Abstract
Objectives
To assess whether reporting “possible cystic fibrosis (CF)” newborn screening (NBS) results via fax plus simultaneous telephone contact with primary care providers (PCPs), versus fax alone, influenced three outcomes: getting a sweat chloride test, age at sweat chloride test, and sweat-testing before 8 weeks old.
Study Design
Retrospective cohort comparison of infants born in Wisconsin whose PCPs received telephone intervention (n=301), versus recent historical controls whose PCP did not (n=355). Intervention data were collected during a longitudinal research and quality improvement effort; de-identified comparison data were constructed from auxiliary NBS tracking information. Parametric and nonparametric statistical analyses tested for group differences.
Results
Most infants (92%) with “possible CF” NBS results whose PCPs lacked telephone intervention ultimately underwent sweat-testing, underlining efficacy for fax-only reporting. Telephone intervention was significantly associated with improvements in infants undergoing sweat-testing at both ≤6 and <8 weeks and a slight, but non-significant, 3.5-day reduction in infants’ age at sweat-testing. The effect of telephone intervention was greater for PCPs whose patients underwent sweat-testing at community-affiliated medical centers versus academic medical centers (p=0.008).
Conclusion
Reporting “possible CF” NBS results via fax plus simultaneous telephone follow-up with PCPs increases the number of infants who have sweat chloride tests before 8 weeks of age, when affected infants are more likely to receive full benefits of early diagnosis and treatment.
Keywords: Neonatal screening, sweat chloride testing, heterozygote, genetic carrier detection, genetic testing, public health genetics, parent-provider communication
INTRODUCTION
All U.S. states, and many countries worldwide, test for cystic fibrosis (CF) as part of routine newborn screening (NBS) on infant blood spots collected shortly after birth. Testing methods for CF vary across NBS programs, but all begin with analyzing for elevated immunoreactive trypsinogen (IRT) and end by requiring a confirmatory sweat chloride test for a large subset of infants with abnormal results. This project investigates how telephone follow-up with primary care providers (PCPs) influences the timing of sweat chloride testing.
In Wisconsin, NBS for CF is a two-tiered process beginning with IRT analysis on all blood spot samples. When IRT is elevated, the sample undergoes targeted genetic analysis for 23 mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The most commonly reported abnormal result is “possible CF,”1 which denotes elevated IRT and the presence of a single CFTR mutation. The CF Foundation recommends all infants with this type of abnormal CF screening result undergo sweat chloride testing by pilocarpine iontophoresis because of the residual chance to have CF caused by the presence of a second mutation that was not included in the NBS mutation panel2, 3. Wisconsin data suggest that these infants have a 2-5% chance to have CF diagnosed through an abnormal sweat chloride test, and that sweat chloride testing should be done by 8 weeks of age in order for infants to receive full benefits of early diagnosis and treatment4-8.
In order for timely sweat chloride testing to occur, however, NBS laboratories must ensure that abnormal results reach a PCP who will take responsibility for informing the parent and ensuring the infant gets a sweat chloride test. Nonetheless, there is no consensus about protocols for reporting and following up after “possible CF” NBS results, possibly because each NBS program is responsible for determining its own protocols for each geographic locale9-12. Reporting and follow-up procedures vary, but can include any combination of the following methods for reporting results9, 11, 12: fax, telephone, certified letter, regular post, and email. Many NBS programs employ nurses or other allied health professionals to contact families directly, while other NBS programs defer such contact entirely to clinicians or support groups13-15.
The usual mechanism for Wisconsin’s NBS laboratory to report “possible CF” NBS results is by fax to PCPs, followed by a copy of the results sent via regular mail. Additional contact with PCPs is not routine but may occur, via follow-up letter or telephone, to look into delays in sweat chloride testing when the NBS laboratory has not received notification of its occurrence. The primary goal of this secondary analysis was to assess whether reporting of “possible CF” NBS results via fax plus simultaneous telephone contact with PCPs, versus fax alone, affected the following three sweat chloride testing outcomes: 1) infants actually getting a sweat chloride test, 2) infants’ age at time of sweat chloride testing, and 3) infants getting a timely sweat chloride test (before 8 weeks of age). A secondary, post hoc analysis was done to determine if the three sweat chloride testing outcomes were influenced by other factors, such as the centers at which infants underwent sweat chloride testing.
METHODS
This secondary using a subset of data from a larger project16 is a retrospective cohort comparison of infants whose PCPs received an immediate telephone intervention, versus recent historical controls from the previous two years whose PCP did not receive the intervention. The telephone intervention was done as part of a longitudinal, statewide research study and quality improvement effort entitled the “Wisconsin Project on Improvement of Communication Processes and Outcomes after Newborn Screening” (henceforth called “the Project”). This Project is a collaboration of the Wisconsin State Laboratory of Hygiene and the Department of Health Services with the Medical College of Wisconsin as a contracted agent. Project Methods are further described elsewhere16. The Project was approved by Institutional Review Boards at the Medical College of Wisconsin and University of Wisconsin—Madison.
