Abstract
A20 (also known as TNFAIP3) is a potent anti-inflammatory signalling molecule that restricts multiple intracellular signalling cascades. Recent studies in three general areas have converged to highlight the clinical and biological importance of A20. First, human genetic studies have strongly linked polymorphisms and mutations in the gene encoding A20 to inflammatory, autoimmune and malignant diseases. Second, studies in gene-targeted mice have revealed that A20 regulates multiple immune cell functions and prevents experimental diseases that closely mimic human conditions. Third, biochemical studies have unveiled complex mechanisms by which A20 regulates ubiquitin-dependent nuclear factor-κB and cell-survival signals. Taken together, these studies are revealing the importance of A20-mediated regulation of ubiquitin-dependent signalling in human disease.
Human genetic studies have implicated a wide variety of immune genes in the pathophysiology of autoimmune and inflammatory diseases. These studies have suggested a role for aberrant immune cell activation in these diseases and have guided recent investigations into the molecular mechanisms underlying disease pathogenesis. From the many polymorphisms analysed, several genes — including the gene encoding the anti-inflammatory signalling molecule A20 (TNFAIP3) — have been linked to susceptibility to multiple diseases. The products of these genes are likely to regulate crucial steps in immune cell homeostasis and should be attractive targets for targeted therapies.
Perhaps the best-characterized molecular pathway for triggering immune cell activation is the canonical nuclear factor-κB (NF-κB) signalling pathway, which leads to the transcription of numerous pro-inflammatory and cell-survival genes1. Excessive NF-κB signalling in multiple cell types has been linked to both human and experimental inflammatory diseases, and to malignant diseases. For example, dysregulated NF-κB signals underlie the pathophysiology of common subtypes of B cell lymphoma and myeloma2, as well as colon carcino-genesis3. Therefore, determining how NF-κB signals are normally restricted is crucial both for understanding the pathophysiology of these diseases and for devising therapeutic strategies.
The NF-κB signalling cascade is prominently regulated by ubiquitylation, a process that can generate a series of post-translational modifications that direct proteins towards distinct biological fates4. For example, the attachment of K48-linked polyubiquitin chains targets proteins for proteasomal degradation, whereas the attachment of K63-linked polyubiquitin chains can result in the recruitment of downstream signalling molecules that propagate signals4,5. Thus, the precise synthesis, recognition and degradation of diverse types of ubiquitin chain must be specified by ubiquitin ligases, ubiquitin-binding proteins and de-ubiquitylating enzymes (DUBs), respectively, to ensure the proper regulation of intracellular signalling pathways. The functions of ubiquitylation in cell signalling have recently been described in several excellent reviews4–6.
The ubiquitin-modifying enzyme A20 has emerged as a potent and unusually complex regulator of ubiquitin-dependent signals. A20 is a pleiotropically expressed cytoplasmic protein, the expression of which is regulated at both the transcriptional and the post-transcriptional level. Induced by NF-κB-dependent signals, A20 in turn restricts the duration and intensity of signalling by several molecules involved in the NF-κB pathway. Hence, the induction of A20 expression constitutes a negative feedback loop for NF-κB signalling7. In addition, post-translational modifications of A20 — including phosphorylation, protein cleavage, glycosylation and ubiquitylation — may serve to support or restrict its activity8,9,10.
A20 restricts NF-κB signalling downstream of tumour necrosis factor receptor 1 (TNFR1), CD40, Toll-like receptors (TLRs), NOD-like receptors (NLRs) and the interleukin-1 receptor (IL-1R)11–19. A20 also promotes cell-survival signals, adding another dimension to its ability to regulate dynamic immune responses14. Of note, the ability of A20 to inhibit cell death may be independent of its role in restricting NF-κB signalling, as decreased NF-κB signalling is typically associated with increased cell death. The molecular mechanisms by which A20 performs these diverse functions are incompletely understood, but are likely to involve the regulation of ubiquitin-dependent signalling complexes (see below).
A20 cleaves polyubiquitin chains, thereby exhibiting DUB activity15,20–22, but also collaborates with ubiquitin-activating E1 enzymes and ubiquitin-carrier E2 proteins to build ubiquitin chains, thus displaying E3 ubiquitin ligase activity22. Furthermore, A20 directly binds to ubiquitin chains23,24. Therefore, A20 interfaces with and modifies ubiquitylated protein substrates in multiple ways and probably uses a variety of biochemical mechanisms to regulate NF-κB and cell-death signals (see below).
In this Review, we examine recent studies in three areas. First, human genetic studies have linked both germline polymorphisms and somatic mutations of TNFAIP3 (which encodes A20) to inflammatory and malignant human diseases. Second, new strains of mice bearing lineage-specific deletions of Tnfaip3 have revealed several cell type-specific functions for A20. Third, biochemical studies have revealed complex mechanisms by which A20 regulates a variety of ubiquitin-dependent signalling pathways in immune cells. Taken together, these studies are revealing a diverse set of genetic, cellular and molecular mechanisms by which A20 prevents disease.
A20: a protein linked to multiple human diseases
Human genetic studies have linked germline single-nucleotide polymorphisms (SNPs) of TNFAIP3 with susceptibility to multiple human diseases25. These diseases include systemic lupus erythematosus (SLE)26–32, rheumatoid arthritis30,33–37, psoriasis38,39, type 1 diabetes37,40, coeliac disease37,41, Crohn's disease42,43, coronary artery disease in type 2 diabetes44, and systemic sclerosis45,46 (FIG. 1). Given the potent anti-inflammatory function of A20, disease-associated TNFAIP3 SNPs might reduce its function or expression. Indeed, one SLE-associated SNP in the coding region of TNFAIP3 causes a phenylalanine to cysteine substitution at residue 127 and reduces A20 function27. Another SLE-associated SNP reduces A20 expression by altering the binding of transcription factors at a putative 3′ enhancer in TNFAIP3 (REF. 31). Other disease-associated TNFAIP3 SNPs are located outside of the coding regions, suggesting that they may also confer susceptibility to disease by reducing A20 expression35,44. Importantly, mice expressing reduced levels of A20 develop spontaneous inflammation, providing direct evidence that reduced A20 expression causes autoimmune or inflammatory disease18,19,43.
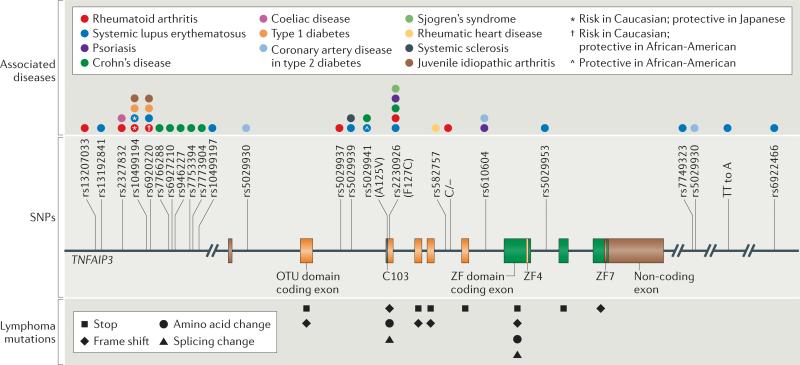
Figure 1. Polymorphisms or mutations in TNFAIP3 and human diseases.
The figure shows a schematic of the TNFAIP3 gene locus, which encodes A20. Exons encoding the amino-terminal OTU domain are shown in orange and exons encoding the carboxy-terminal zinc fingers (ZFs) of A20 are shown in green. The C103, ZF4 and ZF7 motifs are highlighted. Non-coding exons, including AT-rich sequences at the end of exon 9, are shown in brown. Human germline single-nucleotide polymorphisms (SNPs) in the TNFAIP3 locus that are associated with autoimmune and autoinflammatory diseases are indicated (labelled with their reference SNP (rs) numbers)26–46, as are somatic mutations that have been identified in the coding exons of TNFAIP3 in human B cell lymphomas50–54.
Potential benefits of correlating SNPs in specific genes with genetically complex human diseases include the ability to associate genotypes and/or molecular phenotypes with prognostic or therapeutic responses. TNFAIP3 polymorphisms and altered A20 expression have been correlated with therapeutic responses to TNF blockade in the treatment of rheumatoid arthritis, inflammatory bowel disease and psoriasis47–49. Moreover, the presence of certain TNFAIP3 SNPs strongly correlates with the risk of severe renal or haematological complications in patients with SLE31. Therefore, in addition to providing insights into the pathogenesis of these inflammatory disorders, further genetic analyses might link TNFAIP3 polymorphisms with distinct subtypes of these diseases.
