Behavioral activation psychotherapy elements, such as pleasant activities scheduling targeting sadness related to loss of pre-injury function and post-injury anxious avoidance, were also part of routine post-injury care management.33, 36, 37
Care managers were trained in the delivery of evidence-based motivational interviewing (MI) intervention targeting problematic alcohol use and other high risk behaviors associated with injury recurrence.10 All intervention patients with positive blood alcohol concentration (BAC) results on admission, or who at any point during the trial demonstrated symptoms of alcohol abuse/dependence, received the MI intervention.
Patients randomized to the intervention condition discussed PTSD treatment preferences with the collaborative care team thereby informing the choice, timing, and delivery of medication, cognitive behavioral therapy, or combined treatment interventions. The medication intervention component aimed to initiate and maximize adherence to psychopharmacological treatments targeting PTSD and related disturbances, such as insomnia. The NP intervention team member prescribed medications with supervision by the study psychiatrist. Patients were advanced to and maintained on guideline-level therapeutic doses of Serotonin Specific Reuptake Inhibitor (SSRI) anti-depressant agents (e.g., sertraline 75–200mg, paroxetine 10–50mg).19 Patients were also started and advanced on medications targeting insomnia and related symptoms, such as posttraumatic nightmares (e.g., prazosin 1mg, trazadone 50mg).19, 38 The cognitive behavioral therapy intervention was delivered by trained MSW study team members and included psychoeducation, muscle relaxation, cognitive restructuring, and graded exposure.33, 39
The intervention was designed as a stepped care procedure. Symptoms were repeatedly measured in intervention patients and higher intensity care was available for patients with persistent or recurrent symptoms of PTSD. Care managers remained in contact with patients and used standardized scales to periodically reassess symptoms. Patient progression through the stepped care protocol was discussed during weekly measurement-based psychiatric supervision (DZ).
Preparatory training for the care management, behavioral activation, MI, CBT, and medication components consisted of a) didactics focusing on the treatment manual contents, b) role-plays with standardized patients, and c) individual preceptorship sessions with expert supervisors in MI (CD), CBT (AW), and pharmacotherapy (DZ). The collaborative care team maintained detailed clinical notes and charted the nature and duration of all intervention activities.
Usual Care Control Condition
Patients in the control condition underwent informed consent, PTSD screening, baseline surgical ward evaluation, and follow-up interviews. Prior investigation suggests that usual post-injury care includes routine outpatient surgical, and primary care visits, as well as the occasional use of specialty mental health services.31, 33
Assessments: PTSD Symptoms
Two measures were used to assess PTSD symptoms, the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist Civilian Version (PCL-C).34, 40 The CAPS was administered at the one-, six-, and twelve-month follow-up interviews either in person or over the telephone. For both measures a higher score indicates greater symptomatic distress. A cutoff of ≥ 45 and a rule requiring that symptoms occur at least monthly at the moderate intensity level were used to diagnose PTSD symptoms on the CAPS.40 Total PTSD remission defined as a score of < 20 and treatment response defined as a drop of ≥ 10 points were also ascertained with the CAPS.40
The PCL-C was used to screen injured trauma survivors with high PTSD symptom levels into the investigation and to follow PTSD symptoms longitudinally at the one-, three-, six-, nine, and twelve-month follow-up time points. A two-step screening procedure was employed. Patients were first screened as surgical inpatients. Patients with an initial PCL-C score of ≥35 in the surgical ward received a second PCL-C screen in the days and weeks immediately following hospital discharge either in outpatient surgery clinics or over the telephone. Patients who again screened high on the PCL-C with scores ≥35 were randomized into the study.
All research assistants (RAs) were trained in the administration of the interviews. Training included attending workshops, watching training CDs/videos, mock interviews, and observed practice interviews with hospitalized surgical inpatients. Additional training was required for the administration of the CAPS structured clinical interview. Practice CAPS interviews were conducted and RA interviewers received feedback and coaching until they consistently attained 100% reliability on CAPS PTSD criteria when compared to “gold standard” PhD expert raters. During the course of the study, 5% of CAPS interviews were audio-recorded and coded by expert raters. A pooled Kappa of 0.83 was attained for CAPS rating comparisons between study RAs and expert raters.
Assessments: Other Clinical and Injury Characteristics
The nine-item Patient Health Questionnaire (PHQ)41 was used to assess depressive symptoms. The Alcohol Use Disorders Identification Test-Consumption Items (AUDIT-C)42 were used to assess alcohol use. The Medical Outcomes Study Short Form 36 (MOS SF-36) Physical Component Summary (PCS)43 score was used to assess physical health and function. Prior trauma, PTSD symptoms, and pre-injury alcohol abuse/dependence were assessed with items from the National Co-morbidity Survey investigations.44 Previously developed items assessing post-injury health service use were administered at the baseline and at the one-, three-, six-, nine-, and twelve-month follow-up interviews.33 Interview items contained self-report descriptions of current medication usage (i.e., name, dosage, duration). We derived from these items the number of anti-depressant prescriptions, attainment of guideline dosages, and adequate adherence to guideline level doses for ≥ 25 or more days. Other medication categories included medications targeting insomnia related to PTSD (i.e., prazosin or trazadone). The number of psychotherapy visits was also assessed at each follow-up time point. Finally, items assessing satisfaction with general health care services and emotional health care services were included in all interviews.33
The investigation determined injury severity at baseline during the index admission from the medical record International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) Codes using the Abbreviated Injury Scale and Injury Severity Score.45 TBI was also prospectively identified in the medical record and categorized by the following ICD-9-CM codes: 800.0–801.9, 803.0–804.9, 850.0–854.1, and 959.01.8, 46 TBI severity was coded based on a previously validated algorithm for hospitalized inpatients.8, 46 Laboratory toxicology results, insurance status, length of hospital and intensive care unit stays, and other clinical characteristics were abstracted from the trauma registry and electronic medical record.
