Abstract
Objective
Among postmenopausal women who do not use estrogen hormone therapy (HT) we have previously reported that intensive lifestyle intervention (ILS) leads to increases in sex hormone binding globulin (SHBG), and such increases were associated with reductions in fasting plasma glucose (FPG) and 2-hour post-challenge glucose (2HG). Oral HT decreases FPG and increases 2HG, while increasing both SHBG and estradiol (E2). It is unknown if ILS reduces glucose among HT users, if changes in SHBG and E2 might mediate any glucose decreases in HT users, and if these patterns differ from non-HT users.
Methods
We conducted a secondary analysis of postmenopausal women in the Diabetes Prevention Program who used HT at baseline and 1 year follow-up (n=324) and who did not use HT at either time point (n=382). Participants were randomized to ILS, metformin, or placebo administered 850 mg twice a day.
Results
HT users were younger, more often white, and more likely to have had bilateral oophorectomy than non-HT users. Among HT users, ILS reduced FPG (p<0.01) and 2HG (p<0.01), and metformin reduced FPG (p<0.01) but not 2HG (p=0.56), compared to placebo. Associations between SHBG and total E2 with FPG and 2HG were not significant among women randomized to ILS or to metformin. These patterns differed from those observed among women who did not use HT.
Conclusions
We conclude that among glucose intolerant HT users, interventions to reduce glucose are effective but possibly mediated through different pathways than among women who did not use HT.
Keywords: menopause, estrogen, progestogen, hormone therapy, glucose
Introduction
Endogenous estradiol (E2) and sex hormone binding globulin (SHBG) have been associated with alterations in glucose levels.1–4 In the Diabetes Prevention Program (DPP),5 intensive lifestyle modification (ILS) and metformin reduced fasting plasma glucose (FPG) and 2-hour post-challenge glucose (2HG) among glucose-intolerant adults.5 Among postmenopausal DPP participants not using estrogen, ILS led to increases in sex hormone binding globulin (SHBG) which were associated with decreases in both FPG and 2HG.1 Neither ILS nor metformin led to significant changes in E2 levels compared to placebo.1
It has not been reported whether ILS and metformin reduce glucose levels among estrogen hormone therapy (HT) users, and whether SHBG or E2 are associated with glucose in HT users. The impact of ILS and metformin and mechanisms of action might differ between HT users and non-users for several reasons. The protective effects of endogenous SHBG and the detrimental effects of endogenous E2 might be obscured by HT use, which increases SHBG but also increases E2.6 Also, randomization to HT compared to placebo has been associated with both decreases in FPG as well as increases in 2HG.7–12 The mechanisms through which HT use might affect glucose levels are not known. In studies of HT use, the decreases in FPG persisted after adjustment for adiposity, suggesting that exogenous oral HT reduced FPG through other means, such as hepatic gluconeogenesis or hepatic insulin resistance.7, 12 HT use could increase postprandial glucose through reductions in whole-body insulin sensitivity,13 but at least one other study showed no association between HT use and insulin sensitivity.14
While HT use has declined after the publication of trials demonstrating an increased risk of morbidity,15 oral estrogens are still commonly prescribed for relief of menopausal symptoms. Therefore, the metabolic effects of HT remain important to examine, and potential mechanisms are relevant for understanding progression of glucose intolerance in both HT and non-HT users. In the DPP, participants randomized to ILS and metformin had maximal weight loss and reductions in glucose at 1-year after randomization.5 Changes in glucose were associated with changes in weight and insulin sensitivity.16 We examined whether postmenopausal women using oral HT at baseline and 1-year follow-up had declines in glucose, and whether DPP treatments induced changes in E2 and SHBG that were associated with these changes in glucose. We also examined whether these patterns differed among women who did not use oral HT at either baseline or 1-year follow-up (Figure 1). We hypothesized that interventions would lead to reductions in both FPG and 2HG among HT users, but unlike in non-users, changes in SHBG and E2 would not be associated with these reductions. Finally, we examined whether associations between sex hormone changes and glucose changes persisted after consideration of changes in weight and insulin sensitivity.
