Abstract
Pathogenic autoantibodies target aquaporin-4 (AQP4) water channels in individuals with neuromyelitis optica (NMO). Recently, allelic mutations were reported at residue 19 of AQP4 in three cases of NMO, and it was suggested that polymorphisms may influence disease by altering AQP4 supramolecular assembly into orthogonal arrays of particles (OAPs). We analyzed the determinants of OAP formation by human AQP4 to investigate the possible role of polymorphisms in NMO pathogenesis. NMO-associated mutations R19I and R19T in AQP4 did not affect OAP assembly, palmitoylation-dependent regulation of assembly, or NMO autoantibody binding. Residue-19 polymorphisms in AQP4 are thus unlikely to be disease relevant.
Keywords: NMO, AQP4, Water channel, Neuroinflammation
1. Introduction
Aquaporin-4 (AQP4) is a water channel expressed at the plasma membrane in astrocytes in the central nervous system as well as in various peripheral organs such as kidney and skeletal muscle (Frigeri et al., 1995). AQP4 facilitates osmotically driven water transport. Phenotype analysis of AQP4 knockout mice indicated the involvement of AQP4 in brain water balance, neuroexcitation and astrocyte migration (Verkman et al., 2006). AQP4 is the target antigen of autoantibodies in the neuroinflammatory demyelinating disease neuromyelitis optica (NMO), where pathogenic autoantibodies are thought to bind to AQP4, produce astrocyte damage, and initiate a cascade of inflammatory events resulting in myelin loss and neurological impairment (Lennon et al., 2005; Wingerchuk et al., 2007; Jarius et al., 2008).
The AQP4 protein is expressed as two major isoforms: a long isoform, M1, with translational initiation at Met-1, and a shorter isoform, M23, with translational initiation at Met-23 (Yang et al., 1995; Lu et al., 1996). AQP4 can form supramolecular assemblies in membranes called orthogonal arrays of particles (OAPs), which are square arrays of particles visualized by freeze-fracture electron microscopy (Wolburg, 1995). M23 by itself forms large OAPs in transfected cells (Yang et al., 1996), whereas M1 by itself forms few or no OAPs. However, M1 can co-associate with M23 to form mixed-composition OAPs that are smaller than the OAPs formed by M23 alone (Furman et al., 2003). Using multiple independent techniques, we found that M1 and M23 can assemble as heterotetramers, and that OAPs are dynamic structures that are subject to regulation by second messengers and N-terminus palmitoylation at residues Cys-13 and Cys-17 (Crane et al., 2009; Tajima et al., 2010). Truncation and mutagenesis indicated that OAP formation by M23 involves N-terminus M23–M23 inter-tetrameric interactions, and that the inability of M1 to form OAPs involves blocking of the N-terminus interaction by residues just upstream of Met-23 (Crane and Verkman, 2009).
The causative factors in the generation of pathogenic AQP4 autoantibodies in NMO are not known. A genetic susceptibility has been proposed based on increased disease occurrence in related than unrelated persons (Matiello et al., 2010). The possible involvement of AQP4 polymorphisms in NMO was proposed fromtheobservationthat3 out of 172 NMO patients had mutations at Arg-19 (R19I and R19T), whereas control subjects did not (Matiello et al., 2009a, b). Based on data that some AQP4 autoantibodies bind more tightly to OAPs than to individual AQP4 tetramers (Nicchia et al., 2009; Mader et al., 2010; Crane et al., 2011), and on the involvement of the AQP4 N-terminus in OAP formation/disruption(Crane et al., 2009),it was speculated that the residue-19 polymorphisms may alter OAP formation by AQP4 and hence autoantibody binding (Matiello et al., 2009a, b). Here, we tested this possibility by a series of biophysical and biochemical measurements on native human AQP4 and human Arg-19 AQP4 mutants.