Statistical Analyses
We first examined the intervention and control groups for differences in baseline demographic and clinical characteristics using chi-square for categorical variables and t-tests for continuous variables. We also examined patients with and without complete longitudinal data for differences in baseline characteristics.
Our primary analyses evaluated the symptoms of PTSD longitudinally over the course of the year after injury in the intent-to-treat sample of 207 randomized patients. The symptoms of related conditions including depression and alcohol use, physical function, and health service utilization outcomes were also examined in longitudinal intent to treat analyses. To determine if patients in the two groups manifested different patterns of change in PTSD symptoms and other outcomes over the year after injury, we used mixed effects random coefficient regression models employing SAS PROC MIXED for continuous variables and SAS PROC GLIMMIX for categorical variables.
In the models examining the PCL-C, PHQ-9, AUDIT-C, and PCS, repeated measurements of the continuous scale scores at the baseline, one-, three-, six-, nine-, and twelvemonth time points were the dependent variables. For the models examining CAPS PTSD outcomes, the dependent variables were one-, six-, and twelve-month repeated measurements of the continuous CAPS total score and the dichotomized CAPS diagnosis, remission, and treatment response outcomes. Mixed model regression was also used to determine if intervention patients had differential patterns of psychotropic medication usage, psychotherapy visits, or satisfaction with care over the course of the year post-injury.
For all dependent variables we first fit models containing time categories, intervention, intervention by time interactions, and baseline symptomatic or functional status with and without covariates. Our final models adjusted for characteristics that significantly differed across groups either at baseline or longitudinally; the baseline characteristics included in all adjusted models as covariates were marital status, employment, insurance status, and years of education. For all regression models in the event of a non-significant interaction, the interaction term was removed and the models refit. In the event of a significant interaction or main effect of group we examined adjusted simple main effects at each time point. In order to examine the impact of TBI on any observed treatment effects, we fit additional models that included the presence or absence of TBI injuries and their interaction with treatment group and time.
Finally, we performed sensitivity analyses. We included age, gender, and injury severity as additional covariates in all longitudinal outcome analyses, as these had been essential design variables in prior clinical studies.5, 14 We used SAS version 9.2 (SAS Institute) for all analyses.
RESULTS
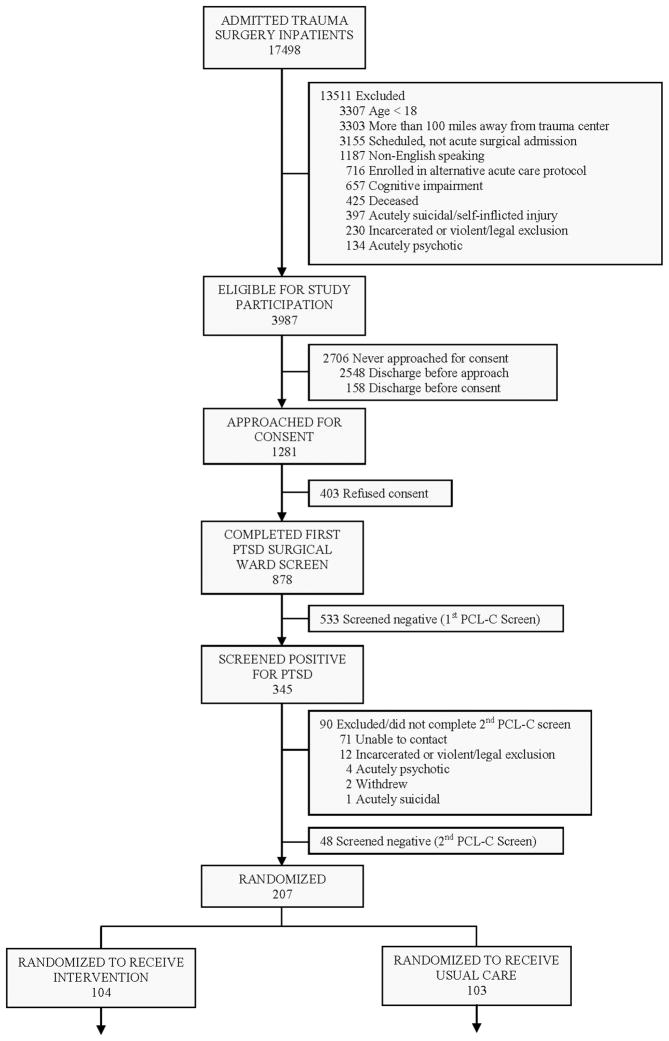
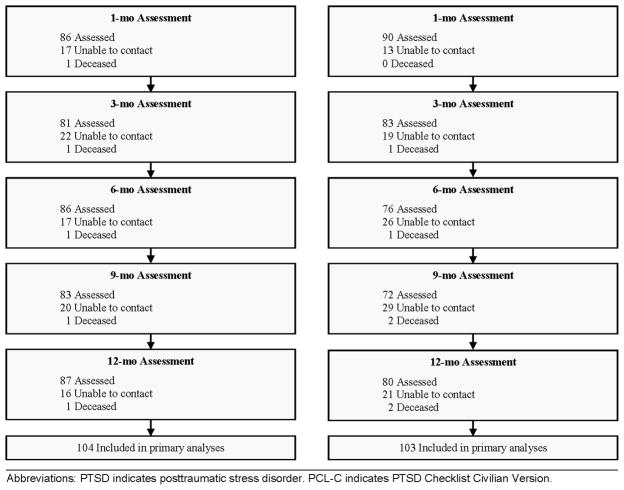
Compared to all other patients admitted to the trauma center during the time period of the investigation, the 207 study patients were more likely to be female, less severely injured, intentionally injured, blood alcohol positive, younger, and had greater length of surgical hospitalization. The investigation achieved ≥ 75% follow-up at each assessment point (Figure 1).
