Abstract
Objective
The objective was to evaluate the relationship between the time course of slow wave EEG activity (SWA) during NREM sleep and insulin sensitivity in adolescents.
Methods
Nine normal weight and nine overweight (BMI ≥ 85th percentile) adolescents (13–18 years of age) participated. None of the participants had a history of sleep disordered breathing, confirmed by sleep study. Participants maintained a regularized sleep wake cycle for five days followed by overnight polysomnography in the lab or at home. An oral glucose tolerance test (OGTT) was administered after a 12 hour fast and within two weeks of the sleep study. Whole body insulin sensitivity (WBISI) and homeostasis model assessment (HOMA-IR) determined insulin resistance. Power spectral analysis quantified slow-wave EEG activity (05–3.9 Hz) and exponential regression evaluated SWA across successive NREM periods.
Results
Those who were insulin resistant and had low insulin sensitivity had less Stages 2, 3 & 4 of NREM sleep, more Stage 1, but did not sleep less than those with low resistance and high sensitivity. SWA power was significantly lower in the first NREM period and the decay rate of SWA across NREM sleep was significantly slower in the low insulin sensitivity group. Similar results were obtained after removing the influence of BMI and Tanner score.
Conclusions
Insulin sensitivity in adolescents is related to SWA power and its time course, not total sleep time, regardless of BMI.
Keywords: sleep, delta EEG activity, children, adolescents, glucose tolerance, insulin sensitivity, metabolism, diabetes
Introduction
Epidemiological studies have shown that reduced total sleep time is associated with impaired glucose tolerance and an elevated risk for the development of type 2 diabetes in adults [1, 2]. Laboratory-based polysomnographic assessments of sleep have confirmed that the incidence of type 2 diabetes was nearly twice as high in those with sleep onset insomnia compared to those with sleep complaints, but no objective evidence of insomnia [3]. Further, the association between insomnia and incidence of diabetes remained after controlling for body mass index (BMI), suggesting that sleep may play a unique role in diabetic risk above that conferred by obesity.
Further, sleep restriction and sleep deprivation produce 30–40% worsening in glucose tolerance and acute insulin response to an oral glucose challenge in healthy adults [4, 5]. More recent studies indicate that a single night of partial sleep deprivation results in significant changes in hepatic and peripheral glucose metabolism and peripheral lipolysis [6]. In a similar study, a single night of total sleep deprivation was associated with impaired glucose tolerance in healthy young men that was equivalent to the level of glucose tolerance in non-sleep deprived diabetic men [5].
Using actigraphs to quantify sleep, Gozal and colleagues have shown that short and disrupted sleep in children is associated with high levels of fasting glucose and low levels of insulin sensitivity, low density lipoproteins and elevated C-reactive protein [7, 8]. Moreover, children with evidence of sleep disordered breathing or sleep apnea showed the greatest sleep disruption and the strongest evidence of impaired insulin sensitivity [9].
However, van Cauter and colleagues have shown that it is NREM sleep, and specifically delta waves EEG activity, that plays the strongest role in regulating leptin, ghrelin and appetite [5]. Most compelling, reducing delta waves results in a significant increase in glucose tolerance [10]. Since delta waves in NREM sleep, also known as slow-wave activity (SWA) are presumed to be a proxy of homeostatic sleep drive and the recovery function of sleep, SWA measures and assessing the baseline time course of SWA may be more strongly related to insulin sensitivity than sleep duration. To date, however, SWA measures have not been included in these studies and they have been restricted to adults. The purpose of the proposed study was to assess the time course of SWA and its relationship to insulin sensitivity in adolescents.
The purpose of the present study was to evaluate the relationship between SWA and subsequent insulin sensitivity in adolescents. Our hypothesis was that those with low SWA would be associated with high insulin resistance and low insulin sensitivity.
2. Methods
The study was approved by the Institutional review Board of the University of Michigan and all participants provided written informed consent (or assent for those under 18 years of age). Twenty 13–18 year olds were recruited for study, but for technical reasons, data were only available for 18 participants (15.5 ± 1.4 years), nine females and nine males. Half of participants were overweight based on a BMI ≥85th percentile [11]. Evidence of sleep disordered breathing by history or polysomnogram was exclusionary, as was current medication use. Tanner maturational development was assessed and all participants were Tanner Stage 3 or higher (4.6 ± 0.6).