2. Materials and methods
2.1. DNA constructs, cell culture and transfections
DNA constructs encoding human AQP4 isoforms were generated by PCR-amplification using whole brain cDNA as template. Myc-tagged AQP4 was generated as previously described. AQP4 mutants were generated by PCR-amplification using either tagged or non-tagged templates. PCR fragments were ligated into mammalian expression vector pcDNA3.1 and fully sequenced. COS-7 (ATCC CRL-1651) and U87MG (ATCC HTB-14) cell cultures were maintained at 37 °C in 5% CO2/95% air in the appropriate medium containing 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin.
2.2. Single particle tracking
COS-7 cells were grown on 18-mm diameter glass coverslips and transfected in Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with DNA encoding Myc-tagged AQP4 isoforms and mutants. 12–24 h after transfection, cells were washed with 2 mL PBS containing 6 mM glucose and 1 mM pyruvate (GP buffer) and incubated for 5 min in blocking buffer. Cells were then incubated for 5 min with 70 ng/mL mouse anti-Myc antibody (Covance, Emeryville, CA) in blocking buffer, rinsed, and incubated for 5 min with 0.1 nM goat F(ab')2anti-mouse IgG-conjugated Qdot 655 (Invitrogen) in blocking buffer. Cells were rinsed extensively and maintained throughout experiments in GP buffer. SPT was performed on a Nikon Eclipse TE2000S inverted epifluorescence microscope (Nikon, Melville, NY) equipped with a Nikon 100x TIRF oil immersion objective (numerical aperture 1.45) and a deep-cooled CCD camera (Hamamatsu EM-CCD, Bridgewater, NJ). Qdot fluorescence was excited using an E460SPUV excitation filter and 475DCXRU dichroic mirror, and detected through a D655/40 m emission filter (Chroma, Rockingham, VT). Data were acquired continuously at 11 ms per frame (91 Hz) for 6 s. Image sequences were analyzed and trajectories constructed as described previously (Crane et al., 2008). Diffusion data are reported as averaged diffusion coefficients and cumulative distributions of range at 1 s, where P (range) is defined as the probability that a particle's range is less than or equal to a given distance at t=1 s.
2.3. Electrophoresis and immunoblotting
48 h prior to lysis, U87MG cells were transfected using Lipofectamine 2000 with DNA encoding AQP4 isoforms and mutants. Cultures were lysed with NativePAGE sample buffer (Invitrogen) containing 1% dodecyl-β-d-maltoside (EMD chemicals, Gibbstown, NJ) for10 min on ice. Lysates were centrifuged at 20,000 g for 30 min at 4 °C and the pellet discarded. Blue-native gel electrophoresis (BN-PAGE) was performed with polyacrylamide native gradient gels (3–9%). 10 μg of protein was mixed with 5% Coomassie Blue G-250 (Invitrogen) and loaded in each lane. Ferritin was used as the molecular mass standard (440 and 880 kDa). Running buffers were: 25 mM imidazole, pH 7 (anode buffer) and 50 mM Tricine, 7.5 mM imidazole, 0.02% Coomassie Blue G-250, pH 7 (cathode buffer). Tricine SDS-PAGE was performed with a 12% running gel and 3% stacking gel. SeeBlue Plus2 Pre-Stained Standard (Invitrogen) was used as a molecular weight marker. Proteins were blotted onto polyvinyl difluoride membranes (Bio-Rad, Hercules, CA). For immunoblot analysis, membranes were blocked with 3% BSA and incubated with rabbit anti-AQP4 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), then rinsed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch), rinsed extensively, and labeled proteins were detected using the ECL Plus enzymatic chemiluminescence kit (Amersham Biosciences, Pittsburgh, PA).
