Abstract
Objective
The stress-vulnerability model of addiction predicts that environmental factors, such as cumulative stress, will result in individual adaptations that decrease self-control, increase impulsivity, and increase risk for addiction. Impulsivity and cumulative stress are risk factors for tobacco smoking that are rarely examined simultaneously in research.
Methods
We examined the indirect and direct effects of cumulative adversity in a community sample consisting of 291 men and women who participated in an assessment of cumulative stress, self-reported impulsivity, and smoking history. Data were analyzed using bootstrapping techniques to estimate indirect effects of stress on smoking via impulsivity.
Results
Cumulative adversity is associated with smoking status via direct effects and indirect effects through impulsivity scores. Additional models examining specific types of stress indicate contributions of traumatic stress and recent life events as well as chronic relationship stressors.
Conclusions
Overall, cumulative stress is associated with increased risk of smoking via increased impulsivity and via pathways independent of impulsivity. These findings support the stress-vulnerability model and highlight the utility of mediation models in assessing how, and for whom, cumulative stress increases risk of current cigarette smoking. Increasing self-control is a target for interventions with individuals who have experienced cumulative adversity.
Keywords: cumulative stress, smoking, tobacco, impulsivity, adversity
INTRODUCTION
Cigarette smoking is one of the leading causes of preventable death and, according to recent research, is a modifiable and preventable risk factor that has a considerable negative impact on life expectancy (Danaei et al. 2010, Lantz et al. 2010). Public health interventions that focused on implementing tobacco control measures and altering public policy have successfully decreased the prevalence of tobacco use from 36% in 1997 to 22% in 2003. However, since 2003, the prevalence rates have remained between 20% and 22% (CDC, 2009). Given these findings, there is a need for research that examines key factors that contribute to current smoking or the maintenance of smoking behavior in community individuals who are not treatment-seeking. Identified risk factors for smoking range across environmental and biological factors such as genetic vulnerability and family history (Colamussi et al., 2007; Hu et al., 2006; Johnson et al., in press; Perkins, Coddington, et al., 2009). Cumulative adversity or the adverse social and psychological factors that accumulate over a person’s lifetime (Turner et al., 1995; Thoits, 2010) is an environmental factor that has been associated with increased risk for addictive behaviors and poor health outcomes (Dube et al., 2003; Turrell et al., 2007; Sinha, 2008;). Stress has been frequently examined in the literature as a risk factor for smoking (Wills et al., 2002, Lloyd, Taylor 2006, Koval, Pederson 1999). However, understanding how, and for whom, an environmental risk factor such as stress increases smoking behaviors is a needed avenue of further research.
Cumulative stress has been conceptualized as a risk factor that can increase individual vulnerability for a wide range of addictive behaviors (Sinha 2008). This vulnerability is typically conceived of as a complex, reciprocal process in which a stressful life event exerts demands on the individual, neural processes evaluate the demand and assess the availability of adaptive resources, and the individual exhibits behavioral, cognitive, and physiological adaptations in response to the stressor (Sinha 2008, Sinha 2001). Thus, a stressor activates an adaptive stress response in the organism as attempts are made to regain homeostasis (McEwen, 2002; 2007). As the stressor becomes prolonged, repeated, or chronic, the greater the magnitude of the resulting stress response in the organism and the greater the risk for persistent homeostatic dysregulation. The accumulating physiological response to stressors may subsequently alter brain motivational pathways (such as the medial prefrontal cortex), which enable the individual to exert self-control and inhibition of impulses (Anestis et al., 2007; Arnsten and Goldman-Rakic, 1998; Fishbein et al., 2006; Hatzinger et al., 2007; Mischel, 1996; Sinha, 2008; Verdejo-Garcia et al., 2007). Shifts in individual differences related to self-control may occur as a result of this cumulative stress experience, and these shifts may increase vulnerability for engagement in addictive behaviors in the face of future stressors. The stress-vulnerability model may be particularly important in understanding risk factors for smoking behaviors. Self-control, or impulsivity, represents a commonly investigated individual difference that is associated with both cumulative stress and addiction, particularly smoking. Self-control pathways may be susceptible to individual adaptations to stress that alter subsequent physiological response and reward sensitivity when future stress is encountered (Sinha 2008).
A longitudinal study on the lifetime cumulative stress and smoking habits in young adults found that distal stressors (those greater than 2 years prior to the onset of smoking) and proximal stressors (those less than 2 years) were independent predictors of heavy smoking onset (Lloyd and Taylor, 2006). In other words, the effect of distal stressors on heavy smoking onset was not explained by the effect of distal stressors increasing proximal stressors that increased the probability of heavy smoking onset. This finding was independent from the presence of lifetime psychiatric or substance-dependence disorders. Together, these findings highlight the importance of cumulative stress in the onset of smoking habits. These data are consistent with temporal examinations of stress and smoking frequency that indicate that recent stress experiences increased smoking behaviors; however, smoking behaviors did not increase the experience of subsequent stressful events (Wills et al., 2002). Despite considerable research on stress and tobacco, the vulnerability hypothesis suggests that it is the cumulative load of stress over the course of the lifetime that predisposes an individual to alterations, such as increased impulsivity, that may subsequently increase the risk for ongoing tobacco use. Rarely have comprehensive measures of cumulative adversity and stress examined risk for tobacco use or other health behaviors (Thoits, 2010), and it appears none have examined the potential mediating role of impulsivity in this relationship.
