Abstract

An efficient synthesis of densely substituted 2-aroyl indolizines via the palladium-catalyzed carbonylative cyclization/arylation is reported. This transformation proceeds via the 5-endo-dig cyclization of 2-propargylpyridine triggered by an aroyl Pd-complex. It produced diversely substituted 2-aroyl indolizines in good-to-excellent yield.
Indolizines have attracted noteworthy attention in recent years due to their profound biological effects.1 Both naturally-occurring and synthetic indolizines have shown great potential in pharmaceutical research as cytotoxins,2 anti-inflammatory agents,3 and 5-HT3 receptor antagonists4. In this regard, transformations that utilize readily available substrates to provide access to diversely substituted indolizines, especially those bearing an electron withdrawing group at the C-2 postion5, are in high demand. The classic Tschichibabin reaction provides straightforward access to C-2 substituted indolizines via the condensation of picolines and α-bromo-acetophenone derivatives. However, the limited availability of starting materials restricts the substitution pattern of the product.6 The [3+2] cycloaddition of pyridinium ylides with alkynes provides another viable route to the indolizine core. However, the regioselectivity issue, as well as the moderate yields caused by necessary oxidation of the formed intermediate, limits its synthetic application.7 The Morita-Baylis-Hillman reaction can also be utilized for the preparation of indolizin-2-yl ketone from an appropriate Michael acceptor. However, it suffers from the narrow range of starting materials that can be used and the usually low reactivity of the substrates.8 In addition to the traditional condensation methods, transition metal catalysis has been widely used for the construction of diversely functionalized, especially heteroatom-substituted indolizines, under mild conditions.9 Nonetheless, the selective introduction of functionality at the C-2 position is still a challenging task.10 Along this line, our group has recently reported a synthesis of 2-aryl indolizines via the palladium catalyzed arylative 5-endo-dig cyclization of 2-propargylpyridine (Scheme 1, eq. 1).11 Herein, we report a Pd-catalyzed cascade carbonylative cyclization/arylation approach to densely substituted 2-aroyl indolizines that proceeds in good-to-excellent yields (eq. 2).
Scheme 1.

Indolizine Synthesis via Palladium Catalyzed Cyclization
Continuing with our efforts in the synthesis of diversely functionalized indolizines, we thought that the employment of benzoyl chloride instead of an aryl halide as electrophile in this cascade cyclization would provide 2-benzoyl indolizine.12 However, the reaction of pivaloate 1a with benzoyl chloride in the presence of a Pd catalyst failed to produce even trace amount of 2a (eq. 3).
Thus, we thought of alternative methods to introduce the benzoyl function at the C-2 position of indolizine. Since (1) an acyl palladium species can be formed via a migratory insertion of carbon monoxide into an aryl-palladium bond; and (2) there have been reported examples on synthesis of diaryl ketones via the palladium catalyzed carbonylative cyclization/arylation cascades,13 we anticipated that under a CO atmosphere, the in situ formed acyl palladium species might trigger the expected carbonylative cyclization/arylation cascade to obtain 2.
 |
(3) |
To test this idea, we examined the carbonylative cyclization of pivaloate 1a and different aryl halides under continuous 10 psi CO supply. Initially we found that the carbonylative cyclization of 1a and iodobenzene in the presence of Pd(PPh3)Cl2 catalyst yielded 2a in quantitative yield (Table 1, entry 1). However, aryl halides bearing electron-withdrawing groups, such as methyl p-iodobenzoate, were not competent reactants under these conditions (entry 2).14 On the contrary, the combination of Pd(OAc)2 catalyst, PCy3 ligand and triethylamine base afforded benzoate 2f in 90% yield (entry 3). Under the same conditions, the yield of 2a was only 19% along with a substantial amount of palladium black produced, which implied decomposition of an excessively reactive catalyst (entry 4). The yield of 2a was improved to 51% by switching ligand to PPh3 (entry 5). The reaction that was performed in a sealed Schlenk tube, which simplified the reaction setup and allowed a higher pressure (20 psi and above), provided 2a in 86% yield at 20 psi CO pressure (entry 6). Finally the screening of different solvents under a reduced temperature revealed that acetonitrile was the optimal choice over DMF and toluene (entries 7–9).