2.4. Quantitative immunofluorescence
NMO serum was obtained from four seropositive individuals who met the revised diagnostic criteria for clinical disease (Wingerchuk et al., 2006). U87MG cells were grown on 12 mm glass coverslips and transfected in Lipofectamine 2000 with DNA encoding AQP4 isoforms and mutants. 12–24 h after transfection, cells were incubated for 20 min in live-cell blocking buffer (PBS containing 6 mM glucose,1 mM pyruvate, 1% bovine serum albumin, 2% goat serum), and then for 30 min with heat-inactivated patient serum, as described (Crane et al., 2011).Cells were then rinsed extensively, fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.1% Triton X-100. Cells were then blocked again and incubated for 30 min with 0.4 μg/mL polyclonal, C-terminal specific rabbit anti-AQP4 antibody (Santa Cruz), then rinsed with PBS. Finally, cells were incubated for 30 min with 4 μg/mL goat anti-human IgG-conjugated Alexa Fluor 488 and goat anti-rabbit IgG-conjugated Alexa Fluor 555 (Invitrogen) in blocking buffer. After incubation with secondary antibodies, cells were rinsed extensively in PBS, and coverglasses were mounted with VectaMount hard-set medium (Vector Laboratories, Burlingame, CA). Quantitative analysis of AQP4-antibody binding was done on a Nikon Eclipse TE2000S inverted epifluorescence microscope equipped with a Nikon 10× air objective (numerical aperture0.3).Green and red dyes were excited and observed through Chroma filter sets #41001 and #42001, respectively. Images were acquired using a CCD camera (Hamamatsu Orca), and intensities determined using custom software.
3. Results
3.1. OAP assembly by human M1 and M23 AQP4
We generated constructs encoding human native AQP4 and various mutants to investigate OAP formation by human AQP4. Fig. 1A shows the topography of human AQP4. The Met-1 and Met-23 start sites in the N-terminal sequence are indicated, as well as the two cysteine residues involved in palmitoylation (Cys-13, Cys-17), the site of polymorphisms (Arg-19), and the key residues just upstream of Met-23 that are involved in the N-terminus association required for OAP formation. OAP assembly was quantified with quantum dot single particle tracking (SPT) and blue-native gel electrophoresis (BN-PAGE).
Fig. 1.
OAP-forming properties of human AQP4. A. Schematic diagram of the N-terminus ofM1 AQP4 showing the human sequence. Sites of cysteine palmitoylation are shown in red. Arg-19, which is mutated in a small subset of NMO patients, is shown in green. Hydrophobic residues that are required for OAP assembly by M23 AQP4 are shown in yellow. Transmembrane domains are shown in light blue. B. Representative single particle trajectories from quantum dot-labeled human AQP4 isoforms M23 (black) and M1 (red). Trajectories were acquired at 91 Hz for 6 s. Bar, 1 μm. C. Cumulative distribution of the diffusion range at 1 s for AQP4 isoforms M23 (black) and M1 (red). Both human (solid) and rat (dotted) diffusion profiles are shown for comparison.
SPT was done in live cells expressing human M23 and M1 AQP4. Representative single particle trajectories showed characteristic confinement of human M23 in OAPs (Fig. 1B), with a median range of 39 nmin 1 s, and average diffusion coefficient of 7×10−11 cm2/s. In contrast, human M1 had a median range of 429 nm in 1 s, with diffusion coefficient of 7×10−10 cm2/s, indicating rapid and unrestricted motion of non-OAP-forming M1 tetramers. The diffusion profiles for human AQP4 were qualitatively similar to those found previously for rat AQP4, but with both human isoforms having a slightly greater range in 1 s (Fig. 1C). We previously showed that mutations in the hydrophobic N-terminus of M23 AQP4 could prevent OAP formation by rat AQP4 (Crane and Verkman, 2009). Mutations at the same residues in human M23 also prevented OAP formation by human AQP4 (data not shown), supporting the conclusion that the same molecular mechanisms are involved in regulated OAP assembly in human and rat AQP4.
