Abstract
Objective
To report the clinical features of 20 pediatric patients with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis.
Study design
Review of clinical data, long-term follow-up, and immunological studies performed in a single center in Spain in the last 4 years.
Results
The median age of the patients was 13 years (range, 8 months-18 years), 70% were female. In 12 patients (60%) the initial symptoms were neurologic, usually dyskinesias or seizures, and in the other 40% psychiatric. One month into the disease, all patients had involuntary movements and alterations of behavior and speech. All patients received steroids, intravenous immunoglobulin (IVIG) or plasma exchange, and 7 rituximab or cyclophosphamide. With a median follow up of 17.5 months, 85% had substantial recovery, 10% moderate or severe deficits, and 1 died. Three patients had previous episodes compatible with anti-NMDAR encephalitis, 2 of them with additional relapses after the diagnosis of the disorder. Ovarian teratoma was identified in two patients, one at onset of encephalitis and the other one year later. Two novel observations (one patient each) include, the identification of an electroencephalographic pattern (“extreme delta brush”) considered characteristic of this disorder, and the development of anti-NMDAR encephalitis after herpes simplex encephalitis (HSE).
Conclusions
The initial symptoms of pediatric anti-NMDAR encephalitis vary from those of the adults (more neurologic and less psychiatric in children), the development of a mono-symptomatic illness is extremely rare (except in relapses), and most patients respond to treatment. Our study suggests a link between post-HSE choreoathetosis and anti-NMDAR encephalitis.
Keywords: Autoimmune encephalitis, Choreoathetosis, Herpes simplex encephalitis, Child, Extreme delta brush
Since its initial description in 20071, anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis has been recognized as the most frequent autoimmune encephalitis in children after acute demyelinating encephalomyelitis (ADEM).2 In a center focused on the etiology and epidemiology of encephalitis (California Encephalitis Project) the frequency of anti-NMDAR encephalitis surpassed that of any viral encephalitis.3 Patients develop serum and cerebrospinal fluid (CSF) antibodies to a restricted epitope region of the NR1 subunit of the NMDAR.4 In cultures of hippocampal neurons, patients’ IgG or CSF produce a substantial decrease of the levels of NMDAR and NMDAR-mediated currents that it is reversible upon removal of patients’ antibodies. A similar effect was obtained when antibodies were injected in vivo into the hippocampus of rats.5,6 In pediatrics, increased awareness of this disorder is largely due to single case reports or small series7,8, with the largest experience being an American series of 32 patients, 8 from a single Institution.9 This and subsequent studies suggested that the younger the patient, the less likely a tumor would be found, and that the disease onset in children may be different from that of adults.9,10 However, in those studies the retrospective diagnosis of some patients and conservative treatment approach due to the novelty of the disease limited the information on symptom onset, treatment, and outcome. To address these issues, we report our experience with 20 pediatric patients with anti-NMDAR encephalitis focusing on disease presentation, spectrum of symptoms, treatment, and relapses. Moreover, the identification of a patient who developed the disorder one month after onset of herpes simplex encephalitis (HSE) provides an explanation for a rare and poorly understood complication of HSE11–13 that often presents with choreoathetosis and occurs without viral reactivation.
Methods
From January 2008 until February 2012 we identified 61 patients (median 22 years; 8 months-76 years) with anti-NMDAR encephalitis whose serum and CSF were referred for antibody testing to Hospital Clinic, University of Barcelona. All patients were suspected to have autoimmune encephalitis after being extensively studied by their physicians. Twenty patients (33%) were younger than 19 years and are the focus of this study. Analysis of serum and CSF for NMDAR antibodies was performed using two different tests, immunohistochemistry with rodent brain tissue and a highly specific cell-based assay, following reported criteria.14 None of the patients had antibodies to other cell surface or synaptic proteins using cell-base assays for the following proteins: AMPA receptor, GABA(B) receptor, mGluR1, mGluR5, LGI1, Caspr2, Glycine receptor (GlyR) and dopamine receptor (D2). All patients were seen by the authors. Disease severity and residual deficits were determined with the "Pediatric Cerebral Performance Category Scale" (PCPC; Table I; available at www.jpeds.com).15 There was not standardized protocol for ancillary tests; all patients underwent electroencephalography (EEG), magnetic resonance imaging (MRI), CSF analysis and extensive bacterial and viral studies, including in all instances herpes simplex virus (HSV) among others. Treatment decisions were based on the physician’s discretion. Three patients have been previously reported as part of a series of patients with relapses of encephalitis.16
The study was approved by the Ethics Committee of the Hospital Clinic. Samples are deposited in a collection of biological samples registered in the Biobank of Institut d’ Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona.