Figure 1.
Patient Flow Through the Trial
The 207 injured surgical inpatients recruited and randomized into the investigation were diverse, predominantly publically insured or uninsured patients with high levels of PTSD, depressive and alcohol use symptoms, and substantial histories of pre-injury trauma (Table 1). Patients in the intervention and control conditions did not substantially differ with regard to the percentage and distribution of TBI (Table 1).
TABLE 1.
Baseline Patient Characteristics
| Characteristics | No. (%) of Patients
|
||
|---|---|---|---|
| All (N = 207) | Intervention (n = 104) | Usual Care (n = 103) | |
| Demographic | |||
| Age, mean (SD), years | 38.5 (13.1) | 39.4 (13.4) | 37.7 (12.8) |
| Female | 99 (47.8) | 54 (51.9) | 45 (43.7) |
| Race/ethnicity | |||
| White | 124 (59.9) | 63 (60.6) | 61 (59.2) |
| Black | 38 (18.4) | 21 (20.2) | 17 (16.5) |
| American Indian | 27 (13.0) | 14 (13.4) | 13 (12.6) |
| Asian | 12 (5.8) | 4 (3.9) | 8 (7.8) |
| Hispanic | 6 (2.9) | 2 (1.9) | 4 (3.9) |
| Education, mean (SD), years | 13.1 (2.2) | 13.2 (2.5) | 12.9 (1.9) |
| Marital status* | |||
| Married/living with partner | 52 (25.1) | 37 (35.6) | 15 (14.6) |
| Employed | 96 (46.4) | 43 (41.3) | 53 (51.5) |
| Insurance | |||
| Private | 45 (21.7) | 22 (21.1) | 23 (22.3) |
| Public | 111 (53.6) | 58 (55.8) | 53 (51.5) |
| None | 51 (24.6) | 24 (23.1) | 27 (26.2) |
| Acute care injury & medical | |||
| Injury severity category | |||
| 0–8 | 46 (22.2) | 21 (20.2) | 25 (24.3) |
| 9–15 | 93 (44.9) | 50 (48.1) | 43 (41.7) |
| ≥ 16 | 68 (32.9) | 33 (31.7) | 35 (34.0) |
| Traumatic brain injury | |||
| None | 128 (61.8) | 64 (61.5) | 64 (62.1) |
| Mild | 55 (26.6) | 29 (27.9) | 26 (25.3) |
| Moderate/severe | 24 (11.6) | 11 (10.6) | 13 (12.6) |
| Intentional injury | 47 (22.7) | 24 (23.1) | 23 (22.3) |
| Number of co-morbid medical conditions | |||
| 0 | 126 (60.9) | 56 (53.8) | 70 (68.0) |
| 1 | 47 (22.7) | 27 (26.0) | 20 (19.4) |
| 2 | 20 (9.6) | 11 (10.6) | 9 (8.7) |
| ≥ 3 | 14 (6.8) | 10 (9.6) | 4 (3.9) |
| Days in intensive care unit | |||
| 0 | 137 (66.2) | 76 (73.0) | 61 (59.2) |
| 1 | 37 (17.9) | 14 (13.5) | 23 (22.3) |
| ≥ 2 | 33 (15.9) | 14 (13.5) | 19 (18.5) |
| Days in hospital, mean (SD) | 9.0 (10.4) | 8.4 (7.7) | 9.5 (12.6) |
| Clinical | |||
| Serious prior traumas before injury admission, mean (SD) | 5.7 (2.8) | 5.9 (2.8) | 5.5 (2.8) |
| Pre-injury PTSD symptoms | 130 (62.8) | 66 (63.5) | 64 (62.1) |
| Baseline 1st PCL-C Screen total score, mean (SD)† | 50.6 (10.5) | 50.5 (10.5) | 50.8 (10.5) |
| Baseline PHQ-9 depression total score, mean (SD)† | 13.9 (5.5) | 13.7 (5.8) | 14.1 (5.2) |
| Pre-injury alcohol abuse or dependence | 74 (36.3) | 36 (35.6) | 38 (36.9) |
| Pre-injury AUDIT-C score, mean (SD) | 3.4 (3.3) | 3.0 (2.9) | 3.8 (3.6) |
| Positive blood alcohol on admission | 73 (35.3) | 30 (28.8) | 43 (41.7) |
| Urine toxicology screen positive for stimulants (amphetamine and/or cocaine) | 23 (11.1) | 14 (13.5) | 9 (8.7) |
| Urine toxicology screen positive for marijuana | 34 (16.4) | 20 (19.2) | 14 (13.6) |
P < 0.05. All other comparisons were not statistically significant.