NBS laboratory protocol for reporting abnormal CF NBS results
The WI NBS program began CF screening in 1985 (on a research basis) and incorporated it into routine practice on all newborns in 1994, using the IRT/DNA (F508del mutation only) method. In 2002, the laboratory replaced single (F508del) mutation screening with a multiple mutation panel of 25 CFTR mutations, which was slightly reduced to 23 CFTR mutations in 2008 in accordance with ACMG recommendations17 (though this did not alter the timing, reporting, or accuracy of CF NBS screening), with an estimated sensitivity of 97%18. The NBS laboratory’s usual practice is to fax and mail a copy of abnormal CF NBS results indicating elevated IRT and a single CFTR mutation to the clinician of record (COR) listed on the NBS card. Infants with abnormal results indicating elevated IRT and 2 CFTR mutations (affected) prompt a fax plus immediate phone call to COR, while infants with highly elevated IRT only (≥170 ng/mL) with no CFTR mutation identified have results reported by mail. The focus of this analysis is on follow-up for elevated IRT, single CFTR mutation NBS results only.
The NBS laboratory also performs auxiliary tracking of abnormal CF NBS results in order to verify sweat chloride testing dates, sweat chloride testing sites, chloride levels, and resulting diagnoses. Wisconsin lists 5 CF referral centers for sweat chloride testing on all abnormal CF NBS reports. Of these 5 medical centers, 4 receive faxes of every abnormal CF NBS result in the state, while 1 medical center requested not to receive them. Additionally, all 5 medical centers’ CF clinics are faxed a “summary report” every 1-2 weeks listing all of the year’s infants with abnormal CF results in the state, the specifics about the NBS results, and space for the CF clinics to fill in and update sweat chloride test information. Once updated, the CF clinics fax the summary report back to the NBS laboratory within a few days, the lab updates their records, and the cycle continues. Medical centers other than those listed on the abnormal CF NBS report do not typically receive the individual abnormal CF results or the faxed summary report, and the sweat chloride test results are usually conveyed by phone to the NBS laboratory.
If there is no record of an infant’s sweat chloride test by the age of 8 weeks, the NBS laboratory will send a reminder letter to the COR. If there is still no record of a sweat chloride test and the COR has not contacted the NBS lab with further information, a representative from the NBS laboratory will phone the COR to reiterate the need for sweat chloride testing. If the COR knows he/she is not the infant’s PCP, a good faith attempt must be made (by the COR) to identify the infant’s PCP or contact the family with the results. At any point, the NBS laboratory may receive information from the COR that they are not PCP, cannot find the PCP, or cannot reach the family. In those cases, the NBS laboratory pursues the case more aggressively (by calling the hospital of birth or having a public health nurse or social worker attempt to contact the family directly). The NBS laboratory continues to follow-up on infants with belated sweat chloride tests for up to 12 months, or until enough information is gathered to “feel confident” that the infant’s need for sweat chloride testing is known by a responsible PCP and will eventually happen. The NBS laboratory did this follow-up, regardless of the Project intervention (so both the intervention and non-intervention groups received the same level of NBS laboratory follow-up, at the same time points) and is therefore unlikely to have biased either cohort.
Source of data and recruitment for the study (“intervention”) group
As part of the Project, NBS results were faxed from the NBS laboratory to the Project team at the same time they were faxed to the COR. Infants were included in the Project if they had NBS results indicating elevated IRT with a single CFTR mutation, and they had birth dates in the 24 month period from December 2007 to November 2009. Infants were excluded if the study team obtained information indicating (a) abnormal NBS results for more than one condition, (b) the gestational age was less than 35 weeks, (c) the calendar age at the time of specimen collection was greater than 180 days; infants were also excluded if the study team discovered that the infant (d) spent more than 5 days in the hospital or neonatal intensive care unit, (e) was re-hospitalized after discharge from the nursery, (f) was undergoing evaluation for another serious medical condition, or (g) the infant’s PCP identified a contraindication to contacting the family, such as psychosocial issues or a language barrier. The study team obtained information about criteria (d), (e), (f), and (g) by speaking directly with the birthing center (nursery, NICU, birthing unit), the COR, or the infant’s PCP. Sometimes we spoke with another representative allied health professional who had access to that information when we phoned.
The sweat chloride test summary report was also faxed to the Project’s study team every 1-2 weeks by the NBS laboratory for data analysis purposes. Diagnoses from sweat chloride testing were most often definitive, resulting in unaffected CF carriers (infants with a normal sweat chloride test) or affected infants with CF (infants with an abnormal sweat chloride test). On occasion, however, sweat chloride testing ends with ambiguous, inconclusive results when chloride levels are in a “borderline” range and further genetic testing or clinical observation is necessary. It is still recommended that all infants with abnormal CF NBS results undergo sweat chloride testing because there is no way to foresee which sweat chloride test result an infant would ultimately have. Infants were therefore included in this analysis regardless of whether or not they underwent sweat chloride testing and despite their resulting diagnoses from sweat chloride testing as confirmed CF carriers, affected with CF, or inconclusive results.
