Abstract
Repeated courses of antenatal steroids in women threatening preterm delivery have beneficial effects on lung maturation, but concern exists about the effects on the developing brain. We aimed to determine whether repeated courses of antenatal corticosteroids increased the risk of neuropathology compared to single courses or no treatment. Single course animals received antenatal steroids at 123dg (n=6). A second course was administered to the repeated course group at 137dg (n=7). Controls received no antenatal intervention (n=5). Baboons delivered naturally at term, after which necropsy was performed. Brains were assessed histologically for parameters of development and neuropathology. Body weights did not differ between the groups (p>0.05); neither did brain weight in relation to body weight. Density of glial fibrillary acidic protein-immunoreactive (IR) astrocytes in white matter was increased in the single (p<0.05) and repeated course (p<0.01) groups compared to controls. Density of myelin-basic protein-IR oligodendrocytes was reduced in the repeated course animals compared to both controls and single course groups (p<0.05); olig2-IR, which labels all cells in the oligodendrocyte lineage, showed no difference between groups. Repeated courses of antenatal corticosteroids have effects on myelination in the developing non-human primate brain which should be taken into account when determining a dosing regimen.
Keywords: Non-human primate, myelination, brain development, repeated courses antenatal steroids
INTRODUCTION
Routine administration of antenatal steroids to mothers who are at risk of delivering prematurely has resulted in improved outcomes for preterm infants. Benefits to the newborn of a single course of antenatal corticosteroids include a reduction of the risk of respiratory distress syndrome and intraventricular hemorrhage, and reduced mortality and reliance on respiratory support (1). There is evidence that the effectiveness of antenatal corticosteroids diminishes with time (2), so if birth has not occurred within seven days of corticosteroid administration it is common practice to administer a second course of corticosteroids (3, 4).
Randomized controlled trials of repeated weekly or bi-weekly courses of antenatal steroids results in improved neonatal respiratory outcomes, however this has been coupled with a reduction in some measures of birth weight and head circumference at birth (5, 6). Several longer-term studies have shown no impact of repeat courses of corticosteroids on physical growth or neurodevelopmental outcome at 2 years of age (7-9), however other studies suggest a higher rate of cerebral palsy in infants exposed to repeat courses of corticosteroids (10) neurodevelopmental abnormalities (11) and behavioural effects (12).
Studies in sheep have shown a dose-dependent effect of corticosteroids on birth weight (13) and that a reduction in brain weight following antenatal corticosteroid administration persists to 3.5 years of age (adulthood in the sheep) (14). Other animal studies have shown that repeat courses of antenatal steroids can have detrimental effects on the developing brain, delaying myelination (15-17) and causing cell death in the hippocampus (18); a single course has been reported to reduce neural cell proliferation in rats (19) and alter cytoskeletal protein and synaptophysin in baboon brains (20).
Therefore in the light of evidence of possible adverse effects on the developing brain, the question as to whether obstetricians should continue to give repeated courses of corticosteroids remains controversial (21-23). A recent meta-analysis (24) concludes that repeated betamethasone restricts intrauterine growth with possible long-term consequences on neurodevelopment.
In this study we had the unique opportunity to investigate the developmental sequelae of single or repeated courses of antenatal corticosteroids compared to no treatment in a non-human primate model, the baboon. We have shown previously that this model is appropriate for studying neuropathology due to its similarity with human brain development albeit the baboon brain reaches a more mature state by birth (25-27). The present experiment was designed to assess the effects of antenatal corticosteroid treatments when gestation was not affected by confounding factors such as hypoxia or malnutrition and when offspring were delivered naturally at term (185 days of gestation (dg) in the baboon). Our aim was to determine whether a single course of corticosteroids at 123-124 dg (66% of gestation, equivalent to ~26 weeks’ gestation in the human (28)) or repeated courses administered at 123-124dg and 137-138dg (74% of gestation, equivalent to ~30 weeks’ gestation in the human (28)) increased the risk of neuropathology compared to no treatment.
METHODS
All animal studies were performed at the Southwest Foundation for Biomedical Research in San Antonio, TX. All animal husbandry, animal handling, and procedures were reviewed and approved to conform to American Association for Accreditation of Laboratory Animal Care guidelines.
