Abstract
Branching morphogenesis occurs during the development of many organs, and the embryonic mouse submandibular gland (SMG) is a classical model for the study of branching morphogenesis. In the developing SMG, this process involves iterative steps of epithelial bud and duct formation, to ultimately give rise to a complex branched network of acini and ducts, which serve to produce and modify/transport the saliva, respectively, into the oral cavity1-3. The epithelial-associated basement membrane and aspects of the mesenchymal compartment, including the mesenchyme cells, growth factors and the extracellular matrix, produced by these cells, are critical to the branching mechanism, although how the cellular and molecular events are coordinated remains poorly understood 4. The study of the molecular mechanisms driving epithelial morphogenesis advances our understanding of developmental mechanisms and provides insight into possible regenerative medicine approaches. Such studies have been hampered due to the lack of effective methods for genetic manipulation of the salivary epithelium. Currently, adenoviral transduction represents the most effective method for targeting epithelial cells in adult glands in vivo5. However, in embryonic explants, dense mesenchyme and the basement membrane surrounding the epithelial cells impedes viral access to the epithelial cells. If the mesenchyme is removed, the epithelium can be transfected using adenoviruses, and epithelial rudiments can resume branching morphogenesis in the presence of Matrigel or laminin-1116,7. Mesenchyme-free epithelial rudiment growth also requires additional supplementation with soluble growth factors and does not fully recapitulate branching morphogenesis as it occurs in intact glands8. Here we describe a technique which facilitates adenoviral transduction of epithelial cells and culture of the transfected epithelium with associated mesenchyme. Following microdissection of the embryonic SMGs, removal of the mesenchyme, and viral infection of the epithelium with a GFP-containing adenovirus, we show that the epithelium spontaneously recombines with uninfected mesenchyme, recapitulating intact SMG glandular structure and branching morphogenesis. The genetically modified epithelial cell population can be easily monitored using standard fluorescence microscopy methods, if fluorescently-tagged adenoviral constructs are used. The tissue recombination method described here is currently the most effective and accessible method for transfection of epithelial cells with a wild-type or mutant vector within a complex 3D tissue construct that does not require generation of transgenic animals.
Keywords: Genetics, Issue 71, Molecular Biology, Cellular Biology, Developmental Biology, Virology, Medicine, Adenovirus, Embryonic, Epithelial rudiments, Extracellular matrix, Mesenchyme, Organ culture, Submandibular gland, ex vivo, cell culture, tissue engineering, embryo, mouse, animal model
Protocol
The protocol contains four major steps, as depicted in Figure 1. All steps are described in full detail. Adenovirus construction and viral purification should be performed in advance of the organ harvesting for use in the genetic transduction of dissected epithelial rudiments. All standard BSL-2 safety precautions should be followed when working with adenoviruses.
1. Mouse Embryonic Submandibular Gland (SMG) Harvesting and Microdissection
Euthanize timed-pregnant female mice (outbred strain CD-1 or ICR) using CO2 narcosis, followed by cervical dislocation and harvest strings of mouse embryos at embryonic day 13 (E13, 3-5 epithelial buds) following procedures approved by the institutional IACUC committee. The day of vaginal plug discovery is designated as E0. Salivary glands can also be harvested and cultured at the E12 stage when there is a single primary bud and stalk with clefts just starting to initiate. SMGs prior to this stage require different culture conditions than those indicated here.
Transfer embryo strings into sterile 10 cm tissue culture dishes filled with 25 ml of DMEM/Ham's F12 Medium (F12) lacking phenol red (Life Technologies) and supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin (pen-strep, Life Technologies).
Using a sterile scalpel (#11 blades) and forceps (#5, Fine Science Tools, 11252-20), remove the embryos from the sacs into a separate 10 cm dish containing 25 ml of DMEM/F12 + pen-strep.
Sever the embryo heads with an angled cut just below the lower jaw.
Isolate the lower mandibles from the heads under a stereo dissecting microscope with a transmitted light base (Nikon SMZ645 or equivalent) using a cut below the upper jaw. Place the tissues on top of an extra piece of glass rather than directly on the glass component of the microscope base.
Collect the lower mandibles into 3 ml of DMEM/F12 + pen-strep in a sterile 35 mm tissue culture dish.
Place a mandible slice on the dissecting microscope stage so that the tongue is on the bottom of the slice but is pointing towards the 12:00 o'clock position.
