Abstract
Vision loss is a major social issue, with more than 20 million people over the age of 18 years affected in the USA alone. Loss of vision is feared more than premature death or cardiovascular disease, according to a recent Society for Consumer Research group survey. The annual direct cost of medical care for the most prevalent eye disease, age-related macular degeneration, was estimated at US$255 billion in 2010 with an additional economic impact of US$88 billion due to lost productivity and the burden of family and community care for visual disability. With the blossoming of human stem cell research, regenerative treatments are now being developed that can help reduce this burden. Positive results from animal studies demonstrate that stem cell-based transplants can preserve and potentially improve vision. This has led to new clinical trials for several eye diseases that are yielding encouraging results. In the next few years, additional trials and longer-term results are anticipated to further develop ocular regenerative therapies, with the potential to revolutionize our approach to ophthalmic disease and damage.
Keywords: bone marrow stem cell, eye disease, human embryonic stem cell, human neural stem cell, induced pluripotent stem cell, limbal stem cell, retinal pigment epithelial stem cell, umbilical cord stem cell
Due to the burden of eye disease, and its relative accessibility, the eye is a prime target for stem cell transplantation therapies, with good surgical access and the ability to visually monitor changes after transplantation being significant advantages [1]. Systemic complications from intraocular agents are rare, and the risks of overgrowth and tumor formation associated with intraocular stem cell transplantation [2–5] are mitigated by the ability of using laser ablation and in extreme cases, evisceration or enucleation [6]. In addition, advanced methods exist to assess the clinical meaningfulness of eye tissue transplant outcome. Quantifiable visualization of the retina with resolution up to a few microns is routine using computerized fundus imaging, laser scanning ophthalmoscopy and optical coherence tomography technologies. Furthermore, visual function can be assessed rapidly, quantitatively and accurately by visual acuity and visual field measurements.
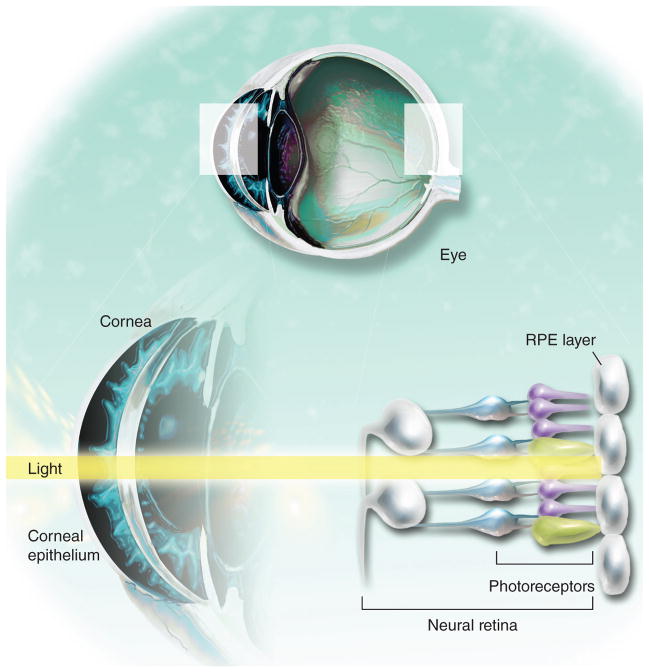
The key sites currently targeted for stem cell transplantation include the cornea, the clear tissue covering the front of the eye that helps focus incoming light, the neural retina, which contains the photoreceptor cells that transduce light into neural electrical signals sent to the visual cortex, and the retinal pigment epithelium (RPE), a single layer of pigmented cells that plays a key role in maintaining the photoreceptor cells and the blood– retina barrier (Figure 1). The neural retina and RPE are CNS tissues, so studies of their replacement with stem cell products serve as a model for stem cell approaches to less accessible areas of the CNS. In this article we survey recent advances in stem cell-based therapies for ocular disease.
Figure 1. Several tissues in the eye are being targeted for stem cell replacement.