Timed gestations were determined by observing characteristic sex skin changes and confirmed by serial fetal ultrasound examinations. Pregnant baboon dams (Papio papio) were randomly assigned to one of the following groups: the single course group (n=6) received a single course of antenatal steroids (6mg betamethasone intramuscularly (IM) or 6mg dexamethasone IM) at 123dg and 124dg (66% term gestation); the repeated course group (n=7) received an additional course of two IM injections at 137dg and 138dg (74% term gestation); a third control group received no placebo injections or sham handling procedures and served as the no antenatal steroid treatment group (n=5). Note that access to betamethasone was stopped by the supplier over a period of time during this study; dexamethasone had to be substituted during the time betamethasone was not available. All dams delivered air-breathing infants naturally at term (185 days). Necropsy was performed at 1-2 days after delivery.
Histology and Immunohistochemistry
Brains were weighed, immersed in 4% paraformaldehyde or 10% formalin in 0.1M phosphate buffer and 14-15 blocks from the right forebrain (at 5mm intervals) were processed to paraffin. Ten sections (8μm thick) were cut from the rostral surface of each block. One section per block was stained with hematoxylin and eosin (H&E) and assessed for gross morphologic changes, including the presence of hemorrhages, lesions or infarcts, neuronal death, axonal injury, gliosis and perivascular cuffing. Immunohistochemistry for rabbit anti cow-glial fibrillary acid protein (GFAP; 1:500, Sigma, St Louis, MO, USA) was used to identify astrocytes, and rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1; 1:1500, Wako, Richmond, USA) to identify microglia/macrophages. To identify cells in the oligodendrocyte lineage, mouse anti-chicken myelin basic protein (MBP, 1:100; Chemicon, Temecula, CA, USA) and rabbit anti-Olig2 polyclonal antibody (1:200; Chemicon, Temecula, CA, USA) were used. All analyses were performed on all brains in the study. Qualitative and quantitative measurements were made on coded slides blinded to the observer.
Analysis
For each animal, all quantitative measurements were made using an image analysis system (Image Pro v4.1, Media Cybernetics, Maryland, USA). All values were calculated as mean of means for each group; measurements of cell numbers are expressed as cells/mm2.
Volumetric measurements
In thionin-stained sections, the cross-sectional area of coronal sections (right hemisphere) was assessed and the total forebrain volume estimated using the Cavalieri principle (29). The total volume of white matter, deep grey matter (basal ganglia, thalamus and hippocampus), ventricle and neocortex were also assessed in this manner. The surface folding index (SFI), which gives an estimation of the expansion of the surface area relative to volume, was then determined (28).
Areal density of astrocytes
GFAP-IR cells were counted (×300; sample area 0.02mm2) in the deep and subcortical white matter (WM) and the neocortex of the frontal/temporal, parietal/temporal and occipital lobes of each brain. Two randomly selected areas in each of the deep and subcortical white matter were selected from each block. In the neocortex, 3 regions (in the dorsal, lateral, and ventral cortex) were sampled in layers 2-4 of each animal.
Assessment of myelination
MBP-IR and Olig2-IR oligodendrocytes were counted (×300) in two randomly selected areas (0.02mm2) in both the deep and subcortical WM in blocks from frontal/temporal, parietal/temporal and occipital lobes. Darkfield images were taken of the corpus callosum to qualitatively assess the extent of myelination within this structure.
Areal density of microglia/macrophages
In Iba1-IR sections, cells were counted in randomly selected areas (×300; sample area 0.02mm2) of both the deep and subcortical WM. In the neocortex (layers 2-4) a section from each lobe was selected and 3 regions (dorsal, lateral, and ventral) sampled in each section (×300; 9 regions/animal).
Hippocampus
Qualitative morphological analysis of the hippocampus, including identification of apoptotic cells was performed in 2 H&E-stained sections per animal. Migration of pyramidal neurons in the cornu ammonis 3 (CA3) region of the hippocampus was assessed quantitatively in 2 thionin-stained sections per animal. An area of CA3, including the stratum oriens and the stratum pyramidale was divided into 2 equal layers. The number of nucleated pyramidal cells in each layer was counted (at a magnification of ×300) and expressed as a percentage of the total number of cells counted (30).
