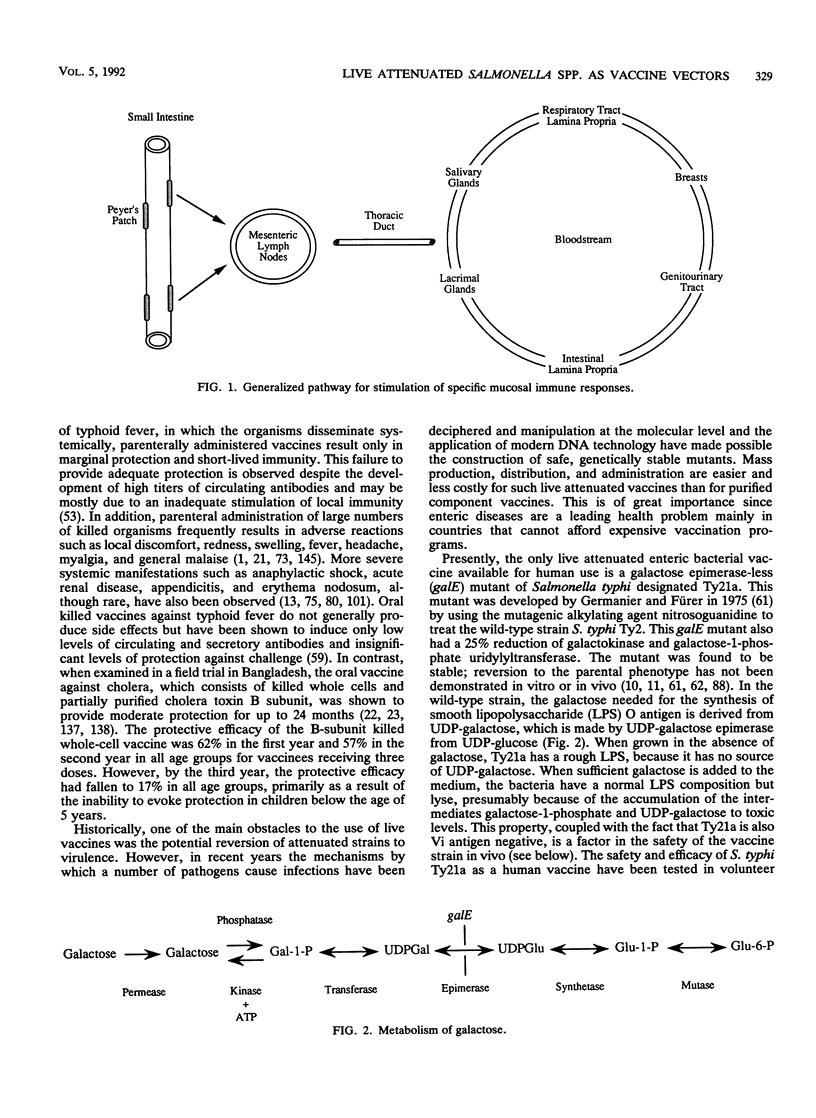
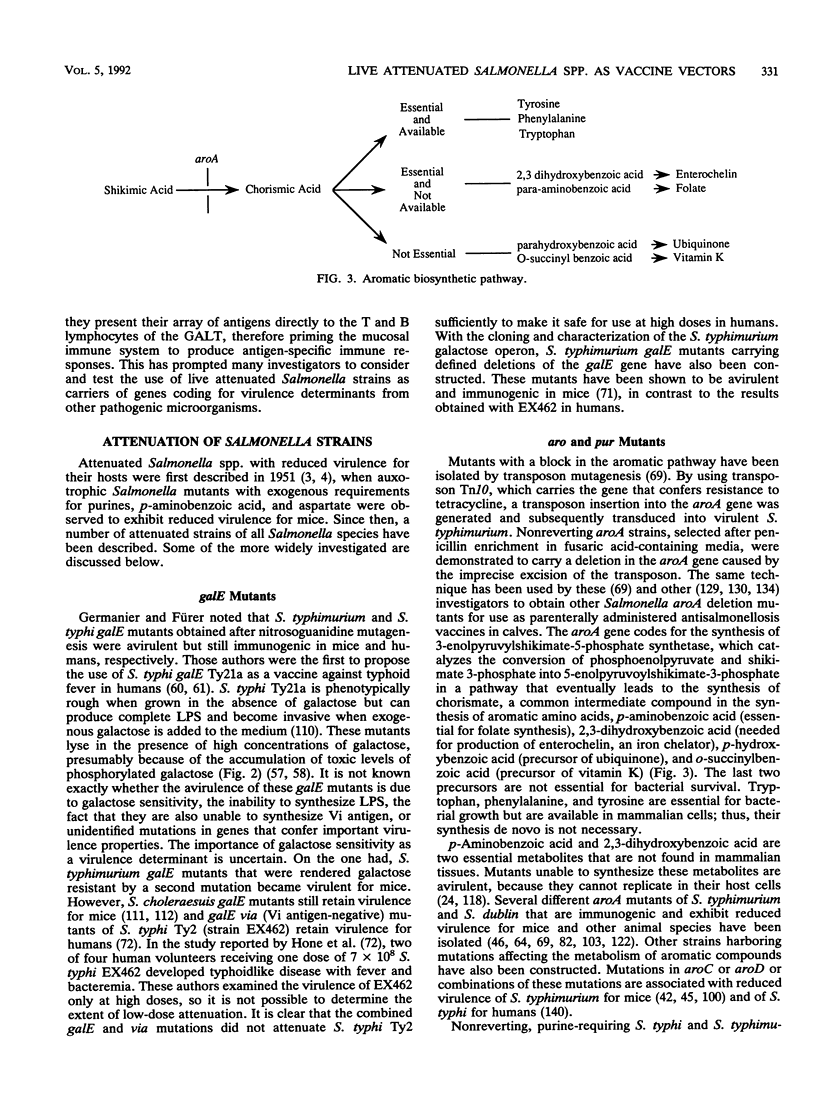
Abstract
A variety of techniques, including the use of live oral vaccines, have been used to deliver antigens to the gut-associated lymphoid tissues in an attempt to initiate production of specific secretory immunoglobulin A for protection against pathogens that colonize or cross mucosal surfaces to initiate infection. A number of attenuated Salmonella mutants are able to interact with the lymphoid tissues in the Peyer's patches but are not able to cause systemic disease. Some of these mutants are effective as live vaccines (i.e., able to protect against infection with the virulent Salmonella parent) and are candidates for use as carriers for virulence determinants of other mucosal pathogens. This has been shown to be an effective means of stimulating significant levels of specific mucosal secretory immunoglobulin A directed against the carrier strains and against a variety of heterologous antigens and has been shown to stimulate production of serum antibodies and cell-mediated responses as well. This review examines the history of this mechanism of vaccine delivery and summarizes the most recent applications of this evolving technology. This is a technique for vaccine delivery with significant potential for influencing the management of infectious diseases on a large scale. It can be used not only for vaccines against enteric bacterial pathogens but also for vaccines against a variety of other bacteria, viruses, and parasites. The results obtained to date are encouraging, and there is great potential for development of safe, effective, affordable vaccines.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHCROFT M. T., RITCHIE J. M., NICHOLSON C. C. CONTROLLED FIELD TRIAL IN BRITISH GUIANA SCHOOL CHILDREN OF HEAT-KILLED-PHENOLIZED AND ACETONE-KILLED LYOPHILIZED TYPHOID VACCINES. Am J Hyg. 1964 Mar;79:196–206. doi: 10.1093/oxfordjournals.aje.a120376. [DOI] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum: the virulence of mutants. Br J Exp Pathol. 1950 Dec;31(6):714–724. [PMC free article] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum; the loss of virulence of certain mutants. Br J Exp Pathol. 1951 Apr;32(2):85–96. [PMC free article] [PubMed] [Google Scholar]
- Bao J. X., Clements J. D. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect Immun. 1991 Oct;59(10):3841–3845. doi: 10.1128/iai.59.10.3841-3845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Kopecko D. J., Formal S. B., Seid R., Guerry P., Powell C. Introduction of Shigella flexneri 2a type and group antigen genes into oral typhoid vaccine strain Salmonella typhi Ty21a. Infect Immun. 1987 Nov;55(11):2797–2801. doi: 10.1128/iai.55.11.2797-2801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A. Immunity to experimental fowl typhoid in chickens induced by a virulence plasmid-cured derivative of Salmonella gallinarum. Infect Immun. 1990 Jul;58(7):2283–2288. doi: 10.1128/iai.58.7.2283-2288.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P. A., Lovell M. A. The association between a large molecular mass plasmid and virulence in a strain of Salmonella pullorum. J Gen Microbiol. 1988 Aug;134(8):2307–2316. doi: 10.1099/00221287-134-8-2307. [DOI] [PubMed] [Google Scholar]