Table 1.
Optimization of Conditions

| ||||||
|---|---|---|---|---|---|---|
| entry | ligand | base | solvent | temp. | COa | yield (%)b |
| 1 | Pd(PPh3)2Cl2/PPh3 | K2CO3 | DMF | 100 | A | 2a > 99c |
| 2 | Pd(PPh3)2Cl2/PPh3 | K2CO3 | DMF | 100 | A | 2f decomp.c |
| 3 | Pd(OAc)2/PCy3 | NEt3 | DMF | 80 | A | 2f 90 |
| 4 | Pd(OAc)2/PCy3 | NEt3 | DMF | 80 | A | 2a 19 |
| 5 | Pd(OAc)2/PPh3 | NEt3 | DMF | 80 | A | 2a 51 |
| 6 | Pd(OAc)2/PPh3 | NEt3 | DMF | 80 | B | 2a 86 |
| 7 | Pd(OAc)2/PPh3 | NEt3 | DMF | 70 | B | 2a 65 |
| 8 | Pd(OAc)2/PPh3 | NEt3 | toluene | 70 | B | 2a 45 |
| 9 | Pd(OAc)2/PPh3 | NEt3 | MeCN | 70 | B | 2a 95 |
A: Reactions were conducted in flask with continuous 10 psi CO supply. B: Reacitons were conducted in sealed Schlenk tube with 20 psi CO.
Isolated yields of 0.5 mmol reactions.
1 equivalent of TBAI was added.
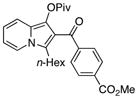
With the optimized conditions in hand, the scope of reaction was examined. It was found that a series of iodoarenes bearing different substituents at various positions smoothly underwent this carbonylative cyclization with 1a, yielding indolizines 2a to 2i in moderate to excellent yields (Table 2, entries 1–9). The cyclization of 3-n-hexyl, n-butyl and cyclohexenyl substituted pivaloates also provided the expected products in good yield (entry 10 to 15). Substrates possessing a functionalized pyridine ring at C-5 produced indolizines 2p, 2q and pyrrolo[1,2-a]quinoline 2r in moderate-to-good yields (entries 16–18). On the contrary, cyclization of 3-methyl substituted pyridine provided 8-methyl 2s in a relative low yield (entry 19). In addition to various pivaloates, a TBDMS ether was equally effective in this reaction, yielding 2t in 80% yield (entry 20). More interestingly, the cyclization of 1-(1-pyridin-2-yl-propargyl)morpholine resulted in the 1-morpholin-1-yl indolizine 2u in 71% yield, which provided a convenient access to the C-2 substituted 1-amino-indolizines.15
Table 2.
Reaction Scope of Carbonylative Cyclization.

| ||
|---|---|---|
| entry | 2 | yield (%) b |
| 1 |
 2a |
95 |
| 2 |
 2b |
77 |
| 3 |
 2c |
78 |
| 4 |
 2d |
99 |
| 5 |
 2e |
59 |
| 6 |
 2f |
61 |
| 7 |
 2g |
69 |
| 8 |
 2h |
64 |
| 9 |
 2i |
64 |
| 10 |
 2j |
80 |
| 11 |
 2k |
80 |
| 12 |
 2l |
64 |
| 13 |
 2m |
81 |
| 14 |
 2n |
67 |
| 15 |
 2o |
72 |
| 16 |
 2p |
90 |
| 17 |
 2q |
85 |
| 18 |
 2r |
53 |
| 19 |
 2s |
35 |
| 20 |
 2t |
80 |
| 21 |
 2u |
71 |
Conditions: Pd(OAc)2 5 mol %, PPh3 10 mol %, Ar-I 1.5 equiv, triethylamine 2 equiv, MeCN 0.2 M, 70 °C, CO 20 psi in sealed Schlenk tube, 12 h.