In support of this vulnerability hypothesis, impulsivity is a well-established correlate of smoking and risk factor for cessation failure (Doran et al., 2007a; Doran et al., 2007b; Krishnan-Sarin et al., 2007; Reynolds et al., 2007; Doran et al., 2009). As a recent factor analysis of behavior and self-report assessments confirms (Meda et al. 2009), impulsivity is a multifactorial construct with divergent assessment forms and methods. At-risk addiction groups differ from normals in self-reported impulsivity, supporting assertions that it is a vulnerability factor for a wide range of addictive behaviors, particularly smoking cessation failure (Krishnan-Sarin et al. 2007, Reynolds et al. 2007, Meda et al. 2009). Self-reported impulsivity is also associated with both structural and functional neuro-adaptations within the prefrontal cortex (Spinella 2004), the same area discussed previously in which stress induced changes have been found. Therefore, self-reported impulsivity represents a potential mediator of stress and smoking that has not previously been examined. Further understanding of the complex nature with which stress and impulsivity may influence one another with regards to smoking risk is needed to understand for whom environmental factors such as stress may be influencing individual difference risk factors such as self-control/impulsivity.
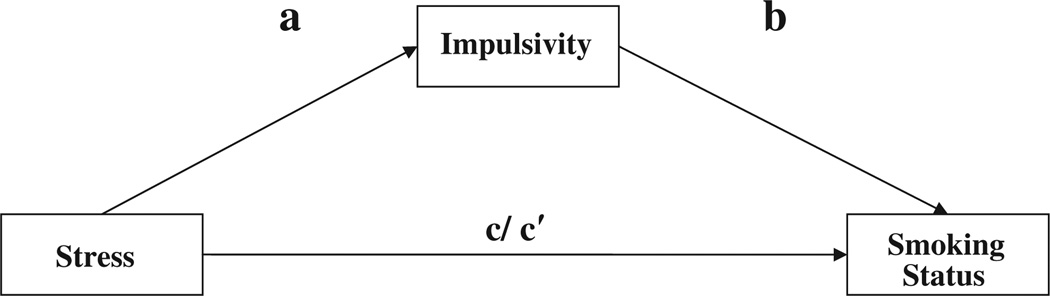
The stress-vulnerability model predicts that environmental factors such as cumulative stress events will increase risk for addiction as well as decrease behavioral control (increased impulsivity). In addition, the stress-vulnerability model hypothesizes that stress will indirectly increase risk for addiction via decreased behavioral control. Given that any smoking behavior is associated with detrimental health outcomes (Tverdal and Bjartveit, 2006; Wong et al., 2010), it is important to consider how a measure of cumulative adversity and stress over the course of the lifespan is associated with risk for continued smoking (Thoits, 2010) and whether individual differences in impulsivity are associated with smoking status when examining a community, non treatment-seeking sample. Applying the stress-vulnerability model to smoking status, we hypothesized that stress would have an indirect effect, via impulsivity, on smoking status as depicted in Figure 1. This hypothesis is supported by recent findings that delay discounting, a behavioral measure of impulsivity, mediated perceived stress effects on cigarette smoking status in adolescents (Fields et al. 2009). Because of the broad nature of the cumulative stress measure, it is also informative to examine what types of stress are associated with smoking status. Consistent with the limbic and striatal processes articulated within the stress-vulnerability model, trauma that occurs during specific neurodevelopmental periods may have particularly salient effects on reward sensitivity (Sinha 2008, Sinha 2001). Therefore, we also hypothesized that specific forms of stress, particularly early traumas, would have a direct effect on smoking status (e.g., when controlling for impulsivity).
Figure 1.
Theoretical mediation model of stress, impulsivity, and smoking status
In this study, we examined the indirect, or statistical mediation, effects of cumulative stress on smoking status by self-reported impulsivity in a community sample of adult men and women. Mediation is a complex phenomenon in which a variety of factors are necessary to establish a variable as a mechanism of change (Nock 2007). These factors can be assessed through experimental manipulation of variables, longitudinal examinations of change, and through statistical associations. The latter approach may be implemented within a cross-sectional sample that retrospectively assesses stressful life events and statically examines whether the associations in a cross-sectional sample support theoretically predicted relationships. Although the cross-sectional design does not allow for longitudinal course to be examined, other studies have examined and identified a longitudinal course association between stress and smoking and between impulsivity and smoking behaviors and found significant associations. Therefore, it is reasonable to model the retrospective report of stressful life events over the course of the lifespan as measured in the present study.