For infants meeting inclusion criteria, the Project’s staff re-faxed the abnormal CF NBS results and a description of the Project to the COR. A Project member then telephoned the COR and confirmed receipt of the NBS results, and confirmed that the COR was the infant’s PCP. In instances when the COR did not know the infant or denied a PCP relationship, the study team would attempt to locate the correct PCP by asking the COR for advice, and then by contacting the birthing facility or its medical record department. If these methods were not successful in finding the PCP, a few days later the Project team contacted the COR again to see if the infant’s parents had made an appointment. IRB stipulations disallowed the Project team from contacting families directly unless we assumed clinical care responsibilities for the infant, which was rarely necessary. Once the PCP and receipt of the NBS results was confirmed, the study team made sure the PCP knew that the infant needed a sweat chloride test, provided answers to any questions the PCP had about the NBS result or sweat chloride testing, and invited the PCP to rehearse how he/she would inform the infant’s family about the NBS results. Data from these PCP interviews and rehearsals will be reported elsewhere. This reporting mechanism of fax plus simultaneous telephone contact with PCPs was the “intervention” for this analysis.
Source of data for the recent historical control (“non-intervention”) group
A comparison group for this analysis was constructed from de-identified data from the NBS laboratory’s tracking records for sweat chloride testing after CF screening. Infants were included in the non-intervention group if they had NBS results indicating elevated IRT with a single CFTR mutation, and they had birth dates in the 24 months prior to the start of the Project from December 2005 through November 2007. There were insufficient data in the NBS laboratory’s tracking records to allow for other exclusion criteria, since these records contained fewer details than the Project’s dataset.
Data Analysis
Sweat chloride test data were analyzed using SPSS19. Nonparametric Pearson correlations and independent samples t-tests were used to test for relationships between variables of interest. Finally, Chi-squared analyses were performed to determine whether associations could be predicted between the intervention and non-intervention groups of infants.
RESULTS
By following the inclusion and exclusion criteria described above, 301 infants qualified for the intervention group and 355 infants qualified for the non-intervention group. Information about the infants’ characteristics and final diagnoses after sweat chloride testing are shown in Table 1. The non-intervention group of infants were slightly more likely to be male and had a higher average IRT level, but these differences were not statistically significant (p=0.083, χ2 and p=0.104, t-test, respectively). Premature infants tend to have higher average IRT levels20; since we were unable to exclude premature infants from the non-intervention group, it is possible their inclusion may account for the slight differences in IRT levels between the groups. Infant gender and IRT level were not significantly associated with any of the outcome variables. Intervention and non-intervention groups did not differ significantly by infants’ diagnoses after sweat chloride testing (p=0.113, χ2).
Table 1.
Infant characteristics and sweat test timing information
| Intervention Group (N = 301) |
Non-Intervention Group (N = 355) |
|||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Year of birth | ||||
| 2005 | 0 | (0) | 19 | (5.4) |
| 2006 | 0 | (0) | 176 | (49.6) |
| 2007 | 11 | (3.7) | 160 | (45.1) |
| 2008 | 154 | (51.2) | 0 | (0) |
| 2009 | 136 | (45.2) | 0 | (0) |
| Sex | ||||
| Female | 166 | (55.1) | 168 | (47.3) |
| Male | 134 | (44.5) | 183 | (51.5) |
| Not reported | 1 | (0.3) | 4 | (1.1) |
| Final Diagnosis | ||||
| Carrier, CF | 260 | (86.4) | 307 | (86.5) |
| Affected, CF | 11 | (3.7) | 15 | (4.2) |
| Inconclusive | 15 | (5.0) | 7 | (2.0) |
| IRT ^ | ||||
| Average ng/mL (SD) | 78.2 | (26.6) | 82.6 | (40.1) |
| Got a sweat test | ||||
| n (%) | 286 | (95.0) | 329 | (92.7) |
| Age (days) at sweat test | ||||
| average (SD; 95% CI) | 31.4 | (29.6; 28.0-34.8) | 34.9 | (36.9; 30.9-38.9) |
| median (range) | 23 | (8-294) | 22 | (8-334) |
| Got a timely sweat test (<8 weeks)◇ | ||||
| n (%) | 257 | (85.4)** | 275 | (77.5) |
Infant’s immunoreactive trypsinogen (IRT) on newborn screen report in ng/ML; (SD) is standard deviation
Compared with infants who got a sweat test at an age ≥8 weeks and infants who did not get a sweat test.
Statistically significant difference (p=0.010, χ2)
Accuracy of PCPs’ names and contact information (as listed by the birthing facility) was not available for this analysis due to de-identification of the historical comparison group and the sub-sample that comprised the intervention group. To compare the results with other NBS programs, however, it may be helpful to review the following updated results about all CF infants included in the Project to date. Of all infants with “possible CF” who met inclusion criteria (n=563), the study team found that the NBS card had accurate PCP information 71% of the time (n=400) or PCP was a partner of the COR 9% of the time (n=52). On 9% of reports (n=49), the PCP’s name was correctly listed but associated with a completely different practice at an incorrect address (so the fax would have been sent to incorrect practice); in 10% of reports (n=59), the PCP was someone entirely different from the clinician listed on the NBS report. Lastly, 1% of the time (n=3) our study team was unable to identify a PCP for the infants and assumed clinical responsibility for them. In short, the NBS results were unlikely to have reached the correct PCP in a timely manner in 19.7% of infants.