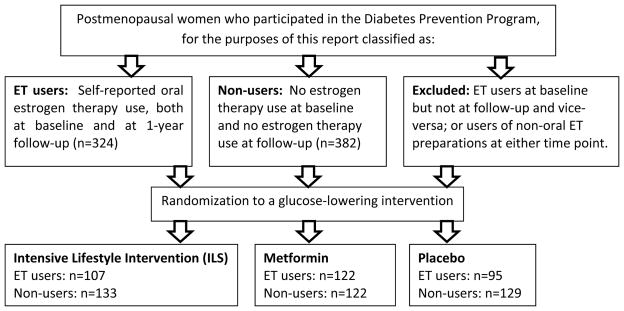
Figure 1.
Secondary analysis design. We conducted an analysis of the effectiveness of glucose-lowering interventions in a randomized trial (the Diabetes Prevention Program), among postmenopausal women who were either 1) oral estrogen users at baseline and at 1-year follow-up or 2) non-estrogen users at baseline and at 1-year follow-up.
Methods
Characteristics of DPP participants have been reported.5 Briefly, the DPP inclusion criteria included age ≥ 25 years, FPG of 95–125 mg/dl and 2HG of 140–199 mg/dl following a 75-glucose load, and body mass index (BMI) ≥ 24 kg/m2 (≥22 kg/m2 for Asian Americans). Written informed consent was obtained from all participants before screening, consistent with the guidelines of each participating center’s institutional review board. Eligible participants recruited between 1996 and 1999 were randomly assigned to one of three interventions: 850 mg metformin twice daily, placebo twice daily, or ILS. The goals of ILS were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a low-calorie, low-fat diet, and to engage in moderate physical activity for at least 150 min/week.5 Weight and waist circumference were measured semiannually, and participants underwent an annual oral glucose tolerance test and semiannual FPG test. At the time of randomization, all women completed a questionnaire about their menses, gynecological history including surgeries, and exogenous HT use. Medication use was assessed every 6 months.
Women were classified as being postmenopausal if they met any of the following criteria: bilateral oophorectomy, lack of menses for at least one year without gynecologic surgery, cessation of menses prior to hysterectomy, cessation of menses within the past year and age ≥ 55 years, and cessation of menses with hysterectomy and age ≥ 55 years. For this report, we included women who were postmenopausal at randomization, had an available stored serum sample for E2 and SHBG measurement, and could be categorized as oral HT users both at randomization as well as at 1 year follow-up (n=324), or as non-HT users both at randomization and 1 year follow-up (n=382). Women who used injection, implant, transdermal, or transvaginal HT were excluded, as were women who used any HT at baseline but not at follow-up and vice-versa. We did not include women enrolled at Native American centers, as these women were not originally consented to participate in ancillary studies.
Baseline and year 1 assays were performed using the same batched assays. Glucose and insulin were measured as previously reported.16 Briefly, women were instructed to consume a usual diet and an oral glucose tolerance test was performed between 7 a.m. and 11 a.m. after an overnight fast. Blood was sampled from a vein before (fasting) and 2 hours after a 75-gram oral glucose load (Trutol 75; Custom Laboratories, Baltimore, MD). Plasma glucose was measured fasting and 2-hours, and plasma insulin was measured fasting. Insulin sensitivity was assessed using inverse fasting insulin levels.16 Insulin measurements were performed by a radioimmunoassay method using an anti–guinea pig antibody that measures total immunoreactive insulin. The assay is a 48-h polyethylene glycol–accelerated method with coefficients of variation (CVs) of 4.5% for high-concentration quality control samples and 6.9% for low-concentration quality control samples. The CV for masked split duplicates in this assay was <8.5%. SHBG, follicle stimulating hormone (FSH), and total E2 were measured as previously reported.1 SHBG was measured at Endoceutics (Quebec City, Canada) using ELISA(Bioline) with interassay coefficients of variation of 7.8 and 5.0 at 18.2 and 63.1 nmol/l, respectively. FSH was measured at Endoceutics using ELISA (Bioline) with interassay coefficients of variation of 3.6 and 4.4 at 27.1 and 72.9 mIU/ml, respectively. E2 was analyzed using gas chromatography/mass spectrometry at Endoceutics.17 The limit of detection was 3.0 pg/ml for total E2 with an interassay coefficient of variation of 17.5 at 4.7 pg/ml. For measurements below the detection limit, values were extrapolated below the lower limit of quantitation using Mass Hunter Workstation software (Agilent, Santa Clara, CA).1 We also performed sensitivity analyses where we assigned these values the equivalent of the lower limit of detection and found a similar pattern of results (not shown). Bioavailable E2 was calculated according to the method described by Sodergard and colleagues (macro courtesy of Frank Stanczyk, University of Southern California) taking the concentrationsof total E2 and SHBG into account and assuming a fixed albumin concentration of 4.0 g/dl.18