RPE : Retinal pigment epithelium.
Stem cell types for eye disease clinical trials
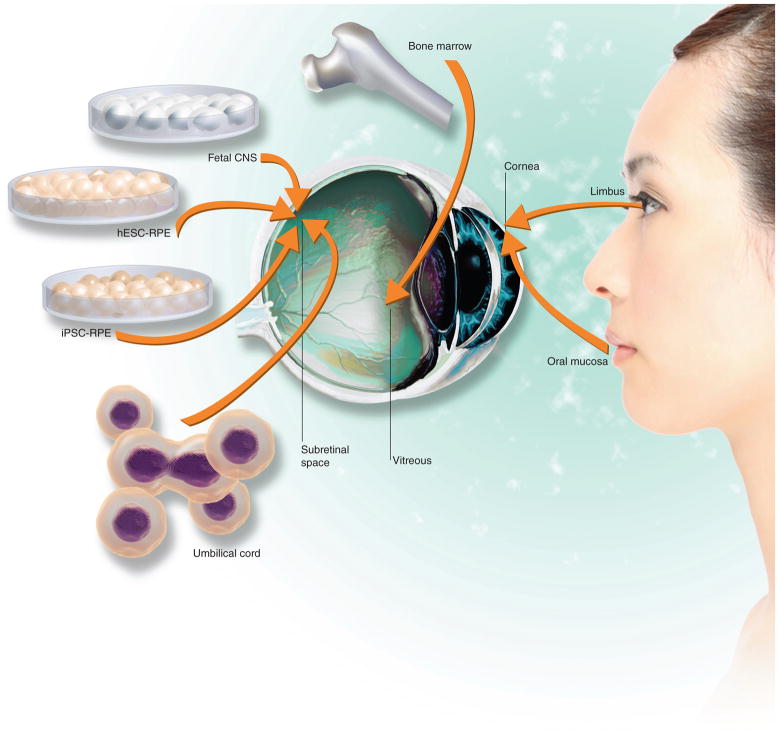
Human stem cells from a wide variety of sources are being explored for eye disease transplantation therapies (summarized in Figure 2). Some of the transplants are aimed to directly replace lost or damaged tissue, while others replace essential functions of a tissue and/or produce beneficial growth and trophic factors to slow the disease progress.
Figure 2. Human stem cell sources explored for eye tissue replacement.
hESC : Human embryonic stem cell; iPSC : Induced pluripotent stem cell; RPE : Retinal pigment epithelium.
Pluripotent stem cells
Pluripotent stem cells are, by definition, able to generate all somatic tissues, including every cell type found in the eye. Pluripotent human embryonic stem cells (hESCs) or the recently developed induced pluripotent stem cells (iPSCs), bring new hope for eye-replacement therapies by producing ocular cells in essentially unlimited amounts. Moreover, iPSCs made from a patient’s own cells could reduce the need for immunoprotective regimens post-transplantation. An important concern in using pluripotent stem cells is unwanted cell overgrowth or tumor formation. This concern is particularly acute for iPSCs, which are produced from a donor somatic cell type by incorporating key genes that create a primitive, pluripotent state [7]. iPSCs readily form tumors when produced using oncogenic, permanently integrating gene-delivery vectors [8,9]. The use of newly developed techniques now enables elimination of exogenous reprogramming factors [10], which is anticipated to reduce tumor threat. In addition, efforts are being made to detect and eliminate residual pluripotent cells contaminating the desired differentiated cell product.
Neural stem cells
Human neural stem cells (NSCs) are typically derived from donated human fetal forebrain tissue. These cells are capable of producing neurons and glia, but have not yet been shown to generate neural retina or retinal pigment epithelium. However, NSCs are capable of producing cells that can substitute several key functions of these tissues and produce specialized trophic factors that could be beneficial [11].
RPE stem cells
The recently discovered stem cell in the adult human RPE layer [12] allows the generation of large numbers of RPE cells in tissue culture, and is being explored for production of other ocular cell types.
