Abstract
Objective
Characterization of clinical heterogeneity in ADHD remains controversial. Neuropsychological and cognitive studies provides one type of validation data, but too often has considered only a narrow range of functional domains.
Method
The current study examined ADHD subtype and presentation differences across a broad range of neurocognitive domains in a large clinically-characterized, community-recruited sample of 498 youth (213 control, 107 ADHD-Primarily Inattentive (ADHD-PI), 137 ADHD-Combined (ADHD-C)), ages 6–17 years. Domains assessed included executive functions, working memory, arousal, processing speed, response variability, and temporal information processing.
Results
Youth with ADHD-C performed worse than youth with ADHD-PI in all domains, consistent with a severity model. Performance among a subgroup with a “restrictive inattentive” presentation indicated potential deficits in processing speed relative to other ADHD-PI youth, but no other effects. When all measures were included in the same model, cognitive control (executive functions, working memory, and memory span), arousal, and response variability each provided uniquely incremental statistical prediction of specific symptom dimensions and of subtype/presentation, but temporal information processing and processing speed did not.
Conclusion
Results suggest the potential to consolidate multiple neurocognitive theories of ADHD, and that such consolidation will apply across putative clinical subtypes or presentations.
Keywords: attention-deficit hyperactivity disorder, subtypes, neuropsychological functioning, symptom dimensions
Attention-deficit hyperactivity disorder (ADHD) is a common, costly, and impairing syndrome characterized by age-inappropriate difficulties with overactivity, impulsivity, inattention and disorganization. The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 2004) specified three subtypes of ADHD: Primarily Inattentive (ADHD-PI), Primarily Hyperactive-Impulsive (ADHD-PH) and Combined (ADHD-C). While these subtype distinctions may have some clinical utility, the etiological relationships between the subtypes as well as their stability over time remains in question (Lahey et al., 2005; Milich, Balentine, & Lynam, 2001; Willcutt et al, in press). As a result, DSM-5 has proposed to retain these designations but downgrade them to specifiers or presentations, and to add a fourth presentation for restrictive-inattentive. This presentation, as proposed at this writing, would be characterized by the presence of 6 or more inattention symptoms, with 2 or fewer symptoms of hyperactivity-impulsivity (http://www.dsm5.org, accessed November 2011). Neuropsychological approaches can provide a potentially useful method for examining the validity of these designations. In particular, as noted by Willcutt et al. (in press), it may be that subtypes or presentations are essentially severity variations, rather than distinct configurations or etiologies. The configuration of neuropsychological scores can help evaluate that argument (see Goth-Owens et al., 2010). However, outside of meta-analyses and reviews, few new investigations have examined multiple (rather than one or two) domains of neuropsychological functioning as a method for exploring subtype validity. In turn, confirmation of patterns of neuropsychological weakness among youth with ADHD can also provide clues to pathophysiology of the disorder and involvement of particular psychological processes as well as neural systems.
Due to the utility of neuropsychological approaches for examining questions of validity such studies of ADHD have proliferated (for one review, see Willcutt et al., 2005). However, a key weakness in the neuropsychological literature concerning ADHD, and particularly regarding performance among the DSM-IV subtypes, has been an excess of single-process hypotheses and studies (Nigg, 2006). Further, failure to consider multi-process hypotheses have thus far prevented more in-depth examination regarding the validity of a categorical versus dimensional view of ADHD. Further validation of the structure of ADHD via measures of neuropsychological functioning may be particularly relevant in light of recent taxometric work highlighting the dimensional structure of ADHD (Marcus & Barry, 2011). If subtypes are essentially arrayed on a severity dimension, then this would undercut further the idea of categorical variation in the disorder. But if subtypes have unique configurations of neuropsychological patterns, this would tend to argue for some degree of categorical variation.
Recent studies have begun exploring multi-process hypotheses by examining ADHD and its putative subtypes across multiple domains of neuropsychological functioning, albeit mainly component operations of executive functioning (Guerts et al., 2005; Loo et al., 2007; Martel, Nikolas, & Nigg, 2007; Riccio et al., 2006; Scheres et al., 2004; Solanto et al., 2007; Wodka et al., 2008). Such studies in some instances seem to support the hypothesis of multiple neurocognitive components contributing to ADHD (Sonuga-Barke, Bitsakou, & Thompson, 2010). Most examinations of subtype differences have focused on the DSM-IV Inattentive and Combined subtypes, because prevalence of the Hyperactive-Impulsive subtype is quite low among school-age children and adolescents when full DSM-IV criteria (including impairment) are required. This literature has yielded mixed findings, often with relatively modest sample sizes, but has suggested that ADHD-C has worse performance in most areas than ADHD-PI (Hinshaw et al., 2002; Klorman et al., 1999; Nigg et al., 2002). Few studies have provided evidence of specific patterns of weakness in ADHD-PI relative to ADHD-C, although processing speed may be an exception (Goth-Owens et al., 2010; Nigg et al., 2002; Solanto et al., 2007).
As noted previously, vanishingly few studies of subtype effects have considered a broad array of hypothesized neuropsychological functions relevant to ADHD in the same study. Inclusion of multiple domains of neurocognitive functioning appears important for more comprehensive exploration of subtype differences, should they exist, and is crucial for future attempts to examine the validity of the categorical/subtype view of ADHD. Even so, the few studies that have employed multiple measures rarely found effects (Barkley, Grodzinksky, & DuPaul, 1992; Bauermeister et al., 2005; Chhabildas, Pennington, & Willcutt, 2001; Faraone, Biederman, Weber, & Russel, 1998; Guerts et al., 2005; Barkley, Murphy & Bush, 2001), suggesting little validity to the subtype classifications. Again, however, sample sizes were small and studies under-powered to find effects in most instances. Therefore, further clarification of these issues is needed in order to ascertain (1) how many constructs are needed to capture ADHD neuropsychological weakness, and (2) which of many candidate measures or constructs (if any) may be most useful in validating any potential distinctions between the ADHD diagnostic subtypes as well as their relationships to ADHD symptom dimensions (Castellanos et al., 2006; Nigg, 2006; Sonuga-Barke, 2003; Willcutt et al., in press).
This is now particularly relevant in light of proposed DSM-5 presentations. While DSM-5 has not yet issued final, published versions, the recent proposal therein for a restrictive inattentive presentation (www.dsm5.org; accessed November 2011), reflects concern that it that heterogeneity within the Inattentive group may obscure potential subtype differences in neuropsychological performance. Although little studied, this hypothesis has received some support from family inheritance studies (Stawicki, von Eye, & Nigg, 2006), as well as from recent evidence of slower processing speed (Goth-Owens et al., 2010) and altered attentional filtering (Carr, Henderson, & Nigg, 2010) in children and adolescents with a “restrictive-Inattentive” presentation.
In all, due to small samples, introduction of possible new presentation in DSM-5, and insufficient coverage of hypothesized ADHD neuropsychological deficits, it remains unclear which components of neurocognition make unique contributions ADHD, its subtypes or presentations, or its symptom dimensions. Past work regarding subtype differences has been mixed, and further examination of these issues is needed in order to more thoroughly vet evidence regarding the validity of the categorical (subtype) and dimensional views of the disorder. The current study sought to more definitively address these issues by examining neuropsychological performance in a large clinically-characterized sample of ADHD and control youth with a broad battery of measures designed to capture a wide range hypotheses about ADHD deficits.
METHOD
Participants
Participants were 498 youth ages 6–17 years (M=10.8 years, SD=2.4 years, 55.0% male). They included 205 sibling pairs and 88 singleton children from a total of 293 families. Sibling non-independence was addressed in the data analysis as explained later. Participants were recruited using mass mailings to parents in the local school districts, public advertisements, and community outreach to local clinics in order to recruit as broad a range of volunteers for the study as possible (and also in order to avoid potential biases inherent in a purely clinic-referred sample). A multi-stage, multi-informant screening process was used to identify cases and non-cases meeting research criteria, among those who volunteered. Informed consent and informed assent were obtained from all participating parents and children. This study was approved by the local Institutional Review Board.
At stage 1, a parent completed a telephone screen to evaluate potential rule-outs, including physical handicap, non-native English speaking, history of intellectual disability, autistic disorder, and prescription of long-acting psychoactive medications (e.g., atomoxetine, bupropion). 902 individual children from 762 families completed the initial telephone screen. Of these, 724 individual children from 588 families were invited to the complete the stage 2 diagnostic assessment. Informed consent was obtained from all participating parents and children provided written assent. For stage 2, parents and teachers completed normative behavioral rating scales including (1) the DSM-IV ADHD Rating Scale (DuPaul et al., 1998), and (2) the Conners’ (1997) Rating Scale Revised Short Form. One parent completed the Kiddie Schedule for Affective Disorders and Schizophrenia-E (KSADS-E; Puig-Antich & Ryan, 1986) with a trained master’s level clinical interviewer. Interviewers all viewed and scored a common set of 20 KSADS-E interviews to ensure reliability across interviewers. Agreement rates were moderate to high for ratings of ADHD symptoms of inattention (kappa=.94) and hyperactivity-impulsivity (kappa=.81) as well as for comorbid DSM-IV diagnoses with a base rate of 5% or greater (kappas ranged from .74-.92).