Subjects were asked to maintain regular sleep/wake sleep schedules for five days prior to study, based on their habitual schedules and confirmed by sleep diary and actigraphy.
Standard polysomnography (PSG) was conducted on a single night in the home (n=8) or in the UM lab (n=10). EEG was recorded from C3 and C4, referenced to the earlobes and connected to a 10-k resistor to minimize nonhomogeneous current flow. The electrode montage also included left and right electro-oculogram (EOG) leads placed on both the upper and lower canthi; a bipolar, chin-cheek electromyography (EMG); leg leads, chest and abdomen respiration bands, and a nasal-oral thermistor. Impedances were maintained below 2 k. Records were scored according to Rechtschaffen and Kales [12] standardized criteria by research assistants trained to better than 90% agreement on an epoch-by-epoch basis. Scoring of respiratory events followed the AASM guidelines [13]. Using adult criteria, where apneas were defined as > 90% reduction in air flow of at least 10 second duration and hypopneas were defined as a 30% drop in flow of at least 10 ssecond duration, with ≥ four percent desaturation, none of the subjects in the present study had an AHI >0. In fact, none of the participants showed respiratory events that lasted 20 seconds or longer and were associated with ≥3% desaturation. EEG arousal was defined as an abrupt shift to higher frequency of > three seconds duration and preceded by > 10 seconds of stable sleep [14].
All electrophysiological signals were transduced by Vitaport III™ described in detail elsewhere [11]. All data were digitized at 256 Hz and analyzed with power spectral analysis (PSA). Although the full EEG spectrum was quantified, statistical analyses for the present paper focused on SWA (0.5 to < 4 Hz) power in each successive period of NREM Stage 2, 3 or 4 sleep. Stage 1 sleep epochs were excluded from the SWA analysis and NREM periods were terminated by either REM or wakefulness. In addition, the %SWA power in each NREM period expressed relative to all night SWA power was computed to control for individual differences in overall power. Exponential regression determined the accumulation and decay rate of SWA across the nigh [15].t High accumulation of SWA in the first NREM periods with rapid dissipation over subsequent NREM sleep time is considered evidence of a strong homeostatic sleep drive.
A two hour oral glucose tolerance test (OGTT) was administered the morning following the sleep study after a 12-hr fast in 15/18 subjects. For logistic reasons, the remaining three subjects had OGTTs within two weeks of the sleep study. Plasma glucose and insulin values were assessed at time 0, and 30, 60, 90 and 120 minutes after the consumption of an oral glucose solution dosed at 1.75 mg/kg up to max of 75 gm [16].
Two different measures of insulin sensitivity were measured to be consistent with the literature. The whole body insulin sensitivity index (WBISI) was computed using the formula: WBISI = (10000/square root (glucose at time 0 × insulin at time 0 × average glucose × average insulin)) [13]. The homeostatic model assessment of insulin resistance (HOMA-IR) [17] was computed using the formula HOMA-IR = (insulin at time 0 (microunits per millileter and glucose at time 0 (millimoles per liter)/22.5. To create a categorical between-group variable for insulin and glucose measures, HOMA-IR and WIBISI were split into tertiles. The highest levels of insulin resistance represent the highest tertile on HOMA-IR and the lowest tertile for WBISI. Analysis of variance (ANOVA) assessed the relationship between SWA in each NREMP, and glucose and insulin measures, controlling for age, and BMI percentile. The decay rate of SWA was also compared across tertiles of WBISI and HOMA-IR.
The three subjects who did not have OGTTs in the morning after sleep study were distributed across the tertile of HOMA-IR. The sleep and insulin data were compared to their respective groups to insure that they fell within one standard deviation of the mean.
3. Results
PSG Measures
The means and standard deviations of select polysomnographic variables and HOMA-IR and WBISI values are shown in Table 1 grouped by HOMA-IR tertiles. ANOVA indicated that only the percentage of Stage 1 (F2,15=2.9, p<.05) and % Stage 2,3,4 (F2,15=5.8, p<.01) differed between tertiles. Multiple comparisons indicated that the 1st tertile (insulin resistant) had significantly more Stage 1 and less Stage 2,3,4 sleep than the 2nd and 3rd tertiles (p<.05). Results were similar with WBISI tertiles, showing significant effects for Stage 1 (F2,15=4.1, p<.04) and Stage 2, 3, 4 combined (F2,15=5.1, p<.02). Those with the lowest insulin sensitivity had more Stage 1 and less Stage 2,3,4 than the middle and highest insulin sensitivity groups (range of p: .02 - .003). Note that total sleep time did not differ by HOMA-IR or WBISI tertile, nor did any of the other PSG measures (F < 1).