Our use of a comprehensive stress interview expands upon prior research to quantify the occurrence of explicit stressful events throughout the lifespan in the context of vulnerability factors such as impulsivity. This may be particularly important in understanding how and for whom cumulative stress experiences increase vulnerability for individual adaptations that mediate stress effects on risk for cigarette smoking.
METHODS
Participants
Recruitment and eligibility
Two hundred and ninety-one individuals, 132 men and 159 women, were drawn from a community sample in and around the New Haven area and recruited via advertisements placed either online or in local newspapers and community center. Eligibility was ascertained via an initial phone screen. All participants were required to be between the ages of 18 and 50 years and able to read and write in English to at least a sixth grade level as assessed via self-report. Exclusion criteria included DSM-IV dependence for any drug other than alcohol or nicotine. Participants using prescribed medications for any psychiatric or medical disorders were also excluded, and all individuals underwent stringent medical assessments including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid function. Any individuals with acute medical conditions requiring immediate medical intervention were excluded from the study. All participants gave both written and verbal informed consent, and the study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedure
Potential subjects completed an initial screening over the telephone to determine eligibility on the basis of inclusion/exclusion criteria. Following initial eligibility, screening participants were scheduled for two 2-h assessment and evaluation sessions. During the first session, participants met with research assistants in order to complete informed consent forms for both the current and subsequent studies as well as complete medical, substance abuse, and psychiatric health assessments including the Cornell Medical Index, the Structured Clinical Interview for the Diagnostic and Statistical Manual (First et al. 1996), and a physical health assessment. During a subsequent intake session, participants again met with the same research assistant in order to complete baseline demographic questionnaires, smoking status, stress (Cumulative Adversity Interview, CAI), and impulsivity (Barratt Impulsiveness Scale, BIS-11). Following this, participants received a physical examination with a research nurse specialist assessing cardiovascular, renal, hepatic, pancreatic, hematopoietic, and thyroid function in order to ensure all participants were in good health. During both appointments, subjects underwent measurement of breath carbon monoxide (CO) levels and urine toxicology screens in order to confirm self-report of alcohol and drug information.
Assessments
Barratt Impulsiveness Scale (BIS-11; Patton, Stanford and Barratt 1995): The BIS-11 is a 30-item self-report questionnaire that provides a total score of general impulsivity. The BIS-11 is widely used in both research and clinical settings and is highly associated with many clinical disorders characterized by impulsive behavior. These include substance abuse (Lejuez et al. 2007, Lane et al. 2007), borderline personality disorder (Fossati et al., 2004), and eating disorders (Nasser et al., 2004). The scale has also been associated with both structural and functional neuro-adaptations within the prefrontal cortex (Spinella 2004). The BIS shows good test–retest reliability and demonstrates high convergent validity with similar self-report measures including the Zuckerman Sensation-seeking Scale, the Eysenck Impulsiveness Scale, and the Behavioral Inhibition/Activation Scales (Stanford et al. 2009). The BIS-11 uses a four-point Likert scale ranging from 1 (rarely/never) to 4 (almost always). Higher scores indicate higher levels of impulsiveness. Scores between 52 and 72 are within normal impulsivity limits (Stanford et al. 2009).
The Cumulative Adversity Interview (Turner and Wheaton, 1995): This 140-item event interview is a comprehensive measure of cumulative adversity and chronic stress that covers major life events, life trauma, and chronic stressors. Recent life events are also included. Recent life events: this section comprises a checklist of 33 items referring to discreet stressful events occurring in the previous 12 months. These are broadly divided into items referring to exits from the social field (i.e., death, divorce, relationships ending) and undesirable events both interpersonal and financial (i.e., being attacked, financial crises, robberies). Major life events: this section includes 11 items relating to social adversities, not typically violent in nature, but that differ from standard life events because of their severity and potentially long-term consequences (Turner, Lloyd 2003). Examples of items are parental divorce and failing a grade in school. Life traumas: this section comprises 34 items relating to life trauma, witnessed violence, and traumatic news. Life trauma includes events that imply force or coercion and include physical, emotional, and/or sexual abuse, including rape and being injured with a weapon. Witnessed violence contains items that involve being present in dangerous or upsetting situations, such as seeing some get shot or attacked with a weapon. Traumatic news is comprised of items that involve not being present but rather hearing news about someone else being killed, abused, or injured. Chronic stressors: this section comprises 62 items relating to the subjective experience of perceived or continuous stressors. Items refer to longer-term interpersonal, social, and financial relationships and responsibilities including work and home environment and relationships with family and significant others. Total scale: a total scale score was computed by standardizing each subscale and summing the scores. This approach ensured that each category of events was weighted equally in the final score. In all cases, a higher score relates to a higher number of stressful events.
Cigarette smoking status was assessed through self-report in an interview and verified via breath CO levels greater than 10 ppm. If CO levels were between 6 to 10 mg/kg, urine cotinine levels were assessed to verify smoking status. Smoking status was coded as present or absent (1,0).