Using one side of two crossed forceps in a scissoring motion, remove and discard the lower 1/3rd of the mandible slice.
Turn the slice 90 ° to the right (if right-handed) and, using a scissoring cut with the forceps, cut the top portion of the mandible slice (without cutting the tongue) halfway into the slice.
Peel the excess tissue away and remove it to expose the SMGs underneath. There will be one on each side near the base of the tongue.
Using one pair of forceps to hold down the tissue by the tongue, use the other forceps to tease the SMG away from the tongue. Repeat for the other gland.
Collect the microdissected salivary glands into 3 ml of DMEM/F12 + pen-strep into a new sterile 35 mm tissue culture dish. Note: the larger submandibular glands (3-5 epithelial buds) along with the connected smaller sublingual gland (1 bud) can be cultured intact by skipping to step 4 and performing steps 4.1, 4.3, and 4.4, as described previously 2,8.
2. SMG Epithelial Rudiment Separation
Place 5 (or more) salivary glands into a glass-bottomed, 50 mm diameter microwell dish (MatTek Corporation, P50G-1.5-14-F) containing 200 μl of Hank's balanced salt solution (HBSS lacking Ca++or Mg++, Life Technologies) containing 0.4% (v/v) dispase (Life Technologies, cat. no. 17105-041) and incubate for 15 min at 37 °C.
After the incubation, using a sterile 200 μl pipette tip, carefully aspirate out the dispase solution without disturbing the glands. Immediately add 200 μl of DMEM/F12 media containing 5% (w/v) BSA (DMEM/F12-BSA, pre-sterilized with a 0.22 μm filter) to neutralize the dispase.
Under a dissecting microscope, using a pair of forceps with fine tips (#5 Dumostar, Fine Science Tools, 11295-20), gently separate the loosened mesenchyme from around the SMG epithelial buds and ducts, taking care to leave the epithelium intact.
Pre-wet a sterile 200 μl pipette tip with DMEM/F12-BSA media and gently pipette out the epithelial rudiments into a new 50 mm diameter microwell dish containing 200 μl of DMEM/F12 + pen-strep. Use a high quality 200 μl pipette tip with a smooth edge.
In the dish that contains the mesenchyme pieces, discard any non-mesenchymal tissue including the sublingual glands and any detached submandibular gland buds.
3. Adenoviral Infection of Epithelial Rudiments
Make 200 μl of viral infection media by diluting the adenovirus of interest in DMEM/F12 + pen-strep in a microcentrifuge tube so that the titer of the resulting solution is 1x108-109 PFUs. The optimal titer required for different viruses may vary. It is important to use cesium chloride (CsCl)-purified viral preparations for optimal uptake efficiency.
Remove the media from the dish containing the five epithelial rudiments. Quickly add 200 μl of the infection media, keeping the rudiments wet at all times. Take necessary precautions when handling the virus and disposing of contaminated pipet tips. Disinfect any virus-contaminated surfaces with 10% bleach solution.
Move the epithelial rudiments far away from each other with forceps to prevent them from sticking together and allow them to settle to the bottom of the plate. Incubate rudiments in viral media for 1 hr at room temperature. During the incubation, separate the rudiments, if they move close together. Excessive rocking or movement will allow the glands to clump together.
After incubation, gently remove the viral-containing media and discard appropriately, taking care not to disrupt the rudiments. Add 200 μl of DMEM/F12 + pen-strep. Repeat this wash at least two more times and replace with 200 μl of DMEM/F12 + pen-strep media. These washes are critical to remove any virus that is not strongly attached to the epithelium and TO prevent infection of the mesenchyme.
Incubate epithelial rudiments with 200 ml of DMEM/F12 containing 2% (v/v) Matrigel (BD Biosciences, 356231) for 20 min. We find that this short incubation in Matrigel minimizes the regression of existing clefts during the initial culture period, but this step can be dispensed with if exposure to Matrigel is not desired.
4. Ex vivo Culture of Recombined SMGs
Place each rudiment, one at a time, using a pre-wet 200 μl pipette tip, on top of a notched (small cut) 13 mm, 0.1 μm Nuclepore Track-Etch membrane filter (Whatman) floating over 200 μl of culture media (5 rudiments /filter) in a glass-bottomed microwell plate. The culture media is: DMEM/F12 + pen-strep containing 50 μg/ml transferrin and 150 μg/ml ascorbic acid, as previously described2,8. Transferrin is known to stimulate survival and branching morphogenesis of salivary gland and other organ explants9. Addition of ascorbic acid has been shown to stimulate SMG branching.10 It is known to act as a cofactor in many hydroxylation reactions of metabolic pathways (such as proline hydroxylation during collagen fiber formation11) and also stimulates fibronectin and laminin production in other organ explants12.