Isolated yields of 0.5 mmol reactions.
Presumably, this palladium catalyzed carbonylative cyclization starts with the formation of the aroyl palladium species 3 (Scheme 2) via a migratory insertion of CO into the aryl-palladium bond, followed by its coordination of the triple bond of 1 to the palladium center. The 5-endo-dig cyclization leads to the formation of indolizinium intermediate 4, which after a proton loss-aromatization produces heteroaryl palladium species 5. The reductive elimination of 5 releases the product 2-benzoylindolizine 2 to regenerate the Pd catalyst.
Scheme 2.
Proposed Mechanism
In summary, the Pd-catalyzed carbonylative cyclization/arylation approach to 2-aroyl indolizines has been developed. This new method allows for the efficient and selective synthesis of important5 2-aroyl indolizines from readily available propargyl pyridines, iodoarenes under a carbon monoxide atmosphere.
Supplementary Material
Acknowledgments
The support of the National Institute of Health (GM-64444 and 1P50 GM-086145) is gratefully acknowledged.
Footnotes
Supporting Information Available Detailed experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Flitsch W. Comprehensive Heterocyclic Chemistry, II. Vol. 8. Pergamon Press; Oxford: 1984. pp. 237–248. [Google Scholar]; (b) Fan H, Peng J, Hamann MT, Hu JF. Chem Rev. 2008;108:264. doi: 10.1021/cr078199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Ishibashi F, Tanabe S, Oda T, Iwao M. J Nat Prod. 2002;65:500. doi: 10.1021/np0104525. [DOI] [PubMed] [Google Scholar]; (b) Pla D, Marchal A, Olsen CA, Francesch A, Cuevas C, Albericio F, Alvarez M. J Med Chem. 2006;49:3257. doi: 10.1021/jm0602458. [DOI] [PubMed] [Google Scholar]; (c) Chittchang M, Batsomboon P, Ruchirawat S, Ploypradith P. ChemMedChem. 2009;4:457. doi: 10.1002/cmdc.200800339. [DOI] [PubMed] [Google Scholar]
- 3.Oslund RC, Cermak N, Gelb MH. J Med Chem. 2008;51:4708. doi: 10.1021/jm800422v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez J, Fake CS, Joiner GF, Joiner KA, King FD, Miner WD, Sanger GJ. J Med Chem. 1990;33:1924. doi: 10.1021/jm00169a016. [DOI] [PubMed] [Google Scholar]
- 5.For biological activity of indolizines bearing an electron withdrawing group at C-2 postition, see: Shen YM, Lv PC, Chen W, Liu PG, Zhu HL, Zhang MZ. Euro J Med Chem. 2010;45:3184–3190. doi: 10.1016/j.ejmech.2010.02.056.Wu XW, Wu ZP, Chen JW, Zhang W, Gu LQ, Huang ZS, An LK, Wang LX, Zhang HB. Euro J Med Chem. 2011;46:4625. doi: 10.1016/j.ejmech.2011.07.042.Warui DM, Baranger AM. J Med Chem. 2009;52:5462. doi: 10.1021/jm900599v.
