Abstract
Dermal exposure to the vesicant sulfur mustard causes marked inflammation and tissue damage. Basal keratinocytes appear to be a major target of sulfur mustard. In the present studies, mechanisms mediating skin toxicity were examined using a mouse skin construct model and a full-thickness human skin equivalent (EpiDerm-FTTM). In both systems, administration of the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide (CEES, 100–1000 µM) at the air surface induced mRNA and protein expression of heat shock proteins 27 and 70 (Hsp27 and Hsp70). CEES treatment also resulted in increased expression of caveolin-1, the major structural component of caveolae. Immunohistochemistry revealed that Hsp27, Hsp70 and caveolin-1 were localized in basal and suprabasal layers of the epidermis. Caveolin-1 was also detected in fibroblasts in the dermal component of the full thickness human skin equivalent. Western blot analysis of caveolar membrane fractions isolated by sucrose density centrifugation demonstrated that Hsp27 and Hsp70 were localized in caveolae. Treatment of mouse keratinocytes with filipin III or methyl-β-cyclodextrin, which disrupt caveolar structure, markedly suppressed CEES-induced Hsp27 and Hsp70 mRNA and protein expression. CEES treatment is known to activate JNK and p38 MAP kinases; in mouse keratinocytes, inhibition of these enzymes suppressed CEES-induced expression of Hsp27 and Hsp70. These data suggest that MAP kinases regulate Hsp 27 and Hsp70; moreover, caveolae-mediated regulation of heat shock protein expression may be important in the pathophysiology of vesicant-induced skin toxicity.
Keywords: caveolin-1, sulfur mustard, MAP kinase, skin, heat shock proteins, caveolae
Introduction
Exposure to the chemical warfare agent sulfur mustard, a highly reactive vesicant, and related analogs including, 2-chloroethyl ethyl sulfide (CEES) or half mustard, is known to result in persistent skin damage that includes keratinocyte necrosis, inflammation and blistering (Papirmeister et al., 1991). Toxicity is thought to be mediated by alkylation of cellular macromolecules including proteins, lipids and nucleic acids (Shakarjian et al., 2010; Laskin et al., 2010). This can result in activation of signal transduction pathways regulating cell growth, differentiation and apoptosis contributing to toxicity (Dillman et al., 2004; Rebholz et al., 2008; Kehe et al., 2009; Pal et al., 2009; Black et al., 2010b).
Heat shock proteins (Hsp’s) are molecular chaperones important in the folding, assembly and degradation of proteins; they are upregulated in response to cellular stresses such as heat shock, inflammation, and electrophilic and oxidative stress (Macario and Conway de Macario, 2007; Liberek et al., 2008). Hsp’s also play critical roles in regulating signal transduction, protein trafficking, cellular proliferation and differentiation (Helmbrecht et al., 2000). Two prominent Hsp’s are 27 kDa Hsp (Hsp27) and 70 kDa Hsp (Hsp70). While Hsp27 is an ATP-independent molecular chaperone that protects against stress-induced aggregation of proteins, Hsp70 is ATP-dependent and functions in protein folding (Macario and Conway de Macario, 2007). In intact skin, Hsp70 expression is observed throughout the epidermis, while Hsp27 is localized primarily in the granular and spinous layers, indicating that this protein is also involved in keratinocyte differentiation (Robitaille et al.; Gandour-Edwards et al., 1994; Trautinger et al., 1995; Trautinger, 2001). Increases in both Hsp27 and Hsp70 have been reported in human and mouse skin, as well as in cultured keratinocytes and skin explants in response to heat shock, UVB light, heavy metals, oxidative stress, and inflammatory mediators (Maytin, 1995; Trautinger, 2001). Hsp’s are thought to protect against cellular stress and toxicity by modulating intracellular signal transduction pathways such as MAP kinase and Nrf2 (Mosser et al., 2000). Hsp27 and Hsp70 are also known to interact with the cytoskeleton and are involved in the regulation of actin and microtubulin polymerization and reorganization (Liang and MacRae, 1997).
Caveolae comprise a subset of membrane lipid rafts important in intracellular signaling, lipid transport and protein trafficking (Cohen et al., 2004). Caveolin-1 is the primary structural protein in caveolae (Liu et al., 2002). Although caveolae play an important role in regulating growth and differentiation in keratinocytes, they do not appear to be required for skin development (Roelandt et al., 2009). Thus, while caveolin-1 knockout mice can generate functional epidermis, aberrant keratinocyte proliferation and differentiation are observed (Capozza et al., 2003; Roelandt et al., 2009). Previous studies have shown that Hsp27 and Hsp70 are present in lipid rafts and co-localize with caveolar proteins (Broquet et al., 2003; Chen et al., 2005; Staubach et al., 2009). Moreover, there appears to be a link between caveolae-mediated cell signaling and cytoskeletal organization which may be important in regulating keratinocyte responses to stress (Head et al., 2006).
In the present studies, we analyzed the effects of CEES on expression of Hsp27 and Hsp70 in a full-thickness human skin equivalent and a mouse keratinocyte skin construct model (Black et al., 2010a) and investigated the role of caveolae in regulating expression of these proteins. CEES was found to markedly upregulate expression of Hsp 27 and Hsp70, a MAP kinase-dependent process in which these Hsp’s were selectively localized in caveolae. Disruption of caveolae blocked CEES-induced changes in expression of the Hsp’s, demonstrating a direct link between sensitivity to CEES and caveolae expression. Hsp expression via the formation of caveolae may be an adaptive response important in protecting cells during stress. Thus, caveolae may play a key role in activation of signaling pathways and protein trafficking in mediating the keratinocytes response to sulfur mustard.
Materials and Methods
Chemicals and reagents
All primary antibodies were affinity-purified. Rabbit polyclonal antibodies to p38, phospho-p38, JNK, phospho-JNK, ERK1/2, and phospho-ERK1/2 were from Cell Signaling Technology (Beverly, MA). Rabbit monoclonal or polyclonal antibodies to caveolin-1 were from Cell Signaling Technology or Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to Hsp70, goat polyclonal antibodies to Hsp27 and β-actin, and horseradish peroxidase-labeled donkey anti-goat secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-labeled goat anti-rabbit secondary antibodies and detergent-compatible protein assay reagents were obtained from Bio-Rad Laboratories (Hercules, CA). MultiScribe Reverse Transcriptase was from Promega (Madison, WI), the RNeasy purification kit from Qiagen (Minneapolis, MN), and the Western Lightning enhanced chemiluminescence kit (ECL) from Perkin Elmer Life Sciences, Inc. (Boston, MA). Rabbit and goat IgG were from ProSci (Poway, CA) and the Vectastain Rabbit and Goat Kits and the Peroxidase Substrate Kit DAB from Vector Labs (Burlingame, CA). SYBR Green Master Mix and other PCR reagents were purchased from Applied Biosystems (Foster City, CA) and precast gradient polyacrylamide gels from Pierce Biotechnology, Inc. (Rockford, IL). Cell culture reagents were from Invitrogen Corp (Carlsbad, CA) and polyester Transwell permeable supports from Corning Life Sciences Inc (Acton, MA). SP600125 and filipin III were from EMD Biosciences (La Jolla, CA) and CEES, SB203580, methyl-β-cyclodextrin (MBCD), protease inhibitor cocktail and all other chemicals from Sigma-Aldrich (St. Louis, MO). CEES (100 mM stock solution) was prepared fresh in absolute ethanol immediately before use and diluted to the appropriate concentrations in PBS.
Mouse keratinocyte skin construct
A three dimensional skin-like structure was generated using PAM212 mouse keratinocytes grown in DMEM supplemented with 10% fetal bovine serum as previously described (Black et al., 2010b). Briefly, cells (1 × 106) were grown on 24-mm Transwell semi-permeable polyester membrane supports (0.4 µm pore size) in 6-well cell culture plates. After reaching confluence, the medium was removed from the upper portion of the Transwell such that the cells were exposed to air at the apical surface and in contact with the medium at the basolateral surface. After 24 hr incubation at 37°C in a humidified CO2 incubator, cells were treated with 1 ml vehicle control or CEES for 2 hr. The Transwells were removed, washed in PBS and immediately replaced in the same 6-well culture plates. In some experiments, the cells were preincubated at 37°C for 30 min or 3 hr prior to CEES treatment at the apical surface with p38 MAP kinase inhibitor (SB203580, 10 µM), JNK inhibitor (SP600125, 20 µM), caveolae inhibitors (filipin, 10 µM or MBCD, 5 mM), or DMSO control.
