Abstract
The amygdala is mostly thought to exert an excitatory influence on the hypothalamic-pituitary-adrenal (HPA) axis, although its role regulating HPA basal tone is less clear, particularly during primate development. The current study examined the effects of neonatal amygdala lesions on basal HPA function and the postnatal testosterone (T) surge of rhesus monkeys reared with their mothers in large outdoor social groups. An early morning basal blood sample was collected at 2.5 months of age, whereas at 5 months samples were collected not only at sunrise, but also at mid-day and sunset to examine the diurnal rhythm of cortisol. At 2.5 months of age sham-operated males exhibited higher cortisol than females, but this sex difference was abolished by neonatal amygdalectomy, with lesioned males also showing lower basal cortisol than controls. Although neonatal amygdalectomy did not alter the postnatal T surge, there was a positive relationship between T and basal cortisol levels. At 5 months of age, neither the sex difference in cortisol, nor its correlation with T levels were apparent any longer. Instead, the diurnal cortisol rhythm of both males and females with amygdalectomy showed a blunted decline from mid-day to sunset compared to controls. These results indicate that neonatal amygdala damage alters basal HPA function in infant rhesus monkeys, affecting males only at early ages (at 2.5 months), while leaving the postnatal T surge intact, and resulting in a flattened diurnal rhythm in both genders at the later ages. Thus, the primate amygdala has a critical influence on the HPA axis in the first few months of life.
Keywords: amygdala, HPA axis, testosterone, sex difference
The amygdala is anatomically positioned to play a critical role in the evaluation of salient and threatening cues from the environment and in the modulation of behavioral, autonomic, and neuroendocrine responses to potential threats (e.g., Freese & Amaral, 2009). The amygdala influences neuroendocrine stress responses through indirect inputs to the hypothalamic paraventricular nucleus (PVN), via direct projections to the bed nucleus of the stria terminalis (Herman, et al., 2003; Freese & Amaral, 2009). Thus, in response to a perceived threat, stressor-specific pathways from the amygdala activate the PVN, yielding a cascade of events beginning with the secretion of corticotrophin releasing hormone (CRH) into the hypophyseal portal blood followed by the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland into the systemic circulation. ACTH then binds to receptors in adrenocortical cells, which in turn increases the synthesis and release of glucocorticoids, such as cortisol in primates (Herman, et al., 2003). A stimulatory role of the amygdala on these neuroendocrine responses has mostly been demonstrated in adult animals. Thus, electrical stimulation of the primate amygdala increases cortisol secretion (Mason, 1959), whereas either complete bilateral lesions of the amygdala nuclei or selective lesions of the central nucleus (CeA) of the amygdala reduces glucocorticoid secretion in response to a stressor in both rodents (Herman, et al., 2003) and primates (Kalin, et al., 2004; Machado & Bachevalier, 2008). Most of these studies, however, have focused on the role of the amygdala regulating HPA axis stress reactivity (for review see Herman, et al., 2003) with little consideration of its role on basal cortisol secretion and its circadian secretory rhythm. Allen and Allen (1975) reported that the rodent amygdala was necessary to maintain ACTH secretion after adrenalectomy, suggesting a potential stimulatory role of the amygdala for tonic (basal) control of the HPA axis in the absence of glucocorticoid regulation. However, a more recent study showed that CRH knockdown in the CeA of adult mice actually increases basal corticosterone (Regev, et al., 2012). In primates, no effects on baseline (pre-stressor) cortisol levels have been reported after adult amygdala lesions in monkeys (Sapolsky, et al., 1991; Kalin, et al., 2004; Machado & Bachevalier, 2008). Even less studied is the role of the amygdala in the regulation of basal HPA axis function during prepubertal development.
Human studies suggest that the basal HPA axis secretory rhythm emerges between 8–12 weeks of age (see review Tarullo & Gunnar, 2006). The few studies that have examined the ontogeny of basal HPA function in monkeys indicated either stable or slight decreases in basal cortisol secretion between 2 and 24 weeks of age (Champoux, et al., 1989; Higley, et al., 1992; Clarke, 1993) with an adult-like diurnal pattern of cortisol secretion already present by one year of age (Sanchez, et al., 2005; Barrett, et al, 2009). Thus, the available developmental data point to a progressive maturation of HPA axis function throughout infancy and raise the question of whether the amygdala plays a critical role in this maturation. Few studies have examined the effects of amygdala lesions on HPA axis function in developing rhesus monkeys, either during the juvenile period (Norman & Spies, 1981) or in infancy (Goursaud, et al., 2006; Raper, et al., 2012 submitted). Two have reported no effects on basal activity (Norman & Spies, 1981; Goursaud, et al., 2006). However, these negative results may have resulted either from studying the juvenile rather than the infant developmental period (Norman & Spies, 1981) or from the lack of true baseline samples in the experimental design (Goursaud, et al., 2006). In the most recent study (Raper, et al., 2012 submitted), lower basal cortisol was found in adult animals with neonatal amygdala lesions, although cortisol was not measured in infancy. Thus, the amygdala’s influence on basal HPA axis functioning during early primate development remains to be directly investigated.
The current study had two main aims: 1) to examine the effects of neonatal neurotoxic amygdala lesions on basal HPA function of rhesus monkeys during infancy, and 2) to determine whether these effects are sexually dimorphic. In adulthood, gonadal hormones modulate the HPA axis activity. Thus, estrogens in females have mostly a stimulatory effect on the basal HPA axis (Burgess & Handa, 1992; Stavisky, et al., 2003), whereas testosterone appears to inhibit corticosteroid secretion, at least in rodents (Seale, et al., 2004). Although the relationship between gonadal hormones and the HPA axis in adults is complex and not clearly understood, a few studies have also reported sex differences in basal cortisol levels during childhood, prior to the pubertal increases in gonadal hormones. Thus, boys have higher basal cortisol levels than girls in some studies (Davis & Emory, 1995; Elmlinger, et al., 2002; Ouellet-Morin, et al., 2010); but opposite findings have also been reported (Essex, et al., 2002; Koupil, et al., 2005; Sondeijker, et al., 2007). The differing results may reflect differences in the age at which boys and girls were sampled. Importantly, Davis and Emory (1995) reported higher cortisol levels in boys than girls during a developmental phase when the hypothalamic-pituitary-gonadal (HPG) axis is temporarily activated in boys, resulting in a transient postnatal testosterone (T) surge (Forest, 1979). A similar HPG activation occurs neonatally in male rhesus monkeys, with elevated T levels from birth through 4 months of age followed by HPG inactivation until puberty (Robinson & Bridson, 1978; Mann, et al., 1989). Furthermore, amygdala androgen receptors (AR; Choate, et al., 1998) are present in higher concentrations in males than females (Pomerantz & Sholl, 1987), suggesting that the amygdala may be an important site for the regulation of the HPG axis and for interactions between the HPG and HPA axes. To investigate this potential regulatory role of the amygdala on HPA and HPG activity during infancy, we measured both basal cortisol and T at 2.5 months (during the postnatal T surge) and at 5 months of age (after the surge, when T was expected to be low) in male and female infant monkeys with and without neonatal amygdala lesions.
