Abstract
Red blood cell (RBC) transfusion is vital for the treatment of a number of acute and chronic medical problems such as thalassemia major and sickle cell anemia 1-3. Due to the presence of multitude of antigens on the RBC surface (~308 known antigens 4), patients in the chronic blood transfusion therapy develop alloantibodies due to the miss match of minor antigens on transfused RBCs 4, 5. Grafting of hydrophilic polymers such as polyethylene glycol (PEG) and hyperbranched polyglycerol (HPG) forms an exclusion layer on RBC membrane that prevents the interaction of antibodies with surface antigens without affecting the passage of small molecules such as oxygen ,glucose, and ions3. At present no method is available for the generation of universal red blood donor cells in part because of the daunting challenge presented by the presence of large number of antigens (protein and carbohydrate based) on the RBC surface and the development of such methods will significantly improve transfusion safety, and dramatically improve the availability and use of RBCs. In this report, the experiments that are used to develop antigen protected functional RBCs by the membrane grafting of HPG and their characterization are presented. HPGs are highly biocompatible compact polymers 6, 7, and are expected to be located within the cell glycocalyx that surrounds the lipid membrane 8, 9 and mask RBC surface antigens10, 11.
Keywords: Immunology, Issue 71, Bioengineering, Pathology, Chemistry, Biochemistry, Hematology, polymers, Blood transfusion, surface antigens, antigen camouflage, RBC modification, hyperbranched polyglycerol, HPG, red blood cells, RBC, whole blood, flow cytometry
Protocol
A. Hyperbranched Polyglycerol Modification (SS-HPG)
Place lyophilized HPG 60 kDa (0.5 g, 0.0083 mmol) in a round bottom flask and dry it overnight under vacuum at 90 °C.
Refrigerate the flask to room temperature, and dissolve the dried HPG in anhydrous pyridine (3 ml).
To functionalize approximately eight hydroxyl groups on HPG with carboxyl groups, add catalytic amount of dimethylaminopyridine (one drop of 5 mg/ml solution in pyridine) to the HPG solution. To this mixture, add succinic anhydride, (0.0067 g, 0.0664 mmol) dissolved in 0.5 ml pyridine drop wise over 10 min. Stir the mixture overnight at room temperature under argon.
Precipitate the mixture in 40 ml of cold acetone (4 °C) in a 50 ml centrifuge tube, and centrifuge using a Beckman J2-MC centrifuge at 27,000 x g for 15 min. Decant the supernatant, and remove residual acetone by flushing with argon at room temperature.
To activate the carboxyl groups with succinimidyl succinate (SS) groups, dissolve the carboxyl -functionalized HPG in 3 ml of anhydrous DMF. Add N-hydroxysuccinimide (0.0077 g, 0.0664 mmol) and N, N'-diisopropylcarbodiimide (0.0084 g, 0.0664 mmol) to the HPG solution and stir the mixture overnight at room temperature under argon.
Purify the SS-HPG by precipitation in cold acetone.
Remove residual acetone by flushing with argon.
Determine the purity of modified HPG and the degree of carboxyl and SS functionalization by proton (1H)- NMR analysis.
B. Whole Blood Collection and Separation of Red Blood Cells (RBCs)
Collect whole blood (40% Hematocrit, 3 ml) from consented healthy human donors into a citrate vacutainer tube.
Centrifuge the citrate tube at 1,000 x g for 4 min.
Remove the supernatant that contains plasma and platelets, and the intermediate buffy coat that contains white blood cells and platelets using a Pasteur pipette.
Transfer packed RBCs (80% Hematocrit, 1.2 ml) into a 12 ml Falcon centrifuge tube, and add PBS buffer (8 ml, pH = 8.0) to wash RBCs.
Mix RBCs by inversion to obtain uniform cell distribution.
Centrifuge the Falcon tube at 1,000 x g for 4 min, and remove the supernatant.
Wash RBCs with PBS two more times by repeating steps 5 and 6.
