Abstract
B7-H1/PD-L1, a member of the B7 family of immune-regulatory cell-surface proteins, plays an important role in the negative regulation of cell-mediated immune responses through its interaction with its receptor, programmed death-1 (PD-1) 1,2. Overexpression of B7-H1 by tumor cells has been noted in a number of human cancers, including melanoma, glioblastoma, and carcinomas of the lung, breast, colon, ovary, and renal cells, and has been shown to impair anti-tumor T-cell immunity3-8.
Recently, B7-H1 expression by pancreatic adenocarcinoma tissues has been identified as a potential prognostic marker9,10. Additionally, blockade of B7-H1 in a mouse model of pancreatic cancer has been shown to produce an anti-tumor response11. These data suggest the importance of B7-H1 as a potential therapeutic target. Anti-B7-H1 blockade antibodies are therefore being tested in clinical trials for multiple human solid tumors including melanoma and cancers of lung, colon, kidney, stomach and pancreas12.
In order to eventually be able to identify the patients who will benefit from B7-H1 targeting therapies, it is critical to investigate the correlation between expression and localization of B7-H1 and patient response to treatment with B7-H1 blockade antibodies. Examining the expression of B7-H1 in human pancreatic adenocarcinoma tissues through immunohistochemistry will give a better understanding of how this co-inhibitory signaling molecule contributes to the suppression of antitumor immunity in the tumor's microenvironment. The anti-B7-H1 monoclonal antibody (clone 5H1) developed by Chen and coworkers has been shown to produce reliable staining results in cryosections of multiple types of human neoplastic tissues4,8, but staining on paraffin-embedded slides had been a challenge until recently13-18. We have developed the B7-H1 staining protocol for paraffin-embedded slides of pancreatic adenocarcinoma tissues. The B7-H1 staining protocol described here produces consistent membranous and cytoplasmic staining of B7-H1 with little background.
Keywords: Cancer Biology, Issue 71, Medicine, Immunology, Biochemistry, Molecular Biology, Cellular Biology, Chemistry, Oncology, immunohistochemistry, B7-H1 (PD-L1), pancreatic adenocarcinoma, pancreatic cancer, pancreas, tumor, T-cell immunity, cancer
Protocol
B7-H1/PD-L1, a member of the B7 family of immune-regulatory cell-surface proteins, plays an important role in the negative regulation of cell-mediated immune responses through its interaction with its receptor, programmed death-1 (PD-1) 1,2. Overexpression of B7-H1 by tumor cells has been noted in a number of human cancers, including melanoma, glioblastoma, and carcinomas of the lung, breast, colon, ovary, and renal cells, and has been shown to impair anti-tumor T-cell immunity3-8.
Recently, B7-H1 expression by pancreatic adenocarcinoma tissues has been identified as a potential prognostic marker9,10. Additionally, blockade of B7-H1 in a mouse model of pancreatic cancer has been shown to produce an anti-tumor response11. These data suggest the importance of B7-H1 as a potential therapeutic target. Anti-B7-H1 blockade antibodies are therefore being tested in clinical trials for multiple human solid tumors including melanoma and cancers of lung, colon, kidney, stomach and pancreas12.
In order to eventually be able to identify the patients who will benefit from B7-H1 targeting therapies, it is critical to investigate the correlation between expression and localization of B7-H1 and patient response to treatment with B7-H1 blockade antibodies. Examining the expression of B7-H1 in human pancreatic adenocarcinoma tissues through immunohistochemistry will give a better understanding of how this co-inhibitory signaling molecule contributes to the suppression of antitumor immunity in the tumor's microenvironment. The anti-B7-H1 monoclonal antibody (clone 5H1) developed by Chen and coworkers has been shown to produce reliable staining results in cryosections of multiple types of human neoplastic tissues4,8, but staining on paraffin-embedded slides had been a challenge until recently13-18. We have developed the B7-H1 staining protocol for paraffin-embedded slides of pancreatic adenocarcinoma tissues. The B7-H1 staining protocol described here produces consistent membranous and cytoplasmic staining of B7-H1 with little background.
1. De-parafinization
Bake slides at 55 °C for 20 min.
In a chemical hood, immerse slides in xylene for 10 min.
Immerse slides in fresh xylene for 10 min.
Immerse slides in 100% ethanol for 5 min.
Immerse slides in fresh 100% ethanol for 5 min.
Immerse slides in 95% ethanol for 5 min.
Immerse slides in 80% ethanol for 5 min.
Immerse slides in deionized water for 5 min.
2. Antigen Retrieval
Immerse slides in Tris-EDTA buffer (pH 9.0) and heat in a pressure cooker to 125 °C for 30 sec, then 90 °C for 10 sec.
Let slides cool for 60 min.
Immerse slides in TBST for 5 min; repeat twice.