In addition to the effects of germline polymorphisms of TNFAIP3 in conferring susceptibility to inflammatory diseases, biallelic somatic mutations of this gene are pathogenic in human lymphomas, suggesting that A20 acts as a tumour suppressor50–54. Biallelic mutations in the coding sequence of TNFAIP3 have been identified in ~18% of B cell lymphomas, including diffuse large B cell lymphoma, mucosa-associated lymphoid tissue-type lymphoma and Hodgkin's lymphoma50,51. Such mutations occur throughout the TNFAIP3 gene and result in the introduction of stop codons, frame shifts, amino acid changes or splicing alterations55,56 (FIG. 1). The expression of TNFAIP3 may also be inhibited by epigenetic methylation of its promoter53,57. The degree to which mutations affect A20 protein stability and/or function has yet to be determined for most lymphomas.
TNFAIP3 mutations are likely to be pathogenic in cancerous transformation, as re-expressing A20 in tumour cells induces apoptosis or cell-cycle arrest50,51,53,54. As A20 deficiency can lead to exaggerated NF-κB signalling and/or increased cell death, and as enhanced NF-κB signalling is linked to several types of human B cell lymphoma, A20-mediated restriction of this crucial signalling cascade may explain the tumour-suppressive function of A20 in B cells. In addition, whether A20 inactivation contributes to other cancer cell types or predicts responses to therapies warrants further investigation. In summary, polymorphisms and mutations of TNFAIP3 have an impact on a wide range of human diseases. These links to human disease provide a compelling rationale for further investigations into the mechanisms by which A20 regulates immune cell functions.
Cellular mechanisms of A20 and disease
A20 is expressed by virtually all cell types, which complicates our understanding of the physiological functions of A20 and its roles in preventing disease. In addition, A20-regulated signals, such as canonical NF-κB signals and cell-survival signals, are shared by multiple ligands and cell types. Given the pleiotropic functions of NF-κB and cell-death signalling in various cell types that contribute to autoimmune and inflammatory diseases, the regulation of these signalling cascades by A20 may contribute to disease pathogenesis (TABLE 1). However, the multiorgan inflammation and perinatal lethality of A20-deficient mice largely prevents detailed studies of the functions of A20 in adult mice14. The recent development of mice with conditionally targeted Tnfaip3 alleles (Tnfaip3flox/flox mice) has enabled lineage-specific and temporally controlled deletions of Tnfaip3, thereby facilitating studies of the functions of A20 in specific immune cell types.
Table 1.
Mouse phenotypes resulting from cell type-specific ablation of A20 expression and the related human diseases
| Cell type | Genetic modification | Mouse phenotype | Related human disease | Refs |
|---|---|---|---|---|
| B cells | Cre-mediated deletion of Tnfaip3 in cells that express CD19 | Germinal centre and plasma cell dysplasia; production of autoantibodies; renal immunoglobulin deposition; B cell resistance to FAS-mediated cell death | Systemic lupus erythematosus | 18,19,60 |
| DCs | Cre-mediated deletion of Tnfaip3 in cells that express CD11c | DC activation; expansion and activation of T cell and myeloid cell populations; colitis; spondyloarthritis | Inflammatory bowel disease | 43 |
| DCs | Cre-mediated deletion of Tnfaip3 in cells that express CD11c | DC activation; expansion of T cell and plasma cell populations; increased uptake of apoptotic cells by DCs; autoantibody production; nephritis | Systemic lupus erythematosus | 62 |
| Macrophages and granulocytes | Cre-mediated deletion of Tnfaip3 in cells that express lysozyme M | Increased IL-6 production; production of collagen-specific autoantibodies; protection against influenza A virus infection | Rheumatoid arthritis | 67,68 |
| Intestinal epithelial cells | Cre-mediated deletion of Tnfaip3 in cells that express villin | Hypersensitivity to experimental colitis | Inflammatory bowel disease | 69 |
| Intestinal epithelial cells | Villin-driven expression of a Tnfaip3 transgene | Protection against DSS-induced colitis | Inflammatory bowel disease | 72 |
| Keratinocytes | Cre-mediated deletion of Tnfaip3 in cells that express keratin 14 | Epidermal hyperproliferation; hair and skin defects; sebaceous gland hyperplasia | ? | 73 |
DC, dendritic cell; DSS, dextran sulphate sodium; IL-6, interleukin-6.
B cell-mediated autoimmunity and lymphomas
The genetic association of TNFAIP3 SNPs with SLE and of somatic TNFAIP3 mutations with B cell lymphomas suggests that A20 might have important functions in B cells. The expression of A20 is transcriptionally induced in B cells and may also be regulated at the post-translational level via cleavage by the paracaspase MALT1 (REFS 9,58,59). Insights into how A20 regulates B cell functions have emerged from studies of mice lacking A20 specifically in B cells (Tnfaip3flox/flox Cd19-Cre mice). Three independent groups have generated such mice18,19,60, and the phenotypes of these mice are largely similar.
A20-deficient B cells display exaggerated NF-κB-mediated responses and are hyperresponsive to multiple stimuli, including lipopolysaccharide (LPS) and B cell receptor (BCR) and CD40 ligation. They produce higher levels of IL-6 following stimulation than wild-type B cells, which may account for the moderate increase in the number of A20-sufficient T cells in Tnfaip3flox/flox Cd19-Cre mice. As these mice age, a progressive increase in spontaneous B cell activation, the expansion of myeloid cell populations and plasma cell hyperplasia are observed18,19. In addition, the expression of A20 in B cells may be important for the development of marginal zone B cells19,60.
Tnfaip3flox/flox Cd19-Cre mice spontaneously develop an autoimmune condition similar to SLE that is characterized by elevated numbers of plasma cells and germinal centre B cells, IgM and IgG autoantibodies, and renal immunoglobulin deposits18,19,60. These phenotypes are observed by 3–4 months of age, suggesting that A20 expression in B cells might prevent an early step in SLE pathogenesis. Although Tnfaip3flox/flox Cd19-Cre mice were not shown to develop renal failure, the severity of the clinical disease in these mice may be more apparent when they are bred onto lupus-prone backgrounds.
The increase in the number of germinal centre B cells in Tnfaip3flox/flox Cd19-Cre mice may be due to the resistance of A20-deficient B cells to FAS-mediated apoptosis18. This resistance may be mediated by the markedly elevated levels of NF-κB-dependent anti-apoptotic proteins, including B cell lymphoma X (BCL-X), in stimulated A20-deficient B cells. This finding was somewhat surprising given the increased sensitivity of A20-deficient fibroblasts and thymocytes to TNF-mediated programmed cell death14. Thus, the regulation of cell survival or death by A20 is likely to be dependent on both the cell type and the context61.
Focused genetic studies — including deep sequencing of the human TNFAIP3 gene — have revealed that a SNP in the 3′ non-coding region confers susceptibility to SLE by reducing A20 expression31. The spontaneous development of autoantibodies in heterozygous Tnfaip3+/– mice may thus genetically mimic the human condition (G. E. Hammer and A.M., unpublished observations). Furthermore, heterozygous Tnfaip3flox/+ Cd19-Cre mice, which express reduced levels of A20 in B cells, also contain increased numbers of germinal centre B cells and develop autoantibodies18. In contrast to many mouse models of autoimmune disease in which homozygous null alleles are used to approximate human heterozygous SNPs, mice with spontaneous SLE-like disease owing to reduced A20 expression may provide a closer genetic approximation to the human condition.
The increased NF-κB signalling and enhanced survival of A20-deficient germinal centre B cells also provide a potential molecular underpinning for the role of A20 in preventing human B cell lymphomas. Several human B cell lymphoma subtypes are thought to arise from germinal centre B cells, and dysregulated NF-κB signalling is a known feature of the oncogenic mutations in these cells. A failure to eliminate germinal centre B cells could also lead to prolonged activation-induced cytidine deaminase (AID) activity and increased numbers of somatic mutations, including oncogenic mutations. Thus, the effects of exaggerated NF-κB signalling in causing increased expression of anti-apoptotic proteins and resistance to cell death suggest several ways in which A20 deficiency may contribute to the transformation of germinal centre B cells. Although mice bearing A20-deficient B cells have not yet been shown to spontaneously develop B cell lymphomas, future studies of potentially collaborative oncogenes may better define the tumour-suppressor functions of A20.
In summary, A20 regulates the homeostasis of germinal centre B cells and marginal zone B cells. In addition, important roles for A20 in other B cell subtypes may become apparent with the generation of mice that lack A20 expression in B cells at other stages of their development. Nevertheless, the studies to date indicate that precisely regulated A20 expression levels in B cells are essential for preventing B cell-mediated autoimmunity and B cell malignancies in mice and humans.
Dendritic cells and immune homeostasis
Early physiological evidence for the importance of A20 in regulating innate immune responses emerged from the observation that mice deficient in both A20 and recombination-activating gene 1 (RAG1; which is required for the development of B and T cells) develop myeloid cell-dependent inflammation, cachexia and perinatal lethality in a similar manner to A20-deficient mice14. In addition to restricting TNF-induced NF-κB signals, A20 has been shown to restrict NF-κB signals triggered by both TLRs and NLRs14–16. Indeed, A20-dependent restriction of TLR signals may be particularly important for immune homeostasis, as mice lacking both A20 and the TLR and IL-1R adaptor MYD88 (myeloid differentiation primary-response protein 88) exhibit markedly less inflammation and prolonged survival compared with mice lacking A20 only17.