Human skin equivalent construct model
EpiDerm-FTTM full-thickness human skin equivalents (EFT-400) and EFT-400-MM medium, supplemented with growth factors, hormones and lipid precursors, were kindly provided by MatTek Corporation (Ashland, MA). The skin equivalents were placed in 6-well plates in 2 ml of EFT-400-MM medium. After overnight incubation at 37° C in a humidified CO2 incubator, 1 ml of PBS containing vehicle control or CEES was added to the apical surface of the tissues. After 2 hr, the skin equivalents were removed from the plates, washed in PBS, immediately replaced in the same 6-well culture dishes and incubated for an additional 2–72 hr. For immunohistochemistry, tissues were fixed in 3% paraformaldehyde in PBS supplemented with 2% sucrose at 4°C for 24 hr, and then removed from the supports, transferred to 50% ethanol and paraffin embedded. Tissue sections (5 µm) were deparaffinized, blocked in 100% serum at room temperature for 2 hr, and then incubated overnight at 4°C with rabbit or goat IgG control, anti-Hsp27 antibody (1:400), anti-Hsp70 antibody (1:500) or anti-caveolin-1 antibody (1:800) followed by incubation with biotinylated anti-rabbit or goat antibodies (1:200) for 30 min at room temperature. Binding was visualized using a Peroxidase Substrate Kit. For PCR and Western blot analysis, the epidermis was removed from unfixed skin equivalents by gentle peeling and immediately analyzed for mRNA or protein expression.
Isolation of caveolae
Caveolar fractions of mouse keratinocytes were prepared as described by Smart et al. (Smart et al., 1995). Briefly, after incubation with 300 µM CEES or control, cells were scraped from the Transwell supports in a sucrose buffer (0.25 M sucrose, 20 mM Tricine, 1 mM EDTA, pH 7.8), centrifuged at 1400 × g for 5 min at room temperature, and resuspended in 0.5 ml sucrose buffer. The cells were then homogenized using a glass Dounce homogenizer and centrifuged at 1000 × g for 10 min at 4°C. The supernatant was collected and the pellet resuspended in an additional 0.5 ml of sucrose buffer, homogenized, and centrifuged. The second supernatant was combined with the first supernatant which was then carefully layered onto 9 ml Percoll solution (30% Percoll in sucrose buffer) and centrifuged at 84,000 × g for 30 min in a Ti-70 rotor using a Beckman Coulter L7–55 ultracentrifuge. The caveolar fraction, visible as a white band approximately 2 cm from the top of the ultracentrifuge tube, was collected and protease inhibitor cocktail (1:100) added.
Western blotting
Lysates were prepared using buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl and 1% Triton X-100 supplemented with 5 µl protease inhibitor cocktail [4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, bestatin hydrochloride, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, EDTA and leupeptin]. Proteins (20 µg) from lysates were separated on 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. After incubation in blocking buffer (5% dry milk Tris-buffered saline containing 0.1% Tween 20) for 1 hr at room temperature, the membranes were incubated overnight at 4°C with anti-Hsp27 (1:400), anti-Hsp70 (1:200) or anti-caveolin-1 (1:1000) antibodies followed by horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature. Protein expression was visualized using enhanced chemiluminescence (ECL) reagents.
Real-time polymerase chain reaction
RNA was isolated using the Versagene RNA purification kit following the manufacturer’s protocol. RNA was converted to cDNA using Superscript reverse transcriptase. The cDNA was diluted 1:10 in RNase-DNase-free water for PCR analysis. For each gene, a standard curve composed of a serial dilution of pooled cDNA from the samples was used as a reference. All values were normalized to GAPDH (n = 3). The control was assigned a value of one and samples calculated relative to control. Real-time PCR was performed on an ABI Prism 7300 Sequence Detection System using 96-well optical reaction plates. SYBR-Green was used for detection of fluorescent signal and the standard curve method was used for relative quantitative analysis. The primer sequences for the genes were generated using Primer Express software (Applied Biosystems) and the oligonucleotides were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The forward and reverse sequences (5’ → 3’) used are listed in Table 1.
Table 1.
Real-time PCR primer sequences.
| Gene | Forward (5’→3’) | Reverse (5’→3’) |
|---|---|---|
| Human | ||
| Caveolin-1 | AGACGAGCTGAGCGAGAAGC | TCGATCTCCTTGGTGTGCG |
| GAPDH | TGGGCTACACTGAGCACCAG | GGGTGTCGCTGTTGAAGTCA |
| Hsp27 | AAGCTAGCCACGCAGTCCAA | CGACTCGAAGGTGACTGGGA |
| Hsp70 | ATGTCGGTGGTGGGCATAGA | CACAGCGACGTAGCAGCTCT |
| Mouse | ||
| Caveolin-1 | ACCGTGCATCAAGAGCTTCC | TAGACGCGGCTGATGCACT |
| GAPDH | TGAAGCAGGCATCTGAGGG | CGAAGGTGGAAGAGTGGGAG |
| Hsp27 | AAGGAAGGCGTGGTGGAGAT | TTCGTCCTGCCTTTCTTCGT |
| Hsp70 | CAGCGAGGCTGACAAGAAGAA | GGAGATGACCTCCTGGCACT |
Statistical analysis
All experiments were repeated 3 times with similar results. Data are expressed as mean ± SEM. Statistical differences between the means were determined using two-way ANOVA and considered significant at p < 0.05.