For PCL-C and PHQ-9 baseline assessments inpatients were asked to report symptoms since the injury event.
Abbreviations: AUDIT-C, The Alcohol Use Disorders Identification Test – Consumption Items; PCL-C, PTSD Checklist Civilian Version; PHQ, Patient Health Questionnaire; PTSD, posttraumatic stress disorder; SD, standard deviation.
Intervention Implementation
Care managers spent a median of 13.2 hours (IQR = 13.3 hours) with each patient over the course of the year after injury. Care manager time intensity in the stepped care procedure gradually decreased over the course of the year. Over 60% of care management activity occurred during the first six months post-injury (Median hours = 9.4, IQR = 8.6 hours). Eighty-four (81%) of the 104 intervention patients received NP medication evaluations. Seventy-eight (75%) of the 104 intervention patients were offered PTSD pharmacotherapy; 48 (62%) of these 78 patients accepted and maintained their medication regimes during the study. The majority (75%) of NP intervention hours also occurred during the first six months after the injury. Eighty intervention patients (77%) received one or more MI session targeting alcohol use and other high risk behaviors. Ninety-three (89%) of the 104 intervention patients were assessed for multisession CBT. Twenty-five of these patients demonstrated adequate CBT readiness,33 and were offered CBT; nine (36%) of these 25 patients received four or more CBT sessions.
Process of Care
When compared to control patients, collaborative care intervention patients were significantly more likely to receive evidence-based PTSD pharmacotherapy. Intervention patients were significantly more likely to have received an adequate dosage of anti-depressant medication (adjusted main effect, Odds Ratio (OR) = 2.32, 95% Confidence Interval (CI) = 1.20, 4.50). Intervention patients were also significantly more likely to use PTSD insomnia medications (adjusted group by time interaction, Wald’s χ2 = 19.57, P < .001). There were no observed significant group, time, or group by time effects for pain medications, benzodiazepines, or psychotherapy visits over the course of the year after injury.
Intervention patients were significantly more likely to report being very satisfied with their general health care (adjusted main effect, OR = 2.00, 95% CI = 1.01, 3.96) and emotional health care services (adjusted main effect, OR = 2.93, 95% CI = 1.84, 4.67) when compared to control patients.
Symptomatic and Functional Outcomes
The intervention group demonstrated clinically and statistically significant reductions in the symptoms of PTSD over the course of the year after injury (Figure 2). Regression analyses demonstrated significant CAPS (adjusted F (2, 185) = 5.50, P < 0.01), and PCL-C (adjusted F(4, 185) = 5.45, P < 0.001) group by time interaction effects over the course of the year. The intervention also achieved a statistically and clinically significant impact on CAPS PTSD treatment response criteria over the course of the year after injury (adjusted main effect, OR = 1.93, 95% CI = 1.00, 3.70). PTSD remission criteria also demonstrated significant reductions over the course of the year (adjusted group by time interaction, F (2,306) = 5.41, P < 0.01). No significant treatment effects were observed for PTSD diagnostic criteria over the course of the year (adjusted main effect, OR = 1.39, 95% CI = 0.77, 2.51).
Figure 2.
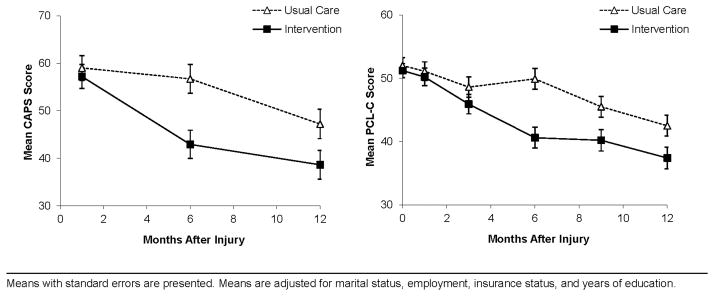
PTSD Symptom Severity by Treatment Group on the Clinician-Administered PTSD Scale (CAPS) and PTSD Checklist Civilian Version (PCL-C).
The intervention achieved clinically and statistically significant symptom reductions on the CAPS and PCL-C continuous PTSD symptom scores at the six-, nine-, and twelve-month post-injury time points (Table 2). Effect sizes for PTSD were temporally associated with treatment intensity, and peaked at the six-month post-injury time point; effect sizes at the six-and twelve-month time points ranged from 0.32 – 0.65 (Table 2).
TABLE 2.