Analyses
In order to test the proposed mediation model (Figure 1), ordinary least squares (OLS) and logistic hierarchical regressions were employed to test a, b, c, and c′ pathways. The a pathway represents unstandardized beta from the OLS regression of stress scale on the mediator, BIS-11 score. The b pathway represents the standardized coefficient from the logistic regression of the mediator, BIS-11, on smoking status, controlling for stress scale score. The ab pathway represents the effect of stress on smoking status via the effect of BIS-11. This pathway is also termed the indirect effect of stress on smoking status. The c pathway represents the logistic regression of the stress scale on smoking status without BIS-11 in the model. The c′ pathway represents the logistic regression of the stress scale on smoking status with the effects of BIS-11-controlled. The c′ pathway is also called the direct effect of stress on smoking status as it represents the effects of stress on smoking independent of impulsivity. Models were run and odds ratios (ORs) computed for total CAI and each of the 4 subscales (life trauma, major life events, recent life events, and chronic stressors) with BIS-11 as the mediating variable and smoking status as the dependent variable. To test the significance of the indirect effects of stress scale (or subscales) on smoking status via BIS-11 impulsivity, we employed the approach by Preacher and Hayes (2004) using the SPSS INDIRECT bootstrapping macro. As indirect effects do not meet normal assumptions for statistical analysis, bootstrapping was used to estimate the significance of the indirect effects. Bias corrected and accelerated 95% confidence intervals (CI) were computed using 10 000 bootstrapped resamples for each indirect effect point estimate. CIs that do not contain a zero value indicate a significant indirect effect. For interpretability and consistency with recommendations in the literature, we report unstandardized OLS coefficients and standardized coefficients for logistic regression (ORs) where applicable. (MacKinnon and Dwyer, 1993).
RESULTS
The sample demographics and the mean scores for the scales are displayed in Table 1. Participants were on average aged 31 years, 45% men, and 66% Caucasian (22% African American, 4% Hispanic). They had on average almost 3 years post high school education, and 29% were current smokers, and 20% of the sample were daily smokers. Approximately 12% of the subjects met criteria for a lifetime diagnosis of alcohol dependence.
Table 1.
Demographics of sample
| Subject variable | N = 291 |
|---|---|
| Agea | 31.00 (9.51) |
| Years of education a | 14.92 (2.27) |
| Gender—% male | 132.00 (45.36%) |
| Race | |
| African American (N% AA) | 66.00 (22.68%) |
| Caucasian (N% Caucasian) | 193.00 (66.32%) |
| Hispanic (N% Hispanic) | 13.00 (4.47%) |
| Asian (N% Asian) | 6.00 (2.06%) |
| Other (N% Other) | 13.00 (4.47%) |
| Marital status | |
| Never married (%) | 200.00 (68.73%) |
| Married (%) | 58.00 (19.93%) |
| Separated (%) | 0.00 (0.00%) |
| Divorced (%) | 29.00 (9.97%) |
| Cohabitating (%) | 4.00 (1.37%) |
| Widowed (%) | 0.00 (0.00%) |
| Total impulsiveness a | 61.63 (11.66) |
| Total CAIa | 0.00 (2.96) |
| Major life events a | 2.17 (1.83) |
| Life traumas a | 6.50 (4.56) |
| Recent life events a | 3.11 (2.63) |
| Chronic stressors a | 10.56 (6.52) |
| Relationship a b | 2.54 (2.94) |
CAI, Cumulative Adversity Interview.
CAI total = standardized major life events + standardized life traumas + standardized recent life events.
Denotes mean values (standard deviations); all other measures reported in frequency (percents).
N = 171.
The results for the mediation model examining the total cumulative stress score, BIS-11 impulsivity, and smoking status are presented in Table 2. Cumulative stress was significantly positively associated with self-reported impulsivity (t = 4.76, b = 1.06, p < 0.001), and BIS-11 self-reported impulsivity was significantly associated with a greater likelihood of being a current smoker (Wald χ2 = 13.57, OR = 1.03, p < 0.001). The OR for impulsivity indicates that a one point increase in BIS-11 score is associated with a 3% increase in the probability that an individual currently smokes. The indirect effect for cumulative stress total score on smoking status via BIS-11 was also significant (a × b = 0.05, 95% CI = 0.02–0.09, p < 0.05), supporting a statistical mediation effect such that greater stress is associated with greater impulsivity that is associated with increased likelihood of current smoking status. The initial effect of cumulative stress total score was significant (Wald χ2 = 17.95, OR = 1.21, p < 0.001), and the direct effect, which controls for BIS-11 score, was also significant (Wald χ2 = 10.08, OR = 1.16, p = 0.002). This indicates that the effects of cumulative stress were not fully mediated by self-reported impulsivity score. The OR for the direct effect of cumulative stress total score indicates that a one point increase in standardized cumulative stress total score was associated with a 16% increase in the probability that an individual currently smokes (see Table 2). Mediation models were subsequently examined for each of the cumulative adversity interview subscales.
Table 2.