Statistical Analysis
Women who used HT at baseline and follow-up were analyzed separately from women who did not use HT at baseline and follow-up. For HT users and non-users, baseline characteristics were described using percentages for categorical variables and means (SD) for normally distributed quantitative variables. Skewed variables, including insulin levels, FSH, SHBG, total and bioavailable E2, were log-transformed before comparison. Changes in glucose, SHBG, total E2, and bioavailable E2 were calculated as year 1 level – baseline level. In order to assess the association between randomization assignment and change in FPG and 2HG as well as change in SHBG and E2 levels between baseline and year 1 follow-up, we used t-tests to compare levels of change between randomization arms. We also compared log-transformed SHBG and E2 levels and found a similar pattern of results. Models substituting the homeostasis model assessment of insulin resistance (HOMA-IR) for 1/fasting insulin had similar results (data not shown).
To determine whether changes in SHBG, bioavailable E2, or total E2 were associated with changes in glucose apart from changes in adiposity, we created a series of multiple linear regression models, with coefficients obtained by using ordinary least squares. Changes in the aforementioned sex hormones and associations with changes in FPG were examined after adjustment for baseline characteristics (age, race/ethnicity, baseline FSH, and baseline glucose levels), as well as changes in waist circumference and changes in 1/fasting insulin levels. We chose to adjust for weight and not physical activity, because in the DPP, reductions in body mass were the strongest predictor of reductions in glucose levels.19 While level of physical activity was associated with body mass, physical activity was not associated with reductions in glucose after adjustment for body mass.19 In other words, changes in weight mediated physical activity effects on glucose. We also chose to adjust for 1/fasting insulin due to previous reports suggesting that associations between sex hormones and glucose were mediated by fasting insulin.2 In the DPP, weight and waist circumference were highly correlated, and we used waist circumference as a proxy for visceral adiposity.20 Similar models were created that examined associations between changes in sex hormones and changes in 2HG. In sensitivity analyses, we examined only women using oral estrogen alone, without progestin. We observed similar results compared to women using oral estrogen and progestin (data not shown). We also examined models that did not include FSH or baseline glucose levels and observed similar results (data not shown). The SAS analysis system was used for all analyses (SAS Institute, Cary, NC).
Results
Baseline characteristics of the postmenopausal cohort of HT users and non-HT users are shown in Table 1. Reflecting DPP recruitment criteria, all women were overweight or obese at baseline and had elevated glucose levels. HT users were slightly younger and more often Caucasian than non-users. Among HT users, the most common cause of menopause was oophorectomy, while among non-users, the most common cause of menopause was natural or non-surgical cessation of menses. HT users weighed less, and had smaller waist circumferences and lower BMIs than non-users. HT users had lower levels of fasting insulin, FPG, and FSH but higher levels of SHBG, total E2, and bioavailable E2 than non-users. Of the 324 women who reported using oral estrogen at baseline and at follow-up, 266 women were estrogen-only users at baseline and 58 women used estrogen-progestin; at year 1 follow-up, 258 women were estrogen-only users and 66 women used estrogen-progestin.
Table 1.
Baseline characteristics of postmenopausal women by oral estrogen therapy (ET) use.