Results
The median age of the patients was 13 years (8 months-18 years); 14 were Caucasian, 5 Hispanic, and 1 Asian. NMDAR antibodies were identified in the CSF of all patients and serum of 9; the serum of two patients was negative and was not available from the other nine. Seventy percent of the patients were female. The ratio female/ male varied according to age, so that 33% of patients younger than 12 years and all above this age were female.
Initial symptom
Eleven patients (55%) developed prodromal symptoms a few days before the onset of the disease, including fever (n=7), headache (6), and vomiting (4). A two year-old girl developed anti-NMDAR encephalitis 1 week after completing treatment with acyclovir for HSE (Appendix 2 and Videos 1–3; available at www.jpeds.com).
In 12 patients (60%) the first symptom was neurologic, usually abnormal movements or seizures, and in the other 8 patients (40%) the first symptom was psychiatric or cognitive dysfunction. Neurologic presentations were more frequent in patients younger than 12 years (67% versus 55%) (Figure 1, A). Three patients were admitted to Psychiatric Units.
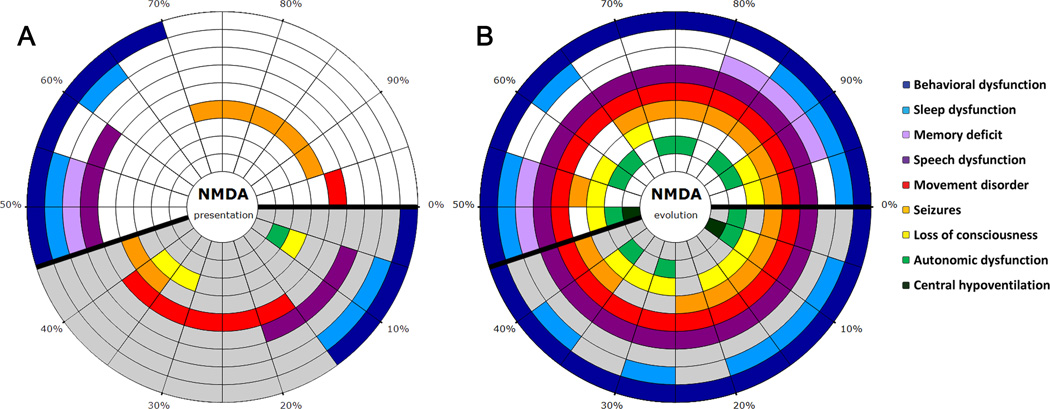
Figure 1.
Symptoms at presentation and during the first month of the disease. A, The initial symptoms of each patient are shown in panel; every radial segment represents one patient. The percentages assist to determine the percentage of patients with a specific symptom or combination of symptoms, each symptom coded with a different color. Patients > 12 years-old are shown in the section with white background, and those ≤ 12 in the section with grey background. Behavioral dysfunction included agitation and aggression (4 patients), psychosis, delusional thoughts, and hallucinations (3), nonspecific behavioral disturbance (3), anxiety (2), stereotyped behavior and obsessions (1), and negativism and autolytic thoughts (1). B, The symptoms during the first month of the disease; patients are represented in the same order as in A.
Symptoms and diagnostic studies during the first four weeks of disease
Figure 1, B shows the predominant symptoms that occurred within the first month of the disease grouped in 9 categories. The median number of symptoms was 5.5 (range 4–8), and all patients developed psychiatric dysfunction, impaired speech, and abnormal movements, usually orofacial dyskinesias and choreoathetosis.
The EEG was abnormal in 18 patients (90%) and the brain MRI in 9 (45%). A list of these findings is shown in Table II. One of the patients had an EEG pattern named “extreme delta brush” (Figure 2; available at www.jpeds.com).17 Extreme delta brush consists of a nearly continuous combination of delta activity with superimposed fast activity, usually in the beta range, in patients who are not under effects of sedation or anesthetics. This pattern resembles the delta brushes described in premature infants but extreme delta brush is more symmetric and synchronous involving predominantly frontal regions.17 We have not been able to review all EEG studies performed in other patients.
