Abstract
Consistent with the ability of severe alcohol intoxication to impair memory, high concentrations of ethanol (60 mM) acutely inhibit long-term potentiation (LTP) in the CA1 region of rat hippocampal slices. To account for this, we hypothesized that local metabolism to acetaldehyde may contribute to the effects of high ethanol on synaptic function. However, sodium azide, a catalase inhibitor, and allyl sulfide, an inhibitor of cytochrome P450 2E1 (CYP2E1), failed to overcome LTP inhibition by 60 mM ethanol. In contrast, LTP was successfully induced in the presence of ethanol plus 4-methylpyrazole (4MP), an inhibitor of alcohol dehydrogenase, suggesting that local metabolism via alcohol dehydrogenase contributes to synaptic effects. Furthermore, exogenously administered acetaldehyde overcame the effects of 4MP on LTP inhibition mediated by ethanol. These observations indicate that acetaldehyde generated by local metabolism within the hippocampus participates in the synaptic dysfunction associated with severe alcohol intoxication.
Keywords: ADH3, alcoholism, blackout, amnesia, CNS, synaptic plasticity
Alcohol ingestion produces a variety of neuropsychiatric symptoms. One of these is called a memory “blackout” and refers to a state in which individuals perform complex acts for which they have no recollection [24]. Ethanol is thought to induce this amnesia by inhibiting long-term potentiation (LTP), a form of synaptic plasticity associated with memory processing [3]. In hippocampal slices, LTP inhibition by acute ethanol requires high concentrations [14,22]. To account for these latter observations, we hypothesized that accumulation of ethanol metabolites may contribute to effects on LTP and represent an effect of ethanol that requires high concentrations. Among ethanol metabolites, acetaldehyde is not only the most prominent but also the most toxic. Because acetaldehyde dehydrogenase (ALDH) is present in the blood brain barrier [25], systemic acetaldehyde is unlikely to reach the brain in significant concentrations [20]. Acetaldehyde accumulation in brain can occur, however, under conditions in which ALDH is impaired or when the capacity to metabolize acetaldehyde is exceeded [11]. Acetaldehyde may be generated de novo in the CNS, and, in cultured astrocytes and rat brain homogenates, accumulates in the presence of ethanol [2,6,8]. Although alcohol dehydrogenase (ADH) converts ethanol to acetaldehyde in the liver, it is thought that brain ethanol is oxidized to acetaldehyde mainly by catalase and cytochrome P450, with ADH playing a minor role if any [27]. In this study, we examined the possible role of acetaldehyde in mediating the effects of ethanol on LTP in CA1 pyramidal neurons.
Male albino rats (postnatal age 28-32 days) were used for all studies. Protocols for animal use were approved by the Washington University Animal Studies Committee in accordance with the NIH guidelines for care and use of laboratory animals.
For electrophysiology, hippocampal slices were transferred to a submerged recording chamber with continuous bath perfusion of artificial cerebrospinal fluid at 2 ml/min at 30°C. Extracellular recordings were obtained from the apical dendritic layer of the CA1 region elicited with 0.1 ms constant current pulses through a bipolar stimulating electrode placed in stratum radiatum. During an experiment, EPSPs were monitored using a half-maximal stimulus based on a baseline input-output curve. After establishing a stable baseline, LTP was induced by applying a single 100 Hz × 1 s high frequency stimulus (HFS) using the same intensity stimulus as used for monitoring. An input-output curve was repeated 60 min following HFS for statistical comparisons of changes in EPSP slopes at half-maximal intensity. Signals were digitized and analyzed using PCLAMP software (Axon Instruments, Union City, CA). All chemicals were used at concentrations that did not significantly suppress baseline EPSPs. Because 30 min administration of 0.3 mM sodium azide suppressed EPSPs (49.8 ± 3.8%, n = 3), we used sodium azide at 0.1 mM.
