Abstract
A growing body of research suggests that rats represent and remember specific earlier events from the past. An important criterion for validating a rodent model of episodic memory is to establish that the content of the representation is about a specific event in the past rather than vague information about remoteness. Recent evidence suggests that rats may also represent events that are anticipated to occur in the future. An important capacity afforded by a representation of the future is the ability to plan for the occurrence of a future event. However, relatively little is known about the content of represented future events and the cognitive mechanisms that may support planning. This article reviews evidence that rats remember specific earlier events from the past, represent events that are anticipated to ccur in the future, and develops criteria for validating a rodent model of future planning. These criteria include representing a specific time in the future, the ability to temporarily disengage from a plan and reactivate the plan at an appropriate time in the future, and flexibility to deploy a plan in novel conditions.
Keywords: animal model, comparative cognition, episodic memory, incidental encoding, prospective cognition, prospective memory
1. Introduction
Memory enables information to be stored and retrieved after seconds to years and is essential for daily life. The loss of memory function is debilitating. Moreover, cognitive decline exerts significant societal costs. Consequently, even small improvements to retain cognitive function can have significant impacts on wellbeing, social engagement, and productivity. Episodic memory is memory for your own unique personal past experiences and the context in which those events occurred (Tulving 1972); whereas episodic memories contain details about earlier events, semantic memory stores generic facts (Tulving 1993). Episodic memory is one of the most vulnerable aspects of cognition. For example, episodic memory is profoundly impaired in patients suffering from Alzheimer's disease, and a decline in episodic memory is one of the earliest symptoms of Alzheimer's (Leube et al. 2008; Storandt 2008). It is noteworthy that deficits in episodic memory in people afflicted with Alzheimer's are deficits in the content of episodic memory, not merely in reports of subjective experiences (Bäckman et al. 1999; Egerhazi et al. 2007; Le Moal et al. 1997; Liscic et al. 2007). Consequently, modeling the content of episodic memory in animals may facilitate development of therapeutic approaches for understanding and intervening in cognitive decline.
Prospective memory is the ability to remember to take some action in the future (McDaniel and Einstein 2007). Because people remember the past, they can remember to take actions in the future based on the past. Indeed, representing the future to simulate and predict possible future events depends on the same neural machinery that is used to remember the past (Schacter et al. 2007), including the medial prefrontal regions, posterior regions in the medial and lateral parietal cortex, the lateral temporal cortex, the medial temporal lobe, and hippocampus (Martin et al. 2011; Schacter et al. 2007). Integration of information from the past is used to construct simulations about future events (e.g., episodic simulation, planning, prediction, and remembering intentions) (Schacter et al. 2008). Consequently, it is not surprising that prospective memory is also impaired in Alzheimer's disease (Blanco-Campal et al. 2009; Driscoll et al. 2005; Jones et al. 2006; Schmitter-Edgecombe et al. 2009; Troyer and Murphy 2007). Moreover, representations of past and future may share functional commonalities. For example, the temporal distance and elaboration of details regarding past and future events play similar roles in episodic memory and prospective cognition (Addis and Schacter 2008; Crystal 2012b; Roberts 2012; Roberts and Feeney 2009; Schacter and Addis 2007), namely with remote events characterized by disparateness of details.
One benefit of studying cognition in animals is that it may provide insight into impairments in cognition observed in people. Developing insight into the origins of such impairments offers a tool to improve the effectiveness of treatments. Significant obstacles nonetheless impede the development of animal models of disordered cognition. Although there is a long history of studying learning and memory in animals, these types of cognitive processes may not match those observed clinically. For example, most preclinical models of Alzheimer's disease focus on general assessments of learning and memory, particularly spatial cognition, rather than on episodic memory. Thus, it is possible that drug-development programs may identify agents effective at the pre-clinical level that subsequently fail when translated to a clinical trial in people. Ultimately, the expansion of the suite of cognitive processes that may be modeled in animals may translate to improved therapies for debilitating memory impairments observed in humans (Crystal 2012a).
2. Remembering the past
In the sections that follow, I examine three questions about remembering the past. (1) Do rats remember past episodes? (2) Can memory be isolated to a specific past episode, rather than general information about remoteness? (3) Can independent, converging lines of evidence be obtained that implicate the use of episodic memory?
2.1 Do rats remember past episodes?
One approach to demonstrating memory for a specific earlier event is to focus on what-where-when memory (Clayton et al. 2003; Clayton and Dickinson 1998); that is, memory for what happened, where it took place, and when in time it occurred. Hence, we evaluated the hypothesis that rats have what-where-when memories while eliminating a number of non-episodic memory hypotheses.
Our approach (Zhou and Crystal 2009, 2011) was to allow rats to encounter different flavors of food at various locations. We provided rats with daily information about the location of a preferred food type (chocolate) that replenished or did not replenish at its previously encountered location. Another flavor (regular chow) was available at all other locations but never replenished. The rats had the opportunity to learn that a preferred flavor would replenish at a recently presented location, but the replenishment was contingent on the time of day at which the flavor was initially encountered. If rats remember what-where-when they encountered the distinctive flavor, then they should revisit the distinctive location at a high rate when the distinctive flavor is about to replenish, but they should inhibit revisits on equivalent trials when the distinctive flavor is not about to replenish. By contrast, a rat without what-where-when memory would not be able to selectively revisit the location baited with the distinctive flavor more in the replenishment condition than in the non-replenishment condition.
In the Zhou and Crystal (2009) study, rats' memory was assessed once per day, either in the morning or in the afternoon (see Figure 1a). Chocolate replenished at a daily unique location at only one of these times of day (morning for some rats; afternoon for other rats). Another flavor (regular chow) was available at all other locations, but chow flavored locations never replenished. The interval between memory encoding (study phase) and memory assessment (test phases) was approximately 2 min. A rat with what-where-when memory could visit the chocolate location selectively on occasions when chocolate was about to replenish despite the fact that the location of chocolate varied randomly across days and the morning and afternoon sessions were presented in random order. Indeed, when the chocolate location was about to replenish, the rats revisited that location at a higher rate relative to equivalent trials in which chocolate did not replenish (Figure 2a). Differential rates of revisiting chocolate-flavored locations was accomplished while rats accurately avoided revisits to depleted chow-flavored locations. These data are consistent with the hypothesis that rats used what-where-when memories to adjust revisit rates to the daily-unique chocolate location. Importantly, what-where-when memory in this study could not be based on the delay between study and test, which was constant in replenishment and non-replenishment conditions. Thus, several sources of vague information about remoteness (i.e., judging relative familiarity of the study items, judging how long ago the study occurred, or timing an interval between study and test) could not be responsible for selective revisits in the replenishment condition because the retention interval was constant in replenish and non-replenish conditions. This approach rules out important non-episodic memory solutions that have been difficult to control in earlier experiments (Babb and Crystal 2006; Clayton and Dickinson 1998; Roberts et al. 2008).
Figure 1.