3.2. AQP4 OAP assembly is not affected by Arg-19 mutation
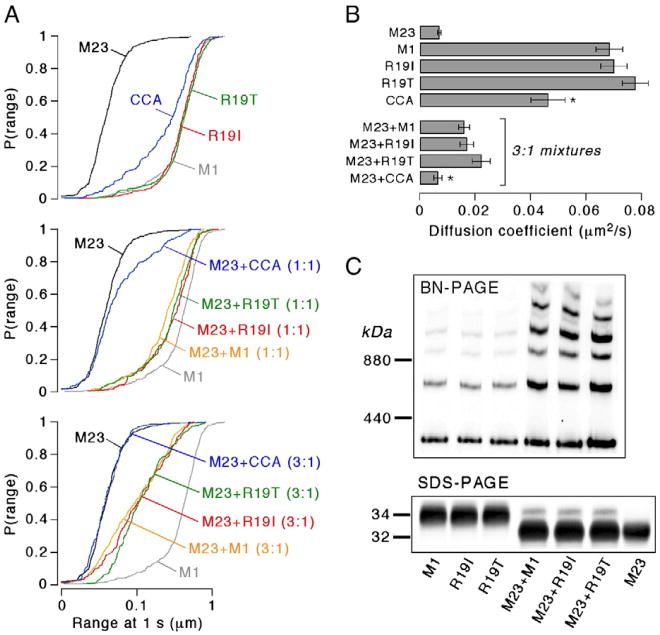
The hypothesis that Arg-19 mutants of M1 AQP4 are significant in NMO involves an altered (increased) propensity for OAP formation compared with the native M1 AQP4, and/or decreased propensity for OAP disruption. It has been proposed that the inability of M1 Arg-19 mutants to disrupt OAPs may be the result of deficient S-palmitoylation at Cys-17 or Cys-13 in the mutants (Matiello et al., 2009a, b). To test these possibilities, we measured the ability of Arg-19 mutants R19I and R19T to form OAPs on their own, and to disrupt OAPs when co-expressed with OAP-forming M23 AQP4.
SPT of human M1 Arg-19 mutants showed diffusion profiles identical to native M1 (Fig. 2A top, 2B). Further, BN-PAGE on cell lysates showed identical patterns for Arg-19 mutants and native M1 (Fig. 2C). Prior SPT studies of co-expressed rat M23 and M1 AQP4 indicated the OAP-disrupting ability of M1 AQP4 (Crane et al., 2009). We found here that human M1 also significantly disrupted OAPs, leading to increased diffusion, corresponding to smaller, more mobile OAPs (Fig. 2A middle, 2B). The Arg-19 mutants, when co-expressed with M23, also disrupted OAP formation, leading to a diffusion profile indistinguishable from that of human native M1 with M23. In contrast, a double alanine mutant at Cys-13 and Cys-17 in M1 (CCA) had greatly reduced ability to disrupt OAP formation by M23 (Fig. 2A bottom, 2B), as previously shown for rat AQP4 (Crane et al., 2009). Co-expression of M23 with native M1 or the Arg-19 mutants produced identical banding patterns on BN-PAGE (Fig. 2C), further supporting the conclusion that the Arg-19 mutations R19I and R19T do not affect OAP formation or disruption.
Fig. 2.
Arg-19 mutations in AQP4 do not affect AQP4 OAP assembly. A. Cumulative distribution of the range at 1 s for M1 Arg-19 mutants R19I (red), R19T (green) and double cysteine mutant CCA (blue). Top panel: diffusion of pure AQP4 isoforms or mutants. Middle panel: Diffusion of 1:1 mixtures of M23 and M1 (orange), or M23 and mutants (same colors as top). Bottom panel: Diffusion of 3:1 mixtures of M23 and M1 or mutants (same colors as middle). Diffusion of pure AQP4 isoforms M23 (black) and M1 (grey) are shown in each panel. B. Diffusion coefficients for pure AQP4 isoforms or M1 mutants and 3:1 mixtures of M23 and M1 or mutants (mean ± S.E., n> 16 cells). * P <0.01 when compared to non-mutated M1. C. AQP4 immunoblot following blue-native gel electrophoresis (top) or Tricine SDS-PAGE (bottom) of lysates from U87MG cells transfected with AQP4 isoforms, M1 mutants, or mixtures composed of 3:1 M23 to M1 (native or mutant).