Place packed-RBCs (100 μl) in 1.6 ml Eppendorf tube, and add PBS buffer (300 μl, pH = 8.0) to obtain 20 % Hematocrit RBCs.
C. HPG Grafting to RBCs
Immediately after precipitation of modified HPG in acetone in step A6, place SS-HPG (150 mg) in a glass vial (1 dram), and dissolve it in PBS buffer (300 μl, pH = 8.0).
Add SS-HPG polymer solution into 20% Hematocrit washed RBCs to obtain a final volume of 400 μl and SS-HPG concentration that ranges from 0.5 mM to 3 mM. For example to prepare RBCs treated with 0.5 mM SS-HPG, remove 36 μl of PBS from the washed RBC and place 36 μl of SS-HPG polymer solution.
Vortex the suspension gently and place the Eppendorf tubes on an orbital shaker at room temperature for 1 hr.
Centrifuge the Eppendorf tubes at 1,000 x g for 4 min, and pipette out the supernatant.
Add 1 ml of PBS to wash RBCs, centrifuge and remove the supernatant.
Add 1 ml saline and wash RBCs twice as in step 5.
Add 300 μl of saline to 100 μl of packed RBCs (80% Hematocrit) to a final concentration of 20 % Hematocrit.
D. Characterization of HPG Modified RBCs
I. Complement mediated lysis
Collect around 8 ml of whole blood from healthy human donors into BD vacutainer glass serum tube, and allow the blood to clot at room temperature for 30 min.
Centrifuge the tube at 2,000 x g for 10 min and collect around 3 ml of serum.
One milliliter of the serum was placed in a water bath at 60 °C for 30 min to prepare heat inactivated serum.
Add 60 μl of fresh serum or heat inactivated serum into an Eppendorf tube that contain 60 μl of 20% Hematocrit HPG modified RBCs or unmodified RBCs.
Incubate RBCs for 1 hr at 37 °C.
To quantify the amount of hemoglobin in the cell suspension, place 5.9 μl of 20% hematocrit of HPG modified RBCs or unmodified cells in triplicate in 96 well plate. Add 294 μl of Drabkin's reagent (a reagent that is used to quantify the amount of hemoglobin spectrophotomerically). Mix cells with Drabkin's reagent by pipetting in and out. Measure the absorbance of the hemoglobin cyanoderivative at 540 nm using SPECTRA MAX 190 plate reader.
To quantify the amount of hemoglobin in the supernatant, centrifuge cells at 13,000 x g for 1 min. Place 50 μl of supernatant in triplicate in a 96 well plate. Add 250 μl of Drabkin's reagent. Mix cells with Drabkin's reagent by pipetting in and out. Measure the absorbance of the hemoglobin cyanoderivative at 540 nm using SPECTRA MAX 190 plate reader.
Quantify the amount of lysed cells from the ratio of hemoglobin in the supernatant (step 7) and the total hemoglobin in the sample (step 6) 8, 11, 12.
II. The camouflage of major and minor antigens using MTS cards
Place 50 μl of HPG modified RBC (10 % Hematocrit) in an Eppendorf tube, centrifuge at 1,000 x g for 1 min, and remove the supernatant.
Add 110 μl of MTS diluent to the cell pellet and homogenize the cell suspension by pipetting in and out.
Add 11 μl of cell suspension to each mini gel column. Avoid touching the gel during the addition.
Locate MTS cards into a centrifuge card holder, and centrifuge at 156 x g for 6 min.
Protection of surface antigens is determined from the location of RBC in the mini gel column, based on the manufacturer description.
III. Aqueous two phase partition measurements
Prepare Dextran 500 kDa/PEG 8 kDa two phase separation system composed of (5% dextran 500k, 4% PEG 8K, 150 mM NaCl, 10 mM phosphate) according to 13.
Centrifuge at 433 x g for 10 min at room temperature to obtain a two phase system (dextran in the bottom).
Separate the two phases carefully.
Aliquot 1.0 ml of the upper PEG phase into a labelled tube (with concave bottom), add 20 μl of 20% hematocrit HPG modified RBC, and mix cells gently by flicking.