While parents completed the semi-structured clinical interview, youth completed an abbreviated testing battery. A three-subtest version of the Wechsler Intelligence Scale for Children – 4th Edition (WISC-IV; Wechsler, 2003; Block Design, Vocabulary, Information) was used to estimate full scale IQ. Reading abilities were assessed via the Wechsler Individual Achievement Test – 2nd Edition (WIAT-II; Wechsler, 2003; Word Reading subtest). Reading disorder was presented as a potential diagnosis to the diagnostic team (see below) if reading achievement was >15 points below IQ and below a standard score of 85 (1 SD below mean). 87/498 youth (17.5%) were coded as having a potential reading disorder based on these methods and 68/498 (13.7%) of parents reported a history of reading disorder on the KSADS-E.
At stage 3, final diagnostic assignment and eligibility were determined by a best-estimate procedure as follows. Data from the KSADS-E and the parent and teacher rating scales, along with IQ and achievement scores, interviewer notes and observations, and history of treatment, were presented to a diagnostic team consisting of a board-certified child psychiatrist and a licensed child clinical psychologist. Both professionals arrived independently at a clinical decision regarding ADHD subtype and comorbid diagnoses. Agreement rates were acceptable for all ADHD subtype diagnoses as well as all diagnoses discussed in this report (all kappas>.88). In all cases of disagreement, consensus was able to be reached upon discussion. Importantly, when reviewing current and lifetime ADHD subtype diagnoses, youth were classified as Combined type by the diagnostic team if they had ever previously been diagnosed as ADHD-C in order to account for diagnostic history (see Lahey et al., 2005).
Exclusion Criteria
Youth were then invited to complete the stage 4 neuropsychological testing visit, provided they were not excluded based upon the following criteria. After the diagnostic assessment, youth were excluded if the diagnostic team identified intellectual disability (based on having a full-scale IQ <70), head injury with a loss of consciousness, history of seizures as ascertained by parent report, autism spectrum disorders, current major depressive episode, lifetime bipolar disorder, lifetime psychosis, or current substance abuse or dependence.
The remaining sample of 498 youth was therefore selected by design to complete the neuropsychological testing battery. This group included 251 ADHD cases and 213 non-ADHD controls as determined by our research clinical team described above. By DSM-IV criteria, the ADHD group was comprised of 107 ADHD-Primarily Inattentive subtype (68 of whom would be identified as having the “restrictive-inattentive” presentation as defined by the proposed DSM-5 criteria, see below), 7 Primarily Hyperactive-Impulsive Subtype, and 137 Combined subtype. Of these 498 youth who completed the testing battery, 34 were classified as having subthreshold (5 symptoms) or situational ADHD symptoms (lack of cross-informant convergence on parent and teacher symptom ratings; n=16 with subthreshold symptoms, n=18 with situational ADHD). These 34 were included for analyses of dimensional symptom scores but not in analysis of diagnostic group effects.
The final sample consisted of 205 sibling pairs and 88 singleton children. Of the 205 sibling pairs, 37 were classified as concordant control pairs, 109 were ADHD-control discordant sibling pairs (52 ADHD-PI/control pairs, 50 ADHD-C/control pairs, and 7 ADHD-PH/control pairs), and 25 were sibling pairs concordant for ADHD. In addition, 26 sibling pairs were comprised of ADHD and subthreshold/situational ADHD youth, and 8 sibling pairs included one child with subthreshold/situational ADHD and one non-ADHD control.
Evaluation of the “Restrictive -Inattentive” Presentation
Included in our purview was consideration, in secondary analyses, of youth with the proposed DSM-5 “restrictive-Inattentive” presentation. To evaluate the validity of this proposed new presentation, youth who met DSM-IV criteria for the ADHD-PI subtype were further divided into two groups; those with current and lifetime history of two or fewer hyperactive-impulsive symptoms and those with current or lifetime history of three or more hyperactive-impulsive symptoms. Lifetime history of hyperactive-impulsive symptoms was estimated based on parent-report on the KSADS-E regarding past history of ADHD symptoms as well as the age of onset of each symptom. This procedure resulted in a group classified as “restrictive inattentive” (n=68).
Medication Status
Of the 498 children, 98 were currently prescribed stimulant medication preparations (19.7% of the entire sample; 36.2 % of the ADHD group). The medications prescribed were Adderall 30.6% (n=30), Concerta 36.7% (n=36), Ritalin (pill or patch) 30.6% (n=30), and Vyvanse 3.1% (n=3). This medication rate (36.2% of ADHD cases) is lower than many clinic-referred or treated samples, but consistent with estimated medication treatment rates of 11–50% in community identified samples of ADHD such as this one (Jensen et al., 1999). Lifetime medication rates were similar to rates of current medication prescription (21.4%). All children completed the neuropsychological testing battery after a minimum washout period of 24 hours for short-acting preparations and 48 hours for long-acting preparations (washout range 24–152 hours, mean=58 hours). Use of longer acting psychoactive prescription medications (including atomextine and guanfacine) was a rule-out.
Measures
The testing battery included tasks chosen to assess a variety of neuropsychological domains deemed especially relevant to ADHD. They were administered in a fixed order as follows:
Working Memory: Stars Task
We developed a computerized task modeled on work by Engle (2002). For each trial of the task, presentations alternated between screens with blue stars and screens with yellow stars. For the blue star presentations, between 1 and 3 blue stars were presented horizontally in the middle of the computer display. For the yellow star presentations, five stars were presented horizontally on the screen, such that only one star was shaded yellow. For the blue stars, participants were instructed to press the keyboard for the number of blue stars shown. For the yellow star presentations, children were instead instructed to remember its position within the row of five stars. Each trial involved a series of alternating blue stars and yellow star presentations and ranged from one block of blue star/yellow star pairings (1-span set) to five blocks of blue star/yellow star pairings (5-span set). At the end of each trial, youth were told to circle on a corresponding page all of the positions in which they had viewed yellow stars, in the order in which they were presented. The span of presentation of trials ranged from children having to remember the position of 1 to 5 yellow stars. Five blocks of each span-type (1,2,3,4 and 5 span) were presented for a total of 25 blocks and 75 total trials. To score this task, 1 and 2 span trials were counted as indexing immediate recall whereas 4 and 5 span trials were considered to index working memory (blocks of 3 were omitted). The total points earned on the working memory trials (4- and 5-span blocks) were retained for analyses.
Memory span and working memory: Spatial span
Children completed a computerized version of the spatial working memory task (see Martinussen & Tannock, 2007) to examine visuospatial span and working memory capabilities. On this task, children were presented with a screen containing 10 squares arranged in a fixed position. Individual squares then changed color (from gray to yellow) in a fixed sequence. A tone sounded at the end of the sequence to note when the sequence was finished. Youth were then instructed to click on the squares in the order in which they changed color. The number of squares in the sequence began at three and increased to nine, with two trials for each sequence length. Youth completed both forward (span) and backward (working memory) recall conditions. The total raw score (i.e., number of correct sequences) for the forward (memory span) backward version (working memory) were retained for analyses.
Memory span and working memory: Digit span
Youth completed the WISC-IV Digit Span task to assess verbal span (forward) and working memory (backward). On this task, children are aurally presented with a series of digits. In forward span, they recalled the digits in order. In backward span, they recalled the digits in reverse order. The span of digits for both forward and backward trials increases, beginning at 2 and ending at a series of 9 digits. Youth complete both sections until they fail to correctly complete two trials of the same span of digits. Raw scores on the forward (memory span) and backward (working memory) trials were retained for analyses.
Interference control: DKEFS Color-Word Interference
This subtest from the Delis-Kaplan Executive Function System (DKEFS; Delis, Kaplan, & Kramer, 2001) was administered to assess interference control; it is similar to the classic Stroop task. Youth complete four trials. On the first trial, children were presented with a series of color patches on a page and instructed to name the colors out loud without skipping any or making any mistakes (Color Naming). The second trial involves reading the color names, again as quickly as possible without making mistakes (Word Reading). On the third trial, youth are presented with color names printed in different-colored ink and instructed to say the color of the ink and avoid reading the word (Inhibition - similar to the interference trial in the classic Stroop paradigm). On the last trial, youth are again presented with color names printed in different colored-ink, yet some names are inside of boxes. Youth are instructed to name the color of the ink for ordinary items, but for those inside boxes, to read the word (Inhibition/Switching). The total completion times for each trial (Color Naming, Word Reading, Inhibition, and Inhibition/Switching) were retained for analyses.
Response suppression/inhibition: Stop Task
The Stop Task (Logan, 1994) was administered to assess response inhibition; it requires the suppression of a prepotent motor response. During this choice reaction time task, participants see an X or an O on a computer screen and respond rapidly with one of two keys to indicate which letter they had seen (called Go Response trials). On 25% of trials, a tone sounds shortly after the X or the O is displayed, indicating that participants are to withhold their response. A stochastic tracking procedure was used; stop signal reaction time (SSRT) was computed as an index of how much warning each participant needs to interrupt a response. Trials are presented across 8 blocks. SSRT was calculated by subtracting the average stop signal delay from the average Go Response time (Logan, 1994).