Schematic representation of experimental design of Zhou and Crystal’s (2009) study. a. Design of Experiment 1. First helpings (study phase; encoding) and second helpings (test phase; memory assessment) of food was presented either in the morning or afternoon, which was randomly selected for each session and counterbalanced across rats. Study and test phases show an example of the accessible arms, which were randomly selected for each rat in each session. Chocolate or chow flavored pellets were available at the distal end of four arms in the study phase (randomly selected). After a 2-min retention interval, the test phase provided chow-flavored pellets at locations that were previously blocked by closed doors. The figure shows chocolate replenished in the test phase conducted in the morning (7 a.m.) but not in the afternoon (1 p.m.), which occurred for a randomly selected half of the rats; these contingencies were reversed for the other rats (not shown). For each rat, one session was conducted per day. b. Phase-shift design of Experiment 2. Light onset occurred at midnight, which was 6 hr earlier than in Experiment 1, and the session occurred in the morning. The horizontal lines emphasize the similarity of the 7-hr gap between light onset and sessions in probe (solid) and training (dashed) conditions in Experiment 1. This design puts the predictions for time-of-day and how-long-ago cues in conflict; performance typical of the morning baseline is expected based on time of day whereas afternoon performance is expected based on how long ago. c. Transfer-test design of Experiment 3. Study phases occurred at the same time of day as in Experiment 1. Test phases occurred at novel times of day (7 hr later than usual). Therefore, early and late sessions had study times (but not test times) that corresponded to those in Experiment 1. The first two sessions in Experiment 3 were one replenishment and one non-replenishment condition, counterbalanced for order of presentation. An early or late session was randomly selected on subsequent days. More revisits to the chocolate location are expected in replenishment compared to non-replenishment conditions if the rats remembered the time of day at which the study episode occurred; revisit rates are expected to be equal in early and late sessions if the rats used the current time of day when the test phase occurred. Study and test phases were as in Experiment 1, except that they were separated by 7-hr delays (shown by horizontal brackets). d. Conflict-test design of Experiment 4. The study phase occurred at 1 p.m. and was followed by a test phase at 2 p.m. These times correspond to the time of day at which a late-session study phase and early-session test phase occurred in Experiment 3, which put predictions for time of day at study and time of day at test in conflict. If rats remembered the time of day at which the study episode occurred, they would be expected to behave as in its late-session, second-helpings baseline; alternatively, if the rats used the current time of day at test, they would be expected to behave as in its early-session, second-helpings baseline. Reproduced with permission from Zhou, W., & Crystal, J. D. (2009). Evidence for remembering when events occurred in a rodent model of episodic memory. Proceedings of the National Academy of Sciences of the United States of America, 106, 9525–9529. © 2009 National Academy of Sciences, U.S.A.
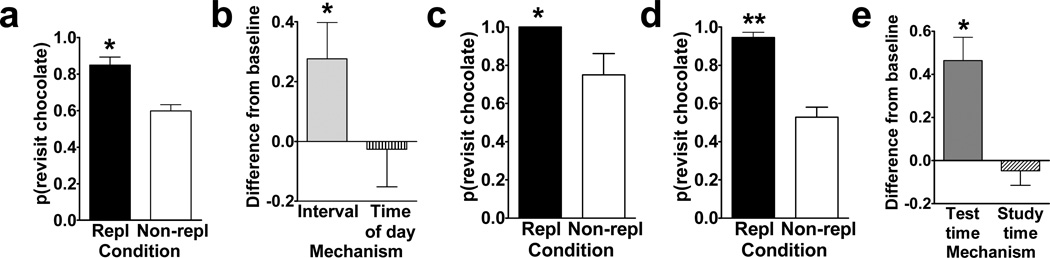
Figure 2.
a. Rats preferentially revisited the chocolate location when it was about to replenish in Experiment 1. The probability of a revisit to the chocolate location in the first four choices of a test phase is plotted for replenishment and non-replenishment conditions. b. Rats used time of day, rather than information about remoteness, to adjust revisit rates in Experiment 2. The figure shows the difference between observed and baseline revisit rates. For the bar labeled interval, the baseline is the probability of revisiting chocolate in the afternoon. The significant elevation above baseline shown in the figure documents that the rats did not use remoteness or an interval mechanism. For the bar labeled time of day, the baseline is the probability of revisiting chocolate in the morning. The absence of a significant elevation above baseline is consistent with the use of time of day. The horizontal line corresponds to the baseline rate of revisiting the chocolate location in Experiment 1. Positive difference scores correspond to evidence against the hypothesis shown on the horizontal axis. c. and d. Rats preferentially revisited the replenishing chocolate location when the study, but not the test, time of day was familiar in Experiment 3. The probability of a revisit to the chocolate location in the first four choices of a test phase is shown for first replenishment and first non-replenishment sessions (c; initial) and for subsequent sessions (d; terminal). e. Rats remembered the time of day at which the study episode occurred in Experiment 4. Rats treated the novel study-test sequence as a late-session test phase, documenting memory of the time of day at study rather than discriminating time of day at test. The figure shows the difference between observed and baseline revisit rates. For the bar labeled test time, the baseline was the probability of revisiting chocolate in the test phase of the early session in Experiment 3. The significant elevation above baseline documents that the rats did not use the time of day at test to adjust revisit rates. For the bar labeled study time, the baseline was the probability of revisiting chocolate in the test phase of the late session in Experiment 3. The absence of a significant elevation above baseline is consistent with memory of the time of day at study. The horizontal line corresponds to the baseline revisit rate to the chocolate location from Experiment 3 (terminal). Positive difference scores correspond to evidence against the hypothesis indicated on the horizontal axis. a–e. Error bars indicate SEM. a, c, and d. The probability expected by chance is 0.41. Repl = replenishment condition. Non-repl = non-replenishment condition. a. * P < 0.001 difference between conditions. b. * P < 0.04 different from baseline. c and d. * P < 0.04 and ** P < 0.0001 difference between conditions. e. * P < 0.001 different from baseline. Reproduced with permission from Zhou, W., & Crystal, J. D. (2009). Evidence for remembering when events occurred in a rodent model of episodic memory. Proceedings of the National Academy of Sciences of the United States of America, 106, 9525–9529. ©2009 National Academy of Sciences, U.S.A.
2.2 Can memory be isolated to a specific past episode?
The central hypothesis in animal models of episodic memory is that, at the time of a memory assessment, the animal remembers a specific earlier event. According to this episodic memory hypothesis, at the time of memory assessment, the rats remembered the earlier study episode and adjusted revisits to chocolate at test accordingly. Although the study described above is consistent with the hypothesis that rats remember the earlier study episode, it is also possible that the rats solved the task by using a remaining piece of information about the remoteness of an earlier event, namely the light onset in the colony which was more remote in the afternoon than in the morning sessions. According to this non-episodic memory explanation, the rats may have been reactive at the time of test based on other available cues without remembering the study episode. Thus, Zhou and Crystal (2009) determined the type of timing mechanism used in what-where-when memory by testing the following two proposals. According to the circadian time-of-day hypothesis, the rats used a circadian signal (i.e., morning vs. afternoon) to adjust revisit rates at the daily-unique chocolate location; this view is consistent with the episodic-memory hypothesis that the rats remember the specific time of day at which the study episode occurred. Alternatively, according to the interval-timing hypothesis, the rats timed the interval from light onset in the colony to the morning and afternoon sessions. We employed a 6-hr phase advance of the colony light cycle (i.e., light onset at midnight instead of 6 a.m.) and immediately tested the rats in the morning session (Zhou and Crystal 2009). Because the magnitude of the phase advance was equal to the spacing between morning and afternoon sessions, an animal may treat the probe as an afternoon session (based on an interval mechanism) or as a morning session (based on a circadian mechanism). Importantly, adjusting the revisit strategy based on the passage of time since light onset can be done without remembering the time at which the study episode occurred, which makes the interval-timing proposal a non-episodic memory hypothesis. Because morning and afternoon sessions occurred 1 and 7 hr, respectively, after light onset in the colony, a 6-hr phase shift of light onset dissociates circadian time-of-day and interval-timing hypotheses. The lights in the colony were turned on 6 hr early and the probe session was conducted at the usual time in the morning (see Figure 1b). The rats treated the probe as a morning session (Figure 2b), which is consistent with episodic memory because an endogenous circadian oscillator is not expected to adjust immediately to a phase shift (Takahashi et al. 2001). These data are significantly different from the predictions of the interval-timing hypothesis, according to which the rats would treat the probe as an afternoon session.