Statistical Analysis
Differences between single course, repeated course, and control animals were assessed using a one-way ANOVA with post-hoc test (Tukey’s test) for histologic parameters; a probability of p<0.05 was considered significant. Linear regression analysis was carried out to determine if there was a correlation between histologic parameters (astrocyte, oligodendrocyte, and microglia/macrophage cell densities) and volumetric measurements; a probability of p<0.05 was considered significant. Results are expressed as mean ± SEM (weights and areas) and mean of means ± SEM (histologic parameters).
RESULTS
Group characteristics
There was no difference between groups (p>0.05) in the age at delivery (control 186.0±0.71; single course 186.2±0.17; repeated course 186.4±0.37) or the ratios of males to females (control 2/3; single course 4/2; repeated course 4/3).
Brain growth and development
Brain and body weights
Body weights at necropsy did not differ between the groups (p>0.05; Table 1). Total brain and cerebellar weights were increased in the single course group compared to both controls and the repeated course group (p<0.05; Table 1); there was no difference between groups in either brain or cerebellar weights when expressed in relation to body weight (p>0.05; Table 1).
Table 1. Effects of prenatal steroids on body and brain weights.
| Term control (n=5) |
Single course (n=6) |
Repeated course (n=7) |
|
|---|---|---|---|
| Body weight at post mortem (g) | 846±68 | 991±74 | 864±44 |
| Total brain weight (g) | 78.1± 1.7 | 94.5±4.7**† | 81.2±2.2 |
| Brain/body weight (%) | 9.7±0.8 | 9.5±0.3 | 9.2±0.3 |
| Cerebellar weight (g) | 5.5±0.4 | 7.0±0.3*† | 5.6±0.2 |
| Cerebellar/body weight (%) | 0.70±0.03 | 0.72±0.03 | 0.64±0.03 |
| Cerebellar/brain weight (%) | 7.2±0.4 | 7.6±0.3 | 7.1±0.2 |
Data are presented as mean±SEM.
p<0.05 compared to controls
p<0.01 compared to controls
p<0.05 compared to repeated course
Brain volumes
The volume of deep grey matter was reduced in the repeated course group compared to the control group (p<0.05; Table 2) but this was not significant when expressed relative to the total forebrain volume. There were no differences in other volumetric parameters between the groups (Table 2). The overall SFI between groups was not significantly different (p>0.05; Table 2).
Table 2. Forebrain volumetric parameters.
| Term control (n=5) |
Single course (n=6) |
Repeated course (n=7) |
|
|---|---|---|---|
| Volume of the (right) forebrain (mm2) | 35338±2068 | 34957±1719 | 31328±1370 |
| Neocortical volume (mm2) | 21240±1283 | 21277±993 | 19787±1032 |
| White matter volume (mm2) | 9415±735 | 9351±419 | 8342±593 |
| Deep grey matter volume (mm2) | 3884±205 | 3558±158 | 3202±176* |
| Ventricular volume (mm2) | 658±93 | 640±55 | 564±70 |
| Neocortex/Total hemispheric volume (%) | 61.2±0.7 | 62.1±1.5 | 64.3±1.3 |
| White matter/Total hemispheric volume (%) | 27.1±1.0 | 27.3±0.5 | 27.0±1.1 |
| Deep grey matter/Total hemispheric volume (%) | 11.2±0.3 | 10.4±0.5 | 10.5±0.6 |
| Ventricular volume/Total hemispheric volume (%) | 1.9±0.2 | 1.9±0.1 | 1.8±0.2 |
| Neocortical volume/White matter volume | 2.3±0.1 | 2.3±0.1 | 2.4±0.1 |
| SFI | 82.0±2.3 | 87.2±3.1 | 79.0±1.9 |
Data are presented as mean±SEM.
p<0.05 compared to controls
Neuropathology
There was no evidence of cerebral infarction, cystic lesions or intraventricular hemorrhage in the cerebral hemispheres of any animal.
Areal density of GFAP-IR astrocytes
There was no difference between groups in the density of GFAP-IR astrocytes in the neocortex (p>0.05, Table 3), however in both the deep and subcortical WM, the density of astrocytes was increased in both the single and the repeated course groups compared to controls (p<0.05; Table 3).