Results
Effects of CEES on expression of Hsp’s
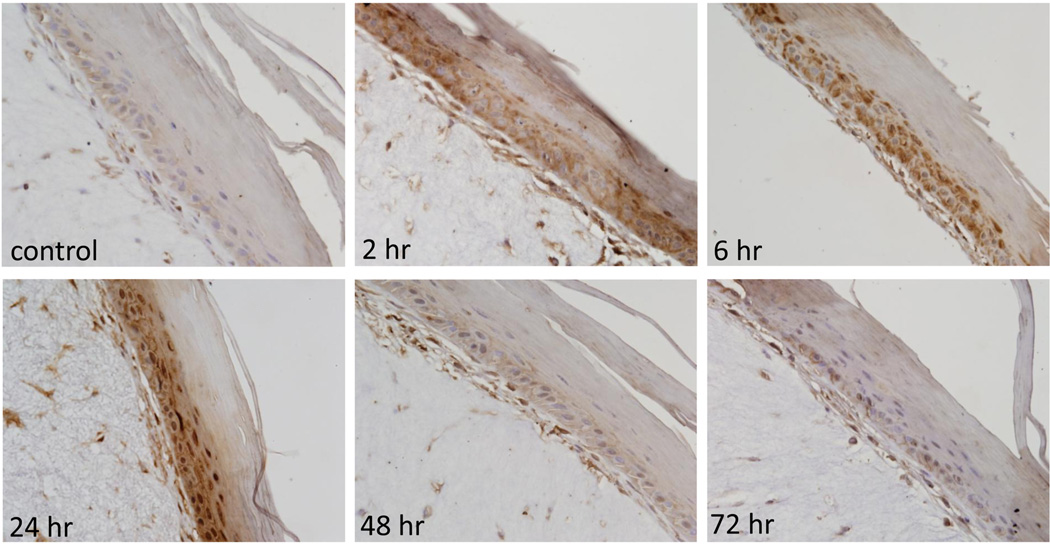
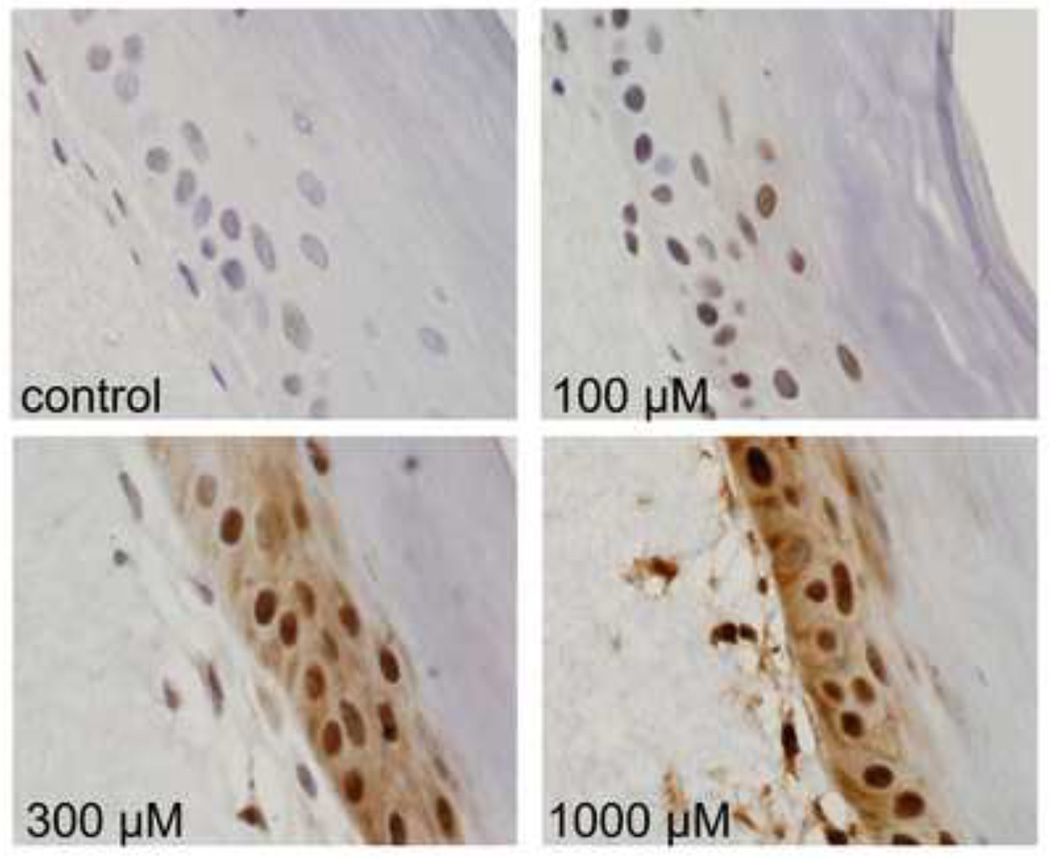
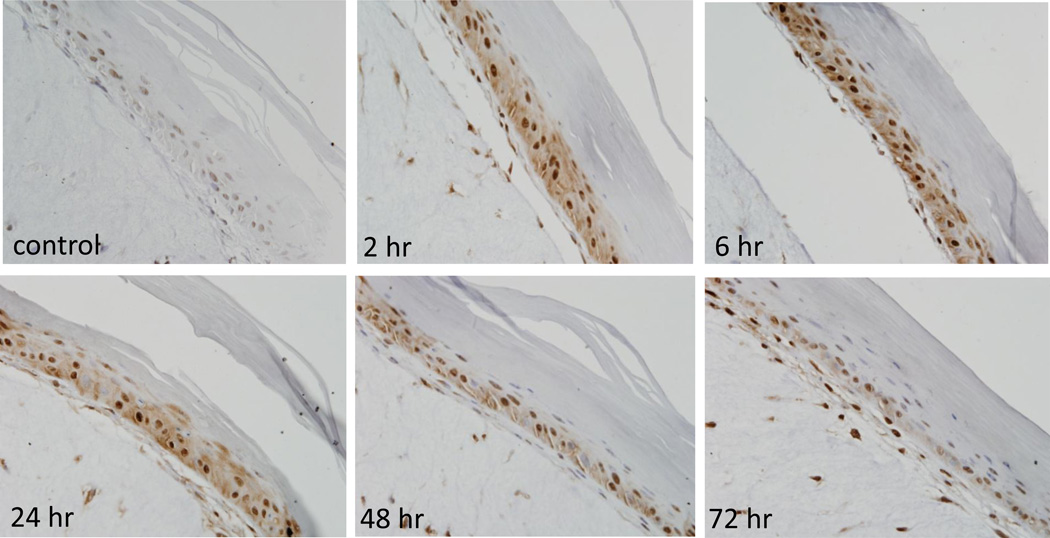
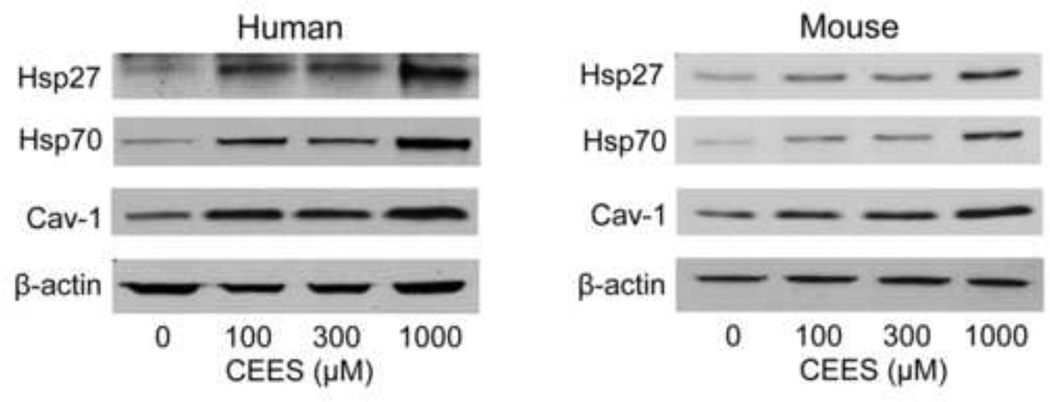
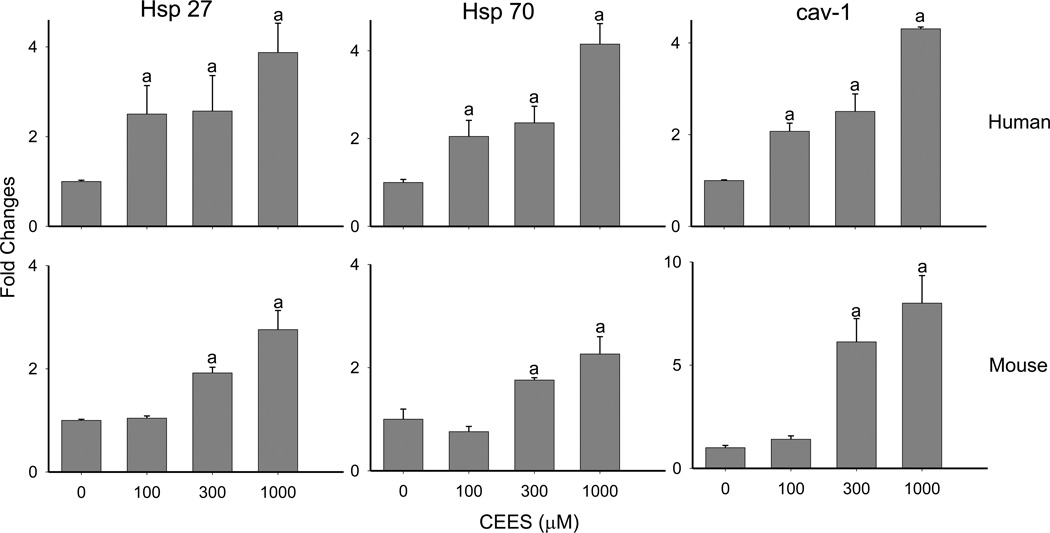
In initial studies we determined if CEES treatment of human and mouse skin construct models altered expression of Hsp27 and Hsp70. Immunohistochemical analysis of the human skin equivalent revealed low constitutive expression of Hsp27 and Hsp70 in control keratinocytes (Figs. 1–4). Treatment of the human skin equivalent with CEES (100–1000 µM) resulted in a time- and concentration-dependent increase in both Hsp27 and Hsp70 in keratinocytes, as well as fibroblasts which was maximal at 24 hr (Fig. 1 and 2). The Hsp’s were identified in both cytosol and nuclei of basal and suprabasal keratinocytes and fibroblasts. CEES-induced upregulation of Hsp27 and Hsp70 was confirmed by western blotting and real time PCR analysis of epidermal sheets isolated from the skin equivalents (Figs. 5 and 6). Maximal effects were noted with 1000 µM CEES. Similar results were observed in the mouse skin construct model. Thus, CEES treatment (100–1000 µM) resulted in concentration-dependent increases in mRNA expression of Hsp27 and Hsp70 24 hr post-treatment (Fig. 6). CEES also upregulated Hsp27 and Hsp70 protein expression with maximal increases detected at 1000 µM CEES (Fig. 5).