Limbal stem cells
One of the earliest stem cells in ocular clinical trials, limbal stem cells produce corneal epithelial cells, which are essential for maintaining the cornea [13].
Umbilical cord stem cells
Umbilical cord tissue is a source of valuable stem cells in the blood and mesenchymal lineages [14]. Although umbilical cord stem cells (UCSCs) do not produce ocular tissues such as neural retina or RPE, UCSCs could slow degeneration through trophic factor release. Banking umbilical cord tissue enables patient-matching.
Bone marrow stem cells
These have a similar potential to UCSCs, but can be obtained from the adult patient, allowing both allogenic and autologous transplantation [14].
RPE & photoreceptor diseases
Several blinding diseases, including age-related macular degeneration (AMD), types of retinitis pigmentosa (RP), Stargardt’s disease and gyrate atrophy (GA), are characterized by dysfunction of the RPE [15], a monolayer of pigmented, polarized, cobblestone epithelium that lies beneath the neural retina, providing essential support [16–19]. Loss or disease can impair essential RPE functions such as the diurnal phagocytosis and replenishment of photoreceptor outer segments, and thus secondarily produce retinal dysfunction. The most common RPE disease, AMD, affects approximately 10 million Americans over the age of 50 years and is the leading cause of blindness in the elderly [20].
Proof of principle for RPE replacement has been shown by pioneering surgical experiments [21–24]. Autologous surgery is challenging; alternatives using RPE from other donors is limited by the amount of tissue available, a need that stem cells can fulfill.
Pluripotent stem cells for retinal & RPE degeneration
RPE arises spontaneously from pluripotent human stem cells, albeit slowly and at low efficiency [25–27]. Supplementing with growth factors that stimulate anterior neural plate fate and RPE specification during normal development [28–30] or small molecules with similar function [31] improves the speed and efficiency of RPE production. RPE derived from pluripotent stem cells using a variety of methods can preserve vision after transplantation into animal models of retinal degeneration [32]. These breakthrough discoveries have led to clinical trials to determine if such visual preservation can occur in humans. In 2011, the US FDA allowed a Phase I/II open-label, multicenter, nonrandomized, prospective study proposed by Advanced Cell Technology, Inc. (ACT) to determine the safety and potential efficacy of subretinal injection of RPE cells, spontaneously produced from hESCs, in patients with late-stage ‘dry’ AMD (the form of AMD without neovascularization; NCT01344993), or Stargard’s disease (NCT01345006 and NCT01469832). Stargardt’s disease is the most common early-onset macular degeneration, a genetic disease in which proteins involved in the visual phototransduction cycle are dysfunctional, causing accumulation of waste materials leading to RPE cell death, hence the rationale for RPE replacement.
hESC-RPE were generated by ACT collaborators according to good manufacturing practice (GMP) and their purity was determined by qPCR and immunostaining for RPE-specific markers [33]. Lack of pluripotency markers and lack of teratoma formation were used to show negligible contaminant residual hESCs. Phagocytosis of labeled beads was used to demonstrate functionality of the GMP-compliant hESC-RPE cells.
This clinical trial enrolling 12 dry AMD and 12 Stargardt’s disease patients to receive uniocular subretinal injection of GMP-compliant hESC-RPE cells, has enrolled the first three patients, receiving a dose of 50,000 cells. Later groups will receive doses up to 200,000 cells. At 4 months after treatment of the first two patients (one with dry AMD, one with Stargardt’s), an early report showed no abnormal growths, teratoma, rejection or inflammation [34]. The AMD patient did not follow the immunosuppression regimen to completion, and no donor cells were detected, potentially indicating that immunosuppression is needed for donor cell survival. Nevertheless, this patient showed visual improvement, from reading 21 letters of the Early Treatment of Diabetic Retinopathy Study eyechart before treatment, to reading 28 letters 3 months after transplantation. Surprisingly, mild improvement was seen also in the untreated eye. For the Stargardt’s disease patient, visual improvement was observed (five letters of the Early Treatment of Diabetic Retinopathy Study chart), including improved color vision and contrast/dark adaptation.