All data are expressed as mean ± s.e.m. For comparisons between two groups Student's t-test was used. P-values of less than 0.05 were considered statistically significant.
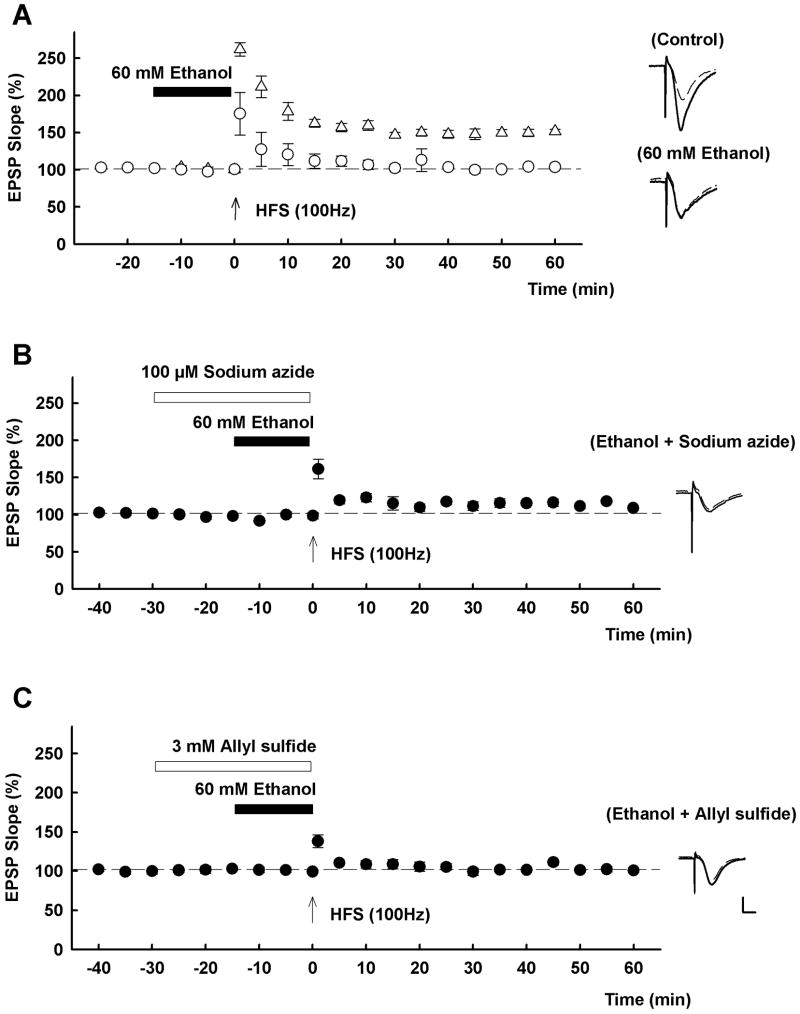
As previously reported [14,15,22], LTP in the CA1 region of hippocampal slices was blocked acutely by 60 mM ethanol (EPSP change; 151.8 ± 3.0% in control slices 60 min following HFS, and 103.6 ± 2.5% of baseline in the presence of ethanol, n = 5 each, p < 0.01 by t-test, Fig 1A.). Because alcohol metabolizing enzymes are expressed in the hippocampus [7], we subsequently examined the effects of inhibitors of ethanol metabolism on the ability of ethanol to inhibit LTP.
Figure 1.
Effects of catalase and CYP2E1 inhibitors on ethanol-mediated LTP inhibition A, In control slices, LTP is readily induced (triangles) by a single 100 Hz × 1 s high frequency stimulation (HFS, arrow). Fifteen min administration of 60 mM ethanol (closed bar) inhibits LTP (open cirecles). B, In the presence of both 60 mM ethanol (closed bar) and 100 μM sodium azide, a catalase inhibitor (open bar), LTP is not induced (closed circles). C, In the presence of both 60 mM ethanol (closed bar) and 3 mM allyl sulfide, a CYP2E1 inhibitor (open bar), LTP is not induced (closed circles). Traces depict EPSPs before (dashed lines) and 60 min after HFS (solid lines). Scale; 1mV, 5 msec.
