Abstract
Inbreeding depression and lack of genetic diversity in inbred mice could mask unappreciated causes of graft failure or remove barriers to tolerance induction. To test these possibilities, we performed heart transplantation between outbred or inbred mice. Unlike untreated inbred mice in which all allografts were rejected acutely (6–16 days post-transplantation), untreated outbred mice had heterogeneous outcomes, with grafts failing early (<4 days post-transplantation), acutely (6–24 days), or undergoing chronic rejection (>75 days). Blocking T cell costimulation induced long-term graft acceptance in both inbred and outbred mice, but did not prevent the early graft failure observed in the latter. Further investigation of this early phenotype established that it is dependent on the donor, and not the recipient, being outbred and that it is characterized by hemorrhagic necrosis and neutrophilic vasculitis in the graft without pre-formed, high titer anti-donor antibodies in the recipient. Complement or neutrophil depletion prevented early failure of outbred grafts, whereas transplanting CD73-deficient inbred hearts, which are highly susceptible to ischemia-reperfusion injury, recapitulated the early phenotype. Therefore, outbred mice could provide broader insight into donor and recipient determinants of allograft outcomes but their hybrid vigor and genetic diversity do not constitute a uniform barrier to tolerance induction.
Keywords: outbred, transplantation, inbreeding depression, innate immunity, complement, neutrophils
Introduction
Inbred mouse strains have been instrumental in uncovering fundamental immunological mechanisms that underlie transplant rejection. The utility of the inbred mouse as a pre-clinical model for testing transplantation tolerance strategies, however, has been more limited (1, 2). Administration of biological agents that readily induce tolerance in inbred mice, for example, often fail in large animals and humans. Failure has been attributed to the evolutionary separation (~ 65 million years) between mice and humans, reduced exposure of laboratory mice housed in barrier facilities to microbial pathogens, and/or the biological consequences of inbreeding (1, 2). The latter include inbreeding depression and restricted genetic diversity within and between inbred mouse strains (3–5).
Known discrepancies between mice and humans that can be attributed to evolutionary separation span both innate and adaptive immunity (6). Humanized mice in which the human immune system has replaced that of the mouse are beginning to elucidate the importance of these differences (7), but it remains unclear whether they are superior to inbred mice as pre-clinical models of organ transplantation. The impact of specific-pathogen free (SPF) housing on the ease by which transplantation tolerance can be induced in laboratory mice, on the other hand, is supported by heterologous immunity studies. Mice previously exposed to microbial pathogens generate memory T cells that cross-react with allogeneic MHC molecules and become resistant to tolerance-inducing agents (8–10). In contrast, naïve laboratory mice harbor less memory T cells and can be readily made to accept MHC-mismatched allografts. As cross-reactive memory constitutes a substantial component of the human alloreactive T cell repertoire (11, 12), heterologous immunity could provide one explanation why transplantation tolerance strategies that succeed in mice often fail in humans.
In addition to their rich repertoire of alloreactive memory T cells, humans are an outbred species characterized by considerable heterozygosity and genetic diversity. Both traits contribute to the vigorous yet diverse immune responses of humans; raising the possibility that outbreeding among humans is an important reason why transplantation tolerance or allograft acceptance strategies do not translate uniformly from inbred mice to patients. Except for one study in which outbred mice were found to require more stringent immunosuppression than their inbred counterparts to achieve islet allograft survival (13), the effect of outbreeding on allograft outcomes, particularly clinically-relevant vascularized grafts, has not been tested yet. Here we investigated the effects of outbreeding in the donor, recipient or both on the survival of vascularized heart allografts in mice.
METHODS
Mice
B6 male mice (6–8 weeks old) (Jackson Laboratory), non-sibling, CD-1 and CF-1 outbred male and female mice (4 weeks old) (Charles River Laboratory; CRL), and BALB/c, FVB, and SJL inbred male mice (6–8 weeks old) (also from CRL) were housed under SPF conditions. B6 CD73−/− mice were a generous gift from Linda F. Thompson (Oklahoma Medical Research Foundation). Serum and tail snips were obtained prior to transplantation. PCR analysis to type MHC II loci was performed at CRL (14). All procedures were IACUC approved.