Table II.
Clinical features, diagnostic tests, treatment, and outcome
| Patient’s age | <12 years | 12–18 years | All patients |
|---|---|---|---|
| Number of patients | 9 | 11 | 20 |
| Female | 3 (33%) | 11 (100%) | 14 (70%) |
| Median age (range), years | 3 (0,7–11) | 15 (13–18) | 13 (0,7–18) |
| Associated Tumor | 0 | 2 (18%), both ovarian teratoma |
2 (10%) |
| Prodomal symptoms | 6 (67%) | 5 (45%) | 11 (55%) |
|
Symptom presentation* - Psychiatric features - Neurological |
3 (33%) 6 (67%) |
5 (45%) 6 (55%) |
8 (40%) 12 (60%) |
| Abnormal EEG** | 8 (89%) | 10 (90%) | 18 (90%) |
| Abnormal MRI*** | 5 (55%) | 4 (36%) | 9 (45%) |
| CSF pleocytosis | 4 (44%) | 10 (91%) | 14 (70%) |
| Hospitalization, median (range) days | 56 (13–90) | 56 (17–336) | 56 (13–336) |
| PCPC maximum, median (range) | 4 (4–5) | 4 (4–6) | 4 (4–6) |
|
Treatment - Steroids - IVIg - Plasma exchange - Rituximab alone - Rituximab combined with Cyclophosphamide - Other∞ |
9 (100%) 6 (67%) 1 (11%) 2 (22%) 1 (11%) 2 (22%) |
11 (100%) 9 (82%) 0 3 (27%) 1 (9%) 1 (9%) |
20 (100%) 15 (75%) 1 (5%) 5 (25%) 2 (10%) 3 (15%) |
| Follow up, median (range), months | 18 (9–149) | 15 (4–103) | 17,5 (4–149) |
|
Delay of treatment from disease onset >1 month >2 months |
3 (33%) 0 |
4 (36%) 2 (18%) |
7 (35%) 2 (10%) |
|
First sign of improvement since disease onset, median (range), days |
20 (7–180) | 46 (25–276) | 40 (7–276) |
|
First sign of improvement since immunotherapy, median (range), days |
7 (2–176) | 15 (2–90) | 11,5 (2–176) |
|
Outcome (PCPC score) 1: Full recovery 2: Mild disability 3: Moderate disability 4: Severe disability 5: Coma 6: Death |
5 3 0 1 0 0 |
7 2 0 1 0 1 |
12 (60%) 5 (25%) 0 2 (10%) 0 1 (5%) |
For patients with relapses, the information used in the table corresponds to the first episode, except treatment that includes all episodes.
See Figure 1A for detailed information about symptom presentation.
Abnormal EEG findings included: 7 patients with generalized slowing, 6 with generalized slowing and focal epileptic activity, 3 with asymmetrical or focal slowing, 1 with generalized epileptiform activity, and 1 with "extreme delta brush"19(Figure 2, online).
Brain MRI abnormalities included: 6 patients with T2/fluid-attenuated inversion recovery (FLAIR) abnormalities in the temporal lobes (one with contrast enhancement, and two with abnormal diffusion weighted images), 1 patient with minimal changes in arterial spin labeled perfusion in insular regions, 1 patient with cystic lesions in the left temporal lobe with perilesional gliosis, and 1 patient with transient brain atrophy attributed to steroids.
One patient received tacrolimus before the diagnosis of anti-NMDAR encephalitis for suspected Rasmussen Encephalitis. Two other patients received oral mycophenolate mofetil after completing second-line immunotherapies.