The studies outlined above suggest that rats can use time of day to judge when an event occurred in what-where-when memory. However, the central hypothesis about episodic memory is that the rats remember when the earlier study event occurred. By contrast, a non-episodic memory alternative hypothesis posits that the rats merely adjust revisit strategies based on the current time of day when a test occurs (Babb and Crystal 2006; Hampton and Schwartz 2004; Naqshbandi et al. 2007). Thus, we next sought to determine if rats remember the time at which the earlier episode occurred (an episodic-memory hypothesis) or, alternatively, if they were merely selectively reactive at the different times of test. Importantly, reactivity at the time of test can occur without a memory of the earlier episode, making this a non-episodic memory alternative. Hence, we determined if it was the time of day at study or at test that was responsible for the different rates of revisiting the chocolate location. The rats had been trained using study and test times that were separated by a small (2 min) delay. Because this delay is too small for rats to discriminate based on a circadian oscillator (Pizzo and Crystal 2004), we increased the delay between study and test to 7 hr (see Figure 1c), which is a value likely to be discriminated based on a circadian oscillator (Pizzo and Crystal 2006). Importantly, when we first introduced the long delay between study and test, the time of day at test was unfamiliar (approximately 7 hr later than usual) whereas the time of day at study was familiar from earlier training. If the rats remembered the study episode, then they should continue to differentially revisit the chocolate locations when their memory was assessed at novel test times, using the familiar study time of day. Alternatively, if the rats were merely reactive to the time of day at test (i.e., without remembering the earlier study episode), then there is no basis for them to revisit chocolate locations at different rates in the morning and afternoon because the test times were unfamiliar; hence, the absence of episodic memory predicts equivalent revisit rates when tested at novel times of day. When tested with novel test times of day after familiar morning or afternoon study times of day, differential rates of revisiting occurred on the very first trial in the morning and afternoon (Figure 2c–d, i.e., complete transfer). These data suggest that at the time of memory assessment, the rats remembered the time of day at which the study episode occurred.
Next, we obtained an additional line evidence for the same episodic-memory conclusion by putting episodic (study time) and non-episodic (test time) hypotheses into conflict. We used a novel combination of study and test times to determine if the rats remembered the study episode or were merely revisiting based on the current time of test. The 7-hr delays between study and test phases produced a 1-hr overlap between the two types of trials, which allowed us to start a trial with a late study phase and end the trial with an early test phase (see Figure 1d). Again we sought to determine if the rats were adjusting revisit rates in the test phase based on the time of day at test (test-time hypothesis; the non-episodic memory proposal) or based on memory of the time of day at which the study phase occurred (study-time hypothesis; the episodic memory proposal). According to the test-time hypothesis, the rats should revisit at the baseline rate that was previously typical for that test time of day. Alternatively, according to the study-time hypothesis, the rats should revisit at the baseline rate that was previously typical for that study time of day. The rats adjusted chocolate revisits based on the time of day at study rather than the time of day at test (Figure 2e). These data suggest that rats remembered the study episode, and the time of day at which the study episode occurred, providing a second line of evidence that converges on the conclusion that rats remember when the earlier study episode occurred.
2.3 Independent converging lines of evidence for episodic memory
An important reason that episodic memory has been difficult to model in animals (and consequently controversial amongst researchers) is that behavioral training likely gives rise to well-learned expectations about the sequence of events. Thus, it is possible that animals may solve an episodic-memory test by using well-learned rules without remembering the episode at memory assessment; this possibility is a major threat to the validity of animal models of episodic memory. A fundamental aspect of episodic memory is that retrieval of information can occur when encoding is incidental and memory assessment is unexpected (Beran 2012; Singer and Zentall 2007; Zentall 2005, 2006; Zentall 2010; Zentall et al. 2001; Zentall et al. 2008; Zhou and Crystal 2011; Zhou et al. 2012). Thus, we tested the hypothesis that rats can answer an unexpected question (via its behavior) after incidental encoding in a hippocampal-dependent manner, consistent with the use of episodic memory (Zhou et al. 2012). Our approach builds on common features of episodic memory in the everyday life of people. Although events are not always known to be important when they occur, people can nonetheless report details about such events; in this situation, the memory assessment is unexpected, and the information is encoded incidentally (if it is encoded at all). For example, bystanders might observe a getaway car outside a crime scene. When the event occurs, it may not be obvious to observers that anything important has happened. However, during the subsequent investigation, important details about the getaway car may be obtained from eyewitnesses. This example highlights that such reports rely on memories for incidental aspects of the earlier episode.
Incidental encoding occurs when apparently unimportant information is stored, but it is not known at the time of encoding that the information may subsequently be quite useful. In other situations, information is explicitly encoded because the information is needed later. When information is encoded for use in an upcoming, expected test of retention, it is possible that success on the test is based on retrieval of a memory of the earlier episode. However, because the test is expected, it is also possible that the explicitly encoded information is used to generate a planned action; according to this view, at the time of the test, the remembered action can occur successfully without remembering the earlier episode. Thus, although explicit encoding and an expected test may yield successful performance, it is difficult to be certain that successful performance is based on a memory of the earlier episode. By contrast, when information is encoded incidentally, the nature of the subsequent memory test is not yet known, which prevents transforming the information into a specific action plan. Hence, if we observe accurate performance on an unexpected test after incidental encoding, it is likely that this performance is based on retrieval of an episodic memory. Most memory assessments in animals rely on explicit training (e.g., Clayton and Dickinson 1998; Roberts et al. 2008; Zhou and Crystal 2009, 2011). When an animal is trained to study some material and then repeatedly tested for retention, it is likely that studying gives rise to the expectation that the test will occur. Thus, whether the animal has episodic memory or not, it can perform accurately when information is encoded explicitly for an expected test.
