Abstract
The central nervous system is derived from the neural plate that undergoes a series of complex morphogenetic movements resulting in formation of the neural tube in a process known as neurulation. During neurulation, morphogenesis of the mesenchyme that underlies the neural plate is believed to drive neural fold elevation. The cranial mesenchyme is comprised of the paraxial mesoderm and neural crest cells. The cells of the cranial mesenchyme form a pourous meshwork composed of stellate shaped cells and intermingling extracellular matrix (ECM) strands that support the neural folds. During neurulation, the cranial mesenchyme undergoes stereotypical rearrangements resulting in its expansion and these movements are believed to provide a driving force for neural fold elevation. However, the pathways and cellular behaviors that drive cranial mesenchyme morphogenesis remain poorly studied. Interactions between the ECM and the cells of the cranial mesenchyme underly these cell behaviors. Here we describe a simple ex vivo explant assay devised to characterize the behaviors of these cells. This assay is amendable to pharmacological manipulations to dissect the signaling pathways involved and live imaging analyses to further characterize the behavior of these cells. We present a representative experiment demonstrating the utility of this assay in characterizing the migratory properties of the cranial mesenchyme on a variety of ECM components.
Keywords: Neurobiology, Issue 71, Cellular Biology, Neuroscience, Medicine, Molecular Biology, Pharmacology, exencephaly, cranial mesenchyme, migration, neural tube closure, cell rearrangement, extracellular matrix, pharmacological treatment
Introduction
Neural tube closure in the cranial region occurs between embryonic day 8.5 (E8.5) and 9.5 in the mouse embryo. Failure to properly close the neural tube in the head results in anencephaly, a common structural birth defect in humans and is incompatible with life. The forces that drive cephalic neural tube closure are generated from both the neural tissue itself and the surrounding epidermis and mesenchyme3. In particular expansion of the cranial mesenchyme is thought to be essential for elevation of the cranial neural folds1,2. The cranial mesenchyme is rich in ECM proteins in particular glycosylated proteins such as heparin sulphate proteoglycans, chondroitin sulfates and hyaluronate4-8.
Unlike in the chicken embryo where neural crest cells emigrate from the dorsal neural tube following neural tube closure, the neural crest in the mouse embryo migrates at the same time that the neural folds begin to rise (after the 5 somite stage). Thus during neurulation in the mouse embryo, the cranial mesenchyme is composed of cells derived from the neural crest and the paraxial mesoderm. Neural crest and paraxial mesoderm populations are induced at different times in development; localized in different positions in the embryo and develop into different structures9,10. The paraxial mesoderm originates from the primitive streak and migrates to the anterior region of the embryo to underlie the presumptive neural plate. The neural crest is induced at the junction of the neural plate and epidermal ectoderm, undergoes an epithelial to mesenchyme transition and delaminates just prior to neural fold elevation in the rodent embryo. Neural crest cells migrate along stereotypic paths in the subectodermal paraxial mesoderm to the branchial arch, frontonasal and periocular mesenchyme. The paraxial mesoderm will contribute to some of the bones of the skull vault and muscles of the face; whereas the neural crest will contribute to other bones of the skull and face in addition to cranial nerves9-11. The paraxial mesoderm and neural crest lineages can be differentially marked by the Mesp1-cre and Wnt1-cre transgenic mouse lines, respectively 9.
The essential role of the cranial mesenchyme in neural tube closure has been inferred from experiments where treatment of rodent embryos with ECM disrupting agents such as hyaluronidase, chondroitinase ABC, heparitinase or Diazo-oxo-norleucine (DON) during neurulation impaired neural tube closure7,12-14. In these experiments, histological analysis of static sections following neurulation revealed associated dysmorphogeneis of the cranial mesenchyme7,12-14. However, since the teratogenic agent had access to multiple tissues, it remains to be determined if the cranial mesenchyme is really the target tissue. In support of the conclusion that this tissue is essential for neurulation, the cranial mesenchyme appears abnormal upon histological analyses in some mouse mutants with exencephaly15-17. Still, in most cases, the effect of the mutation on the cellular behavior of the cranial mesenchyme has not been addressed.