Methods
Subjects
Twenty-eight infant rhesus monkeys (Macaca mulatta) were selected from middle-ranking multiparous mothers living in large social groups at the Yerkes National Primate Research Center (YNPRC) Field Station (Lawrenceville, GA), Emory University. The social groups were housed in 38 × 38 m outdoor compounds with indoor housing and capture area. Social groups consisted of 20–30 adult females with their immature offspring and two unrelated adult males. Infants were divided into two treatment groups; neonatal amygdala lesion (Neo-A; males = 9, females = 7) and sham-operated controls (Neo-C; male = 6, females = 6). Infants in group Neo-A received MRI-guided bilateral neurotoxic lesion of the amygdala at an average of 25.6 ± 0.8 days of age (range: 20–30 days). Infants in group Neo-C received a sham surgery (see below) at an average of 24 ± 1.6 days of age (range: 12–33 days). All neuroimaging and surgical procedures were performed at the YNPRC Main Station (Atlanta, GA).
To minimize the risk of maternal rejection, four days prior to surgery, mother-infant pairs received a one hour separation-trial that simulated the manipulations that the mother and infant would receive prior to the imaging and surgical procedures (see below). The separation-trial required isolation of the mother-infant pair away from their social group and separating the infant from the mother, shaving the infant’s head, cleaning the area with alcohol and betadine, and placing the infant away from the mother in a temperature-controlled isolette incubator for one hour. The infant was then reunited with the mother and the mother-infant pair was returned to their social group. Two days prior to surgery, the mother-infant pair was transported from the YNPRC Field Station to the YNPRC Main Station. Once all surgical and post-surgical procedures were completed, the mother-infant pairs were transported back to the YNPRC Field Station where they were reintroduced to their social group using a staged procedure. This staged reintroduction began with mother-infant pairs being housed overnight in the small indoor housing area adjacent to the larger group housing area. Housing in this area provides full visual but restricted physical contact between the mother-infant pair and other group members and allows for re-establishment of dominance hierarchy before being released into the social group. The following morning, researchers monitor the release of the mother-infant pair into the social group, all reintroductions were successful and without incidents.
All procedures were approved by the Animal Care and Use Committee of Emory University in Atlanta, GA and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Imaging and surgical procedures
Magnetic Resonance Imaging (MRI) Procedure
The neurotoxic surgical technique utilized MR images of each animal for pre-surgical location of injection sites and pre-surgical calculations of stereotaxic coordinates of each injection site. On the day of the surgery, the infant was removed from their mother, anesthetized (Ketamine hydrochloride, 100mg/ml), intubated and given isoflurane (1–2% to effect). An intravenous drip of dextrose and 0.45% sodium chloride was placed to maintain normal hydration during MRI and surgery. The animal’s head was shaved and secured in a nonferromagnetic stereotaxic apparatus. After the animal was aligned in the center of the magnet, the brain was imaged in each of two stereotaxic planes (sagittal and coronal) using a Siemens 3.0T/90 cm whole body scanner and a 3″ circular surface coil. Following a sagittal localizer, a high resolution T1-weighted scan (spin-echo sequence, echo time [TE] = 11ms, repetition time [TR] = 450ms, 12cm field of view [FOV], 256×256 matrix) was acquired throughout the brain at 1 mm in the coronal plane. This series was used to estimate the coordinates of injection sites. Additionally, three fluid attenuated inversion recovery (FLAIR) scans (3D T2-weighted fast spoiled gradient [FSPGR]-echo sequence, TE = 2.6ms, TR = 10.2ms, 25 flip angle, 12 cm FOV, 256×256 matrix) were obtained in the coronal plane at 3.0 mm (each offset of 1 mm posteriorly) throughout the brain. The FLAIR images reveal tissue T2 prolongation with cerebrospinal fluid suppression and were used with the post-surgical FLAIR images to estimate the lesion extent.
Injection coordinates
A T1 coronal image representing the largest body of the amygdala, roughly at the level of the chiasm and the middle portion of the anterior commissure, was selected. From this image, four injection sites were located 1 mm dorsal, 1 mm ventral, 1 mm lateral, and 1 mm medial to the center point of the amygdala, allowing diffusion of ibotenic acid through the entire amygdala. Two additional images, one immediately anterior and one immediately posterior to the central image, were used to determine 2–3 additional injection sites spaced 2-mm apart depending on the size of the amygdala at these levels for each subject. Coordinates for each injection site were determined by measuring the distance of the target site to each of three referents for each monkey: Anterior/Posterior Coordinates were calculated from the zero point determined from the tips of the ear bars; Medial/Lateral Coordinates were calculated from the midline of the brain; and Dorsal/Ventral Coordinates were calculated from the dorsal/ventral coordinates of the earbars. These MRI coordinates were then translated into stereotaxic coordinates.
Surgical Procedure
After the imaging procedure, the animals were kept anesthetized and immediately transported to the surgical suite. The scalp was disinfected with Nolvasan Solution and a local anesthetic (Bupivicaine 0.25% concentration, 1.5ml) was injected subcutaneously along the midline (beginning at the supra-orbital ridge and ending at the occipital notch) to reduce the pain during skin incision. Under aseptic conditions, the skin was opened and connective tissue was gently displaced laterally to expose the skull. Two small craniotomies were made bilaterally, in front of bregma and above the amygdala, and the dura was cut and retracted to expose the brain. Injections were made simultaneously in the two hemispheres using 30-gauge needles attached to 10 μl Hamilton syringes. The needles were lowered slowly at each injection site, and 0.6–0.8 μl of ibotenic acid (PH 7.8–7.9, 10 mg/ml concentration) was manually injected at a 0.2 μl/min rate. After injection, the needles were left in place for an additional 3-minute period to allow complete diffusion of the neurotoxin at the tip of the needle and minimize its spread in the needle track during retraction of the needles. After the last injection, the dura was closed with silk sutures, the bone opening was covered with Surgicel NU-KNIT (absorbable hemostat), and connective tissues and skin were sutured at the midline. Neo-C animals receiving sham-surgeries were treated in the same way as the experimental animals except that no needles were lowered and no injections were administered.
Postoperative care
Upon complete recovery from anesthesia, the infants were taken to the nursery and placed in an isolette incubator ventilated with oxygen. They were given banamine (1mg/kg for 3 days), dexamethasone (0.5mg/kg for 3 days) and antibiotic (rocephin, 25mg/kg for 7 days) after surgery to prevent pain, edema, and infection, respectively.
Mother-infant Reunions and Cross-fostering
The day after surgery, after ensuring that the animal was alert, it was returned to the mother. The mother-infant reunions were monitored constantly via a secured internet web camera. In most of the cases, the mothers immediately retrieved their infants and the infants were observed nursing on the mother that same day. However, for a few infants that demonstrated difficulty nursing on the mother during the first reunion, the mother-infant pairs received additional overnight separations for nutrition and monitoring purposes followed by morning reunions until the infant was able to nurse on the mother adequately.
In three cases (one Neo-C male and two Neo-A animals, one male and female), the mother refused to take the infant back after surgery. Repeated attempts were made to reunite those infants with their mother at the YNPRC Main Station, but in each case the mother refused to cradle and nurse the infant. In two cases (Neo-A female and Neo-C male), another reunion was attempted at the YNPRC Field Station upon reintroduction of the mother-infant pairs to their social group. However, in both cases the mother still rejected its infant, but the “abandoned” infant was adopted and raised by another adult female that already had an infant during the birth season and, thus, raised two infants that year. In these two cases of mother-infant ‘twin’ pairs, the infants’ growth patterns were not different from other subjects of the study. The third case, Neo-A male, was successfully cross-fostered to a mother that had recently lost its infant.