Reaction time variability
The within-child variability of the reaction time on the Go Response trials from the Stop task was retained as a measure of response variability.
Signal detection (arousal): Continuous Performance Task
A version of the identical pairs continuous performance task (Cornblatt et al., 1988) similar to that used by Halperin et al. (1991) was used to examine vigilance and sustained attention. In this task, children were presented with a rapid series of four-digit numbers. Youth were told to press a red button each time they saw a repeat of the exact digits (e.g., 2524 followed by a second 2524). The task was divided into 5 blocks of 288 paired trials. There were three pair types: (1) pairs of distinct digits (e.g., 6923 and 2524) called ‘stim trials,’ (2) digit pairs that differed by only one number (e.g., 2524 and 2534), called ‘catch trials,’ and (3) digit pairs that matched exactly, which was the target (e.g., 2524 and 2524), called ‘pair trials.’ Children received an accuracy score for each pair type. Hit rates (i.e., correct button presses out of total pair trials) and false alarm rates were then computed (i.e., button presses on both stim and pair trials). These scores were then used to compute d-prime, a sensitivity index expressed in units of standard deviation. A d-prime score was computed for each of the five blocks and then averaged. A higher d-prime score traditionally indicates greater sensitivity in distinguishing the targets (pair trials) from the non-targets (catch and stim trials). However, this was reverse-scored for data analysis so that higher scores for all measures indexed worse performance.
Temporal information processing: Tapping Task
A computerized tapping task was administered to assess temporal information processing abilities (Toplak & Tannock, 2005). Four conditions were presented. Participants were instructed to tap along at the same rate by pressing a red button. Two conditions (slow rate of 1000 ms between taps; fast rate of 400ms between taps) for each stimulus presentation modality (visual and auditory) were administered to each child, for a total of four conditions. Youth first had to tap along with the stimulus presentation for 30 seconds. After that time, the stimulus presentation stopped and participants were instructed to keep tapping for an additional 30 seconds. A detrended standard deviation (Ivry & Hazeltine, 1995) was computed for each condition for each individual child. Larger values indicate greater deviation from the target tapping rate and thus poorer temporal information accuracy. These four detrended standard deviations were then averaged and retained for analyses.
Processing speed and set shifting: DKEFS Trailmaking Task
The DKEFS Trailmaking task (Delis, Kaplan, & Kramer, 2001) was administered to assess cognitive-control and set-shifting abilities. Youth completed five conditions. The first trial, Visual Scanning, required children to scan two pages of numbers and letters, while marking only the number ‘3s.’ The second trial, Number Sequencing, required youth to connect a series of numbers, in order (sequencing 1–16). The third trial, Letter Sequencing, again requires examinees to connect a series of letters, in alphabetical order (sequencing A-P). The fourth trial then requires youth to alternate sequencing between numbers and letters across two pages (e.g., connecting 1-A-2-B, etc). The fifth and final trial, Motor Speed, instructs examinees to connect open circles along a path as quickly as possible. The total completion times for number sequencing and letter sequencing (processing speed) and number-letting sequencing (set-shifting) were retained for analyses.
Validity Checks
All scores from each task were subjected to several validity criteria to ensure that the participants were completing the tasks correctly and that the data was providing an accurate measure of each neuropsychological construct. For the Stars Task, responses to the blue star trials had to be correct for the yellow star response to be counted as correct, and the total accuracy for the 1-star trials had to be greater than the accuracy for the 5-star trials. Similarly, for the Digit Span and Spatial Span tasks, the forward raw score had to be greater than or equal to the backward raw score in order for the child’s data to be included. On the DKEFS Color-Word Interference and Trailmaking tasks, youth had to accurately complete the practice portion of each trial as well as complete the task within the 180 second time-frame in order to have their data included. On the Stop Task, blocks were only counted if youth had both (1) Go trial accuracy (e.g., identification of X and O) greater than 75%, and (2) probability of stopping between 25%-75%, Each child had to have at least one valid block to have their data included. For the CPT, blocks were counted if pair trial accuracy was greater than “stim trial” accuracy. To be included, each child had to have at least one valid block of the five. For the Tapping task, for a tap to be included as part of the detrended standard deviation calculation, the tapping reaction time had to fall between 200–600 ms for the 400 ms trials and between 500 and 1500 ms for the 1000 ms trials.
Based on these criteria, invalid data for each child for each task were then removed from analyses (and subsequently coded as missing, see below). In general, invalid data were low and ranged from 0% to 5% for each task. For each child and for each task, data validity was coded as yes/no and then examined in relation to ADHD diagnostic status, gender, and age to determine whether data validity differed between children with ADHD versus controls, by sex, and by age. All validity codes were unrelated to demographic or diagnostic variables (all ps>.15). Missing data (i.e., children did not complete task combined with data ruled invalid) was also generally low (ranged from 1% to 11%) on tasks and was unrelated to demographic or diagnostic variables (all ps>.36).
Data Analytic Strategy and Plan
Data Reduction
All scores were transformed such that higher scores were indicative of worse performance (i.e., slower reaction times, worse accuracy) and then standardized via z-transformation on the mean and standard deviation of the entire sample, both to enable appropriate tests of statistical interactions and to simplify data presentation. The goal of the current study was to examine performance across a broad set of neurocognitive measures that have been hypothesized to be relevant to ADHD. At the same time, we did not wish to use an excessive number of redundant indicators in the analyses. We therefore constructed a set of latent factors that captured variance common among measures that tapped into similar domains of neuropsychological functioning, while removing the error variance associated with each individual measure, so as to maximize our estimate of true effect sizes.
To do this, confirmatory factor analyses were examined based upon prior conceptualizations of putative neurocognitive domains. Competing conceptual models were evaluated in relation to simpler models (including a 1-factor model) to evaluate the robustness and validity of the final solution. In these models, residual variances of measures from the same task were allowed to correlate (e.g., color naming, inhibition, inhibition/switching from the DKEFS Color-Word Interference). We began by testing a primary seven-factor model that captured the conceptual domains underlying the different tasks: inhibition factor, working memory, signal detection (arousal), response variability, temporal information processing, memory span, and processing speed. We then tested the fit of equivalent models (4, 5, 6 and 7 factor solutions as well as hierarchical models). Alternative models were generated by examination of modification indices as well as the relationships between factors while also considering the conceptual basis underlying each neurocognitive domain.
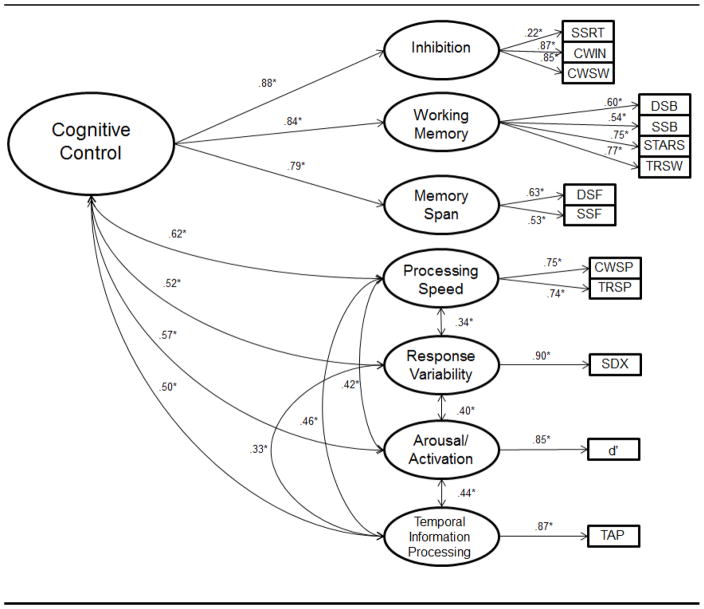
Models were fit using maximum-likelihood with robust chi-square error techniques. Fit was evaluated using four recommended indices: the chi-square value, the Comparative Fit Index (CFI; >.90=adequate), the Tucker Lewis Index (TLI; >.90=acceptable), and the root mean square error of approximation (RMSEA; <.05=good, .05-.08=adequate, .08-.10=marginal, >.10=poor). Model fit statistics are presented in Table 1 for the best fitting 4, 5, and 6 factor models (Inhibition and working memory factors were combined in the 6, 5, and 4 factor models; the best-fitting 5-factor and 4 factor models combined the span and speed factors, while the best-fitting 4-factor model moved tapping onto the executive factor (with inhibition and working memory)). As can be seen in Table 2, most of the models as well as the hierarchical models had comparable and reasonably acceptable fit, suggesting no “best” reduction strategy empirically. Hierarchical model #4 (H4) was selected as the final model for further analysis, due having (a) the lowest BIC value of all models and (b) the conceptually appealing retention of separate inhibition, working memory, and short term span factors while also acknowledging the commonalities in these domains accounted for by a higher-order factor, which we termed “cognitive control.” Figure 1 depicts this final model, with its factor loadings, and correlations between factors. Table 2 contains a list of latent variables in the model and their respective indicators. Factor scores from this model were retained for the study analyses. All analyses were conducted in MPlus version 6.0 (Muthen & Muthen, 2011) with the cluster option to enable appropriate parameter estimation while taking into account the non-independence of sibling data.