| Women with oral ET use at baseline and at follow-up | Women with no ET use at baseline and no ET use at follow-up | |
|---|---|---|
| n=324 | n=382 | |
| Age (years)* | 56.5 (7.6) | 58.7 (9.0) |
| Race/ethnicity* | ||
| Caucasian | 66 | 53 |
| African-American | 16 | 28 |
| Hispanic | 13 | 16 |
| Asian | 4 | 3 |
| Type of menopause* | ||
| Bilateral oophorectomy | 42 | 20 |
| Natural menopause | 39 | 67 |
| Age ≥ 55 years and hysterectomy | 19 | 16 |
| Years since final menstrual period | 14 (9) | 15 (10) |
| Baseline weight (kg)* | 87.6 (17.8) | 91.0 (19.7) |
| Baseline waist circumference (cm)* | 101 (14) | 104 (14) |
| Baseline BMI (kg/m2)* | 33.3 (6.5) | 34.6 (6.8) |
| Baseline fasting insulin levels (IU/l)* | 23.4 (12.0) | 26.4 (16.0) |
| Baseline fasting plasma glucose (mg/dl)* | 104 (7) | 107 (8) |
| Baseline 2-hour glucose | 166 (18) | 164 (17) |
| Baseline follicle stimulating hormone (IU/l)* | 34.8 (22.5) | 55.3 (26.6) |
| Baseline sex hormone binding globulin (nmol/l)* | 85.3 (77.0) | 33.2 (18.3) |
| Baseline total estradiol (pg/ml)* | 17.6 (14.8) | 8.5 (8.0) |
| Baseline bioavailable estradiol (pg/ml)* | 8.6 (9.4) | 6.0 (6.4) |
Means (SD) or percentages shown; medians (interquartile ranges or IQR) shown for sex hormones.
indicates significant difference (p<0.05) between ET users and non-users.
Among HT users, there were no significant differences between women randomized to ILS (n=107), metformin (n=122), and placebo (n=95), with the exception that there were slightly more African-American women in the metformin arm than the placebo arm (9% vs. 5%, p<0.05). Of the oral estrogens that women reported using at baseline and follow-up, the most common was conjugated equine estrogen (Premarin), followed by 17-β-estradiol (Estrace), followed by esterified estrogen (Estratab, Menest) and estropipate (Ogen, Ortho-Est). Of the 211 women who had a hysterectomy, none were using progestin at baseline or follow-up. Of the 113 women who did not report a hysterectomy, all were using a progestin at baseline and follow-up. Among non-users, there were no significant differences between women randomized to ILS (n=133), metformin (n=122), or placebo (n=129) exception that women randomized to metformin had slightly lower FSH levels than women randomized to placebo (51.5 IU/l vs. 59.3 IU/l, p<0.05).
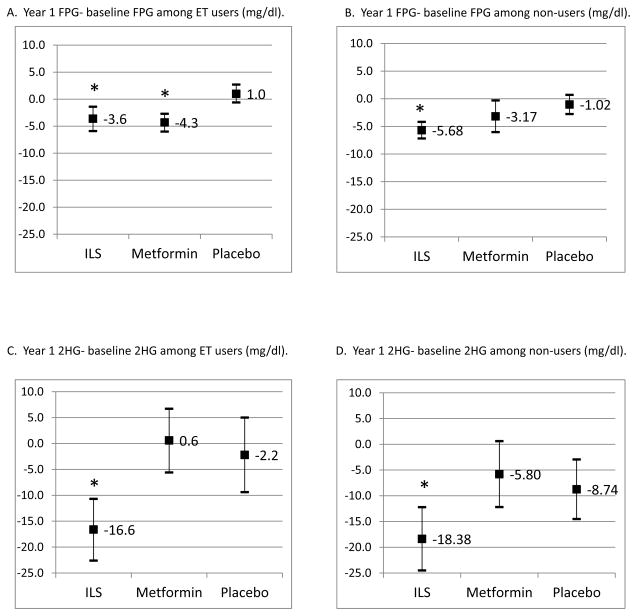
Among both HT users and non-users, women randomized to ILS and metformin had declines in waist circumference compared to placebo (p<0.01 for all comparisons). The association between randomization arm and decreased waist circumference persisted after adjustment for age, race/ethnicity, and FSH levels (results not shown). Changes in glucose levels between baseline and year 1 by randomization arm are presented in Figure 2. Among HT users, women randomized to ILS had significant reductions in FPG (p<0.01) and 2HG (p<0.01) compared to placebo. Among HT users, women randomized to metformin had significant reductions in FPG (p<0.01) but not 2HG (p=0.56) compared to placebo. This pattern of results was similar across different types of estrogen (results not shown). Among non-users, women randomized to ILS had significant reductions in FPG (p<0.01) and 2HG (p=0.03) compared to placebo, but women randomized to metformin did not have significant reductions in FPG (p=0.20) or 2HG (p=0.50) compared to placebo. These associations between randomization arm and glucose levels persisted after adjustment for age, race/ethnicity, and FSH levels (results not shown).