3. Staining
Wipe off solutions around the tissue on the slides. Mark a circle around the tissue with a hydrophobic pen.
Place the slides in a humidity chamber and avoid the drying of the tissue on the slide throughout the entire staining process. Place one drop (approximately 200 μl or more to cover the tissue area completely) of peroxidase block within the hydrophobic circle on the slide and incubate slides in peroxidase block for 5 min.
Rinse slides with TBST, then place in TBST for 5 min.
Place one drop of BLOCK ACE blocking buffer on each slide and incubate for 15 min.
Rinse slides with TBST, then place in TBST for 5 min.
Place one drop of Avidin solution on each slide and incubate for 15 min.
Rinse slides with TBST, then place in TBST for 5 min.
Place one drop of Biotin solution on each slide and incubate for 15 min.
Rinse slides with TBST, then place in TBST for 5 min.
Incubate slides with 1:1,000 dilution of mouse anti-B7-H1 monoclonal antibody (clone 5H1) or a mouse IgG1 κ isotype control in 200 μl TBST at 4 °C overnight for 22 hr+/- 1 hr. The final antibody concentration in TBST is 2.57 μg/ml.
Rinse slides with TBST, then place in TBST for 5 min. Repeat twice.
Incubate slides with a biotin conjugated rat anti-mouse IgG1 secondary antibody at a 1:500 concentration (in 200 μl 2% goat serum solution) for 30 min.
Rinse slides with TBST, then place in TBST for 5 min. Repeat twice.
Place one drop of Streptavidin-Biotin Complex (must be prepared at least 30 min prior to use) on each slide and incubate for 15 min.
Rinse slides with TBST, then place in TBST for 5 min. Repeat twice.
Place one drop of Amplification Reagent (diluted 2:1 with TBST) on each slide and incubate for 4 min.
Rinse slides with TBST, then place in TBST for 5 min. Repeat twice.
Place one drop of Streptavidin-HRP on each slide and incubate for 15 min.
Rinse slides with TBST, then place in TBST for 5 min. Repeat twice.
Place one drop of 3,3-Diaminobenzidine (DAB) substrate and chromogen on each slide and develop the color for 2 min.
Immediately after the color development, wash slides with ddH2O for 1-2 min.
Incubate slides with one drop of hematoxylin for 90 sec.
Wash with ddH2O for 1-2 min.
Wash with soapy tap water for 1-2 min.
Wash with ddH2O for 1-2 min.
4. Dehydration and Mounting
Immerse the slides in 80% ethanol for 3 min.
Immerse the slides in 95% ethanol for 3 min.
Immerse the slides in 100% ethanol for 5 min. Repeat.
Immerse the slides in xylene for 5 min. Repeat.
Add one drop of Cytoseal 60 to the slide and place the coverslip.
Table 1 lists the reagents used in the above described procedures.
Representative Results
A successful immunohistochemical staining of B7-H1 will demonstrate the heterogeneous expression of B7-H1 (brown signals DAB color development) in pancreatic adenocarcinoma. Some pancreatic adenocarcinoma cells have predominantly membranous expression of B7-H1 (Figure 1A) whereas others have either predominantly cytoplasmic expression of B7-H1 or little expression of B7-H1 (Figures 2 and 3). Normal pancreatic duct epithelium expresses little B7-H1 (Figure 4). Staining with a mouse IgG1 κ isotype control antibody instead of the anti-B7-H1 antibody shows little background staining (Figure 1B).
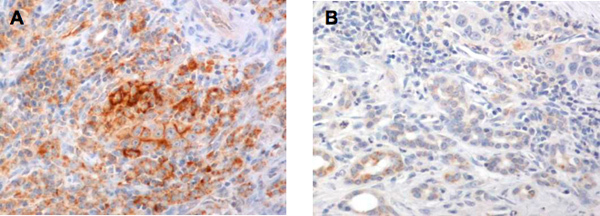 Figure 1. A. Immunohistochemical staining with the anti-B7-H1, clone 5H1 antibody shows membranous expression on some pancreatic adenocarcinoma cells. B. Immunohistochemical staining with the isotype control IgG1 shows little DAB color development on the same pancreatic adenocarcinoma tissue.
Figure 1. A. Immunohistochemical staining with the anti-B7-H1, clone 5H1 antibody shows membranous expression on some pancreatic adenocarcinoma cells. B. Immunohistochemical staining with the isotype control IgG1 shows little DAB color development on the same pancreatic adenocarcinoma tissue.
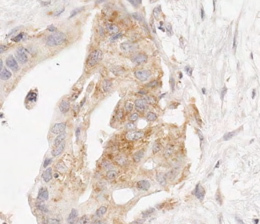 Figure 2. B7-H1 expression in the cytoplasm of pancreatic adenocarcinoma cells.