Table 1.
Fit statistics from confirmatory factor analyses: Final model and alternative models for data reduction.
| Model | χ2 | df | CFI | TLI | AIC | BIC | RMSEA | 95%CI | SRMR |
|---|---|---|---|---|---|---|---|---|---|
| 7-factor | 89.92 | 52 | .98 | .97 | 16222.61 | 16504.72 | .038 | .024–.051 | .025 |
| 6-factor | 95.11 | 58 | .98 | .98 | 16217.60 | 16474.45 | .035 | .032–.048 | .027 |
| 5-factor | 103.42 | 63 | .98 | .97 | 16217.02 | 16452.81 | .036 | .023–.048 | .029 |
| 4-factor | 107.01 | 66 | .98 | .98 | 16216.04 | 16439.20 | .035 | .022–.047 | .029 |
| 1-factor | 386.20 | 70 | .87 | .84 | 16504.93 | 16681.78 | .090 | .081–.099 | .053 |
| Hierarchical Models | χ2 | df | CFI | TLI | AIC | BIC | RMSEA | 95%CI | SRMR |
|---|---|---|---|---|---|---|---|---|---|
| Model H1: 7 lower factors/1 higher-order factor (inhibition, working memory) | |||||||||
| 94.28 | 56 | .98 | .97 | 16219.91 | 16485.17 | .037 | .023–.050 | .027 | |
| Model H2: 7-lower factors/1 higher-order factor (inhibition, working memory, tapping) | |||||||||
| 98.17 | 60 | .98 | .98 | 16217.89 | 16466.39 | .036 | .022–.048 | .027 | |
| Model H3: 7 lower factors/1 higher-order factor (inhibition, working memory, span) | |||||||||
| 102.33 | 60 | .98 | .97 | 16219.10 | 16461.86 | .038 | .025–.051 | .028 | |
| Model H4: 7 lower factors/1 higher-order factor (inhibition, working memory, speed) | |||||||||
| 94.88 | 60 | .98 | .98 | 16213.43 | 16431.86 | .034 | .020–.047 | .027 | |
| Model H5: 7 lower factors/1 higher-order factor (inhibition, working memory, span, speed) | |||||||||
| 101.91 | 63 | .98 | .98 | 16214.18 | 16449.98 | .035 | .022–.044 | .028 | |
Note. CFI=comparative fit index, TLI=Tucker-Lewis index, AIC=Akaike’s Inforamation Criterion, BIC=Bayesian Information Criterion, RMSEA=root mean square error of approximation, SRMR=standardized root mean square residual.
Table 2.
Neuropsychological latent variables and corresponding manifest variables.
| Latent Variable | Manifest Variable(s) | Abbreviation |
|---|---|---|
| Inhibition* | Stop-signal reaction time | SSRT |
| Color-Word Inhibition time | CWIN | |
| Color-Word Inhibition/Switching time | CWSW | |
| Working Memory* | Digit span backward | DSB |
| Spatial span backward | SSB | |
| Stars task working memory trials score | STARS | |
| Trails number-letter switching time | TRSW | |
| Memory Span* | Digit span forward | DSF |
| Spatial span forward | SSF | |
| Processing Speed | Color-Word naming time | CWSP |
| Trails sequencing time | TRSP | |
| Response Variability | Go reaction time standard deviation | SDX |
| Arousal | Mean d prime | d′ |
| Temporal Information Processing | Mean tapping detrended standard deviation | TAP |
Note.
indicates latent variable loading on second-order variable of Cognitive Control.
Figure 1.
Confirmatory factor analysis of neuropsychological performance: 7-factor theoretical model and factor loadings.
Note. ** indicates estimate p<.01. SSRT=stop signal reaction time, Inhibition = Inhibition trial from DKEFS Color-Word Interference, Inh/Switch = Inhibition/Switching trial from DKEFS Color-Word Interference, Spatial-B = spatial span backwards, Digits-B = digit span backward, Stars WM = working memory trials from Stars task, NL Seq = number-letter sequencing from DKEFS Trailmaking, Stop RVar = response variability from Stop Task, a400 = detrended standard deviation from auditory 400ms tapping trial, v400 = detrended standard deviation from visual 400 ms tapping trial, a1000 = detrended standard deviation from auditory 1000ms tapping trial, v1000 = detrended standard deviation from visual 1000ms tapping trial, C Naming = color naming from DKEFS Color-Word Interference, Visual Scan = visual scanning from DKEFS Trailmaking, Number Seq = number sequencing from DKEFS Trailmaking, M Speed = motor speed from DKEFS Trailmaking, Dprime1–5 = d-prime scores from blocks 1–5 of continuous performance test.
RESULTS
Demographic and Descriptive Statistics
Demographic and descriptive statistics are presented in Table 3. Examination of symptom counts and rating scale scores indicated that our diagnostic procedures effectively discriminated both ADHD children from controls as well as the ADHD subtypes from each other. In line with expectations, ADHD groups had more males than the non-ADHD group. The proportion of males in the ADHD-C group was also higher than the ADHD-PI group, consistent with past work (Gaub & Carlson, 1997). ADHD children were slightly younger than controls.
Table 3.
Descriptive and demographic statistics.
| Control | ADHD-PI | ADHD-C | p | |
|---|---|---|---|---|
| N | 213 | 107 | 137 | |
| % Male | 42.3a | 57.0b | 75.9c | <.001 |
| % Caucasian | 76.5 | 74.8 | 70.8 | .49 |
| % African-American | 8.9 | 5.6 | 9.5 | .50 |
| % Latino | 3.8 | 2.8 | 8.8 | .08 |
| % Mixed/Biracial | 9.4 | 13.1 | 10.9 | .60 |
| Age (SD) | 11.0 (2.4)a | 11.3 (2.3)a | 9.9 (2.2)b | <.001 |
| Income+ | 79.0 (47.4)a | 75.2 (36.4)a | 55.9 (38.0)b | <.001 |
| % Stimulant Medication | 1.4a | 28.0b | 45.3c | <.001 |
| KSAD Diagnostics | ||||
| Inattention Symptoms (SD) | .74 (1.4)a | 7.4 (1.5)b | 7.6 (1.6)b | <.001 |
| Hyperactive Symptoms (SD) | .62 (1.2)a | 1.8 (1.7)b | 6.2 (2.0)c | <.001 |
| % ODD (current) | 5.2a | 18.9b | 38.7c | <.001 |
| % CD (current) | 0a | 2.8ab | 5.8b | .009 |
| % MDD (lifetime) | 5.0 | 12.4 | 7.3 | .060 |
| % LD (lifetime) | 7.0a | 23.4b | 20.0b | <.001 |
| % Social Phobia (lifetime) | 4.1a | 12.5b | 10.2b | .015 |
| % Specific Phobia (lifetime) | 6.4 | 9.6 | 8.0 | .580 |
| % GAD (lifetime) | 9.6 | 11.5 | 14.6 | .354 |
| % Enuresis (lifetime) | 14.2a | 21.2ab | 30.7b | .001 |
| % Tourette’s/Tics (lifetime) | 3.2 | 3.8 | 8.0 | .100 |
| Parent Conners’ Rating Scale | ||||
| Cognitive Problems T Score | 48.2 (7.6)a | 72.8 (10.2)c | 69.9 (10.7)b | <.001 |
| Hyperactivity T Score | 48.9 (8.2)a | 57.2 (11.9)b | 71.2 (12.3)c | <.001 |
| Teacher Conners’ Rating Scale | ||||
| Cognitive Problems T Score | 49.6 (8.5)a | 66.7 (11.0)b | 68.5 (13.4)b | <.001 |
| Hyperactivity T Score | 49.6 (8.8)a | 57.7 (10.5)b | 62.2 (12.2)b | <.001 |
Note. p-values indicate 3-group significance test. Superscript letters indicate significant pairwise differences at p<.05. Conners’ T scores and standard deviations provided reflect age and sex norms.
Income reported in thousands
Differences in rates of comorbid disorders also varied across subtype in patterns that were consistent with expectations from the literature. Age, gender, and rates of current stimulant medication were included as covariates in all analyses examining group differences. Of note, results were unchanged when covarying lifetime medication rate instead of current medication rate.
DSM-IV Subtype Differences
Subtype Differences
In order to evaluate potential subtype differences in performance (ADHD versus control; ADHD-PI versus ADHD-C), regression models were used. Group comparisons were coded using orthogonal contrast codes (as in Nigg et al., 2004). The first code compared ADHD youth to non-ADHD controls (Contrast 1: ADHD-PI=1/3, ADHD-C=1/3, Control=-2/3) and the second compared ADHC-C to ADHD-PI (Contrast 2: ADHD-PI=-1/2, ADHD-C=1/2, Control=0). Note, ADHD-H is omitted from this analysis due to the very low n. These contrasts were entered into a regression model simultaneously; each neuropsychological domain factor score served as a separate dependent variable. Interactions between subtype diagnosis and gender as well as between subtype diagnosis and age were examined in a series of 2×2 interactions (e.g., Contrast 1 × Sex, Contrast 2 × sex, Contrast 1 × age, Contrast 2 × age) as well based on previous work (Nigg et al., 2004).