Remove as much media on top of the filter as possible after adding each epithelial rudiment. Too much media on top of the filter may cause the filter to sink, while less media makes placement of the rudiments and mesenchyme much easier. Excess media can be removed either with a pipette tip or by using forceps to grip the media between the tips.
Place mesenchyme pieces derived from three to four glands on top and/or around each epithelial rudiment. Remove any extra media that is surrounding the glands to avoid movement of the mesenchyme away from the rudiments. Mesenchyme derived from more glands is not beneficial but less can be detrimental to epithelial rudiment growth.
Incubate recombined SMGs in a humidified incubator (95% air/5% CO2) at 37 °C for 48 -72 hr, as desired.
Acquire brightfield and/or fluorescent images of live glands at 0 hr (if desired), 2 hr after recombination and every 24 hr thereafter on an inverted, upright, or stereo light microscope fitted with a digital camera using a 4X or 5X magnification objective to capture the entire recombined gland in a single frame of view. Images of the salivary gland show the best contrast using either the brightfield setting or the appropriate phase ring placed part way into the light path (which is not possible on fully-automated microscopes). Standard phase contrast is not necessary since the gland is thick and creates contrast.
At the desired time-points, recombined SMGs can be fixed for 20 minutes with fixative solution made of 2% paraformaldehyde (PFA) in 1X PBS containing 5% (w/v) sucrose (to better preserve internal cellular structure by keeping the cells in an isosmotic state). Unlike 4% PFA, 2% PFA will preserve a significant amount of the GFP signal if the fixative is completely removed after fixation. Glands can be stored for up to 1 week in 1X PBS in the dark at 4 °C until whole mount immunocytochemistry and confocal imaging is performed, as reported previously3,6,13-15. Ideally, immunocytochemistry and imaging should be performed soon after fixation to obtain the best quality images. Glands may also be lysed in RIPA buffer for SDS-PAGE followed by Western blotting analysis.
Representative Results
The flow of the major experimental steps is outlined in Figure 1. An example of an intact SMG, an isolated epithelial rudiment, and its corresponding mesenchyme are shown in Figure 2. Brightfield images of recombined SMGs, which continue to undergo branching morphogenesis when cultured ex vivo for the indicated times, are shown in Figure 3. Recombined glands grown for 48 hr expressing epithelial GFP are shown in Figure 4. Confocal images of recombined SMGs fixed and imaged after 72 hr in culture followed by immunocytochemistry, are shown in Figure 5. The epithelial marker, E-cadherin, demarcates the GFP-expressing epithelial cell population from the surrounding mesenchyme. Detection of the basement membrane protein, perlecan, localized at the periphery of the epithelium demonstrates at least partial reconstitution of the basement membrane in recombined glands. Figure 6. Parasympathetic submandibular ganglia undergo neurite outgrowth and innervate the glands in recombination cultures as demonstrated previously.19
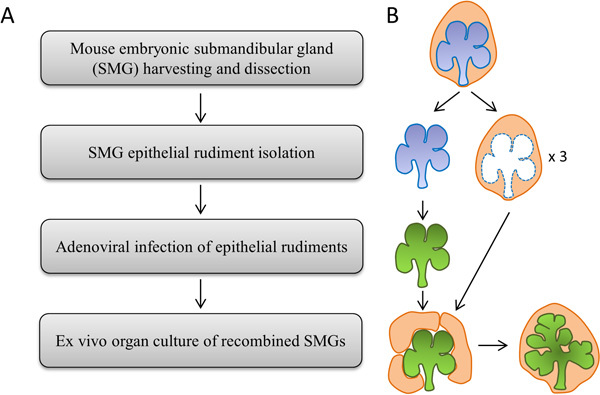 Figure 1. (A) Flowchart and (B) Schematic depicting the major experimental steps.
Figure 1. (A) Flowchart and (B) Schematic depicting the major experimental steps.
