Abstract
Background
Identify whether the bacterial protein, Integration Host Factor (IHF), is present within sputum solids collected from Cystic Fibrosis (CF) patients and thus might contribute to the structural stability of biofilms within the lungs.
Methods
The presence of IHF in sputum was determined by immunohistochemistry. The role of IHF in stabilizing biofilms within sputum was tested in vitro wherein anti-IHF was used to attempt to dissolve sputum solids.
Results
Thirty-seven of 44 sputum samples (84%) were positive for anti-IHF staining. Treatment with anti-IHF or DNase of 6 representative samples, dissolved sputum solids significantly better than treatment with normal saline in vitro, and strong synergism was observed when these agents were used in combination.
Conclusions
IHF was detected in the majority of sputum samples from patients with CF and in vitro treatment with anti-IHF induced dissolution of sputum solids. These data support further investigation of IHF as a potential therapeutic target for patients with CF.
Keywords: Cystic Fibrosis, biofilms, Integration Host Factor, airway clearance, Pseudomonas aeruginosa
INTRODUCTION
Cystic fibrosis is a lethal genetic disorder that causes significant morbidity and early mortality (2, 3). Based on the most recent data in the Cystic Fibrosis Foundation (CFF) Patient Registry, the median predicted age of survival is 38 years of age (4) and lung disease contributes to nearly 85% of observed mortality (5). Lung pathogenesis results from dehydration of the airway surface liquid and impaired mucociliary clearance (6–9). As a result, individuals with CF have difficulty clearing pathogens from the lungs and experience chronic pulmonary infections and inflammation (6, 10, 11). This pathogenesis remains the major cause of significantly decreased life span in CF patients (6, 10, 11).
One of the most clinically relevant bacteria chronically present in the lungs of CF patients is the Gram-negative opportunistic pathogen Pseudomonas aeruginosa (11–14). A major cause of the persistence of P. aeruginosa in patients with CF, and thus the establishment of chronic infection and progressive lung destruction, is that these bacteria form and reside within highly recalcitrant biofilm structures (15–17).
The foundation of the biofilm structure is the extracellular polymeric substance (EPS) that acts as a barrier to both effectors of the immune system and antimicrobial agents. Recent work has highlighted extracellular DNA (eDNA) as a key component in many pathogenic biofilms (18, 19). It was recently showed that members of the DNABII family of proteins are critical for the integrity of the EPS matrix of biofilms formed by the human pathogen nontypeable Haemophilus influenzae (NTHI) which contains eDNA (18). Integration Host Factor (IHF) is one of the members of this family of proteins, existing as a heterodimer of homologous subunits that binds and compacts double-stranded DNA (20). We have shown previously that antiserum directed against isolated E. coli IHF (anti-IHF), either used alone or combined with other treatment modalities such as DNase or traditional antibiotics, is highly effective in its ability to debulk biofilms and render the bacteria within more susceptible to the action of antibiotics, respectively (18).
Since IHF has been proven to be an important element of the structure of biofilms formed by NTHI and multiple other human pathogens, and because biofilms within the respiratory tract of patients with CF make treatment significantly more difficult, we were interested in determining if the IHF protein might also be present within clinical specimens obtained from CF specimens, thus providing evidence that IHF may prove to be a reasonable target for the development of novel therapies for patients with CF.
MATERIALS AND METHODS
Sputum samples were collected from 44 from patients with CF (ages 4 – 63 years) during a routine clinic visit and under an approved IRB between December 2010 and May of 2012. Inclusion criteria were all CF patients who were able to produce a sputum sample. Exclusion criteria were CF patients who had undergone a lung transplant. A portion of the sample was sent to the Clinical Microbiology lab at Nationwide Children’s Hospital for routine culture.