- Barrow P. A., Simpson J. M., Lovell M. A., Binns M. M. Contribution of Salmonella gallinarum large plasmid toward virulence in fowl typhoid. Infect Immun. 1987 Feb;55(2):388–392. doi: 10.1128/iai.55.2.388-392.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R., Levine M. M., Young C., Rooney J., Levine S., Clements M. L., O'Donnell S., Hugues T., Germanier R. Immunogenicity of Ty21a attenuated "Salmonella typhi" given with sodium bicarbonate or in enteric-coated capsules. Dev Biol Stand. 1983;53:9–14. [PubMed] [Google Scholar]
- Bowen J. C., Alpar O., Phillpotts R., Roberts I. S., Brown M. R. Preliminary studies on infection by attenuated Salmonella in guinea pig and on expression on herpes simplex virus. Res Microbiol. 1990 Sep-Oct;141(7-8):873–877. doi: 10.1016/0923-2508(90)90123-8. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Chikami G. K., Fierer J., Guiney D. G. Plasmid-mediated virulence in Salmonella dublin demonstrated by use of a Tn5-oriT construct. Infect Immun. 1985 Nov;50(2):420–424. doi: 10.1128/iai.50.2.420-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Thole J. E., Sathish M., Bosecker B. A., Sela S., de Carvalho E. F., Esser R. E. Protein antigens of Mycobacterium leprae. Res Microbiol. 1990 Sep-Oct;141(7-8):859–871. doi: 10.1016/0923-2508(90)90122-7. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Kay B. A., Khan M. U., Yunus M., Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986 Jul 19;2(8499):124–127. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Clements J. D., El-Morshidy S. Construction of a potential live oral bivalent vaccine for typhoid fever and cholera-Escherichia coli-related diarrheas. Infect Immun. 1984 Nov;46(2):564–569. doi: 10.1128/iai.46.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Hartzog N. M., Lyon F. L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988 Jun;6(3):269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D. Use of attenuated mutants of Salmonella as carriers for delivery of heterologous antigens to the secretory immune system. Pathol Immunopathol Res. 1987;6(2):137–146. doi: 10.1159/000157055. [DOI] [PubMed] [Google Scholar]
- Cohen S., Powell C. J., Dubois D. R., Hartman A., Summers P. L., Eckels K. H. Expression of the envelope antigen of dengue virus in vaccine strains of Salmonella. Res Microbiol. 1990 Sep-Oct;141(7-8):855–858. doi: 10.1016/0923-2508(90)90121-6. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd 1984 Kreshover lecture. Genetic analysis of Streptococcus mutans virulence and prospects for an anticaries vaccine. J Dent Res. 1986 Aug;65(8):1034–1045. doi: 10.1177/00220345860650080101. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Goldschmidt R. M., Fletchall N. B., Kelly S. M. Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine. 1988 Apr;6(2):155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M., Gulig P. A., Nakayama K. Selective delivery of antigens by recombinant bacteria. Curr Top Microbiol Immunol. 1989;146:35–49. doi: 10.1007/978-3-642-74529-4_4. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Nakayama K., Kelly S. M. Recombinant avirulent Salmonella vaccine strains with stable maintenance and high level expression of cloned genes in vivo. Immunol Invest. 1989 Jan-May;18(1-4):583–596. doi: 10.3109/08820138909112265. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M., Guenounou M., Ronco E., Vacheron F., Nauciel C. Impairment of lymphocyte proliferative responses and interleukin-2 production in susceptible (C57BL/6) mice infected with Salmonella typhimurium. Immunology. 1986 Jun;58(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Chatfield S., Higgins C. F., Hayward C., Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989 Jul;57(7):2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Chatfield S., Pickard D., Bester J., O'Callaghan D., Maskell D. Construction and characterization of vaccine strains of Salmonella harboring mutations in two different aro genes. J Infect Dis. 1988 Dec;158(6):1329–1335. doi: 10.1093/infdis/158.6.1329. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sellwood R., Maskell D., Sweeney K., Liew F. Y., Beesley J., Hormaeche C. In vivo properties of a cloned K88 adherence antigen determinant. Infect Immun. 1986 Apr;52(1):344–347. doi: 10.1128/iai.52.1.344-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. H., Gilley R. M., Staas J. K., Moldoveanu Z., Meulbroek J. A., Tice T. R. Biodegradable microspheres: vaccine delivery system for oral immunization. Curr Top Microbiol Immunol. 1989;146:59–66. doi: 10.1007/978-3-642-74529-4_6. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. Nature. 1959 Oct 10;184(Suppl 15):1168–1169. doi: 10.1038/1841168a0. [DOI] [PubMed] [Google Scholar]
- Fahey K. J., Cooper G. N. Oral immunization against experimental salmonellosis I. Development of temperature-sensitive mutant vaccines. Infect Immun. 1970 Mar;1(3):263–270. doi: 10.1128/iai.1.3.263-270.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C., Levine M. M., Rodriguez H., Contreras R. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J Infect Dis. 1989 Apr;159(4):766–769. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fierer J., Chikami G., Hatlen L., Heffernan E. J., Guiney D. Active immunization with LD842, a plasmid-cured strain of Salmonella dublin, protects mice against group D and group B Salmonella infection. J Infect Dis. 1988 Aug;158(2):460–463. doi: 10.1093/infdis/158.2.460. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Weiss W. R., Norris K. A., Seifert H. S., Kumar S., So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990 Dec;4(12):2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest B. D., LaBrooy J. T., Attridge S. R., Boehm G., Beyer L., Morona R., Shearman D. J., Rowley D. Immunogenicity of a candidate live oral typhoid/cholera hybrid vaccine in humans. J Infect Dis. 1989 Jan;159(1):145–146. doi: 10.1093/infdis/159.1.145. [DOI] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E. Immunity in experimental salmonellosis. II. Basis for the avirulence and protective capacity of gal E mutants of Salmonella typhimurium. Infect Immun. 1971 Dec;4(6):663–673. doi: 10.1128/iai.4.6.663-673.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habasha F. G., Smith B. P., Schwartz L., Ardans A., Reina-Guerra M. Correlation of macrophage migration-inhibition factor and protection from challenge exposure in calves vaccinated with Salmonella typhimurium. Am J Vet Res. 1985 Jul;46(7):1415–1421. [PubMed] [Google Scholar]