Age and timeliness of sweat chloride testing
Although most infants in both groups underwent sweat chloride testing, 26 infants (7%) in the non-intervention group and 15 infants (5%) in the intervention group did not (Table 1). This difference was not statistically significant (p=0.218, χ2). Reasons for infants not getting a sweat chloride test were as follows: parent declined based on genetic testing of infant’s affected sibling’s known mutations (n=4: intervention group, n=3; non-intervention group, n=1), parent declined based on parental carrier testing (n=7: intervention group, n=3; non-intervention group, n=4), parent declined based on infant’s genetic testing prior to sweat chloride testing (n=1, non-intervention group only), parent declined because of infant’s severe medical condition (n=2, intervention group only), parent officially declined but did not give a reason “active decline” (n=4: intervention group, n=3; non-intervention group, n=1), parent did not schedule a sweat chloride test for the infant and did not give a reason “passive decline” (n=18: intervention group, n=5; non-intervention, n=13), infant lost to follow up most often because the family moved (n=5: intervention group, n=1; non-intervention group, n=4).
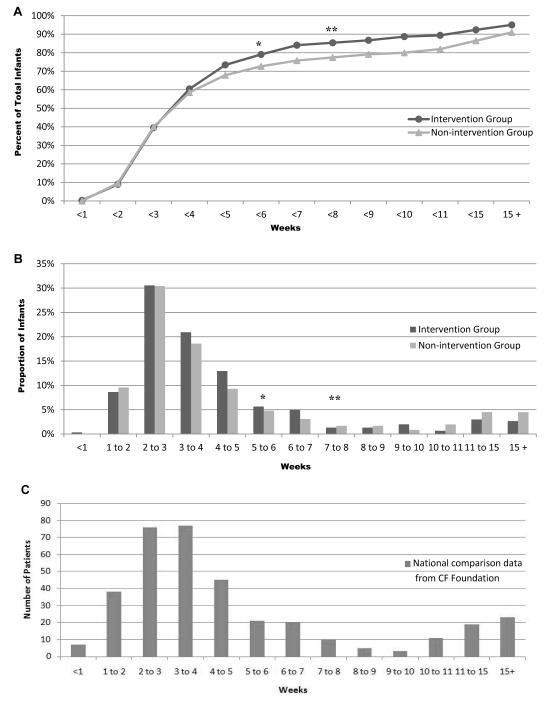
The telephone intervention was associated with a slight, but non-significant, reduction in average age at time of sweat chloride testing (p=0.204, t-test). Moreover, the telephone intervention was associated with a significant improvement in the proportion of infants who had a timely sweat chloride test (<8 weeks of age) (Table 1, Figure 1A and 1B). Additionally, further analysis showed that telephone intervention also made an impact on sweat chloride testing at ≤6 weeks (infant ≤48 days old), with a significant improvement in the proportion of infants having a sweat chloride test by this time point (84% and 76% for the intervention and non-intervention groups, respectively; p=0.009, χ2). For comparison, the CF Foundation has provided Figure 1C illustrating sweat chloride test timing for infants nationwide who were diagnosed with CF via NBS and reported to the Foundation’s Patient Registry.
Figure 1.
Time from birth to first recorded sweat test in infants with abnormal CF NBS results. A, Total percentage of all infants, and B, proportion of infants by intervention group to get a sweat test by the indicated (week) time point. C, Total number of affected infants born in the U.S. in 2010 diagnosed via NBS and reported to the CF Foundation patient registry (n=355), graphed by week of initial sweat chloride test.
* Cumulative difference significant, (p=0.009)
** Cumulative difference significant, (p=0.010)
Post hoc analysis of sweat chloride test timing at academic versus community-affiliated medical centers
Since this analysis is also part of a quality improvement effort, we were interested in assessing post hoc comparisons of other factors that might be associated with differences in timeliness or age at sweat chloride testing. We examined timing of sweat chloride testing at 9 sweat chloride testing centers in Wisconsin and Minnesota for which we had data. We categorized the sweat chloride testing centers into two categories: academic medical centers or community-affiliated medical centers. Four of the sweat chloride testing centers were academic medical centers that operated out of facilities that are responsible for teaching and research (3 in Wisconsin, 1 in Minnesota). The remaining 5 centers were categorized as community-affiliated medical centers (4 in Wisconsin, 1 in Minnesota). The sweat chloride test site was not reported for 3 infants in the intervention group and 6 infants in the non-intervention group. We did not group sweat chloride testing sites by volume or whether the laboratory was CFF certified since the data for all sweat chloride tests performed at each site were not uniformly available.
Infants who underwent sweat chloride testing at community-affiliated medical centers (n=138) were significantly influenced by the Project’s intervention in getting timely sweat chloride tests before 8 weeks of age (Table 2). These infants had a mean difference in age at sweat chloride test of approximately 10 days earlier compared with non-intervention infants at the same centers which, though not significant, trended toward significance (p=0.077, t-test). Infants who underwent sweat chloride testing at academic medical centers (n=468) were not significantly influenced by the Project’s intervention regarding their age at the time of sweat chloride test or getting timely sweat chloride tests before 8 weeks of age (p=0.802, t-test and p=0.611, χ2, respectively).
Table 2.