Based on the proposed role of catalase in local brain metabolism of ethanol, we initially examined whether catalase inhibitors would overcome the inhibitory effects of 60 mM ethanol on LTP induction. However, in the presence of 100 μM sodium azide, a catalase inhibitor [27], 60 mM ethanol still inhibited LTP (EPSP slope; 109.1 ± 2.8% of baseline, n = 5, p = 0.181 vs. ethanol alone by t-test, Fig. 1B). Administration of sodium azide alone did not affect LTP induction (EPSP slope; 149.3 ± 4.0 % of baseline, n = 3, data not shown). Similarly, administration of 150 μM sodium azide also failed to overcome ethanol-mediated LTP inhibition (EPSP slope; 102.5 ± 8.3% of baseline, n = 4, p = 0.889 vs. ethanol alone by t-test, data not shown).
Ethanol can also be metabolized in brain by the cytochrome P450 enzyme, CYP2E1 [21]. Similar to sodium azide, we found that 3 mM allyl sulfide, an inhibitor of CYP2E1, failed to overcome the effects of 60 mM ethanol on LTP (EPSP slope; 100.8 ± 1.8% of baseline, n = 5, p = 0.385 vs. ethanol alone by t-test, Fig. 1C). Administration of allyl sulfide alone did not affect LTP induction (EPSP slope; 150.2 ± 8.7% of baseline, n = 3, data not shown). A combination of allyl sulfide and sodium azide also failed to overcome the effects of 60 mM ethanol on LTP (EPSP slope: 99.8 ± 1.8% of baseline, n = 5, p = 0.240 vs. ethanol alone by t-test, data not shown).
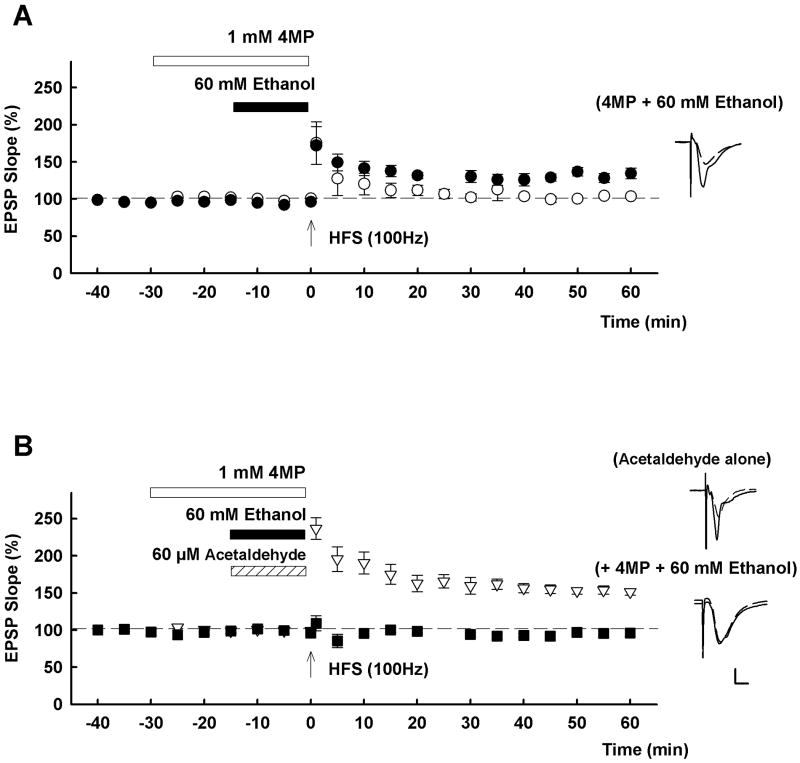
In contrast to the catalase and CYP2E1 inhibitors, LTP was successfully induced when 1mM 4-methylpyrazole (4MP), an inhibitor of ADH [4], was administered with 60 mM ethanol (EPSP slope; 134.5 ± 7.3% of baseline, n = 6, p < 0.01 vs. ethanol alone by t-test Fig. 2A), suggesting that acetaldehyde generation via ADH contributes to the effects of high ethanol. Administration of 1mM 4MP alone did not affect LTP induction (EPSP slope; 141.9 ± 2.0 % of baseline, n = 3, data not shown). The effects of 4MP on LTP inhibition were concentration dependent, with lower concentrations (0.1 mM and 0.3 mM) failing to alter the effects of 60 mM ethanol on LTP (EPSP slope; 91.7 ± 7.0% and 116.1 ± 6.4% of baseline n = 5 each, p = 0.115 and 0.103 vs. ethanol alone by t-test, respectively, data not shown).