The portion of the sample brought to the lab was further split, and half was used to determine the effect of anti-IHF and/or DNase on sputum solids while the remaining portion was embedded in OCT compound (Fisher Scientific, Pittsburgh, PA), snap frozen over liquid nitrogen and stored at −80° C until further analysis. Ten micron serial sections were cut using a Microm rotary cryotome, adhered to glass slides (Mercedes Medical, Sarasota, FL) and stored at −80° C. Sections were then immunostained to determine the relative presence of IHF within biomasses (or solids) present in CF sputum. Briefly, slides were air-dried, fixed in cold acetone, equilibrated in buffer (0.05M Tris-HCl, 0.15M NaCl and 0.05% Tween 20, pH 7.4), and blocked with image-iT FX signal enhancer (Molecular Probes, Eugene, OR) and with Background Sniper (BioCare Medical, Concord, CA). Sections were then incubated with a 1:200 dilution of polyclonal rabbit anti-IHF (E. coli) overnight at 4° C, in a humidified chamber. Slides were rinsed and incubated with goat anti-rabbit IgG conjugated to AlexaFluor 594 (Invitrogen, Eugene, OR) for 30 minutes at room temp, counterstained with DAPI to detect eDNA and cover-slipped using ProLong Gold antifade reagent (Molecular Probes, Eugene, OR). Use of naive rabbit serum in place of immune serum and use of secondary antibody alone served as negative control preparations. Sections were viewed with a Zeiss LSM 510 Meta confocal system attached to a Zeiss Axiovert 200 inverted microscope (Carl Zeiss Inc., Thornwood, NY).
For further in vitro analysis, equal volumes of sputum recovered from six patients (50 μl aliquots) were added to each of 4 wells in an 8-well chamber slide (Fisher Scientific, Pittsburgh, PA). Normal saline was added to the negative control well and either saline that contained a 1:10 dilution of DNase (Dornase Alfa, Pulmozyme, San Francisco, CA), saline that contained a 1:50 dilution of rabbit anti-IHF serum or saline that contained both DNase (1:10) and rabbit anti-IHF serum (1:50) were added to the wells. Chamber slides were incubated at 37° C for 6 hours as time–lapse images were collected at 15 minute intervals to visually assess dissociation of sputum solids.
To quantify this dissociation, equal volumes of sputum (50 μl) were added to each of 5 wells of a 24-well plate. Normal saline was added to one well as a negative control. The other wells received either naive serum (negative control), DNase (1:10), Anti-IHF (1:10), or a combination of DNase plus anti-IHF (both 1:10) diluted in saline. Changes in the optical density of the solution surrounding sputum solids was monitored using an Infinite M200Pro kinetic plate reader (Tecan, Durham, N.C.). Absorbance readings at 600 nm were collected every 5 minutes for 1 hour while incubating at 37°C including at t = 0 and at the endpoint (t = 60 minutes). A total of 6 sputum samples were assessed in this manner. For statistical analysis of changes in OD values, three blinded evaluators were asked to select 5 defined regions of each well which contained only the solution surrounding sputum solids. Changes in OD for each of these five defined areas at t = 0 were compared to those obtained at t = 60 for each well, and a Mixed Model was used to test for group effects. A p-value ≤ 0.05 was considered significant.
RESULTS
Clinical characteristics
Patients enrolled in the study ranged in age from 4 to 63 years of age. There were 21 female and 23 males (48% and 52% respectively). FEV1 of the patients ranged from 29–115%. Of the samples collected for this study, 10 samples were collected from patients who were experiencing an exacerbation at the time of their visit. These ten samples were not distinguishable from other samples in regards to immunolabeling for the presence of IHF, FEV1 values, microbiology culture results or other patient demographics.
Microbiology of sputum samples collected
The microorganisms identified from the sputum samples which underwent microbiologic analysis (39 total) are shown in Table 1 wherein Pseudomonas aeruginosa (PA) was the most prominent microorganism cultured. Seventy-seven percent of the sputum samples were culture-positive for this microbe. Staphylococci were also very prevalent within the samples collected from this patient pool. Methicillin sensitive Staphlococcus aureus (MSSA) and methicillin resistant Staphlococcus aureus (MRSA) were cultured from 38% and 28% of all samples, respectively. Of the sputum samples collected, 73% were culture-positive for more than one microorganism (Table 2), an observation which indicated that CF infected lungs are populated by multiple organisms, any of which may play a role in the pathogenesis of this disease.
TABLE 1.