- Hackett J., Kotlarski I., Mathan V., Francki K., Rowley D. The colonization of Peyer's patches by a strain of Salmonella typhimurium cured of the cryptic plasmid. J Infect Dis. 1986 Jun;153(6):1119–1125. doi: 10.1093/infdis/153.6.1119. [DOI] [PubMed] [Google Scholar]
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Hoertt B. E., Ou J., Kopecko D. J., Baron L. S., Warren R. L. Novel virulence properties of the Salmonella typhimurium virulence-associated plasmid: immune suppression and stimulation of splenomegaly. Plasmid. 1989 Jan;21(1):48–58. doi: 10.1016/0147-619x(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hone D. M., Attridge S. R., Forrest B., Morona R., Daniels D., LaBrooy J. T., Bartholomeusz R. C., Shearman D. J., Hackett J. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988 May;56(5):1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D., Attridge S., van den Bosch L., Hackett J. A chromosomal integration system for stabilization of heterologous genes in Salmonella based vaccine strains. Microb Pathog. 1988 Dec;5(6):407–418. doi: 10.1016/0882-4010(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Hone D., Morona R., Attridge S., Hackett J. Construction of defined galE mutants of Salmonella for use as vaccines. J Infect Dis. 1987 Jul;156(1):167–174. doi: 10.1093/infdis/156.1.167. [DOI] [PubMed] [Google Scholar]
- Iwarson S., Larsson P. Intradermal versus subcutaneous immunization with typhoid vaccine. J Hyg (Lond) 1980 Feb;84(1):11–16. doi: 10.1017/s0022172400026462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Mestecky J., Childers N. K., Michalek S. M. Liposomes containing anti-idiotypic antibodies: an oral vaccine to induce protective secretory immune responses specific for pathogens of mucosal surfaces. Infect Immun. 1990 Jun;58(6):1932–1936. doi: 10.1128/iai.58.6.1932-1936.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joekes A. M., Gabriel J. R., Goggin M. J. Renal disease following prophylactic inoculation. Nephron. 1973;9(3):162–170. doi: 10.1159/000180146. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L., Ristow S., Yilma T., Moss B. Accidental human vaccination with vaccinia virus expressing nucleoprotein gene. Nature. 1986 Feb 13;319(6054):543–543. doi: 10.1038/319543a0. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Katz J., Michalek S. M., Curtiss R., 3rd, Harmon C., Richardson G., Mestecky J. Novel oral vaccines: the effectiveness of cloned gene products on inducing secretory immune responses. Adv Exp Med Biol. 1987;216B:1741–1747. [PubMed] [Google Scholar]