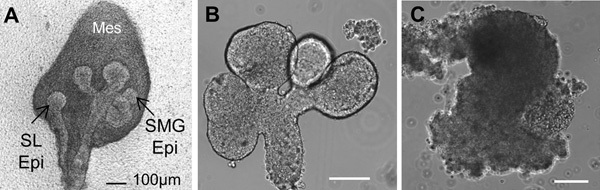 Figure 2. Brightfield images of (A) a whole embryonic day 13 (E13) salivary gland showing the sublingual gland epithelium (SL Epi) and the submandibular gland epithelium (SMG Epi) surrounded by mesenchyme (mes). (B) Isolated epithelial rudiment, and (C) Separated Mesenchyme. Scale bars = 100 μm.
Figure 2. Brightfield images of (A) a whole embryonic day 13 (E13) salivary gland showing the sublingual gland epithelium (SL Epi) and the submandibular gland epithelium (SMG Epi) surrounded by mesenchyme (mes). (B) Isolated epithelial rudiment, and (C) Separated Mesenchyme. Scale bars = 100 μm.
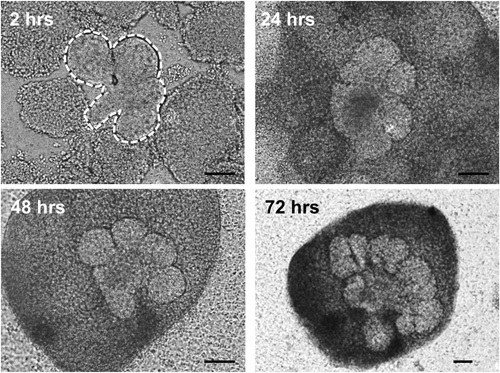 Figure 3. Brightfield images of epithelial rudiments (outlined by white dashed lines) surrounded by mesenchyme pieces grown in ex vivo culture for the indicated times. Epithelial cells undergo branching morphogenesis and mesenchymal condensation is evident as early as 24 hr in culture. Scale bars = 100 μm
Figure 3. Brightfield images of epithelial rudiments (outlined by white dashed lines) surrounded by mesenchyme pieces grown in ex vivo culture for the indicated times. Epithelial cells undergo branching morphogenesis and mesenchymal condensation is evident as early as 24 hr in culture. Scale bars = 100 μm
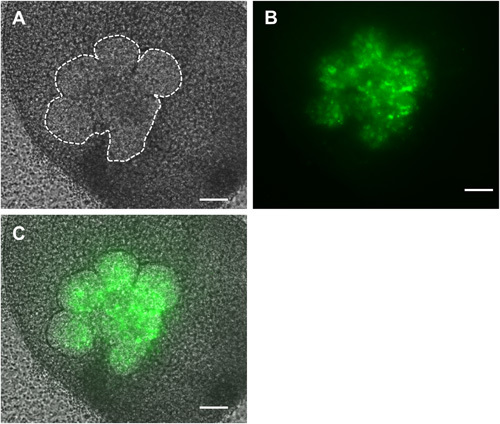 Figure 4. Brightfield (A), fluorescent (B) and overlaid (C) images of recombined SMGs in which only the epithelial cells (outlined in white dashed lines) were infected with GFP-expressing adenovirus and cultured ex vivo for 48 hr. Scale bars = 100 μm.
Figure 4. Brightfield (A), fluorescent (B) and overlaid (C) images of recombined SMGs in which only the epithelial cells (outlined in white dashed lines) were infected with GFP-expressing adenovirus and cultured ex vivo for 48 hr. Scale bars = 100 μm.
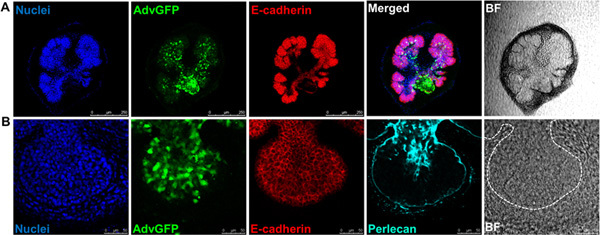 Figure 5. Confocal images or brightfield images (BF) of recombined SMGs labeled with (A) nuclei (DAPI, blue), adenoviral GFP (green), the epithelial marker. E-cadherin (red), scale bars = 250 μm , or, (B) nuclei (DAPI, blue), adenoviral GFP (green), the epithelial marker, E-cadherin (red), and the basement membrane marker, Perlecan (cyan), scale bars = 50 μm. Dashed white lines outline epithelium. Click here to view larger figure.