Microorganisms isolated from CF sputum samples
| CF Sputum Culture Results | Number of Patients | % |
|---|---|---|
| Pseudomonas aeruginosa | 30 | 77 |
| - non-mucoid | 11 | 28 |
| - mucoid | 10 | 26 |
| - both | 9 | 23 |
| Staphylococcus aureus | 26 | 67 |
| - MSSA | 15 | 38 |
| - MRSA | 11 | 28 |
| - both | 0 | 0 |
| Stenotrophomonas | 7 | 19 |
| Serratia | 1 | 3 |
| Aspergillus | 3 | 8 |
| E. coli | 1 | 3 |
| Achromobacter | 2 | 5 |
| Candida | 1 | 3 |
| non-candidal yeast | 1 | 3 |
TABLE 2.
Number of microorganisms isolated from CF sputum samples (for those
| CF sputum culture positive for: | Number of patients | % | Most common bacterial combinations |
|---|---|---|---|
| 1 organism | 10 | 28 | MPA |
| 2 organisms | 14 | 38 | PA / MSSA |
| 3 organisms | 10 | 27 | PA / MPA / MSSA or PA / MPA / MRSA PA / MPA / MRSA / Aspergillus |
| >3 organisms | 3 | 8 | PA / MPA / MSSA / Stenotrophomonas MPA / MSSA / Aspergillus/Stenotrophomonas |
Two strain variants of Pseudomonas aeruginosa, mucoidal and non-mucoidal, have been implicated in CF lung infections with the mucoidal variants being associated with more severe cases (12, 16, 21). In our study, we identified both variants, and in some cases both types were present within the same sample. Mucoidal Pseudomonas alone was observed in 26% (10/39) of the sputum samples, while non-mucoidal Pseudomonas was identified as the sole Pseudomonas variant in 28% (11/39) of the samples. Additionally, 23% (9/39) of the samples had both variants identified.
DNABII proteins were located at the vertices of bent and crossed strands of eDNA contained within sputum samples recovered from CF patients
To determine whether members of the DNABII family of DNA binding proteins were in sputum samples recovered from patients with CF, we examined whether rabbit polyclonal antiserum directed against purified E. coli IHF (henceforth referred to as ‘anti-IHF’) would label frozen sections of sputum samples. We have previously found that while anti-IHF has significant avidity for virtually all IHF and HU proteins across genera, it is nonetheless highly specific for the DNABII family of proteins (18)(data not shown). Overall, we found that anti-IHF cross-reacting species were detected in 37 of 44 (84%) of CF sputum samples analyzed here, regardless of which bacterial species were present in the sample.
In terms of the spatial arrangement of IHF present within these sputum samples, in Figure 1, eDNA within the sputum sample can be seen as white strands, appearing in the form of an interwoven lattice or mesh. We found positive immunolabeling with anti-IHF antibody at virtually every visualized vertice of eDNA observed within the sample, as shown by punctate fluorescence signals (red) positioned wherever strands of eDNA crossed one another. Use of naive rabbit serum in combination with Alexa-Fluor conjugated goat-anti rabbit IgG, or of Alexa Fluor conjugated goat anti-rabbit IgG alone, did not result in this characteristic labeling of vertices (data not shown). Based on these observations, strands of eDNA present in the sputum of patients with CF were noted to be in association with at least one extracellular DNABII protein. This observation was identical to what we have observed in biofilms formed by NTHI both in vitro and in vivo (18) wherein the presence of a protein that was highly immunoreactive with antiserum directed against isolated E. coli IHF was present at each observed vertice of crossed strands of the abundant eDNA contained within the biofilm. These data suggest a potential common theme for biofilms formed in vivo within the mammalian airway (and perhaps other organ systems as well) regardless of the specific microbe involved or disease induced.
Figure 1.