Symptomatic and Functional Outcomes for Patients in Collaborative Care (n = 104) Versus Usual Care Control (n = 103) Conditions
| Outcome Assessment | Baseline
|
1 Month
|
3 Months
|
6 Months
|
9 Months
|
12 Months
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean† (95% CI) | Mean† (95% CI) | Mean† (95% CI) | Mean† (95% CI) | Mean† (95% CI) | Mean† (95% CI) | ||||||||||
|
| |||||||||||||||
| CC | UC | CC | UC | ES§ | CC | UC | CC | UC | ES§ | CC | UC | CC | UC | ES§ | |
| CAPS‡ | ---- | 57.2 (52.3,62.2) | 59.0 (53.9,64.2) | 0.08 | ---- | 42.9 (37.0,48.8) | 56.7** (50.7,62.7) | 0.53 | ---- | 38.6 (32.5,44.6) | 47.2* (41.2,53.3) | 0.32 | |||
| PCL-C‡,¶ | 51.2 (48.9,53.4) | 52.0 (49.5,54.5) | 50.2 (47.4,52.9) | 51.1 (48.2,54.0) | 0.07 | 45.9 (42.9,48.9) | 48.6 (45.5,51.8) | 40.6 (37.3,43.9) | 49.9** (46.6,53.1) | 0.65 | 40.2 (36.8,43.5) | 45.5** (42.2,48.7) | 37.4 (34.0,40.7) | 42.5* (39.3,45.7) | 0.34 |
| PHQ-9‡,¶ | 13.4 (12.3,14.6) | 14.2 (13.0,15.5) | 12.5 (11.3,13.7) | 13.2 (11.9,14.5) | 0.12 | 11.7 (10.5,13.0) | 13.0 (11.5,14.5) | 8.7 (7.4,10.1) | 11.3** (9.9,12.8) | 0.43 | 9.7 (8.3,11.2) | 11.4 (9.9,12.9) | 8.4 (7.1,9.7) | 10.1 (8.6,11.7) | 0.26 |
| AUDIT-C‡,|| | 3.1 (2.5,3.8) | 3.9 (3.2,4.6) | 1.4 (1.0,1.8) | 1.9 (1.2,2.5) | 0.19 | 1.9 (1.4,2.4) | 2.7* (2.0,3.4) | 2.0 (1.4,2.6) | 2.8 (2.1,3.5) | 0.28 | 2.4 (1.8,3.0) | 2.6 (1.9,3.2) | 2.0 (1.5,2.6) | 2.4 (1.8,3.0) | 0.13 |
| SF-36 PCS‡,|| | 49.1 (46.5,51.7) | 50.6 (47.8,53.4) | 34.6 (32.0,37.3) | 32.4* (29.9,34.9) | 0.32 | 39.0 (36.3,41.7) | 34.8** (32.4,37.2) | 42.4 (39.6,45.3) | 37.8** (35.2,40.4) | 0.56 | 43.2 (40.2,46.1) | 39.8* (37.2,42.5) | 43.7 (41.0,46.5) | 41.2 (38.5,43.9) | 0.26 |
Means are adjusted for marital status, employment, insurance status, and years of education; t-tests were used to compare the symptoms and functional status of intervention and control groups.
P value
<0.05;
<0.01.
Effect sizes were calculated as the difference in group means divided by the pooled standard deviation.
For PCL-C and PHQ-9 baseline assessments patients were asked to report symptoms since the injury event.
For AUDIT-C and SF-36 PCS baseline assessments patients were asked to report symptoms prior to the injury event.
Abbreviations: AUDIT-C, The Alcohol Use Disorders Identification Test – Consumption Items; CAPS, Clinician-Administered PTSD Scale; CC, collaborative care; CI, confidence interval; ES, effect size; PCL-C, PTSD Checklist Civilian Version; PHQ-9, Patient Health Questionnaire 9 Item Depression Scale; SF-36 PCS, Medical Outcomes Study Short Form Health Survey 36 Physical Component Summary Score; UC, usual care.
Intervention patients demonstrated significant improvements in physical function (adjusted β = 3.10, SE = 0.99, P < 0.01) over the course of the year. The intervention effects for physical function achieved statistical significance at the three-, six-, and nine-month post-injury time points (Table 2).
For alcohol consumption (adjusted β = −0.52, SE = 0.30, P = 0.08) and depression (adjusted β = −1.4, SE = 0.76, P = 0.07), trend level effects were observed. Regression revealed that patients with TBI responded equally well to the stepped care intervention as those without TBI. Analyses revealed no significant interaction of TBI by treatment group for PTSD, physical function, or other conditions. Finally, sensitivity analyses did not substantially alter the magnitude, pattern, or significance of the observed treatment effects.
DISCUSSION
This investigation documents the effectiveness of a stepped collaborative care intervention in reducing PTSD symptoms in surgically hospitalized trauma survivors over the course of the year after physical injury. Intervention patients had a significantly more favorable course of PTSD recovery over the year, as evidenced by diminished symptom severity. PTSD treatment effects were temporally associated with intervention intensity and were optimized at the six-month post-injury time point. At the twelve-month post-injury study endpoint intervention patients continued to demonstrate clinically and statistically significant reductions in PTSD symptoms when compared to usual care control patients. Intervention patients received higher quality posttraumatic care that included more frequent prescriptions for adequate dosages of evidence-based PTSD pharmacotherapy and also greater satisfaction with general medical and emotional health care services.
A series of prior descriptive investigations have established an association between PTSD symptoms and a broad spectrum of functional impairment, including role and physical limitations.6, 12, 13, 15, 17 To our knowledge, this is the first clinical trial with acutely injured trauma survivors to demonstrate improvements in physical function among patients receiving stepped collaborative care interventions. A substantial proportion of the low-income, diverse surgical inpatients recruited into the trial were not working prior to the injury event; therefore we were not able to directly target return to work as part of the stepped collaborative care intervention procedure.