Transplantation and Tissue Processing
Vascularized, heterotopic cardiac transplantation was performed as described (15). Vascular anatomy was similar between inbred and outbred mice and surgical mortality was equivalent in the two groups (3.8% and 4.3%, respectively). Grafts were monitored daily and harvested upon cessation of palpable heartbeat or after 75 days. Serum was collected at harvest and graft tissue was snap frozen and embedded in OCT (Sakura Finetek), or paraffin and stained with hematoxylin and eosin (H&E). Histological analysis was performed by AJD in a blinded fashion. Donor splenocytes and thymocytes were harvested at time of surgery.
Mouse Treatment
15 mg/kg MR1 (anti-CD40L mAb) and 15 mg/kg CTLA-4-Ig (BioExpess and BioXCell) were given i.p. on days 0, 2, 4, and 6 to induce long-term graft survival. C3 was depleted by injecting 10 units cobra venom factor (CVF) (Quidel Corporation) in 3 divided doses i.p. one day before transplantation. Neutrophils were depleted by injecting 250ug anti-Ly6G (1A8) antibody (BioXCell) i.v. one day prior to transplantation (16). Adenosine analog 5′-N-ethylcarboxamidoadenosine (NECA) (Sigma) was administered to CD-1 donors (0.1 mg/kg) and CD-1 recipient mice (0.01 mg/kg) i.p. 4 hours prior to transplantation.
Agglutination Assay
A panel of RBCs from 10 outbred mice were incubated with pre-transplant serum (diluted 1:2) from 7 outbred mice that had accelerated graft loss for 30 minutes at 37°C, centrifuged at 400g, resuspended, and examined for agglutination. If agglutination was absent, the plate was washed and 10μg anti-mouse-Ig (Chemicon) added to each well before re-assessing for agglutination.
Flow Cytometry
To measure pre-formed antibodies to donor MHC, serum was diluted 1:2 and incubated with thymocytes from their respective donors for 20 minutes at 4°C, stained with anti-IgM and anti-IgG antibodies (BD Biosciences) and analyzed on an LSRII cytometer.
Immunofluorescence
Antibody deposits were examined in allografts transplanted between outbred mice. As a positive control, we transplanted BALB/c hearts into C57Bl/6 mice pre-sensitized by transplanting BALB/c skin grafts10 days earlier. Allografts were harvested at 6 or 12 hours post transplantation and frozen in OCT. 6 μm sections were fixed, blocked (Vectabond ABC Blocking Kit, Vector Laboratories), and stained with FITC-conjugated anti-IgG or anti-IgM (Zymed Laboratories) at room temperature for 1 hour. To identify neutrophils, sections were incubated with unconjugated anti-Ly6G and biotin conjugated CD31 (eBioscience) overnight at 4°C. After incubating with secondary antibodies, the tissue was counterstained with DAPI (Molecular Probes).
Enzyme-linked Immunosorbent Assay (ELISA)
C3 serum levels were measured pre-transplantation and on days 1, 5, 7, and 11 after transplantation using mouse C3 ELISA kit (Kamiya Biomedical) per manufacturer’s instructions.
Statistical Analysis
Flow cytometry data is shown as mean fluorescent intensity (MFI) and standard error of mean (SEM), and analyzed by unpaired Student’s T-test. Presence or absence of early graft failure was analyzed using a two-sided Fischer’s exact test. Median survival time (MST) comparisons were performed by Gehan-Breslow-Wilcoxon test.
Results
Genetic diversity of outbred mouse stocks
CD-1 and CF-1 outbred mouse stocks, defined as closed populations of genetically variable animals that are bred to maintain maximum heterozygosity (17), were used as either donors or recipients of heart grafts. Mice from either stock displayed outbred vigor judged by greater body weight and larger litter size than inbred strains (http://www.criver.com). To determine their diversity, we genotyped class II H-2 loci by PCR. Of 12 alleles that were genotyped, four (p, b, q, and u) were represented in the CD-1 (n = 364) and five (p, b, q, u, and k) in the CF-1 stock (n = 79), resembling the diversity of an isolated ‘island’ population (18). The extent of heterozygosity at these loci was 0.60 and 0.74 in the CD-1 and CF-1 stocks, respectively, which is similar to the average heterozygosity of feral mice and humans (18). Therefore, the outbred mouse stocks used in this study are somewhat limited in their diversity but maintain a significant degree of heterozygosity typical of outbred populations.