Factor score means for each group, and the results of regression models in which the group contrast codes were used to predict neuropsychological factor scores (e.g., cognitive control, including its constituent factors of inhibition, working memory, and memory span, processing speed, arousal, response variability, and temporal information processing) are all presented in Table 4. All eight separate regression models (one for domain score as well as the higher order cognitive control factor score) yielded reliable differences between ADHD and control youth, with Cohen’s d effect sizes ranging from .32-.61. ADHD children performed significantly worse than non-ADHD controls, consistent with prior literature. Additionally, ADHD-C youth always performed worse than ADHD-PI youth for all neuropsychological performance domains, with effect sizes ranging from.21-.44.
Table 4.
Regression models predicting DSM-IV diagnostic status: Main effects and interactions with sex and age.
| Mean Scores | Contrast Effects and Interactions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Control | ADHD-PI | ADHD-C | C1 | C2 | C1xSex | C2xSex | C1xAge | C2xAge |
| Cognitive Control | −.24 (.04) | .06 (.06) | .35 (.14) | .39** | .35** | .13 | .19** | .06 | .03 |
| Inhibition | −.18 (.11) | .11 (.09) | .36 (.10) | .37** | .34** | .11 | .22** | .02 | .04 |
| Working Memory | −.13 (.07) | .04 (.12) | .32 (.11) | .41** | .36** | .07 | .17* | .09 | .17* |
| Memory Span | −.20 (.09) | .16 (.14) | .29 (.18) | .29** | .26** | .10 | .11 | .08 | .21** |
| Processing Speed | −.21 (.05) | .19 (.18) | .33 (.10) | .36** | .27** | .08 | .09 | .08 | .25** |
| Arousal | −.19 (.08) | .03 (.09) | .25 (.15) | .41** | .36** | .08 | .07 | .02 | .01 |
| Response Variability | −.20 (.07) | .06 (.10) | .40 (.10) | .33** | .28** | .03 | .04 | .01 | −.02 |
| Temporal Processing | −.21 (.07) | .02 (.10) | .24 (.09) | .39** | .29** | .10 | .20** | .01 | .03 |
Note. Contrast 1 compares ADHD (n=251) against non-ADHD controls (n=213); Contrast 2 compares ADHD-Combined subtype (n=137) against ADHD-Primarily Inattentive subtype (n=107). Contrast 1 × sex examines interactions between ADHD/non-ADHD diagnostic status and sex in predicting neuropsychological performance. Contrast 2 × sex examines interactions between ADHD subtype diagnosis (ADHD-PI vs. ADHD-C) and sex in predicting neuropsychological performance. Contrast 1 × age examines interactions between ADHD/non-ADHD diagnostic status and age in predicting neuropsychological performance. Contrast 2 × age examines interactions between ADHD subtype diagnosis and age in predicting neuropsychological performance. Values displayed are standardized beta weights from linear regression models. Age, gender, and medication status were included as covariates in all models but their results are not shown for simplicity of presentation.
DSM-IV Subtype x Gender Interactions
Interactions between ADHD/control diagnosis (contrast 1) and sex of child were all non-significant, but the interactions between sex and ADHD-subtype (ADHD-PI vs. ADHD-C, contrast 2) were significant for cognitive control (β=.19, p=.002) and temporal information processing (β=.20, p<.001). Within the domain of cognitive control, subtype x sex interactions were significant for both inhibition and working memory. Examination of the mean factor scores by gender indicated that for girls, ADHD-PI and ADHD-C groups performed similarly on cognitive control (ADHD-PI=.46, ADHD-C=.55, p=.32), inhibition (ADHD-PI=.55, ADHD-C=.59, p=.65), working memory (ADHD-PI=.44, ADHD-C=.51, p=.52) and temporal information processing (ADHD-PI=.44, ADHD-C=.47, p=.74).
However, for boys, ADHD-C performed worse than ADHD-PI on cognitive control (ADHD-PI =.14, ADHD-C=.68, p<.001), inhibition (ADHD-PI =.16, ADHD-C=.60, p=.002), working memory (ADHD-PI =.20, ADHD-C=.54, p=.021), and temporal information processing (ADHD-PI=.02, ADHD-C=.50, p<.001) (recall that all factors are scaled so that higher scores = worse performance). Thus, the subtype effects for these neuropsychological domains appeared to be due to effects in boys, suggesting that the distinction among these subtypes (or presentations) may have more validity for boys than girls. However, even among boys, it appears as primarily a severity distinction.
DSM-IV Subtype x Age Interaction
Similarly, interactions between ADHD/control diagnosis (contrast 1) and age of child were all non-significant (all ps>.11). However, interactions between age and ADHD-subtype (ADHD-PI vs. ADHD-C, contrast 2) were significant for working memory, (β=.17, p=.004), memory span, (β=.22, p<.001), and arousal (β=.25, p<.001). We explored these differences by examining performance by subtypes for those youth below (n=251) and above (n=247) the median age (median age =10.0 years). Results indicated that subtype differences were significant only among those ages 11–17 years for each domain (working memory: ADHD-C=.36, ADHD-PI=.02, p=.011; memory span ADHD-C=.33, ADHD-PI=.09, p=.037; arousal ADHD-C=.35, ADHD-PI=.05, p=.026), again reflecting a severity model in which the ADHD-C group always performed worse than the ADHD-PI group. By contrast, significant subtype differences were not apparent in these domains for those youth ages 6–10 years (working memory: ADHD-C=.28, ADHD-PI=.19, p=.14; memory span ADHD-C=.26, ADHD-PI=.22, p=.33; arousal ADHD-C=.29, ADHD-PI=.25, p=.27).
Analysis of DSM-5 “Restrictive-Inattentive” Presentation
Orthogonal contrast codes were again used in linear regression models to examine neuropsychological performance differences between (1) those youth in the ADHD-PI group with 2 or fewer hyperactive-impulsive symptoms (“restrictive inattentive presentation” n=68) and those with 3 or more hyperactive-impulsive symptoms (“regular-inattentive” n=39) as well as (2) the restrictive-inattentive presentation youth and the ADHD-C group. Note, for these comparisons, three contrast codes were entered as follows: Contrast 1: restrictive-inattentive=−1/2, regular-inattentive=1/2, ADHD-C=0; Contrast 2: restrictive-inattentive=−1/2, regular-inattentive=0, ADHD=C=1/2; Contrast 3: restrictive-inattentive=0, regular-inattentive=−1/2, ADHD-C=1/2). However, contrast 3 involving comparisons between the regular-inattentive group and ADHD-C was not interpreted due to its overlap with previous analyses comparing ADHD-PI to ADHD-C. Results are presented in Table 5. The “restrictive-inattentive” and ADHD-C groups did not differ significantly in measures of processing speed (Table 5). By contrast, however, those with the “restrictive-inattentive presentation” were significantly slower on measures of processing speed compared to the “DSM-5 regular inattentive” (i.e., the remaining DSM-IV PI group) subgroup (b=.16, p=.026), consistent with prior work in an overlapping sample suggesting a potential significant deficit in speed among these youth (Goth-Owens et al., 2010). No other differences among ADHD-PI youth (i.e., restrictive-inattentive vs. regular-inattentive) emerged for any other neuropsychological domain (all ps>.09). However, consistent with a severity model, those in the ADHD-C group performed worse than those in the restrictive-inattentive group in all other domains.
Table 5.
Regression models predicting Restrictive-Inattentive, Regular-Inattentive, and ADHD-C: Main effects and interactions with sex and age.
| Mean Scores | Contrast Effects and Interactions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Restrictive | Regular | ADHD-C | C1 | C2 | C1xSex | C2xSex | C1xAge | C2xAge |
| Cognitive Control | .07 (.03) | .10 (.05 | .35 (.14) | .03 | .27** | .04 | .20** | .09 | .07 |
| Inhibition | .08 (.04) | .13 (.05) | .36 (.10) | .07 | .21** | .02 | .18* | .05 | .08 |
| Working Memory | .04 (.03) | .06 (.03) | .32 (.11) | .02 | .23** | .01 | .15* | .06 | .04 |
| Memory Span | .15 (.08) | .18 (.09) | .29 (.18) | .02 | .14* | .02 | .03 | .09 | .11 |
| Processing Speed | .32 (.06) | .09 (.10) | .33 (.10) | .16* | .01 | .04 | .02 | .11 | .09 |
| Arousal | .08 (.07) | .01 (.09) | .25 (.15) | .09 | .18** | .05 | .07 | .16** | .10 |
| Response Variability | .04 (.08) | .11 (.06) | .40 (.10) | .12 | .29** | .02 | .05 | .05 | .04 |
| Temporal Processing | .07 (.06) | .01 (.09) | .24 (.09) | .11 | .20** | .11 | .12 | .09 | .08 |
Note. Contrast 1 compares the two ADHD-PI subgroups - the Restrictive-Inattentive presentation (n=68) and the Regular-Inattentive; Contrast 2 compares Restrictive-Inattentive presentation (n=68) to the ADHD-Combined subtype (n=137).