Given the central role for dendritic cells (DCs) in initiating immune responses, the functions of A20 in DCs have been investigated via the generation of mice with a DC-specific deletion of Tnfaip3 (Tnfaip3flox/flox Cd11c-Cre mice)43,62 and via short hairpin RNA-mediated knockdown of Tnfaip3 expression in DCs63–65. Tnfaip3flox/flox Cd11c-Cre mice rapidly develop profound disturbances in immune homeostasis, suggesting that A20 expression in DCs has major roles in regulating immune homeostasis, although these mice do not develop the cachexia and perinatal lethality observed in globally A20-deficient mice14,43,62. A20-deficient DCs are spontaneously activated in Tnfaip3flox/flox Cd11c-Cre mice and produce high levels of pro-inflammatory cytokines. Spontaneous activation of B cells, T cells and myeloid cells, as well as the development of lymphadenopathy and splenomegaly, was observed in young (~4–6-week-old) Tnfaip3flox/flox Cd11c-Cre mice. DCs from these mice drive the rapid activation and proliferation of normal adoptively transferred naive T cells and prevent the induction of anergy and the deletion of antigen-specific T cells43.
Although the ligands that trigger the activation of A20-deficient DCs under basal conditions are unknown, A20 is known to be a crucial regulator of TLR and IL-1R signals15,17. Breeding of Tnfaip3flox/flox Cd11c-Cre mice with Myd88flox/flox mice revealed that A20 restricts MYD88-dependent signals in DCs that lead to enhanced IL-6 expression and T cell clonal expansion under basal conditions43. Persistent DC and T cell activation in these Tnfaip3flox/floxMyd88flox/flox Cd11c-Cre mice indicated that A20 also restricts MYD88-independent signals that drive these phenotypes43. Thus, even under basal conditions, DCs probably receive a variety of potentially stimulatory signals. A20 restricts these intracellular signals and prevents aberrant immune activation.
Surprising and potentially informative differences were observed in the phenotypes of two independently generated strains of Tnfaip3flox/flox Cd11c-Cre mice. In one strain, increased levels of B cell-activating factor (BAFF) and increased numbers of plasma cells were associated with elevated levels of serum immunoglobulin and double-stranded DNA-specific autoantibodies62. These mice developed nephritis, antiphospholipid syndrome and autoimmune arthritis, which are phenotypes that resemble human SLE. By contrast, the other strain of mice developed lymphocyte-dependent colitis, seronegative arthritis, enthesitis and ankylosing spondylitis. This phenotype mimics a stereotypical syndrome of human inflammatory bowel disease with associated arthritides43. One intriguing potential explanation for these phenotypic differences could reside in divergent luminal microbiota in the two colonies66.
DC-based immunization strategies have been used for vaccinating humans, and approaches for optimizing DC-based immunization have focused on increasing the immunogenicity of transferred DCs. In this context, it is notable that A20-deficient DCs induce enhanced antigen-specific immune responses in Tnfaip3flox/flox Cd11c-Cre mice43. In addition, knockdown of Tnfaip3 expression in bone marrow-derived DCs leads to enhanced antigen-specific immune responses that overcome regulatory T cell-mediated suppression63 and result in enhanced immune responses to tumours63,64 and HIV65. Hence, the attenuation of A20 expression in DCs could significantly improve the efficacy of DC-based vaccines.
A20, myeloid cells and autoimmunity
Macrophages share a number of innate immunostimulatory functions with DCs, although their localization and subspecialization distinguishes their physiological functions from those of DCs. Studies with mice in which expression of A20 was ablated in macrophages and granulocytes (Tnfaip3flox/flox Lysm-Cre mice) revealed that A20 expression in these cells is necessary for maintaining innate immune homeostasis67. Indeed, these mice developed spontaneous polyarthritis that was associated with collagen-specific autoantibodies and increased systemic and local cytokine production, reminiscent of human rheumatoid arthritis. This phenotype involved IL-6- and TLR4–MYD88-dependent signals, but was not ameliorated by broad-spectrum antibiotics or by the genetic elimination of adaptive immune cells or of TNF signals67. Thus, A20-deficient myeloid cells appear to drive erosive polyarthritis via IL-6- and TLR4-dependent signals. These mice also exhibited increased osteoclast function, which probably contributed to the osteoporotic changes. Hence, A20 expression in myeloid cells restricts signals that drive arthritis and osteoporosis. These findings provide pathophysiological insights into how A20 deficiency may lead to arthritis in mice and possibly humans.
In addition to rendering mice susceptible to inflammatory disease, selective loss of A20 in myeloid cells protected mice against influenza A virus infection68. This finding shows that enhanced A20-dependent inflammatory responses may be protective, rather than detrimental, during influenza A virus infection. It also provides a tantalizing hint that the evolutionary preservation of hypomorphic A20 expression and the associated increased susceptibility to inflammatory disease may have resulted from an evolutionary pressure to resist certain types of infection.
A20 and intestinal immune homeostasis
In addition to being expressed by immune cells, A20 is expressed by intestinal epithelial cells (IECs), suggesting that it might have a role in the response to microorganism-derived molecules in the intestinal lumen. A20 ablation in IECs (in Tnfaip3flox/flox villin-Cre mice) renders mice hypersensitive to dextran sulphate sodium (DSS)-induced colitis and TNF-induced inflammation69. The sensitivity of these mice to DSS-induced colitis is rescued by TNFR1 deficiency, suggesting that A20 may protect IECs from TNF-induced apoptosis during acute damage. Tnfaip3flox/flox villin-Cre mice exhibited more severe intestinal damage when rendered deficient of MYD88, consistent with previous studies showing that TLR-mediated sensing of commensal bacteria contributes to intestinal health70. The effect of A20 loss in IECs was most dramatic during intestinal recovery immediately after the removal of DSS treatment, suggesting a role for A20 in tissue repair. Complementary studies using enforced expression of A20 in IEC cell lines suggested that A20 mediates IEC tolerance to LPS71. Moreover, transgenic expression of A20 in IECs protects mice against DSS-induced colitis by supporting tight junctions between epithelial cells and preserving intestinal barrier function72. Hence, A20 expression in IECs may help to maintain mucosal immune homeostasis during episodes of gross inflammation.
A20 and skin pathology
The differentiation of keratinocytes is dependent on NF-κB signalling, and increased expression of NF-κB-dependent gene products is associated with skin inflammation and psoriasis. Although the pathophysiology of human psoriasis remains somewhat enigmatic, recent genome-wide association studies have strongly linked polymorphisms in TNFAIP3 with susceptibility to psoriasis38,39,49.
Mice in which A20 expression is specifically ablated in keratinocytes (Tnfaip3flox/flox Krt14-Cre mice) exhibited keratinocyte hyperproliferation, dishevelled hair and sebocyte hyperplasia73. These phenotypes were consistent with dysregulated ectodysplasin A receptor (EDAR)-mediated signalling, and in vitro studies suggested that A20 can inhibit EDAR-triggered NF-κB signalling in a manner independent of its DUB activity73. No spontaneous inflammation was observed in these mice, suggesting that A20 does not restrict basal pro-inflammatory signals in keratinocytes. Alternatively, cylindromatosis protein (CYLD), another DUB, might partly compensate for the loss of A20 expression and prevent exaggerated NF-κB signalling activity in these cells under steady-state conditions. CYLD is highly expressed in the skin, and mutations in this gene cause the skin disease cylindromatosis74. Compensation for the loss of A20 by CYLD would also be consistent with the ability of CYLD to remove ubiquitin chains from proteins similar to those de-ubiquitylated by A20 (REF. 75). Given the crucial roles of A20 in restricting NF-κB signals, challenging Tnfaip3flox/flox Krt14-Cre mice with pro-inflammatory ligands might reveal whether A20 expression by keratinocytes restricts the duration or intensity of skin inflammation.
A20 and endothelial cells
A20 was originally cloned from endothelial cells, and heterologous expression of A20 in endothelial cells suppresses inflammation in neighbouring tissues (including transplanted tissues)76–78. The role of A20 in endothelial cells might also contribute to links between human TNFAIP3 polymorphisms and vascular disease; for example, SNPs in TNFAIP3 are associated with increased atherosclerotic disease in patients with type 2 diabetes44 (FIG. 1). In addition, a genome-wide screen for genes regulating susceptibility to atherosclerosis in apolipo-protein E-deficient mice identified a mutation in the coding sequence of Tnfaip3, and transgenic expression of A20 reversed this susceptibility79,80. One pathophysiological suggestion from these studies was that A20 expression in endothelial cells might suppress the expression of adhesion proteins for monocytes at sites of atherosclerotic plaque formation. Given the emerging evidence that type 2 diabetes and atherosclerosis are associated with chronic low levels of inflammation in humans, the anti-inflammatory functions of A20 may help to prevent these diseases. Endothelial cell-specific deletion of Tnfaip3 may provide further insight into these issues.