Lesion Assessment
The extent of lesion was assessed in vivo using postsurgical neuroimaging procedures, following previously published protocols (Nemanic, et al, 2002). Six to eight days following the surgical procedures, Neo-A infants were again separated from their mother and given a second MRI session for acquisition of both high resolution T1 and FLAIR images, using the same protocols as described for the pre-surgical MRI (see above). Sham-operated animals did not receive this follow-up MRI, but were separated from their mothers and placed in an isolette incubator for the same amount of time. All infants were then re-united with their mothers.
The extent of lesion was estimated using pre- and post-surgery FLAIR images. Presurgical T1 images were also used to help identify the borders of each structure. The pre- and post-surgery FLAIR images were matched to digital drawings of coronal sections from a normalized rhesus monkey template brain (J. Bachevalier, unpublished atlas). Hypersignals were identified on the post-surgical FLAIR images and plotted onto corresponding coronal drawings from the template brain using Adobe Photoshop CS5 software. These drawings were imported into image analysis program Image-J® (version 1.44) to measure the surface area (in pixels squared) of damage for intended targets, as well as all areas sustaining unintended damage (entorhinal and perirhinal cortex and hippocampus). The surface area of hypersignal on each section through each hemisphere were summed and then multiplied by image thickness (i.e. 1mm) to calculate a total volume of damage. The volume of amygdala damage was then divided by the normal volume of the amygdala (obtained from the template brain in a similar manner) and multiplied by 100 to estimate a percentage of the total volume damage.
Procedures for Assessment of Basal HPA Axis Function
Training and capture procedures
Due to the subjects’ young age (2.5 and 5 months) they depend on their mothers, which carry the infants during procedures. Thus, all mother-infant pairs were trained to quickly separate from their social group and enter an indoor area. Once inside, mothers were trained to enter a transfer box with their infant and, then, the pair was placed into a cage from which the infant could be separated from the mother. Mothers learned quickly to voluntarily remove their infant off their ventrum, leaving it in the cage while returning to the transfer box. Infants were then carefully removed from the cage, wrapped in a fleece cloth and gently held while a second researcher took a blood sample via femoral venipuncture from the unanesthetized infant. Blood samples were collected within 10 minutes from the initial disturbance (i.e., researchers approaching the social group). Blood samples collected under these training/habituation conditions and within 10 minutes reflect basal hormone levels since elevations in plasma cortisol concentrations are minimal/undetectable, as demonstrated by previous studies of rhesus monkeys done at the YNPRC, including infants (McCormack, et al., 2009; Sanchez, et al., 2010). In addition, infants experiencing these procedures exhibit normal development (Wilson, Gordon, & Collins, 1986).
Blood Sampling
At 2.5 months of age, an early morning basal blood sample was collected within 30 minutes from sunrise (0654–0722 hrs) to assess basal cortisol plasma concentrations. No additional afternoon or evening samples were collected at this early age to avoid multiple disturbances to the mother-infant pair. At 5 months of age, the diurnal rhythm of cortisol secretion was examined by collecting blood samples at sunrise (0710–0756 hrs), mid-day (1220–1335 hrs), and sunset (1843–2000 hrs) using time charts published by the United States Naval Meteorology and Oceanography Command (http://aa.usno.navy.mil/data/docs/RS_OneYear.php) to determine exact daylight times (Sanchez et al., 2005). These daylight diurnal time points were selected instead of clock times because the animals live under natural lighting conditions that affect their circadian cortisol secretory rhythms.
All blood samples were collected in pre-chilled plastic 2 ml vacutainer tubes containing EDTA (3.6mg) and immediately placed on ice. Samples were centrifuged at 3,000 rpm for 15 minutes in a refrigerated centrifuge (at 4°C). Plasma was pipetted into sterile eppendorfs and stored at −80°C until assayed.
Plasma Hormonal Assays
All assays were performed by the YNPRC Biomarker Core Laboratory. Plasma concentrations of cortisol were assayed in duplicate by R.I.A. using commercially available kits (DSL kit: Diagnostic Systems Laboratories, Webster, TX). The sensitivity of the DSL assay was 1.25μg/dl and intra- and interassay coefficients of variation were <10%. Plasma T levels were also assayed in duplicate by R.I.A using commercially available kits (DSL kit: Diagnostic Systems Laboratories, Webster, TX). The sensitivity of the DSL assay was 0.05ng/ml and intra- and interassay coefficients of variation were <7%.
Data Analysis
Of a total of 112 blood samples collected, only seven took more than 10 minutes to collect (five samples were collected within 11–12 minutes of initial disturbance; 2.5 months: n=3 samples; 5 months: n=2 samples) and were kept in the analysis; the other two were collected within 15 and 16 minutes from disturbance (2.5 months) and were excluded from the cortisol analysis. A Neo-C male exhibited T levels two standard deviations higher than the group mean; thus, this sample was excluded from the T analysis as an outlier to avoid bias in any group or sex differences in T. At 5 months, diurnal blood samples could not be collected from one Neo-C male due to illness. Lastly, one Neo-A male had a history of chronic illness (unrelated to the lesion) and thus its data were excluded from the analysis at both 2.5 and 5 months.
Preliminary analyses were performed to ascertain if surgical, research manipulations factors, or the time from disturbance until collection of blood samples had any influence on basal cortisol levels. Two Hierarchical Linear Model (HLM) Regression analyses were performed on basal cortisol levels at 2.5 months and 5 months of age separately. Any factor that explained a significant portion of the variance in cortisol levels was used as a covariate in subsequent analyses of group differences.
Early morning cortisol levels at 2.5 months were analyzed using a General Linear Model (GLM) ANOVA with Group (2; Neo-C, Neo-A) and Sex (2; male, female) as the between subjects factors, cortisol levels as the dependent variable and time to collect the sample as covariate. Interaction effects were examined with post-hoc comparisons using independent t-tests. Testosterone levels at 2.5 and 5 months of age were analyzed using a GLM ANOVA with Group (2) and Sex (2) as the between subjects factors and T level in the morning as the dependent variable. Lastly, the relationship between cortisol and testosterone levels were examined for each age (2.5, 5 months) separately using HLM Regression accounting for “time to collect the cortisol sample” and group differences in cortisol level. Regression analyses were also separated by sex, such that tests were performed for all males (Neo-A and Neo-C) and all females separately.
Diurnal rhythm at 5 months of age was analyzed using a Repeated Measures ANOVA with between subjects factors Group (2) and Sex (2), within subjects factor Time of the Day (3; sunrise, mid-day, sunset), and time to collect the sample as covariate. Comparisons of Time of Day across the between subjects factors were assessed by repeated measures contrasts. The percent change of cortisol concentrations throughout the day was calculated as percent decline between sunrise and mid-day or between mid-day and sunset ([sunrise − mid-day]/sunrise*100 or [mid-day − sunset]/mid-day*100, respectively) for each subject. The percent decline was analyzed by GLM ANOVA with Group (2) and Sex (2) as the between subjects factors and percent decline in cortisol level as the dependent variables. Significance level was set at p < 0.05 for all analyses and effect sizes were eta squared for ANOVA’s and cohen’s d for t-tests.
Lastly, we investigated potential correlations between the extent of amygdala lesion and cortisol levels at 2.5 and 5 months of age. Partial correlations were performed for cortisol and extent of amygdala damage correcting for the “amount of time it took to collect the blood sample”.