Literature review suggests that this is the first stepped collaborative care trial to specifically target TBI patients with PTSD and related co-morbidities. The intervention appears to have been equally effective in reducing PTSD symptoms, and physical limitations, among injured patients with and without TBI.
One large-scale primary care collaborative trial targeting PTSD and related anxiety disorders found six- and twelve-month PTSD treatment effects comparable to the current investigation.47 The PTSD treatment effects observed in the current investigation are towards the middle end of the continuum reported for effective multisession early psychological PTSD interventions developed in efficacy trials.21 A possible explanation for this observation is that in order to enhance the generalizability of the investigative results to populations of surgically hospitalized patients, this pragmatic trial included subjects regardless of patient characteristics that are typically excluded in many PTSD clinical trials including injury co-morbidity, concurrent active substance abuse/dependence, and histories of multiple prior traumas. A meta-analysis of 37 primary care intervention trials reported comparable or lower collaborative care treatment effects for depression (0.25; 95% CI = 0.18, 0.31),29 as was observed for PTSD in the current trial twelve months after injury.
The stepped care intervention approach implemented in this trial suggests that primary reliance upon lower intensity treatments embedded within care management procedures, yields the greatest potential overall population impact.48, 49 Early stepped care intervention components readily deliverable to large populations of trauma center patients include problem solving around each patients unique constellation of post-injury concerns and behavioral activation elements embedded within routine post-injury care management.36 Similarly, early pharmacologic intervention targeting initial PTSD related insomnia may have greater potential breadth of applicability, treatment effects, and overall population impact, when compared to sustained treatment with SSRI pharmacologic agents.22, 38 More intensive and challenging to deliver procedures such as session-based cognitive behavioral therapies, could be reserved for smaller subgroups of injured patients with recalcitrant or recurrent PTSD symptoms.36, 50 Future pragmatic trials of stepped care interventions targeting PTSD could also test whether intervention elements with greater breadth of applicability could be delivered earlier on after the injury with sustained six- and twelve-month treatment effects.
There are important considerations in interpreting the results of this investigation. Because this was a multifaceted intervention, the study did not yield information regarding which components of the stepped care procedure were most influential in reducing symptoms and improving function. Aspects of the stepped care intervention including the two-staged PTSD screening procedure and the intensive care management treatment protocol may be too resource intensive to ever have broad reach across US trauma centers; subsequent investigation could develop more efficient screening and stepped care intervention procedures with greater breadth of applicability and overall population impact.48, 51 An additional limitation of the study is that the investigation did not follow-up with injured patients who had initial low symptom scores on the PCL-C. Prior investigations suggest that early PTSD screening efforts may miss some individuals who develop high PTSD symptom levels in the months after the event.52 Finally, the TBI results can be seen as preliminary, as the overall sample size combined with a rate of TBI of approximately 40% limited our ability to assess TBI interaction effects.
Beyond these considerations, this investigation contributes to an evolving literature on early multidisciplinary PTSD interventions for injured trauma survivors treated initially in surgical inpatient settings and that require the establishment of continuous care through surgical and primary care outpatient services.18 With regard to the trauma center context, the investigation integrated many components of practical clinical trials.53 This included the recruitment of a diverse clinical sample from a Level I trauma center practice setting, intervention delivery by social work and nurse practitioner acute care providers, and the measurement of a broad range of policy relevant outcomes.54 The American College of Surgeons has demonstrated the capacity to mandate screening and intervention procedures for alcohol use problems at US trauma centers based on the results of empiric investigations.10, 54, 55 By establishing the feasibility and effectiveness of the stepped collaborative care model in diminishing PTSD symptoms and improving post-injury physical function, the investigative findings could influence the development of guidelines for the sustainable implementation of PTSD screening and intervention procedures at trauma centers nationwide.54
Acknowledgments
Support/Funding: This work was supported by National Institute of Mental Health Grants R01/MH073613 and K24/MH086814 (Dr. Zatzick).
The investigators would like to acknowledge the contribution of Kari Stephens, PhD, in the rating of the clinical interviews and Jeffrey Love, BA, Melanie-Kukana Maglaya, BS, and Stephanie O’Malley, BA, for their contribution to the preparation of the manuscript, tables, and figures for submission and publication. All four individuals acknowledged are currently employed with the University of Washington in the Department of Psychiatry and Behavioral Sciences and received no additional compensation for their work on the manuscript.
Biographies
Douglas Zatzick is responsible for conception and design of the study as well as the data analyses. He was directly involved in all drafting and revising of the manuscript as well as gave the final approval for submission.
Gregory Jurkovich made a substantial contribution to the acquisition of data for the project. He was also involved in the drafting and revising of the manuscript and gave his approval to the final submission.
Frederick Rivara made a substantial contribution to the analysis and interpretation of the data. He was also involved in the revising of the manuscript and gave his approval to the final submission.
Joan Russo made a substantial contribution to the analysis and interpretation of the data. She was also involved in the drafting and revising of the manuscript and gave her approval to the final submission.
Amy Wagner made a substantial contribution to the acquisition of data for the project. She was also involved in the drafting of the manuscript and gave her approval to the final submission.