Figure 2.
Changes in fasting plasma glucose (FPG) and 2-hour post-challenge glucose (2HG) between baseline and year 1 follow-up, among estrogen therapy (ET) users and non-users. Means and 95% confidence intervals shown; * indicate significant differences from placebo arm.
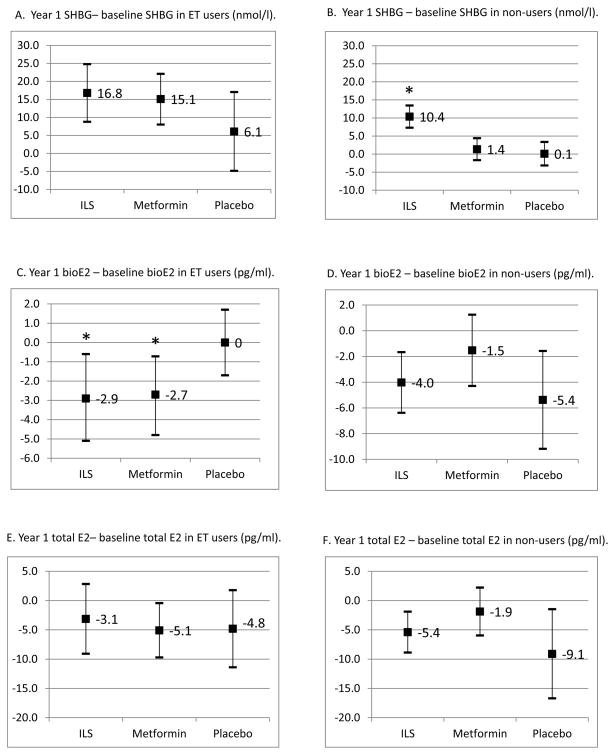
Changes in SHBG, total E2, and bioavailable E2 between baseline and year 1 by randomization arm are presented in Figure 3. Among HT users, women randomized to ILS or metformin did not have significant changes in SHBG (p=0.11 and p=0.16, respectively) or total E2 (p=0.46 and p=0.15, respectively) compared to placebo. However, women randomized to ILS (p=0.046) and metformin (p=0.042) did have significant reductions in bioavailable E2 compared to placebo. Among non-users, women randomized to ILS had significant increases in SHBG compared to placebo (p<0.01), but women randomized to metformin (p=0.58) did not have significant changes in SHBG. Among non-users, women randomized to ILS or metformin did not have significant changes in total E2 (p=0.36 and p=0.10, respectively) or bioavailable E2 (p=0.54 and p=0.11, respectively). This pattern of associations between randomization arm and SHBG and E2 levels remained similar after adjustment for age, race/ethnicity, and FSH levels (results not shown).
Figure 3.
Changes in select sex hormone measures (sex hormone binding globulin or SHBG, bioavailable estradiol or bioE2, total estradiol or total E2) between baseline and year 1 follow-up, among estrogen therapy (ET) users and non-users. Means and 95% confidence intervals shown; * indicate significant differences from placebo arm.
Table 2 shows the associations between changes in SHBG, total E2, and bioavailable E2 with changes in glucose. Among HT users who were randomized to ILS, there were no associations between changes in SHBG or E2 with changes in FPG or changes in 2HG. Among HT users who were randomized to metformin, reductions in bioavailable E2 were associated with reductions in 2HG. This association persisted after further adjustment for changes in 1/fasting insulin (p-value=0.04), suggesting that the association between bioavailable E2 and 2HG was not mediated through adiposity or 1/fasting insulin. However, overall, significant reductions in 2HG among metformin users vs. placebo did not occur (Figure 2).
Table 2.