- Khan R. I. Anaphylactoid reaction to typhoid-paratyphoid A and B vaccine. Trop Geogr Med. 1971 Mar;23(1):115–116. [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Differences in delayed-type hypersensitivity responses in various mouse strains in the C3H lineage infected with Salmonella typhimurium, strain SL3235. J Immunol. 1984 Sep;133(3):1190–1196. [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985 Mar;47(3):605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Hornick R. B., Snyder M. J., Woodward W., Gilman R. H., Libonati J. P. Attenuated, streptomycin-dependent Salmonella typhi oral vaccine: potential deleterious effects of lyophilization. J Infect Dis. 1976 Apr;133(4):424–429. doi: 10.1093/infdis/133.4.424. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Ferreccio C., Black R. E., Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987 May 9;1(8541):1049–1052. doi: 10.1016/s0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Modern vaccines. Enteric infections. Lancet. 1990 Apr 21;335(8695):958–961. doi: 10.1016/0140-6736(90)91013-z. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- McFarland W. C., Stocker B. A. Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb Pathog. 1987 Aug;3(2):129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Mel D. M., Arsic B. L., Radovanovic M. L., Kaljalovic R., Litvinjenko S. Safety tests in adults and children with live oral typhoid vaccine. Acta Microbiol Acad Sci Hung. 1974;21(1-2):161–166. [PubMed] [Google Scholar]
- Michalek S. M., Childers N. K., Katz J., Denys F. R., Berry A. K., Eldridge J. H., McGhee J. R., Curtiss R., 3rd Liposomes as oral adjuvants. Curr Top Microbiol Immunol. 1989;146:51–58. doi: 10.1007/978-3-642-74529-4_5. [DOI] [PubMed] [Google Scholar]
- Miller I. A., Chatfield S., Dougan G., Desilva L., Joysey H. S., Hormaeche C. Bacteriophage P22 as a vehicle for transducing cosmid gene banks between smooth strains of Salmonella typhimurium: use in identifying a role for aroD in attenuating virulent Salmonella strains. Mol Gen Genet. 1989 Jan;215(2):312–316. doi: 10.1007/BF00339734. [DOI] [PubMed] [Google Scholar]
- Mittermayer C. H. Lethal complications of typhoid-cholera-vaccination. (Case report and review of the literature). Beitr Pathol. 1976 Jul;158(2):212–224. doi: 10.1016/s0005-8165(76)80197-7. [DOI] [PubMed] [Google Scholar]
- Morris Hooke A., Sordelli D. O., Cerquetti M. C., Bellanti J. A. Differential growth characteristics and immunogenicity of tight and coasting temperature-sensitive mutants of Pseudomonas aeruginosa. Infect Immun. 1987 Jan;55(1):99–103. doi: 10.1128/iai.55.1.99-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkur T. K., McDowell G. H., Stocker B. A., Lascelles A. K. Protection against experimental salmonellosis in mice and sheep by immunisation with aromatic-dependent Salmonella typhimurium. J Med Microbiol. 1987 Aug;24(1):11–19. doi: 10.1099/00222615-24-1-11. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Nagy L. K., Walker P. D., Bhogal B. S., Mackenzie T. Evaluation of Escherichia coli vaccines against experimental enteric colibacillosis. Res Vet Sci. 1978 Jan;24(1):39–45. [PubMed] [Google Scholar]