Figure 2.
Involvement of ADH in ethanol-mediated LTP inhibition. A, LTP inhibition is overcome by 4MP (closed circles). For reference, the effects of 60 mM ethanol alone from Figure 1A are again depicted as open circles. B, While 60 μM acetaldehyde alone (hatched bar) does not inhibit LTP induction (triangles), LTP is not induced (squares) in the presence of both 60 mM ethanol and 60 μM acetaldehyde with 4 MP (open bar). Traces depict EPSPs before (dashed lines) and 60 min after HFS (solid lines). Scale; 1mV, 5 msec.
Consistent with a possible role of endogenous acetaldehyde in the inhibitory effects of ethanol on LTP, we found that exogenously administered acetaldehyde (60 μM) overcame the effects of 1 mM 4MP on LTP inhibition mediated by 60 mM ethanol (EPSP slope; 101.9 ± 2.2 % of baseline, n = 5, p < 0.01 vs. 4MP alone with ethanol by t-test, Fig. 2B). However, administration of 60 μM acetaldehyde alone did not affect LTP induction (EPSP slope; 151.3 ± 4.3% of baseline, n = 5, p = 0.925 vs. control LTP by t-test, Fig. 2B).
In the present study, we examined whether the acute effects of ethanol on LTP involve synthesis of acetaldehyde locally in the hippocampus. A role for acetaldehyde in ethanol's effects has been suspected but remains controversial [5,17]. Despite significant elevation of systemic acetaldehyde following ethanol loading (over 50 μM in men with ALDH2*1/*2 after 0.75g/kg ethanol loading) [16], it is unclear whether systemic acetaldehyde affects CNS function. For instance, a moderate systemic dose of ethanol does not inhibit LTP induction in vivo [1], and systemic acetaldehyde does not mimic ethanol's effects on brain neurosteroid levels [4]. Other evidence, however, suggests the importance of brain ethanol metabolism [2,8, 27], raising the possibility that locally-generated acetaldehyde contributes to ethanol-mediated neuronal changes.
Moderate levels of ethanol may impair cognitive function if acetaldehyde accumulates in the brain. Consistent with this, disulfirum, an inhibitor of ALDH, facilitates the effects of low doses of ethanol on LTP in vivo [1]. If acetaldehyde acts locally within the same neurons in which it is generated from ethanol, levels of acetaldehyde measured in the plasma or even in the cerebrospinal fluid are unlikely to predict actions of acetaldehyde in the brain, even though significant acetaldehyde levels are observed in plasma after ethanol loading in humans [16].
In the present study, we found that ADH plays a key role in hippocampal acetaldehyde formation. While it is uncertain how ethanol is metabolized in the brain, some evidence indicates that brain metabolism may involve catalase, at least in certain cells such as catecholaminergic neurons [26]. In the striatum, however, ethanol oxidation is not prevented completely by catalase inhibitors [2,8,26], indicating that pathways such as CYP2E1 [12] or ADH [2,7] may be important. We found that sodium azide, a catalase inhibitor, and allyl sulfide, a CYP2E1 inhibitor, failed to overcome the effects of 60 mM ethanol on LTP induction, suggesting that although these enzymes are expressed in the hippocampus [18], they do not play major roles in ethanol-mediated LTP inhibition in area CA1.