Note: although a third contrast code comparing Regular-Inattentive vs. ADHD-C was entered, it is not interpreted due to its overlap with prior results. Contrast 1 × sex examines interactions between ADHD subgroup membership and sex in predicting neuropsychological performance. Contrast 2 × sex examines interactions between Restrictive-Inattentive vs. ADHD-C by sex in predicting neuropsychological performance. Values displayed are standardized beta weights from linear regression models. Age, gender, and medication status were included as covariates in all models but their results are not shown for simplicity of presentation.
DSM-5 Presentation x Sex Interaction
When examining interactions with sex, a similar pattern emerged as did when examining differences among the DSM-IV ADHD subtypes. Contrast 2 (Restrictive-Inattentive vs. ADHD-C) x sex interactions were significant for cognitive control (including inhibition and working memory) indices. Specifically, males in the ADHD-C group performed significantly worse than males with the “Restrictive-Inattentive” presentation – a subtype presentation difference did not emerge for females. Additionally, the Contrast 1 (Restrictive-Inattentive vs. Regular-Inattentive) x sex interaction was significant for temporal information processing. Examination of mean scores indicated that males with the “Restrictive Inattentive” presentation performed significantly worse those with the “Regular-Inattentive” presentation on these measures (Restrictive-Inattentive=.16, Regular-Inattentive=.01).
DSM-5 Presentation x Age Interaction
A significant interaction was observed between DSM-5 presentation Contrast 1 (Restrictive-Inattentive vs. Regular-Inattentive) and age in predicting arousal (β=.16, p=.031). Examination of the means for youth above and below the median age (10 years) revealed that Restrictive-Inattentive youth ages 11–17 years had a significantly worse arousal score compared to their Regular-Inattentive age-mates (Restrictive Inattentive=.39, Regular Inattentive=.12, p=.035), a difference that was not present for younger children (Restrictive Inattentive=.31, Mild-Combined=.27, p=.54). No significant contrast 2 (Restrictive-Inattentive vs. Combined) x age interactions emerged for any of the neuropsychological domains (all ps>.12).
Neuropsychological Domains as Unique Contributors to ADHD
Diagnostic Status
Logistic regression was employed next in order to assess which neuropsychological domains uniquely predict ADHD diagnostic status (i.e., ADHD vs. control and ADHD-PI vs. ADHD-C). In these models, necessarily, the dependent and independent variables were reversed from the preceding analyses: group status became the outcome variables, and neuropsychological measures were independent variables (“predictors”). All neuropsychological factor scores were entered simultaneously into the model in addition to covariates (age, gender, and stimulant medication status). The first model predicted ADHD versus control group membership, and the second model predicted subtype grouping (ADHD-C vs. ADHD-PI). In the first model, of the seven lower-order domains, only inhibition, (b=2.01, p<.001, OR=3.09, Cohen’s d=.62), and arousal (b=1.66, p=.003, OR=3.4, Cohen’s d=.67) uniquely associated with ADHD diagnosis. The second-order factor of cognitive control also significantly distinguished ADHD and non-ADHD youth (b=1.94, p<.001, OR=3.62, Cohen’s d=.71) In the second model (ADHD-C versus ADHD-PI), inhibition (b=1.74, p=.001, OR=2.8; Cohen’s d=.57), arousal(b=1.61, p=.003, OR=2.9, Cohen’s d=.59), response variability (b=1.54, p=.015, OR=2.6, Cohen’s d=.53), and the higher-order factor of cognitive control (b=1.78, p=.004, OR=3.5, Cohen’s d=.69), each uniquely contributed to statistical prediction of subtype diagnosis.
Restrictive-Inattentive Presentation
Similarly, we next used logistic regression to examine which (if any) of the neuropsychological domains distinguished youth with the restrictive-inattentive presentation from other ADHD youth. Group status (restrictive-Inattentive presentation vs. other ADHD youth) was the outcome measure. In this model, only processing speed (b=1.68, p=.018, OR=2.1, Cohen’s d=.41) significantly predicted outcome.
Additional Covariates
Past work has shown that youth with learning disorder may also show impairments in neuropsychological performance, particularly with working memory (Rogers et al., 2011). As such, we re-analyzed group differences with the inclusion of WIAT-II reading score and lifetime stimulant medication as additional covariates. The pattern of group differences and the magnitude of the effect sizes remained the same with the inclusion of these measures as covariates. Notably, however, effect sizes regarding working memory performance decreased for both the ADHD-control comparison (d changed from .51 to .29), and for the ADHD-C vs. ADHD-PI comparison (d changed from .39 to .27) with the inclusion of WIAT-II reading score as a covariate. These decreases, however, were not significant, based on their overlapping 95% confidence intervals. The attenuation of effect sizes here suggest that reading ability may partially but not entirely account for ADHD-related and subtype differences in neuropsychological performance.
Symptom Dimensions
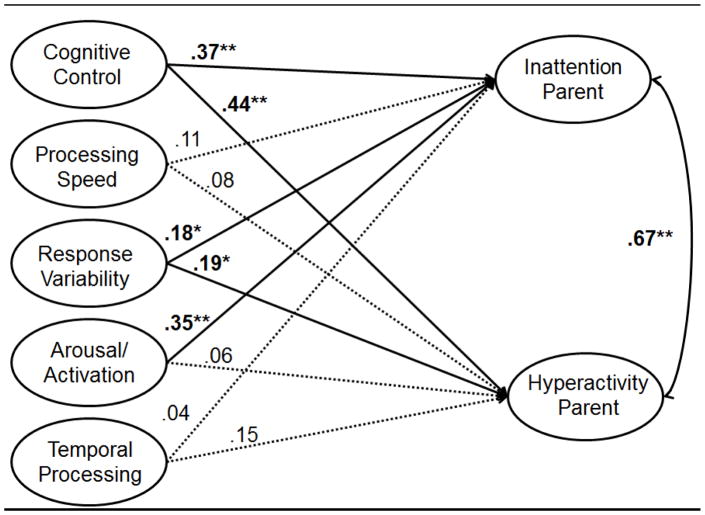
Latent variables for the ADHD symptom dimensions of inattention and hyperactivity-impulsivity were constructed using the 18 DSM-IV items from the ADHD Rating Scale (DuPaul et al., 1998). Relationships between the latent neurocognitive factors created in our confirmatory factor analyses and latent variables representing each of the ADHD symptom dimensions were examined (9 item indicators on the inattentive symptom dimension latent variable, and 9 item indicators on the hyperactivity-impulsivity dimension latent variable). Note, all analyses were done at the latent level and factor scores were not used. Separate models were computed for parent and teacher report. Note, in this model, only paths from the second-order factor of cognitive control were estimated to reduce model complexity. Gender, age, and stimulant medication status were again covariates. The resulting SEM model examining relationships between neuropsychological functioning and parent report of ADHD symptoms provided an adequate fit to the data [χ2=1475.84, df=635, CFI=.96, TLI=.95, RMSEA=.048]. Cognitive control and response variability each significantly contributed to both inattentive and hyperactive-impulsive symptoms. Low arousal was uniquely related to inattentive symptoms (see Figure 2). Temporal information processing and processing speed did not uniquely predict inattention or hyperactive-impulsive symptoms. Thus, neuropsychological functioning in the full model accounted for 29% of the variance in inattentive symptoms (Cohen’s d=1.3) and 23% of the variance in hyperactive-impulsive symptoms (Cohen’s d=1.1).
Figure 2.
Structural equation model of relationship between neuropsychological performance domains and parent-report of ADHD symptoms of inattention and hyperactivity.
Note. ** indicates estimate p<.01. Item loadings for individual neuropsychological measures and for inattention and hyperactivity symptoms not shown for ease of presentation (see Figure 1 for individual measure loadings for the neuropsychological factors). All factor loadings for ADHD symptoms >.75. Bolded paths indicate significant relationships between neuropsychological domain(s) and ADHD symptom dimensions.
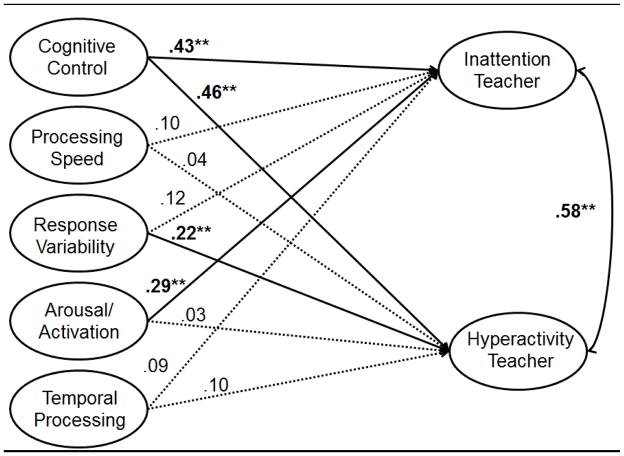
A similar pattern of results emerged for teacher report of ADHD symptom dimensions. Again, the model predicting teacher reports of inattention and hyperactivity provided an adequate fit to the data [χ2=1522.39, df=635, CFI=.94, TLI=.93, RMSEA=.052]. Again, temporal information processing and processing speed were non-contributory. As with parent report, cognitive control was again related to both symptom dimensions and arousal uniquely predicted inattention. However, unlike parent report, response variability uniquely predicted teacher-rated hyperactivity-impulsivity (see Figure 3). As can be seen from Figure 3, neuropsychological functioning in the full model accounted for 27% of the variance in teacher report of inattentive symptoms (Cohen’s d=1.2) and 26% of the variance in teacher-rated hyperactive-impulsive symptoms (Cohen’s d=1.2)
Figure 3.