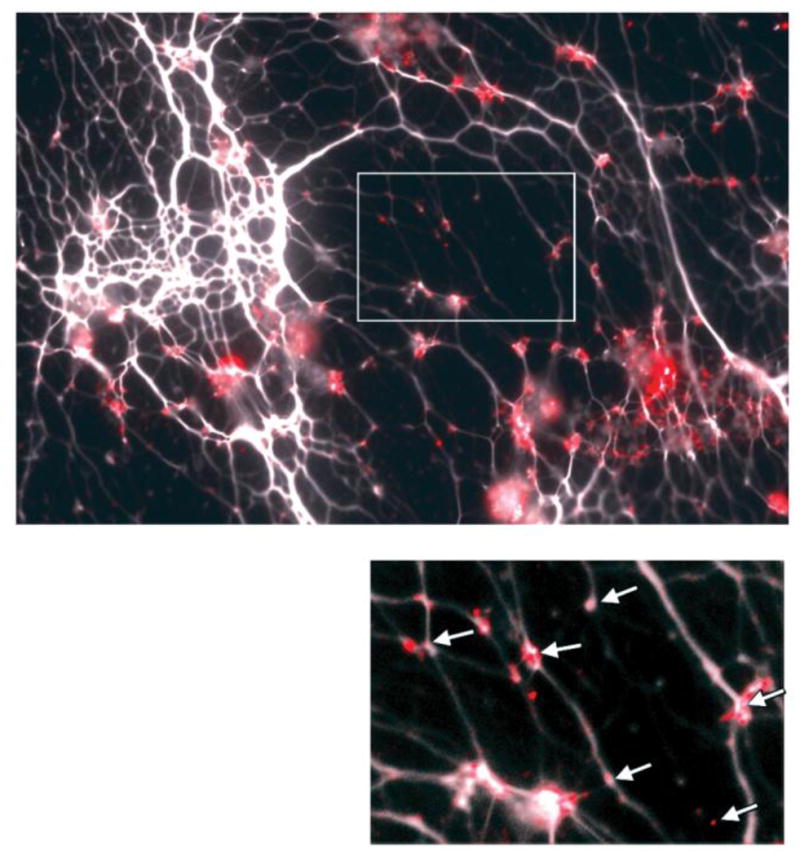
Representative image of immunohistochemical labeling for IHF (see punctate area of red fluorescent labeling in image and as indicated by arrows in inset) in OCT-embedded CF sputum samples. 10 micron section. Note: both Pseudomonas aeruginosa and methicillin resistant Staphlococcus aureus (MRSA) were cultured from this sample.
CF patient sputum was destabilized by treatment with anti-IHF which also further enhanced treatment with DNase
Based on our previous work to date, and the considerable strategic presence of IHF at vertices of crossed strands of the eDNA lattice present within CF sputum samples as observed here, collectively these data suggested that a DNABII family member was involved in the formation and perhaps maintenance of the structural integrity of this abundant eDNA-containing lattice. We have previously shown the ability of anti-IHF to disrupt the EPS of biofilms formed by numerous human pathogens in vitro (18). To determine if exposure to anti-IHF might similarly result in a loss of structural integrity of the biofilm matrix contained within CF sputum samples, we treated equal volumes of 6 representative sputum samples ex vivo with either saline, naive rabbit serum (negative control), anti-IHF, DNase or with a combination of DNase plus anti-IHF. Furthermore, we collected images of the samples at both t = 0 and at t = 60 mins, and observed a reduction of the visible sputum mass from all treatment groups compared to the control groups (Figure 2). However, there was greater observed dissolution of the sputum sample when incubated with the combination of anti-IHF and DNase (Figure 2, last column). These results suggested a loss of structural integrity of the sputum sample as mediated by incubation with anti-IHF that was equal to or greater than when incubated with a current common treatment modality (DNase). However, the dissolution of the sputum sample when incubated with the combination of anti-IHF plus DNase was notable and suggested the potential for synergistic use of these agents in the treatment of patients with CF.
Figure 2.
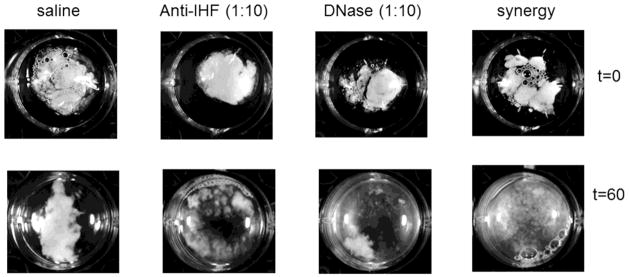
Representative images taken before and after 1 hour incubation with either saline, anti-IHF (1:10), DNase (1:10), or a combination of anti-IHF and DNase demonstrating the disruption of the sputum sample and increase in turbidity of the incubation solution.