In contrast to catalase and CYP2E1 inhibitors, the effects of 60 mM ethanol were overcome by the ADH inhibitor, 4MP. A recent study has shown that 4MP inhibits ethanol-mediated phosphorylation of extracellular signal-regulated kinase (ERK) in the nucleus accumbens [23], suggesting a role for ADH in the actions of ethanol in this region. Furthermore, Mori et al. have shown that ADH3, but not ADH1, is expressed in the hippocampus [17]. ADH3 is a more recently discovered member of the ADH family [9,19] and was originally thought to play little role in ethanol metabolism because of its low affinity for ethanol. Recent evidence, however, suggests a more significant role in alcohol metabolism [10], and ADH3 is expressed in pyramidal neurons [7]. We found that 1 mM, but not 0.1-0.3 mM 4MP overcame the effects of 60 mM ethanol. This is consistent with the low sensitivity of ADH3 to pyrazoles [10]. The ability of 4MP to overcome the effects of ethanol (Fig. 2A), suggests that local metabolism of ethanol to acetaldehyde is pivotal for LTP inhibition. Furthermore, the ability of 60 μM acetaldehyde administered with ethanol plus 4MP to restore LTP inhibition further implicates acetaldehyde even though acetaldehyde alone does not inhibit LTP at this concentration (Fig. 2B). While this latter result seems contradictory, it supports the idea that ethanol inhibits LTP via multiple actions, one of which is mediated by acetaldehyde; other actions of ethanol are uncertain but likely include partial NMDA receptor antagonism [13-15,22]. In the experiment administering ethanol and acetaldehyde with 4MP (Fig. 2B), post-tetanic potentiation (PTP), is also depressed. This suggests that acetaldehyde combined with ethanol and 4MP may have additional actions, including effects on glutamate release.
Taken together, our results indicate that acetaldehyde formed regionally within the hippocampus modulates the synaptic and possibly the cognitive dysfunction associated with alcohol intoxication. Future studies should determine whether systemically administered ethanol disrupts memory acquisition via metabolism to acetaldehyde.
Highlights (for review).
Ethanol induces amnesia via inhibition of LTP, a form of synaptic plasticity.
In this study, 4-methylpyrazole was used as an alcohol dehydrogenase inhibitor.
4-methylpyrazole allowed LTP in the presence of ethanol.
Local metabolism to acetaldehyde may contribute to the ethanol-mediated amnesia.
Acknowledgments
This work was supported by NIH grants AA017413, MH07791 and GM47969, and the Bantly Foundation.