ACT has recently enrolled additional patients in the USA and Europe [101]. It remains to be determined if visual gains observed are due to implanted cells or to a placebo effect, and whether immunosuppression is essential for transplant survival; while the retina has immune privilege [35], this is often compromised in a diseased eye, especially when the RPE, essential for the blood–retina barrier, is damaged. Nevertheless, the preliminary results are promising and this pioneering hESC clinical trial will be widely watched.
Coming soon: transplanting a patch of hESC-derived RPE monolayer
Given that the RPE is organized in vivo as a tight, polarized monolayer, it is possible that a transplant of pre-polarized RPE cells will integrate and function better than a cell suspension. Several groups are working to create a suitable matrix that maintains a stable RPE monolayer patch for transplantation. Preclinical studies in pigs using a hESC-derived RPE polarized monolayer growing on a coated, nonbiodegradable polyester insert have been completed by a team led by Peter Coffey at the Institute of Ophthalmology in London, UK and UC Santa Barbara, and collaborators at the London Project to Cure Blindness, in partnership with Pfizer [36,37], and a clinical trial is anticipated.
Patient-derived RPE
Transplantation of patient-matched RPE cells reduces the necessity of immunosuppression. This could be accomplished using iPSCs generated from patients. At the 2012 International Stem Cell Research meeting in Yokohama, Japan, Masayo Takahashi of the Laboratory for Retinal Regeneration at the Riken Center in Kobe announced a clinical trial for early 2013 enrolling five AMD patients, using GMP-compliant iPSC-derived RPE cells [28,29,38]; this is the first announced clinical trial using cells derived from iPSCs. At the same meeting, Peter Coffey also reported production of GMP-compliant, iPSC-derived RPE. Another approach toward immune-matching being developed in our laboratories at the Neural Stem Cell Institute utilizes the adult human RPE stem cell that can be derived from living patients for autologous transplantation of this tissue-specific stem cell.
NSCs for AMD
In preclinical studies by StemCells Inc. and collaborators, NSCs isolated from second trimester brain tissue were selected and grown into a defined cell line (HuCNS-SCs). These cells were transplanted into the subretinal space of the Royal College of Surgeons rat, which has an RPE defect that prevents normal phagocytosis of the photoreceptor outer segments, and is a widely used model of retinal degeneration. The implanted NSCs significantly improved photoreceptor survival and vision [39]. Interestingly, these cells did not differentiate into RPE or other retinal cell types, but were still beneficial, potentially by substituting for RPE functions, such as phagocytosis and/or by producing trophic factors that slowed the photoreceptor degeneration. In June 2012, StemCells Inc. announced the initiation of a Phase I/II safety and preliminary efficacy trial.
UCSCs for RP & AMD
UCSCs transplanted into the subretinal space of the Royal College of Surgeons rat were found to slow vision loss [40]. Based on these data, in 2007, Centocor Biotech (currently Janssen Biotech, Inc., a subsidiary of Johnson & Johnson) began a Phase I clinical trial using their patented UCSC line, CNTO 2476, to evaluate safety and efficacy outcomes in patients with RP (NCT00458575). In 2010, the study was terminated citing an ‘internal business decision’. In 2010, Janssen Biotech, Inc. began a Phase I/II clinical trial (NCT01226628) transplanting CNTO 2476 into the subretinal space of patients with AMD, administered using a microcatheter, to determine whether UCSCs are safe and can slow degeneration and preserve vision in this disease.