Summary of mediation by self-reported impulsivity (BIS-11) on smoking status (10 000 bootstrap samples)
| Independent variable | Effect of IV on M (a)b | Effect of M on DV (b)c | Total effect (c)c | Direct effect (c′)c | Indirect effect (a × b) (95% CI) | |
|---|---|---|---|---|---|---|
| 1. | CAI total | 1.06* | 1.03* | 1.21* | 1.16* | 0.05* (0.02–0.09) |
| 2. | CAI totala | 0.90* | 1.05* | 1.12* | 1.08 | 0.04* (0.01–0.08) |
| 3. | Trauma | 0.63* | 1.05* | 1.17* | 1.14* | 0.03* (0.01–0.05) |
| 4. | Major life events | 0.88* | 1.06* | 1.20* | 1.16* | 0.05*(0.01–0.10) |
| 5. | Recent life events | 0.85* | 1.05* | 1.15* | 1.10 | 0.04* (0.02–0.09) |
| 6. | Chronic stressors | 0.40* | 1.05* | 1.05* | 1.03 | 0.02* (0.01–0.04) |
| 7. | Relationship stress | 0.70* | 1.04* | 1.20* | 1.18* | 0.02 (0.00–0.07) |
IV, independent variable; DV, dependent variable; CAI, Cumulative Adversity Interview; M, mediator, self-reported impulsivity as measured by the BIS-11; CI, confidence interval.
Model includes two covariates: years of education and alcohol dependence.
Unstandardized ordinary least squares regression coefficients.
Odds ratios from logistic regression.
p < 0.05.
Life trauma was significantly positively associated with impulsivity (t = 4.31, b = 0.63, p < 0.001), and impulsivity was significantly associated with greater likelihood of current smoking (Wald χ2 = 12.60, OR = 1.05, p < 0.001). ORs for impulsivity indicate that a one point increase in BIS-11 score was associated with a 5% increase in the probability that an individual smokes. The indirect effect for life trauma on smoking status via impulsivity was significant (a × b = 0.03, 95% CI = 0.01–0.05, p < 0.05), supporting the significance of the indirect effect of trauma on smoking status via greater impulsivity. However, the direct effect of life trauma on smoking status was also significant (Wald χ2 = 15.58, OR = 1.14, p < 0.001), indicating that trauma was not fully mediated by impulsivity. ORs indicate that a one point increase in trauma score, in this case the occurrence of a single additional traumatic event, was associated with an increased likelihood of an individual smoking by 14%.
Major life events were significantly associated with greater impulsivity (t = 2.37, b = 0.88, p = 0.02), and impulsivity was associated with greater likelihood of current smoking status (Wald χ2 = 18.86, OR = 1.06, p < 0.001. The OR for impulsivity indicates that a one point increase in BIS-11 score was associated with a 6% increase in the probability that an individual currently smokes. The indirect effect was significant (a × b = 0.05, 95% CI = 0.01–0.10, p < 0.05), supporting the partial mediation of major life events stress on smoking status via higher impulsivity. However, the significant direct effect (Wald χ2 = 4.04, OR = 1.16, p < 0.04) indicates that major life events stress had an effect on smoking status independent of self-reported impulsivity. The OR indicates that a one point increase in the major life events scale, or the occurrence of one major life event, was associated with a 16% increase in the probability that an individual smokes.
Recent life events were significantly associated with higher self-reported impulsivity (t = 3.31, b = 0.85, p = 0.001), and impulsivity was significantly associated with current smoking status (Wald χ2 = 17.57, OR = 1.05, p < 0.001). The OR for impulsivity indicates that a one point increase in BIS-11 scores was associated with a 5% increase in the probability that an individual smokes. The indirect effect was significant (a × b = 0.04, 95% CI = 0.02–0.09, p < 0.05), supporting the significance of an indirect effect of recent life events on smoking status via higher impulsivity. Although the initial effect of recent life events on smoking status was significant (Wald χ2 = 7.83, OR = 1.15, p = 0.005), the direct effect of recent life events on smoking status, when impulsivity was controlled for, was no longer significant (Wald χ2 = 3.74, OR = 1.10, p = 0.05). This indicates that recent life events stress was fully statistically mediated by self-reported impulsivity.
Chronic stress, or the subjective experience of difficult ongoing conditions of daily life, was significantly positively associated with impulsivity (t = 3.91, b = 0.40, p < 0.001), and impulsivity was associated with current smoking status (Wald χ2 = 17.91, OR = 1.05, p < 0.001). The OR for impulsivity indicates that a one point increase in BIS-11 was associated with a 5% increase in the probability that an individual smokes. The indirect effect was also significant (a × b = 0.02, 95% CI = 0.01–0.04, p < 0.05), indicating that chronic stress was mediated by increased impulsivity in its association with smoking status. Although the initial association of chronic stress was significantly associated with smoking status (Wald χ2 = 5.99, OR = 1.05, p = 0.01), the direct effect was not significant (Wald χ2 = 2.19, OR = 1.03, p = 0.14). This supports the full statistical mediation of chronic stress effects on smoking status via impulsivity.