Table 3. Forebrain neuroglial cell densities.
| Term control (n=5) |
Single course (n=6) |
Repeated course (n=7) |
|
|---|---|---|---|
| GFAP-IR cells – Cortex | 5±2 | 6±2 | 3±1 |
| Subcortical WM | 305±23 | 480±31* | 473±27* |
| Deep WM | 277±18 | 445±35* | 548±55** |
| Olig2-IR cells – Subcortical WM | 311±16 | 309±14 | 285±25 |
| Deep WM | 356±13 | 358±11 | 355±32 |
| MBP-IR cells – Subcortical WM | 126±15 | 136±5 | 83±9*† |
| Deep WM | 179±12 | 170±12 | 136±6**† |
| Iba1-IR cells – Cortex | 66±3 | 67±2 | 60±3 |
| Subcortical WM | 103±6 | 113±6 | 104±6 |
| Deep WM | 148±8 | 165±10 | 148±14 |
All measurements cells/mm2. Data are presented as mean±SEM.
p<0.05 compared to controls
p<0.01 compared to controls
p<0.05 compared to single course
Assessment of myelination
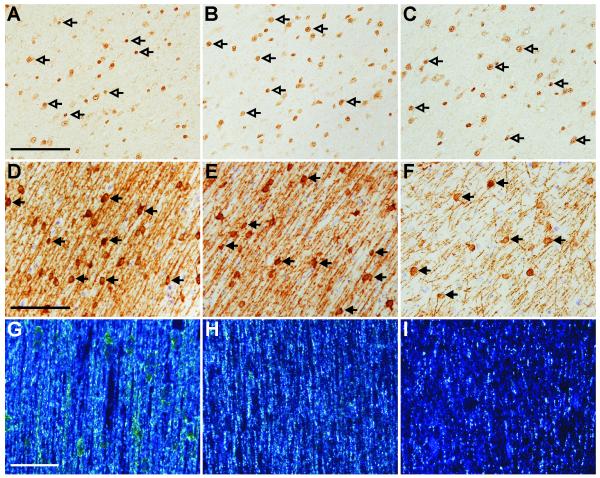
There was no difference in the areal density of Olig2-IR cells between groups in either region. This is illustrated by comparing controls (Figure 1A), single (Figure 1B), and repeated (Figure 1C) courses of corticosteroids. MBP-IR cell density in repeated course animals was lower than controls and single course animals in both the deep and subcortical WM (p<0.05; Table 3), illustrated for the subcortical WM by comparing MBP-IR from repeated course animals (Figure 1E) with controls (Figure 1F) and single course animals (Figure 1E). The intensity of IR for MBP, as assessed by darkfield illumination in the corpus callosum was markedly reduced in the repeated course group (Figure 1I), but minimally reduced in the single course group (Figure 1H) compared to controls (Figure 1G).
Figure 1. Effect of steroids on myelination.
Areal density of Olig2-IR oligodendrocytes (open arrows) did not differ between control (A), single course (B) and repeated course (C) groups, demonstrated in the subcortical white matter. The areal density of MBP-IR cells (closed arrows) in the white matter was reduced in the repeated course animals (F) compared to both the control animals (D) and the single course animals (E). Intensity of MBP-IR as viewed under darkfield illumination was reduced in the repeated course groups (I) compared to the control (G) and the single course (H) groups. Scale bars: A-C=100μm; D-F=100μm; G-I=50μm.
Areal density of microglia/macrophages
There was no difference between groups in the density of Iba1-IR cells in cortex, deep or subcortical WM (p>0.05; Table 3)
Hippocampus
Morphological analysis of the hippocampus showed no consistent abnormalities in the structure of CA1-3 pyramidal cells or of dentate granule cells in corticosteroid-treated animals compared to controls. Apoptotic cells were observed infrequently (approx 1.4 cells per mm2) in CA1-3 region of controls (Figure 2A) and corticosteroid treated animals (Figure 2B-C). There was no difference between corticosteroid groups in the percentage of cells that had migrated into the innermost half of CA3 (single course 96.90.7%, repeated course 93.51.0% P>0.05); however there was a higher percentage (p<0.05) in single course animals than in controls (89.23%).
Figure 2.
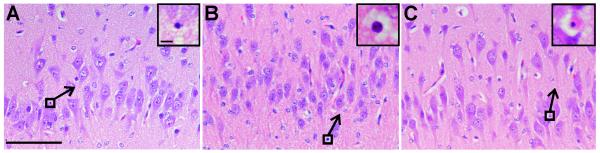
Haemotoxylin and eosin staining showing similar levels of apoptosis in the hippocampus across control (A), single course (B), and repeated course (C) animals. Inset shows apoptotic cells (from boxed region) at higher magnification. Scale bars: A-C=100μm; A-C inset=5μm.