The studies above indicate that the level of A20 expression in specific cell types influences the type of inflammation that occurs in vivo and the susceptibility to inflammatory diseases. Reduced A20 expression in several cell types may thus collaborate with additional genes or environmental triggers to define disease phenotypes (TABLE 1). Moving forward, analyses of mice with reduced or absent expression of A20 in other specific cell types (such as T cells) or combinations of cell types (such as both B and T cells) should provide new insights into disease pathologies and potential therapeutic strategies.
A20: biochemical mechanisms of action
A20 and the regulation of ubiquitin-dependent signalling
The A20 protein exhibits DUB, E3 ubiquitin ligase and ubiquitin-binding activities in cell-free and cellular systems (FIG. 2). Its DUB activity is mediated by an amino-terminal motif containing a catalytic cysteine residue (C103) in its OTU domain, whereas zinc finger 4 (ZF4) in the carboxy-terminal domain of A20 binds ubiquitin and supports E3 ubiquitin ligase activity15,22,23,81,82. These biochemical roles — considered together with emerging evidence of the crucial roles of ubiquitylation in regulating protein–protein interactions, protein stability and intra-cellular signals — suggest that A20 regulates intracellular signals by regulating ubiquitylated signalling complexes.
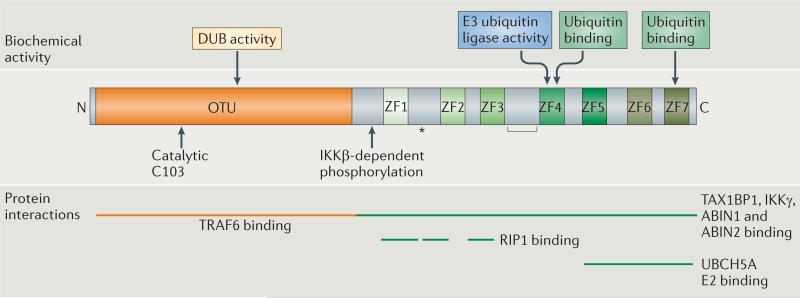
Figure 2. Biochemical characteristics of A20 protein function.
The de-ubiquitylating (DUB) activity of A20 is mediated by the catalytic cysteine at position 103 (C103) within the OTU domain. A20 also contains seven zinc fingers (ZFs), which mediate its E3 ubiquitin ligase activity (via ZF4) and its ubiquitin-binding activity. Indeed, A20 binds to ubiquitylated E2 enzymes such as UBCH5A via ZF4–ZF7, to K63-linked polyubiquitin chains via ZF4 (REF. 23) and to linear polyubiquitin chains via ZF7 (REFS 24,90,91). A20 also interacts with substrates such as receptor-interacting protein 1 (RIP1) via ZF1–ZF3 (with ZF1 being crucial for binding)22,23, with E3 enzymes such as TNFR-associated factor 6 (TRAF6) via the OTU domain, and with ubiquitin-binding proteins such as TAX1-binding protein 1 (TAX1BP1), IκB kinase-γ (IKKγ), A20-binding inhibitor of NF-κB activation 1 (ABIN1) and ABIN2 via the ZF domain. Some of these latter interactions may occur through the mutual binding of A20 and the interacting protein to ubiquitin chains. The regions that mediate the interaction of A20 with the E3 enzymes RING-finger protein 11 (RNF11) and ITCH, as well as with itself, have not been clearly defined. In addition to their other functions, the C103 and ZF4 motifs have been shown to support the degradation of E2 enzymes81. A20 also undergoes post-translational modifications; for example, a site of A20 phosphorylation by IKKβ is indicated. Human A20 is cleaved by the paracaspase MALT1 at the site indicated by an asterisk. The site at which mouse A20 is cleaved has not been precisely determined, but the region where it is cleaved is indicated by a bracket.
The attachment of polyubiquitin chains to signalling proteins stimulates the recruitment of ubiquitin-binding proteins. The attachment of K48-linked polyubiquitin chains has long been known to target proteins for proteasomal degradation, whereas polyubiquitin chains linked via other lysine residues, such as K63-linked chains, can recruit downstream signalling proteins, thereby propagating signals. The DUB activity of A20 cleaves anchored K63-linked polyubiquitin chains, so this biochemical function may help to restrict ubiquitin-dependent signals. During TNF signalling, the conjugation of receptor-interacting protein 1 (RIP1; also known as RIPK1) with K63-linked polyubiquitin chains leads to the recruitment of the signalling molecules TAK1-binding protein 2 (TAB2), TGFβ-activated kinase 1 (TAK1) and IκB kinase-γ (IKKγ; also known as NEMO) to form a complex with RIP1. This in turn leads to the phosphorylation of NF-κB inhibitor-α (IκBα) and the activation of NF-κB4. The increased ubiquitylation of RIP1 in stimulated A20-deficient cells indicates that A20 is crucial for restricting RIP1 ubiquitylation22 (FIG. 3). In this context, the DUB activity of A20 probably restricts TNF-induced NF-κB signalling by removing K63-linked polyubiquitin chains from RIP1.
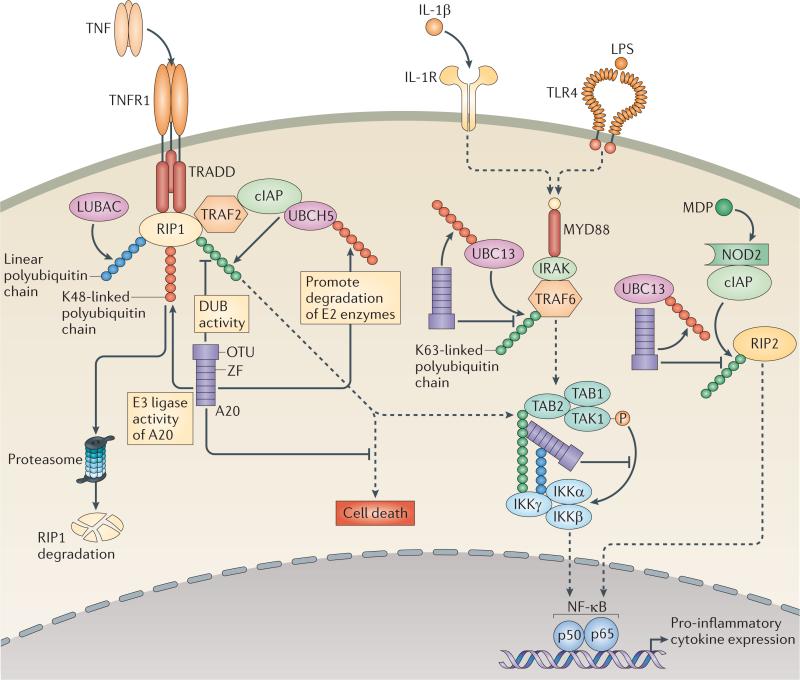
Figure 3. A20-dependent regulation of ubiquitin-dependent signalling pathways.
A20 regulates multiple ubiquitin-dependent innate immune signalling cascades, including those downstream of tumour necrosis factor receptor 1 (TNFR1), interleukin-1 receptor (IL-1R), Toll-like receptor 4 (TLR4) and nucleotide-binding oligomerization domain (NOD) proteins. During these signalling cascades, E2 enzymes, such as UBCH5 and UBC13, and E3 ligases, such as cellular inhibitor of apoptosis (cIAP) proteins, TNFR-associated factor 6 (TRAF6), and perhaps TRAF2, collaborate to build polyubiquitin chains. These polyubiquitin chains can be K48-linked, K63-linked or joined by other linkages, and are attached to substrates such as receptor-interacting protein 1 (RIP1), TRAF6, IκB kinase-γ (IKKγ) and RIP2. A distinct enzymatic complex known as LUBAC (linear ubiquitin chain assembly complex) builds linear polyubiquitin chains on RIP1 and IKKγ116, 117. In addition, unanchored ubiquitin chains (that is, chains that are not covalently attached to signalling proteins) are integral to these cascades. A20 may regulate these signalling complexes by cleaving K63-linked ubiquitin chains from RIP1, TRAF6 and/or IKKγ through the de-ubiquitylating (DUB) activity of its OTU domain. A20 can also regulate these signalling pathways by supporting the degradation of the E2 enzymes UBCH5 and UBC13, thereby inhibiting E3 ligase activity that is dependent on these E2 enzymes. Finally, A20 can build K48-linked polyubiquitin chains on RIP1, which leads to its degradation. In addition, the ZF4 and ZF7 domains of A20 have been shown in vitro to bind to K63-linked ubiquitin chains and linear ubiquitin chains, respectively, on IKK complexes and TNFR complexes and may use these interactions to facilitate the inhibition of these signalling complexes. Although it is not shown in the figure, the ubiquitin-binding activities of A20 might also compete with those of other ubiquitin-binding proteins and/or help A20 to function as an adaptor protein for other regulators, such as A20-binding inhibitor of NF-κB activation 1 (ABIN1), ITCH, RING-finger protein 11 (RNF11) or TAX1-binding protein 1 (TAX1BP1). In addition to restricting TNFR, IL-1R, TLR and NOD signals, A20 regulates signals triggered by the T cell receptor9 (not shown) and CD40 (not shown). Finally, A20 restricts TNF-induced apoptosis, possibly by restricting the ubiquitylation of caspase 8 (REFS 14,88) (not shown). IRAK, IL-1R-associated kinase; LPS, lipopolysaccharide; MDP, muramyl dipeptide; NF-κB, nuclear factor-κB; TAB, TAK1-binding protein; TAK1, TGFβ-activated kinase 1; TRADD, TNFR1-associated death domain protein; ZF, zinc finger.