Add 0.5 ml of the upper phase that contain cells to 0.5 ml of a lower phase, and mix the system gently by flicking.
Place the tube on the bench for ~ 2 min for the system to separate, and determine the location of cells in the system. Localization of RBCs (in the upper PEG phase, in the lower dextran phase or in both) is function to the degree of modification and molecular weight of HPG.
IV. Osmotic fragility measurements
Prepare NaCl solutions with concentrations that range from 0 to 0.9 (w/v) %.
Place 0.4 ml of NaCl solutions into 1.5 ml Eppendorf tubes.
Add 20 μl of RBCs (20% hematocrit) into the NaCl solutions.
Mix cells carefully by inversion and place them in a water bath at 37 °C for 30 min.
Suspend cells carefully by inversion. Take 50 μl of RBC suspension and add it to a 1 ml Drabkin's reagent, placed in a cuvette. Mix cells with Drabkin's reagent by pipetting in and out. Measure the absorbance of the hemoglobin cyanoderivative at 540 nm using BECKMAN COULTER DU 730 UV/VIS reader.
To quantify the amount of hemoglobin in the supernatant, centrifuge cells at 1,000 x g for 1 min. Add 200 μl of supernatant to 1 ml of Drabkin's reagent in a cuvette. Mix cells with Drabkin's reagent by pipetting in and out. Measure the absorbance of the hemoglobin cyanoderivative at 540 nm using BECKMAN COULTER DU 730 UV/VIS reader.
Calculate the amount of cells lysed in different NaCl solution from the ratio of the amount hemoglobin in the supernatant (step 6) to the total amount of hemoglobin in the cell suspension (step 5).
V. Flow cytometry measurements - Protection of Rhesus-D (RhD) antigen
Add 5 μl of control or HPG modified RBCs (20% hematocrit) in triplicate to PBS buffer supplemented with 0.5% BSA to a total volume of 25 μl.
Add 25 μl of FITC monoclonal anti-Rhesus D (RhD) purchased from Quotient Biodiagnostics (PA, USA).
Incubate the mixture for 30 min at 37 °C in a water bath.
Wash the mixture with 1.5 ml PBS buffer two times. Centrifuge at 1,000 x g for 1 min to remove the supernatant.
Suspend RBCs into 1 ml saline, and transfer the suspension into 4 ml flow BD flow cytometry tube.
Analyze using FACSCanto II flow cytometer, acquiring 5,000 events on the RBC control gate, and use whole events for analysis.
VI. Flow cytometry measurements - Expression of CD47
Add 1.54 μl of control or HPG modified RBCs (20 % hematocrit) in triplicate to PBS buffer supplemented with 0.5% BSA to a total volume of 44 μl.
Add 6 μl of phycoerythrin (PE) labelled mouse anti-human CD47 purchased from BD Biosciences (NJ, USA).
Incubate the mixture for 30 min at 37 °C in a water bath.
Wash the mixture with 1.5 ml PBS buffer for two times. Centrifuge at 1,000 x g for 1 min to remove the supernatant.
Suspend RBCs into 1 ml saline, and pass through 26 G 5/8 needle to minimize cell clumping.
Transfer the suspension into 4 ml flow BD flow cytometry tube.
Analyze using FACSCanto II flow cytometer, acquiring 10,000 events on the RBC control gate.
Representative Results
Camouflage of Rhesus D antigen and CD47 RBC surface protein were quantified by flow cytometry using fluorescent labelled monoclonal antibodies, and a representative result is given in Figure 1. In case of HPG-grafted RBCs (grey), the intensity of the signal decreased (peak shifted to left) compared to the control RBCs (red & green) indicating a reduction in binding to antibodies to cell surface which indicate the masking of surface proteins.