Blocking T cell costimulation induces long-term allograft acceptance in outbred mice
We hypothesized that a standard tolerogenic regimen that induces allograft acceptance in inbred mice would be less effective in outbred mice. This was tested by comparing the survival of heart allografts transplanted between disparate inbred mouse strains (BALB/c to B6, n = 41) to that of allografts transplanted between non-sibling outbred mice (CD-1 to CD-1, n = 33, or CF-1 to CD-1, n = 28). Recipients were either left untreated or received a combination of CTLA4-Ig and anti-CD154 (MR1) to block T cell costimulation at the time of transplantation. As expected, T cell costimulation blockade induced 100% allograft acceptance (graft survival > 75 days) in the inbred group while all untreated recipients rejected their allografts acutely (MST = 8 days) (Fig. 1a). In contrast, graft outcomes were not uniform in the outbred group (Fig 1b). In untreated mice, grafts failed either very early (1 – 4 days after transplantation, 10/35 or 29%), acutely (6 – 24 days, 19/35 or 54%), or underwent chronic rejection (> 75 days, 6/35 or 17%) with graft MST remaining similar to that of the inbred group (10 vs 8 days, p = 0.52). In treated, outbred mice, the response was dichotomous with 7/26 (27%) losing their grafts very early and 19/26 (73%) achieving long-term graft survival. The rate of graft acceptance in the treated, outbred group was significantly greater than that in the untreated outbred group (73% vs 17%, p < 0.0001). Treated, outbred recipients that did not manifest the unusual early graft failure went on to have 100% (19/19) long-term allograft acceptance similar to the treated inbred group. Allograft acceptance after costimulation blockade occurred independent of outbred stock combination used (69% in CD-1 to CD-1 and 80% in CF-1 to CD-1, n = 16 and 10, respectively, p = 0.67). Histopathological analysis revealed mild cellular infiltration, minimal fibrosis, and focal chronic vasculopathy (intimal thickening) in long-term accepted allografts in the treated inbred and outbred groups, while long-term surviving allografts in the untreated outbred group exhibited chronic rejection, ranging from severe to focal (Fig. 1c). These data indicate that genetic diversity and heterozygosity (hybrid vigor) lead to more heterogeneous graft outcomes in untreated mice but do not constitute a uniform barrier to graft acceptance after induction therapy.
Fig 1. Effect of costimulation blockade on allograft survival in inbred and outbred mouse groups.

Survival of (a) inbred Balb/c allografts transplanted to inbred B6 recipients (I to I) and (b) outbred (CD-1 or CF-1) allografts transplanted to outbred CD-1 recipients (O to O) was assessed in the presence or absence of recipient treatment with costimulation blockade (MR-1 + CTLA4-Ig). (c) Histopathology (H&E) of allografts that survived > 75 days in treated I to I group (left panel) and either treated or untreated O to O groups (right panels). Magnification = 2×. Insets show evidence of chronic allograft vasculopathy in all groups (magnification = 30×).
Increased early graft failure in outbred mice
An unexpected finding that emerged from the above experiments is that all treated, outbred mice that lost their allografts (27%) did so between 1 and 4 days after transplantation (Fig. 1b). This accelerated graft loss was also observed in a similar proportion (29%) of untreated mice in the outbred group (Fig. 1b), but in none (0/41) of the untreated or treated mice in the inbred group (p < 0.0001; Fig. 1a). Early graft failure was not influenced by the particular outbred stock combination used (33% in CD-1 to CD-1 and 21% in CF-1 to CD-1, p = 0.40). The gross morphology and histopathology of early graft failure were distinct from classical acute cellular rejection (Fig. 2). Allografts that failed very early were significantly enlarged and were dark in color. Histopathology revealed extensive hemorrhagic necrosis with neutrophilic margination and focal neutrophilic arteritis without mononuclear cell infiltration (Fig. 2, left panels). In contrast, acutely rejected hearts in the untreated outbred group exhibited typical severe acute lymphocytic infiltration with arteritis (Fig 2, right panels). These findings suggest that the unusually high early graft failure observed exclusively in the outbred group is caused by an inflammatory or innate immune process.
Fig 2. Histopathology of early graft failure.
Representative pathology of cardiac allografts transplanted between untreated outbred mice that failed < 4 days (left panels) or between 6 and 24 days (right panels) after transplantation is shown. Gross appearance of allografts is shown in the top panels, H&E stained tissue sections in the middle panels (magnification = 4×), and immunofluorescent stained tissue section in the bottom panel. Insets in left middle panel highlight areas of neutrophil clot and neutrophilic vasculitis, while inset in right middle panel demonstrates lymphocytic arteritis typical of untreated acute cellular rejection. Note large area of hemorrhagic necrosis (*) in the early graft failure but not acute cellular rejection phenotype. Ly6G, CD31 and DAPI identify neutrophils, endothelial cells, and nuclei, respectively (bottom panel; magnification = 20×).