The assembly of K63-linked polyubiquitin chains also supports TNFR-associated factor 6 (TRAF6)-mediated activation of NF-κB during IL-1R and TLR signalling, and the removal of these chains by A20 may be a mechanism by which A20 restricts TLR signals4,15,17,20,83,84 (FIG. 3). Furthermore, A20-restricts the ubiquitylation of RIP2 (also known as RIPK2) during NOD signalling16 and the ubiquitylation of TBK1 and IKKε (also known as inducible IKK) during double-stranded RNA-induced signalling85, which suggests other potential targets for the DUB activity of A20 (FIG. 3). A20 also restricts NF-κB signalling triggered by the T cell receptor (TCR), BCR and CD40, so A20 may also de-ubiquitylate targets in these pathways9,18,19,86. These studies are consistent with the notion that the DUB activity of A20 restricts cellular activation signals by cleaving activating K63-linked polyubiquitin chains from target signalling proteins.
In addition to limiting ubiquitylation by cleaving polyubiquitin chains, A20 has been shown to inhibit ubiquitin chain synthesis by interfering with E2–E3 binding81. A20 inhibits the interactions of the E2 enzymes UBCH5C and UBC13 with TRAF2 and cellular inhibitor of apoptosis (cIAP) proteins, which are RING-finger E3 ubiquitin ligases81. These findings suggest that A20 could inhibit multiple E2–E3 combinations, and hence a broad array of ubiquitylation events. Whether A20 restricts substrate ubiquitylation by cleaving polyubiquitin chains or by inhibiting ubiquitin ligase activity, the restriction of the ubiquitylation of signalling proteins appears to be an important mechanism by which A20 regulates immune signals.
Several questions concerning the DUB activity of A20 remain. First, A20 selectively cleaves unanchored K48-linked polyubiquitin chains (that is, free chains that are not covalently attached to signalling proteins), but not K63-linked polyubiquitin chains, to monoubiquitin20,21. However, A20 removes K63-linked polyubiquitin chains from TRAF6. It does this without disassembling the chains, but rather by cleaving the entire polyubiquitin chain at the ubiquitin–TRAF6 junction20. Although recombinant A20 exhibits these specificities in vitro, the physiological targets of A20 in cells are incompletely defined, and it may collaborate with other proteins to define target specificity. Second, the C103 motif of A20 appears to be required for A20 to destabilize E2 enzymes and inhibit E2–E3 interactions81. However, it remains to be determined how the DUB activity of A20 would support the ubiquitylation and degradation of E2 enzymes. Another OTU domain-containing protein, otubain 1, uses residues other than the catalytic cysteine to inhibit the function of E2 enzymes, so it is possible that A20 utilizes its OTU domain in this manner87. Third, it is currently unclear how the DUB activity of A20 overlaps with that of other DUBs (such as CYLD) that target similar substrates. Such DUBs could remove different types of ubiquitylation chain from common substrates. Alternatively, distinct DUBs could target the same ubiquitylated substrates in distinct cell types, after distinct stimuli or at different times after ligand binding. Future biochemical studies aimed at better understanding how different ubiquitin chains are targeted for deubiquitylation, together with genetic studies aimed at understanding the epistatic relationships between distinct DUBs, could begin to shed light on this issue. Finally, as the DUB activity of A20 clearly depends on its catalytic C103 residue, the physiological role of this DUB activity could be clarified by using knock-in mice expressing A20 proteins mutated at C103.
A20 also regulates cell death. The roles of A20 in regulating cell survival are complex, and may be cell type specific, as A20 inhibits TNF-induced death in fibroblasts but supports FAS-mediated death in activated B cells14,18. The anti-apoptotic functions of A20 are not likely to be secondary to decreased NF-κB signalling, as more A20-deficient fibroblasts die in the presence of cycloheximide (which abrogates NF-κB-dependent protein synthesis), and NF-κB-dependent genes tend to have anti-apoptotic functions. The molecular mechanisms by which A20 inhibits apoptosis are incompletely understood but may involve A20 de-ubiquitylating an activated, ubiquitylated form of caspase 8 (REF. 88). By contrast, the mechanism by which A20 supports cell death in activated B cells may involve A20 inhibiting NF-κB-dependent expression of the anti-apoptotic protein BCL-X. The cell death-regulating functions of A20 add an important additional dimension to the A20-mediated regulation of pro-inflammatory NF-κB signals, as the survival of immune cells and stromal cells has distinct effects in inflammatory, autoimmune and infectious diseases. How the anti-inflammatory and cell death-regulating functions of A20 are integrated in vivo and in specific cell types will be an important area of future investigation.
In addition to the N-terminal DUB motif, the C-terminal domain of A20 probably has crucial roles in regulating ubiquitin-dependent signals (FIG. 2). The C-terminal domain contains seven zinc fingers, and early studies showed that enforced expression of this domain inhibits NF-κB signalling89. More recent studies revealed that ZF4 functions as an E3 ligase, supporting the K48-linked ubiquitylation and proteasomal degradation of RIP1 (REF. 22) (FIG. 3). The evidence that A20 both disassembles K63-linked polyubiquitin chains and builds K48-linked chains suggests that these two enzymatic functions would result in the de-activation and degradation of RIP1, thereby terminating the role of this signalling molecule in propagating TNF-induced NF-κB signalling. These findings also raise interesting questions regarding whether and how these two biochemical activities might be coordinated. For example, K63-linked polyubiquitin chains might be exchanged for K48-linked chains in a synchronized ubiquitin-editing process on a single lysine residue in RIP1 (FIG. 3).
The ZF4 motif of A20 also binds directly to ubiquitin chains and supports the binding of A20 to ubiquitylated E2 enzymes23. Combined with the ability of A20 to bind to RIP1, this interaction could help to explain how ZF4 supports the ubiquitylation of RIP1. Interestingly, the ZF4 motif was also shown to be required for A20-mediated inhibition of E2–E3 interactions in cells81, implying that the ZF4 motif can inhibit ubiquitylation. Thus, the ZF4 motif might support or restrict ubiquitylation in distinct contexts. It also remains possible that the ZF4 motif of A20 performs an E3 ubiquitin ligase-independent function. As with the catalytic C103 residue, the physiological importance of ZF4 in regulating various cell signals and immune functions should become clearer with genetic interrogation of this motif via targeted mutation of ZF4.
Recent experiments indicate that the ZF7 motif of A20 also regulates NF-κB signalling by directly restricting the TAK1-dependent activation of IKKγ24. This activity is mediated via the binding of the ZF7 motif to linear ubiquitin chains90,91 (FIG. 3). The presence of truncated mutants of A20 lacking ZF7 in B cell lymphomas reinforces the importance of this motif. Thus, A20 may use several biochemical activities to regulate ubiquitylated signalling complexes. These observations raise interesting questions regarding how the functions of the C103, ZF4 and ZF7 motifs collaborate to restrict NF-κB signals. For example, in other proteins, spatially separated ubiquitin-binding motifs recognize distinct conformations of polyubiquitin chains through multipartite inter actions92,93, so the multiple ubiquitin-binding motifs in A20 may collaborate to recognize substrates bearing specific ubiquitin chains. In addition, the other five zinc fingers of A20 may have important functions complementary to those of ZF4 and ZF7. The complex biochemical mechanisms by which A20 regulates ubiquitylated signalling proteins provide clues as to how ubiquitin-dependent signals are generally regulated and also suggest novel approaches for the therapeutic manipulation of these signalling cascades through the targeting of these motifs. For example, selective inhibition of the C103-based DUB activity or the ZF4- or ZF7-based ubiquitin-binding motifs of A20 might preferentially affect the regulation of TNF-induced versus TLR-induced NF-κB signals. In addition, selective inhibition of A20 interactions with K63-linked versus linear ubiquitin chains may favour distinct ubiquitin-dependent signals.