Jin Wang made a substantial contribution to the analysis and interpretation of the data. She was also involved in the drafting and revising of the manuscript and gave her approval to the final submission.
Chris Dunn made a substantial contribution to the concept and design of the study. He was also involved in the drafting of the manuscript and gave his approval to the final submission.
Sarah Peregrine Lord made a substantial contribution to the acquisition of data for the project. She was also involved in the drafting and revising of the manuscript and gave her approval to the final submission.
Megan Petrie made a substantial contribution to the acquisition of data for the project. She was also involved in the drafting of the manuscript and gave her approval to the final submission.
Stephen O’Connor made a substantial contribution to the interpretation of the data. He was also involved in the revising of the manuscript and gave his approval to the final submission.
Wayne Katon made a substantial contribution to the conception and design of the study. He was also involved in the drafting and revising of the manuscript and gave his approval to the final submission.
References
- 1.Rivara FP, Grossman DC, Cummings P. Injury prevention. First of two parts. N Engl J Med. 1997;337(8):543–548. doi: 10.1056/NEJM199708213370807. [DOI] [PubMed] [Google Scholar]
- 2.Bonnie RJ, Fulco CE, Liverman CT. Reducing the burden of injury: advancing prevention and treatment. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 3.Bergen GS, National Center for Health Statistics (U.S.) Injury in the United States : 2007 chartbook. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2008. [Google Scholar]
- 4.Shih RA, Schell TL, Hambarsoomian K, et al. Prevalence of posttraumatic stress disorder and major depression after trauma center hospitalization. J Trauma. 2010;69(6):1560–1566. doi: 10.1097/TA.0b013e3181e59c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zatzick D, Rivara FP, Nathens AB, et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med. 2007;37(10):1469–1480. doi: 10.1017/S0033291707000943. [DOI] [PubMed] [Google Scholar]
- 6.Holbrook TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: 12-month and 18-month follow-up results from the trauma recovery project. J Trauma. 1999;46(5):765–773. doi: 10.1097/00005373-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bryant RA, O’Donnell ML, Creamer M, et al. The psychiatric sequelae of traumatic injury. Am J Psychiatry. 2010;167(3):312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 8.Zatzick D, Rivara F, Jurkovich G, et al. Multi-site investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Arch Gen Psychiatry. 2010;67(12):1291–1300. doi: 10.1001/archgenpsychiatry.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoge C, McGurk D, Thomas J, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 10.Gentilello LM, Rivara FP, Donovan DM, et al. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230(4):473–480. doi: 10.1097/00000658-199910000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbrook TL, Hoyt DB, Coimbra R, et al. Long-term posttraumatic stress disorder persists after major trauma in adolescents: New data on risk factors and functional outcome. J Trauma. 2005;58(4):764–769. doi: 10.1097/01.ta.0000159247.48547.7d. [DOI] [PubMed] [Google Scholar]
- 12.Ramchand R, Marshall GN, Schell TL, et al. Posttraumatic distress and physical functioning: A longitudinal study of injured survivors of community violence. J Consult Clin Psychol. 2008;76(4):668–676. doi: 10.1037/0022-006X.76.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zatzick D, Jurkovich G, Fan MY, et al. The association between posttraumatic stress and depressive symptoms, and functional outcomes in adolescents followed longitudinally after injury hospitalization. Arch Pediatr Adolesc Med. 2008;162(7):642–648. doi: 10.1001/archpedi.162.7.642. [DOI] [PubMed] [Google Scholar]
- 14.Zatzick D, Jurkovich G, Rivara F, et al. A national US study of posttraumatic stress disorder, depression, and work and functional outcomes after injury hospitalization. Ann Surg. 2008;248(3):429–437. doi: 10.1097/SLA.0b013e318185a6b8. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell ML, Holmes AC, Creamer MC, et al. The role of post-traumatic stress disorder and depression in predicting disability after injury. Med J Aust. 2009;190(7 Suppl):S71–74. doi: 10.5694/j.1326-5377.2009.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 16.deRoon-Cassini TA, Mancini AD, Rusch MD, et al. Psychopathology and resilience following traumatic injury: A latent growth mixture model analysis. Rehabil Psychol. 2010;55(1):1–11. doi: 10.1037/a0018601. [DOI] [PubMed] [Google Scholar]
- 17.Michaels AJ, Michaels CE, Moon CH, et al. Posttraumatic stress disorder after injury: Impact on general health outcome and early risk assessment. J Trauma. 1999;47(3):460–467. doi: 10.1097/00005373-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JC, deRoon-Cassini TA, Brasel KJ. Posttraumatic stress disorder: a primer for trauma surgeons. J Trauma. 2010;69(1):231–237. doi: 10.1097/TA.0b013e3181e16e2a. [DOI] [PubMed] [Google Scholar]
- 19.Ursano RJ, Bell C, Eth S, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. American Psychiatric Association; 2004. [PubMed] [Google Scholar]