Figure 1. Effects of CEES on Hsp27 expression in a full-thickness human skin equivalent.
EpiDerm-FTTM was exposed on the air surface with CEES (1000 µM) or control. Tissues were collected 2–72 hr later and stained with anti-Hsp27 antibody. Binding was visualized using a peroxidase DAB substrate kit. Original magnification, 400×.
Figure 4. Effects of CEES on Hsp70 expression in a full-thickness human skin equivalent.
EpiDerm-FTTM was exposed to CEES (100–1000 µM) or control. Tissues were collected 24 hr later and stained with antibodies to Hsp70. Binding was visualized using a peroxidase DAB substrate kit. Original magnification, 1000×.
Figure 2. Effects of CEES on Hsp70 expression in a full-thickness human skin equivalent.
EpiDerm-FTTM was exposed on the air surface with CEES (1000 µM) or control. Tissues were collected 2–72 hr later and stained with anti-Hsp70 antibody. Binding was visualized using a peroxidase DAB substrate kit. Original magnification, 400×.
Figure 5. CEES upregulates Hsp27, Hsp70 and caveolin-1 protein expression in a full-thickness human skin equivalent and in a mouse keratinocyte skin construct.
EpiDerm-FTTM or mouse keratinocyte skin constructs were exposed to CEES (100–1000 µM) or control. After 24 hr, epidermal sheets from the skin equivalents and keratinocytes from the skin construct were collected and analyzed for Hsp27, Hsp70 and caveolin-1 (cav-1) protein expression by Western blotting. β-actin was used as a control for equal protein loading.
Figure 6. CEES upregulates Hsp27, Hsp70 and caveolin-1 mRNA expression in a full-thickness human skin equivalent and in a mouse keratinocyte skin construct.
EpiDerm-FTTM or mouse keratinocyte skin constructs were exposed to CEES (100–1000 µM) or control. After 24 hr, epidermal sheets from the skin equivalents and keratinocytes from the skin construct were collected and analyzed for Hsp27, Hsp70 and caveolin-1 (cav-1) mRNA expression by real-time PCR. Data are presented as fold change in gene expression relative to control cells. aSignificantly (p < 0.05) different from control.