Infants’ sweat test timing by sweat test center groupings
| Sweat Test Site Grouping | Intervention Group (N = 286) |
Non-intervention Group (N = 329) |
||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Academic Medical Centers | 220 | (77.0) | 248 | (75.4) |
| Age (days) at sweat test | ||||
| average (SD; 95% CI) | 31.8 | (33.8; 27.6-36.0) | 32.5 | (33.8; 28.3-36.7) |
| median (range) | 22 | (8-294) | 22 | (8-334) |
| Got a timely sweat test (<8 weeks) ◇ | ||||
| n (%) | 195 | (88.6) | 216 | (87.1) |
| Community-Affiliated Medical Centers | 63 | (22.0) | 75 | (22.8) |
| Age (days) at sweat test | ||||
| average (SD; 95% CI) | 30.7 | (19.0; 26.0-35.4) | 40.9 | (42.1; 31.4-50.4) |
| median (range) | 27 | (10-138) | 23 | (11-198) |
| Got a timely sweat test (<8 weeks) ◇ | ||||
| n (%) | 59 | (93.7)** | 58 | (77.3) |
Compared with infants who got a sweat test at an age ≥8 weeks and infants who did not get a sweat test.
Statistically significant difference (p=0.010, χ2)
We also examined sweat chloride test timing for the individual centers. When sweat chloride test centers were compared individually, one community-affiliated medical center was significantly influenced by the Project’s intervention for infants receiving a timely sweat chloride test (p=0.043, χ2), while another academic medical center trended toward significance (p=0.064, χ2). Site-specific statistical comparisons for 4 centers were not possible because they performed sweat chloride testing on fewer than 10 infants in the sample.
DISCUSSION
Two-tiered NBS methods for CF are sufficient to identify most infants with CF, but timely sweat chloride testing is crucial for “possible CF” infants who may have the disease despite only having a single CFTR mutation detected by NBS. Through our statewide quality improvement and follow-up research project, we assessed whether reporting “possible CF” NBS results via fax plus simultaneous telephone contact with PCPs, versus fax alone, affected sweat chloride test timing.
This secondary analysis suggests three implications about adding simultaneous PCP telephone calls to the usual fax reporting of “possible CF” NBS results. First, most infants with the “possible CF” result are being evaluated by sweat chloride test without the telephone follow-up, suggesting that the fax-only mechanism and “as-needed” follow-up is generally efficacious. Second, it appears that fax plus simultaneous telephone follow-up with PCPs improves the timeliness of sweat chloride testing (at both ≤6 and <8 weeks of age), such that infants with the “possible CF” result may be more likely to receive benefits of early detection. Thirdly, there may be small-area variations in follow-up after NBS for CF, as seen in the observation that the fax plus simultaneous telephone follow-up, versus fax alone, has a greater effect on PCPs referring to certain sweat chloride testing centers. It is unclear from the site-specific results why certain sweat chloride test sites were more influenced by our intervention. Future research is needed to study the characteristics or practices of PCPs who refer to these centers, or the scheduling protocols among these sites.
Early identification of infants affected with CF via NBS is shown to increase survival21, lead to substantial growth benefits4, 5, 7, 22, improve nutritional status4, 5, 8, 22, and reduce the number of long-term therapies required7, hospitalizations22, and chronic P aeruginosa infections in childhood7, 22. However, there is discrepancy about what defines “early”23. Sims and colleagues7 contend that NBS for CF has the greatest benefit in infants diagnosed presymptomatically, since significant morbidity may occur even if not clinically evident before 8-12 weeks of age. A review of 10 studies published since 2001 all make recommendations or statements that infants can successfully be sweat-tested by or before 8 weeks of age5, 7, 24-30; 8 of the 10 recommend sweat chloride testing ≤6 weeks. To adopt the most inclusive definition of “early diagnosis” we chose our cut-point at <8 weeks of age.
Infants in both of our study groups had a comparable age at sweat chloride testing to reports from the CF Foundation’s registry data, other US states, and countries worldwide. Infants in both cohorts commonly got a sweat chloride test between 3-5 weeks of age, compared with studies from Michigan31 and Massachusetts26 that reported 2-4 weeks of age (mean 3.8, median 2.9) and a median of 4 weeks of age, respectively, while another study from Colorado32 reported 6-7 weeks (mean 6.2). A survey of 26 European NBS programs33 reported that infants commonly got diagnosed via sweat chloride testing at 5-6 weeks old (median 5.3), with 16 NBS programs reporting ≤6 weeks of age and 8 NBS programs reporting ranges from >6 to 17 weeks (frequently ~8-10 weeks).
Adding simultaneous PCP telephone calls to the traditional fax reporting method for “possible CF” results showed a modest, positive effect on sweat chloride test occurrences and significantly impacted infants getting a timely sweat chloride test. Infants in both groups had high incidences of getting a sweat chloride test and having the correct PCPs listed on the NBS card. It is possible that it might be more difficult to see the effects of the immediate telephone intervention among groups already faring well with high baseline rates of occurrence. It is probable that the addition of immediate telephone follow-up with PCPs would minimize the delays associated with inaccurate or missing PCP information, since the inaccuracies would be resolved in a timelier manner by immediately seeking out the correct PCP for the infant. However, one may presume that states or countries with lower rates of correct PCPs listed on the NBS card, lower rates of getting a sweat chloride test, and longer time to sweat chloride test would benefit more from immediate follow-up with PCPs, as it may quickly identify the PCP inaccuracy for more infants, allow the inaccuracy to be remedied sooner, clarify misconceptions about sweat chloride test timing, and ensure that the correct PCP receives the NBS results (thereafter, we assume, parents would be informed sooner and the sweat chloride test would occur in a timelier manner).