Associations between changes (Δ) in glucose (dependent variable) and sex hormone binding globulin (SHBG), bioavailable estradiol (E2), and total E2. ET indicates estrogen therapy, ILS indicates intensive lifestyle change, and FPG indicates fasting plasma glucose. Adjusted β coefficients (p-values) shown.*
| Women with oral ET use at baseline and at follow-up | Women with no ET use at baseline and no ET use at follow-up | |||
|---|---|---|---|---|
| ILS | Metformin | ILS | Metformin | |
| Association between Δ SHBG and Δ FPG | −0.01 (0.69) | −0.04 (0.12) | −0.12 (<0.01) | −0.05 (0.75) |
| Association between Δ bioavailable E2 and Δ FPG | 0.14 (0.51) | 0.08 (0.55) | 0.23 (0.02) | 0.14 (0.56) |
| Association between Δ total E2 and Δ FPG | 0.10 (0.36) | 0 (0.99) | 0.13 (0.048) | 0.08 (0.60) |
| Association between Δ SHBG and Δ 2-hour glucose | −0.07 (0.39) | −0.14 (0.11) | −0.39 (0.04) | −0.08 (0.82) |
| Association between Δ bioavailable E2 and Δ 2-hour glucose | 0.02 (0.96) | 1.1 (0.04) | 0.62 (0.14) | −0.04 (0.94) |
| Association between Δ total E2 and Δ 2-hour glucose | −0.13 (0.60) | 0.43 (0.06) | 0.37 (0.19) | −0.07 (0.83) |
Adjusted for age, race/ethnicity, baseline follicle stimulating hormone levels, baseline glucose levels, and changes in waist circumference.
Among non-users (Table 2), increases in SHBG were associated with declines in FPG and 2HG after adjustment for waist circumference, and these associations persisted after further adjustments for change in 1/fasting insulin (p<0.05 for both associations). Among non-users, declines in total and bioavailable E2 were associated with declines in FPG, and this association persisted after further adjustment for change in 1/fasting insulin (p<0.05). However, overall, significant reductions in total E2 and bioavailable E2 among women randomized to interventions vs. placebo did not occur (Figure 3). Among non-users, changes in E2 were not associated with changes in 2HG. When we examined the non-users, excluding women who had undergone oophorectomy, ILS still led to declines in SHBG compared to placebo (p<0.01), and declines in SHBG were still associated with significant declines in FPG (p=0.01) although the association with declines in 2HG was attenuated (p=0.14).
Discussion
In a secondary analysis from the Diabetes Prevention Program, a randomized placebo-controlled trial, we observed that ILS led to reductions in both fasting and post-prandial glucose among HT users and non-users. However, ILS effects on SHBG and E2 differed between HT users and non-users, and SHBG and E2 had associations with glucose that differed by HT use. These findings suggest that the roles of SHBG and E2 in influencing glucose levels depend upon whether these hormones are exogenous or endogenous. Among HT users, ILS did not change SHBG, and SHBG was not associated with changes in FPG or 2HG. In contrast, among non-users, ILS led to increases in SHBG which were associated with decreases in glucose even after adjustment for adiposity and insulin sensitivity. Among HT users, ILS led to significant reductions in bioavailable E2 compared to placebo, although these changes were not associated with declines in glucose. Among non-users, ILS did not change E2 levels, although decreases in E2 were associated with decreases in FPG. Among HT users, the declines in FPG observed among metformin users did not appear to be associated with changes in SHBG, bioavailable E2, or E2. Among non-users, metformin had minimal impact upon glucose levels. In other words, the relationship between E2, SHBG and glucose were dissociated in HT users compared to non-users, even as interventions were still effective in reducing glucose in both HT users and non-users.
Our findings are notable for several reasons. First, we found that lifestyle modification can still reduce postprandial glucose levels in the context of HT use. In the Postmenopausal Estrogen/Progestin Intervention Study, randomization to HT was associated with significant reductions in FPG and fasting insulin, but with elevations in 2HG.7 The effects of exogenous estrogen on 2HG were not mitigated by lifestyle behaviors.7 Our results may have differed in that the DPP examined a glucose intolerant population, and any additional negative effects of HT on glucose tolerance may not have been as apparent. Also, women were randomized to lifestyle change which in turn resulted in significant reductions in weight and glucose compared to placebo. Second, we found that SHBG was not associated with reductions in glucose levels in HT users, although SHBG was associated with reductions in both FPG and 2HG in non-users.1 Among non-users, hepatic steatosis may be a common antecedent of SHBG and glucose, or SHBG may influence hepatic glucose production apart from steatosis.21 HT use itself was associated with almost a tripling of SHBG levels, and it is possible that further increases in SHBG resulting from ILS had minimal effects on glucose levels. Third, we found that bioavailable E2 declined among HT users randomized to ILS and metformin compared to placebo, although we did not observe these changes among non-users. Baseline E2 levels were lower among non-users compared to HT users, and it is possible that further reductions in bioavailable E2 were minimized by these low levels. However, among HT users, these reductions in bioavailable E2 were not significantly associated with reductions in glucose. Even though reductions in bioavailable E2 were associated with reductions in 2HG among metformin users, metformin users did not have statistically significant declines in 2HG overall.