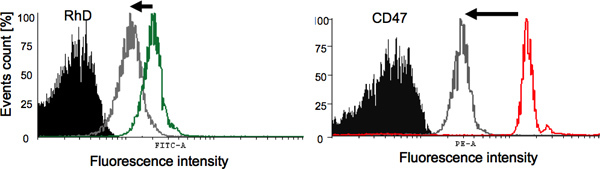 Figure 1. Evaluation of the camouflage of surface antigens and proteins using flow cytometry. A) Rhesus D (RhD) protection: Black shaded peak represents the negative control (non-modified RBCs treated with PE-labelled IgG1), the green peak represent the positive control (non-modified RBCs treated with anti-RhD fluorescent antibody), and the grey peak represents HPG modified RBCs treated with the same amount of anti-RhD fluorescent antibody. B) CD47: Black shaded peak represents the negative control (non-modified RBCs treated with PE-labelled IgG1), the red peak represent the positive control (non-modified RBCs treated with anti-CD47 fluorescent antibody), and the grey peak represents HPG modified RBCs treated with the same amount of anti-CD47 fluorescent antibody. Click here to view larger figure.
Figure 1. Evaluation of the camouflage of surface antigens and proteins using flow cytometry. A) Rhesus D (RhD) protection: Black shaded peak represents the negative control (non-modified RBCs treated with PE-labelled IgG1), the green peak represent the positive control (non-modified RBCs treated with anti-RhD fluorescent antibody), and the grey peak represents HPG modified RBCs treated with the same amount of anti-RhD fluorescent antibody. B) CD47: Black shaded peak represents the negative control (non-modified RBCs treated with PE-labelled IgG1), the red peak represent the positive control (non-modified RBCs treated with anti-CD47 fluorescent antibody), and the grey peak represents HPG modified RBCs treated with the same amount of anti-CD47 fluorescent antibody. Click here to view larger figure.
Discussion
Universal donor RBCs have great potential in enhancing blood availability and safety for blood transfusion therapy. RBCs are also considered promising drug delivery vehicles due to their long circulation and inherent biocompatibility 14, 15. Experiments presented in this paper evaluate the in vitro characteristics of HPG modified RBCs. The in vitro properties and in vivo circulation of HPG modified RBCs have been investigated in our group recently 8, 11. The partition of RBCs in Dextran500K/PEG8K aqueous two phase system, supplemented with NaCl and sodium phosphate, depends on the erythrocyte surface charge and on surface glycoprotein composition 16. Depending on the extent of RBC glycocalyx modification with HPGs, modified RBCs tend to partition from the lower dextran to the upper PEG phase. The two phase system provides a facile evaluation of the extent of RBC surface modification. Lysis of RBCs in autologous serum is significant test to investigate, whether HPG modified RBCs triggers complement activation, since introducing new materials to cells and biological surfaces renders them foreign and subject to immune system mediated lysis and clearance 17. The osmotic fragility experiment, on the other hand, indicates whether mechanical properties and deformability of RBC membrane has been compromised as a result of HPG grafting. The deformability of RBCs is important for the physiological function of O2 delivery to different parts of the body. In this experiment modified RBCs are exposed to deformation stress by treating with different concentrations of NaCl, and quantifying percent of lysed cells.
Flow cytometry is used to evaluate the extent of surface antigen and surface protein protection (Figure 1) by reacting a particular antigen on the surface of RBC with its corresponding fluorescent- labelled antibody. The extent of Rhesus D antigen protection is evaluated using flow cytometry. The masking of antigens is evident from the decrease in antibody binding to the RBCs. Rhesus D antigen masking is also evaluated by MTS cards. MTS cards are widely used in hematology laboratories in hospitals for phenotyping of RBCs. For example, when A-group RBC is located in A-type MTS card, cells agglutinate as a result of reaction with the corresponding monoclonal antibody. However, B-group blood does not agglutinate and travels to the bottom of the mini gel. The concept of agglutination was used to evaluate the level of protection that HPG grafting provides to RBCs. HPG-grafted RBCs penetrates the mini gel column, depending on the level of surface antigen protection.
Disclosures
No conflicts of interest declared.