Zentall (Singer and Zentall 2007; Zentall et al. 2001; Zentall et al. 2008) developed techniques to ask an animal an unexpected question after incidental encoding. Zentall and colleagues (2001) trained pigeons in a symbolic matching task that was designed to determine if pigeons can answer the nonverbal question "Did you just peck or did you just refrain from pecking?" In one part of the experiment, the birds were trained to classify line orientations; the birds were trained on a symbolic matching task in which a line orientation (vertical vs. horizontal line) sample was followed by the requirement to peck or withhold pecking, followed by the selection of one of two colors (red vs. green). Note that the presentation of one line orientation signaled that a particular behavior (i.e., pecking or its absence) was required, which was then followed by the requirement to select one color to obtain reward. In another part of the experiment, the pigeons were provided with conditions that would elicit pecking or the absence of pecking, but without the requirement (and hence without an expectation) that a report about the pecking behavior would be required. In this part of the experiment, one color (e.g., yellow) was paired with food (which elicited pecking) and another color (e.g., blue) was presented but not paired with food (which elicited the absence of pecking). In the test, the sample stimuli that elicit pecking or the absence of pecking (i.e., yellow or blue) but that do not elicit the expectation of a question about pecking were presented. Next, the red and green comparison stimuli were presented, thereby unexpectedly providing the birds with the opportunity to report about their recent behavior (pecking vs. not pecking). When the pigeons were first asked the unexpected question, they reported accurately whether they had been pecking or not. In a further test, the birds were presented with a novel event that would elicit pecking (i.e., a new stimulus that occasioned generalized pecking) or a novel event that would elicit the absence of pecking (i.e., presentation of no stimulus on the test). Again the birds were unexpectedly asked whether they had recently pecked (i.e., by presentation of the red and green comparison stimuli), and they again accurately reported whether they had pecked or not. In further tests, Zentall and colleagues have controlled for residual proprioceptive cues that may be present when the unexpected question occurs (Singer and Zentall 2007) and shown that pigeons can also report about the location of their pecking response when unexpectedly asked (Zentall et al. 2008).
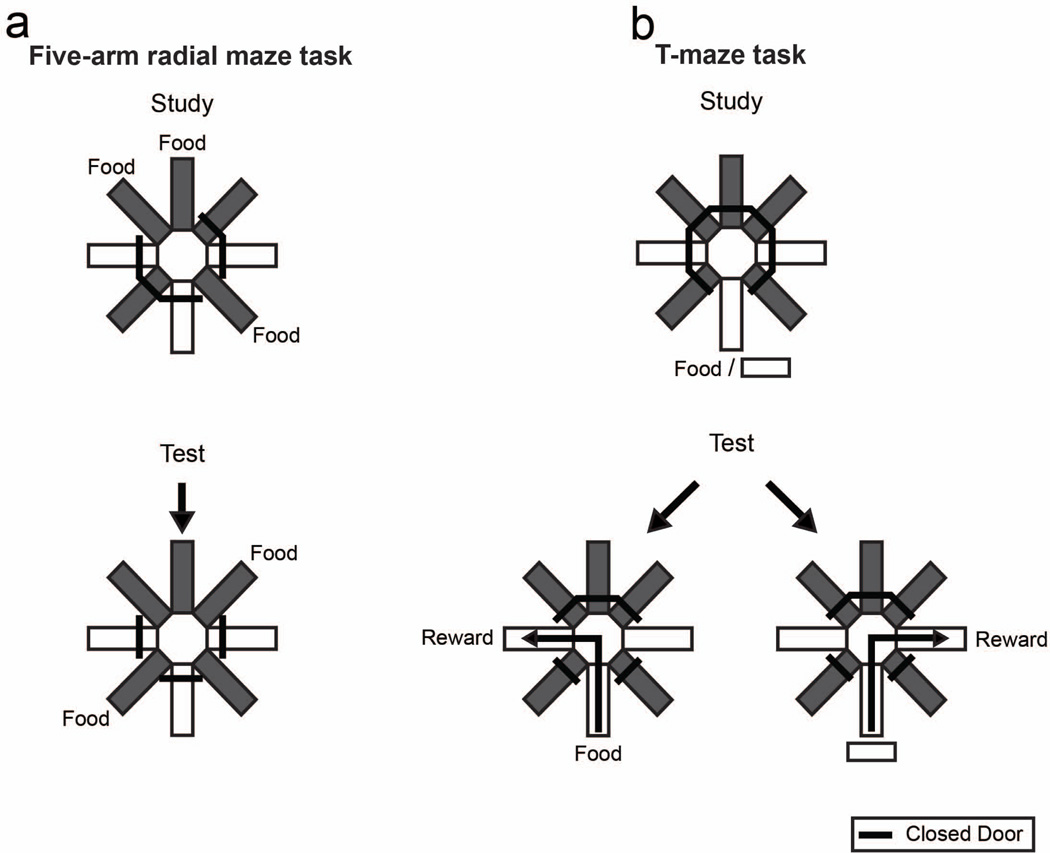
We recently developed techniques to test the hypothesis that rats can answer an unexpected question (via their behavior) after incidental encoding, and we used temporary inactivation of the hippocampus, an important processing center for episodic memory (Corkin 2002; Eichenbaum 2001; Ergorul and Eichenbaum 2004; O'Brien and Sutherland 2007; Tulving and Markowitsch 1998; Vargha-Khadem 1997), to test the hypothesis that answering an unexpected question requires episodic memory (Zhou et al. 2012). Importantly, at the time of encoding, it was not possible for the rat to know that the information would subsequently be needed (i.e., encoding was incidental) or that it would be requested (i.e., the test was unexpected). To determine if rats can answer an unexpected question after incidental encoding, we needed to train them to report about a recent event (in our case food vs. no food). Separately, we provided the rats with the opportunity to encounter food while foraging but where there was no expectation of being asked about the presence or absence of food. Next, we gave the rats the opportunity to incidentally encode the presence or absence of food while foraging and subsequently confronted them with the unexpected opportunity to report if they remembered encountering food or no food. Accordingly, we arranged for incidental encoding by using two types of tasks embedded within the same radial maze (Figure 3). In one task, the rats foraged for food at multiple locations (five-arm radial maze task; Figure 3a). In a second task, the rats learned the "reporting" skill (T-maze task; Figure 3b) that would be used later in the unexpected question. In the T-maze task, rats were rewarded for selecting left/right turns after being presented with food or no-food, respectively. Because the animals received extensive training, the T-maze task involved explicit encoding for the purpose of answering an expected question. Thus, presentation of food or no food may have generated an action plan to turn left or right. Formally, an action plan based on semantic memory of a rule (e.g., if food → turn left) may be formed when food/no-food occurs, but subsequently, the animal need only remember the to-be-performed response (left turn) without remembering the study episode. Thus, successful performance on the T-maze task does not specifically implicate the use of episodic memory. The purpose of the five-arm task was to provide the rats with an opportunity to search for food where there is no expectation of being asked about the presence of food. When foraging, the rat may encode the locations of food to avoid revisiting these locations or it may maintain a to-do-list of locations that have not yet been visited (Cook et al. 1985; Kesner 1989). However, there was no expectation of being asked about the presence of food; thus, there was no reason for the rat to specifically plan to turn left/right. In this respect, the presence or absence of food during initial foraging is incidental to successfully obtaining additional food in foraging.
Figure 3.
Schematic representation of experimental design of training from Zhou, Hohmann and Crystal’s (2012) study. a. Five-arm task. Each rat was presented with study and test phases, separated by a brief retention interval (1 trial/day). An example of the accessible arms in the study phase and corresponding test phase is shown. Accessible arms were randomly selected for each rat on each session. Grey shading in the figure identifies arms used in the five-arm radial maze task. Doors to T-maze arms (shown in white) were closed. b. T-maze task. Sample and choice phases were separated by a brief retention interval. In the sample phase, each rat was either given food (6 pellets) or no food (0 pellets). In the choice phase, each rat was rewarded with 6 pellets after turning left or right. Food and no-food samples led to reward in opposite sides of the T maze (counterbalanced across rats). Six trials were conducted per day with a random order of food and no-food samples. Doors to the five-arm radial maze were closed. a–b. All arms of the actual maze were white. Reproduced with permission from Zhou, W., Hohmann, A. G., & Crystal, J. D. (2012). Rats answer an unexpected question after incidental encoding. Current Biology, 22, 1149–1153. © 2012 Elsevier Ltd.