To our knowledge, no reports have examined the impact of metformin in postmenopausal women without polycystic ovarian syndrome. Among HT users in this report, metformin had favorable effects on FPG with less marked reductions in 2HG. Metformin effects upon FPG did not appear to be mediated by metformin effects upon E2 levels, since total E2 and bioavailable E2 were not associated with FPG among HT users. Thus, sex hormone effects do not appear to be an important pathway for metformin effects on glucose among postmenopausal women using exogenous estrogen. Among non-HT users, metformin effects upon FPG and 2HG were minimal, consistent with other DPP studies demonstrating reduced effectiveness of metformin in older populations.22 While metformin has decreased sex steroid levels in women with polycystic ovarian syndrome,23 sex steroid levels including estradiol were higher in women with polycystic ovarian syndrome than the postmenopausal women in this report. Thus, for different reasons compared to HT users, sex hormones do not appear to be an important pathway for metformin effects on glucose among postmenopausal women not using estrogen.
Limitations of this report are that women were not randomized to HT use as well as to lifestyle intervention. To assess optimally the interactions between HT use and glucose-reduction interventions, randomization to HT use as well as to ILS, metformin, and placebo would have been necessary, although such a study is unlikely to be conducted. As HT use was strongly associated with surgical menopause, particularly bilateral oophorectomy, this would have required additional adjustment for indications of surgical menopause, which would have been logistically challenging. Therefore, despite uniform inclusion criteria for weight and glucose elevations, HT users differed from non-users regarding demographic characteristics, anthropometrics, and metabolic measurements, and these characteristics as well as other unmeasured characteristics may have led to the different roles of E2 and SHBG in glucose changes. It is possible that residual confounding from adiposity or insulin sensitivity occurred as the proxy measures used were not direct measures. HT was assessed via recording of medications and may have over- or underestimated HT use. While overweight and obese women tend to have lower FSH levels than normal weight women in the postmenopause,24 some of the non-estrogen users may have been misclassified as being postmenopausal as opposed to premenopausal, and in turn, this may have increased or decreased observed associations. Although the analyses did not differ when we examined estrogen users only vs. estrogen-progestin users, progestin type may have altered the hormonal milieu. Finally, the women studied were already overweight and glucose intolerant, and our results may not extend to healthier women. Strengths of our report include its randomized design for glucose-reducing interventions, interventions which successfully reduced waist circumference, and performance of post-challenge as well as fasting glucose.
Conclusions
Our hypothesis-generating report suggests that glucose-lowering interventions might act via different mechanisms in estrogen users and non-users, and specifically that glucose-reduction interventions are effective among HT users, and that the role of E2 and SHBG in glucose change differs depending upon whether women use HT. Intervention-associated increases in SHBG may be less important among women who use HT in part due to significant elevations in SHBG associated with HT. While there were reductions in bioavailable E2 among HT users with lifestyle change, the clinical significance of this finding is not clear. Metformin may be less effective for reducing post-challenge glucose among postmenopausal women than ILS, but this effect is observed among non-users as well as HT users and is likely not due to adverse effects of HT.
Acknowledgments
Financial support: National Institutes of Health U01DK048489, R01DK083297, and K23DK071552
The Investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies. A complete list of centers, investigators, and staff can be found in reference 5. We thank Frank Stanczyk (University of Southern California, Los Angeles) for provision of the macro enabling calculation of bioavailable E2. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; collection, management, analysis, and interpretation of the Diabetes Prevention Program. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other agencies.
Footnotes
Conflicts of interest: None declared.