Relationship between myelination and brain volumes
There were positive correlations between the density of MBP-IR cells in the WM and the volume of the right hemisphere (r2=0.31; p<0.03), the deep grey matter (r2=0.31; p<0.03), and the neocortex (r2=0.23; p<0.03). These correlations occurred across all groups, and are consistent with normal brain growth. There were no other correlations across all parameters tested.
DISCUSSION
These studies were designed to determine whether single or repeated courses of antenatal corticosteroids in fetuses not exposed to any other factors such as hypoxia or infection, affect brain development at normal term delivery. The main finding of our study was that repeated courses have a greater effect on the brain than a single course, delaying myelination and causing a reduction in the volume of the deep grey matter; this reduction however was not reflected in an altered brain/body weight ratio. Both single and repeated courses lead to astrogliosis in the deep and subcortical white matter suggesting that steroids might be causing some brain injury although there were no overt signs of cell death or infarction at birth. Alterations could be present at the microstructural and/or neurochemical level but we were unable to address these issues in the present study. A strength of the study is that it was performed with non-human primates where, as in humans, major events in brain development occur in utero and the brain has similar proportions of grey and white matter. Furthermore the dosing regimes were similar to those used in humans in an appropriate timeframe to observe any intrauterine developmental alterations; we acknowledge that the baboon brain is more developed than the human brain at birth. In a companion study on the lung by our group it has been shown that lung volumes are increased and there was a decrease in mesenchyme in the alveolar walls suggesting antenatal corticosteroids may have an impact on the connective tissue matrix.
Overall brain growth was not affected by single or repeated course corticosteroids
The body weight of baboons at postmortem was not different between single, repeated or no antenatal corticosteroid-treated animals. Whole brain and cerebellar weights of the single course infants were significantly greater than both control and repeated course infants although this was not significant when expressed in relation to body weight. Volumetric measurements did not exhibit any differences between groups apart from a reduction in the deep grey matter volume in the repeated course group compared to controls. Again this was not significant when expressed in relation to body weight. Furthermore, there was no difference between groups in SFI indicating that gyral formation was not affected. Taken together it would appear that these regimens of antenatal steroid treatment did not have a marked effect on overall brain growth. Other animal studies have found differences in body and brain weight in the short (31) and long-term (14) but these involved different dosing regimens and species from those used here. We note that intrauterine growth restriction can result from prenatal corticosteroid-administration in humans but this usually follows several courses of corticosteroids (24), as compared with the two courses administered in the present study.
Repeated courses of corticosteroids delay myelination
We identified that repeated but not single courses of antenatal steroids affected myelination in the developing subcortical and deep white matter as indicated by lower numbers of MBP-IR cells compared to controls. Furthermore, qualitative assessment of IR for MBP in the corpus callosum also revealed a marked reduction in the repeated course group compared to controls. As it was only possible to examine the effects of steroids at one time point we are unable to determine whether myelination will recover to control levels postnatally. We do know, however, that the density of Olig-2 positive cells was not different between groups at term. As Olig-2 marks all cells of the oligodendrocyte lineage, we suggest that myelination is most likely to be delayed rather than permanently reduced; long term studies will be required to unequivocally determine the outcome.
Although this is the first study to describe alterations to myelination in a non-human primate as a result of repeated exposure to antenatal corticosteroids, previous studies have shown that repeated courses to fetal sheep prior to term, delay myelination in the corpus callosum at term (15). A similar regimen delays myelination in the optic nerve during gestation but recovery occurs by term. The authors suggest that regional differences in the effects of corticosteroids on the central nervous system might be due to the greater maturity of the optic nerve where 10% of the fibres are myelinated at the time of the first injection of corticosteroids (30). Myelination in the baboon has already begun in the deep cerebral white matter at about the time of the first course of corticosteroids (28) and with a single course, myelination is not different from controls at term. However it appears that in primates, exposure to an additional course of corticosteroids affects vulnerable pre-oligodendrocytes, delaying their maturation into myelinating oligodendrocytes and resulting in a deficit in MBP-IR cells at term. Oligodendrocytes are known to be at particular risk of damage or altered function during the peak period of myelination (32).