3.3. NMO-IgG binding is not affected by Arg-19 mutation
Effects of Arg-19 mutations R19I and R19T on NMO-IgG binding were measured. Prior studies have shown that NMO-IgG appears to bind better to M23 than to M1 AQP4 (Nicchia et al., 2009; Mader et al., 2010). We recently showed that this difference is due to OAP formation by M23, resulting in increased binding affinity (Crane et al., 2011). If mutation at residue 19 of M1 were involved in NMO disease pathogenesis, NMO-IgG binding would be increased in cells expressing M23 and the M1 Arg-19 mutants compared with M23 and native M1. We measured the binding of NMO-IgG in patient serum to AQP4 M1 (native and Arg-19 mutants) and M23 individually, and in combination. For the combination study, AQP4 mixtures were expressed in a ratio of three M23 to one M1 (native or mutant), as found in human brain.
Fig. 3A shows representative fluorescence micrographs of NMO-IgG staining of U87MG cells expressing M23 or M1 AQP4, or the M1 mutants R19I and R19T, each individually. Green fluorescence (NMO patient serum) was normalized to red fluorescence (anti-AQP4) to quantify NMO-IgG binding. Fig. 3B shows concentration-dependent binding of NMO patient serum to the native human M1 and M23 isoforms compared to M1 mutants R19I and R19T. As expected, binding to M23 was greater than to M1 AQP4, but binding to Arg-19 mutants was not different than that to native M1 (Fig. 3B, left). Fitting to a single-site binding model yielded an apparent IC50 of 1.4% serum for M23, compared to 11–16% serum for native M1 and the Arg-19 mutants. NMO-IgG binding was significantly greater to U87MG cells co-expressing M23 and M1 than to M1 alone (Fig. 3B, right). As found for the Arg-19 M1 AQP4 mutants expressed individually, NMO-IgG binding to mixtures of M23 and the Arg-19 mutants was indistinguishable from binding to mixtures of M23 with native M1. Effective IC50 for binding to 3:1 mixtures M23 and M1 (native or mutant) was 2.5–2.9% serum. Similar measurements done using a fixed concentration of serum from three other NMO patients also showed no effect of the Arg-19 mutations on NMO-IgG binding to M1 alone or in combination with M23 (Fig. 3C).
Fig. 3.
Arg-19 mutations in AQP4 do not affect NMO autoantibody binding. A. Fluorescence micrographs showing M1 and M23 expressing U87MG cells stained with 10% NMO serum (green), and with reference AQP4 antibody (red). B. Binding curves for NMO patient serum to M23 or M1 AQP4, and M1 Arg-19 mutants. Left panel: fraction of NMO-IgG bound to cells expressing pure M23 (black circles), M1 (open circles), R19I (red) or R19T (green). Right panel: fraction of NMO-IgG bound to cells expressing 3:1 mixtures of M23 to M1 (black), R19I (red) or R19T (green). Circles represent mean ± S.E., n =5). Curves represent fit to single-site binding model. C. Binding of NMO serum (measured at 10%) from three additional different NMO patients to M1, R19I and R19T alone and in combination with M23 (mean ± S.E., n =3, differences not significant).