A20 and the collaborative regulation of ubiquitin-dependent signals
Several lines of evidence suggest that A20 may collaborate with other proteins to regulate cell activation and survival signals. A20 physically interacts with several proteins that bind to ubiquitin, including A20 itself, A20-binding inhibitor of NF-κB activation 1 (ABIN1; also known as TNIP1), ABIN2 (also known as TNIP2), TAX1-binding protein 1 (TAX1BP1) and IKKγ. In addition, A20 interacts with various proteins that function directly or collaboratively as E3 enzymes, such as TRAF2, TRAF6, cIAP1, cIAP2, ITCH and RING-finger protein 11 (RNF11)12,13,94–98 (FIG. 2). The overlapping effects on TNF signalling in cells lacking several of these proteins suggest that these interactions may be physiologically relevant. Complexes containing various combinations of these proteins could recognize diverse ubiquitylated signalling proteins and modify them in tandem with E2 enzymes. How each of these proteins collaborates with A20 to regulate cell signals and immune responses is poorly understood, but biochemical and genetic tools are emerging to address these questions.
ABIN1 was identified as a binding partner of A20 via a yeast two-hybrid experiment, and enforced expression of ABIN1 suppresses TNF-induced NF-κB signals12. ABIN1 appears to cooperate with A20 to inhibit TRAF3-dependent signals that lead to type I IFN production85. ABIN1 deficiency in mice leads to late embryonic lethality and TNF-dependent cell death, demonstrating that ABIN1, similarly to A20, preserves perinatal survival and restricts TNF-induced cell death14,99,100. ABIN1-deficient mice that survive to adulthood develop spontaneous autoimmunity and inflammation, a phenotype grossly resembling that of A20-deficient mice100. Thus, ABIN1 and A20 are both crucial for preserving immune homeostasis in adult mice. Part of this anti-inflammatory function may be due to the restriction by ABIN1 of MYD88-dependent TLR signalling and NOD2 signalling — functions that are again shared with A20 (REFS 16,17,100). ABIN1 has also recently been shown to restrict the TLR-induced expression of CCAAT/enhancer-binding protein(C/EBP)-dependent genes rather than NF-κB- dependent genes101. Hence, ABIN1 binds to A20 in cells, and ABIN1 deficiency resembles A20 deficiency in several regards. However, it remains to be determined whether and how A20 and ABIN1 collaborate to regulate cell signalling.
Similarly to polymorphisms of TNFAIP3, polymorphisms of TNIP1 (the gene encoding ABIN1) are strongly linked with susceptibility to SLE28,102 and psoriatic arthritis103. Attempts to establish epistatic relationships between human SNPs in the genes encoding A20 and ABIN1 have not yet been successful, but the power to detect such associations may be limited by clinical variables and genetic haplotypes. Additional similarities between the functions of A20 and ABIN1 in human cells are suggested by the presence of somatic mutations in TNIP1 in human lymphomas104,105. Thus, ABIN1 may share the tumour-suppressive function of A20 in human lymphocytes.
Little is known about the biochemical functions of ABIN1. Unlike A20, ABIN1 does not have recognizable DUB or ubiquitin ligase motifs and is not known to exhibit enzymatic ubiquitin-modifying functions. As ABIN1 binds to polyubiquitin chains with high affinity, one model for how A20 and ABIN1 collaborate is that ABIN1 binds to both ubiquitylated signalling proteins and A20, thereby bringing A20 into close proximity with its ubiquitylated targets101,106. This adaptor function could provide a biochemical underpinning for the biological similarities observed in A20- and ABIN1-deficient animals, as well as for the correlation of SNPs in the genes encoding A20 and ABIN1 with susceptibility to SLE, psoriasis and other diseases28,38,102–105. An alternative model for ABIN1 function is suggested by the observation that ABIN1 and IKKγ contain similar ubiquitin-binding motifs94,99,106. ABIN1 might thus compete with IKKγ for binding to polyubiquitin chains in activated signalling complexes. As the recruitment of IKKγ is essential for the propagation of most canonical NF-κB signals, ABIN1-mediated displacement of IKKγ from signalling complexes could inhibit NF-κB signalling. A20 also binds to ubiquitin chains with high affinity, although A20 uses ubiquitin-binding motifs that are distinct from the motif used by ABIN1. Whether and how A20 may also compete with IKKγ for ubiquitin binding, and whether such competition involves ABIN1, remains to be determined. Further definition of the types of ubiquitin chain involved and the specific functions of these ubiquitin-binding motifs are needed to address these issues.
In addition to binding to ABIN proteins, A20 interacts with various other proteins, including TRAF2, TRAF6, ITCH, RNF11 and TAX1BP1 (REFS 12,13,94–98) (FIG. 2). The phenotypes of mice and cells lacking these proteins partially overlap with the phenotypes of A20 and ABIN1 deficiency14,100,107–111, and TAX1BP1, RNF11 and ITCH have been shown to support A20 function during TNF and TLR signalling85,99,111,112. Intriguingly, all of these proteins bind to ubiquitin and/or help to build ubiquitin chains. It remains to be determined how A20 interacts with these proteins, whether they collaborate in a single complex or in multiple complexes, and how they regulate ubiquitylated signalling complexes. Nevertheless, it is likely that A20 functions in larger ubiquitin-editing protein complexes that have a high degree of specificity and regulation.
Therapeutic implications of A20 biology
The broad (and growing) links between polymorphisms in TNFAIP3 and inflammatory and malignant diseases suggest that aberrant A20-dependent signals are pathogenic in many conditions. Loss of A20 function is directly implicated in the pathogenesis of human lymphomas, and reduced A20 function or expression has been linked with susceptibility to SLE in humans. Reduced or absent expression of A20 also causes spontaneous inflammatory and autoimmune diseases in mice. The direct correlation of hypomorphic A20 expression with both human and experimental diseases argues that such mouse models are excellent models for understanding the role of A20 in human disease. Moreover, this correlation suggests that increasing the expression and/or function of A20 is a promising therapeutic strategy. One indication of the potential of A20 as a buttress against destructive inflammation is the resistance to experimental colitis observed in mice with enforced A20 expression in IECs, which has no apparent effect on normal intestinal function72. In the case of B cell lymphomas, re-expression of A20 is directly cytotoxic for human B cell lymphomas bearing TNFAIP3 mutations but does not affect cell lines with intact A20 loci and normal NF-κB expression51. The challenge for finding effective therapies is to devise strategies that enhance A20 expression. As current therapeutic approaches based on antibodies or small molecules are more effective in reducing the functions of proteins rather than restoring them, targeting proteins that inhibit A20 function could be a more tractable, if somewhat indirect, therapeutic approach. Recent studies have identified microRNAs that target TNFAIP3 mRNA, so inhibition of these microRNAs could enhance A20 expression113–115. Regardless of the approach, the genetic links between SNPs in TNFAIP3 and rheumatoid arthritis, SLE, psoriasis, coeliac disease, Crohn's disease, type 2 diabetes, atherosclerosis and lymphomas — as well as the potential use of A20 modulation in vaccine development and transplantation63–65,76–78 — suggest that approaches that either enhance or suppress A20 expression may be promising arenas for therapeutic development.
Ubiquitylation
The covalent attachment of single ubiquitin molecules or ubiquitin chains to proteins to regulate their interactions with proteasomal components and other proteins. Protein ubiquitylation occurs in three enzymatic steps that require a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2) and a ubiquitin ligase (E3), respectively. The ligase catalyses the formation of an isopeptide bond between the carboxyl terminus of ubiquitin and an amino group belonging to a lysine residue of the target protein.
Single-nucleotide polymorphisms
(SNPs). Variations in DNA sequence in which one of the four nucleotides is substituted for another (for example, C for A). SNPs are the most frequent type of polymorphism in the genome.
Apoptosis
A common form of cell death. Many physiological and developmental stimuli cause apoptosis, and this mechanism is frequently used to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation.
Activation-induced cytidine deaminase
(AID). An enzyme that is required for two crucial events in the germinal centre: somatic hypermutation and class-switch recombination.
Dextran sulphate sodium (DSS)-induced colitis
A commonly used experimental model of colitis induced in mice by ingestion of the sulphated polysaccharide DSS. This model causes acute colonic epithelial damage and inflammation via unknown mechanisms.
Tight junctions
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight-junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludens proteins.
Ectodysplasin A receptor
(EDAR). A member of the TNFR family that can activate NF-κB-, JNK- and caspase-independent cell signalling pathways. EDAR pathways are important for the development of ectodermal structures, such as hair follicles, sweat glands and teeth.
Cellular inhibitor of apoptosis
A family of proteins (comprising cIAP1, cIAP2, XIAP and NAIP) that contain a BIR domain and that can act as E3 ubiquitin ligases and as inhibitors of caspases.
MicroRNAs
Endogenous single-stranded RNA molecules of approximately 21–23 nucleotides in length that regulate the expression of other genes by binding to mRNAs and inducing RNA degradation.