Early graft failure is dependent on donor’s outbred status
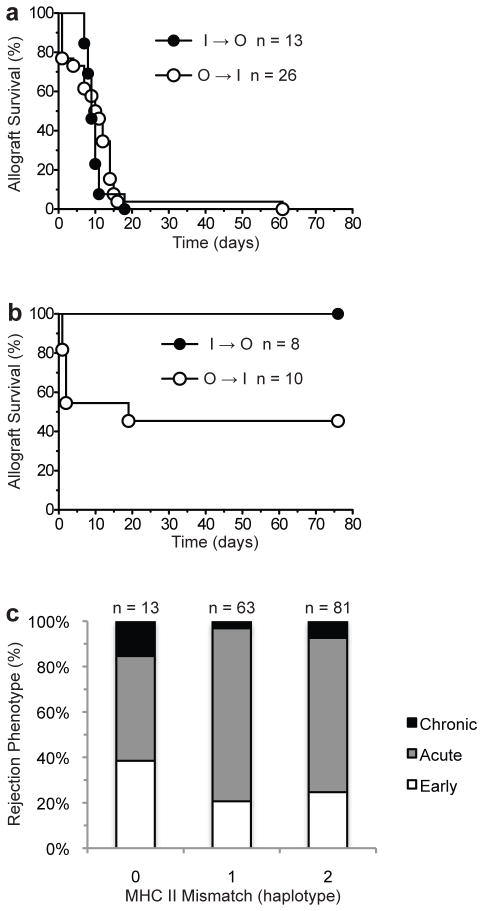
Data from mouse and human studies suggest that allograft outcomes are not only determined by the recipient’s genetic background but also by that of the donor. To investigate whether recipient or donor outbred status is responsible for early graft failure, we transplanted inbred (BALB/c) hearts into outbred CD-1 mice and vice versa. Donors and recipients were weight-matched to avoid unanticipated surgical complications that could arise from transplanting grafts into size-mismatched recipients. In untreated recipients, early graft failure was observed in 7/26 (27%) of inbred recipients of outbred hearts (outbred to inbred, O to I, group) but in none of the outbred recipients of inbred grafts (inbred to outbred, I to O, group) (Fig. 3a). The donor outbred status effect was also observed in immunosuppressed recipients. Costimulation blockade induced 100% allograft acceptance in the inbred to outbred group, while 5/10 (50%) of grafts failed in the first 4 days in a similarly treated outbred to inbred cohort (Fig. 3b). Analysis of all outbred to outbred or outbred to inbred transplantation experiments showed that graft outcome (early failure, acute rejection, and chronic rejection) did not correlate with the degree of MHC class II mismatch between donor and recipient (Fig. 3c).
Fig 3. Early graft failure is dependent on donor outbred status.
Survival of cardiac allografts transplanted from inbred to outbred (BALB/c to CD-1; I to O) or outbred to inbred (CD-1 to BALB/c; O to I) mice in the absence (a) or presence of costimulatory blockade (b). Note complete absence of early graft failure phenotype in recipients of allografts from inbred donors. (c) Degree of MHC II mismatch between all donor-recipient pairs used in this study as well as contemporaneous experiments involving outbred mice. Note that the degree of MHC II haplotype disparity between donor and recipient does not correlate with graft loss or rejection phenotype.
Since CD-1 animals were originally derived from the Swiss mouse stock (17), we then asked whether early graft failure is determined by genetic determinants specific to the Swiss background. To answer this question, we performed heart transplants using inbred Swiss strains as either donors (SJL to C57Bl/6, n = 23) or as both donors and recipients (SJL to FVB, n = 9). Of these, only 1/23 (3%) (p = 1.0 compared to I to I group) in the former and none in the latter group exhibited the early graft failure phenotype, indicating that the Swiss background of CD-1 mice does not account for the high incidence of early graft failure observed in the outbred to inbred (Fig. 2a & 2b) or in the outbred to outbred (Fig. 1b) transplantation experiments.