These results were presented in abstract form at the 2012 American Diabetes Association Scientific Sessions in Philadelphia, Pennsylvania.
Contributor Information
Catherine Kim, University of Michigan, Ann Arbor, MI
Shengchun Kong, University of Michigan, Ann Arbor, MI
Gail A. Laughlin, University of California, San Diego
Sherita H. Golden, Johns Hopkins University, Baltimore, MD
Kieren J. Mather, Indiana University, Indianapolis, IN
Bin Nan, University of Michigan, Ann Arbor, MI
John F. Randolph, Jr., University of Michigan, Ann Arbor, MI
Sharon L. Edelstein, George Washington University, Rockville, MD
Fernand Labrie, Laval University, Quebec City, Canada and Al Imam Mohammed Ibn Saud Islamic University, Riyadh, Saudi Arabia
Elizabeth Buschur, University of Michigan, Ann Arbor, MI
Elizabeth Barrett-Connor, University of California, San Diego
References
- 1.Kim C, Nan B, Laughlin G, et al. Endogenous sex hormone changes in postmenopausal women in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalyani R, Franco M, Dobs A, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–35. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh J, Barrett-Connor E, Wedick N, Wingard D. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Ding E, Song Y, Manson J, Rifai N, Buring J, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–84. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 5.Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrinoudaki I, Armeni E, Rizos D, et al. Sex hormones in postmenopausal women receiving low-dose hormone therapy: the effect of BMI. Obesity (Silver Spring) 2011;19:988–93. doi: 10.1038/oby.2010.232. [DOI] [PubMed] [Google Scholar]
- 7.Espeland M, Hogan P, Fineberg S, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators Diabetes Care. 1998;21(10):1589–95. doi: 10.2337/diacare.21.10.1589. [DOI] [PubMed] [Google Scholar]
- 8.Troisi R, Cowie C, Harris M. Hormone replacement therapy and glucose metabolism. Obstet Gynecol. 2000;96:655–70. doi: 10.1016/s0029-7844(00)00980-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Howard B, Cowan L, et al. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in American Indian postmenopausal women: the Strong Heart Study. Diabetes Care. 2002;25:500–4. doi: 10.2337/diacare.25.3.500. [DOI] [PubMed] [Google Scholar]
- 10.van Genugten R, Utzschneider K, Tong J, et al. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes. 2006;55:3529–35. doi: 10.2337/db06-0577. [DOI] [PubMed] [Google Scholar]
- 11.Davidson M, Maki H, Marx P, et al. Effects on continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med. 2000;160(21):3315–25. doi: 10.1001/archinte.160.21.3315. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya A, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Sites C, L’Hommedieu G, Toh M, Brochu M, Cooper B, Fairhurst P. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:2701–7. doi: 10.1210/jc.2004-1479. [DOI] [PubMed] [Google Scholar]
- 14.Godsland I, Manassiev N, Felton C, et al. Effects of low and high-dose oestradiol and dydrogesterone therapy on insulin and lipoprotein metabolism in healthy postmenopausal women. Clin Endocrinol (Oxf) 2004;60:541–9. doi: 10.1111/j.1365-2265.2004.02017.x. [DOI] [PubMed] [Google Scholar]
- 15.Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 16.Kitabchi A, Temprosa M, Knowler W, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–14. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labrie F, Martel C, Balser J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: the role of the ovary? Menopause. 2011;18(1):30–43. doi: 10.1097/gme.0b013e3181e195a6. [DOI] [PubMed] [Google Scholar]
- 18.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 19.Hamman R, Wing R, Edelstein S, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray G, Jablonski K, Fujimoto W, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87(5):1212–8. doi: 10.1093/ajcn/87.5.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peter A, Kantartzis K, Machann J, et al. Relationship of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59(12):3167–73. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–81. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel S, Iqbal N, Kaul S, et al. Effects of metformin and leuprolide acetate on insulin resistance and testosterone levels in nondiabetic postmenopausal women: a randomized, placebo-controlled trial. Fertil Steril. 2010;94(6):2161–6. doi: 10.1016/j.fertnstert.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tepper P, Randolph J, Jr, McConnell D, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among Women in the Study of Women’s Health across the Nation (SWAN) J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]