Figure 5. Confocal images or brightfield images (BF) of recombined SMGs labeled with (A) nuclei (DAPI, blue), adenoviral GFP (green), the epithelial marker. E-cadherin (red), scale bars = 250 μm , or, (B) nuclei (DAPI, blue), adenoviral GFP (green), the epithelial marker, E-cadherin (red), and the basement membrane marker, Perlecan (cyan), scale bars = 50 μm. Dashed white lines outline epithelium. Click here to view larger figure.
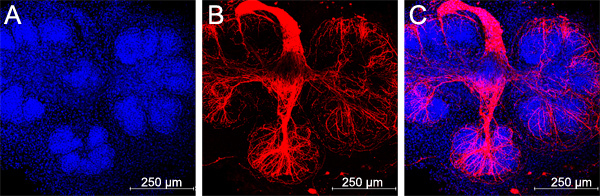 Figure 6. Parasympathetic submandibular ganglia undergo neurite outgrowth and innervate the glands in recombination cultures, scale bars = 250 μm.
Figure 6. Parasympathetic submandibular ganglia undergo neurite outgrowth and innervate the glands in recombination cultures, scale bars = 250 μm.
Discussion
The ex vivo epithelial-mesenchymal recombination technique was first published for submandibular salivary glands in 198116. In this protocol, we expand upon the original method, using adenoviral infection to manipulate epithelial cell gene expression within the context of a recombined gland. We show that a percentage of the epithelial cells are infected with the adenovirus, whereas the percentage of cells that are infected depends upon the properties of the viral promoter, viral titer, and viral purity. We have found that it is necessary to use CsCl gradient-purified viruses for efficient epithelial transduction, the purification of which is not described here. Since we have also been able to infect the mesenchyme cells prior to recombination (data not shown), independent genetic manipulation of the mesenchymal population is also possible. We recently used this adenoviral transfection/tissue recombination method to identify a role for the polarity protein, PAR-1b, in the control of the placement of basement membrane by the epithelium in developing salivary glands. Kinase-dead PAR-1b adenovirus and wild-type PAR-1b adenovirus were both used in this study13 to infect epithelial rudiments and recombined with E13 mesenchymes. The ability to use mutant viruses to interrogate functions of specific molecules greatly enhances the utility of this method.
There is currently a lack of effective tools to target specific molecules within the epithelial cell population in intact embryonic salivary glands without affecting the mesenchymal compartment. Although multiple studies have used pharmacological inhibitors to manipulate signal transduction in intact organ cultures, these inhibitors affect both the epithelium and mesenchyme. To directly assess effects of inhibitors on the epithelium, epithelial rudiments must be cultured in the absence of mesenchyme in either Matrigel or laminin-111 gels6,7. Small inhibitory RNAs (siRNAs) are an effective method to down-regulate epithelial gene expression since siRNAs are preferentially taken up by the epithelial cells in the presence of most lipid carriers13,14,17,18. However, neither of these methods for interfering with protein levels or function allow overexpression of a wild-type or mutant gene. Attempts at traditional (non-viral) transfection of embryonic whole SMG cultures have met with limited success in that only a small number of epithelial cells can be targeted (M.L., unpublished data). In comparison, adenoviral transduction represents an effective method for specifically transfecting salivary epithelial cells.
Parasympathetic nerves have recently been shown to be critical for normal salivary gland branching by maintaining a keratin 5-positive progenitor cell population19. An advantage of this tissue recombination method over the mesenchyme-free epithelial rudiment culture method is that the parasympathetic ganglion can be maintained in the recombination culture system. By keeping careful track of the approximate location of the parasympathetic ganglion, which is located in the mesenchyme adjacent to the primary epithelial duct19, it is possible to ensure that each rudiment ends up being associated with a ganglion. However, we find that by combining 3-4 glands worth of mesenchymes with every epithelial rudiment, each rudiment is associated with a ganglion sufficient to innervate the buds to some extent (Figure 6), although not always to the same as in the intact unmodified glands.
There are alternate methods for infecting the epithelium in the context of the intact gland. We previously reported that microinjection can be used to introduce adenovirus into epithelial cells in intact glands20,21. However, microinjection is difficult to control, may physically damage the glands, and typically leads to infection of only a localized subset of cells20. Adeno-associated viruses (AAV) have been shown to be useful for transfection of distinct cell populations within the context of intact SMG organ explants22. The serotype scAAV2 was shown to be substantially more efficient at specific transduction of the epithelium of intact SMGs over other recombinant AAV vectors23. Since AAVs are not widely commercially available, nor does the expertise to prepare AAV exist in most laboratories, the adenoviral transduction and tissue recombination protocol provided here is more generally accessible to most laboratories than is the use of AAV vectors.