We have devised an ex vivo explant assay to directly examine the consequence of genetic mutation or pharmacological manipulation on the behavior of cranial mesenchyme cells15. This assay is similar to that published by Tzahor et al 2003 to access the differentiation potential of the cranial mesenchyme that underlies the rhombomeres18 except we have modified the explant dissection to study the migratory properties of more anterior populations of cranial mesenchyme that underlie the anterior neural plate. Our method is also a modification of explant assays performed in the chicken embryo to analyze the migratory behavior of the neural crest with key differences. Previous preparations have explanted the neural crest or the more posterior paraxial mesoderm19,20. Furthermore, during neural fold elevation in the chicken embryo, the neural crest has not yet emigrated from the dorsal neural tube and thus explants taken of the anterior paraxial mesoderm would not contain neural crest cells. In our assay, cranial mesenchyme explants consisting of paraxial mesoderm, neural crest and surface ectoderm are prepared and plated on a substrate. Experimental manipulation including isolation of explants from genetic mutants, plating explants on different ECM or pharmacological treatments can be performed. Cells migrate from the explant and the distance, number and behavior can be analyzed and compared between treatment groups. In addition, this preparation is amendable to analyses of cellular migration by live imaging techniques. After the migration experiment, explants can be fixed and subjected to immunohistochemical analyses to further elucidate the effect of treatments. On the whole, the protocol presented here is a simple ex vivo assay to investigate the behavior of the cranial mesenchyme. As a representative experiment, we utilize this assay to examine the migration of cranial mesenchyme on different extracellular substrates.
Protocol
1. Preparation of ECM Coated Culture Dishes or Coverslips
Prepare ECM stock solution by dissolving ECM substrate in PBS (Dulbecco's Phosphate-Buffered Saline, Invitrogen, #14040-133). Filter using a 0.2 mm syringe filter (VWR, #28145-495) and freeze in aliquots at -80 °C.
Dilute ECM stock solution in PBS to desired concentration. For MaxGel, dilute in DMEM (Dulbecco's Modified Eagle Medium, Invitrogen, #11995-065).
If immunofluorescence will be performed, sterilize 12 mm circular glass coverslips (VWR, #89015-724) by dipping in ethanol and allow to air dry. This should be done in the sterile laminar hood. If immunofluorescence will not be performed skip to step 1.5.
Place sterilized coverslips in each well of a 24 well plate (Corning, #3526).
Add 0.5 ml of diluted substrate solution to each well of 24 well plate and incubate at room temperature for 3 hr.
Aspirate off the ECM solution and wash three times in PBS.
Aspirate off PBS and use immediately or wrapped in parafilm and stored at 4 °C for up to 2 weeks.
2. Preparation of Dissection Tools
Sylgard plates with added charcoal are prepared per the manufacture's protocol. The resistant black background of these plates provides support for pinning down the embryo and the color offers good contrast which facilitates visualizing the translucent embryo.
To prepare plates weigh directly into a tri-pour beaker on a balance 150 g Part A liquid 15 g Part B and 1 g charcoal powder (World Precision Instruments, #SYLG184). Mix together using a wooden popsicle stick or tongue depressor. Pour into glass or plastic 100 cm dishes. Use a small butane torch held about 10 to 12 inches above the dishes to burn off any bubbles. Place lids on plates and let sit for at least 24 hr to set.
To prepare dissection tools insert a Minutien pin (0.1 mm diameter) into a pin holder (Fine Scientific Tools, #26002-10 and #26018-17, respectively) alternatively 26 gauge needles (BD, # 309597) bent at right angles with the aid of forceps may be used as dissecting instruments. Sharpened Student Dumont #5 Forceps (Fine Science Tools, #91150-20) using a sharpening stone (Fine Scientific Tools, #29008-22).
Sterilize all dissection tools with 70% ethanol before use.
3. Collection of Embryos
Euthanize the timed pregnant mouse at 8.5 days post coitum according to animal use committee regulations and remove uterus.
Place uterus in a plastic Petri dish with sterile PBS.
Wash uterus once with sterile PBS.
Under a dissection microscope carefully remove embryo from deciduas using a pair of forceps.
Remove extraembryonic membranes and save for genotyping if needed.
Stage embryo by counting somites. The ideal stages to make explants to analyze cellular behavior during neural fold elevation would be between the 6 and 10 somite stages when the neural folds are just beginning to elevate and before the major morphogenesis of the cranial mesenchyme takes place.