Bone marrow stem cells for photoreceptor diseases
Bone marrow-derived stem cells have been shown to rescue retinal degeneration in mouse models [41,42]. Based on this promising work, clinical trials were started to determine the safety and efficacy of these cells in patients with eye disease. One was conducted to evaluate the short-term (10 months) safety of a single transplantation of 10 × 106 bone marrow-derived mononuclear stem cells in three patients with RP and two patients with cone–rod dystrophy, an early-onset genetic disease involving degeneration of both cones and rods [43,44]. No detectable structural or functional toxicity was found, and further studies are ongoing: in RP patients in Brazil (NCT01560715) and Thailand (NCT01531348); and in Brazil in both AMD (NCT01518127) and ischemic retinopathy (NCT01518842) patients.
Corneal repair
The corneal epithelium is essential for maintaining a clear ocular surface. Corneal damage, for example due to alkali burns, can destroy the corneal epithelium, resulting in opacification and blindness [13]. The limbus, a ring of tissue at the edge of the cornea, contains stem cells that divide and differentiate into the corneal epithelium over the lifetime of an individual. In a remarkable series of studies, it was shown that limbal stem cells could be harvested from a healthy area of the limbus in an individual with a damaged cornea and expanded in vitro to form a stratified epithelium that stained positive for a corneal-specific marker. Once mounted on a soft contact lens, these cells can be transplanted to regenerate the patient’s cornea [45,46], leading in most cases to vision restoration. In a much-anticipated recent report, Graziella Pellegrini and Michele De Luca in Modena, Italy, presented the results of a 10-year follow-up of patients who underwent such autologous limbal stem cell transplantation procedures [47]. They showed that permanent restoration of a transparent, renewing corneal epithelium was observed in 76.6% of eyes and was stable at 10 years [47]. This remarkable, life-altering result demonstrates the enormous potential of autologous stem cell therapy. For patients in which the limbus is completely destroyed, allogeneic transplantation using limbal tissue from an allogenic donor is a suitable, although less successful, alternative [48].
Currently, there are a number of clinical trials ongoing for corneal transplantation of limbal stem cells from both autologous (NCT00845117, NCT01619189 and NCT01123044) and allogeneic sources (NCT00736307, NCT01619189, NCT01237600 and NCT01562002), transplanted alone (NCT00845117 and NCT01237600), or on amniotic membranes (NCT00736307, NCT01619189, NCT01123044 and NCT01562002). Other stem cell types being tested for this application include cultured oral mucosal epithelial stem cells (NCT01489501 and NCT00491959) and bone marrow-derived mesenchymal stem cells (NCT01562002).
Future perspective
Increasing the ocular target cell repertoire
Vision improvement after transplantation of photoreceptors in animal models [49–52], has spurred efforts to produce human photoreceptors from hESCs and iPSCs in sufficient purity and quantity for transplantation [52–55]. Protocols for the generation of other neural retinal cell types, such as ganglion cells, are also being developed to replace those lost in glaucoma and other optic nerve disorders. In addition to CNS tissue, non-neural ocular elements, such as trabecular tissue that regulates fluid homeostasis, are target tissues for modeling in stem cell cultures and could be used to aid eye repair and slow or prevent disease.
Multilayered transplants
The normal 3D configuration of eye tissues should be recapitulated to ensure the best possible outcome. In a notable series of experiments, Yoshika Sasai’s group has shown that mouse and human pluripotent stem cells can generate differentiated, 3D structures similar to the embryonic eye cup, containing RPE and neural retina with the appropriate layering and orientation [56,57]. It will be exciting to see how such multicellular growths might provide a more sophisticated 3D transplant, incorporating multiple retinal layers, which could be especially important for patients who have lost both RPE and photoreceptor cells or who have otherwise suffered extensive loss of neural retina.