4. Discussion
The occurrence of familial NMO suggests a genetic component to the disease. Given the critical role of AQP4 autoantibodies in disease pathogenesis, coding changes in the AQP4 gene are potential candidates for disease-relevant mutations. Sequence analysis of the AQP4 open reading frame in affected individuals identified two different amino acid substitutions at Arg-19 in two related and one sporadic case of NMO (Matiello et al., 2009a, b). This finding prompted the hypothesis that the Arg-19 polymorphisms enhance AQP4 autoantibody formation or binding by facilitating AQP4 OAP assembly or stability. To test this hypothesis directly, we generated and characterized a series of constructs containing the human AQP4 isoforms with various mutations and epitope tags. We found that the OAP-forming properties of the human AQP4 isoforms were similar to those of rat AQP4, both for the isoforms expressed individually and in combination, as is found in astrocytes. The two Arg-19 mutations reported to be associated with NMO, R19I and R19T, did not affect the OAP forming or disrupting ability of M1 AQP4, nor did they affect NMO autoantibody binding. Notwithstanding the caveat that we did not study every possible function of AQP4 that could, in principle, be affected by Arg-19 mutation, we conclude that the Arg-19 polymorphisms are unlikely to be involved in NMO pathology though a mechanism involving altered OAP formation or NMO-IgG binding. The postulated link between the Arg-19 polymorphism and NMO pathology, altered OAP formation with Arg-19 mutation, was ruled out by the biophysical and biochemical measurements that probe OAP structure, as well as by the NMO autoantibody binding measurements that probe the key disease-relevant downstream consequence of OAP structure.
Given the low incidence of familial NMO and the significant discordance of AQP4 seropositivity amongst affected family members (Matiello et al., 2010), it seems unlikely that a single coding mutation in AQP4 would have major influence on AQP4 autoantibody formation. Similar to multiple sclerosis, where a low incidence of familial disease is due to the compound influence of more than a dozen non-HLA disease susceptibility genes (Oksenberg and Baranzini, 2010), familial clustering in NMO is more likely secondary to various non-AQP4 immune susceptibility genes. For example, an association has been reported between the HLA-DPB1*0501 allele and AQP4 autoantibody positivity in Japanese patients (Matsushita et al., 2009). One prior preliminary study suggested an association between AQP4 and susceptibility to multiple sclerosis (Ban et al., 2007). To date, however, no risk allele for multiple sclerosis has been linked to NMO. Various reports have considered possible associations of AQP4 polymorphisms with a variety of neurological conditions, including sudden infant death syndrome (Opdal et al., 2010), migraine (Rubino et al., 2009), temporal lobe epilepsy (Heuser et al., 2010) and stroke-associated brain edema (Kleffner et al., 2008), with largely weak or negative conclusions. One study surveying random AQP4 polymorphisms in normal subjects reported various loss-of-function and gain-of-function AQP4 polymorphisms (Sorani et al., 2008); however, the conclusions may not be correct because the effect of polymorphisms on AQP4 expression and plasma membrane targeting were not accounted for. Thus, though the possible clinical significance AQP4 polymorphisms has received increasing attention, to date compelling evidence is lacking for association between an AQP4 polymorphism and human disease.
Acknowledgments
We thank Drs. Marcello Matiello and Brian Weinshenker of the Mayo Clinic for the original suggestion to study AQP4 polymorphisms and for useful advice during the course of the study. This work was supported, in whole or in part, by grants from the Guthy-Jackson Charitable Foundation, and grants EY13574, EB00415, DK35124, HL73856, DK86125 and DK72517 from the National Institutes of Health, and grant RG4320 from the National Multiple Sclerosis Society.