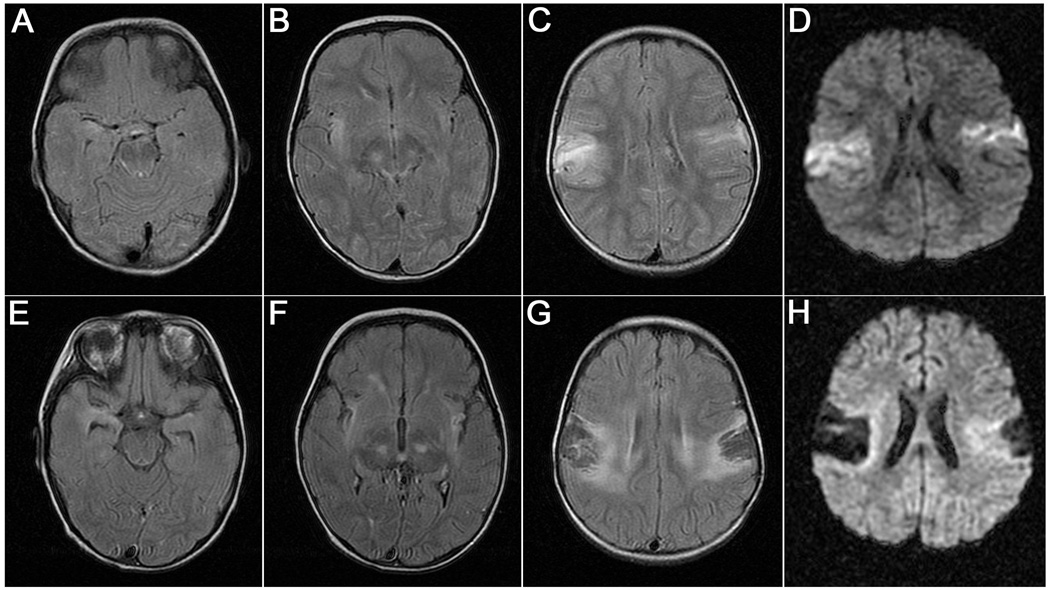
In one patient, the MRI obtained when the patient developed HSE showed increased T2/FLAIR signal in opercular regions, medial temporal lobes, and posterior aspect of the basal ganglia (Figure 3, A–D). In contrast to these rapid and irreversible viral-induced abnormalities, the subsequent development of anti-NMDAR encephalitis did not result in additional changes to the MRI (Figure 3, E–H).
Figure 3.
MRI findings in a patient who developed herpes simplex encephalitis (HSE) followed by anti-NMDAR encephalitis. A–D, The MRI findings during the first week of HSE. A-C, Increased T2-FLAIR signal was demonstrated in the right medial temporal lobe, right insula, posterior basal ganglia, and bilateral opercular regions, and D, increased signal in diffusion weighted images (DWI) . E–H, The MRI obtained during admission for anti-NMDAR encephalitis, one month after HSE onset, showed no additional changes other than the interval evolution of areas of encephalomalacia in opercular regions and hippocampal atrophy.
The initial CSF study showed lymphocytic pleocytosis in 14 (70%) patients (median 30 cells/µL; range 5–140). Only one patient had elevated CSF protein concentration. CSF specific oligoclonal bands were identified in 3 of 6 (50%) patients. One patient underwent a brain biopsy that was normal.
In all patients the CSF polymerase chain reaction (PCR) for HSV-1 was negative. Four patients had specific viral or bacteriological findings including, a throat swab positive for H. influenza; a CSF PCR positive for Human Herpesvirus 6; serum IgM and IgG antibodies to mumps virus, and a nasopharyngeal aspirate positive for Enterovirus.
All patients had tumor screening with MRI of the abdomen and pelvis, or abdominal or testicular ultrasound. An ovarian mass suggesting a teratoma was identified in 2 patients, leading to unilateral oophorectomy; in one of the patients (17 year-old) pathological studies demonstrated a mature teratoma, and in the other (13 year-old) a benign follicular cyst.
Treatment
During the first episode of encephalitis, 19 (95%) patients received first-line immunotherapies (one patient was only treated at third relapse). All patients received at least a short course of high-dose steroids (median 1, range 1–3 courses), followed in 13 patients by oral steroid tapering for a median of 12 weeks (range 3–47). In addition, 14 patients received intravenous immunoglobulin (IVIG; median 2 cycles, range 1–12) and one patient had plasmapheresis. In one patient, steroids were stopped because of worsening symptoms of psychosis; no side effects of first line therapies occurred in the other patients.