Bioengineered eye tissues
In order to build ocular structures, such as the trabecular meshwork or the RPE monolayer, bioengineers are incorporating biocompatible materials with stem cell products [58]. For example, Bruch’s membrane, the thick matrix that underlies the RPE, is damaged in AMD, leading to defective exchange of nutrients and cell products with the underlying choroidal vasculature [59]. In approximately 10% of patients with AMD, Bruch’s membrane is compromised such that the choroidal vasculature invades into the retina, causing extensive loss of central vision; so-called ‘wet’ AMD. To help repair the tissue and prevent disease progression, RPE cells can be delivered on a bioengineered Bruch’s membrane capable of preserving and restoring the relationship between the RPE and the choriocapillaris. Several laboratories are working toward the establishment of such matrix materials with different properties of chemical composition, thickness, porosity, topography and biodegradation [60–62].
Disease modeling: GA
iPSCs can be generated from patients with eye diseases to study disease etiology and produce ‘disease in a dish’ models valuable for drug screening. For example, GA is a rare, blinding genetic disease caused by dysfunction of a vitamin B6-dependent enzyme that results in deterioration of retina, choroid and RPE [63]. Administration of vitamin B6 is reported to benefit a number of patients. Recently, iPSCs were derived in David Gamm’s laboratory from GA donor dermal fibroblasts and differentiated into RPE. Treatment of the iPSC-derived RPE with vitamin B6 helped determine the dose needed to restore enzymatic activity, aiding treatment of the donor [64]. Such iPSC models will be enormously valuable assets in the fight against eye diseases.
Conclusion
Most ocular stem cell translational studies are at early stages – basic research, preclinical and Phase I/II trials – with successful longer term results from autologous limbal cell transplant serving as a beacon for what we might achieve with stem cell approaches. Preserving and restoring vision are key outcomes to be gained, balanced with necessary caution surrounding the risks associated with transplanting living cells with the potential to divide and undergo metaplastic changes. Progress will be swifter as these first trials yield data, allowing the development of more sophisticated therapies that are envisioned to combine stem cells, bioengineered products and small molecules for a new generation of regenerative therapies.
Key points.
Eye disease is highly prevalent worldwide, with multiple eye tissues affected.
The eye is a prime location for transplantation therapies, with easy surgical access and post-transplantation monitoring, as well as sensitive visual tests for measuring outcomes and a localized safety profile.
Stem cell-based transplantation to replace the function of lost cells is a promising therapy for patients with eye diseases.
Encouraging results from animal studies demonstrate stem cell-based therapies can preserve and restore vision.
A variety of stem cells hold promise for different ocular applications, with some in clinical trials.
Human embryonic stem cell-derived retinal pigment epithelium replacement is pioneering the use of human pluripotent stem cells for stem cell-based CNS repair.
The future of stem cell therapy includes the use of human stem cells as disease models, enabling a new pathway for drug discovery.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Braunstein R. AMD: its potential health and economic impact around the world. World Ophthalmology Congress Abstracts, ISRET-SA 232; 2012. [Google Scholar]
- 2.Chaudhry GR, Fecek C, Lai MM, et al. Fate of embryonic stem cell derivatives implanted into the vitreous of a slow retinal degenerative mouse model. Stem Cells Dev. 2009;18(2):247–258. doi: 10.1089/scd.2008.0057. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Zhong X, Yan J, et al. Pluripotent embryonic stem cells developed into medulloepithelioma in nude mice eyes. Yan Ke Xue Bao. 2002;18(1):37–44. [PubMed] [Google Scholar]
- 4.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45(12):4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Bogt KE, Swijnenburg Rj, Cao F, Wu JC. Molecular imaging of human embryonic stem cells: keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle. 2006;5(23):2748–2752. doi: 10.4161/cc.5.23.3533. [DOI] [PubMed] [Google Scholar]
- 6.US FDA Cellular, Tissue and Gene Therapies Advisory Committee. Cellular and Gene Therapies for Retinal Disorders (CTGTAC Meeting #52). Cellular, Tissue and Gene Therapies Advisory Committee, FDA; USA. 2011. [Google Scholar]