- 20.Holbrook TL, Galarneau MR, Dye JL, et al. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 21.Roberts NP, Kitchiner NJ, Kenardy J, et al. Multiple session early psychological interventions for the prevention of post-traumatic stress disorder. Cochrane Database Syst Rev. 2009;(3):CD006869. doi: 10.1002/14651858.CD006869.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Shalev AY, Ankri YL, Israeli-Shalev Y, et al. Prevention of posttraumatic stress disorder by early treatment: Results from the Jerusalem Trauma Outreach and Prevention Study. Arch Gen Psychiatry. 2012;69(2):166–176. doi: 10.1001/archgenpsychiatry.2011.127. [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Mental Health. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence: a workshop to reach consensus on best practices. Bethesda, MD, Washington, D.C: National Institute of Mental Health : U.S. Government Printing Office; 2002. [Google Scholar]
- 25.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 27.Wells KB, Sherbourne C, Schoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. JAMA. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 28.Roy-Byrne P, Craske MG, Sullivan G, et al. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: A randomized controlled trial. JAMA. 2010;303(19):1921–1928. doi: 10.1001/jama.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 30.Zatzick D, Roy-Byrne P, Russo J, et al. Collaborative interventions for physically injured trauma survivors: A pilot randomized effectiveness trial. Gen Hosp Psychiatry. 2001;23(3):114–123. doi: 10.1016/s0163-8343(01)00140-2. [DOI] [PubMed] [Google Scholar]
- 31.Zatzick D, Roy-Byrne P, Russo J, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61(5):498–506. doi: 10.1001/archpsyc.61.5.498. [DOI] [PubMed] [Google Scholar]
- 32.Kassam-Adams N, Felipe Garcia-Espana J, Marsac ML, et al. A pilot randomized controlled trial assessing secondary prevention of traumatic stress integrated into pediatric trauma care. J Trauma Stress. 2011;24(3):252–259. doi: 10.1002/jts.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zatzick D, Rivara F, Jurkovich G, et al. Enhancing the population impact of collaborative care interventions: Mixed method development and implementation of stepped care targeting posttraumatic stress disorder and related comorbidities after acute trauma. Gen Hosp Psychiatry. 2011;33(2):123–134. doi: 10.1016/j.genhosppsych.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weathers F, Ford J. Psychometric review of PTSD Checklist (PCL-C, PCL-S. PCL-M, PCL-PR) In: Stamm B, editor. Measurement of stress, trauma, and adaptation. Lutherville: Sidran Press; 1996. pp. 250–251. [Google Scholar]
- 35.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 36.Geiss Trusz S, Wagner A, Russo J, et al. Assessing barriers to care and readiness for cognitive behavioral therapy in early acute care PTSD interventions. Psychiatry. 2011;74(3):207–223. doi: 10.1521/psyc.2011.74.3.207. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AW, Zatzick DF, Ghesquiere A, et al. Behavioral activation as an early intervention for posttraumatic stress disorder and depression among physically injured trauma survivors. Cogn Behav Pract. 2007;14(4):341–349. [Google Scholar]
- 38.Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Bryant RA, Harvey AG, Dang ST, et al. Treatment of acute stress disorder: A comparison of cognitive-behavioral therapy and supportive counseling. J Consult Clin Psychol. 1998;66(5):862–866. doi: 10.1037//0022-006x.66.5.862. [DOI] [PubMed] [Google Scholar]
- 40.Weathers F, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babor TF, De La Fuente JR, Saunders J, et al. The alcohol use disorders identification test: guidelines for use in primary health care. Geneva: World Health Organization; 1989. [Google Scholar]
- 43.Ware JE, Snow KK, Kosinski M. SF-36 health survey: manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 44.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 45.Johns Hopkins Health Services Research and Development Center. Determining injury severity from hospital discharges: A program to map ICD-9-CM diagnoses into AIS and ISS severity scores. The Johns Hopkins University Press; 1989. [Google Scholar]
- 46.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282(10):954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 47.Craske MG, Stein MB, Sullivan G, et al. Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Arch Gen Psychiatry. 2011;68(4):378–388. doi: 10.1001/archgenpsychiatry.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koepsell TD, Zatzick DF, Rivara FP. Estimating the population impact of preventive interventions from randomized trials. Am J Prev Med. 2011;40(2):191–198. doi: 10.1016/j.amepre.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zatzick D, Koepsell T, Rivara FP. Using target population specification, effect size, and reach to estimate and compare the population impact of two PTSD preventive interventions. Psychiatry. 2009;72(4):346–359. doi: 10.1521/psyc.2009.72.4.346. [DOI] [PubMed] [Google Scholar]
- 50.Shalev AY, Ankri YL, Peleg T, et al. Barriers to receiving early care for PTSD: results from the Jerusalem trauma outreach and prevention study. Psychiatr Serv. 2011;62(7):765–773. doi: 10.1176/ps.62.7.pss6207_0765. [DOI] [PubMed] [Google Scholar]
- 51.Lagomasino IT, Zatzick DF, Chambers DA. Efficiency in mental health practice and research. Gen Hosp Psychiatry. 2010;32(5):477–483. doi: 10.1016/j.genhosppsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163(10):1777–1783. doi: 10.1176/ajp.2006.163.10.1777. [DOI] [PubMed] [Google Scholar]
- 53.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 54.American College of Surgeons Committee on Trauma. Resources for the optimal care of the injured patient: 2006. Chicago: American College of Surgeons Committee on Trauma; 2006. [Google Scholar]
- 55.Terrell F, Zatzick DF, Jurkovich GJ, et al. Nationwide survey of alcohol screening and brief intervention practices at US Level I trauma centers. J Am Coll Surg. 2008;207(5):630–638. doi: 10.1016/j.jamcollsurg.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]