Acknowledgments
This research was funded by the Canadian Blood Services (CBS) and the Canadian Institutes of Health Science (CIHR) Research Partnership Fund. The authors thank the LMB Macromolecule Hub at the UBC Centre for Blood research for the use of their research facilities. The infrastructure facility is supported by the Canada foundation for Innovation (CFI) and the Michael Smith Foundation for Health Research (MSFHR). R. Chapanian is a recipient of (CIHR/CBS) postdoctoral fellowships in Transfusion Science and a recipient of MSFHR research trainee post doctoral fellowship. J.N. Kizhakkedathu is a recipient of MSFHR Career Investigator Scholar Award.
References
- Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim. Biophys. Acta. 2002;1561(2):147–158. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- Murad KT, Mahany KL, Brugnara C, Kuypers FA, Eaton JW, Scott MD. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly(ethylene glycol. Blood. 1999;93(6):2121–2127. [PubMed] [Google Scholar]
- Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: Stealth erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 1997;94(14):7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G, Reid ME. Blood groups: the past 50 years. Transfusion. 2010;50(2):281–289. doi: 10.1111/j.1537-2995.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- Murad KL, Gosselin EJ, Eaton JW, Scott MD. Stealth cells: Prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood. 1999;94(6):2135–2141. [PubMed] [Google Scholar]
- Kainthan RK, Hester SR, Levin E, Devine DV, Brooks DE. In vitro biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials. 2007;28(31):4581–4590. doi: 10.1016/j.biomaterials.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Kainthan RK, Janzen J, Levin E, Devine DV, Brooks DE. Biocompatibility testing of branched and linear polyglycidol. Biomacromolecules. 2006;7(3):703–709. doi: 10.1021/bm0504882. [DOI] [PubMed] [Google Scholar]
- Chapanian R, Constantinescu I, Brooks DE, Scott MD, Kizhakkedathu JN. In vivo circulation, clearance, and biodistribution of polyglycerol grafted functional red blood cells. Biomaterials. 2012;33(10):3047–3057. doi: 10.1016/j.biomaterials.2011.12.053. [DOI] [PubMed] [Google Scholar]
- Chapanian R, Constantinescu I, Rossi NAA, Medvedev N, Brooks DE, Scott MD, Kizhakkedathu JN. Influence of polymer architecture on antigens Camouflage, CD47 protection and complement mediated lysis of surface grafted red blood cells. Biomaterials. 2012;33(31):7871–7883. doi: 10.1016/j.biomaterials.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Rossi NAA, Constantinescu I, Brooks DE, Scott MD, Kizhakkedathu JN. Enhanced cell surface polymer grafting in concentrated and nonreactive aqueous polymer solutions. J. Am. Chem. Soc. 2010;132(10):3423–3430. doi: 10.1021/ja909174x. [DOI] [PubMed] [Google Scholar]
- Rossi NAA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31(14):4167–4178. doi: 10.1016/j.biomaterials.2010.01.137. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Smirnov MD, Domogatsky SP. Hemolytic complement activity assay in microtitration plates. J. App. Biochem. 1985;7(3):223–227. [PubMed] [Google Scholar]
- Walter H, Brooks DE, Fisher D. Partitioning in aqueous two-phase systems: theory, methods, uses, and applications to biotechnology. London: Academic Press Inc; 1985. [Google Scholar]
- Rossi L, Serafini S, Pierige F, Antonelli A, Cerasi A, Franternale A, et al. Erythrocyte-based drug delivery. Expert Opin. Drug Deliv. 2005;2(2):311–322. doi: 10.1517/17425247.2.2.311. [DOI] [PubMed] [Google Scholar]
- Walter H, Krob EJ, Brooks DE. Membrane surface properties other than charge involved in cell separation by partition in polymer, aqueous 2-phase systems. Biochemistry. 1976;15(14):2959–2964. doi: 10.1021/bi00659a004. [DOI] [PubMed] [Google Scholar]
- Muzykantov VR, Murciano JC, Taylor RP, Atochina EN, Herraez A. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal Biochem. 1996;241(1):109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]