- 7▪.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi: 10.1038/nature10761. Recent review covering various aspects of the induced pluripotent stem cell technology, including an up-to-date summary of different methods of induced pluripotent stem cell derivation and their application in disease modeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Mostoslavsky G. Concise review: the magic act of generating induced pluripotent stem cells: many rabbits in the hat. Stem Cells. 2012;30(1):28–32. doi: 10.1002/stem.742. [DOI] [PubMed] [Google Scholar]
- 11.Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron. 2011;70(4):597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 12▪.Salero E, Blenkinsop TA, Corneo B, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10(1):88–95. doi: 10.1016/j.stem.2011.11.018. Describes the identification of a multipotent stem cell population in the adult human retinal pigment epithelium, that can be differentiated into retinal pigment epithelium or, surprisingly, into mesenchymal lineages. [DOI] [PubMed] [Google Scholar]
- 13.O’Callaghan AR, Daniels JT. Concise review: limbal epithelial stem cell therapy: controversies and challenges. Stem Cells. 2011;29(12):1923–1932. doi: 10.1002/stem.756. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander M, Dorrell MI, Ritter MR, et al. Progenitor cells and retinal angiogenesis. Angiogenesis. 2007;10(2):89–101. doi: 10.1007/s10456-007-9070-4. [DOI] [PubMed] [Google Scholar]
- 15.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 16.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 17.Bonilha Vl, Rayborn ME, Bhattacharya SK, et al. The retinal pigment epithelium apical microvilli and retinal function. Adv Exp Med Biol. 2006;572:519–524. doi: 10.1007/0-387-32442-9_72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10(9):802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharti K, Miller SS, Arnheiter H. The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24(1):21–34. doi: 10.1111/j.1755-148X.2010.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75– 80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 21.Del Priore LV, Tezel TH, Kaplan HJ. Maculoplasty for age-related macular degeneration: reengineering Bruch‘s membrane and the human macula. Prog Retin Eye Res. 2006;25(6):539–562. doi: 10.1016/j.preteyeres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26(6):598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23▪.Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26(5):516–554. doi: 10.1016/j.preteyeres.2007.02.002. Review on surgical techniques currently used in retinal pigment epithelium transplantation, both in suspension or as a patch of retinal pigment epithelium/choroidal sheet, summarizing biological and artificial substrates investigated as matrices for the retinal pigment epithelium. [DOI] [PubMed] [Google Scholar]
- 24.Tezel TH, Del Priore LV, Berger AS, Kaplan HJ. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. 2007;143(4):584–595. doi: 10.1016/j.ajo.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6(3):217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 26.Lund Rd, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8(3):189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 27.Corneo B, Temple S. Sense and serendipity aid RPE generation. Cell Stem Cell. 2009;5(4):347–348. doi: 10.1016/j.stem.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 29.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458(3):126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5(4):396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Osakada F, Jin Zb, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122(Pt 17):3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 32.Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227(2):457–466. doi: 10.1002/jcp.22814. [DOI] [PubMed] [Google Scholar]
- 33.Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27(9):2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 34▪.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. Encouraging report on the results of the first clinical trial using human embryonic stem cell-derived progeny. Human embryonic stem cell-derived retinal pigment epithelium were transplanted in one patient with severe age-related macular degeneration and one patient with Stargardt’s macular dystrophy. [DOI] [PubMed] [Google Scholar]
- 35.Taylor AW, Kaplan HJ. Ocular immune privilege in the year 2010: ocular immune privilege and uveitis. Ocul Immunol Inflamm. 2010;18(6):488–492. doi: 10.3109/09273948.2010.525730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr Aj, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- 37.Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214(2):347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Jin Zb, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS ONE. 2011;6(2):e17084. doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Mcgill TJ, Cottam B, Lu B, et al. Transplantation of human central nervous system stem cells – neuroprotection in retinal degeneration. Eur J Neurosci. 2012;35(3):468–477. doi: 10.1111/j.1460-9568.2011.07970.x. Neural stem cells have been used in clinical trials for various neurological disorders. This paper describes how they can also protect host photoreceptors and preserve visual function after transplantation in an animal model of retinal degeneration, forming the basis for a clinical trial of neural stem cells for retinal disease. [DOI] [PubMed] [Google Scholar]