Wash embryo in PBS. Place in a black sylgard plate with DMEM that contains no phenol red (Invitrogen, #21063-029). Remove the extraembryonic membranes and save for genotyping if needed.
4. Preparation of Explants
Refocus the microscope at the highest possible magnification over the head region of the embryo.
Make cuts with a dissection needle in each hand. Use one needle to hold the tissue in place and the other to make cuts.
Cut off the head of the embryo anterior to the rhombomeres.
Cut along the midline to bisect the head into two equal halves.
Cut around the outer fringes of the head and midline. These cuts will remove the premigratory crest and surface ectoderm at the outer border and the prechordal plate at the midline. The remaining tissue will be the explant and will contain the migrating cranial neural crest, paraxial mesoderm and surface ectoderm.
As the tissue is cut, remove dissected debris from the dish using a sterile pasteur pipette. This prevents confusing the explant with the dissected debris.
Gently wash the explant by pipeting up and down with a 20 μl pipette tip and pipetman. This will remove loosely attached cells and prevent jagged edges on the explants.
Carefully pick up each explant in approximately 10 μl of liquid using a 20 μl pipette tip and pipetman and place at the center of the coated culture well.
Add 20 μl of culture media supplemented with 15% FBS to cover the explant.
Place plate in a humidified tissue culture incubator (5% CO2, 37 °C) for 1-2 hr to allow the explant to attach to the bottom of the dish. View periodically to ensure that the explant does not dry out.
When the explant has attached, carefully add to each well 250 μl DMEM supplemented with 10% FBS that has been warmed to 37 °C in a waterbath. Once explants have attached they will not move when plate is gently shaken under microscope.
Return dish to tissue culture incubator.
After 24 hr, the explants will be firmly attached and cells will begin to emigrate. At this time pharmacological agents can be added to the media.
48 hr after plating, cells will have migrated from the explants. The distance migrated can be visualized and imaged. We typically stop the culture at this point since explants begin to show signs of decreased viability with longer incubations. A vital stain such as acridine orange or trypan blue can also be used to check viability of the explant.
At this point images can be acquired to document the distance and number of cells that migrate from the explant. Alternatively, if explants were plated on coverslips, they can be fixed and immunohistochemical analyses performed to visualize proteins of interest.
Critical steps in the protocol
Cut explants of approximately the same size.
Clear all dissection debris off the dissecting plate as you are dissecting to avoid confusing debris with the desired explanted tissue.
When plating explants in wells or on coverslips be sure to place droplet of media with explant in center of well.
Allow explant sufficient time to attach before flooding the well with media.
Treatment conditions should be standardized for every test substrate and pharmacological agent used.
Two explants can be made from each embryo (one from the left and one from the right of the neural tube).
When testing migration of cells from mutant mouse lines under different experimental conditions, put one mutant explant in the control group and the other in the treated group.
Troubleshooting
If explants fail to attach, increase the allotted time for attachment. In our experience, approximately half of the explants do not attach and larger explants adhere more efficiently.
If explants attach to the periphery of the well they will be hard to image. Be sure to place explants in center of the well.
Ideally explants will be approximately the same size. However, discrepancies in explant size can be corrected for using the diameter of the explants as a correction factor when quanitating migrations of cells from explants.
If contamination occurs be sure sterile working conditions are being used. All tools and work surfaces should be sterilized with 70% ethanol. Pipets and Petri dishes should be new and sterile.
If explants are getting lost during the dissection, periodically remove debris to prevent losing explant.
Mastering the dissection is time consuming and takes practice. Optimal magnification, sharp dissection tools and needles help.
Representative Results
The appearance of cells migrating from cranial mesenchyme explants is shown in Figure 2. We tested migration on various ECM substrates that are present in the cranial mesenchyme during neurulation including Fibronectin (100 mg/ml; Sigma #F1141), Laminin (100 mg/ml; Sigma #L2020), MaxGel ECM (1:500 dilution in DMEM; Sigma #E0282) and Hyaluronic acid (1 mg/ml; Sigma, # 53747). Cells fail to migrate from the explant when no ECM is present (Data not shown) and all substrates tested supported migration of the cells. Both the shape as well as the sizes of cells that migrate from the explants depends upon the substrate. This is expected as previous studies demonstrate that the mechanical forces generated by interaction of cells with different ECM affect both the shape and migratory properties of cells21.