Role of antibodies in pathogenesis of early graft failure
The histopathology of early graft failure described above bears striking resemblance to that of hyperacute rejection observed in sensitized transplant recipients who harbor pre-formed anti-bodies against donor ABO or HLA antigens (19, 20). We therefore tested for such antibodies in the pre-transplantation serum of mice that went on to develop early graft failure. Neither pre-transplantation serum from 7 such recipients (all outbred) nor serum from additional 10 untransplanted outbred mice caused agglutination of blood from a panel of 10 unrelated outbred mice (data not shown), indicating absence of significant pre-formed hemagglutinins. These results are consistent with reported lack of demonstrable expression or function of blood group antigens in mice (21, 22). We then tested whether recipients that went on to develop early graft failure had pre-formed IgG or IgM antibodies against donor MHC by incubating pre-transplantation serum with donor thymocytes. As shown in Fig. 4a, no anti-donor IgG antibodies were detected while a small increase in IgM antibodies over that found in pre-transplantation serum of one cohort of mice (O to I) that developed acute rejection could be identified, with the caveat that all groups had very low IgM levels to start with. These data indicate the absence of significant pre-formed IgG or IgM anti-MHC antibodies in recipients that develop early graft failure but do not rule out the presence of antibodies directed at other tissue antigens. To explore the latter possibility, we assessed antibody binding to graft tissue 6 and 12 hrs after transplanting outbred hearts into inbred recipients. IgM and IgG could not be detected at 6 & 12 hrs in grafts that already displayed early histopathologic evidence of hemorrhagic necrosis (Fig. 4b). Grafts harvested from prior experiments that had already developed conspicuous hemorrhagic necrosis at 24 hrs after transplantation stained strongly with fluorescein-conjugated anti-IgM or anti-IgG antibodies (micrographs not shown). The presence of significant tissue damage and hemorrhage at the 24 hr time point, however, makes it likely that fluorescein conjugated antibodies bound to complement and Ig in the extravasated blood rather than in the heart tissue. These data therefore rule out the presence of significant, pre-formed, anti-donor antibodies in recipients that develop early graft failure but do not exclude a contribution of low titer antibodies to the observed pathology.
Fig 4. Lack of significant, preformed anti-donor antibodies in recipients that exhibited early graft failure.
(a) Pre-formed, anti-donor thymocyte IgG (left panel) and IgM (right panel) antibodies in serum of recipients of outbred allografts that went on to develop early graft failure. Serum was obtained prior to transplantation in all mice except the sensitized group. MFI of isotype control antibody was subtracted in each case to determine net binding of IgG or IgM antibodies to donor thymocytes. (b) IgG and IgM deposits in cardiac allografts harvested at indicated time points after transplantation. Top panels (positive control) show IgG and IgM deposits in BALB/c cardiac allografts 12 hrs after transplantation to sensitized C57Bl/6 mice. Middle and bottom panels show lack of IgG and IgM deposits in outbred allografts (CD-1 to CD-1) harvested at 6 and 12 hrs after transplantation. These grafts had evidence of interstitial congestion, focal neutrophilic margination, and platelet fibrin thrombi at 6 hrs, and hemorrhagic necrosis with moderate neutrophilic margination at 12 hrs (H&E micrographs), consistent with early graft failure phenotype (magnification = 2×, inset magnification = 30×).
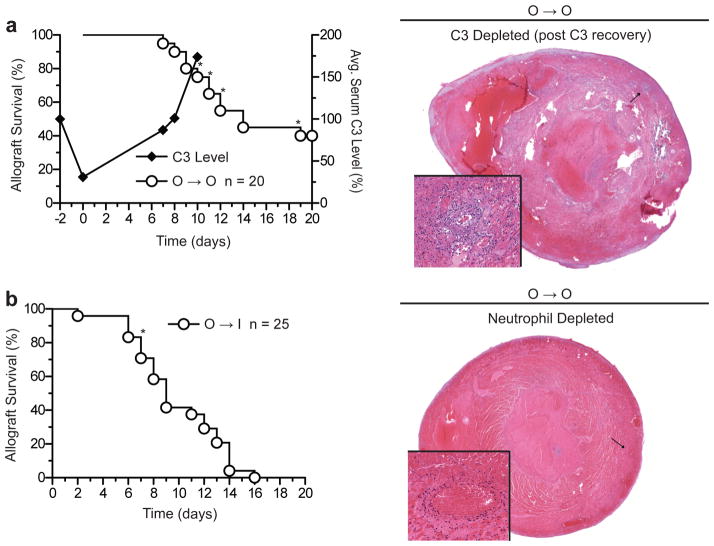
Early graft failure is dependent on complement activation and neutrophils
Because of the characteristics of the early graft failure phenotype observed so far, we sought to investigate innate mechanisms that could contribute to this process. We first depleted circulating C3 in outbred donor and recipient mice by administering cobra venom factor (CVF) around the time of transplantation. We found that C3 depletion completely abrogated early graft failure (n = 0/20, p = 0.02 compared to untreated O to O group) (Fig. 5a), but as C3 levels returned to baseline, 5 heart grafts failed between days 8 and 18 after transplantation with histopathologic manifestations of hemorrhagic necrosis (Fig. 5a). These results establish a cause-effect relationship between the complement cascade and early graft failure. They also confirm that the early hemorrhagic necrosis phenotype is not a reflection of an unanticipated high surgical failure rate but is a consequence of a biological process dependent on complement activation.