Table 1.
Means and standard deviations (in brackets) for key anthropometrics and sleep variables in groups expressed in tertiles of HOMA-IR values from the OGTT
| HOMA-IR Tertiles
| ||||
|---|---|---|---|---|
| 1 (n=4) Highest Resistance | 2 (n=8) Medium Resistance | 3 (n=6) Lowest Resistance | Significant LS Means | |
| Age | 15.3 (1.2) | 15.5 (1.4) | 15.6 (1.7) | |
| BMI % tile | 95.8 (5.2) | 72.9 (22.2) | 61.5 (29.2) | 1 vs 3 p<.03 |
| Waist | 41.5 (3.1) | 34.6 (4.3) | 32.3 (5.0) | 1 vs 2 p<.05; 1 vs 3 p<.02 |
| Neck | 15.5 (1.6) | 14.7 (0.8) | 12.9 (0.9) | 1 vs 3 p<.05 |
| Hip | 47.0 (5.6) | 40.3 (1.8) | 35.0 (2.0) | 1 vs 2 p<.04; 1 vs 3 p<.005 |
| HOMA-IR | 13.1 (6.2) | 5.6 (1.0) | 3.0 (2.2) | 1 vs 2 p<.01; 1 vs 3 p<.002 |
| WBISI | 0.9 (0.4) | 1.6 (0.2) | 4.1 (2.1) | 1 vs 2 p<.03; 1 vs 3 p<.01 |
| Tanner Score | 4.3 (0.7) | 4.6 (0.5) | 4.7 (0.5) | |
| SLEEP MEASURES | ||||
| Total Sleep Time | 468.1 (74.9) | 470.5 (44.4) | 463.3(99.5) | |
| Sleep Latency | 17.7 (18.0) | 23.2 (17.5) | 9.3 (7.1) | |
| Sleep Efficiency | 75.0 (14.6) | 88.9 (7.2) | 90.9 (8.7) | |
| % Stage 1 | 8.9 (4.9) | 4.1 (3.3) | 2.8 (2.4) | 1 vs 2 p<.03; 1 vs 3 p<.02 |
| % Stage 2,3,4 | 57.2 (8.7) | 76.4 (3.5) | 71.9 (4.1) | 1 vs 2 p<.001; 1 vs 3 <.001 |
| % Awake | 20.8 (16.1) | 3.9 (1.8) | 7.1 (8.3) | 1 vs 2 p<.008; 1 vs 3 p<.03 |
| % REM | 12.8 (9.1) | 14.8 (1.7) | 17.8 (3.6) | |
| REM latency | 109.6 (35.9) | 116.8 (81.2) | 86.7 (26.2) | |
| EEG Arousal/hr | 2.6 (1.2) | 2.3 (0.6) | 2.8 (1.1) | |
Sleep Stages Expressed as minute of each stage/total sleep period × 100
LS Means: Least-squares multiple comparisons computed after significant ANOVA; shading indicates variables with significant differences between HOMA-IR tertiles
SWA Measures
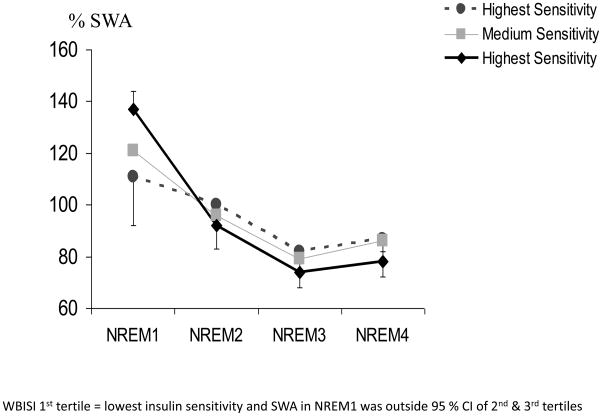
The time course of SWA also varied as a function of HOMA-IR and WBISI tertiles. Figure 1 illustrates the %SWA and its decay over NREM sleep. The decay rate of SWA was significantly slower (−.085) in those with the lowest insulin sensitivity (1st tertile WBISI) and outside the 95% confidence interval of the 2nd (−.15) and 3rd tertile (−.18) groups. The decay rate of SWA did not differ between the middle and high insulin sensitivity groups. Similarly, for HOMA-IR tertiles, those who were the most insulin resistant had the slowest SWA decay rate and were outside the 95% confidence interval of the other two tertiles.