- 40▪.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25(3):602–611. doi: 10.1634/stemcells.2006-0308. Umbilical tissue-derived stem cells are shown here to rescue photoreceptors in animal models of retinal degeneration, opening the route to a clinical trial in age-related macular degeneration patients. [DOI] [PubMed] [Google Scholar]
- 41.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 42.Otani A, Dorrell Mi, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114(6):765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a Phase I trial. Retina. 2011;31(6):1207–1214. doi: 10.1097/IAE.0b013e3181f9c242. A 10-month safety report on the use of bone marrow-derived stem cells as therapy for retinal diseases, encouraging further trials to determine efficacy. [DOI] [PubMed] [Google Scholar]
- 44.Moore AT. Cone and cone-rod dystrophies. J Med Genet. 1992;29(5):289–290. doi: 10.1136/jmg.29.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 46.Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72(9):1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 47▪.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. 10-year follow-up report, showing permanent, stable restoration of the corneal epithelium in patients with severe eye burns who underwent autologous limbal stem cell transplantation. [DOI] [PubMed] [Google Scholar]
- 48.Wylegala E, Dobrowolski D, Tarnawska D, et al. Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol. 2008;18(6):886–890. doi: 10.1177/112067210801800605. [DOI] [PubMed] [Google Scholar]
- 49.Mohand-Said S, Hicks D, Simonutti M, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29(5):290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- 50.Kwan AS, Wang S, Lund RD. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol. 1999;159(1):21–33. doi: 10.1006/exnr.1999.7157. [DOI] [PubMed] [Google Scholar]
- 51.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West EL, Pearson RA, Barker SE, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28(11):1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips JB, Blanco-Sanchez B, Lentz JJ, et al. Harmonin (Ush1c) is required in zebrafish Muller glial cells for photoreceptor synaptic development and function. Dis Model Mech. 2012;4(6):786–800. doi: 10.1242/dmm.006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamba DA, Reh TA. Microarray characterization of human embryonic stem cell – derived retinal cultures. Invest Ophthalmol Vis Sci. 2011;52(7):4897–4906. doi: 10.1167/iovs.10-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue F, Johkura K, Shirasawa S, et al. Differentiation of primate ES cells into retinal cells induced by ES cell-derived pigmented cells. Biochem Biophys Res Commun. 2010;394(4):877–883. doi: 10.1016/j.bbrc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 57.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):763–778. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 59.Castellarin AA, Nasir M, Sugino IK, Zarbin MA. Progressive presumed choriocapillaris atrophy after surgery for age-related macular degeneration. Retina. 1998;18(2):143–149. doi: 10.1097/00006982-199818020-00008. [DOI] [PubMed] [Google Scholar]
- 60.Sodha S, Wall K, Redenti S, Klassen H, Young MJ, Tao SL. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J Biomater Sci Polym Ed. 2012;22(4–6):443–456. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 61.Mcusic AC, Lamba DA, Reh TA. Guiding the morphogenesis of dissociated newborn mouse retinal cells and hES cell-derived retinal cells by soft lithography-patterned microchannel PLGA scaffolds. Biomaterials. 2011;33(5):1396–1405. doi: 10.1016/j.biomaterials.2011.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thieltges F, Stanzel BV, Liu Z, Holz FG. A nanofibrillar surface promotes superior growth characteristics in cultured human retinal pigment epithelium. Ophthalmic Res. 2011;46(3):133–140. doi: 10.1159/000324045. [DOI] [PubMed] [Google Scholar]
- 63.Simell O, Takki K. Raised plasma-ornithine and gyrate atrophy of the choroid and retina. Lancet. 1973;1(7811):1031–1033. doi: 10.1016/s0140-6736(73)90667-3. [DOI] [PubMed] [Google Scholar]
- 64.Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29(8):1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.Advanced Cell Technology. www.advancedcell.com.