Results
Lesion Assessment
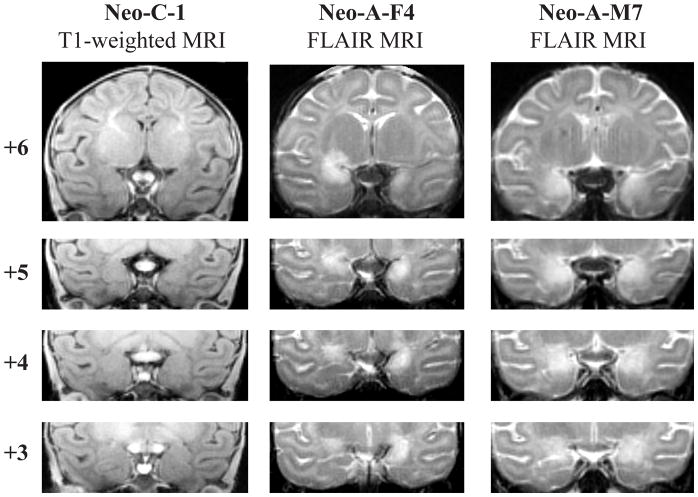
Table 1 displays the volume of damage estimated from the FLAIR MR-images for each monkey in group Neo-A. The extent of bilateral amygdala damage in twelve cases was substantial and bilateral (mean: 84.82% and 92.80% on the right and left, respectively). Case Neo-A-F5 had more moderate but bilateral damage (Neo-A-F5: right: 61.6%, left: 58.4%) and cases Neo-A-F3 and Neo-A-M4 had asymmetrical amygdala damage (right vs left: 100% vs 32.2% and 50.5% vs 84.9%, respectively). Only in one case (Neo-A-F1) was the damage restricted to the right hemisphere (right: 82.3%). The extent of unintended damage to the perirhinal and entorhinal cortices, temporal cortical areas TE, TEO, TG, anterior portion of the hippocampus, and tail of the putamen were negligible in 13 cases. In two cases, Neo-A-F1 and Neo-A-F3, moderate damage to the entorhinal cortex was found in the right hemisphere (18.3% and 21.8%, respectively). Lastly, moderate damage to the tail of the putamen was noted in two cases (Neo-A-F5 and Neo-A-F6). Figure 1 illustrates an example of the extent of bilateral amygdala damage in two cases (Neo-A-F4 and Neo-A-M7) as reflected by the location and extent of hypersignals seen in post-surgical FLAIR images.
Table 1.
Intended and unintended damage after neurotoxic lesions of the amygdala
| Subjects | Intended Damage
|
Unintended Damage
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala
|
Hippocampus
|
Entorhinal
|
Perirhinal
|
Area TE
|
Area TG
|
|||||||||||||||||||
| Rt% | Lf% | X% | W% | Rt% | Lf% | X% | W% | Rt% | Lf% | X% | W% | Rt% | Lf% | X% | W% | Rt% | Lf% | X% | W% | Rt% | Lf% | X% | W% | |
| Neo-A-F1 | 82.3 | 0.0 | 41.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 18.3 | 0.0 | 9.2 | 0.0 | 3.8 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8.8 | 0.0 | 4.4 | 0.0 |
| Neo-A-F2 | 65.7 | 98.7 | 82.2 | 64.8 | 0.0 | 5.7 | 2.9 | 0.0 | 0.6 | 5.5 | 3.0 | 0.03 | 0.5 | 1.9 | 1.2 | 0.01 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | 1.7 | 0.0 |
| Neo-A-F3 | 100 | 32.2 | 66.1 | 32.2 | 2.5 | 0.0 | 1.2 | 0.0 | 21.8 | 2.9 | 12.4 | 0.6 | 6.7 | 1.0 | 3.8 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 10.8 | 0.0 | 5.4 | 0.0 |
| Neo-A-F4 | 90.9 | 89.3 | 90.1 | 81.1 | 1.9 | 0.0 | 1.0 | 0.0 | 12.3 | 0.0 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 3.0 | 0.0 |
| Neo-A-F5 | 61.6 | 58.4 | 60.0 | 36.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.8 | 0.0 | 3.3 | 0.0 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.6 | 0.0 | 1.8 | 0.0 |
| Neo-A-F6 | 100 | 97.7 | 98.8 | 97.7 | 2.4 | 7.9 | 5.1 | 0.2 | 1.3 | 2.0 | 1.6 | 0.03 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.7 | 0.0 | 5.3 | 0.0 |
| Neo-A-F7 | 98.3 | 99.0 | 98.6 | 97.3 | 4.3 | 2.1 | 3.2 | 0.1 | 7.3 | 2.5 | 4.9 | 0.2 | 2.8 | 5.5 | 4.2 | 0.2 | 0.0 | 0.6 | 0.3 | 0.0 | 9.6 | 0.0 | 4.8 | 0.0 |
| Neo-A-M1 | 100 | 80.6 | 90.3 | 80.6 | 8.9 | 9.0 | 9.0 | 0.8 | 8.3 | 15.8 | 12.0 | 1.3 | 4.1 | 0.2 | 2.2 | 0.01 | 0.0 | 0.0 | 0.0 | 0.0 | 11.8 | 0.0 | 5.9 | 0.0 |
| Neo-A-M2 | 66.8 | 89.1 | 77.9 | 59.5 | 0.0 | 2.7 | 1.4 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.9 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.4 | 0.0 | 5.2 | 0.0 |
| Neo-A-M3 | 70.3 | 90.8 | 80.6 | 63.9 | 3.1 | 5.3 | 4.2 | 0.2 | 2.1 | 31.4 | 16.8 | 0.7 | 0.1 | 7.2 | 3.7 | 0.01 | 0.1 | 0.0 | 0.1 | 0.0 | 7.1 | 0.0 | 3.5 | 0.0 |
| Neo-A-M4 | 50.5 | 84.9 | 67.7 | 42.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-A-M5 | 95.9 | 97.1 | 96.5 | 93.1 | 0.0 | 12.1 | 6.1 | 0.0 | 5.0 | 4.4 | 4.7 | 0.2 | 1.6 | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.4 | 0.0 | 1.7 | 0.0 |
| Neo-A-M6 | 77.3 | 92.3 | 84.8 | 71.3 | 4.4 | 0.0 | 2.2 | 0.0 | 0.0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.7 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-A-M7 | 90.9 | 98.9 | 94.9 | 90.0 | 5.1 | 0.6 | 2.9 | 0.03 | 1.4 | 0.0 | 0.7 | 0.0 | 1.4 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-A-M8 | 100 | 87.0 | 93.5 | 87.0 | 6.4 | 0.4 | 3.4 | 0.03 | 7.1 | 0.0 | 3.6 | 0.0 | 2.2 | 0.9 | 1.6 | 0.02 | 0.1 | 0.0 | 0.03 | 0.0 | 13.0 | 0.0 | 6.5 | 0.0 |
| Neo-A-M9 | 61.8 | 93.2 | 77.5 | 57.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.4 | 0.0 | 0.0 | 0.5 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
L%: percent damage in the left hemisphere; R%: percent damage in the right hemisphere; X%: average damage to both hemispheres; W%: weighted average damage to both hemispheres (W%={L%×R%)/100). Neo-A-F: female amygdala lesion subject, Neo-A-M: male amygdala lesion subject.
Figure 1.
Four coronal MR images through the amygdala: T1-weighted images in one sham-operated control (Neo-C-1) and Fluid Attenuated Inversion Reversal (FLAIR) images in two representative cases with neonatal amygdala lesions (Neo-A-F4 and Neo-A-M7). The numerals to the left of each coronal section indicate the distance in millimeters from the interaural plane. Black arrows point to the hypersignal resulting from the cell death from neurotoxic injections.