While this tissue recombination method recapitulates epithelial-mesenchymal interactions to some extent, it is also possible to infect the epithelium with adenovirus and then grow the epithelium outside of its native context. As previously mentioned, we infected intact salivary epithelial rudiments with adenovirus and then grew the cultures in Matrigel with exogenously added growth factors17,20,21. Salivary gland organ explants can also be cultured on nanofiber scaffolds, although these scaffolds do not recapitulate the full function of the mesenchyme in their current form15. While neither of these methods recapitulates the native mesenchyme, such methods allow for imitation of specific properties of the mesenchyme in ex vivo culture.
Each ex vivo culture method has its own advantages and disadvantages but is applicable for addressing specific scientific questions. While the recombined glands do not undergo as much branching morphogenesis as do intact salivary gland organ explants, organ explants, in general, only recapitulate in vivo branching morphogenesis for a few days. A solution to this problem is of course to create transgenic animals containing targeted gene expression within the epithelial compartment. Since there is a lack of known promoters that are activated specifically in early developing SMG epithelium, and transgenic technology remains significantly more expensive than the described methods, the tissue recombination experiments described here constitute a viable model system to study both epithelial and mesenchymal cell signaling in early developing salivary gland cells within their tissue context.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Dr. Deirdre Nelson for helpful comments and for critical reading of the manuscript. This work was funded by NIH grants DE019244, DE019197, and DE021841 to M.L., F32DE02098001 to S.J.S, and C06 RR015464 to the University at Albany, SUNY.
References
- Tucker AS. Salivary gland development. Seminars in cell & developmental biology. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sakai T, Onodera T. Embryonic organ culture. Curr. Protoc. Cell Biol. 2008;Chapter 19:Unit 19. doi: 10.1002/0471143030.cb1908s41. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Sequeira SJ, Larsen M, DeVine T. Extracellular matrix and growth factors in salivary gland development. Frontiers of oral biology. 2010;14:48–77. doi: 10.1159/000313707. [DOI] [PubMed] [Google Scholar]
- Zheng C, et al. Transient detection of E1-containing adenovirus in saliva after the delivery of a first-generation adenoviral vector to human parotid gland. J. Gene Med. 2010;12:3–10. doi: 10.1002/jgm.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, et al. Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Developmental biology. 2003;255:178–191. doi: 10.1016/S0012-1606(02)00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MP, et al. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–5778. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Rebustini IT, Hoffman MP. ECM and FGF-dependent assay of embryonic SMG epithelial morphogenesis: investigating growth factor/matrix regulation of gene expression during submandibular gland development. Methods Mol. Biol. 2009;522:319–330. doi: 10.1007/978-1-59745-413-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen AM, Thesleff I. Transferrin and tooth morphogenesis: retention of transferrin by mouse embryonic teeth in organ culture. Differentiation. 1987;34:25–31. doi: 10.1111/j.1432-0436.1987.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Naka T, et al. Modulation of branching morphogenesis of fetal mouse submandibular gland by sodium ascorbate and epigallocatechin gallate. In Vivo. 2005;19:883–888. [PubMed] [Google Scholar]
- Bornstein P, Traub W. The chemistry and biology of collagen. In: Neurath H, Hill RL, editors. The Proteins. 3rd. Vol. 4. Academic Press; 1979. pp. 412–632. [Google Scholar]
- Zhou L, Higginbotham EJ, Yue BY. Effects of ascorbic acid on levels of fibronectin, laminin and collagen type 1 in bovine trabecular meshwork in organ culture. Curr. Eye Res. 1998;17:211–217. doi: 10.1076/ceyr.17.2.211.5608. [DOI] [PubMed] [Google Scholar]
- Daley WP, et al. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development. 2012;139:411–422. doi: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Developmental biology. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira SJ, et al. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials. 2012;33:3175–3186. doi: 10.1016/j.biomaterials.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H, Mizuno T. Mesenchymal control over elongating and branching morphogenesis in salivary gland development. Journal of embryology and. 1981;66:209–221. [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13:721–735. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]
- Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol. Ther. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Hsu JC, et al. Viral gene transfer to developing mouse salivary glands. J. Dent. Res. 2012;91:197–202. doi: 10.1177/0022034511429346. [DOI] [PMC free article] [PubMed] [Google Scholar]