Given that the chronic stress scale has considerable heterogeneity in content but the primary focus of the scale and its effects are the daily social and relationships strains (Turner et al., 1995), we performed a follow-up analysis on the chronic stress scale in which the domain of “chronic relationship stress” was examined within the mediation model. The results of this model, using the 171 subjects who reported currently being involved with a significant other, are presented in Table 2. Although chronic relationship stress was significantly positively associated with impulsivity (t = 2.41, b = 0.70, p = 0.02) and impulsivity was significantly associated with current smoking status (Wald χ2 = 4.40, OR = 1.04, p = 0.04), the indirect effect of chronic relationship stress was not significant (a × b = 0.02, 95% CI = 0.00–0.07). However, the direct effect of chronic relationship stress on smoking status, controlling for self-reported impulsivity, was significant (Wald χ2 = 8.16, OR = 1.18, p = 0.004). The OR indicates that a one point increase, or the occurrence of one additional relationship stressor, was associated with an 18% increase in the probability that an individual currently smokes.
Prior research has indicated that demographic variables may play a role in the mediation of stress by impulsivity (Fields et al., 2009). In a post hoc analysis, we examined whether age, sex, years of education, or lifetime history of alcohol dependence better accounted for these findings. Age and sex did not significantly contribute to the model (X2 = 2.23, p = 0.33). However, years of education (X2 = 27.23, p < 0.01) and alcohol dependence (X2 = 19.63, p < 0.01) did significantly contribute to the model. Therefore, the model was rerun with these variables as covariates and is presented in line two of Table 2. The indirect effect of stress on smoking status via impulsivity remained significant (path ab = 0.04, 95% CI = 0.01–0.08, p < 0.05) with education and alcohol dependence in the model. The only change was a reduction of the direct effect of stress on smoking status to nonsignificance. This finding is understandable given that both years of education and alcohol dependence are strongly associated with cumulative stress and history of adversity. Overall, these findings suggest the robust nature of the stress-vulnerability model with regards to mediation by impulsivity.
There is an additional question of the directionality of the proposed mediation model. Nicotine is believed to induce alterations in neurobiology that disposes individuals to future impulsive behaviors. In post hoc analysis, we examined whether cumulative stress may be mediated by smoking status in predicting impulsivity. This model was also viable, and the indirect effect was significant (a × b = 0.24; p < 0.05). The direct effect of stress on impulsivity remained; however, when controlling for smoking status (path c′: t = 3.66, b = 0.83, p = 0.003). This suggests that the effects of stress on impulsivity were not wholly accounted for by nicotine-induced adaptations in smokers.
DISCUSSION
The results of the tested stress-vulnerability models support the significance of both indirect effects, via self-reported impulsivity, and direct effects of cumulative stress and adversity on current smoking status in adults. Across models, the presence of greater cumulative stress was associated with higher levels of self-reported impulsivity. This greater impulsivity was associated with a greater likelihood of current smoking status. The meaningfulness of these findings is highlighted in the direct effect OR for cumulative stress. The cumulative load of one additional point on the standardized total adversity score was associated with a 16% increase in the probability that an individual smoked, independent of self-reported impulsivity.
In addition to cumulative stress, subscales for life trauma and major life events were associated with significant indirect and direct effects on smoking status. Scales of more recent stressors such as recent life events and chronic stressors were associated with significant indirect, but not significant direct, effects on smoking status. Interestingly, chronic relationship stressors were associated with a significant direct effect, but not a significant indirect effect, on smoking status.
These findings support the assertions of the stress-vulnerability theory (Sinha 2008) and highlight the possible influence of cumulative stress in manifesting individual differences such as impulsivity. The direct and indirect pathways may reflect different pathways of stress effects on reward sensitivity and long-lasting effects on behavioral control. Effects of stress in early development, particularly stress related to trauma, are known to affect brain development and alter stress and reward sensitivity (Sinha 2008; Meaney et al., 2002) and maturation of the prefrontal self-control systems (Gratton and Sullivan, 2011, Pruessner et al. 2004). These effects are known to have lasting behavioral, physiological, and neuroendocrine effects on subsequent stressors and reward sensitivity, thereby increasing vulnerability for addictive behaviors. In addition, these early stressors may result in individual adaptations to stressful circumstances with negative implications for stress regulation, especially in prefrontal, executive cognitive functioning, and impulse control systems. This combined impact may represent the direct (alterations in limbic and striatal pathways) and indirect (prefrontal and self-control systems) effects observed in the cumulative adversity and distal (trauma and major life events) stress scales. In contrast, more recent life events and chronic stressors may exacerbate vulnerability for addiction via self-control or indirect effects.