At last follow up all patients had received immunotherapy: 20 had first-line therapies (steroids, IVIG and/or plasma exchange), and 7 (35 %) second-line therapies (rituximab alone or combined with cyclophosphamide) (Table II). The reasons for using second-line therapies included unsatisfactory response to first-line drugs in six patients and multiple relapses in one. The median number of treatments with rituximab was 4 weekly doses (range 4–6) and the median number of cycles of cyclophosphamide was 5.5 monthly doses (range 4 –7). Although 18 patients (90%) were treated with antiepileptic drugs, none of them developed chronic or recurrent seizures and at the last follow-up only one continued with antiepileptic medication. Abnormal movements were symptomatically treated with a variety of medications (tetrabenazine, piracetam), none of them clearly effective.
Disease severity and outcome
At the peak of the disease the median degree of disability was 4 in the PCPC scale (all patients had ≥ 4, and 1 died [PCPC=6]). Nine patients were admitted to Pediatric Intensive Care Units, two of them requiring mechanical ventilation. The median time of hospitalization was 56 days (13–336).
After a median follow up of 17.5 months (4–149), 17 (85%) patients had substantial improvement (PCPC of 1 or 2: 60% complete recovery and 25% minimal residual deficits), 2 (10%) moderate or severe disability (PCPC of 3 or 4) and 1 died. The two patients with moderate or severe disabilities (follow-up of 4 and 9 months) are still improving at the time of writing this manuscript.
The median time from symptom onset until the first sign of improvement was 40 days (7–276), and from the start of immunotherapy until the first sign of improvement 11.5 days (2–176). Full recovery was achieved in 12 patients; this occurred between 8 and 12 months after symptom onset in 8 patients, and 3 and 5 months in 4 patients. For the 17 patients with substantial improvement, the last symptoms to improve in 16 patients (94%) were related to executive functions; in one patient the last symptom to improve was gait ataxia.
The patient who died was a 15 years old girl who presented with psychiatric symptoms (auditory hallucinations, memory disturbance, and anxiety) and subsequently developed abnormal movements, episodes of bradycardia and hypertension, and coma requiring mechanical ventilation and vasoactive drugs. She developed multiple organ failure and intestinal perforation; during surgery multiple areas of ischemia were noted in the liver and intestine. No tumor was identified; she was treated with intravenous steroids and IVIG without effect, and died 8 weeks after onset of encephalitis.
Before the diagnosis of anti-NMDAR encephalitis, three patients (15%) had at least one episode of encephalitis that had been classified as “encephalitis lethargica” (Table III). In retrospect, these episodes were likely prior episodes of anti-NMDAR encephalitis; in all instances symptoms included dyskinesias, language dysfunction, and psychiatric manifestations. Since the diagnosis of anti-NMDAR encephalitis, one of these three patients has developed a new relapse, and another patient three relapses. In the latter patient the two episodes preceding the diagnosis of anti-NMDAR encephalitis were not treated with immunotherapy, episode #3 (when the diagnosis of anti-NMDAR encephalitis was made) and episodes # 4 and 5 were treated with first-line immunotherapy, and episode #6 with rituximab and cyclophosphamide followed by mycophenolate mofetil. At last follow-up, 8 months after rituximab, the patient had substantial neurologic improvement without further relapses (Table III).
Table III.