Role of caveolae in CEES-induced alterations in Hsp27 and Hsp70 expression
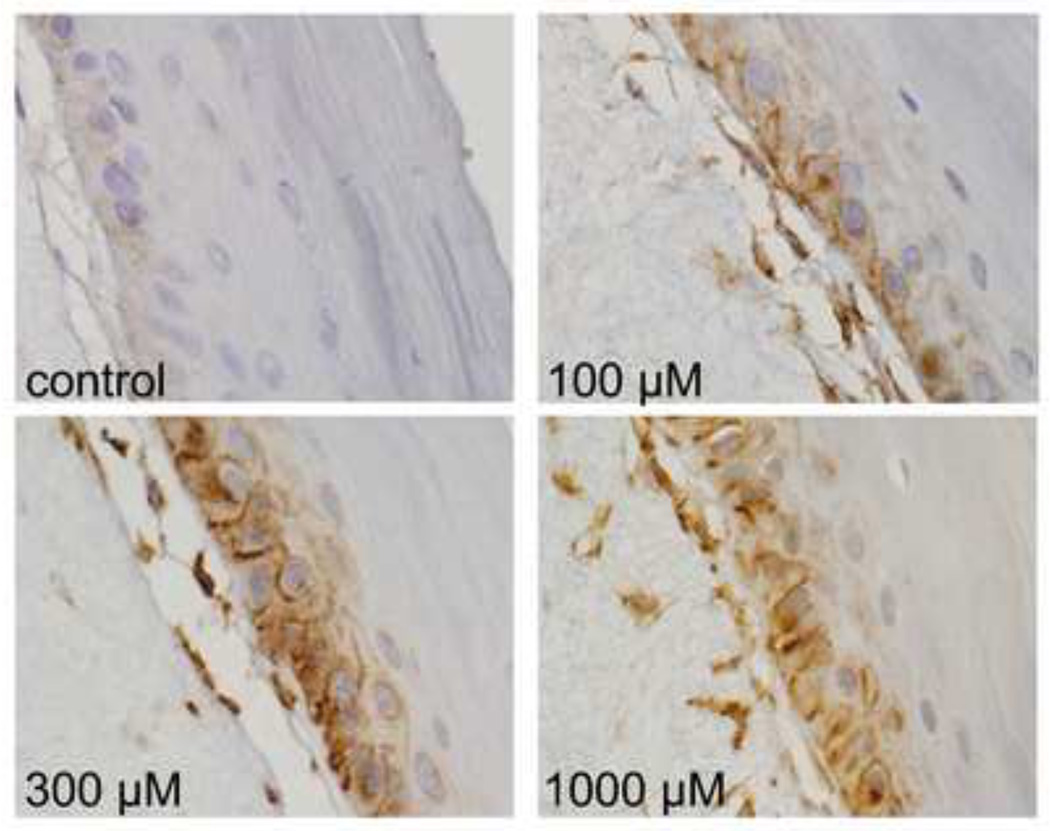
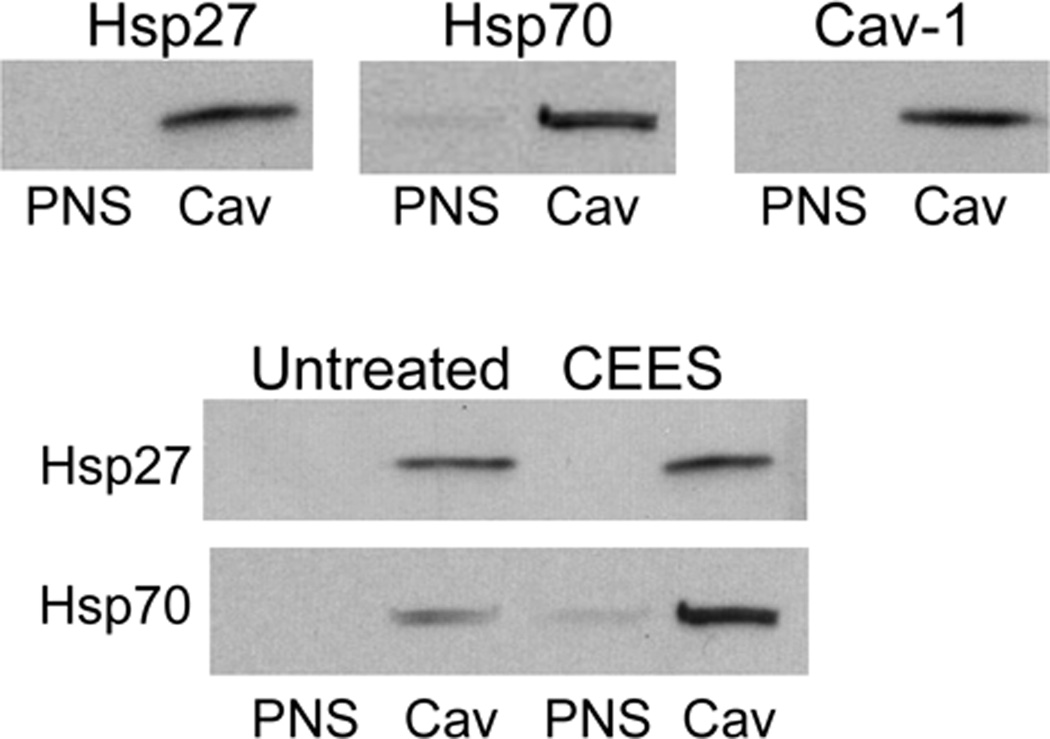
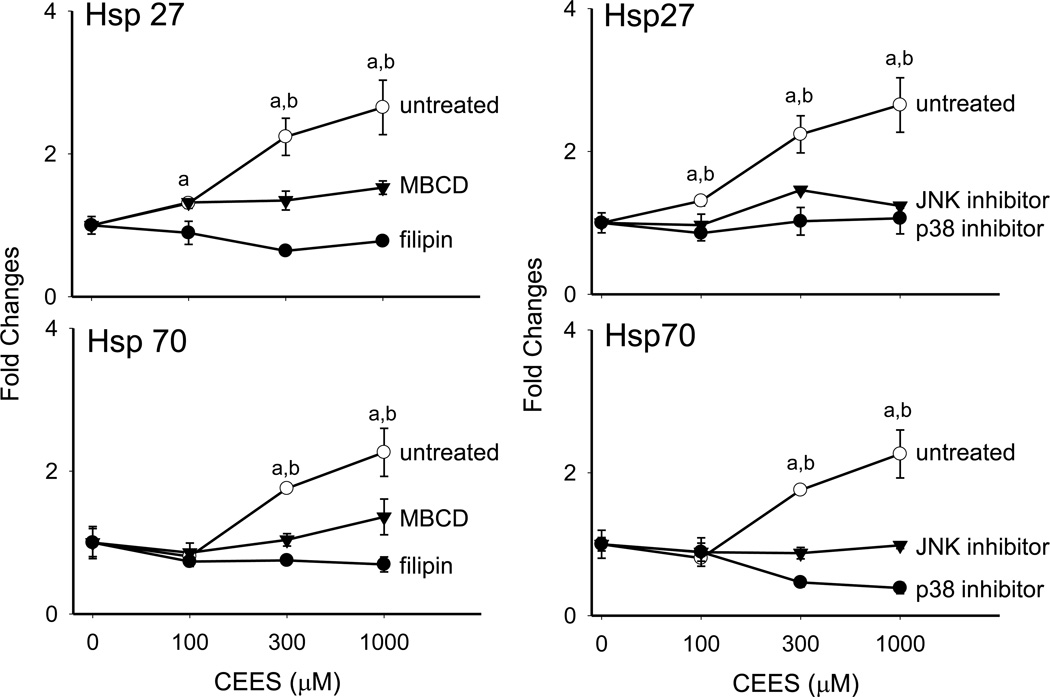
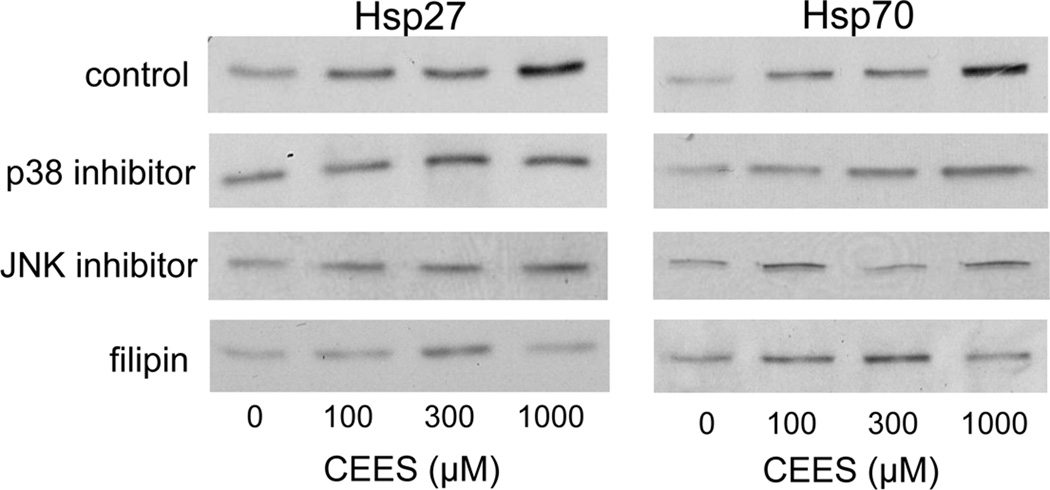
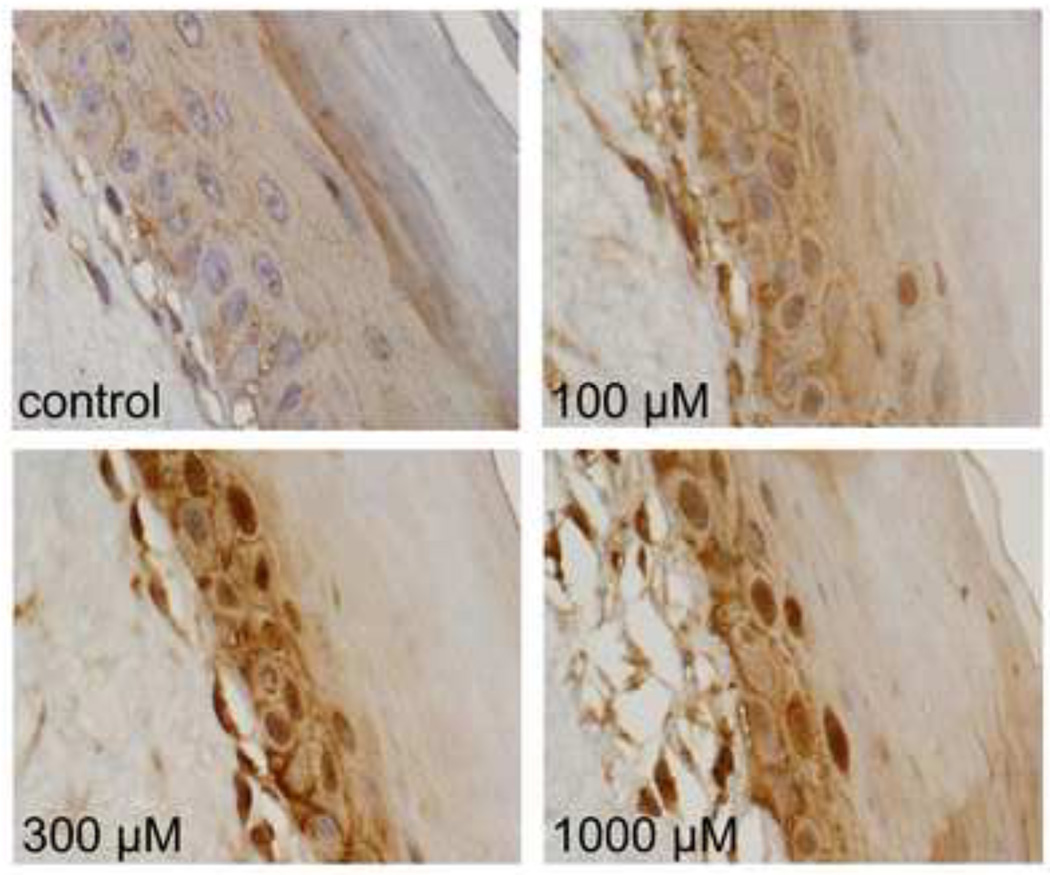
In further studies, we determined if plasma membrane caveolae play a role in mediating CEES-induced increases in expression of Hsp27 and Hsp70. Analysis of tissue sections by immunohistochemistry revealed low constitutive expression of caveolin-1, the major structural component of caveolae, in basal keratinocytes and fibroblasts in human skin equivalents. CEES treatment (100–1000 µM) upregulated expression of this protein in both cell types; maximal expression was evident after 24 hr with 300–1000 µM CEES (Fig. 7). CEES-induced caveolin-1 expression was extranuclear, and predominantly noted as punctate staining of keratinocyte membranes throughout the basal layer (Fig. 7). CEES treatment was also found to up regulate caveolin-1 mRNA (up to 4-fold after 24 hr) and protein expression (Figs. 5 and 6) in isolated epidermal sheets from the human skin equivalents. In the mouse skin construct model, CEES also caused a concentration-dependent increase in caveolin-1 mRNA (up to 8-fold after 24 hr) and protein (Figs. 5 and 6). In these cells, maximal increases were detected at 1000 µM CEES. In further experiments, caveolae were isolated from untreated and CEES (300 µM)-treated keratinocytes from the mouse skin construct model and analyzed for Hsp27 and Hsp70 expression. Interestingly, both Hsp’s were found to be selectively expressed in caveolae (Fig. 8, upper panel). Moreover, CEES treatment resulted in increases in Hsp27 and Hsp70 that were also selectively localized in caveolae (Fig. 8, lower panel). Treatment of the keratinocytes filipin (20 µM) or methyl-β-cyclodextrin (MBCD; 5 mM), which disrupt caveolar integrity (Simons and Toomre, 2000), inhibited CEES-induced increases in expression of mRNA and protein for Hsp27 and Hsp70 (Fig. 9, left panel, Fig. 10, and not shown).