Basal Cortisol
Preliminary analyses were performed to ascertain if surgical, research manipulation factors, or the time from disturbance until collection of the blood sample had any influence on basal cortisol levels. As shown in Table 2, none of the surgical or research manipulation factors accounted for a significant amount of the variance in cortisol level at either age. Therefore, since cortisol levels were not influenced by factors such as surgical procedure, handling, or amount of time infants were separated from their mother, these variables were not included as covariates in the analysis of group differences. However, because a significant portion of the variance in cortisol levels at each age could be explained by “time to collect the sample”, this variable was used as a covariate in subsequent analysis of group differences.
Table 2.
Surgical and Research Manipulation Factors
| Factor | Definition | Measurement | Interaction with Cortisol levels
|
|
|---|---|---|---|---|
| 2.5 months | 5 months | |||
| Separation from mother | Total time separated from mother from birth until reunion after surgery | Hours | R2 = 0.138 F(1,25)=0.04, p=0.95 |
R2 = 0.077 F(1,25)=1.71, p=0.20 |
| Handling | Number of times subjects were handled for research (e.g. blood draw), veterinary (e.g. physical exam), and general care (e.g. weight) procedures | Frequency | R2 =0.167 F(1,24)=0.84, p=0.37 |
R2 = 0.101 F(1,24=0.60, p=0.45 |
| Surgical Stress Score* | Measures of stressors during surgery | Cumulative Score | R2 = 0.169 F(1,23)=0.05, p=0.82 |
R2 = 0.101 F(1,23)=0.47, p=0.98 |
| Length of Surgery | Total time it took to complete the surgical procedure | Hours | R2 = 0.262 F(1,22) = 2.8, p=0.11 |
R2 = 0.101 F(1,22)=0.51, p=0.97 |
| Rate of Isoflurane | Total amount of isoflurane per hour of procedure (pre-surgery MRI, surgery, post-surgery MRI) | % Iso/hr | R2 = 0.297 F(1,21)= 1.0, p=0.32 |
R2 = 0.132 F(1,21)=0.71, p=0.48 |
| Time to Collect the blood sample | Time from group disturbance until the blood sample was obtained | Minutes | R2 = 0.718 F(1,20)=29.9, p<0.001 |
R2 = 0.345 F(1,20)=6.2, p=0.022 |
adapted from Anand & Aynsley-Green (1988)
At 2.5 months of age, Neo-C males had higher basal cortisol levels than did females. However, this sex difference was not evident in animals with amygdala lesions as comparable basal cortisol levels were seen in males and females (Figure 2a). This was confirmed by a significant Group by Sex interaction effect (F[1,25]=4.9, p=0.039, η2=0.20). Bonferroni corrected post-hoc comparisons indicated that Neo-C males differed significantly from Neo-C females (t[8]=2.58, p=0.032, d=1.63), Neo-A males (t[11]=2.89, p=0.015, d=1.65), and Neo-A females (t[9]=2.9, p=0.018, d=1.70), whereas Neo-A males and females had comparable cortisol levels (t[13]=0.473, p=0.64, d=0.24).
Figure 2.
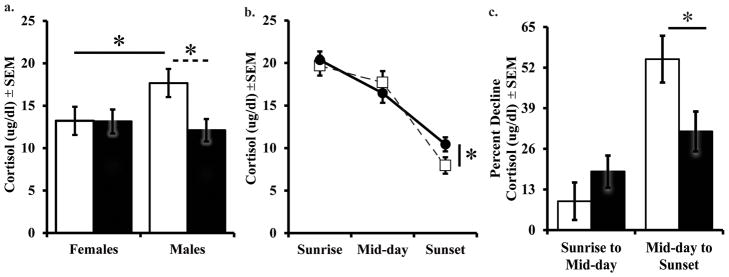
Basal plasma cortisol levels during early morning at 2.5 months (a), diurnal rhythm at 5 months (b), and Percent Decline in the diurnal rhythm at 5 months (c). Sham-operated controls (Neo-C) is represented with open bars or open squares with dashed lines, neonatal amygdala lesion animals (Neo-A) are represented with black bars or black circles with solid lines. * indicates a significance of p < 0.05.
Analysis of the diurnal cortisol rhythm at 5 months revealed no Group (F[1,19]=0.001, p=0.986, η2=0.001) or Sex effect (F[1,19]=2.51, p=0.13, η2=0.12), but a significant Group by Time of day interaction (F[2,38]=3.43, p=0.043, η2=0.15), reflecting a flattened diurnal cortisol rhythm in Neo-A animals, as compared to Neo-C animals (Figure 2b). Repeated contrasts showed no group differences in cortisol decline from sunrise to mid-day (F[1,19]=1.13, p=0.30), but a steeper decline from mid-day to sunset in Neo-C than Neo-A animals (F[1,19]=6.98, p=0.009). This group effect was most evident when the percent decline in cortisol levels (midday to sunset) was calculated (F[1,26]=5.56, p=0.028, η2=0.20; Figure 2c).
To further investigate whether the Sex difference found at 2.5 months of age was truly no longer present at 5 months, an additional a priori planned GLM ANOVA was conducted only for sunrise cortisol at 5 months. Results revealed no Group (F[1,26]=0.08, p=0.78, η2=0.004), or Sex (F[1,26]=0.01, p=0.94, η2=0.001), or Group by Sex interaction effects (F[1,26]=1.79, p=0.20, η2=0.08) on sunrise cortisol at 5 months. These results confirm the lack of Sex effects at this age, when the postnatal T surge is over (as shown below).
Testosterone
As shown in Figure 3a, Neo-A and Neo-C males both demonstrated a normal “postnatal T surge” at 2.5 months of age with comparable levels of T (F[1,26]=0.062, p=0.806, η2=0.003). In addition, all males had significantly higher T levels than did females (F[1,26]=23.32, p<0.001, η2=0.52). Unlike cortisol, there was no Group or Group by Sex interaction for T levels (F[1,26]=0.37, p=0.55, η2=0.02). When time to collect the sample and group (Neo-A and Neo-C) differences in cortisol were corrected for, regression analyses revealed that T levels during the postnatal T surge accounted for a significant percentage of the variance in basal cortisol among all male subjects (R2 change=0.34, F(1,8)=9.3, p=0.016; Figure 3b). The same regression analysis was used for all female subjects (Neo-A and Neo-C) but revealed no relationship between cortisol and T levels (R2 change=0.02, F(1,8)=0.38, p=0.55).
Figure 3.
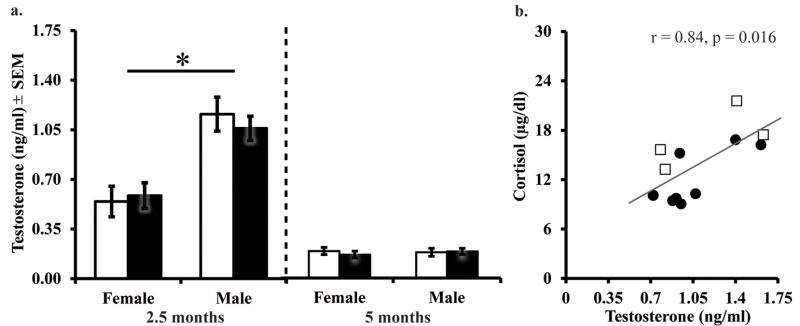
Early morning testosterone levels 2.5 and 5 months of age (a) and correlation of testosterone and cortisol at 2.5 months of age in the males only (b). All abbreviations as in Figure 2.