During the conduct of our in vitro dissolution assays, we made the observation that as the sputum sample was disrupted and the material that made up the sample was dispersed by treatment, there was a discernable increase in the turbidity of the incubation solution. In an effort to quantify this noted increase in turbidity of the surrounding solution as the central semi-solid sputum mass was disrupted or dissolved, we measured the optical density of the samples using a kinetic plate reader which measured the absorbance at multiple points per well. We found that in 83% (5/6) of the samples measured in this manner after ~25 mins incubation, there was a notable increase in the optical density in the treatment wells compared to their respective absorbance readings at t = 0. OD readings of treated wells increased further over time, but maximized, reaching a plateau at ~1 hour of incubation (data not shown). The resulting mean absorbance readings obtained after 60 minutes of incubation with either saline, anti-IHF, DNase or a combination of anti-IHF plus DNase were graphed to demonstrate the relative dissolution of sputum solids upon treatment. An increase in turbidity was noted for samples treated with either anti-IHF, DNase or a combination of these two agents, compared to saline. This increase in turbidity was statistically significant at 60 minutes of incubation (p ≤ 0.05) for samples treated with either anti-IHF or DNAse (Figure 3), however combined treatment with both agents resulted in even greater increase in turbidity when compared to saline (p = 0.0005).
Figure 3.
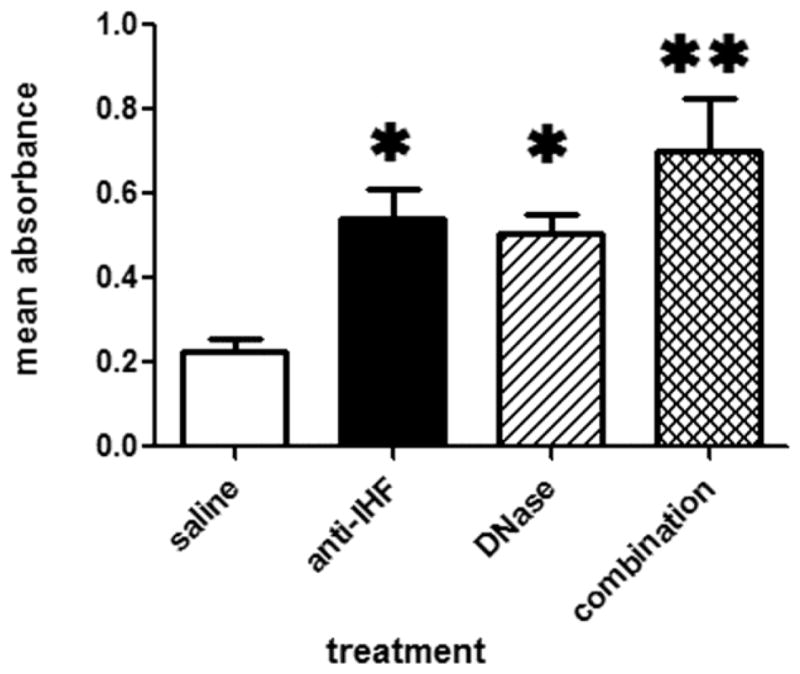
Mean absorbance readings of solution surrounding sputum solids as obtained after incubation of six divided CF sputum samples for one hour with either saline, anti-IHF (1:10), DNase (1:10) or a combination of these latter two agents. *denotes significant difference (p < ≤ 0.05) compared to saline, whereas ** denotes significance at p ≤ 0.001.
DISCUSSION
It is well known that a high concentration of eDNA in the sputum of CF patients increases mucus viscosity and reduces mucociliary clearance (7, 22, 23). Chronic airway obstruction is associated with impaired clearance of these highly viscoelastic secretions (7, 24, 25). Therefore, improving clearance of airway secretions by decreasing the high viscoelasticity of the sputum would have a significant beneficial clinical effect. Mucolytic agents, such as N-acetylcysteine, which are directed against mucus glycoproteins, have been associated with both irritation of the airways and induction of bronchospasm with no consistent beneficial effects (22, 26). Due to these drawbacks, these mucolytic agents are not routinely used in the treatment of CF. Instead, it has been found that there are more clinical benefits using agents such as DNase and hypertonic saline for daily airway clearance. Administration of hypertonic saline, an osmotic agent which may improve airway hydration, or DNase, which breaks down DNA and thereby also decreases mucus viscosity (7, 22, 27), have shown beneficial effects. Their utility is not however without questions, as there have been negative effects associated with use of hypertonic saline, such as decreased lung function, bronchospasm and increased cough in some patients (24). DNase has also had antecdotal variable clinical success, although it is generally tolerated well (22, 24, 27). There is certainly a need for additional airway clearance therapies which may either be used alone, or perhaps in combination with established modalities.