Figure 7. Effects of CEES on caveolin-1 expression in a full-thickness human skin equivalent.
EpiDerm-FTTM was exposed to CEES (100–1000 µM) or control. Tissues were collected 24 hr later and stained with antibodies to caveolin-1 (cav-1). Protein expression was visualized using a peroxidase DAB substrate kit. Original magnification, 1000×.
Figure 8. Localization of Hsp27 and Hsp70 in caveolae.
Mouse keratinocyte skin constructs were exposed to CEES (300 µM) or control. After 24 hr, caveolar (Cav) and post-nuclear supernatant (PNS) subcellular fractions were isolated from the cells using sucrose density gradient centrifugation as described in the Materials and Methods. Upper panel: Cav and PNS fractions were assayed for Hsp27 and Hsp70 by Western blotting. The relative purity of the caveolar fractions was determined by Western blot analysis using caveolin-1 antibodies. Lower panel: Effect of CEEES on Hsp27 and Hsp70 expression in cav and PNS subcellular fractions isolated from control and CEES-treated mouse keratinocytes.
Figure 9. Effects of caveolae and MAP kinase inhibitors on CEES-induced expression of Hsp27 and Hsp70.
Mouse keratinocyte skin constructs were preincubated with the caveolae inhibitors, filipin (10 µM), and MBCD (5 mM), or control for 30 min, or JNK (SP600125, 20 µM) and p38 (SB203580, 10 µM) MAP kinase inhibitors or control for 3 hr, and then exposed to CEES (0, 100, 300 or 1000 µM). After 24 hr, mRNA was isolated from the cells and analyzed by real-time PCR. Data are presented as fold change in gene expression relative to untreated cells. Left panel: Effects of caveolae inhibition on Hsp27 and Hsp70 expression. aSignificantly (p < 0.05) different from control (filipin); bSignificantly different (p < 0.05) from control (MBCD). Right panel: Effects of MAP kinase inhibition on Hsp27 and Hsp70 expression. aSignificantly (p < 0.05) different from control (p38 inhibitor); bSignificantly different (p < 0.05) from control (JNK inhibitor).
Figure 10. Effects of inhibitors on CEES-induced protein expression of Hsp27 and Hsp70.
Mouse keratinocyte skin constructs were preincubated with the caveolae inhibitors, filipin (10 µM), and MBCD (5 mM), or control for 30 min or JNK (SP600125, 20 µM), and p38 (SB203580, 10 µM) MAP kinase inhibitors or control for 3 hr then exposed to CEES (0, 100, 300 or 1000 µM). After 24 hr total cellular lysates were prepared and Hsp27 and Hsp70 protein expression analyzed by Western blotting.
Role of MAP kinase signaling in CEES-induced alterations in heat shock proteins
Both sulfur mustard and CEES are known to activate MAP kinase signaling in intact skin and cultured keratinocytes (Dillman et al., 2004; Rebholz et al., 2008; Pal et al., 2009). CEES (100–1000 µM) also activates JNK and p38 MAP kinases in mouse keratinocytes in the skin construct model (Black et al., 2010b). To assess the role of JNK and p38 MAP kinases in regulating CEES-induced alterations in expression of Hsp27 and Hsp70, we used specific inhibitors of these enzymes. Treatment of the mouse keratinocytes with the p38 MAP kinase inhibitor, SB203580, or the JNK inhibitor, SP600125, suppressed CEES-induced expression of Hsp27 and Hsp70 mRNA and protein (Fig. 9, right panel, and Fig. 10). p38 kinase inhibition was more effective than JNK inhibition in suppressing expression of caveolin-1 mRNA and protein.
Discussion
Dermal vesicants such as sulfur mustard or CEES are known to induce oxidative stress (Shakarjian et al., 2010; Laskin et al., 2010). A characteristic response to oxidative stress is the upregulation of Hsp’s, an important class of molecular chaperones which function to minimize tissue injury (Helmbrecht et al., 2000). Previous studies have demonstrated that Hsp27 and Hsp70 are rapidly upregulated in human and mouse skin, as well as cultured keratinocytes in response to UVB light, ozone, heavy metals and inflammatory mediators (Maytin, 1995; Trautinger, 2001; Valacchi et al., 2004; Matsuda et al., 2010). We found that CEES also effectively upregulates expression of these Hsp’s in a full-thickness human skin equivalent and in a mouse keratinocyte skin construct. In the full-thickness human skin equivalent model, Hsp27 was evident in both basal and suprabasal layers of the epidermis. This is consistent with reports demonstrating that Hsp27 is increased in these cell layers in mouse skin during wound repair (Laplante et al., 1998). Overexpression of Hsp27 has been shown to upregulate cellular actin levels and to stimulate wound contraction during the healing process (Lavoie et al., 1993). These data suggest that increases in Hsp27 are key to triggering tissue repair. Hsp27 is also associated with the epidermal differentiation markers, involucrin, filaggrin, and transglutaminase suggesting that it contributes to the differentiation process (Robitaille et al.; Jonak et al., 2002).
Following CEES exposure, Hsp70 is also markedly increased in basal and suprabasal keratinocytes. Studies with mice overexpressing or lacking Hsp70 have demonstrated that Hsp70 is critical for epidermal repair following exposure to UVB light (Kwon et al., 2002; Matsuda et al., 2010). Additionally, in vivo delivery of Hsp70 accelerates the rate of full-thickness wound closure in mice (Kovalchin et al., 2006). In clinical studies, decreased Hsp70, as well as Hsp27, have been linked to delayed and impaired healing of chronic wounds commonly associated with diabetes (Atalay et al., 2009). Similarly, in diabetic mice, Hsp70 expression is delayed following wounding, leading to an increased healing time (McMurtry et al., 1999). These data suggest that Hsp70, like Hsp27, is critical for the early stages of wound repair. The mechanisms by which Hsp27 and Hsp70 contribute to this process are not known. As chaperones, these Hsp’s may protect critical proteins modified by vesicants. For example, recent studies have shown that both sulfur mustard and CEES alkylate components of the cytoskeleton, resulting in keratin aggregation and actin polymerization (Dillman et al., 2003; Hess and FitzGerald, 2007; Sayer et al., 2009). Heat shock proteins are key to maintaining the structure and organization of cytoskeletal filaments including actin and keratins (Liang and MacRae, 1997; Loffek et al., 2010). Increases in Hsp27 and Hsp70 in keratinocytes following CEES exposure may be important in maintaining cytoskeletal organization, thereby promoting cellular survival and wound repair.