Clinical features of episodes of relapses
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Total number of episodes of encephalitis | 2 | 3 | 6 |
| Time between episodes (months) | 31 | 108 and 36 | 13, 49, 16, 5 and 12 |
|
Number of episodes before the diagnosis of anti-NMDAR encephalitis - Age (years) - First symptom - Course - PCPC maximum - Immunotherapy - Outcome |
1 13 Leg dystonia Full syndrome 4 Steroids, IVIg Full recovery (PCPC 1) in 8 months |
1 8 dyskinesias Full syndrome 4 Steroids Full recovery (PCPC 1) in 6 months |
2 18 and 19 behavior/ speech disorder Full syndrome / Full syndrome 4 / 4 No treatment / No treatment Partial recovery; slow improvement in both episodes (PCPC 2) |
|
Episode that led to the diagnosis of anti- NMDAR encephalitis - Age (years) - First symptom - Course - PCPC maximum - Anti-NMDAR antibodies - Immunotherapy - Response |
15 Speech disorder Only speech dysfunction 3 CSF 1:80, serum NA Steroids Full recovery (PCPC 1) in 1 month |
17 Behavior Full syndrome 4 CSF 1:20, serum NA Steroids Full recovery (PCPC 1) in 6 months |
23 behavior and speech disorder behavior and speech symptoms 3 CSF 1:160, serum 1:1600 Steroids, monthly IVIg for long period of time Recovered to previous baseline,(PCPC 2, in less than 1 month |
|
Number of episodes after the diagnosis of anti-NMDAR encephalitis - Age (years) - First symptom - Course - PCPC maximum - Anti-NMDAR antibodies - Immunotherapy - Response |
0 - - - - - - - |
1 20 aphasia only aphasia 3 CSF 1:80, serum 1:400 Steroids Still improving (1 month of follow up) |
3 24; 25; 26 All 3 episodes: behavioral and speech deficits (more severe psychiatric symptoms in last episode) 3 / 3 / 4 Between relapses (CSF NA, serum 1:1600) Last episode (CSF 1:80, serum 1:3200), After second-line drugs (CSF 1:20, serum 1:1600) Steroids, IVIg. Last episode also: rituximab, cyclophosphamide and myophenolate mofetil. Recovery to previous baseline (PCPC, 2), episodes #4 and 5 in 1 month, episode #6 in 3 months. |
|
Follow up since first episode/ since last relapse (months) |
62/ 31 | 149 / 1 | 103 / 8 |
NA: not available.
A 14 year old girl whose neurologic symptoms of anti-NMDAR encephalitis resolved in 8 months, developed 4 months later (1 year from the diagnosis of encephalitis) acute abdominal pain without neurologic symptoms. A large ovarian mass was identified and removed, with pathological studies demonstrating an inmature teratoma. Review of the tumor screening performed 1 year earlier with computed tomography, MRI, and ultrasound of abdomen and pelvis confirmed that no tumor was visible at the time she developed encephalitis.
Discussion
Our study suggests that the presentation of anti-NMDAR encephalitis in children can be different from that reported in adults. Although 60% of children presented with seizures, abnormal movements, and focal neurologic deficits, previous experience with large series of predominantly young adults demonstrated that 70% presented with psychosis and other psychiatric symptoms.14 In the current study, patients older than 18 years were excluded, but a similar trend was noted comparing the symptom presentation of patients older and younger than 12 years: those older than 12 presented more often with psychiatric symptoms (45% versus 33%). Subsequently, during the first month of the disease all patients developed abnormal movements, psychiatric symptoms, and language dysfunction. Similar features were recently described by Titulaer et al in a series of 568 patients of all ages in which 95.6% developed at least 3 of the groups of symptoms shown in Figure 1, and only 0.7% had a monosymptomatic illness.10 In patients who develop the full syndrome, there is usually a gradual progression of symptoms from those mentioned above towards decrease level of consciousness, autonomic instability, and hypoventilation. Overall, these findings suggest caution in accepting the diagnosis of anti-NMDAR encephalitis in monosymptomatic cases or when symptoms do not fit with the expected syndrome. A prudent approach to these cases is re-assessment of CSF and serum for antibodies. However, clinical relapses can be monosymptomatic and less severe than the initial presentation16, as occurred in one of our patients who over a period of 3 years developed 4 episodes of isolated behavioral and language dysfunction.
The frequency of CSF alterations in this study appears lower than in prior series (70% versus 91% in adults.14 This may be due to an earlier recognition of the disease resulting in prompt diagnosis and reduction of repeat spinal taps that may have shown alterations. The MRI and EEG findings are in most respects similar to those reported in adults, including in one of the patients a unique EEG pattern (“extreme delta brush”) recently described in 30% of adults with anti-NMDAR encephalitis.17
Eighty-five percent of the patients had remarkable clinical improvement or full recovery. The delay between treatment and first sign of improvement was 11.5 days, but in most cases the recovery was achieved 8–12 months after symptom onset. It has been postulated that the prolonged duration of the disease and slow response to immunotherapy are due in part, to the production of antibodies within the CNS as well as systemically. This is supported by the almost constant detection of intrathecal synthesis of antibodies14,18,19 (identified in all 9 patients studied here), and the demonstration of parenchymal and meningeal infiltratres of plasma cells, which are long-lived and difficult to eliminate.20 By abrogating systemic B-cells, rituximab would prevent the entry of these cells in the CNS and subsequent development into antibody-producing plasma cells.21 Cyclophosphamide, which can penetrate the blood-brain-barrier, affects T and B cells, and increases anti-inflammatory cytokines contributing to immunosuppression.22 In the current study, all 7 patients that received rituximab (2 with cyclophosphamide) responded to treatment without further relapses, including one patient who had had 5 previous episodes. None of the patients had significant side effects of the treatment.