Other conclusions emerge from our observations. Most PCPs are aware of the need for sweat chloride testing when they receive “possible CF” results, but some may be unsure about the recommended timing or unaware of the NBS benefits to infants diagnosed <8 weeks of age resulting from prompt sweat chloride testing. Others may be influenced by the historical but erroneous misconception that infants do not sweat adequately before 8 weeks of age. Anecdotally, a number of PCPs asked our Project’s study team about this during the phone call: “When should the baby get the sweat chloride test?” PCPs and parents can benefit from clear, concise communication about next steps after receiving an abnormal CF NBS result. Perhaps an addition to the faxed report suggesting a preferred time frame for follow-up sweat chloride testing could be beneficial. Since data was not routinely collected about incorrect PCPs listed on NBS reports or PCPs who received advice about sweat chloride test timing during the intervention call, we were unable to substantiate the impact of these factors. It is also possible that the simple act of simultaneous telephone contact with PCPs at the time of results reporting via letter or fax, highlighting the results and providing opportunities for PCPs to ask questions, may signify or reinforce the need for prompt follow-up with families. Reporting “possible CF” results with a similar approach and attitude to “(Affected) elevated IRT, 2 mutations” results (and expecting similar responsiveness) may prompt hesitation; however, the overarching concern is that one cannot foresee which of these infants with “possible CF” is actually affected, so all results need to be treated with equal concern. It is unclear from this analysis exactly which features of the immediate PCP calls are most influential on sweat chloride test timing, and future research is needed to examine these factors separately.
Though exact reasons for the efficacy of these immediate PCP calls may warrant further study, we point to the potential benefit of implementing these calls (infants benefiting from early CF diagnosis) which likely outweighs the potential cost. Using a conservative Project estimate of time to complete these PCP calls (30 to 50 minutes per infant), we calculate using a WI birth rate of 70,000 infants screened and approximately 250 abnormal CF NBS results identified34 that 208 hours of work would be required to follow-up on all of these abnormal results (0.1 FTE). To be fair, some infants require less work while others require more, and states with considerably higher birth rates may require up to 0.5 FTE. There are several allied health professionals appropriate for this type of employment including public health nurses or genetic counselors (professions with higher salaries compared to other appropriate professions such as medical or research assistants) to estimate a generous cost per infant calculation. Using the WI birth rate, average WI salary for a genetic counselor, and a range of work hours required (0.1 to 0.5 FTE), we estimate the probable cost of implementing these immediate PCP calls to be $0.08 to $0.42 per newborn screened. Based on recently published data35, this would add 1-6% to the cost of IRT/DNA screening. Consequently, the justification appears compelling, given the potential value of early detection in affected infants and the relatively low cost to implement these PCP calls.
Study limitations are expected in this type of population comparison. The lack of a randomized control trial design might be construed as such, though we feel the recent historical controls in the immediate 2-year period preceding the intervention are adequate for comparison. However, there is some asymmetry in data available for exclusion criteria between study groups. As a result, the non-intervention group is more heterogeneous than the intervention group since we were unable to exclude infants based on specific criteria listed above (with additional health complications) from the non-intervention group. It is possible that infants requiring longer hospitalizations or re-admission would take longer to get a sweat chloride test due to medical complications or low birth weight; alternatively, these infants receiving more medical care and attention may get sweat chloride testing sooner than their non-hospitalized counterparts. Additionally, Wisconsin and other states have reported changes in sweat chloride test timing over the years, potentially impacting studies utilizing historical controls. However, those fluctuations were attributed to specific changes in age recommendations for testing36 or to differences in infants with differing numbers of mutations (2 mutations identified, versus 1 mutation or elevated IRT only)37. We feel confident that secular changes would be minimal or random, since this analysis occurred during a period of no significant changes in NBS reporting or methodology and we only studied infants with 1 CFTR mutation identified on NBS in both cohorts.
Other potential limitations stem from our definitions of “academic” and “community” medical centers or low samples sizes for four sweat chloride test sites, restricting our capability to analyze site-specific data for those centers. We did not have adequate data on referring PCPs to consider alternative infant groupings based on type or size of PCP practice, hospital affiliation, zip code, or medical philosophy, which may influence sweat chloride test timing. We were unable to compare cohorts based on infants’ race/ethnicity or zip code data, or include infants with non-English speaking parents, potentially limiting generalizability.
Limitations notwithstanding, we show that simultaneous telephone follow-up with PCPs at the time of faxed reporting of “possible CF” NBS results has significant benefits in increasing by 8% the proportion of all infants getting a timely sweat chloride test. Furthermore, telephone intervention shows a significant 16% increase in timely sweat chloride tests at community-affiliated medical centers, even exceeding the proportion of timely sweat chloride tests at academic medical centers. Information reported by us here and elsewhere indicates that this follow-up can be done with only a small amount of additional labor and cost16, 38. Based on this analysis, we can recommend the use of fax plus simultaneous telephone follow-up for NBS programs that can afford this extra labor and that wish to increase the benefits of CF screening in newborns.