As indicated above, vesicant-induced injury involves oxidative stress, a process that can result in protein and DNA oxidation and the formation of lipid peroxidation products (Pal et al., 2009; Black et al., 2010b). Protein damage resulting from oxidative stress such as structural denaturation and thiol oxidation results in increased expression of Hsp’s which target damaged molecules for degradation (Kalmar and Greensmith, 2009). Hsp’s also modulate cellular redox state, an activity that may also help to protect against further oxidative injury (Kalmar and Greensmith, 2009). In this regard, both Hsp27 and Hsp70 have been shown to maintain glutathione in its reduced form, and to decrease intracellular free iron concentrations, thus preventing excessive production of reactive oxygen species (Arrigo et al., 2005; Guo et al., 2007). Hsp70 has also been shown to control activation of histone H2AX, a critical signaling molecule in the repair of double strand breaks in DNA induced by oxidative stress (Gabai et al., 2010). This activity is consistent with our findings that Hsp70 localizes in the nucleus following CEES treatment. We have previously shown that CEES induces expression of the activated form of H2AX in the full-thickness human skin equivalent (Black et al., 2010a), and it may be that upregulation of Hsp70, as well as Hsp27, which can also localize in the nucleus, is key to regulating H2AX activation and subsequent DNA repair following exposure to vesicants.
Caveolae are specialized membrane lipid rafts that control a variety of biochemical signaling molecules and cellular processes including growth and differentiation (Cohen et al., 2004). The present studies demonstrate that Hsp27 and Hsp70 are localized, not only in the nucleus, but also in caveolar fractions of keratinocytes. CEES treatment was found to upregulate caveolar-associated expression of Hsp70, as well as Hsp27, although to a lesser extent. These data are in agreement with previous studies showing Hsp expression in detergent-resistant fractions containing lipid rafts and caveolae in many tissues and cell types (Nedellec et al., 2002; Triantafilou et al., 2002; Broquet et al., 2003; Chen et al., 2005; Lancaster and Febbraio, 2005). These latter studies also showed that cellular stressors such as heat shock, calcium depletion, endotoxin, and glucocorticoids increase Hsp expression in the lipid raft fractions. Caveolae are associated with cytoskeletal and other structural proteins including actin, annexin II, filamin, and dynamin which are thought to be important in regulating caveolar functioning (Stahlhut and van Deurs, 2000; Viola and Gupta, 2007). Hsp27 has been reported to associate with actin in lipid rafts fractions, possibly controlling actin and microtubule depolymerization (Piotrowicz and Levin, 1997). Thus, increases in Hsp27 following CEES-induced keratinocyte stress may be important in minimizing damage to caveolar-associated cytoskeletal proteins critical to caveolae function. Hsp70, on the other hand, appears to be primarily involved in responses to oxidative stress and innate immunity (Macario and Conway de Macario, 2007). In this regard, caveolar localization of Hsp70 has been shown to downregulate NADPH oxidase, thereby reducing reactive oxygen species production (Bocanegra et al., 2010). Hsp70, as well as Hsp27, have been detected in the extracellular environment, and it has been proposed that caveolae function as vehicles by which Hsp’s are transported to the cell membrane for externalization (Broquet et al., 2003). It is possible that Hsp70 localized in caveolae functions to prevent oxidative damage to proteins and lipids while extracellular Hsp70 acts as a danger signal to the innate immune system (Vega et al., 2008).
Of interest was our finding that CEES treatment also upregulated keratinocyte expression of caveolin-1, the major structural protein in caveolae (Cohen et al., 2004). Increases in caveolin-1 after vesicant exposure may be important in wound healing and in regulating keratinocyte growth and differentiation (Sando et al., 2003; Zheng and Bollinger Bollag, 2003; Roelandt et al., 2009). Upregulation of caveolin-1 has been shown to be important in protecting against radiation-induced DNA damage by activating repair mechanisms (Zhu et al., 2010). Caveolar localization of Hsp70 may allow it to interact with caveolin-1, thereby facilitating repair of DNA.
An unexpected finding in our studies was that Hsp27 and Hsp70 are not only localized in caveolae, but are also regulated by these structures. Thus, disruption of caveolae by cholesterol depletion markedly decreased CEES-induced Hsp27 and Hsp70 gene and protein expression. These results are in accord with previous reports showing that disruption of caveolae results in decreased heat shock-induced expression of Hsp’s (Broquet et al., 2003; Chen et al., 2005). It has also been shown that disruption of caveolae interferes with cellular release of Hsp70, providing additional support for the idea that caveolar internalization of Hsp70 is important in its transport to the cell membrane (Broquet et al., 2003; Lancaster and Febbraio, 2005).
A question remains as to the mechanism by which CEES upregulates Hsp27 and Hsp70. In previous studies we demonstrated that CEES activates JNK and p38 MAP kinase signaling pathways in the mouse skin equivalent model (Black et al., 2010b). Using inhibitors of these MAP kinases, we found that both JNK and p38 MAP kinase regulate expression of Hsp27 and Hsp70. Consistent with these results are studies showing that activation of Hsp27 and Hsp70 are p38 MAP kinase-dependent (Kato et al., 1999; Garmyn et al., 2001; Kim et al., 2005a; Kim et al., 2005b; Dasari et al., 2006; Banerjee Mustafi et al., 2009). Interestingly, activation of the p38 MAP kinase-Hsp27 signaling pathway has been shown to result in the formation of blisters in autoimmune blistering diseases (Berkowitz et al., 2005; Berkowitz et al., 2006; Berkowitz et al., 2008a; Berkowitz et al., 2008b). Using a pemphigus vulgaris mouse model, inhibition of p38 MAP kinase was found to prevent blister formation, as well as cytoskeletal reorganization and loss of cell-cell adhesion (Berkowitz et al., 2005; Berkowitz et al., 2006). It may be that inhibitors of p38 MAP kinase, as well as JNK kinase, can suppress sulfur mustard-induced epidermal damage and subsequent skin injury including blistering. In this regard, an inhibitor of p38 MAP kinase has been shown to downregulate sulfur mustard-induced cytokine release in human epidermal keratinocytes (Dillman et al., 2004).
In summary, the present studies showed that CEES exposure upregulates expression of Hsp27, Hsp70 and caveolin-1 in a full-thickness human skin equivalent and a mouse keratinocyte skin construct model. Moreover, Hsp27 and Hsp70 are localized in caveolar subcellular fractions and expression of these proteins is regulated by both caveolae and JNK and p38 MAP kinase signaling. Further studies are needed to determine if localization of the Hsp’s in the caveolae are important in protecting keratinocytes from vesicant-induced cellular damage. It is possible that caveolae-associated protein transport and control of signaling pathways play key roles in modulating cellular responses to vesicant exposure in the skin.
Figure 3. Effects of CEES dose on Hsp27 expression in a full-thickness human skin equivalent.
EpiDerm-FTTM was exposed to CEES (100–1000 µM) or control. Tissues were collected 24 hr later and stained with antibodies to Hsp27. Binding was visualized using a peroxidase DAB substrate kit. Original magnification, 1000×.
Acknowledgements
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant number U54AR055073. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This work was also supported in part by National Institutes of Health grants CA100994, CA093798, CA132624, ES004738, ES005022, GM034310, AI084138 and AI51214.
List of Abbreviations
- CEES
2-chloroethyl ethyl sulfide
- cav-1
caveolin-1
- Hsp27
heat shock protein 27
- Hsp70
heat shock protein 70
- MBCD
methyl-β-cyclodextrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement: One of the authors (P.J. Hayden) is employed by MatTek Corporation, manufacturer of the EpiDerm-FTTM full-thickness skin equivalent used in the experiments. The other authors have no conflicts of interest to declare.