As noted in previous series and case reports7,9, most children with anti-NMDAR encephalitis do not have an underlying tumor. However, some patients (usually older than 12 years) do have a teratoma, and as occur in young adults, the tumor may be detectable after the patient has recovered from encephalitis, similar to one of our patients.23 The frequency and duration of tumor surveillance in children is a question that needs to be answered in future studies.
A 2 year-old girl developed anti-NMDAR encephalitis four weeks after HSE. Her symptoms were remarkably similar to the choreoathetosis and orofacial dyskinesias that occur in some patients during the first month of onset of HSE. This complication has an unclear etiology.11–13 In these cases viral reactivation seems unlikely because CSF and brain viral studies are negative, the MRI studies do not show new necrotic-hemorrhagic lesions, and symptoms are refractory to acyclovir. The abnormal movements may last several months or years and are refractory to antiepileptics and dopamine receptor antagonists.11 These observations have suggested a post-infectious immune-mediated etiology.11–13 Taken together, the biphasic course of symptoms of our patient, along with the CSF findings (negative PCR HSV, positive NMDAR antibodies), no additional changes in the brain MRI, and lack of response to acyclovir but improvement after rituximab and cyclophosphamide, suggest that some patients with “post-HSE choreoathetosis” may in fact have anti-NMDAR encephalitis. This link between both disorders is supported by a recent study showing IgG NMDAR antibodies in serum or CSF of 11% of patients with past history of HSE.24
Our study was not prospective and did not have a uniform systematic treatment approach (e.g., same criteria and timing to change from first line immunotherapy to second line immunotherapy, and duration of treatments). Future studies should address these issues in the context of a clinical trial. In addition, studies with larger number of patients will provide predictor factors of the response to treatment and relapses.
Findings from this study suggest that in children the first symptom of anti-NMDAR encephalitis may be different from that of the adults (more neurologic in children, more psychiatric in adults); the development of a monosymptomatic illness is extremely rare (except in relapses), and although the disease is potentially lethal, most patients respond to immunotherapy. Moreover, second line immunotherapy, mostly including rituximab is often effective and well tolerated. A notable feature not previously reported in children is the EEG pattern named extreme delta brush. Further studies are needed to determine the frequency of NMDAR antibodies in patients with “post-HSE choreoathetosis” and whether this disorder is in fact anti-NMDAR encephalitis.
Supplementary Material
Extreme delta brush. A 14 year-old previous healthy girl presented with secondarily generalized seizures. Brain MRI was normal and initial EEG showed slow activity in right temporal lobes. She was dignosed with temporal lobe epilepsy and treatment with levetiracetam was initiated. Two weeks later, she was readmitted to the hospital for severe obsessive-compulsive behavior and visual hallucinations. Levetiracetam was discontinued and oral carbamazepine was started. EEG during the first 48 hours of admission showed a pattern consistent with “extreme delta brush”2, including continuous combination of delta frequency transients with superimposed fast activity in the beta range, symmetrically involving all head regions, with frontal preference. Screening for an underlying tumor was negative and she was treated with IVIG and steroids. At the last follow-up, 10 months after symptom onset, she was fully recovered.
Acknowledgments
Supported by the National Institutes of Health (RO1NS077851 and RO1MH094741to J.D.), the National Cancer Institute (RO1CA89054 to J.D.), Fundació la Marató de TV3 (to J.D.), Fondo de Investigaciones Sanitarias (PI11/01780 to J.D. and PS09/0193 to F.G.), a fellowship from the Dutch Cancer Society (KWF 2009-4451), and a McKnight Neuroscience of Brain Disorders award (to J.D.). J.D. has received a research grant from Euroimmun, and receives royalties from the Editorial Board of Up-To-Date and from patents for the use of Ma2 and NMDA receptor as autoantibody tests.