Acknowledgments
The authors wish to thank Stephanie A. Christopher and Karen Kennedy-Parker for recruiting, advice, and help with data management, and Jenelle L. Collins for her data entry assistance. Additionally, we thank Dr. Bruce Marshall and the Cystic Fibrosis Foundation for providing and approving Figure 1C for publication.
Financial Disclosure and Disclosure of funding
The project is funded by NIH grants K01-HL072530 and R01-HL086691. Authors A La Pean and MH Farrell wrote the first draft of the manuscript and no form of payment was given to any of the authors to produce the manuscript. Acknowledged individuals SA Christopher and JL Collins are funded by NIH project grants already referenced, and K Kennedy-Parker is funded by the Wisconsin Newborn Screening Laboratory at the Wisconsin State Laboratory of Hygiene.
List of Abbreviations Used in this Manuscript
- NBS
newborn screening
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- PCP(s)
primary care provider(s)
- IRT
immunoreactive trypsinogen
- COR
clinician of record
Footnotes
The authors and those individuals named in the Acknowledgments section have no conflicts of interest to disclose.
REFERENCES
- 1.National Newborn Screening and Genetics Resource Center National Newborn Screening Information System (NNSIS) 2008 report and ongoing reports from 2010. Oct 26; Available from: http://nnsis.uthscsa.edu/xreports.aspx?XREPORTID=10&FORMID=13&FCLR=1.
- 2.Comeau AM, Accurso FJ, White TB, Campbell PW, 3rd, Hoffman G, Parad RB, et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics. 2007 Feb;119:e495–518. doi: 10.1542/peds.2006-1993. [DOI] [PubMed] [Google Scholar]
- 3.Wisconsin State Laboratory of Hygiene Health Professionals Guide to Newborn Screening, Cystic Fibrosis. 2011 Mar 14; Available from: http://www.slh.wisc.edu/newborn/guide/cystic_fibrosis.dot.
- 4.Salvatore D, Buzzetti R, Baldo E, Forneris MP, Lucidi V, Manunza D, et al. An overview of international literature from cystic fibrosis registries 2. Neonatal screening and nutrition/growth. J Cyst Fibros. Mar;9:75–83. doi: 10.1016/j.jcf.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, et al. Wisconsin Cystic Fibrosis Neonatal Screening Study Group Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001 Jan;107:1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Farrell PM, Lai HJ, Li Z, Kosorok MR, Laxova A, Green CG, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005 Sep;147:S30–36. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics. 2007 Jan;119:19–28. doi: 10.1542/peds.2006-1498. [DOI] [PubMed] [Google Scholar]
- 8.Marcus MS, Sondel SA, Farrell PM, Laxova A, Carey PM, Langhough R, et al. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr. 1991 Sep;54:578–585. doi: 10.1093/ajcn/54.3.578. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Lloyd-Puryear MA, Tonniges TF. Examination of communication practices between state newborn screening programs and the medical home. Pediatrics. 2003;111:e120–e126. doi: 10.1542/peds.111.2.e120. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh PL, Wang CJ, Therrell BL, Sprinz PG, Bauchner H. Communication of positive newborn screening results for sickle cell disease and sickle cell trait: variation across states. Am J Med Genet C Semin Med Genet. 2008 Feb 15;148C:15–22. doi: 10.1002/ajmg.c.30160. [DOI] [PubMed] [Google Scholar]
- 11.Abhyankar S, Lloyd-Puryear MA, Goodwin R, Copeland S, Eichwald J, Therrell BL, et al. Standardizing newborn screening results for health information exchange; Paper presented at: American Medical Informatics Association 2010 Symposium; Washington DC. 2010; [PMC free article] [PubMed] [Google Scholar]
- 12.Mandl KD, Feit S, Larson C, Kohane IS. Newborn screening program practices in the United States: Notification, research and consent. Pediatrics. 2002;109:269–273. doi: 10.1542/peds.109.2.269. [DOI] [PubMed] [Google Scholar]
- 13.Beucher J, Leray E, Deneuville E, Roblin M, Pin I, Bremont F, et al. Psychological effects of false-positive results in cystic fibrosis newborn screening: a two-year follow-up. J Pediatr. 2010;156:771–776. doi: 10.1016/j.jpeds.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Lang CW, McColley SA, Lester LA, Ross LF. Parental Understanding of Newborn Screening for Cystic Fibrosis After a Negative Sweat Test. Pediatrics. 2011 Feburary;127:276–283. doi: 10.1542/peds.2010-2284. 2011. [DOI] [PubMed] [Google Scholar]
- 15.Moran J, Quirk K, Duff AJA, Brownlee KG. Newborn screening for CF in a regional paediatric centre: The psychosocial effects of false-positive IRT results on parents. Journal of Cystic Fibrosis. 2007;6:250–254. doi: 10.1016/j.jcf.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Farrell MH, Christopher SA, Tluczek A, Kennedy-Parker K, La Pean A, Eskra KL, et al. Improving communication between doctors and parents after newborn screening. Wis Med J. 2011;110:221–227. [PMC free article] [PubMed] [Google Scholar]