- 6.(a) Tschitschibabin AE. Ber Dtsch Chem Ges. 1927;60:1607. [Google Scholar]; (b) Kostik EL, Abiko A, Oku A. J Org Chem. 2001;66:2618. doi: 10.1021/jo0011639. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, Sun JW. J Org Chem. 2012;77:1191. doi: 10.1021/jo2023312. [DOI] [PubMed] [Google Scholar]
- 7.(a) Katritzky AR, Qiou G, Yang B, He HY. J Org Chem. 1999;64:7618. [Google Scholar]; (b) Bora U, Saikia A, Boruah RC. Org Lett. 2003;5:435. doi: 10.1021/ol020238n. [DOI] [PubMed] [Google Scholar]; (c) Rotaru AV, Druta ID, Oeser T, Müller TJJ. Helv Chim Acta. 2005;88:1798. [Google Scholar]
- 8.(a) Bode ML, Kaye PT. J Chem Soc Perkin Trans 1. 1990:2612. [Google Scholar]; (b) Basavaiah D, Rao AJ. Chem Commun. 2003:604. [PubMed] [Google Scholar]; (c) Specowius V, Bendrath F, Winterberg M, Khurshid A, Langer P. Adv Synth Catal. 2012;354:1163. [Google Scholar]
- 9.(a) Kel’in AV, Sromek AW, Gevorgyan V. J Am Chem Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]; (b) Seregin I, Gevorgyan V. J Am Chem Soc. 2006;128:12050. doi: 10.1021/ja063278l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Y, Song Z, Yan B. Org Lett. 2007;9:409. doi: 10.1021/ol062766v. [DOI] [PubMed] [Google Scholar]; (d) Chuprakov S, Gevorgyan V. Org Lett. 2007;9:4463. doi: 10.1021/ol702084f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hardin AR, Sarpong R. Org Lett. 2007;9:4547. doi: 10.1021/ol701973s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Barluenga J, Lonzi G, Riesgo L, López LA, Tomás M. J Am Chem Soc. 2010;132:13200. doi: 10.1021/ja106751t. and Ref. (11), (15) [DOI] [PubMed] [Google Scholar]
- 10.For the regioselectivity of the direct C-H functionalization of indolizine, see: Renard M, Gubin J. Tetrahedron Lett. 1992;33:4433.Shipman M. Science of Synthesis. 2000;10:777.Park CH, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org Lett. 2004;6:1159. doi: 10.1021/ol049866q.Seregin IV, Ryabova V, Gevorgyan V. J Am Chem Soc. 2007;129:7742. doi: 10.1021/ja072718l.Petit A, Flygare J, Miller AT, Winkel G, Ess DH. Org Lett. 2012;14:3680. doi: 10.1021/ol301521n.
- 11.(a) Chernyak D, Skontos C, Gevorgyan V. Org Lett. 2010;12:3242. doi: 10.1021/ol1011949. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chernyak D, Gevorgyan V. Org Lett. 2010;12:5558. doi: 10.1021/ol102447s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For palladium catalyzed acylation reactions using acyl chlorides as electrophile, see: Tohda Y, Sonogashira K, Hagihara N. Synthsis. 1977:777.Negishi E-i, Bagheri V, Chatterjee S, Luo F-T, Miller JA, Stoll AT. Tetrahedron Lett. 1983;24:5181.Renaldo AF, Labadie JW, Stille JK. Org Synth. 1989;67:86.Kabalka GW, Malladi RR, Tejedor D, Kelley S. Tetrahedron Lett. 2000;41:999.
- 13.For selected reports on the palladium catalyzed carbonylative cyclization/arylation cascade, see: Arcadi A, Cacchi S, Carnicelli V, Marinelli F. Tetrahedron. 1994;50:437.Dai G, Larock RC. Org Lett. 2001;4:193. doi: 10.1021/ol010230y.Hu Y, Zhang Y, Yang Z, Fathi R. J Org Chem. 2002;67:2365. doi: 10.1021/jo010839c.Dai D, Larock RC. Org Lett. 2002;4:193. doi: 10.1021/ol010230y.
- 14.See Supporting Information for details.
- 15.For synthesis of 2-nonsubstituted 1-aminoindolizine via the A3 coupling reaction, see: Yan B, Liu Y. Org Lett. 2007;9:4323. doi: 10.1021/ol701886e.Bai Y, Zeng J, Ma J, Gorityala BK, Liu XW. J Comb Chem. 2010;12:696. doi: 10.1021/cc100086h.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