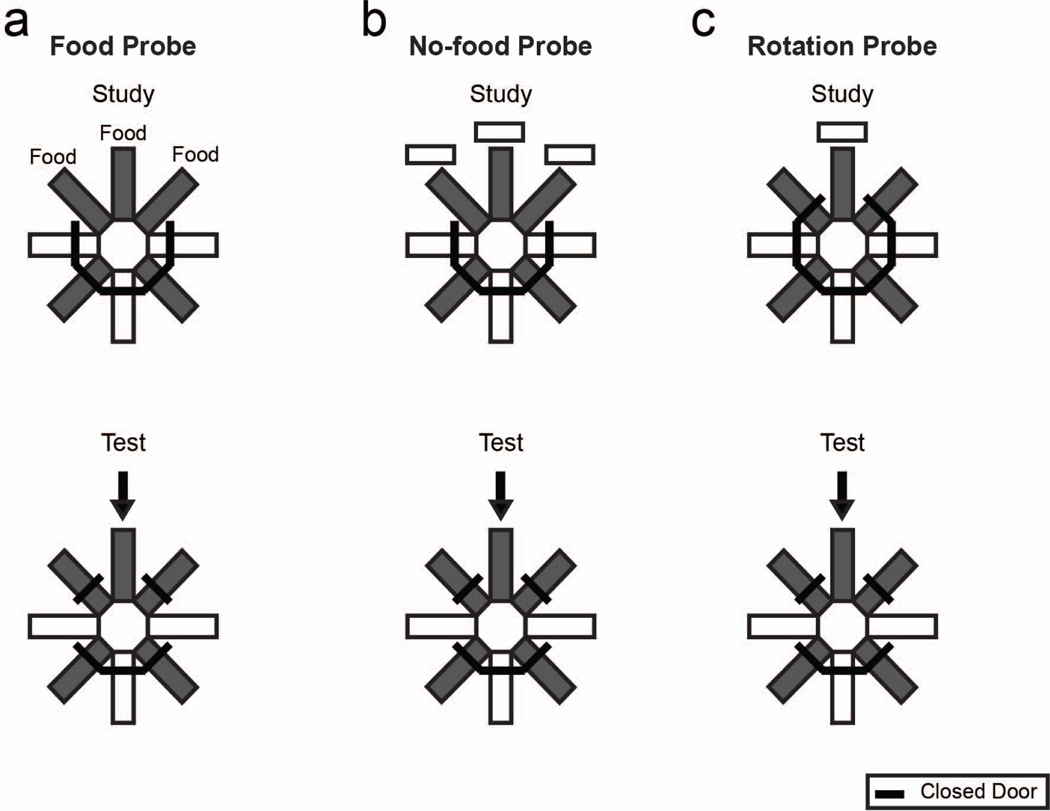
To generate incidental encoding and an unexpected question, rats began foraging for food and then were unexpectedly confronted with the opportunity to report whether they had recently encountered food or no food (Figure 4a–b). Importantly, when unexpectedly confronted with the opportunity to report if it had recently encountered food or no food, to answer the unexpected question successfully, the rat would need to retrieve a memory of the earlier episode. Thus, a rat with episodic memory would be able to answer an unexpected question by retrieving a memory of the episode, despite the fact that the importance of the earlier encounter was not known at the time of encoding. By contrast, a rat without episodic memory would be unable to answer an unexpected question after incidental encoding; hence, the probability of left and right turns is expected to be equal in the absence of episodic memory. The dissociation of episodic memory is unique to unexpected questions after incidental encoding because both a rat with episodic memory and one without it would be able to answer an expected question after explicit encoding.
Figure 4.
Schematic representation of experimental design of probes from Zhou, Hohmann and Crystal’s (2012) study. a. Food and b no-food probes started with a study phase in the five-arm-radial-maze using arms situated 135°, 180° and 225° opposite to the sample arm. In the food probe, rats encountered one pellet at each of the three arms. In the no-food probe, rats visited these three arms but did not receive food pellets. Next, two choice arms from the T-maze were opened. c. The rotation probe was identical to T-maze training (Figure 3B), except the sample was presented in the arm 180° opposite to that used in T-maze training. a–c. All arms of the actual maze were white. Reproduced with permission from Zhou, W., Hohmann, A. G., & Crystal, J. D. (2012). Rats answer an unexpected question after incidental encoding. Current Biology, 22, 1149–1153. © 2012 Elsevier Ltd.
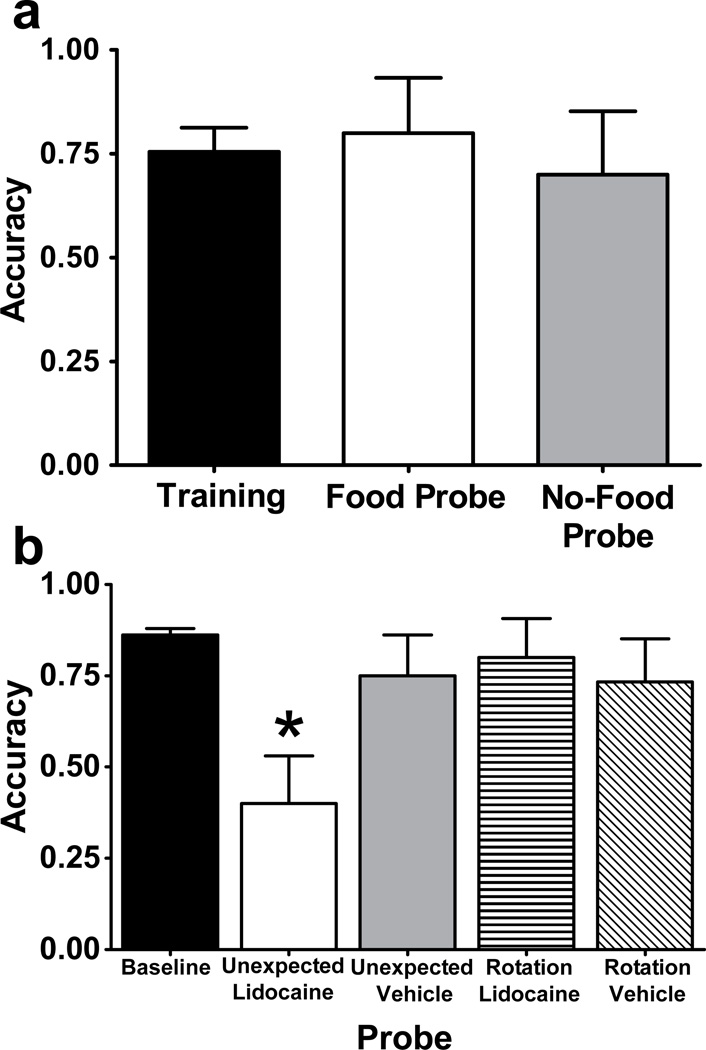
Rats answered unexpected questions (about the presence and absence of food; Figure 4a–b) with a level of accuracy similar to that observed in training (Figure 5a). We hypothesized that answering an unexpected question requires episodic memory. Thus, we next experimentally manipulated their ability to answer an unexpected question by temporarily inactivating the CA3 region of the hippocampus with bilateral infusions of lidocaine. To assess accuracy in answering an expected question, we used a control procedure (rotation probe, Figure 4c) that preserved features of the T-maze task while equating other aspects of the no-food probe (e.g., extent of rotation)1. Hippocampal inactivation selectively eliminated the ability of rats to answer an unexpected question but did not affect performance on control conditions with an expected question (Figure 4b–c and 5b). These experiments suggest that rats remember an earlier episode after incidental encoding based upon hippocampal-dependent episodic memory.