Structural equation model of relationship between neuropsychological performance domains and teacher-report of ADHD symptoms of inattention and hyperactivity.
Note. ** indicates estimate p<.01. Item loadings for individual neuropsychological measures and for inattention and hyperactivity symptoms not shown for ease of presentation (see Figure 1 for individual measure loadings for the neuropsychological factors). All factor loadings for ADHD symptoms >.75. Bolded paths indicate significant relationships between neuropsychological domain(s) and ADHD symptom dimensions.
DISCUSSION
Past work examining ADHD subtype differences in neuropsychological functioning has been limited by small samples, limited coverage of the variety of hypothesized neuropsychological domains that may be involved in the pathophysiology of ADHD, and did not consider the DSM-5 restrictive inattentive group. The current study sought out to address these issues by examining subtype performance differences across a broad battery of neuropsychological measures within a large clinically-characterized sample of ADHD and control youth and considering the DSM-5 presentations specifier.
Results were consistent with past work in demonstrating moderate differences in performance between ADHD and control youth across an array of neuropsychological domains when those domains are considered in isolation, with moderate to large effect sizes (Willcutt et al., 2005). Results examining the key question of ADHD subtype/presentation consistently demonstrated that ADHD-C youth performed worse than ADHD-PI youth in all neuropsychological domains. The consistency of this pattern of results across multiple neurocognitive domains suggests that the DSM-IV ADHD subtypes simply reflect the severity of behavioral and neuropsychological weaknesses among ADHD children rather than unique configurations suggesting distinct etiology. Although youth classified with the restrictive/inattentive presentation appeared to have slower processing speed than other youth in the ADHD-PI group, the pattern of performance between this group and ADHD-C further supported a severity model of subtype performance.
In general, these results are in line with some past work demonstrating performance differences between subtypes, particularly on measures of executive functioning (Hinshaw et al., 2002; Klorman et al., 1999; Nigg et al., 2002; Solanto et al., 2007). Moreover, the current findings are in line with results of a recent meta-analysis demonstrating subtype differences on measures of response inhibition and response variability consistent with a severity model (Willcutt et al., in press). Although findings from the current study may at first seem contradictory of those from past work that failed to find performance differences between the subtypes (Barkley, Grodzinksky, & DuPaul, 1992; Bauermeister et al., 2005; Faraone, Biederman, Weber, & Russel, 1998; Guerts et al., 2005; Murphy, Barkley, & Bush, 2001), the lack of any unique patterns of deficits among the ADHD-PI group here supports the conclusions of past work indicating that there are no distinct neuropsychological deficits among this subtype (Chhabildas et al., 2001). Further, prior work has mostly relied on substantially smaller samples than we employed here, indicating that power limitations may have prevented the detection of these subtype differences in previous research.
Importantly, the effect sizes that emerged herein are similar to those have been previously reported in prior studies and meta-analysis that have examined only one or two neurocognitive constructs (Willcutt et al., 2005, estimated effect sizes between .46 and .69; similar to our findings when using similar measures). However, effect sizes were somewhat larger when examining the combined influence of the various measures, particularly when examining SEM models predicting ADHD symptom dimensions of inattention and hyperactivity-impulsivity. In these models, the effect sizes were large (Cohen’s d ranged from 1.1–1.3) and substantially greater than previous reports. The use of a latent variable approach in the current study allowed us to capture the common variance among single neurocognitive measures, which can often have less than adequate reliabilities. By removing this error variance and the associated problems with unreliability, these effect sizes demonstrate that these constructs generally account for a substantial proportion of the variance in ADHD symptom dimensions.
Interactions between diagnostic subtype and gender also emerged in a manner consistent with previous research (Nigg et al., 2002). Subtype differences, albeit still in a manner consistent with a severity model, were greater for males. Recent work has also demonstrated potential gender differences (O’Brien et al., 2010), such that females with ADHD-C may be more impaired than their male counterparts. Examination of within-subtype gender differences in symptom scores provides some evidence of this in the current study. Teacher ratings of inattention were significantly higher for ADHD-C girls compared to ADHD-C boys (p=.02). Alternatively, parent and teacher hyperactivity ratings among ADHD-PI boys were higher than ADHD-PI girls (both ps<.04). These findings provide some preliminary support for the notion that females, while comprising fewer cases of ADHD, tend to demonstrate greater cognitive deficits and impairments due to inattention than their male counterparts with the disorder (Gaub & Carlson, 1997), potentially because they possess a greater genetic or biological “load” (Faraone et al., 2000; Rhee et al., 1999; Smalley et al., 2000). This idea has been supported by previous research indicating that females with ADHD tend to have more affected family members than do males with the disorder (Smalley et al., 2000). Still, previous research examining subtype differences has either exclusively focused on males, or have been restricted by very small samples of females. Thus, questions regarding the moderating influence of gender on subtype performance differences remain an important direction for future research.
In addition, age also appeared to moderate the current results, such that subtype differences were more pronounced for older youth (ages 11–17) relative to younger youth (ages 6–10). These differences in subtype performance by age suggest that for younger children, subtype differences may not be as pronounced and may reflect a delay in the development of these abilities rather than a deficit, for at least some ADHD children. However, youth with ADHD-C may have more persistent deficits in neurocognitive skills, accounting for the larger subtype difference. In all, moderation of subtype differences by both age and gender suggest potential validity issues with categorical/subtype view of ADHD, consistent with recent work (Marcus & Barry, 2011). Therefore, our results are more consistent with a dimensional understanding of ADHD, such that greater severity in symptomatology is related to greater impairments across a variety of neuropsychological domains.
The proposed inclusion of a “restrictive-inattentive” presentation in DSM-5 raises the need for renewed neuropsychological investigation of this long-neglected group. We preliminarily examined this presentation by further subdividing youth with a potential “restrictive inattentive presentation” from those who symptoms reflect a “regular-inattentive” presentation. While the “restrictive-inattentive” group showed significantly slower processing speed compared to the “regular-inattentive” group, the “restrictive-inattentive” group did not perform significantly worse than the ADHD-C group on any measure. Therefore, these preliminary analyses do not appear to support a unique configural pattern of deficit for youth with the “restrictive-inattentive presentation.” Inclusion of additional “sluggish cognitive tempo” items as well as prospective assessment of inattentive and hyperactive-impulsive symptoms may be better able to identify youth with this restrictive symptom presentation for future studies of potential differences in neuropsychological performance (Bauermeister et al., in press). Additionally, given the subtype difference that emerged in males only, it may be beneficial for future research to follow-up in examining neurocognitive performance among the “restrictive inattentive” males specifically, particularly since preliminary analyses herein indicated that males with this presentation may show impairments in temporal information processing relative to other ADHD-PI youth.
All of the preceding, however, mirrors past literature in considering only one neuropsychological domain at one time. Are all of these domains necessary to characterize ADHD or its heterogeneity? When they were considered simultaneously, temporal information processing and processing speed did not uniquely associate with ADHD subtype or with either symptom dimension. However, cognitive control and arousal did uniquely contribute in additive fashion to diagnostic assignment. These measures, along with response variability, also uniquely predicted subtype or presentation. A similar pattern also emerged when predicting both parent and teacher ratings of ADHD symptom domains of inattention and hyperactivity-impulsivity, in that only this subset of measures was needed to account for relevant neuropsychological variation.
The consistency of findings regarding the unique role of these operations supports the notion that these neurocognitive domains (i.e., cognitive control, particularly inhibition, arousal, and response variability) each make important contributions in distinguishing ADHD from control youth as well as to the heterogeneity among children with ADHD. This is consistent with previous work that has emphasized the potential importance of each of these domains singly and jointly (see Castellanos et al., 2006; Epstein et al., 2011). Importantly, however, our results did not replicate recent findings supporting the inclusion of temporal information processing in a “triple pathway model” of neuropsychological heterogeneity in ADHD (Sonuga-Barke, Bitsakou, & Thompson, 2010). This may be due to the fact that we only included a tapping task (i.e., a timing reproduction task) while Songua-Barke and colleagues measured several different aspects of temporal processing, including duration discrimination and time anticipation. It may be the case that ADHD youth with various symptom presentations (i.e., ADHD-C vs. ADHD-PI) differ in one or all of these areas (Toplak, Dockstader, & Tannock, 2006). The current findings also largely support prior theories that have emphasized the involvement of each of these neurocognitive domains (particularly response inhibition, low arousal, and response variability) in the disorder, and the importance of inclusion of these constructs in models seeking to identify and specify the pathophysiological mechanisms that give rise to ADHD. Theories of ADHD should account for how these mechanisms may contribute uniquely to ADHD.