Figure 1.
Decay of SWA across NREM periods expressed relative to total SWA and by tertiles of WBISI values from the OGTT.
WBISI 1st tertile = lowest insulin sensitivity and SWA in NREM1 was outside 95 % CI of 2nd & 3rd tertiles
The %SWA power also differed by insulin resistance and sensitivity tertiles as evidenced by a significant NREM period by tertile interaction, (HOMA-IR: F3,48=3.0, p<.04; WBISI: F3,48=5.2, p<.02 ,respectively). Multiple comparisons indicated that it was only %SWA in the first NREM period that distinguished between groups. Insulin resistance and low sensitivity was associated with significantly lower %SWA in the 1st NREM period than that observed in either the 2nd or 3rd tertiles (p<.001).
As a final set of analyses, the relationship between SWA power and %SWA in the first NREM period and HOMA-IR and WBISI tertiles was also assessed after removing the potential influence of BMI and Tanner score in an ANCOVA model. Removing the influence of BMI, %SWA remained statistically different between insulin resistance and insulin sensitivity tertiles (HOMA-IR: p<.001; WBISI: p<.02). Similarly, SWA power remained significant after removing BMI (HOMA-IR: p<.001; WBISI: p<.04).
Tanner score also did not account for the relationship between insulin and SWA measures. SWA power in the first NREM period was still significantly different by tertiles of HOMA-IR (p<.05) and WBISI (p<.04) after using Tanner as a covariate. The %SWA in the first NREM period continued to differ between tertiles of HOMA-IR after removing the influence of Tanner score (p<.05), but fell short of significance for WBISI (p<.07).
4. Discussion
The results indicated that the time course of SWA power was associated with low insulin sensitivity and higher insulin resistance in adolescents. Significantly lower %SWA in the 1st NREM period with a slower decay rate across the night was found in those with the highest HOMA-IR and the lowest WBISI, outside the 95% confidence interval of the other tertiles. Although higher BMI was associated with lower SWA, the relationship between low SWA and insulin resistance remained significant after controlling for BMI. Tanner score also did not account for the relationship between insulin resistance and SWA and the effects were still significant after controlling for Tanner statistically. However, with the exception of three adolescents who scored at Tanner stage 3, the adolescent groups were all Tanner Stage 4 and 5, beyond the developmental stage at which puberty influences insulin sensitivity [18]. Findings with WBISI were not as robust and the probability fell short of significance after using Tanner as a covariate.
Previous studies of the relationship between insulin sensitivity and sleep in children and adolescents have mostly focused on those with sleep disordered breathing (SDB). All participants in the present study were screened for SDB by history and polysomnogram, minimizing this potential contributor [9]. The results of this study point out that the relationship between insulin resistance and sleep in adolescents is evident even after controlling for these known influences and risk factors.
These results reported here extend the ground-breaking work of van Cauter’s group [10] that identified a unique contribution of delta waves during NREM sleep in metabolic function to adolescents. Further, this study included power spectral measures of SWA and demonstrated that it is a low accumulation and a slow dissipation of SWA across NREM sleep that characterizes low insulin sensitivity and high insulin resistance. We did not confirm earlier reports [1, 2] that short sleep was associated with insulin resistance; as there was no significant relationship between total sleep time and the insulin measures, adding additional weight to the suggestion that it is SWA, not short sleep that is associated with metabolic function.
Numerous studies have shown that SWA and its response to sleep deprivation is indicative of homeostatic sleep drive and the recovery function of sleep [15, 19, 20]. The findings in the present study are consistent with impaired sleep homeostasis associated with reduced insulin sensitivity. While those with medium levels of insulin resistance also should significantly lower accumulation of SWA than the low resistant group, they did not differ in the dissipation of SWA. Accumulation and dissipation of SWA are often interpreted to reflect sleep drive and recovery [19], respectively. Interpreting our data in this context, adolescents with medium insulin resistance may have a reduced sleep drive, but sleep is still recuperative. It may only be in those individuals with high insulin resistance that homeostatic recovery is impaired. This hypothesis can only be tested by directly manipulating SWA effects on insulin sensitivity.