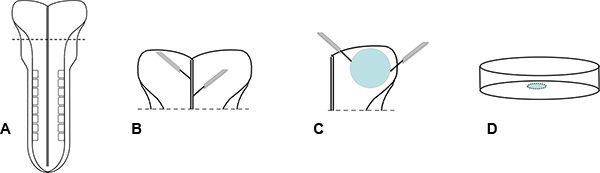 Figure 1. Preparation of explants. (A) To prepare explants, E8.5 embryos are dissected from deciduas and extraembryonic tissues. (B) Embryo heads are dissected from the body anterior to the rhombomeres. Explants are prepared by removing tissue from the midline and the outer edges of the head to remove the prechordal plate at the midline and the premigratory neural crest and surface ectoderm at the border, respectively. (C) Explants are prepared by cutting away tissue from the midline and outer edges of the head to remove the prechordal plate at the midline and the premigratory neural crest and surface ectoderm at the borders. (D) Explants are placed in a small volume of media on ECM coated dishes/coverslips and allowed to attach before the addition of more media.
Figure 1. Preparation of explants. (A) To prepare explants, E8.5 embryos are dissected from deciduas and extraembryonic tissues. (B) Embryo heads are dissected from the body anterior to the rhombomeres. Explants are prepared by removing tissue from the midline and the outer edges of the head to remove the prechordal plate at the midline and the premigratory neural crest and surface ectoderm at the border, respectively. (C) Explants are prepared by cutting away tissue from the midline and outer edges of the head to remove the prechordal plate at the midline and the premigratory neural crest and surface ectoderm at the borders. (D) Explants are placed in a small volume of media on ECM coated dishes/coverslips and allowed to attach before the addition of more media.
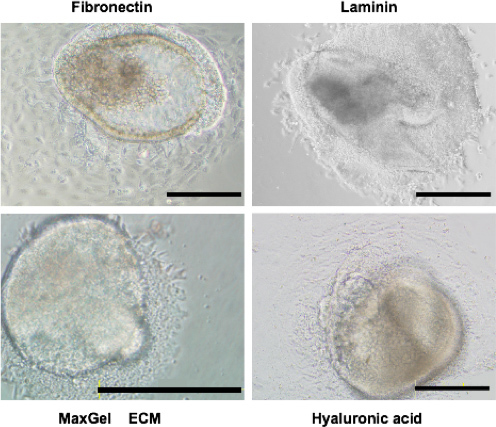 Figure 2. Migration of cranial mesenchyme cells from explants plated on different substrates. Explants were plated on Fibronectin, Laminin, MaxGel ECM or Hyaluronic acid and photographed after 48 hr in culture. Size bar = 200 mm.
Figure 2. Migration of cranial mesenchyme cells from explants plated on different substrates. Explants were plated on Fibronectin, Laminin, MaxGel ECM or Hyaluronic acid and photographed after 48 hr in culture. Size bar = 200 mm.
Discussion
The method applied here provides a powerful assay to examine the behavior of cranial mesenchyme cells. In addition to the static analyses presented here, live imaging experiments in bright field or in combination with expression of GFP-labeled proteins can be employed to examine the behaviors of cells in real time as they migrate from the explant. For live imaging experiments, explants could be labeled with DiI or to differentiate migration of neural crest from the paraxial mesoderm, ROSA-YFP;Mesp1-cre or ROSA-YFP;Wnt1-cre or other transgenic lines could be used. Cranial mesenchyme-ECM interactions can also be examined utilizing this explant assay. Here we plate explants on different ECM substrates that are present in the cranial mesenchyme to show that cells migrate on these to different degrees and that the ECM influences the morphology of the cells. Furthermore, explants can be embedded in a three-dimensional matrix to access the behaviors of cells in this context. This explant assay is amendable to analysis of the effect of genetic and pharmacological manipulations on cranial mesenchyme behaviors to analyze intrinsic and extrinsic cues that can regulate and modify migration. For example, we utilized this assay in our studies to discern the role of excess Hsp90 secretion in the abnormal cellular behaviors in Hectd1 mutant cranial mesenchyme15. In these experiments, we treated explants with anti-Hsp90 antibody, Hsp90 protein, geldanamycin and DMA (dimethyl amelioride) to block secretion of Hsp90 to demonstrate that the abnormal behavior of Hectd1 mutant cells is due to excess extracellular Hsp9015. Similar approaches could be used to pharmacologically dissect additional pathways underlying normal or abnormal morphogenesis of the cranial mesenchyme. Once the assay is completed, explants and migrating cells can be analyzed by a host of methods. For example, immunohistochemical analyses can be employed to examine localization of proteins critical for cell movements. Transcriptome analyses could also be employed to determine how treatments affect gene expression.