References
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2005;7:414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10:85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee Mustafi S, Chakraborty PK, Dey RS, Raha S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones. 2009;14:579–589. doi: 10.1007/s12192-009-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz P, Chua M, Liu Z, Diaz LA, Rubenstein DS. Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signaling in the skin. Am J Pathol. 2008a;173:1628–1636. doi: 10.2353/ajpath.2008.080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz P, Diaz LA, Hall RP, Rubenstein DS. Induction of p38MAPK and HSP27 phosphorylation in pemphigus patient skin. J Invest Dermatol. 2008b;128:738–740. doi: 10.1038/sj.jid.5701080. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci U S A. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010a;249:178–187. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Joseph LB, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010b;245:352–360. doi: 10.1016/j.taap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra V, Manucha W, Pena MR, Cacciamani V, Valles PG. Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens. 2010;28:143–155. doi: 10.1097/HJH.0b013e328332b778. [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003;162:2029–2039. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res. 2005;81:522–529. doi: 10.1002/jnr.20575. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF, 3rd, McGary KL, Schlager JJ. Sulfur mustard induces the formation of keratin aggregates in human epidermal keratinocytes. Toxicol Appl Pharmacol. 2003;193:228–236. doi: 10.1016/j.taap.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, McGary KL, Schlager JJ. An inhibitor of p38 MAP kinase downregulates cytokine release induced by sulfur mustard exposure in human epidermal keratinocytes. Toxicol In Vitro. 2004;18:593–599. doi: 10.1016/j.tiv.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY, Yaglom JA. HSP72 depletion suppresses gammaH2AX activation by genotoxic stresses via p53/p21 signaling. Oncogene. 2010;29:1952–1962. doi: 10.1038/onc.2009.480. [DOI] [PubMed] [Google Scholar]
- Gandour-Edwards R, McClaren M, Isseroff RR. Immunolocalization of low-molecular-weight stress protein HSP 27 in normal skin and common cutaneous lesions. Am J Dermatopathol. 1994;16:504–509. doi: 10.1097/00000372-199410000-00005. [DOI] [PubMed] [Google Scholar]
- Garmyn M, Mammone T, Pupe A, Gan D, Declercq L, Maes D. Human keratinocytes respond to osmotic stress by p38 map kinase regulated induction of HSP70 and HSP27. J Invest Dermatol. 2001;117:1290–1295. doi: 10.1046/j.0022-202x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- Guo S, Wharton W, Moseley P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12:245–254. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JF, FitzGerald PG. Treatment of keratin intermediate filaments with sulfur mustard analogs. Biochem Biophys Res Commun. 2007;359:616–621. doi: 10.1016/j.bbrc.2007.05.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Klosner G, Kokesch C, D FO, H HO, Trautinger F. Subcorneal colocalization of the small heat shock protein, hsp27, with keratins and proteins of the cornified cell envelope. Br J Dermatol. 2002;147:13–19. doi: 10.1046/j.1365-2133.2002.04667.x. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Iwamoto I. Selective stimulation of Hsp27 and alphaB-crystallin but not Hsp70 expression by p38 MAP kinase activation. Cell Stress Chaperones. 1999;4:94–101. [PMC free article] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci U S A. 2005a;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005b;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006;14:129–137. doi: 10.1111/j.1743-6109.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Kwon SB, Young C, Kim DS, Choi HO, Kim KH, Chung JH, Eun HC, Park KC, Oh CK, Seo JS. Impaired repair ability of hsp70.1 KO mouse after UVB irradiation. J Dermatol Sci. 2002;28:144–151. doi: 10.1016/s0923-1811(01)00156-6. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L. Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem. 1998;46:1291–1301. doi: 10.1177/002215549804601109. [DOI] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Heck DE, Laskin DL. Oxidants and antioxidants in the mechanism of sulfur mustard injury. Ann N Y Acad Sci. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. EMBO J. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- Loffek S, Woll S, Hohfeld J, Leube RE, Has C, Bruckner-Tuderman L, Magin TM. The ubiquitin ligase CHIP/STUB1 targets mutant keratins for degradation. Hum Mutat. 2010;31:466–476. doi: 10.1002/humu.21222. [DOI] [PubMed] [Google Scholar]
- Macario AJ, Conway de Macario E. Molecular chaperones: multiple functions, pathologies, and potential applications. Front Biosci. 2007;12:2588–2600. doi: 10.2741/2257. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Hoshino T, Yamashita Y, Tanaka K, Maji D, Sato K, Adachi H, Sobue G, Ihn H, Funasaka Y, Mizushima T. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J Biol Chem. 2010;285:5848–5858. doi: 10.1074/jbc.M109.063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytin EV. Heat shock proteins and molecular chaperones: implications for adaptive responses in the skin. J Invest Dermatol. 1995;104:448–455. doi: 10.1111/1523-1747.ep12605702. [DOI] [PubMed] [Google Scholar]
- McMurtry AL, Cho K, Young LJ, Nelson CF, Greenhalgh DG. Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res. 1999;86:36–41. doi: 10.1006/jsre.1999.5700. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedellec P, Edling Y, Perret E, Fardeau M, Vicart P. Glucocorticoid treatment induces expression of small heat shock proteins in human satellite cell populations: consequences for a desmin-related myopathy involving the R120G alpha B-crystallin mutation. Neuromuscul Disord. 2002;12:457–465. doi: 10.1016/s0960-8966(01)00306-6. [DOI] [PubMed] [Google Scholar]
- Pal A, Tewari-Singh N, Gu M, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free Radic Biol Med. 2009;47:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC Press, Inc.; 1991. [Google Scholar]
- Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Rebholz B, Kehe K, Ruzicka T, Rupec RA. Role of NF-kappaB/RelA and MAPK pathways in keratinocytes in response to sulfur mustard. J Invest Dermatol. 2008;128:1626–1632. doi: 10.1038/sj.jid.5701234. [DOI] [PubMed] [Google Scholar]
- Robitaille H, Simard-Bisson C, Larouche D, Tanguay RM, Blouin R, Germain L. The small heat-shock protein Hsp27 undergoes ERK-dependent phosphorylation and redistribution to the cytoskeleton in response to dual leucine zipper-bearing kinase expression. J Invest Dermatol. 130:74–85. doi: 10.1038/jid.2009.185. [DOI] [PubMed] [Google Scholar]
- Roelandt T, Giddelo C, Heughebaert C, Denecker G, Hupe M, Crumrine D, Kusuma A, Haftek M, Roseeuw D, Declercq W, Feingold KR, Elias PM, Hachem JP. The "caveolae brake hypothesis" and the epidermal barrier. J Invest Dermatol. 2009;129:927–936. doi: 10.1038/jid.2008.328. [DOI] [PubMed] [Google Scholar]
- Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol. 2003;120:531–541. doi: 10.1046/j.1523-1747.2003.12051.x. [DOI] [PubMed] [Google Scholar]
- Sayer NM, Whiting R, Green AC, Anderson K, Jenner J, Lindsay CD. Direct binding of sulfur mustard and chloroethyl ethyl sulphide to human cell membrane-associated proteins; implications for sulfur mustard pathology. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;878:1426–1432. doi: 10.1016/j.jchromb.2009.11.030. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying Y-S, Mineo C, Anderson RGW. A detergent-free method for purifying caveolae membrane from tissue culture cells. P.N.A.S. USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–337. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9:2820–2835. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- Trautinger F. Heat shock proteins in the photobiology of human skin. J Photochem Photobiol B. 2001;63:70–77. doi: 10.1016/s1011-1344(01)00203-2. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Kindas-Mugge I, Dekrout B, Knobler RM, Metze D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: an immunohistological study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Valacchi G, Pagnin E, Corbacho AM, Olano E, Davis PA, Packer L, Cross CE. In vivo ozone exposure induces antioxidant/stress-related responses to murine lung and skin. Free Radic Biol Med. 2004;36:673–681. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- Zheng X, Bollinger Bollag W. Aquaporin 3 colocates with phospholipase d2 in caveolin-rich membrane microdomains and is downregulated upon keratinocyte differentiation. J Invest Dermatol. 2003;121:1487–1495. doi: 10.1111/j.1523-1747.2003.12614.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Yue J, Pan Z, Wu H, Cheng Y, Lu H, Ren X, Yao M, Shen Z, Yang JM. Involvement of Caveolin-1 in repair of DNA damage through both homologous recombination and non-homologous end joining. PLoS One. 2010;5:e12055. doi: 10.1371/journal.pone.0012055. [DOI] [PMC free article] [PubMed] [Google Scholar]