There are additional limitations to the present study that should be noted. The sample size is small and not all participants were studied in the laboratory. Moreover, the OGTT was not available immediately after sleep in all participants and an IVGTT and clamp studies may provide more precise estimations of insulin resistance [13]. However, data from the three subjects who fell into this category was within one standard deviation of their respective group means, suggesting that they were not statistical outliers.
Additionally, our recording methods included only a nasal oral thermistor, not the recommended pressure transducer and we used AASM guidelines [13] for scoring respiratory events. We may have, therefore, underestimated more subtle sleep disordered breathing among this group and cannot completely rule out its potential influence on results [21]. In addition, there are other variables that could play a key role in moderating the relationship between SWA and insulin sensitivity including diet, physical fitness, stress and pro-inflammatory cytokines. Nevertheless, this pilot study is encouraging, but awaits replication and more direct manipulation of SWA to provide a definitive comment on the role of SWA homeostasis in insulin sensitivity in adolescents. If replicated, SWA measures may be useful in identifying those adolescents at highest risk for type 2 diabetes.
Acknowledgments
Research supported by pilot funding from the University of Michigan Diabetes Research and Training Center (NIDDK - DK020572), the Michigan Clinical Research Unit under the Michigan Institute of Clinical Health Research (NIH - UL1RR024986) and NIMH- MH77744. We thank Jared Fordyce and the staff of the Sleep & Chronophysiology Laboratory and Surair Bashir from the Department of Endocrinology for assistance with data collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diaetes mellitus and impaired glucose intolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi HK, Araujo AB, McKinlay JB. Sleep Duration as Risk Factor for the Development of Type 2 Diabetes Mellitus. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 3.Vgontas A, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler E. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32(11):902–909. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief Communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger ad appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 6.Donga E, van Dijk M, van Dijk G, et al. A Single Night of Partial Sleep Deprivation Induces Insulin Resistance in Multiple Metabolic Pathways in Healthy Subjects. Journal of Endrocinology and Metabolism. 2010 doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Bhattacharjee R, Kheirandish-Gozal L, et al. Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. International Journal of Pediatrics. 2010 doi: 10.1155/2010/846098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spruyt K, Molfese DL, Gozal D. Sleep Duration, Sleep Regularity, Body Weight, and Metabolic Homeostasis in School-aged Children. Pediatrics. 2011;127:e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disorder sleep. Respiratory Physiology and Neurobiology. 2011;178:465–474. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. PNAS. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, Ogden DL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 12.Rechtschaffen A, Kales A. A manual of standardized terminology: techniques and scoring systems for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Services/Brain Research Institute; 1968. [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Scholle S, Wiater A, Scholle H. Normative values of polysomnographic parameters in childhood and adolescence: Arousal events. Sleep Medicine. 2012;13:243–251. doi: 10.1016/j.sleep.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Armitage R, Hoffmann R, Conroy D, Arnedt JT, Brower K. Effects of a 3-hr Sleep Delay on Sleep Homeostasis in Alcohol Dependent Adults. Sleep. 2012;35:273–278. doi: 10.5665/sleep.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 17.Quon MJ. Limitations of the fasting glucose to insulin ratio as an index of insulin insensitivity. J Clin Endocrinol Metab. 2001;86(10):4615–4617. doi: 10.1210/jcem.86.10.7952. [DOI] [PubMed] [Google Scholar]
- 18.Kelly L, Lane C, Weigensberg M, Toledo-Corral C, Goran M. Pubertal changes of insulin sensitivity, acute insulin response and β-cell function in overweight Latino youth. Pediatrics. 2011;158(3):442–446. doi: 10.1016/j.jpeds.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scandanavica. 2007;115:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 20.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Lin C-H, Guilleminault C. Current hypopnea scoring criteria underscore pediatric sleep disordered breathing. Sleep Medicine. 2011;12:720–729. doi: 10.1016/j.sleep.2011.04.004. [DOI] [PubMed] [Google Scholar]