One significant limitation of this technique is that it does not model behaviors in three-dimensions as they would occur in vivo. Modification of the protocol where explants are embedded in a three-dimensional matrix (e.g. matrigel) along with expression of fluorescent protein-marked cellular compartments could be employed necessary to address this issue. Even if these modifications were performed, further experiments to correlate behaviors seen in this ex vivo assay with those in vivo would be necessary. Other limitations include the large number of embryos required to generate sufficient numbers to generate statically significant data. Most importantly, the dissection is relatively simple, it does require practice to master.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work is supported by R01-HD058629 to I.E.Z.
References
- Copp AJ. Neurulation in the cranial region--normal and abnormal. Journal of anatomy. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Gerrelli D, Greene ND, Copp AJ. Mechanisms of normal and abnormal neurulation: evidence from embryo culture studies. The International Journal of Developmental Biology. 1997;41:199–212. [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Tuckett F, Morriss-Kay GM. The distribution of fibronectin, laminin and entactin in the neurulating rat embryo studied by indirect immunofluorescence. J. Embryol. Exp. Morphol. 1986;94:95–112. [PubMed] [Google Scholar]
- Tuckett F, Morriss-Kay G. Alcian blue staining of glycosaminoglycans in embryonic material: effect of different fixatives. The Histochemical Journal. 1988;20:174–182. doi: 10.1007/BF01746681. [DOI] [PubMed] [Google Scholar]
- Morris-Wiman J, Brinkley LL. The role of the mesenchyme in mouse neural fold elevation. II. Patterns of hyaluronate synthesis and distribution in embryos developing in vitro. The American Journal of Anatomy. 1990;188:133–147. doi: 10.1002/aja.1001880204. [DOI] [PubMed] [Google Scholar]
- Morris-Wiman J, Brinkley LL. The role of the mesenchyme in mouse neural fold elevation. I. Patterns of mesenchymal cell distribution and proliferation in embryos developing in vitro. The American Journal of Anatomy. 1990;188:121–132. doi: 10.1002/aja.1001880203. [DOI] [PubMed] [Google Scholar]
- Morris-Wiman J, Brinkley LL. Changes in mesenchymal cell and hyaluronate distribution correlate with in vivo elevation of the mouse mesencephalic neural folds. Anat. Rec. 1990;226:383–395. doi: 10.1002/ar.1092260316. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech. Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. Journal of anatomy. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Tuckett F, Solursh M. The effects of Streptomyces hyaluronidase on tissue organization and cell cycle time in rat embryos. J. Embryol. Exp. Morphol. 1986;98:59–70. [PubMed] [Google Scholar]
- Morriss-Kay G, Tuckett F. Immunohistochemical localisation of chondroitin sulphate proteoglycans and the effects of chondroitinase ABC in 9- to 11-day rat embryos. Development. 1989. pp. 106–787. [DOI] [PubMed]
- Tuckett F, Morriss-Kay GM. Heparitinase treatment of rat embryos during cranial neurulation. Anat. Embryol. (Berl) 1989;180:393–400. doi: 10.1007/BF00311170. [DOI] [PubMed] [Google Scholar]
- Sarkar AA, Zohn IE. Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior of the cranial mesenchyme. The Journal of Cell Biology. 2012;196:789–800. doi: 10.1083/jcb.201105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev. Biol. 2007;306:208–221. doi: 10.1016/j.ydbio.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Tzahor E, et al. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103:155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- Newgreen D. Spreading of explants of embryonic chick mesenchymes and epithelia on fibronectin and laminin. Cell and tissue Research. 1984;236:265–277. doi: 10.1007/BF00214227. [DOI] [PubMed] [Google Scholar]
- Peyton SR, Ghajar CM, Khatiwala CB, Putnam AJ. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochemistry and Biophysics. 2007;47:300–320. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]