The “postnatal T surge” was over by 5 months of age, as indicated by the low T levels in both male groups, Neo-C (M=0.18 ng/ml±0.03) and Neo-A (M=0.19 ng/ml±0.02) and a significant Age effect (F[1,23]=66.55, p<0.001, η2=0.74). Neonatal amygdala lesions did not affect T levels at this age, as shown by the lack of a Group effect (F[1,26]=0.16, p=0.70, η2=0.01; Figure 3a). The end of the “postnatal T surge” is also evident by the lack of a sex differences in T levels at 5 months of age (F[1,26]=0.07, p=0.79, η2=0.003). Lastly, the regression analysis preformed at 2.5 months of age was repeated at 5 months of age on males and females separately. At 5 months of age, T level no longer accounted for the variance in morning cortisol level among all males (R2 change=0.03, F(1,9)=0.3, p=0.60), and the same nonsignificant relationship was found among all females (R2 change=0.05, F(1,8)=0.86, p=0.38).
Correlations between extent of amygdala damage and cortisol
There were no significant correlations between cortisol levels and the amount of amygdala damage at either age (2.5 or 5 months of age) in Neo-A animals.
Discussion
The present results demonstrate that neonatal amygdala lesions yielded alterations in basal cortisol secretion in infant rhesus monkeys, affecting early morning levels in males only at the early age (2.5 months) at the time of the postnatal T surge. At 5 months of age, after the postnatal T surge, neonatal amygdala lesions did not alter early morning basal cortisol levels, but resulted in a flattened diurnal rhythm driven by higher cortisol levels secreted at night in the amygdala-lesioned animals. This later effect of neonatal amygdalectomy was evident in both males and females.
As with previous reports of blunted cortisol response to stressors after amygdala lesions in adulthood (Herman, et al., 2003; Kalin, et al., 2004; Machado & Bachevalier, 2008), the lower basal cortisol levels in amygdalectomized males at 2.5 months of age suggest that the amygdala has an excitatory effect on the HPA axis in early infancy, at least on its basal activity. These results are consistent with those of an earlier study indicating that neonatal amygdala lesions reduced basal cortisol levels in adulthood (Raper, et al., 2012, submitted), and extend these findings by showing that the effects of neonatal amygdala lesions on basal cortisol levels are already present in infancy. Nonetheless, these findings contrast with the lack of effects on basal glucocorticoid secretion reported in other studies, including adult-onset amygdala lesions in rodents or primates (Sapolsky, et al., 1991; Herman, et al., 2003; Kalin, et al., 2004; Machado & Bachevalier, 2008), juvenile rhesus monkeys (Norman & Spies, 1981), or in a previous study of infant rhesus with neonatal lesions (Goursaud, et al., 2006). These contrasting results may reflect differences in age at lesion and procedures between studies, including time of day for sample collection. Previous primate studies examined basal cortisol samples taken around mid-morning or mid-day (Kalin, et al., 2004; Goursaud, et al., 2006; Machado & Bachevalier, 2008), but we found that neonatal amygdalectomy affected basal cortisol levels during the early morning (at 2.5 months) or at sunset (at 5 months; see discussion below).
Interestingly, a significant sex difference in basal cortisol levels was detected in Neo-C animals at 2.5 months (i.e., males exhibited higher cortisol levels than females) that was eliminated by the neonatal amygdala lesions. Thus, at 2.5 months, Neo-A males showed lower basal cortisol levels than Neo-C males. Although such a sex dimorphism in basal cortisol during infancy has not been previously reported in human or non-human primates (Champoux, et al., 1989; Higley, et al., 1992; Clarke, 1993; Jonetz-Mentzel & Wiedemann, 1993; Knutsson, et al, 1997; Netherton, et al, 2004), our findings indicate that it may be associated with the T surge in male rhesus infants at this early age given the strong correlation found between cortisol and T levels.
After birth, when infants’ HPG-axis is abruptly released from the inhibitory effect of the mothers’ high estrogen levels, male infants experience a rise in T levels occurring around birth until approximately day 5 in rodents (Forest, 1979; Weisz & Ward, 1980), 4 months in monkeys (Robinson & Bridson, 1978; Mann, et al., 1989), and 7–9 months in humans (Forest, 1979; Bouvattier, et al., 2002), followed by a quiescent phase until puberty. This “postnatal T surge” is known to affect the normal development of male genitalia and reproductive function later in life (Mann, et al., 1989; Bouvattier, et al., 2002; Boas, et al., 2006). The positive relationship between T and cortisol occurring during the T surge at 2.5 months together with its absence at 5 months when T levels are low suggests that the elevated postnatal androgen levels stimulate the HPA axis in male infants whose amygdala is intact, but is not present in males with amygdala damage. A similar positive relationship between T and cortisol levels was found in castrated adult male rhesus monkeys that exhibited lower morning cortisol levels than intact males (Smith & Norman, 1987). These findings are in line with those of a study in newborn human infants reporting sex differences in HPA axis activity, with boys exhibiting higher salivary cortisol response to a mild examination stressor than girls (Davis & Emory, 1995), although this study did not focus on basal HPA function nor directly assessed T levels. Although these findings seem contrary to the inhibitory role of T previously described in adult male rodents (Seale, et al., 2004) and the lower basal cortisol reported in post-pubertal boys than in girls (Jonetz-Mentzel & Wiedemann, 1993; Knutsson, et al., 1997; Netherton, et al., 2004), at least for these latter studies we have to consider that after puberty, the sex differences are also influenced by increases in estrogen, and boys show lower cortisol than girls only when estrogen is highest in the menstrual cycle (Wolfram, Bellingrath, & Kudielka, 2011). Altogether, these findings suggest that gonadal hormones in both males and females play an important role when investigating sexual differences in HPA axis function, and that circulating testosterone levels could represent an important factor modulating its activity prior to puberty.
Although neonatal amygdalectomy eliminated sex differences in basal cortisol seen at 2.5 months, it did not affect the postnatal T surge. This suggests that the amygdala’s role in the development of the HPA axis and its sexual dimorphism is not due to a direct effect on T secretion, but possibly by mediating the stimulatory effect of T on basal cortisol release. Although the specific mechanism of these interactions is unknown, they could be potentially mediated via AR found in high density in the amygdala (Choate, et al., 1998) and whose expression is higher in males than females (Pomerantz, & Sholl, 1987). Although, the reduced basal cortisol levels in amygdalectomized males could be due to a lack of normal T binding to ARs in the amygdala, there are other mechanisms by which the lesions could influence the effects of HPG axis on HPA function. Follow up studies are needed to address this important question about the mechanisms involved.
An alternative possibility is that androgen increases from adrenarche, which occurs in rhesus monkey infants and overlaps in males with increased neonatal testicular secretion, could have influenced basal cortisol directly. During adrenarche, maturation of the adrenal cortex zona reticularis leads to increased release of adrenal androgens, dehydroepiandrosterone (DHEA) and its conjugate DHEAS, with a peak release reported between 6 and 8 weeks after birth (review see Conley, et al., 2011a, 2012). Increased secretion of DHEA and DHEAS reflect adrenal cortex activation, but their increased secretion is unlikely to be reflected in the testosterone we measured at 2.5 months of age, as suppression of GnRH by agonist (Mann, et al., 1989) or antagonist (Wallen, et al., 1995) or neonatal castration (Conley, et al., 2011b) eliminates the neonatal rise in T (Mann, et al., 1989; Wallen, et al., 1995), without altering DHEA and DHEAS secretion. Further evidence of the independence of adrenarche and gonadarche is that previous studies have not found a correlation between T and adrenal androgens in male rhesus monkeys during adrenarche (Conley, et al., 2011b), suggesting that the overlap in the timing of the postnatal T surge and adrenarche is unlikely to produce a functional interaction between testicular and adrenal androgens. However, the possibility of interactions between these two neuroendocrine systems at this age has not been fully explored and needs to be examined in future studies.