Acknowledgements
Work from the authors’ laboratory was supported by the US National Institutes of Health.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Baltimore D. NF-κB is 25. Nature Immunol. 2011;12:683–685. doi: 10.1038/ni.2072. [DOI] [PubMed] [Google Scholar]
- 2.Rui L, Schmitz R, Ceribelli M, Staudt LM. Malignant pirates of the immune system. Nature Immunol. 2011;12:933–940. doi: 10.1038/ni.2094. [DOI] [PubMed] [Google Scholar]
- 3.Greten FR, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komander D, Rape M. The ubiquitin code. Ann. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JT, et al. A20 blocks endothelial cell activation through a NF-κB-dependent mechanism. J. Biol. Chem. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- 8.Hutti JE, et al. IκB kinase β phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Mol. Cell. Biol. 2007;27:7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coornaert B, et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-κB inhibitor A20. Nature Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- 10.Shrikhande GV, et al. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLoS ONE. 2010;5:e14240. doi: 10.1371/journal.pone.0014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opipari AW, Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J. Biol. Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- 12.Heyninck K, et al. The zinc finger protein A20 inhibits TNF-induced NF-κB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-κB-inhibiting protein ABIN. J. Cell Biol. 1999;145:1471–1482. doi: 10.1083/jcb.145.7.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-κB activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee EG, et al. Failure to regulate TNF-induced NF-κ and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. This study unveiled the profound anti-inflammatory functions of A20 in mice, and the requirement for A20 in restricting TNF-induced NF-κB signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nature Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 16.Hitotsumatsu O, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turer EE, et al. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 2008;205:451–464. doi: 10.1084/jem.20071108. References 15 and 17 revealed a crucial role for A20 in restricting basal MYD88-dependent TLR signals that drive a major component of the spontaneous inflammation seen in A20-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavares RM, et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33:181–191. doi: 10.1016/j.immuni.2010.07.017. This study showed that A20 limits the induction of BCL-X expression and promotes the FAS-mediated death of activated B cells, providing a potential molecular underpinning for the tumour-suppressive function of A20 in human B cell lymphomas (see references 50–54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y, et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood. 2011;117:2227–2236. doi: 10.1182/blood-2010-09-306019. References 18 and 19 showed that B cell-specific expression of A20 restricts autoantibody production and germinal centre B cell numbers, providing potential pathophysiological mechanisms underlying human genetic studies linking A20 polymorphisms with SLE (see references 26–31) [DOI] [PubMed] [Google Scholar]
- 20.Lin SC, et al. Molecular basis for the unique deubiquitinating activity of the NF-κB inhibitor A20. J. Mol. Biol. 2008;376:526–540. doi: 10.1016/j.jmb.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komander D, Barford D. Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem. J. 2008;409:77–85. doi: 10.1042/BJ20071399. [DOI] [PubMed] [Google Scholar]
- 22.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. This study demonstrated that two distinct motifs in the A20 protein exhibit de-ubiquitylase and ubiquitin ligase activity towards RIP1, providing an unusual and novel biochemical mechanism for the restriction of NF-κB signalling. [DOI] [PubMed] [Google Scholar]
- 23.Bosanac I, et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell. 2010;40:548–557. doi: 10.1016/j.molcel.2010.10.009. This study demonstrated that the ZF4 motif of A20 directly binds to ubiquitin and ubiquitylated E2 enzymes. [DOI] [PubMed] [Google Scholar]
- 24.Skaug B, et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. This study demonstrated that the seventh zinc finger of A20, ZF7, binds to ubiquitin and directly inhibits TAK1-mediated activation of IKKγ activity. This function occurs independently of the C103 DUB motif of A20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Graham RR, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nature Genet. 2008;40:1059–1061. doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musone SL, et al. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nature Genet. 2008;40:1062–1064. doi: 10.1038/ng.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han JW, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nature Genet. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 29.Cai LQ, et al. A single-nucleotide polymorphism of the TNFAIP3 gene is associated with systemic lupus erythematosus in Chinese Han population. Mol. Biol. Rep. 2010;37:389–394. doi: 10.1007/s11033-009-9818-6. [DOI] [PubMed] [Google Scholar]
- 30.Shimane K, et al. The association of a nonsynonymous single-nucleotide polymorphism in TNFAIP3 with systemic lupus erythematosus and rheumatoid arthritis in the Japanese population. Arthritis Rheum. 2010;62:574–579. doi: 10.1002/art.27190. [DOI] [PubMed] [Google Scholar]
- 31.Adrianto I, et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nature Genet. 2011;43:253–258. doi: 10.1038/ng.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musone SL, et al. Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes Immun. 2011;12:176–182. doi: 10.1038/gene.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plenge RM, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nature Genet. 2007;39:1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson W, et al. Rheumatoid arthritis association at 6q23. Nature Genet. 2007;39:1431–1433. doi: 10.1038/ng.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsby LM, et al. Functional evaluation of TNFAIP3 (A20) in rheumatoid arthritis. Clin. Exp. Rheumatol. 2010;28:708–714. [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes LB, et al. Most common single-nucleotide polymorphisms associated with rheumatoid arthritis in persons of European ancestry confer risk of rheumatoid arthritis in African Americans. Arthritis Rheum. 2010;62:3547–3553. doi: 10.1002/art.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyre S, et al. Overlapping genetic susceptibility variants between three autoimmune disorders: rheumatoid arthritis, type 1 diabetes and coeliac disease. Arthritis Res. Ther. 2010;12:R175. doi: 10.1186/ar3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair RP, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nature Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strange A, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nature Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung EY, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 2009;10:188–191. doi: 10.1038/gene.2008.99. [DOI] [PubMed] [Google Scholar]
- 41.Trynka G, et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-κB signalling. Gut. 2009;58:1078–1083. doi: 10.1136/gut.2008.169052. [DOI] [PubMed] [Google Scholar]
- 42.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammer GE, et al. Dendritic cell expression of A20 preserves immune homeostasis and prevents colitis and spondyloarthritis. Nature Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. This study (together with reference 62) demonstrated that A20 expression in DCs is crucial for preserving immune homeostasis. In this study, mice with A20-deficient DCs developed seronegative arthritis, colitis and spondyloarthritis, resembling a stereotypical human syndrome associated with inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boonyasrisawat W, et al. Tag polymorphisms at the A20 (TNFAIP3) locus are associated with lower gene expression and increased risk of coronary artery disease in type 2 diabetes. Diabetes. 2007;56:499–505. doi: 10.2337/db06-0946. [DOI] [PubMed] [Google Scholar]
- 45.Dieudé P, et al. Association of the TNFAIP3rs5029939 variant with systemic sclerosis in the European Caucasian population. Ann. Rheum. Dis. 2010;69:1958–1964. doi: 10.1136/ard.2009.127928. [DOI] [PubMed] [Google Scholar]
- 46.Koumakis E, et al. Candidate gene study in systemic sclerosis identifies a rare and functional variant of TNFAIP3 locus as a risk factor for individual polyautoimmunity. Arthritis Rheum. 2012;64:2746–2752. doi: 10.1002/art.34490. [DOI] [PubMed] [Google Scholar]
- 47.Koczan D, et al. Molecular discrimination of responders and nonresponders to anti-TNFα therapy in rheumatoid arthritis by etanercept. Arthritis Res. Ther. 2008;10:R50. doi: 10.1186/ar2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arsenescu R, et al. Signature biomarkers in Crohn's disease: toward a molecular classification. Mucosal Immunol. 2008;1:399–411. doi: 10.1038/mi.2008.32. [DOI] [PubMed] [Google Scholar]
- 49.Tejasvi T, et al. TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. J. Invest. Dermatol. 2011;132:593–600. doi: 10.1038/jid.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato M, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 51.Compagno M, et al. Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. References 50 and 51 showed that the reintroduction of A20 into A20-deficient lymphomas suppressed tumour cell growth and apoptosis, suggesting that A20 is a tumour suppressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novak U, et al. The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood. 2009;113:4918–4921. doi: 10.1182/blood-2008-08-174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honma K, et al. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 2009;114:2467–2475. doi: 10.1182/blood-2008-12-194852. [DOI] [PubMed] [Google Scholar]
- 54.Braun FC, et al. Tumor suppressor TNFAIP3 (A20) is frequently deleted in Sézary syndrome. Leukemia. 2011;25:1494–1501. doi: 10.1038/leu.2011.101. References 50–54 demonstrated that biallelic somatic mutations of the gene encoding A20 are frequently present in human B and T cell lymphomas. [DOI] [PubMed] [Google Scholar]
- 55.Malynn BA, Ma A. A20 takes on tumors: tumor suppression by an ubiquitin-editing enzyme. J. Exp. Med. 2009;206:977–980. doi: 10.1084/jem.20090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hymowitz SG, Wertz IE. A20: from ubiquitin editing to tumour suppression. Nature Rev. Cancer. 2010;10:332–341. doi: 10.1038/nrc2775. [DOI] [PubMed] [Google Scholar]
- 57.Chanudet E, et al. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia. 2010;24:483–487. doi: 10.1038/leu.2009.234. [DOI] [PubMed] [Google Scholar]