Fig 5. Early graft failure is dependent on complement and neutrophils.
Survival of cardiac allografts after complement depletion in both donors and recipients (CD-1 to CD-1; O to O) (a) or after neutrophil depletion in the recipients (CD-1 to BALB/c; O to I) (b). Transplantation was performed on day 0. Average C3 levels in recipient blood are depicted as % of baseline level (right-hand y-axis in (a)). *Grafts that exhibited early graft failure phenotype by gross morphology and/or histopathology. H&E micrographs confirmed hemorrhagic necrosis and neutrophilic vasculitis in these grafts (magnification = 2×, inset magnification = 30×).
Since neutrophil infiltration and neutrophilic vasculitis were prominent features of grafts that failed very early (Fig. 2), we then investigated the effect of neutrophil depletion on the incidence of early graft failure. As shown in Fig. 5b, only 2/25 (8%) of neutrophil-depleted inbred recipients of outbred allografts developed hemorrhagic necrosis, which is significantly less than the incidence observed in control, neutrophil-replete mice (7/26 or 27%, p = 0.03). Of the two grafts that had hemorrhagic necrosis in the neutrophil-depleted group, one failed on day 2 with significant neutrophil infiltration, suggesting incomplete neutrophil depletion of the recipient. The other failed on day 7, again with conspicuous neutrophil but no mononuclear cell infiltration, suggesting that early graft failure phenotype could have been precipitated by return of neutrophils to the circulation. These data indicate that neutrophils are important contributors to the pathogenesis of early graft failure.
Increasing the transplanted tissue’s susceptibility to ischemia-reperfusion injury recapitulates early graft failure
The pathology and mechanisms of early graft failure described so far suggest that donor factors, possibly related to susceptibility of donor tissues to ischemia-reperfusion (I/R) injury, contribute to the early graft failure phenotype. To test this hypothesis, we investigated whether transplanting heart allografts from inbred CD73−/− donors, known to have exaggerated tissue I/R responses (23), recapitulates early graft loss otherwise typical of outbred grafts. CD73 is an ectonucleotidase expressed on endothelial cells that downregulates I/R injury by catalyzing the hydrolyis of extracellular AMP to the anti-inflammatory metabolite adenosine (24). We found that CD73−/− B6 hearts transplanted to BALB/c mice exhibit high incidence of early graft failure (4/18 or 22%) similar to that of outbred allografts transplanted to inbred recipients (7/26 or 27%, p = 1.0). Histopathology confirmed that early graft failure was due to hemorrhagic necrosis and neutrophilic vasculitis. Conversely, treating donors and recipients with NECA, a broad adenosine receptor agonist, abrogated early graft loss in the outbred to outbred model (n = 0/14, p = 0.02). These results suggest that donor factors that underly susceptibility to I/R injury also play a role in the pathogenesis of early graft failure after transplantation.
Discussion
It has been postulated that hybrid vigor and genetic diversity of the outbred human population could explain why immune therapies that are successful in inbred mouse models sometimes fail in humans (1). In this study, we found that costimulation blockade induces long-term allograft acceptance in outbred mouse stocks implying that hybrid vigor cannot account for the discrepancy between humans and inbred mice but does not completely rule out the contribution of genetic diversity. The outbred mouse stocks used in this study have been bred to maintain maximum heterozygosity (thus, hybrid vigor) but are still somewhat limited in their genetic diversity compared to the human population (18). It is possible then that costimulation blockade may fail to induce allograft acceptance if a more diverse outbred mouse population was studied. Such a population, known as Diversity Outbred (DO) mice, has been recently generated but remains limited in its availability to investigators (http://jaxmice.jax.org/strain/009376.html) (25). Investigating allograft outcomes in these mice in the future should resolve whether simultaneous presence of maximal heterozygosity (hybrid vigor) and allelic variation (diversity) impedes allograft acceptance. Moreover, it is possible that the contribution of these genetic variables is only apparent when outbred mice are housed in non-SPF facilities to increase the repertoire of alloreactive memory T cells through heterologous immunity.