Even if myelination reaches normal levels postnatally, it is possible that maturational delays might influence functional outcomes. Myelin thickness affects axonal conduction velocity (33) and thus influences latency of action potentials in cerebral fiber tracts; this could affect synchrony of neuronal firing. Synchronous activity of neurons is thought to be important for refinement of connections (34). A recent study of developing human brain using magnetic resonance imaging tractography supports the concept that appropriate development of myelinated axons plays an important role in optimizing connectivity (35). A delay in myelination at critical periods of brain development could conceivably affect connectivity and underlie long-term deficits in function.
As indicated above it seems most likely that myelination is affected via effects of glucocorticoids on myelin synthesis rather than the death of oligodendrocytes. Glucocorticoids can act via genomic or non-genomic mechanisms, or alternatively by inducing effects on membranes. Glucocorticoids have been shown to act on post-transcriptional processes influencing the production of specific components of myelin including glycerol phosphate dehydrogenase, myelin basic protein and proteolipid protein (36).
Neuropathology
Both single and repeated courses of corticosteroids caused astrogliosis in the subcortical and deep white matter compared to controls suggesting that there might be some underlying injury that had stimulated a gliotic response. However this was not evidenced by any infarction, hemorrhage, overt signs of axonal damage, or increased microglial invasion when assessed at postmortem. There was no evidence of cortical cell death or gliosis. Qualitative examination of the hippocampus showed that there was no increase in apoptotic cell numbers in corticosteroid-treated animals compared to controls. We acknowledge that unbiased stereological counting of hippocampal pyramidal neurons is required to unequivocally determine whether cell death has occurred. A study of repeated corticosteroid administration in macaques has demonstrated that cell death occurred in the hippocampus of animals delivered prematurely; in animals delivered at term alterations in the hippocampus were still present, but to a lesser degree (18). It is possible in this study that injury had occurred earlier in response to exogenous steroids and any cells or cellular debris removed prior to postmortem. Currently we cannot determine whether the accelerated migration of CA3 pyramidal cells after a single course of corticosteroids has any physiological significance.
Conclusion
In conclusion we have shown that, in a non-human primate model at term, repeated courses of antenatal corticosteroids affect brain development more significantly than a single course particularly in relation to myelination. Whether myelination recovers postnatally with further development is unknown however the integrity of the oligodendrocyte lineage would provide a basis for this to occur. The functional significance of a maturational delay in myelination is not known but could have long lasting effects on brain function. Overall, brain growth in relation to body growth was not affected by corticosteroid treatment but there was evidence of astrogliosis in both groups of treated animals suggesting that there might be some underlying brain injury. In relating our findings to the human infant, we acknowledge that there are limitations in our study, including the small number of animals and the short duration of the study. However, our results suggest that repeated courses of corticosteroids are associated with a higher risk of brain alterations than a single course. Adverse effects on brain development will need to be balanced against the requirement to promote lung development when deciding whether to give repeated courses.
Acknowledgements
We acknowledge the major role that Professor Terrie Inder has played in the premature baboon project. We are grateful to Ms. Rachael O’Dowd for her technical assistance and Dr. Michelle Loeliger for advice with tissue preparation and analysis.