- 58.Thome M. Multifunctional roles for MALT1 in T-cell activation. Nature Rev. Immunol. 2008;8:495–500. doi: 10.1038/nri2338. [DOI] [PubMed] [Google Scholar]
- 59.Malinverni C, et al. Cleavage by MALT1 induces cytosolic release of A20. Biochem. Biophys. Res. Commun. 2010;400:543–547. doi: 10.1016/j.bbrc.2010.08.091. [DOI] [PubMed] [Google Scholar]
- 60.Hövelmeyer N, et al. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur. J. Immunol. 2011;41:595–601. doi: 10.1002/eji.201041313. [DOI] [PubMed] [Google Scholar]
- 61.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nature Rev. Mol. Cell Biol. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 62.Kool M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. Along with reference 43, this study demonstrated the importance of A20 expression to DC functions. Interestingly, these mice developed systemic autoimmunity, a distinct immunological phenotype from that of the mice described in reference 43. [DOI] [PubMed] [Google Scholar]
- 63.Song XT, et al. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nature Med. 2008;14:258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breckpot K, et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J. Immunol. 2009;182:860–870. doi: 10.4049/jimmunol.182.2.860. [DOI] [PubMed] [Google Scholar]
- 65.Hong B, et al. Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells. J. Clin. Invest. 2011;121:739–751. doi: 10.1172/JCI42656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matmati M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nature Genet. 2011;43:908–912. doi: 10.1038/ng.874. This study showed that mice with A20-deficient myeloid cells spontaneously develop severe polyarthritis, providing potential pathophysiological mechanisms that may underlie the link between TNFAIP3 polymorphisms and rheumatoid arthritis. [DOI] [PubMed] [Google Scholar]
- 68.Maelfait J, et al. A20 (Tnfaip3) deficiency in myeloid cells protects against influenza A virus infection. PLoS Pathog. 2012;8:e1002570. doi: 10.1371/journal.ppat.1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vereecke L, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. This study showed that mice with a specific ablation of A20 in IECs show increased susceptibility to experimental colitis, providing potential pathophysiological mechanisms that may underlie the link between TNFAIP3 polymorphisms and inflammatory bowel disease (see references 42 and 43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rakoff-Nahoum S, Medzhitov R. Role of the innate immune system and host–commensal mutualism. Curr. Top. Microbiol. Immunol. 2006;308:1–18. doi: 10.1007/3-540-30657-9_1. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Ouyang Y, Guner Y, Ford HR, Grishin AV. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J. Immunol. 2009;183:1384–1392. doi: 10.4049/jimmunol.0803987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolodziej LE, et al. TNFAIP3 maintains intestinal barrier function and supports epithelial cell tight junctions. PLoS ONE. 2011;6:e26352. doi: 10.1371/journal.pone.0026352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lippens S, et al. Keratinocyte-specific ablation of the NF-κB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis. Cell Death Differ. 2011;8:1845–1853. doi: 10.1038/cdd.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun LD, et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nature Genet. 2010;42:1005–1009. doi: 10.1038/ng.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bach FH, et al. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nature Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 77.Ferran C. Protective genes in the vessel wall: modulators of graft survival and function. Transplantation. 2006;82(Suppl. 1):S36–S40. doi: 10.1097/01.tp.0000231445.62162.d5. [DOI] [PubMed] [Google Scholar]
- 78.Zhu C, et al. Development of anti-atherosclerotic tissue-engineered blood vessel by A20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials. 2008;29:2628–2636. doi: 10.1016/j.biomaterials.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Idel S, Dansky HM, Breslow JL. A20, a regulator of NFκB, maps to an atherosclerosis locus and differs between parental sensitive C57BL/6J and resistant FVB/N strains. Proc. Natl Acad. Sci. USA. 2003;100:14235–14240. doi: 10.1073/pnas.1835672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfrum S, Teupser D, Tan M, Chen KY, Breslow JL. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-κB target genes. Proc. Natl Acad. Sci. USA. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shembade N, Ma A, Harhaj EW. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. This study described a role for A20 in disrupting the functions of E2 and E3 enzymes, thereby limiting ubiquitin ligase activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans PC, et al. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem. J. 2004;378:727–734. doi: 10.1042/BJ20031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng L, et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 84.Ea CK, Sun L, Inoue J, Chen ZJ. TIFA activates IκB kinase (IKK) by promoting oligomerization and ubiquitination of TRAF6. Proc. Natl Acad. Sci. USA. 2004;101:15318–15323. doi: 10.1073/pnas.0404132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao L, et al. ABIN1 protein cooperates with TAX1BP1 and A20 proteins to inhibit antiviral signaling. J. Biol. Chem. 2011;286:36592–36602. doi: 10.1074/jbc.M111.283762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Düwel M, et al. A20 negatively regulates T cell receptor signaling to NF-κB by cleaving Malt1 ubiquitin chains. J. Immunol. 2009;182:7718–7728. doi: 10.4049/jimmunol.0803313. [DOI] [PubMed] [Google Scholar]
- 87.Nakada S, et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 88.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl Acad. Sci. USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verhelst K, et al. A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 2012 Aug 28; doi: 10.1038/emboj.2012.240. (doi:10.1038/emboj.2012.240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tokunaga F, et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation. EMBO J. 2012 Aug 28; doi: 10.1038/emboj.2012.241. (doi:101038/emboj.2012.241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T, et al. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J. Mol. Biol. 2009;386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sims JJ, Cohen RE. Linkage-specific avidity defines the lysine 63-linked polyubiquitin-binding preference of Rap80. Mol. Cell. 2009;33:775–783. doi: 10.1016/j.molcel.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heyninck K, Van Huffel S, Kreike M, Beyaert R. Yeast two-hybrid screening for proteins interacting with the anti-apoptotic protein A20. Methods Mol. Biol. 2004;282:223–241. doi: 10.1385/1-59259-812-9:223. [DOI] [PubMed] [Google Scholar]
- 95.Mauro C, et al. ABIN-1 binds to NEMO/IKKγ and co-operates with A20 in inhibiting NF-κB. J. Biol. Chem. 2006;281:18482–18488. doi: 10.1074/jbc.M601502200. [DOI] [PubMed] [Google Scholar]
- 96.Shembade N, et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nature Immunol. 2008;9:254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- 97.Li L, Soetandyo N, Wang Q, Ye Y. The zinc finger protein A20 targets TRAF2 to the lysosomes for degradation. Biochim. Biophys. Acta. 2009;1793:346–353. doi: 10.1016/j.bbamcr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-κB signalling. EMBO J. 2009;28:513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oshima S, et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature. 2009;457:906–909. doi: 10.1038/nature07575. This study demonstrated a crucial function for ABIN1, a protein that may bind to and support A20, in restricting TNF signals and preserving embryonic survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nanda SK, et al. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J. Exp. Med. 2011;208:1215–1228. doi: 10.1084/jem.20102177. This study showed that ABIN1 restricts TLR as well as TNF signals and prevents autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou J, et al. A20-binding inhibitor of NF-κB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein β activation and protects from inflammatory disease. Proc. Natl Acad. Sci. USA. 2011;108:e998–e1006. doi: 10.1073/pnas.1106232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gateva V, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nature Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bowes J, et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann. Rheum. Dis. 2011;70:1641–1644. doi: 10.1136/ard.2011.150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong G, et al. A20, ABIN-1/2, and CARD11 mutations and their prognostic value in gastrointestinal diffuse large B-cell lymphoma. Clin. Cancer Res. 2011;17:1440–1451. doi: 10.1158/1078-0432.CCR-10-1859. [DOI] [PubMed] [Google Scholar]
- 105.Yan Q, et al. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica. 2012;97:595–598. doi: 10.3324/haematol.2011.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner S, et al. Ubiquitin binding mediates the NF-κB inhibitory potential of ABIN proteins. Oncogene. 2008;27:3739–3745. doi: 10.1038/sj.onc.1211042. [DOI] [PubMed] [Google Scholar]
- 107.Lin WJ, et al. Crucial role for TNF receptor-associated factor 2 (TRAF2) in regulating NFκB2 signaling that contributes to autoimmunity. Proc. Natl Acad. Sci. USA. 2011;108:18354–18359. doi: 10.1073/pnas.1109427108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lomaga MA, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perry WL, et al. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nature Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 110.Iha H, et al. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-κB activation. EMBO J. 2008;27:629–641. doi: 10.1038/emboj.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-α-, IL-1- and LPS-mediated NF-κB and JNK signaling. EMBO J. 2007;26:3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parvatiyar K, Barber GN, Harhaj EW. TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1–IKKi kinases. J. Biol. Chem. 2010;285:14999–15009. doi: 10.1074/jbc.M110.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim SW, et al. MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor α-induced protein 3 (TNFAIP3, A20). Proc. Natl Acad. Sci. USA. 2012;109:7865–7870. doi: 10.1073/pnas.1200081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 2012;287:21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gantier MP, et al. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Res. 2012;40:8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iwai K. Diverse ubiquitin signaling in NF-κB activation. Trends Cell Biol. 2012;22:355–364. doi: 10.1016/j.tcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012;10:23. doi: 10.1186/1741-7007-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]