A potentially interesting observation in the outbred to outbred transplantation group (Fig. 1b) is the occurrence of spontaneous long-term allograft survival (>75 days), albeit with significant chronic rejection, in approximately 17% of untreated recipients. The reason for this phenomenon was not addressed in this study but could be related to matching between donors and recipients at non-H-2 loci. By performing a large number of heart transplants between inbred mouse strains matched at either H-2 or non-H-2 loci, Peugh et al found spontaneous long-term graft survival in 25% of recipients matched at H-2 but mismatched at non-H-2 loci (and vice versa) (26), suggesting that non-H-2 loci modulate the rejection response. Identifying such loci may be a difficult undertaking at present as it is likely that many loci with additive or opposite effects (quantitative trait loci) exist. Moreover, the spontaneous long-term allograft survival phenotype observed in our study all but disappeared when only donors or recipients were outbred (Fig. 3a), implying a complex interplay between donor and recipient determinants. This warrants careful analysis in the future.
A principal finding in our study is the identification of a dramatic, early graft failure phenotype characterized by neutrophilic vasculitis and hemorrhagic necrosis in 29 of a total of 97 outbred grafts (30%), but in only 1 of a total of 94 inbred grafts (1%), transplanted to either inbred or outbred mice. All transplants reported in this study were contemporaneous and were performed by the same microsurgeon (Q.L.). Moreover, surgical mortality, which occurred in the first day after transplantation due to failure of the vascular anastomosis, was equivalent in the outbred and inbred graft groups (3.8% and 4.3%, respectively). These facts make it less likely that the early graft failure phenotype was a consequence of high technical failure rate in mice that received outbred grafts. The most compelling evidence that early graft failure is a bona fide biological phenotype is its abrogation when specific biological mediators were eliminated (for example, after complement or neutrophil depletion) and its reappearance at later time points when the mediators had rebounded.
The timing and histopathologic hallmarks of the early graft failure phenotype and its dependence on complement but not T cell costimulation strongly indicate that it is caused by innate or inflammatory mechanisms. Although we did not identify the initial trigger of the inflammatory cascade that led to acute hemorrhagic necrosis, one possibility is the binding of complement-fixing, low titer antibodies that evaded detection by either serology or immunofluorescence. Carroll and colleagues have shown that natural IgM antibodies present in low titers initiate ischemia-reperfusion injury in the heart, intestine, and skeletal muscles (27). These antibodies bind to self-antigens exposed after tissue ischemia and are potent activators of the complement cascade (28). Alternatively, IgM antibodies with anti-donor allospecificities may have been present in recipients that exhibited the early graft failure phenotype. Another possibility is that hemorrhagic necrosis was triggered by the alternative pathway of complement activation, which is independent of antibody binding to the affected tissue. The role of the alternative pathway in the pathogenesis of ischemia reperfusion injury has been established in a variety of experimental models (29).
The predominance of the neutrophil among inflammatory cells infiltrating grafts undergoing hemorrhagic necrosis is not surprising. Neutrophils are attracted to sites of ischemia-reperfusion injury via many mediators, including products of complement activation. The early graft failure phenotype observed in our experiments bears striking resemblance to accelerated rejection of cardiac allografts by IFNγ-deficient mice (30), and to accelerated rejection of IFNγ receptor-deficient renal allografts by wildtype recipients (31). In both cases, microscopic examination of the grafts revealed intense neutrophilic infiltration and parenchymal necrosis. As in our model, neutrophil depletion prevented accelerated rejection, further underscoring the importance of the neutrophil as a mediator of early inflammatory events after organ transplantation.
Another principal finding in our study is that the early graft failure phenotype was dependent on the breeding status of the donor and not the recipient. That donor factors, independent of MHC matching, influence graft outcomes has been documented in both mice and humans (32). Several studies have pointed to the role of the donor complement system in influencing graft outcomes. For example, donor C3 deficiency leads to prolonged survival of kidney allografts while donor deficiency of decay-accelerating factor (DAF) accelerates cardiac allograft rejection in mice (33, 34). What donor factors influenced graft outcomes in our study is unclear. We tested whether differences in expression of the complement regulatory molecules DAF and Crry among donors correlate with presence or absence of early graft failure but did not find a significant correlation (Reichenbach, Lakkis & Heeger, unpublished). Our observation that inbred CD73−/− donors recapitulate early graft failure suggests that determinants of tissue susceptibility to I/R in the donor influence graft outcomes after transplantation. This warrants future studies to test whether CD39, CD73, and adenosine receptor expression in outbred mice correlates with graft outcomes.