References
- Ban M, Walton A, Goris A, Gray J, Compston A, Sawcer S. Polymorphisms in the neuromyelitis optica auto-antigen AQP4 and susceptibility to multiple sclerosis. J Neurol. 2007;254:398–399. doi: 10.1007/s00415-006-0392-8. [DOI] [PubMed] [Google Scholar]
- Crane JM, Van Hoek AN, Skach WR, Verkman AS. Aquaporin-4 dynamics in orthogonal arrays in live cells visualized by quantum dot single particle tracking. Mol Biol Cell. 2008;19:3369–3378. doi: 10.1091/mbc.E08-03-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Verkman AS. Determinants of aquaporin-4 assembly in orthogonal arrays revealed by live-cell single-molecule fluorescence imaging. J Cell Sci. 2009;122:813–821. doi: 10.1242/jcs.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Bennett JL, Verkman AS. Live cell analysis of aquaporin-4M1/M23 interactions and regulated orthogonal array assembly in glial cells. J Biol Chem. 2009;284:35850–35860. doi: 10.1074/jbc.M109.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108:2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Nat Acad Sci U S A. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser K, Nagelhus EA, Tauboll E, Indahl U, Berg PR, Lien S, et al. Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res. 2010;88:55–64. doi: 10.1016/j.eplepsyres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Jarius S, Aboul-Enein F, Waters P, Kuenz B, Hauser A, Berger T, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffner I, Bungeroth M, Schiffbauer H, Schabitz WR, Ringelstein EB, Kuhlenbaumer G. The role of aquaporin-4 polymorphisms in the development of brain edema after middle cerebral artery occlusion. Stroke. 2008;39:1333–1335. doi: 10.1161/STROKEAHA.107.500785. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, et al. The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Nat Acad Sci U S A. 1996;93:10908–10912. doi: 10.1073/pnas.93.20.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One. 2010;5:e10455. doi: 10.1371/journal.pone.0010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiello M, Schaefer-Klein J, Hebrink D, Kingsbury D, Lennon V. Two different Arg19 mutations in the N-terminus of aquaporin-4 suggest a molecular mechanism for susceptibility to neuromyelitis optica. Neurology. 2009a;72:A119–A120. [Google Scholar]
- Matiello M, Schaefer-Klein J, Hebrink D, Kingsbury D, Lennon V, Weinshenker B. Genetic analysis of neuromyelitis optica: two different Arg19 mutations in the N-terminus of aquaporin-4 suggest a molecular mechanism for susceptibility to NMO. Mult Scler. 2009b;15:S69. [Google Scholar]
- Matiello M, Kim HJ, Kim W, Brum DG, Barreira AA, Kingsbury DJ, et al. Familial neuromyelitis optica. Neurology. 2010;75:310–315. doi: 10.1212/WNL.0b013e3181ea9f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Matsuoka T, Isobe N, Kawano Y, Minohara M, Shi N, et al. Association of the HLA-DPB1*0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders. Tissue Antigens. 2009;73:171–176. doi: 10.1111/j.1399-0039.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, et al. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia. 2009;57:1363–1373. doi: 10.1002/glia.20855. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Baranzini SE. Multiple sclerosis genetics–is the glass half full, or half empty? Nat Rev Neurol. 2010;6:429–437. doi: 10.1038/nrneurol.2010.91. [DOI] [PubMed] [Google Scholar]
- Opdal SH, Vege A, Stray-Pedersen A, Rognum TO. Aquaporin-4 gene variation and sudden infant death syndrome. Pediatr Res. 2010;68:48–51. doi: 10.1203/PDR.0b013e3181df4e7c. [DOI] [PubMed] [Google Scholar]
- Rubino E, Rainero I, Vaula G, Crasto F, Gravante E, Negro E, et al. Investigating the genetic role of aquaporin4 gene in migraine. J Headache Pain. 2009;10:111–114. doi: 10.1007/s10194-009-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorani MD, Zador Z, Hurowitz E, Yan D, Giacomini KM, Manley GT. Novel variants in human Aquaporin-4 reduce cellular water permeability. Hum Mol Genet. 2008;17:2379–2389. doi: 10.1093/hmg/ddn138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M, Crane JM, Verkman AS. Aquaporin-4 (AQP4) associations and array dynamics probed by photobleaching and single-molecule analysis of green fluorescent protein-AQP4 chimeras. J Biol Chem. 2010;285:8163–8170. doi: 10.1074/jbc.M109.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H. Orthogonal arrays of intramembranous particles: a review with special reference to astrocytes. J Hirnforsch. 1995;36:239–258. [PubMed] [Google Scholar]
- Yang B, Ma T, Verkman AS. cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. Evidence for distinct transcriptional units. J Biol Chem. 1995;270:22907–22913. doi: 10.1074/jbc.270.39.22907. [DOI] [PubMed] [Google Scholar]
- Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]