The source of the eDNA in sputum samples, as well as within biofilms formed during disease, is derived from both the host as well as the pathogens involved. However anti-IHF, as used here, mediates its effect through action on eDNA whose origin is bacterial, as BLAST searches of the human genome to date fail to show significant amino acid sequence identity between the DNABII proteins and any human protein. Microbes incorporate bacterial DNA (and associated proteins such as the DNABII proteins) into the EPS that surrounds and protects them when resident within a biofilm (18, 19). The presence of an abundant amount of eDNA within the lungs of CF patients and the effect that this matrix component has both on mucus viscosity as well as the stability of the biofilm matrix provides additional strong support to the role of eDNA in the symptomology and chronicity of CF. We have shown that a member(s) of the DNABII family that cross reacts with anti-IHF plays an important role in the structural integrity of the biofilm matrix formed by numerous bacterial species by stabilizing the eDNA within the matrix (18). In the present study, we examined whether any members of the DNABII family were present in association with eDNA within sputum of patients with CF.
Upon examining sputum from 44 patients, microbiological cultures were obtained in 39 of those and supported evidence which shows that CF is truly a polymicrobial disease; 72% (28/39) of the samples examined herein contained more than one microorganism. Pseudomonas aeruginosa was cultured most often in the samples, however we were able to identify a total of 6 bacterial species along with fungal pathogens. By immunohistochemical analysis, an anti-IHF cross-reacting species was detected in 84% of samples regardless of which bacterial agents were cultured. This immunolabeling showed the presence of anti-IHF crossreacting proteins positioned at the vertices of crossed strands of abundant eDNA present within the sputum samples. Thus, at least one (or both known) DNABII family members were likely critical components in the maintenance of the structural integrity of biofilms formed in the lungs of CF patients by P. aeruginosa, as well as perhaps other pathogens. There were no detectable differences in any patient characteristics between those that generated sputum samples which yielded positive labeling for IHF and those that yielded negative labeling results based on any of the outcome measures used herein.
This observation suggested that DNABII family members could be viable targets for either immune-mediated clearance or perhaps that mediated by novel therapeutic agents, or a combination of agents. Recently, we have demonstrated the ability of antibodies directed against IHF to debulk biofilms formed by numerous bacterial species in an in vitro assay (18). Furthermore, we showed that when we immunized animals with native IHF after they had already developed robust biofilms in the middle ear following direct challenge of that anatomical space with NTHI, the antibodies induced were able to destabilize and debulk these pre-existing biofilms, thus mediating more rapid resolution of experimental otitis media in the chinchilla host that was statistically significant (18). To determine if we could similarly debulk or dissociate sputum samples by incubation with anti-IHF, here we compared the effect of treatment of CF sputum with anti-IHF in either the absence or presence of DNase. To that end we demonstrated that anti-IHF induced disruption of the sputum biomass to a degree that was approximately equivalent to that mediated by treatment with DNase alone. However, when these two treatments were used in concert, we observed a strong synergistic effect wherein almost complete dissolution of the sputum sample was mediated. Moreover, we observed a measurable increase in the turbidity of the incubation solution as early as ~25 minutes into incubation with the combination of anti-IHF plus DNase and almost complete disruption of the semi-solid sputum sample mass at 1 hour. This effect was highly statistically significant compared to treatment with saline alone. Dissolution of sputum samples by either anti-IHF or DNase was measured as an increase in turbidity in 83% of the samples tested in this manner. Whereas, due to small sample size, sputum rheology was not measured in the present study, this parameter of evaluation will be added to future work.
CF remains a life-shortening condition (2, 3). Disease management and expanded newborn screening have led to a delay in disease progression (28–30), however continued research is still required to identify novel therapies that can alter the course of CF lung disease. Directing the immune response against the DNABII family of proteins which stabilize the eDNA present within biomasses contained in CF sputum, and/or targeting this bacterial protein for direct novel therapeutic interventions, may lead to significantly improved mucociliary clearance and increased susceptibility of resident bacteria to either host immune responses or to other existing treatment modalities (e.g. DNase or antibiotics).