Figure 2. B7-H1 expression in the cytoplasm of pancreatic adenocarcinoma cells.
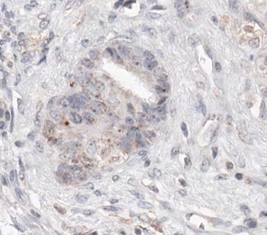 Figure 3. Some pancreatic adenocarcinoma cells express little B7-H1.
Figure 3. Some pancreatic adenocarcinoma cells express little B7-H1.
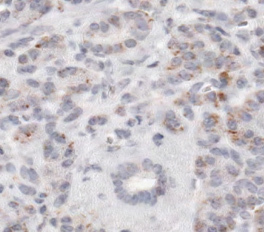 Figure 4. B7-H1 expression on normal pancreatic ductal epithelium.
Figure 4. B7-H1 expression on normal pancreatic ductal epithelium.
Discussion
Immunohistochemical staining of B7-H1 on cryosections has been reported in prior studies for a variety of cancers3,4. However, clinical tumor samples usually are available only in the form of paraffin-embedded blocks. The 5H1 monoclonal antibody has more recently been successfully applied to the staining of paraffin embedded tumor tissues including renal cell carcinoma, cervical carcinoma, bladder cancer and melanoma13-18. B7-H1 staining on paraffin-embedded9,19 or cryostat20 sections of pancreatic adenocarcinoma tissues with other monoclonal antibodies has also been reported. Here, we have shown successful staining of paraffin-embedded pancreatic adenocarcinoma tissues with the 5H1 monoclonal antibody to B7-H1. The expression of B7-H1 in pancreatic adenocarcinoma is heterogeneous, similar to that in many other types of malignancies such as melanoma4,21. Both membranous and cytoplasmic expression of B7-H1 are observed with adenocarcinoma cells (Figures 1 and 2). Membranous expression of B7-H1 is consistent with its biological function. The role of cytoplasmic B7-H1 in neoplastic tissues remains to be explored. Normal pancreatic duct epithelial or acinar cells do not express B7-H1 (Figure 4).
All the steps in this protocol take place at room temperature, except for the overnight incubation at 4 °C. Antigen retrieval conditions used in this B7-H1 staining procedure were not reported in the aforementioned publications9,19. The amplification step was also not employed by these studies. We found that the critical steps in this protocol include the antigen retrieval, the overnight incubation with the primary antibody, the amplification, and the DAB color development. The dilution of the primary antibody might need to be titrated slightly for each different batch of 5H1. Amplification has been kept exactly at 4 min with a diluted amplification reagent. These modifications give sufficient amplification of positive signals without increasing background. The development time is typically 2 min, but we routinely monitor the development under a microscope to ensure optimal staining with minimal background. With each round of staining, we include one slide from a previous batch and one slide of tonsil tissue as positive controls. Paracortical staining of B7-H1 in tonsil has been previously described4. If the background is high or if it takes more than 5 min to develop typical signals in these control slides, we recommend replacing the reagents for the critical steps and checking all staining steps.
The use of this staining technique allows for qualitative and semi-quantitative analysis of B7-H1 expression and localization on neoplastic pancreatic adenocarcinoma cells. With modification of the dilution of the primary antibody, the length and dilution of the amplification step, and the time of color development, this protocol may be applied to other tissue types. We have modified this protocol and used it for breast cancer and sarcoma tissues. As B7-H1 and its binding to PD-1 provide a major tumor-induced immunosuppressive signal in the tumor's microenvironment, this staining technique will facilitate our understanding of the role of the tumor's microenvironment in tumor initiation, progression and anti-tumor immune response.
Disclosures
No conflicts of interest declared.
Acknowledgments
E.B., K.M.B, and H.X. contribute equally. This study was supported by the NCI SPORE in Gastrointestinal Cancers P50 CA062924, NIH K23 CA148964, Lustgarten Foundation, Viragh Foundation, ASCO Young Investigator Award, NIH 5T32 CA0090701-28 Training Grant, Sol Goldman Pancreatic Cancer Center, and National Pancreas Foundation.
References
- Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J. Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J. Mol. Med. (Berl) 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J. Cancer Res. Clin. Oncol. 2008;134:1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, WB M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M, Yagita H, Miura S. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int. J. Oncol. 2009;35:741–749. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr. Top Microbiol. Immunol. 2011;344:245–267. doi: 10.1007/82_2010_81. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Webster WS, Cheville JC, Lohse CM, Dong H, Leibovich BC, Kuntz SM, Sengupta S, Kwon ED, Blute ML. B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in patients with renal cell carcinoma. Urology. 2005;66:10–14. doi: 10.1016/j.urology.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin. Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, vander Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin. Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J. Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]