The model of hemorrhagic necrosis described in this manuscript underscores the importance of the donor in shaping graft outcomes and provides an opportunity to gain better understanding of the inflammatory events that lead to graft injury. Interrupting these events could prevent the adverse consequences of ischemia-reperfusion injury on early graft function and possibly temper innate responses that adversely affect long-term allograft outcomes (35).
Acknowledgments
We acknowledge Dr. Linda Thompson (Oklahoma Medical Research Foundation) for critical reading of the manuscript and Dr. Peter Heeger (Mount Sinai School of Medicine) for assistance with complement regulatory molecule measurements. This work was funded by NIH grant AI064343 to DMR, WDS, and FGL. DKR was funded by NIH/NIAID T32 grant AI 074490.
Abbreviations
- CVF
cobra venom factor
- O
outbred
- I
inbred
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.von Herrath MG, Nepom GT. Lost in translation: barriers to implementing clinical immunotherapeutics for autoimmunity. J Exp Med. 2005;202:1159–1162. doi: 10.1084/jem.20051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth B, Charlesworth D. The genetic basis of inbreeding depression. Genet Res. 1999;74:329–340. doi: 10.1017/s0016672399004152. [DOI] [PubMed] [Google Scholar]
- 4.Frankham R. Conservation genetics. Annu Rev Genet. 1995;29:305–327. doi: 10.1146/annurev.ge.29.120195.001513. [DOI] [PubMed] [Google Scholar]
- 5.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 6.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 7.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 8.Brehm M, Markees T, Daniels K, Greiner D, Rossini A, Welsh R. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infection. J Immunol. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 9.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macedo C, Orkis EA, Popescu I, Elinoff BD, Zeevi A, Shapiro R, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 13.Simeonovic CJ, Prowse SJ, Lafferty KJ. Reversal of diabetes in outbred mice by islet allotransplantation. Diabetes. 1986;35:1345–1349. doi: 10.2337/diab.35.12.1345. [DOI] [PubMed] [Google Scholar]
- 14.Saha BK. Typing of murine major histocompatibility complex with a microsatellite in the class II Eb gene. J Immunol Methods. 1996;194:77–83. doi: 10.1016/0022-1759(96)00065-8. [DOI] [PubMed] [Google Scholar]
- 15.Corry RJ, Winn HJ, Russel PS. Primarily vascularized allografts of hearts in mice: The role of H-2D, H-2K, and non H-2 antigens. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Zecher D, van Rooijen N, Rothstein D, Shlomchik W, Lakkis F. An Innate Response to Allogeneic Nonself Mediated by Monocytes. J Immunol. 2009;183:7810–7816. doi: 10.4049/jimmunol.0902194. [DOI] [PubMed] [Google Scholar]
- 17.Chia R, Achilli F, Festing MFW, Fisher EMC. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37:1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 18.Rice MC, O’Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980;283:157–161. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- 19.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 20.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after human renal homotransplantation. N Engl J Med. 1968;278:642–648. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller G. Survival of Mouse Erythrocytes in Histoincompatible Recipients. Nature. 1963;199:573–575. doi: 10.1038/199573a0. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Lin XH, Kominato Y, Hata Y, Noda R, Saitou N, et al. Murine equivalent of the human histo-blood group ABO gene is a cis-AB gene and encodes a glycosyltransferase with both A and B transferase activity. J Biol Chem. 2001;276:13701–13708. doi: 10.1074/jbc.M010805200. [DOI] [PubMed] [Google Scholar]
- 23.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, et al. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome. 2008;19:382–389. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peugh WN, Superina RA, Wood KJ, Morris PJ. The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics. 1986;23:30–37. doi: 10.1007/BF00376519. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks S, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol. 2003;15:487–492. doi: 10.1016/s0952-7915(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 30.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-gamma. Am J Path. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halloran PF, Afrouzian M, Ramassar V, Urmson J, Zhu L-F, Helms LMH, et al. IFN-g acts directly on rejecting renal allografts to prevent thrombosis during acute rejection. Am J Pathol. 2001;158:215–226. doi: 10.1016/s0002-9440(10)63960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 33.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 34.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan W-H, Lalli PN, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181:4580–4589. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD. Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2010;21:1987–1997. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]