Figure 5.
a. Rats answered unexpected questions after incidentally encoding the presence or absence of food. Baseline data come from the first daily T-maze trial in the terminal five days before probe testing. Food and no-food probes were each conducted once per rat. b. Temporary inactivation of CA3 of the hippocampus before encoding impaired accuracy in answering an unexpected question relative to baseline but did not interfere with answering the expected question (rotation probe). Accuracy was selectively reduced by lidocaine in the unexpected probe relative to baseline and other probes. Baseline data come from the first daily T-maze trial in sessions before and after surgery. Each rat was tested once in each probe condition with the order counterbalanced according to a Latin Square design. Error bars represent 1 SEM. * p < 0.01 difference between the unexpected + lidocaine probe and baseline. Reproduced with permission from Zhou, W., Hohmann, A. G., & Crystal, J. D. (2012). Rats answer an unexpected question after incidental encoding. Current Biology, 22, 1149–1153. © 2012 Elsevier Ltd.
3. Planning for the future
Because representing the future to simulate and predict possible future events depends on the same neural machinery that is used to remember the past, it has been proposed that integration of information from the past is used to construct simulations about future events (Schacter et al. 2007, 2008). We recently provided evidence that rats remember to perform an intended future action, which suggests that rats posses at least a precursor to planning (Wilson and Crystal 2012).
People "remember to remember." The hallmark of prospective memory is that, as the time to execute a remembered plan draws near, a deleterious effect on ongoing behavior occurs because greater attentional resources are diverted to the now activated prospective memory (Hicks et al. 2005; Kliegel et al. 2001; Marsh et al. 2006; Marsh et al. 1998; Smith 2003; Smith et al. 2007). According to this working model of prospective memory, when people form a prospective memory, they temporarily put the memory representation into an inactive state while engaging in other activities. Later, the representation is reactivated in the future. Ultimately, successful activation of the memory representation yields an action at an appropriate future time2. Prospective memory failures may occur when the memory representation fails to be reactivated at an appropriate time.
3.1 Prospective memory in the rat
We recently developed an animal model of prospective memory (Wilson and Crystal 2012). The basic insight is that prospective memory produces a selective deficit in performance at the time when anticipation of a future event is greatest. To provide an ongoing activity, rats were trained in a temporal bisection task for 90 min per day. In bisection trials a 2- or 8-s signal was presented, and a small reward was delivered if the rat pressed the correct lever to classify the signal as short or long. To provide an anticipated future event, rats in the meal group earned an 8-g meal when the bisection task ended, whereas other rats in the no-meal group received no additional food. The meal was earned by interrupting a photobeam located inside a food trough, but photobeam breaks were only effective 90 min after the start of the bisection task. Rats in the meal group may remember to collect the meal, whereas rats in the no-meal group did not learn to remember an additional action beyond the bisection task. If rats have prospective memory, then the meal group should exhibit a negative side effect on ongoing task performance at a late time point (when the representation is most likely activated). If rats do not have prospective memory, then any change in performance from early to late time points should be equivalent for both meal and no-meal groups.
Wilson and Crystal (2012) showed that performance in the ongoing task declined near the meal time in the meal group but not in the no-meal group, consistent with prospective memory. Temporal sensitivity (i.e., the steepness of the psychophysical functions) declined near the meal time in the meal group but not in the no-meal group, as predicted by the prospective-memory hypothesis. Performance in the ongoing task was examined at early and late time points (Figure 6). Temporal sensitivity decreased from early to late time points for the meal group but not for the no-meal group, as predicted by prospective memory. Because ongoing task performance is relatively constant throughout the session when a representation of a meal is absent, our findings suggest that the approaching meal produced the observed performance decline in the meal group. Food-trough visits increased as a function of time in the meal group but not in the no-meal group (Figure 6), which suggests that the meal group anticipated the arrival of the meal, as expected.
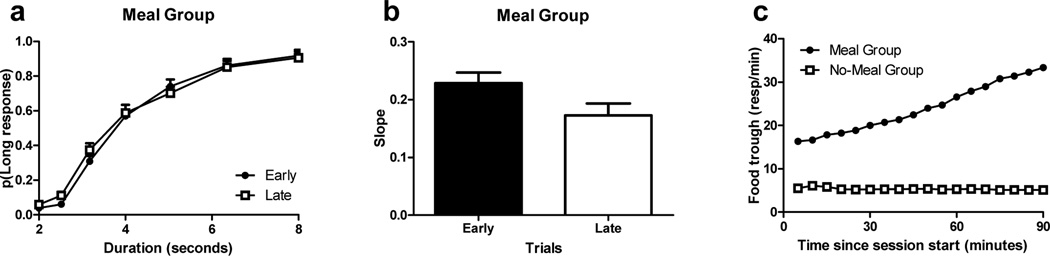
Figure 6.
Performance in an ongoing task was selectively impaired near the time of an anticipated future event. The probability of judging an interval as long (a) increased as a function of the interval duration, as expected. Performance, as measured by the slope of the probability function (b) declined immediately before the end of the daily session in the meal group (a) but not in the no-meal group (not shown). Importantly, the interaction between early vs. late time points and duration was significant for the meal group (a, p < 0.001) but not for the no-meal group (p = 0.1), and these group differences were significant as documented by the three-way interaction (p < 0.009). Similarly, the slope of the psychophysical function was smaller (i.e., poor performance) at the late relative to early time points (p = 0.009) in the meal group but not in the no-meal group (p = 0.8), and these group differences were significant as documented by the interaction (p = 0.03). The meal group anticipated the arrival of the meal, as documented by the increase in food-trough responses before the meal whereas the increase in food-trough responses was absent in the no-meal group (c). These data document a selective impairment in performance in an ongoing task near the time of an anticipated future event (but not at other times). (a–c) Error bars indicate SEM. Reproduced from Wilson, A. G. & Crystal, J. D. (2012). Prospective memory in the rat. Animal Cognition. 15, 349–358. © 2011 Springer-Verlag.
We proposed that prospective memory produced the decline in ongoing task performance as the meal approached (Wilson and Crystal 2012). According to this view, rats formed a representation of the meal but inactivated it at the early time point, when the meal was distant. As the expectation of the meal grew, more attentional resources were recruited to maintain the representation of the forthcoming meal, which impaired ongoing task performance. To support the representational account, we ruled out four non-representational hypotheses (attentional limit, response competition, contrast, and fatigue). A non-representational hypothesis is unlikely to explain our data because (1) an attentional limit imposed by judging intervals and anticipating the meal predicts impaired bisection performance throughout the entire pre-meal window, which is contrary to our data; (2) response competition (trough visits cause a decline in bisection performance) predicts a negative correlation between trough visits and bisection performance, which is contrary to our data; (3) contrast (diminished reward value in the bisection task in anticipation of higher reward value during the meal) predicts a decline in motivation to lever press measured by latencies, which is contrary to our data; and (4) fatigue (more behavioral output in meal than no-meal group) also predicts an increase in latencies to lever press, which is contrary to our data (for details see Wilson and Crystal 2012). Another possible explanation for disruption is that a forthcoming meal may produce arousal, although this is unlikely for the following reason. Although arousal is associated with access to food (Bizo and White 1994; Killeen et al. 1978), timing theories based on arousal propose that the rate of previously presented food sets a single level of arousal (Killeen and Fetterman 1988). Thus, if arousal produces deleterious effects, the impact should be equivalent at early and late timepoints (during which the rate of reward did not change). Nevertheless, a direct test of the hypothesis that arousal may change dynamically will require assessments of deleterious effects (before, during, and after) experimental manipulations of arousal.