These findings also highlight the importance of examining mediation models in order to increase understanding of how, and for whom, cumulative stress increases risk for current cigarette smoking. Consistent with Lloyd and Turner’s (2006) findings, cumulative stress and adversity is an important predictor of increased risk for smoking. These findings extend the findings by Fields and colleagues (Fields et al. 2009) who found full mediation of the perceived stress and smoking status relationship by delay discounting. In our findings, self-reported impulsivity partially mediated the effects of cumulative stress on smoking status. When subscales of cumulative stress were examined, the present findings suggest that more distal stress events, such as trauma and major life events, are only partially mediated by impulsivity, whereas more recent stressors, such as recent life events and chronic stressors, are fully mediated by impulsivity. Fields et al. (2009) used the Perceived Stress Scale, which assesses the self-reported level of stress appraisal within the last month. This time frame is more similar to the chronic stress scale or recent life events, both of which were also fully mediated by impulsivity. It may be that the effects of very recent stress and impulsivity are more complex and potentially reciprocal in their effects on one another. In addition, unlike the analysis of Fields et al. (2009), mediation by impulsivity remained significant when controlling for demographic variables.
Future research will need to examine longitudinal methods in the assessment of cumulative and recent stress in association with impulsive behaviors to better ascertain the relationship captured within the indirect effect of the model. In addition, future research should examine these mediation models using alternative conceptualizations of impulsivity, including delay discounting, as mediators of cumulative stress and adversity.
An unexpected finding resulting from post hoc analyses of the chronic stress scale was the unique role that chronic relationship stress may play in promoting addiction. Relationship stress, unlike the other recent stressor scales, was associated with direct, but not indirect, effects on smoking status. An increase of one relationship stressor increased the probability of an individual being a smoker by 18%, independent of impulsivity. The salience of interpersonal stress as a pathway for ongoing effects of cumulative adversity on daily life was previously speculated as a vulnerability factor for psychopathology by the authors of the cumulative stress measure (Turner et al., 1995). In reference to effects from chronic relationship stressors, they stated “for example, being abused by a parent or losing a parent during childhood may increase risk of interpersonal difficulties and hence relationship strains in adulthood” (p. 112). The possible effects of chronic interpersonal stress on subsequent vulnerability for addiction are also supported by human and animal models in which early maternal separation, social isolation, and social defeat stress in animals and childhood maltreatment in humans affect neurobiological pathways and vulnerability for addiction (Braun et al. 2000, De Bellis 2002, De Bellis 2005). Future research is needed to explore the specific impacts of cumulative interpersonal stressors on addictive behaviors as the present findings suggests that these stressors may have differential, or more homogenous, effects on addiction when examined alone. In addition, future research should clarify the potentially reciprocal influence of cumulative stress on the occurrence of chronic interpersonal stress and how these two factors may influence the maintenance of addictive behaviors.
Overall, these findings extend prior research that has established the effect of stress and the effect of impulsivity on cigarette smoking by examining a statistical mediation model of cumulative stress and adversity and self-reported impulsivity on smoking status. These findings support the stress-vulnerability theory and emphasize the role that cumulative stress and adversity may play in the development of individual differences in risk factors such as impulsivity for smoking. Cumulative adversity and stress is generally not a modifiable risk factor. However, understanding that some of this risk may be conveyed via loss of self-control represents a pathway that can be targeted for intervention in individuals who have experienced significant cumulative adversity. It also emphasizes the importance in examining cumulative stress and adversity as experienced over the lifespan when developing and testing models of how and for whom stress affects smoking. These findings do not preclude the examination of impulsivity as a moderator of chronic stressors on smoking behaviors nor do these findings preclude the reciprocal impact of smoking on individual differences in impulsivity. Indeed, our analysis suggests that stress may increase impulsivity via tobacco’s effects. This may be due to alterations in neurobiology and cognitive flexibility associated with chronic smoking (Durazzo et al., 2010). However, it is important to note that smoking did not fully mediate the association between cumulative stress and impulsivity, suggesting that the complex relationships between these variables require further longitudinal examinations. For example, additional factors, such as genetic variations that were not examined within this model, may contribute to smoking risk.
One limitation in considering the generalizability of these findings is that this was a nontreatment-seeking community sample of individuals who were not currently taking any psychiatric medications and did not meet for dependence on any other substances besides alcohol or tobacco. Although some individuals had alcohol use disorders, these models may not represent the effects found in other samples seeking treatment for substance use disorders. A key limitation of the current findings is the cross-sectional nature of the sample. The causal mechanisms of change need to be further examined within a longitudinal framework, particularly the indirect pathway of impulsivity on smoking. This cross-sectional analysis provides incremental evidence that history of cumulative adversity is directly and indirectly associated with current smoking. Given the current positive findings of cumulative stress and impulsivity on current smoking, future longitudinal studies that assess the effects of both cumulative stress and impulsivity on development and maintenance of smoking behavior are warranted.
ACKNOWLEDGEMENTS
Funding was provided by NIH grants UL1-DE019586 (Sinha), PL1-DA024859 (Sinha), K08-DA029641(Ansell), and the NIH Roadmap for Medical Research Common Fund. NIH had no further role in study design; in the collection, analysis, or interpretation of the data; in the writing of the report or in the decision to submit the paper for publication. R.S. is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo-Smith Kline Pharmaceuticals.
Footnotes
CONFLICT OF INTEREST
No conflict of interest declared.