We thank physicians and family members of patients who provided clinical information. We also thank Myrna R. Rosenfeld for the critical review of the manuscript and Bart M. Titulaer for designing and developing Figure 1.
Abbreviations
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- HSE
herpes simplex encephalitis
- HSV
herpes simplex virus
- IVIG
intravenous immunoglobulin
- MRI
magnetic resonance imaging
- NMDAR
N-methyl-D-aspartate receptor
- PCPC
Pediatric Cerebral Performance Category
- PCR
polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Reference List
- 1.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 3.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The Frequency of Autoimmune N-Methyl-D-Aspartate Receptor Encephalitis Surpasses That of Individual Viral Etiologies in Young Individuals Enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA Receptor Encephalitis Antibody Binding Is Dependent on Amino Acid Identity of a Small Region within the GluN1 Amino Terminal Domain. J Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 7.Luca N, Daengsuwan T, Dalmau J, Jones K, deVeber G, Kobayashi J, et al. Anti-N-methyl-D-aspartate receptor encephalitis: a newly recognized inflammatory brain disease in children. Arthritis Rheum. 2011;63:2516–2522. doi: 10.1002/art.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bseikri MR, Barton JR, Kulhanjian JA, Dalmau J, Cohen RA, Glaser CA, et al. Anti-N-methyl D-aspartate receptor encephalitis mimics viral encephalitis. Pediatr Infect Dis J. 2012;31:202–204. doi: 10.1097/INF.0b013e31823d52eb. [DOI] [PubMed] [Google Scholar]
- 9.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Titulaer MJ, McCracken L, Gabilondo I, Martinez-Hernandez E, Graus F, Balice-Gordon R, et al. Clinical features, treatment and outcome of 500 patients with anti-NMDA receptor encephalitis [Meeting Abstracts 1] Neurology. 2012;78:PL01.001. [Google Scholar]
- 11.Hargrave DR, Webb DW. Movement disorders in association with herpes simplex virus encephalitis in children: a review. Dev Med Child Neurol. 1998;40:640–642. doi: 10.1111/j.1469-8749.1998.tb15431.x. [DOI] [PubMed] [Google Scholar]
- 12.De Tiège X, Rozenberg F, Des Portes V, Lobut JB, Lebon P, Ponsot G, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology. 2003;61:241–243. doi: 10.1212/01.wnl.0000073985.71759.7c. [DOI] [PubMed] [Google Scholar]
- 13.De Tiège X, De Laet C, Mazoin N, Christophe C, Mewasingh LD, Wetzburger C, et al. Postinfectious immune-mediated encephalitis after pediatric herpes simplex encephalitis. Brain Dev. 2005;27:304–307. doi: 10.1016/j.braindev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 16.Gabilondo I, Saiz A, Galan L, Gonzalez V, Jadraque R, Sabater L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77:996–999. doi: 10.1212/WNL.0b013e31822cfc6b. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. "Extreme delta brush": a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruss H, Dalmau J, Harms L, Holtje M, Ahnert-Hilger G, Borowski K, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 19.Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin MP, Cravens PD, Winger R, Kieseier BC, Cepok S, Eagar TN, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009;66:1016–1020. doi: 10.1001/archneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 22.Elkhalifa A, Weiner H. Cyclophosphamide Treatment of MS: Current Therapeutic Approaches and Treatment Regimens. Int MS J. 2010;17:12–18. [PubMed] [Google Scholar]
- 23.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruss H, Finke C, Holtje M, Hofmann J, Klingbeil C, Probst C, et al. NMDA receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012 Jul 16; doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extreme delta brush. A 14 year-old previous healthy girl presented with secondarily generalized seizures. Brain MRI was normal and initial EEG showed slow activity in right temporal lobes. She was dignosed with temporal lobe epilepsy and treatment with levetiracetam was initiated. Two weeks later, she was readmitted to the hospital for severe obsessive-compulsive behavior and visual hallucinations. Levetiracetam was discontinued and oral carbamazepine was started. EEG during the first 48 hours of admission showed a pattern consistent with “extreme delta brush”2, including continuous combination of delta frequency transients with superimposed fast activity in the beta range, symmetrically involving all head regions, with frontal preference. Screening for an underlying tumor was negative and she was treated with IVIG and steroids. At the last follow-up, 10 months after symptom onset, she was fully recovered.