3.2 Evaluation of strengths and weaknesses
Our initial attempt to document prospective memory in rats can be evaluated with respect to its strengths and weaknesses.
The major strength of the model is that it provides a method for evaluating the existence of a representation of a future event that would otherwise be behaviorally silent. The observation that anticipating the arrival of the meal produces a deleterious effect on ongoing behavior suggests that rats form a prospective memory of the future meal. Because the model permits study of a representation of a future event, it may be possible to use the model to study the biological basis of human memory disorders. For example, failures of prospective memory (i.e., forgetting to act on an intention at an appropriate time in the future) are a common feature of aging (Aberle et al. 2010; Craik 1986; d'Ydewalle et al. 2001; Driscoll et al. 2005; Henry et al. 2004) and negatively impacts both health (e.g., forgetting to take medications (Woods et al. 2009)) and independence (Mateer et al. 1987) (e.g., forgetting to lock one's home, turn off the stove, etc.). Prospective memory is impaired in a number of clinical populations, including patients with mild cognitive impairment (Schmitter-Edgecombe et al. 2009; Troyer and Murphy 2007), Alzheimer's disease (Blanco-Campal et al. 2009; Jones et al. 2006; Troyer and Murphy 2007), Parkinson disease (Foster et al. 2009; Raskin et al. 2011), traumatic brain injury (Henry et al. 2007; Mateer et al. 1987; McCauley et al. 2009), and HIV infection (Carey et al. 2006; Woods et al. 2009; Woods et al. 2006). Thus, the animal model of prospective memory may be a valuable tool to explore the biological bases of prospective memory disorders.
There are potential limitations of the model, especially given that not many predictions of the model have been tested. Rats show a cognitive side effect of prospective memory –representation of a future event (Addis and Schacter 2008; Martin et al. 2011; Roberts and Feeney 2009; Schacter and Addis 2007; Schacter et al. 2007, 2008). However, it is not known if rats represent a specific time point in the future. By contrast, human prospective cognition includes the ability to specify a time point for both episodic memories about the past and planning for a specific time in the future–rather than merely general knowledge about remoteness–(Crystal 2012b; Roberts 2012; Roberts and Feeney 2009; Schacter and Addis 2007). The specificity in time is particularly important for time-based prospective memory in which people reactivate a memory representation at an appropriate point in time; by contrast, temporal specificity may be less important in event-based prospective memory in which people reactivate a memory representation when a particular event occurs (McDaniel and Einstein 2007).
Prospective memory in people involves forming a plan, inactivating the representation, and then reactivating it in the future. It is not known if rat prospective memory can withstand a disengagement from the plan (i.e., inactivation), or alternatively if continual engagement is required.
People show a high degree of flexibility in planning. Although rats show a cognitive side effect of planning to obtain a meal in the future, it is not known if this ability is limited to conditions in which it is extensively trained, which would be the case if prospective memory in rats is limited to a learned fixed sequence of actions based on reflexive mechanisms.
4. Criteria to validate planning in rats
The review of limitations of the initial prospective memory model, suggests steps that are needed to test the model. In the section below, three criteria for validating an animal model of prospective memory are outlined.
First, the techniques that we have used to establish that rats remember a specific point in the past (reviewed above) can be adapted to test the hypothesis that rats represent a specific point in time in the future (Crystal 2012b). Second, tests are required to determine if rats can temporarily disengage from a plan and subsequently reactivate the plan at an appropriate time in the future. Distractor tasks can be used to require the rat to disengage from the plan. Beran and colleagues (2012) have recently provided an excellent demonstration of prospective memory in a language trained chimpanzee, which included a clearer demonstration of disengagement (see also Evans and Beran 2012). Third, tests are required to determine if rats exhibit some degree of flexibility to deploy a learned plan in a novel context.
5. Conclusions
A substantial body of research strongly suggests that rats use episodic memory to remember the content of specific earlier events. The content of these representations include the time of occurrence of the earlier event, where it occurred in space, and what flavor of food was encountered (for evidence of flavor specificity see Babb and Crystal 2006). Moreover, rats use episodic memory to retrieve information about events that were not known to be important at the time of encoding when they are unexpectedly asked to report about this information. Our approach provides an animal model of prospective memory (Wilson and Crystal 2012), yet relatively little is known about the content of represented future events and the basic cognitive mechanisms that may support planning in rats. This review has highlighted some directions to explore, including the temporal specificity of prospective cognition, the ability to disengage and subsequently reactivate a representation of a future event, and the range of flexibility or creativity within prospective cognition.
A multi-method approach is needed to fully explore the elements of prospective cognition in rats. It is possible that rats have some aspects of prospective cognition, but in some significant ways it may be limited relative to prospective cognition in humans or other animals. The use of multiple approaches is likely to provide a more complete picture of the representations used in prospective cognition. Maintaining a representation of a future event is a prerequisite for planning yet fully developed planning may not be implicated. For example, other studies of planning (Cheke and Clayton 2012; Correia et al. 2007; Mulcahy and Call 2006; Naqshbandi and Roberts 2006; Raby et al. 2007) document that some animals take action now for a future need that is dissociated from their current motivational needs (Suddendorf and Corballis 1997, 2007). According to this mental time travel approach, an animal forms a representation in which it envisions itself in a future scenario. By contrast, in our approach, rats were food restricted and participated in two tasks that both provided food. Thus, our approach clearly did not seek to dissociate motivational states. Moreover, no evidence for planning was obtained in experiments that dissociated motivational states in rats (Naqshbandi and Roberts 2006). Thus, it is possible that rats exhibit a precursor to planning only in a limited sense, and they may not be capable of more robust planning; alternatively, a multi-method approach with refinements in techniques may reveal more robust planning in future research. Although significant progress has been made using the mental-time-travel framework, it has recently been argued that future-oriented cognition should also be evaluated outside this framework (Crystal 2012b; Raby and Clayton 2009; Zentall 2006; Zentall 2010). One advantage of our approach to model prospective memory in rats outside the mental-time-travel framework is that it may provide insight into the evolution of planning to act in the future across a wide array of species by focusing on deleterious side-effects of a prospective memory representation. Preserving the ability to evaluate prospective cognition in a wide range of species will be valuable for future research that seeks to exploit rodent models of human diseases with impaired cognition.
Highlights.