Limitations
The battery tested here did not include measures of reward sensitivity, delay aversion, or temporal discounting of reward; that domain may also contribute uniquely to ADHD (Sonuga-Barke,. 2003; Sonuga-Barke, Bitsakou, & Thompson, 2010; Wilson et al., 2010). We relied on composite variables that may be difficult to compare to studies of specific measures. To remedy this, data on the individual measures are made available in the online supplement (Table S-1) and indicate the same pattern of effects (although with predictably smaller effect sizes than the more reliable composite scores validated by confirmatory factor analysis).
Conclusion
In conclusion, the current study demonstrated robust differences in neurocognitive performance between ADHD and non-ADHD youth, but these differences are cumulatively accounted for by only a subset of the putative neuropsychological measures cited as ADHD-relevant. ADHD subtypes and presentations differ primarily only in terms of severity, and do not seem to reflect configuraly distinct conditions. Finally, composite and latent variables indicate a stronger relationship between ADHD symptom domains and cumulative neuropsychological weaknesses than reported in single measures studies, accounting for between 23 and 30% of the variance in each symptom domain. However, a restrictive inattentive group remains in need of further evaluation. Of the multiple neuropsychological domains associated with ADHD, not all are needed to account for the disorder, but a subset appear to contribute additively to its severity.
Supplementary Material
Acknowledgments
The project was supported by Award Number R01-MH070004-01A2 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors also thank all participating children and their families for making this work possible.
References
- American Psychiatric Association. [accessed November 2011];Website for Diagnostic and Statistical Manual of Mental Disorders. (1). 2011 www.dsm5.org.
- Barkley RA, Grodzinksy G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: A review and research report. Journal of Abnormal Child Psychology. 1992;20:163–188. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001;15:351–360. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Barkley RA, Bauermeister JA, Martinez JV, McBurnett K. Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: Neuropsychological and psychosocial correlates. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-011-9602-7. (in press) [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Matos M, Reina G, Salas CC, Martinez JV, Cumba E, Barkley R. Comparison of the DSM-IV combined and inattentive types of ADHD in a school-based sample of Latino/Hispanic children. Journal of Child Psychology and Psychiatry. 2005;46:166–179. doi: 10.1111/j.1469-7610.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- Carr L, Henderson J, Nigg JT. Cognitive control and attentional selection in adolescents with ADHD versus ADD. Journal of Clinical Child and Adolescent Psychology. 2010;39:726–740. doi: 10.1080/15374416.2010.517168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Science. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Connors CK. Connors’ Rating Scales Revised. Toronto, Ontario, Canada: Multi Health Systems, Inc; 1997. [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP) I: New findings about sustained attention in normal families. Psychiatry Research. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. The ADHD rating scale IV:Checklists, norms, and clinical interpretation. New York: Guilford Press; 1998. [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Atonini AN, Brinkman WB, Froelich T, Simon JO, Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biedeerman J, Mick E, Williamson S, Wilens T, Spencer T, Weber W, Jetton J, Kraus I, Pert J, Zallen B. Family study of girls with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2000;157:1077–1083. doi: 10.1176/appi.ajp.157.7.1077. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Weber W, Russell RL. Psychiatric, neuropsychological, and psychosocial features of DSM-IV subtypes of attention-deficit/hyperactivity disorder: Results from a clinically referred sample. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:185–193. doi: 10.1097/00004583-199802000-00011. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: A meta-analysis of analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Goth-Owens TL, Martinez-Torteya C, Martel MM, Nigg JT. Processing speed weakness in children and adolescents with non-hyperactive but inattentive ADHD (ADD) Child Neuropsychology. 2010;16:577–591. doi: 10.1080/09297049.2010.485126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant J. ADHD subtypes: Do they differ in their executive functioning profile? Archives of Clinical Neuropsychology. 2005;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Sharma V, Greenblatt E, Schwartz ST. Assessment of the continuous performance test: Reliability and validity in a non-referred sample. Psychological Assessment. 1991;3:603–608. [Google Scholar]
- Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:3–18. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Carte ET, Sami N, Treuting JJ, Zupan BA. Pre-adolescent girls with attention-deficit hyperactivity disorder II: Neuropsychological performance in relation to subtypes and individual classification. Journal of Consulting and Clinical Psychology. 2002;70:1099–1111. doi: 10.1037//0022-006x.70.5.1099. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Kettle L, Roper MT, Sloan MT, Dunclan MK, Hoven C, Bird HR, Bauermeister JJ, Paybe JD. Are stimulants overprescribed? Treatment of ADHD in four U.S. communities. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:797–804. doi: 10.1097/00004583-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Klorman R, Hazel-Fernandez LA, Shaywitz SE, Fletcher JM, Marichone MA, Holahan JM, Steubing KK, Shaywitz BB. Executive function deficits in attention-deficit hyperactivity disorder are independent of oppositional defiant and reading disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1148–1155. doi: 10.1097/00004583-199909000-00020. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham W, Loney J, Lee SS, Willcutt W. Instability of DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Logan GD. A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Loo SK, Humphrey LA, Tapio T, Moilanen IK, McGough JJ, McCracken JT, Yang MJ, Dang J, Taanila A, Ebeling H, Jarvelin MR, Smalley SL. Executive functioning among Finnish adolescents with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1594–104. doi: 10.1097/chi.0b013e3181575014. [DOI] [PubMed] [Google Scholar]
- Marcus DK, Barry TD. Does attention-deficit hyperactivity disorder have a dimensional latent structure? A taxometric analysis. Journal of Abnormal Psychology. 2011;120:427–442. doi: 10.1037/a0021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nikolas M, Nigg JT. Executive function in adolescents with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1437–1444. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Tannock R. Working memory impairments in children with attention deficit hyperactivity disorder with and without comorbid language learning disorders. Journal of Clinical and Experimental Neuropsychology. 2006;28:1073–1094. doi: 10.1080/13803390500205700. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD Combined Type and ADHD Inattentive Type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8:463–488. [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 6. Los Angeles, CA: Muthen & Muthen; 2011. [Google Scholar]
- Nigg JT. What causes ADHD? Understanding what goes wrong and why. New York: The Guilford Press; 2006. [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: Results for parents and siblings of children with ADHD Combined and Inattentive subtypes. Journal of Abnormal Psychology. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit hyperactivity disorder. Archives of Clinical Neuropsychology. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS) Pittsburgh, Pennsylvania: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- Rhee SH, Waldman ID, Hay DA, Levy F. Sex differences in genetic and environmental influences on DSM-III-R attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 1999;8:24–41. doi: 10.1037/0021-843X.108.1.24. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Homack S, Jarratt KP, Wolfe ME. Differences in academic and executive function domains among children with ADHD Predominately Inattentive and Combined types. Archives of Clinical Neuropsychology. 2006;21:657–667. doi: 10.1016/j.acn.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rogers M, Hwang H, Toplak M, Weiss M, Tannock R. Inattention, working memory, and academic achievement in adolescents referred for attention-deficit/hyperactivity disorder. Child Neuropsychology. 2011;17:444–458. doi: 10.1080/09297049.2010.544648. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Guerts H, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant J. Executive functioning in boys with ADHD: primarily an inhibition deficit? Archives of Clinical Neuropsychology. 2004;19:569–594. doi: 10.1016/j.acn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Del’Homme M, NewDelman J, Gordon E, Kim T, Liu A, McCracken JT. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Vail L, Newcorn JH. Neurocognitive functioning in AD/HD Predominately Inattentive and Combined subtypes. Journal of Abnormal Child Psychology. 2007;35:729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. The dual pathway model of AD/HD: An elaboration of neuro developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway mode: Evidence for the dissociation of timing, inhibition, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Stawicki J, Nigg JT, von Eye A. Family psychiatric history evidence on the nosological relations of DSM-IV ADHD Combined and Inattentive subtypes: New data and meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47:935–945. doi: 10.1111/j.1469-7610.2006.01628.x. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: Findings to date and new models. Journal of Neuroscience Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Tannock R. Tapping and anticipation performance in attention-deficit hyperactivity disorder. Perceptual and Motor Skills. 2005;100:659–675. doi: 10.2466/pms.100.3.659-675. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 4. San Antonio, TX: Psychological Corporation; 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 2. San Antonio, TX: Psychological Corporation; 2003. Wechsler Individual Achievement Test. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rhode LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention-deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. doi: 10.1037/a0027347. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: Application in young children. Journal of Child Psychology and Psychiatry. 2010;52:256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka EL, Mostofksy SH, Prahame C, Larson JCG, Loftis C, Denckla MB, Mahone EM. Process examination of executive function in ADHD: Sex and subtype effects. The Clinical Neuropsychologist. 2008;22:826–841. doi: 10.1080/13854040701563583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.