All sources: NIH Grant HL52636, NIH Grant P51 RR13986
Abbreviations
- dg
days of gestation
- GFAP
glial fibrillary acidic protein
- H&E
haemotoxylin and eosin
- Iba1
ionized calcium-binding adaptor protein 1
- IM
intramuscularly
- IR
immunoreactive
- MBP
myelin basic protein
- SFI
surface folding index
- WM
white matter
REFERENCES
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;19:CD004454. doi: 10.1002/14651858.CD004454.pub2. database online. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Aust N Z J Obstet Gynaecol. 2003;43:101–106. doi: 10.1046/j.0004-8666.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians - a survey of clinical practice. Aust N Z J Obstet Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828x.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- 4.Empana JP, Anceschi MM, Szabo I, Cosmi EV, Breart G, Truffert P. Antenatal corticosteroids policies in 14 European countries: factors associated with multiple courses. The EURAIL survey. Acta pædiatr. 2004;93:1318–1322. [PubMed] [Google Scholar]
- 5.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2009;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 7.Peltoniemi OM, Kari MA, Lano A, Yliherva A, Puosi R, Lehtonen L, Tammela O, Hallman M. Two-year follow-up of a randomised trial with repeated antenatal betamethasone. Arch Dis Child Fetal Neonatal Ed. 2009;94:F402–F406. doi: 10.1136/adc.2008.150250. [DOI] [PubMed] [Google Scholar]
- 8.Asztalos EV, Murphy KE, Hannah ME, Willan AR, Matthews SG, Ohlsson A, Kelly EN, Saigal S, Ross S, Delisle M-F, Amankwah K, Guselle P, Gafni A, Lee SK, Armson BA, Sananes R, Tomat L, Multiple Courses of Antenatal Corticosteroids for Preterm Birth Study Collaborative Group Multiple Courses of Antenatal Corticosteroids for Preterm Birth Study: 2-Year Outcomes. Pediatrics. 2010;126:e1045–e1055. doi: 10.1542/peds.2010-0857. [DOI] [PubMed] [Google Scholar]
- 9.Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. New England Journal of Medicine. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 10.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 11.Spinillo A, Viazzo F, Colleoni R, Chiara A, Maria Cerbo R, Fazzi E. Two-year infant neurodevelopmental outcome after single or multiple antenatal courses of corticosteroids to prevent complications of prematurity. Am J Obstet Gynecol. 2004;191:217–224. doi: 10.1016/j.ajog.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 12.French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami M, Jobe AH, Newnham JP, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–184. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 14.Moss TJM, Doherty D, Nitsos I, Sloboda D, Harding R, Newnham J. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192:146–152. doi: 10.1016/j.ajog.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 15.Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Develop Neurosci. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 16.Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz P, Witte O, Schwab M. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:142–151. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- 17.Dunlop SA. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Neonatal Med. 1997;6:309. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 19.Scheepens A, van de Waarenburg M, van den Hove D, Blanco CE. A single course of prenatal betamethasone in the rat alters postnatal brain cell proliferation but not apoptosis. J Physiol. 2003;552:163–175. doi: 10.1113/jphysiol.2003.043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83:F154–F157. doi: 10.1136/fn.83.2.F154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newnham JP, Moss TJM, Nitsos I, Sloboda DM. Antenatal corticosteroids: the good, the bad and the unknown. Curr Opin Obstet Gynecol. 2002;14:607–612. doi: 10.1097/00001703-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29:227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011 doi: 10.1111/j.1600-0412.2011.01132.x. Accepted Article. [DOI] [PubMed] [Google Scholar]
- 25.Rees SM, Camm EJ, Loeliger M, Cain S, Dieni S, McCurnin D, Shaul PW, Yoder B, McLean C, Inder TE. Inhaled nitric oxide: effects on cerebral growth and injury in a baboon model of premature delivery. Pediatr Res. 2007;61:552–558. doi: 10.1203/pdr.0b013e318045be20. [DOI] [PubMed] [Google Scholar]
- 26.Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, Coalson J, Rees SM. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–1653. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- 27.Loeliger M, Inder TE, Dalitz PA, Cain S, Camm EJ, Yoder B, McCurnin D, Shaul PW, Clyman R, Rees SM. Developmental and neuropathological consequences of ductal ligation in the preterm baboon. Pediatr Res. 2008;65:209–214. doi: 10.1203/PDR.0b013e31818d6d0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S. The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol. 2004;63:1297–1309. doi: 10.1093/jnen/63.12.1297. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 30.Rees S, Stringer M, Just Y, Hooper SB, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res. 1997;103:103–118. doi: 10.1016/s0165-3806(97)81787-7. [DOI] [PubMed] [Google Scholar]
- 31.Huang WL, Beazley LD, Qiunlivan JA, Evans SF, Newham JP, Dunlop SA. Effect of Corticosteroids on Brain Growth in Fetal Sheep. Obstet Gynecol. 1999;94:213–218. doi: 10.1016/s0029-7844(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 32.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 34.Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 35.Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran J-P, Grant PE. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Cole R, Chiappelli F, de Vellis J. Differential regulation of oligodendrocyte markers by glucocorticoids: post-transcriptional regulation of both proteolipid protein and myelin basic protein and transcriptional regulation of glycerol phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1989;86:6807–6811. doi: 10.1073/pnas.86.17.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]