The diurnal rhythm of cortisol secretion has not previously been fully characterized in socially-housed, mother-reared, infant rhesus monkeys. Previous research in rhesus monkeys has mostly focused on the impact of early experience on HPA stress reactivity (Champoux, et al., 1989; Clarke, 1993; Capitanio, et al., 2005; Barrett, et al., 2009). Of the few studies that have characterized the diurnal cortisol rhythm during rhesus development (Boyce, et al., 1995; Sanchez, et al., 2005; Barrett, et al., 2009), most have studied juveniles and reported a steep decline in cortisol level between early morning and afternoon, and between afternoon and night (Sanchez, et al., 2005; Barrett, et al., 2009). Our results show that although the diurnal rhythm is already present at 5 months of age, characterized by high cortisol levels in the early morning (at sunrise), there is a nonsignificant decline from sunrise to afternoon (mid-day), followed by a steeper cortisol decline from afternoon to bedtime (sunset). These results suggest that the rhythm is still immature at this age, consistent with the lack of clear cortisol decline from mid-morning to mid-afternoon reported in human infants and toddlers (Larson, et al, 1998; Watamura, et al., 2004). In humans, this immature pattern of cortisol secretion has been attributed to napping (Larson, et al., 1998) and immature self-regulation (e.g., low effortful control and behavioral inhibition: Watamura, et al, 2004; Geoffroy, et al, 2006; Tarullo & Gunnar, 2006; Gunnar, et al, 2011). With age, children’s ability to self-regulate increases and napping periods decrease, resulting in behavioral changes that parallel a steeper decline in cortisol secretion from mid-morning to mid-afternoon (Watamura, et al., 2004). It is possible that similar mechanisms influence the immature pattern of cortisol secretion seen in infant macaques in this study, although this possibility will need to be addressed in future studies.
Although the effects of neonatal amygdala lesions on early morning cortisol seen at 2.5 months were not detected at 5 months, the lesions resulted in a blunted cortisol decline from mid-day to sunset, driven by increased cortisol levels at sunset, in Neo-A as compared to the Neo-C group. Of the few previous studies that have examined the effects of adult, juvenile, or neonatal amygdala lesions on primate basal HPA function (Norman & Spies, 1981; Kalin, et al, 2004; Goursaud, et al., 2006; Machado & Bachevalier, 2008; Raper, et al., 2012 submitted) only one has previously reported effects, in particular lower early morning cortisol compared to controls (Raper, et al., 2012 submitted), in adults with neonatal lesions. One important difference with the studies that did not detect differences in basal cortisol is that the levels were measured during mid-morning or afternoon, where we did not detect changes, either. Regardless of the direction of changes in the diurnal cortisol pattern, both studies point to an important influence of the amygdala on basal HPA function in primates. This evidence supports reports of amygdala regulation of tonic (basal) HPA axis activity in rodents (Allen & Allen, 1975; Regev, et al., 2012). Consistent with our findings, Regev and colleagues (2012) recently showed that CRF knockdown in CeA of mice leads to increased basal corticosterone secretion specifically close to the bedtime/sleep phase of the diurnal rhythm, (“lights on” for rodents, when glucocorticoid levels are low). Altogether, these findings suggest that either disruption of just the CeA or the complete amygdala early in development leads to alterations in HPA axis daytime rhythm, in addition to the more widely reported stimulatory role of this region on HPA stress reactivity.
In addition to developmental factors, the different effects of the lesions on early morning cortisol at 2.5 and 5 months of age could be explained by the low T levels exhibited by all groups at 5 months. These low T levels could, in fact, explain the lack of a sex difference in cortisol secretion at this older age, and the lack of a positive relationship between T and cortisol. Thus, our findings at 2.5 months suggest, that when T levels are high, the amygdala mediates their stimulatory action on the HPA axis function, so that amygdala lesions lead to lower cortisol in males. However, at 5 months, when T levels are low in all groups, the amygdala seems to have an inhibitory role on cortisol secretion instead, at least during the trough of diurnal activity. Altogether, our findings suggest a different (opposite) mechanism of amygdala regulation of basal cortisol secretion, under a low versus a high gonadal hormone context. Future examination of the effects of neonatal amygdalectomy on HPA axis functioning during the juvenile, adolescence and adulthood periods will further help elucidate some of the relationships between amygdala, gonadal hormones, and basal HPA activity.
In summary, results from the current study demonstrate the importance of normal amygdala development and its influence on basal HPA functioning. For example, damage to the amygdala early in life leads to changes in basal cortisol secretion in infant rhesus monkeys, eliminating the normative sex difference seen during the postnatal T surge and leading to a flattened diurnal rhythm at 5 months of age. The long-term effects of neonatal amygdala lesions on this neuroendocrine axis and its interactions with the HPG axis are currently being evaluated and will shed light on the developmental consequences during the juvenile, adolescent, and adult periods.
Acknowledgments
Authors are grateful to Amy Henry, Trina Villarreal, Shannon Stephens, M.A., Christen Merte, Patrick McFarland, Cassie Lyons, Rebecca Roberts, M.A., and Sara Dicker for their invaluable assistance with handling mother-infant reunions, reintroductions to the social groups as well as data collection. We also thank all members of the Bachevalier Laboratory who have helped with the neuroimaging and surgical procedures on the infant monkeys and Sarah Pruett, PhD in the Yerkes BioMarker Core Laboratory for assistance with the hormone assays. This research was supported by the National Institute for Mental Health (MH050268). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIMH, or the National Institutes of Health. The studies were also supported by the Center for Behavioral Neuroscience (NSF IBN 9876754), and Integrated Training in Psychobiology and Psychopathology Fellowship (NIMH T32 MH732505), as well as by the National Center for Research Resources to the Yerkes National Research Center (P51 RR00165; YNRC Base grant) which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Footnotes
Conflicts of Interest
None of the authors have any conflicts of interest in the conduct or reporting of this research.