- 17.Grody WW, Cutting GR, Klinger KW, Richards CS, Watson MS, Desnick RJ. Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genet Med. 2001 Mar-Apr;3:149–154. doi: 10.1097/00125817-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Baker MW, Groose M, Hoffman G, Rock M, Levy H, Farrell PM. Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J Cyst Fibros. Jul;10:278–281. doi: 10.1016/j.jcf.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SPSS Inc. Version GradPack 18. PASW Statistics; Chicago, Illinois: 2009. computer program. [Google Scholar]
- 20.Korzeniewski SJ, Young WI, Hawkins HC, Cavanagh K, Nasr SZ, Langbo C, et al. Variation in immunoreactive trypsinogen concentrations among Michigan newborns and implications for cystic fibrosis newborn screening. Pediatr Pulmonol. 2011 Feb;46 doi: 10.1002/ppul.21330. [DOI] [PubMed] [Google Scholar]
- 21.Lai HJ, Cheng Y, Farrell PM. The survival advantage of patients with cystic fibrosis diagnosed through neonatal screening: evidence from the United States Cystic Fibrosis Foundation registry data. J Pediatr. 2005 Sep;147:S57–63. doi: 10.1016/j.jpeds.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Venkata JA, Jones KL. Benefits of newborn screening for cystic fibrosis in Shreveport, Louisiana, Cystic Fibrosis Center. J La State Med Soc. Nov-Dec;163:316–319. [PubMed] [Google Scholar]
- 23.Farrell PM. The meaning of “early” diagnosis in a new era of cystic fibrosis care. Pediatrics. 2007 Jan;119:156–157. doi: 10.1542/peds.2006-3074. [DOI] [PubMed] [Google Scholar]
- 24.Baumer JH. Evidence based guidelines for the performance of the sweat test for the investigation of cystic fibrosis in the UK. Arch Dis Child. 2003 Dec;88:1126–1127. doi: 10.1136/adc.88.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008 Aug;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parad RB, Comeau AM, Dorkin HL, Dovey M, Gerstle R, Martin T, et al. Sweat testing infants detected by cystic fibrosis newborn screening. J Pediatr. 2005 Sep;147:S69–72. doi: 10.1016/j.jpeds.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Taccetti G, Festini F, Braccini G, Campana S, de Martino M. Sweat testing in newborns positive to neonatal screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed. 2004 Sep;89:F463–464. doi: 10.1136/adc.2003.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp JK, Rock MJ. Newborn screening for cystic fibrosis. Clin Rev Allergy Immunol. 2008 Dec;35:107–115. doi: 10.1007/s12016-008-8082-1. [DOI] [PubMed] [Google Scholar]
- 29.Eng W, LeGrys VA, Schechter MS, Laughon MM, Barker PM. Sweat-testing in preterm and full-term infants less than 6 weeks of age. Pediatr Pulmonol. 2005 Jul;40:64–67. doi: 10.1002/ppul.20235. [DOI] [PubMed] [Google Scholar]
- 30.Mishra A, Greaves R, Massie J. The relevance of sweat testing for the diagnosis of cystic fibrosis in the genomic era. Clin Biochem Rev. 2005 Nov;26:135–153. [PMC free article] [PubMed] [Google Scholar]
- 31.Kleyn M, Korzeniewski S, Grigorescu V, Young W, Homnick D, Goldstein-Filbrun A, et al. Predictors of insufficient sweat production during confirmatory testing for cystic fibrosis. Pediatr Pulmonol. Jan;46:23–30. doi: 10.1002/ppul.21318. [DOI] [PubMed] [Google Scholar]
- 32.Hammond KB, Turcios NL, Gibson LE. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J Pediatr. 1994 Feb;124:255–260. doi: 10.1016/s0022-3476(94)70314-0. [DOI] [PubMed] [Google Scholar]
- 33.Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007 Jan;6:57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 34.National Newborn Screening and Genetics Resource Center National Newborn Screening Status Report. 2012 Feb 9; Available from: http://genes-r-us.uthscsa.edu/nbsdisorders.pdf.
- 35.Wells J, Rosenberg M, Hoffman G, Anstead M, Farrell PM. A decision-tree approach to cost comparison of newborn screening strategies for cystic fibrosis. Pediatrics. Feb;129:e339–347. doi: 10.1542/peds.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM. Newborn screening for cystic fibrosis in Wisconsin: nine-year experience with routine trypsinogen/DNA testing. J Pediatr. 2005 Sep;147:S73–77. doi: 10.1016/j.jpeds.2005.08.004. 2005. [DOI] [PubMed] [Google Scholar]
- 37.Comeau AM, Parad RB, Dorkin HL, Dovey M, Gerstle R, Haver K, et al. Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics. 2004 Jun;113:1573–1581. doi: 10.1542/peds.113.6.1573. [DOI] [PubMed] [Google Scholar]
- 38.Christopher S, Collins J, Farrell M. Effort required to contact primary care providers after newborn screening identifies sickle trait. Journal of the National Medical Association. 2011 doi: 10.1016/s0027-9684(15)30219-4. accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]