- Nakamura M., Sato S., Ohya T., Suzuki S., Ikeda S., Koeda T. Plasmid-cured Salmonella enteritidis AL1192 as a candidate for a live vaccine. Infect Immun. 1985 Nov;50(2):586–587. doi: 10.1128/iai.50.2.586-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Sato S., Ohya T., Suzuki S., Ikeda S. Possible relationship of a 36-megadalton Salmonella enteritidis plasmid to virulence in mice. Infect Immun. 1985 Mar;47(3):831–833. doi: 10.1128/iai.47.3.831-833.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L. Genetic control of Salmonella typhimurium-induced depression of delayed-type hypersensitivity to sheep erythrocytes in mice. Infect Immun. 1988 Feb;56(2):310–313. doi: 10.1128/iai.56.2.310-313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Some galE mutants of Salmonella choleraesuis retain virulence. Infect Immun. 1986 Dec;54(3):635–640. doi: 10.1128/iai.54.3.635-640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Test of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun. 1987 Apr;55(4):955–962. doi: 10.1128/iai.55.4.955-962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P. L., Chiba Y., Beutner K. R., Morag A. Vaccination by non-parenteral routes: characteristics of immune response. Dev Biol Stand. 1976;33:19–26. [PubMed] [Google Scholar]
- Ohta M., Kido N., Fujii Y., Arakawa Y., Komatsu T., Kato N. Temperature-sensitive growth mutants as live vaccines against experimental murine salmonellosis. Microbiol Immunol. 1987;31(12):1259–1265. doi: 10.1111/j.1348-0421.1987.tb01359.x. [DOI] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. Infectivity and antigenicity of streptomycin-dependent Salmonella typhosa. J Infect Dis. 1967 Feb;117(1):101–107. doi: 10.1093/infdis/117.1.101. [DOI] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Ballou W. R., Baron L. S., Majarian W. R., Brey R. N., Hockmeyer W. T., Young J. F., Cryz S. J., Ou J., Lowell G. H. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988 Apr 15;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- Salas-Vidal E., Plebañski M., Castro S., Perales G., Mata E., López S., Arias C. F. Synthesis of the surface glycoprotein of rotavirus SA11 in the aroA strain of Salmonella typhimurium SL3261. Res Microbiol. 1990 Sep-Oct;141(7-8):883–886. doi: 10.1016/0923-2508(90)90125-a. [DOI] [PubMed] [Google Scholar]
- Schödel F., Will H. Construction of a plasmid for expression of foreign epitopes as fusion proteins with subunit B of Escherichia coli heat-labile enterotoxin. Infect Immun. 1989 Apr;57(4):1347–1350. doi: 10.1128/iai.57.4.1347-1350.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel F., Will H. Expression of hepatitis B virus antigens in attenuated Salmonellae for oral immunization. Res Microbiol. 1990 Sep-Oct;141(7-8):831–837. doi: 10.1016/0923-2508(90)90118-a. [DOI] [PubMed] [Google Scholar]
- Sigwart D. F., Stocker B. A., Clements J. D. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989 Jun;57(6):1858–1861. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A., Sandström G., Tärnvik A. Immunization of mice with an attenuated Salmonella typhimurium strain expressing a membrane protein of Francisella tularensis. A model for identification of bacterial determinants relevant to the host defence against tularemia. Res Microbiol. 1990 Sep-Oct;141(7-8):887–891. doi: 10.1016/0923-2508(90)90126-b. [DOI] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Stocker B. A., Hoiseth S. K., Johnson E. Aromatic-dependent Salmonella dublin as a parenteral modified live vaccine for calves. Am J Vet Res. 1984 Nov;45(11):2231–2235. [PubMed] [Google Scholar]