List of Contributing Authors:
Dr. Jessica Raper, Graduate Student, Department of Psychology, Emory University, 36 Eagle Row, Atlanta, GA 30322, Phone #: 404-727-8334, jraper@emory.edu
Dr. Jocelyne Bachevalier, Professor of Psychology, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30329, Phone #: 404-727-9765, jbachev@emory.edu
Dr. Kim Wallen, Professor of Psychology, Yerkes National Primate Research Center, Emory University, 36 Eagle Row, Atlanta, GA 30322, Phone #: 404-727-4125, kim@emory.edu
Dr. Mar Sanchez, Professor of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, GA 30329, Phone #: 404-712-2393, mmsanch@emory.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen JP, Allen CF. Amygdalar participation in tonic ACTH section in the rat. Neuroendo. 1975;19:115–125. doi: 10.1159/000122432. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJS, Aynsley-Green A. Measuring the severity of surgical stress in newborn infants. J Pediatr Surg. 1988;23:297–305. doi: 10.1016/s0022-3468(88)80193-3. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34:1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boas M, Boisen KA, Virtanen HE, Kaleva M, Suomi AM, Schmidt IM, Damgaard IN, Kai CM, Chellakooty M, Skakkebaek NS, Toppari J, Main KM. Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1962 normal boys. Eur J Endocrinol. 2006;154:125–129. doi: 10.1530/eje.1.02066. [DOI] [PubMed] [Google Scholar]
- 5.Bouvattier C, Carel JC, Lecointre C, David A, Sultan C, Bertrand AM, Morel Y, Chaussain JL. Postnatal changes in T, LH, and FSH in 46,XY infants with mutations in the AR gene. J Clin Endocr Metab. 2002;87:29–32. doi: 10.1210/jcem.87.1.7923. [DOI] [PubMed] [Google Scholar]
- 6.Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev Psychobiol. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- 7.Burgess LH, Handa RJ. Chronic estrogen-induced alteration in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 8.Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- 9.Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. Hormonal effects of early rearing conditions in the infant rhesus monkey. Am J Primatol. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- 10.Choate JV, Slayden OD, Resko JA. Immunocytochemical localization of androgen receptors in brains of developing and adult male rhesus monkeys. Endocrine. 1998;8:51–60. doi: 10.1385/ENDO:8:1:51. [DOI] [PubMed] [Google Scholar]
- 11.Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- 12.Conley AJ, Bernstein RM, Nguyen AD. Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. J Endocrinol. 2012;214:121–131. doi: 10.1530/JOE-11-0467. [DOI] [PubMed] [Google Scholar]
- 13.Conley AJ, Moeller BC, Nguyen AD, Stanley SD, Plant TM, Abbott DH. Defining adrenarche in the rhesus macaque (Macaca mulatta), a non-human primate model for adrenal androgen secretion. Mole Cell Endocrinol. 2011a;336:110–116. doi: 10.1016/j.mce.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley AJ, Plant TM, Abbott DH, Moeller BC, AD, Stanley SD. Adrenal androgen concentrations increase during infancy in male rhesus macaques (Macaca mulatta) Am J Physiol Endocrinol Metab. 2011b;301:E1229–E1235. doi: 10.1152/ajpendo.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M, Emory E. Sex differences in neonatal stress reactivity. Child Devel. 1995;66:14–27. doi: 10.1111/j.1467-8624.1995.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Elmlinger MW, Kühnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol, and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40:1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- 17.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychi. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 18.Forest MG. Plasma androgens (testosterone and 4-androstenedione) and 17-hydroxyprogesterone in the neonatal, prepubertal, and peripubertal periods in the human and the rat: Differences between species. J Steroid Biochem. 1979;11:543–548. doi: 10.1016/0022-4731(79)90080-3. [DOI] [PubMed] [Google Scholar]
- 19.Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York: Gilford Press, Inc; 2009. pp. 1–42. [Google Scholar]
- 20.Geoffroy MC, Côté SM, Parent S, Séguin JR. Daycare attendance, stress, and mental health. Can J Psychiat. 2006;51:607–615. doi: 10.1177/070674370605100909. [DOI] [PubMed] [Google Scholar]
- 21.Goursaud APS, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Research. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Gunnar MR, Kryzer E, Van Ryzin MJ, Phillips DA. The import of the cortisol rise in child care differs as a function of behavioral inhibition. Devel Psychol. 2011;47:792–803. doi: 10.1037/a0021902. [DOI] [PubMed] [Google Scholar]
- 23.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuoendocrin. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychi. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 25.Jonetz-Mentzel L, Wiedemann G. Establishment of reference ranges for cortisol in neonates, infants, children, and adolescents. Eur J Clin Chem Clin. 1993;31:525–529. [PubMed] [Google Scholar]
- 26.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knutsson U, Dahlgren J, Marcus C, Rosberg S, Brönnegard M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythm in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J Clin Endocr Metab. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- 28.Koupil I, Mann V, Leon DA, Lundberg U, Byberg L, Vågerö D. Morning cortisol does not mediate the association of size at birth with blood pressure in children born from full-term pregnancies. Clin Endocrinol. 2005;62:661–66. doi: 10.1111/j.1365-2265.2005.02275.x. [DOI] [PubMed] [Google Scholar]
- 29.Larson MC, White BP, Chochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Devel Psychobiol. 1998;33:327–337. [PubMed] [Google Scholar]
- 30.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal, or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann DR, Gould KG, Collins DC, Wallen K. Blockade of neonatal activation of the pituitary-testicular axis: Effect on peripubertal luteinizing hormone and testosterone secretion and on testicular development in male monkeys. J Clin Endocr Metab. 1989;68:600–607. doi: 10.1210/jcem-68-3-600. [DOI] [PubMed] [Google Scholar]
- 32.Mason JW. Plasma 17-hydroxycorticosteroid levels during electrical stimulation of the amygdaloid complex in conscious monkeys. Am J Physiol. 1959;196:44–48. doi: 10.1152/ajplegacy.1958.196.1.44. [DOI] [PubMed] [Google Scholar]
- 33.McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Meth. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 35.Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relations to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 36.Norman RL, Spies HG. Brain lesions in infant female rhesus monkeys: Effects on menarche and first ovulation and on diurnal rhythms of prolactin and cortisol. Endocrinology. 1981;108:1723–1729. doi: 10.1210/endo-108-5-1723. [DOI] [PubMed] [Google Scholar]
- 37.Ouellet-Morin I, Tremblay RE, Boivin M, Meaney M, Kramer M, Côté SM. Diurnal cortisol secretion at home and in child care: a prospective study of 2-year-old toddlers. J Child Psychol Psyc. 2010;51:295–303. doi: 10.1111/j.1469-7610.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 38.Pomerantz SM, Sholl SA. Analysis of sex and regional differences in androgen receptors in fetal rhesus monkey brain. Devel Brain Res. 1987;36:151–154. doi: 10.1016/0165-3806(87)90074-5. [DOI] [PubMed] [Google Scholar]
- 39.Raper J, Wilson M, Sanchez M, Machado C, Bachevalier J. Neonatal amygdala lesions in rhesus monkeys alter emotional and neuroendocrine responses to an acute stressor: Human Intruder Paradigm. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.10.008. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychi. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Robinson JA, Bridson WE. Neonatal hormone patterns in the macaque. I. Steroids. Biol Reprod. 1978;19:773–778. doi: 10.1095/biolreprod19.4.773. [DOI] [PubMed] [Google Scholar]
- 42.Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotrophin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Devel Psychopath. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychi. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocr. 2004b;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith CJ, Norman RL. Circadian periodicity in circulating cortisol is absent after orchidectomy in rhesus macaques. Endocrinology. 1987;121:2186–2191. doi: 10.1210/endo-121-6-2186. [DOI] [PubMed] [Google Scholar]
- 47.Sondeijker FEPL, Ferdinand RF, Oldehinkel AJ, Veenstra R, Tiemeier H, Ormel J, Verhulst FC. Disruptive behaviors and HPA-axis activity in young adolescent boys and girls from the general population. J Psychi Res. 2007;41:570–578. doi: 10.1016/j.jpsychires.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Stavisky RC, Waston SL, Anthony MS, Manuck SB, Adams MR, Kaplan JR. Influence of estradiol on cortisol secretion in ovariectomized cynomologus macaques (Macaca fascicularis) Am J Primatol. 2003;60:17–22. doi: 10.1002/ajp.10076. [DOI] [PubMed] [Google Scholar]
- 49.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Devel Psychobiol. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- 51.Wallen K, Maestripieri D, Mann DR. Effects of neonatal testicular suppression with a GnRH antagonists on social behavior in group-living juvenile rhesus monkeys. Horm Behav. 1995;29:322–337. doi: 10.1006/hbeh.1995.1023. [DOI] [PubMed] [Google Scholar]
- 52.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 53.Wilson ME, Gordon TP, Collins DC. Ontogeny of lutenizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- 54.Wolfram M, Bellingrath S, Kudielka BM. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology. 2011;36:905–912. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]