Footnotes
Disclosure: CFZ serves on the Scientific Advisory Board of Sage Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe K, Yamaguchi S, Sugiura M, Saito H. The ethanol metabolite acetaldehyde inhibits the induction of long-term potentiation in the rat dentate gyrus in vivo. Br J Pharmacol. 1999;127:1805–1810. doi: 10.1038/sj.bjp.0702738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon CM, Rogan F, Amit Z. Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem Pharmacol. 1992;44:93–98. doi: 10.1016/0006-2952(92)90042-h. [DOI] [PubMed] [Google Scholar]
- 3.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Boyd KN, O'Buckley TK, Morrow AL. Role of acetaldehyde in ethanol-induced elevation of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in rats. Alcohol Clin Exp Res. 2008;32:1774–1781. doi: 10.1111/j.1530-0277.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa M, Salamone JD, Segovia KN, Pardo M, Longoni R, Spina L, Peana AT, Vinci S, Acquas E. Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neurosci Biobehav Rev. 2012;36:404–430. doi: 10.1016/j.neubiorev.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Eysseric H, Gonthier B, Soubeyran A, Bessard G, Saxod R, Barret L. Characterization of the production of acetaldehyde by astrocytes in culture after ethanol exposure. Alcohol Clin Exp Res. 1997;21:1018–1023. [PubMed] [Google Scholar]
- 7.Galter D, Carmine A, Buervenich S, Duester G, Olson L. Distribution of class I, III and IV alcohol dehydrogenase mRNAs in the adult rat, mouse and human brain. Eur J Biochem. 2003;270:1316–1326. doi: 10.1046/j.1432-1033.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill K, Menez JF, Lucas D, Deitrich RA. Enzymatic production of acetaldehyde from ethanol in rat brain tissue. Alcohol Clin Exp Res. 1992;16:910–915. doi: 10.1111/j.1530-0277.1992.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 9.Haseba T, Hirakawa K, Tomita Y, Watanabe T. Characterization of high Km alcohol dehydrogenase from mouse liver. In: Hirai H, editor. Electrophoresis '83. Berlin and New York: Walter de Gruyter & Co; 1984. pp. 393–400. [Google Scholar]
- 10.Haseba T, Ohno Y. A new view of alcohol metabolism and alcoholism--role of the high-Km Class III alcohol dehydrogenase (ADH3) Int J Environ Res Public Health. 2010;7:1076–1092. doi: 10.3390/ijerph7031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heap L, Ward RJ, Abiaka C, Dexter D, Lawlor M, Pratt O, Thomson A, Shaw K, Peters TJ. The influence of brain acetaldehyde on oxidative status, dopamine metabolism and visual discrimination task. Biochem Pharmacol. 1995;50:263–270. doi: 10.1016/0006-2952(94)00539-x. [DOI] [PubMed] [Google Scholar]
- 12.Hipólito L, Sánchez MJ, Polache A, Granero L. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–727. doi: 10.2174/138920007782109797. [DOI] [PubMed] [Google Scholar]
- 13.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- 15.Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Kim SW, Bae KY, Shin HY HY, Kim JM, Shin IS, Youn T, Kim J, Kim JK, Yoon JS. The role of acetaldehyde in human psychomotor function: a double-blind placebo-controlled crossover study. Biol Psychiatry. 2010;67:840–845. doi: 10.1016/j.biopsych.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Mori O, Haseba T, Kameyama K, Shimizu H, Kudoh M, Ohaki O, Arai Y, Yamazaki M, Asano G. Histological distribution of class III alcohol dehydrogenase in human brain. Brain Res. 2000;852:186–190. doi: 10.1016/s0006-8993(99)02201-5. [DOI] [PubMed] [Google Scholar]
- 18.Moreno S, Mugnaini E, Cerù MP. Immunocytochemical localization of catalase in the central nervous system of the rat. J Histochem Cytochem. 1995;43:1253–1267. doi: 10.1177/43.12.8537642. [DOI] [PubMed] [Google Scholar]
- 19.Parés X, Vallee BL. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981;98:122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- 20.Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog Neurobiol. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Ronis MJ, Korourian S, Blackburn ML, Badeaux J, Badger TM. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44:157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinci S, Ibba F, Longoni R, Spina L, Spiga S, Acquas E. Acetaldehyde elicits ERK phosphorylation in the rat nucleus accumbens and extended amygdala. Synapse. 2010;64:916–927. doi: 10.1002/syn.20811. [DOI] [PubMed] [Google Scholar]
- 24.White M. What happened? Alcohol, memory blackouts and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- 25.Zimatkin SM. Histochemical study of aldehyde dehydrogenase in the rat CNS. J Neurochem. 1991;56:1–11. doi: 10.1111/j.1471-4159.1991.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 26.Zimatkin SM, Lindros KO. Distribution of catalase in rat brain: aminergic neurons as possible targets for ethanol effects. Alcohol Alcohol. 1996;31:167–174. doi: 10.1093/oxfordjournals.alcalc.a008128. [DOI] [PubMed] [Google Scholar]
- 27.Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]