- Stabel T. J., Mayfield J. E., Tabatabai L. B., Wannemuehler M. J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun. 1990 Jul;58(7):2048–2055. doi: 10.1128/iai.58.7.2048-2055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A. Aromatic-dependent Salmonella as live vaccine presenters of foreign epitopes as inserts in flagellin. Res Microbiol. 1990 Sep-Oct;141(7-8):787–796. doi: 10.1016/0923-2508(90)90112-4. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Hoiseth S. K., Smith B. P. Aromatic-dependent "Salmonella sp." as live vaccine in mice and calves. Dev Biol Stand. 1983;53:47–54. [PubMed] [Google Scholar]
- Strugnell R. A., Maskell D., Fairweather N., Pickard D., Cockayne A., Penn C., Dougan G. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene. 1990 Mar 30;88(1):57–63. doi: 10.1016/0378-1119(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Forrest B., Morona R., Attridge S. R., LaBrooy J., Tall B. D., Reymann M., Rowley D., Levine M. M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990 Jun;58(6):1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Hone D. M., Curtiss R., 3rd, Kelly S. M., Losonsky G., Guers L., Harris A. M., Edelman R., Levine M. M. Comparison of the safety and immunogenicity of delta aroC delta aroD and delta cya delta crp Salmonella typhi strains in adult volunteers. Infect Immun. 1992 Feb;60(2):536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Ebersole J. L., Smith D. J., Stack W. Adjuvants for secretory immune responses. Ann N Y Acad Sci. 1983 Jun 30;409:637–649. doi: 10.1111/j.1749-6632.1983.tb26905.x. [DOI] [PubMed] [Google Scholar]
- Terakado N., Sekizaki T., Hashimoto K., Naitoh S. Correlation between the presence of a fifty-megadalton plasmid in Salmonella dublin and virulence for mice. Infect Immun. 1983 Jul;41(1):443–444. doi: 10.1128/iai.41.1.443-444.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Chung R., Berman S., Keren D., Kapfer C., Formal S. B. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076-1C). J Infect Dis. 1984 Feb;149(2):133–136. doi: 10.1093/infdis/149.2.133. [DOI] [PubMed] [Google Scholar]
- Van de Verg L., Herrington D. A., Murphy J. R., Wasserman S. S., Formal S. B., Levine M. M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990 Jun;58(6):2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Serie C., Germanier R., Lackany A., Cerisier Y., Guerin N., Sallam S., Geoffroy P., el Tantawi A. S., Guesry P. A controlled field trial of liver oral typhoid vaccine Ty21a. Bull World Health Organ. 1980;58(3):469–474. [PMC free article] [PubMed] [Google Scholar]
- Wahdan M. H., Sérié C., Cerisier Y., Sallam S., Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982 Mar;145(3):292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Newton S., Judd A., Stocker B., Robinson W. S. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4726–4730. doi: 10.1073/pnas.86.12.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Cheynier R., Desportes I., Leonard R., Fouchard M., Reveil B., Ittele D., Lurhuma Z., Mbayo K. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988 Apr 21;332(6166):728–731. doi: 10.1038/332728a0. [DOI] [PubMed] [Google Scholar]