REFERENCES
- Anestis MD, Selby EA, Joiner TE. The role of urgency in maladaptive behaviors. Behavior Research and Therapy. 2007;45:3018–3029. doi: 10.1016/j.brat.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Archives of General Psychiatry. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 2000;95(1):309–318. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress-and cue-induced cigarette craving: effects of a family history of smoking. Drug and Alcohol Dependence. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJ, Ezzati M. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Med. 2010;7(3):e1000248. doi: 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27(1–2):155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10(2):150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- Doran N, McChargue D, Cohen L. Impulsivity and the reinforcing value of cigarette smoking. Addict Behav. 2007a;32(1):90–98. doi: 10.1016/j.addbeh.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology. 2007b;194(2):279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology. 2009;207(3):365–373. doi: 10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7(10):3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prevention Medicine. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Fields S, Leraas K, Collins C, Reynolds B. Delay discounting as a mediator of the relationship between perceived stress and cigarette smoking status in adolescents. Behav Pharmacol. 2009;20(5–6):455–460. doi: 10.1097/FBP.0b013e328330dcff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Herman-Stahl M, Eldreth D, et al. Mediators of the stress-substance-use relationship in urban male adolescents. Prevention Science. 2006;7:113–126. doi: 10.1007/s11121-006-0027-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Version (SCID-I/P) New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- Fossati A, Barratt ES, Carretta I, Leonardi B, Grazioli F, Maffei C. Predicting borderline and antisocial personality disorder features in nonclinical subjects using measures of impulsivity and aggressiveness. Psychiatry Research. 2004;125:161–170. doi: 10.1016/j.psychres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gratton A, Sullivan RM. Role of prefrontal cortex in stress responsivity. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Dusseldorf: Elsevier; 2011. p. 838. [Google Scholar]
- Hatzinger M, Brand S, Perren S, von Wyl A, von Klitzing K, Holsboer- Trachsler E. Hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: importance of gender and associations with behavioral/emotional dif.culties. Journal of Psychiatric Research. 2007;41:861–870. doi: 10.1016/j.jpsychires.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Chen LS, Breslau N, et al. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. doi: 10.1111/j.1360-0443.2010.03074.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval JJ, Pederson LL. Stress-coping and other psychosocial risk factors: a model for smoking in grade 6 students. Addict Behav. 1999;24(2):207–218. doi: 10.1016/s0306-4603(98)00037-9. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33(5):717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Lantz PM, Golberstein E, House JS, Morenoff J. Socioeconomic and behavioral risk factors for mortality in a national 19-year prospective study of U.S. adults. Soc Sci Med. 2010;70(10):1558–1566. doi: 10.1016/j.socscimed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Reynolds EK, Daughters SB, Curtin JJ. Risk factors in the relationship between gender and crack/cocaine. Exp Clin Psychopharmacol. 2007;15(2):165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DA, Taylor J. Lifetime cumulative adversity, mental health and the risk of becoming a smoker. Health (London) 2006;10(1):95–112. doi: 10.1177/1363459306058990. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediating effects in prevention studies. Eval Rev. 1993;17(2):144–158. [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27(1–2):127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20(5–6):390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: the good and bad sides of the response to stress. Metabolism. 2002;51:2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiology. Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mischel W. From Good Intentions to Willpower. New York: Guilford Press; 1996. [Google Scholar]
- Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43(3):303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Nock MK. Conceptual and design essentials for evaluating mechanisms of change. Alcohol Clin Exp Res. 2007;31(10 Suppl):4s–12s. doi: 10.1111/j.1530-0277.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp Clin Psychopharmacol. 2007;15(3):264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella M. Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci. 2004;114(1):95–104. doi: 10.1080/00207450490249347. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Pers Indiv Differ. 2009;47:385–395. [Google Scholar]
- Thoits PA. Stress and health: Major findings and policy implications. Journal of Health and Social Behavior. 2010;51:S41–S53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Cumulative adversity and drug dependence in young adults: racial/ethnic contrasts. Addiction. 2003;98(3):305–315. doi: 10.1046/j.1360-0443.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Cohen S, Kessler R, Underwood GL, editors. Measuring Stress. New York: Oxford University Press; 1995. pp. 29–58. [Google Scholar]
- Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. Am Sociol Rev. 1995;60(1):104–125. [Google Scholar]
- Turrell G, Lynch JW, Leite C, Raghunathan T, Kaplan GA. Socioeconomic disadvantage in childhood and across the life course and all-cause mortality and physical function in adulthood: evidence from the Alameda County Study. Journal of Epidemiology and Community Health. 2007;61:723–730. doi: 10.1136/jech.2006.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tobacco Control. 2006;15:472–480. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM. Stress and smoking in adolescence: a test of directional hypotheses. Health Psychol. 2002;21(2):122–130. [PubMed] [Google Scholar]
- Wong KY, Seow A, Koh WP, Shankar A, Lee HP, Yu MC. Smoking cessation and lung cancer risk in an Asian population: findings from the Singapore Chinese Health Study. British Journal of Cancer. 2010;103:1093–1096. doi: 10.1038/sj.bjc.6605782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug and Alcohol Dependence. 2007;91:213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]