Acknowledgments
This work was supported by a grant from the NIH/NIDCD R01 DC 011818 to LOB and SDG. We thank Jennifer Neelans for assisting in the preparation of this manuscript, and Wei Wang and Igor Dvorchik for expert statistical advice and analysis.
Footnotes
Data from this manuscript were presented at North American Cystic Fibrosis Conference (NACFC), Anaheim, California, November 2011 (1).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustave JE, Jurcisek JA, Goodman SD, McCoy KS, Bakaletz LO. Biofilms recovered from the sputum of CF patients contain the bacterial protein Integration Host Factor (IHF). North American Cystic Fibrosis Conference (NACFC); November 3–5, 2011; Anaheim, California. [Google Scholar]
- 2.Zemanick ET, Harris JK, Conway S, et al. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy. J Cyst Fibros. 2010;9(1):1–16. doi: 10.1016/j.jcf.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335(3):179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation. Patient Registry 2010: annual data report. 2010. [Google Scholar]
- 5.Cystic Fibrosis Foundation. Patient Registry 2005: annual data report. Bethesda: 2005. [Google Scholar]
- 6.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 7.Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax. 2010;65(1):51–56. doi: 10.1136/thx.2009.116970. [DOI] [PubMed] [Google Scholar]
- 8.Puchelle E, Bajolet O, Abely M. Airway mucus in cystic fibrosis. Paediatr Respir Rev. 2002;3(2):115–119. doi: 10.1016/s1526-0550(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 10.McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic Pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr. 2008;153(6):752–757. doi: 10.1016/j.jpeds.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60(3):539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bentzmann S, Plesiat P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol. 2011;13(7):1655–1665. doi: 10.1111/j.1462-2920.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 13.Stuart B, Lin JH, Mogayzel PJ., Jr Early eradication of Pseudomonas aeruginosa in patients with cystic fibrosis. Paediatr Respir Rev. 2010;11(3):177–184. doi: 10.1016/j.prrv.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 15.Lujan AM, Macia MD, Yang L, Molin S, Oliver A, Smania AM. Evolution and adaptation in Pseudomonas aeruginosa biofilms driven by mismatch repair system-deficient mutators. PLoS One. 2011;6(11):e27842. doi: 10.1371/journal.pone.0027842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Wang S, Wang D, Parsek MR, Wozniak DJ. The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunology & Medical Microbiology. 2012;65(2):377–380. doi: 10.1111/j.1574-695X.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SD, Obergfell KP, Jurcisek JA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4(6):625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 19.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 20.Swinger KK, Rice PA. IHF and HU: flexible architects of bent DNA. Curr Opin Struct Biol. 2004;14(1):28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Ratjen F, Munck A, Kho P, Angyalosi G. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax. 2010;65(4):286–291. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 22.Shah PL, Scott SF, Knight RA, Marriott C, Ranasinha C, Hodson ME. In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax. 1996;51(2):119–125. doi: 10.1136/thx.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 24.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 25.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361(9358):681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 26.Ratjen F, Wonne R, Posselt HG, Stover B, Hofmann D, Bender SW. A double-blind placebo controlled trial with oral ambroxol and N-acetylcysteine for mucolytic treatment in cystic fibrosis. Eur J Pediatr. 1985;144(4):374–378. doi: 10.1007/BF00441781. [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, VanDevanter DR, Rasouliyan L, et al. Trends in the use of routine therapies in cystic fibrosis: 1995–2005. Pediatr Pulmonol. 2010;45(12):1167–1172. doi: 10.1002/ppul.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abman SH, Ogle JW, Harbeck RJ, Butler-Simon N, Hammond KB, Accurso FJ. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J Pediatr. 1991;119(2):211–217. doi: 10.1016/s0022-3476(05)80729-2. [DOI] [PubMed] [Google Scholar]
- 29.Rock MJ. Newborn screening for cystic fibrosis. Clin Chest Med. 2007;28(2):297–305. doi: 10.1016/j.ccm.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Wang SS, FitzSimmons SC, O’Leary LA, Rock MJ, Gwinn ML, Khoury MJ. Early diagnosis of cystic fibrosis in the newborn period and risk of Pseudomonas aeruginosa acquisition in the first 10 years of life: A registry-based longitudinal study. Pediatrics. 2001;107(2):274–279. doi: 10.1542/peds.107.2.274. [DOI] [PubMed] [Google Scholar]