Rats' memory for the past and planning for the future support the following
Memory of a specific earlier episode including what-where-when information
Memory of an incidentally encoded event using hippocampal-dependent episodic memory
A selective impairment in performance near the time of an anticipated future event
Criteria for validating a rodent model of future planning are outlined
Acknowledgements
This article is in honor of the contributions of Tony Wright to the study of comparative cognition. Supported by National Institute of Mental Health grant R01MH080052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We argued that the rotation probe does not require episodic memory for the following reasons. First, after the study phase, there is nothing unexpected about the test. Second, the study phase is identical to training (despite using a different start location) for a rat that relies on a response-mediated strategy; our rats had received extensive training in the T-maze task prior to probe testing, by which point they would likely rely on a striatal-response system (De Leonibus et al. 2011; Packard 1999; Packard and McGaugh 1996; Yin and Knowlton 2004). Thus, the ability to solve the rotation probe was not expected to require an intact hippocampus because well-trained habits have previously been suggested to be striatal-dependent (Packard 1999; Packard and McGaugh 1996). Moreover, it is unlikely that rats expected “unexpected” questions for the following reasons. First, the rats had received many 5-arm study phases that were not followed by an assessment of food/no-food. Second, right- and left-turn responses in the 5-arm task had an equivalent history of reinforcement, which was produced by the random selection of arm baiting in the study phase of the 5-arm task. Third, hippocampal inactivation eliminated the ability to answer the unexpected but not the expected question. By contrast, the striatum, which underlies habit learning, may mediate the ability of rats to answer the expected question (De Leonibus et al. 2011; Packard 1999; Packard and McGaugh 1996; Yin and Knowlton 2004). Finally, although radial maze tasks typically use baited locations, eating is incidental to efficient navigation (Timberlake and White 1990).
Prospective memory is distinguished from prospective coding, the latter of which has been more extensively studied in animals (e.g., Roitblat 1980; Zentall 2005). For example, in a delayed symbolic matching to sample experiment, a pigeon is presented with a sample (e..g, red or green) which predicts the subsequently rewarded choice (e.g, vertical vs. horizontal lines). An animal may solve this matching task by maintaining a representation of the studied sample (a retrospective code). Alternatively, an animal may solve this task by using a transformation rule (e.g., if red then choose vertical) to translate a code for the presented sample into a code for the forthcoming correct choice and from that point onward maintain a representation of the to-be-selected choice (a prospective code). Note that in both retrospective and prospective coding, the animal is hypothesized to maintain a memory code throughout the retention delay. However, the subsequent action (a correct response after the delay) is not fundamentally different whether the code was retrospective or prospective, except for the use of the transformation rule. By contrast, prospective memory is proposed to involve activating a representation, inactivating the representation, and then subsequently reactivating the representation at a later time. Inactivation and reactivation processes are not involved in prospective coding, unlike prospective memory.
References
- Aberle I, Rendell PG, Rose NS, McDaniel MA, Kliegel M. The age prospective memory paradox: Young adults may not give their best outside of the lab. Developmental Psychology. 2010;46:1444–1453. doi: 10.1037/a0020718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Discrimination of what, when, and where is not based on time of day. Learning & Behavior. 2006;34:124–130. doi: 10.3758/bf03193188. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Current Biology. 2006;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer's disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- Beran, Michael J. Animal memory: Rats can answer unexpected questions about past events. Current Biology. 2012;22:R491–R493. doi: 10.1016/j.cub.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Perdue BM, Bramlett JL, Menzel CR, Evans TA. Prospective memory in a language-trained chimpanzee (Pan troglodytes) Learning and Motivation. 2012;43:192–199. doi: 10.1016/j.lmot.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizo LA, White KG. The behavioral theory of timing: Reinforcer rate determines pacemaker rate. Journal of the Experimental Analysis of Behavior. 1994;61:19. doi: 10.1901/jeab.1994.61-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Campal A, Coen RF, Lawlor BA, Walsh JB, Burke TE. Detection of prospective memory deficits in mild cognitive impairment of suspected Alzheimer's disease etiology using a novel event-based prospective memory task. Journal of the International Neuropsychological Society. 2009;15:154–159. doi: 10.1017/S1355617708090127. [DOI] [PubMed] [Google Scholar]
- Carey CL, Paul Woods S, Rippeth JD, Heaton RK, Grant I and the, H.I.V.N.R.C.G. Prospective Memory in HIV-1 Infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke LG, Clayton NS. Eurasian jays (Garrulus glandarius) overcome their current desires to anticipate two distinct future needs and plan for them appropriately. Biology Letters. 2012;8:171–175. doi: 10.1098/rsbl.2011.0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nature Reviews Neuroscience. 2003;4:685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in the radial maze. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:453–469. [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient H.M.? Nature Reviews Neuroscience. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Correia SPC, Dickinson A, Clayton NS. Western scrub-jays anticipate future needs independently of their current motivational state. Current Biology. 2007;17:856–861. doi: 10.1016/j.cub.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Hagendorf FKH, editor. Human memory and cognitive capabilities: Mechanisms and performances. North Holland: Elsevier; 1986. [Google Scholar]
- Crystal JD. Animal models of human cognition. In: Vonk J, Shackelford T, editors. Oxford Handbook of Comparative Evolutionary Psychology. Oxford: Oxford University Press; 2012a. [Google Scholar]
- Crystal JD. Prospective cognition in rats. Learning and Motivation. 2012b;43:181–191. doi: 10.1016/j.lmot.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ydewalle G, Bouckaert D, Brunfaut E. Age-related differences and complexity of ongoing activities in time- and event-based prospective memory. American Journal of Psychology. 2001;114:411–423. [PubMed] [Google Scholar]
- De Leonibus E, Costantini VJA, Massaro A, Mandolesi G, Vanni V, Luvisetto S, Pavone F, Oliverio A, Mele A. Cognitive and neural determinants of response strategy in the dual-solution plus-maze task. Learning & Memory. 2011;18:241–244. doi: 10.1101/lm.2074311. [DOI] [PubMed] [Google Scholar]
- Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19:28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Egerhazi A, Berecz R, Bartok E, Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behavioural Brain Research. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for 'what', 'where', and 'when'. Learning & Memory. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Monkeys exhibit prospective memory in a computerized task. Cognition. 2012;125:131–140. doi: 10.1016/j.cognition.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovš G, Hershey T. Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23:347–358. doi: 10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Schwartz B. Episodic memory in nonhumans: What, and where, is when? Current Opinion in Neurobiology. 2004;14:192–197. doi: 10.1016/j.conb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19:27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Kliegel M, Theodorou G, Summers F. Traumatic brain injury and prospective memory: Influence of task complexity. Journal of Clinical and Experimental Neuropsychology. 2007;29:457–466. doi: 10.1080/13803390600762717. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Marsh RL, Cook GI. Task interference in time-based, event-based, and dual intention prospective memory conditions. Journal of Memory and Language. 2005;53:430–444. [Google Scholar]
- Jones S, Livner Å, Bäckman L. Patterns of prospective and retrospective memory impairment in preclinical Alzheimer's disease. Neuropsychology. 2006;20:144–152. doi: 10.1037/0894-4105.20.2.144. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Retrospective and prospective coding of information: role of the medial prefrontal cortex. Experimental Brain Research. 1989;74:163–167. doi: 10.1007/BF00248289. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychol. Rev. 1988:95. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Hanson SJ, Osborne SR. Arousal: Its genesis and manifestation as response rate. Psychological Review. 1978;85:571–581. [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO. Varying the importance of a prospective memory task: Differential effects across time - and event-based prospective memory. Memory. 2001;9:1–11. doi: 10.1080/09658210042000003. [DOI] [PubMed] [Google Scholar]
- Le Moal S, Reymann JM, Thomas V, Cattenoz C, Lieury A, Allain H. Effect of normal aging and of Alzheimer's disease on episodic memory. Dementia And Geriatric Cognitive Disorders. 1997;8:281–287. doi: 10.1159/000106645. [DOI] [PubMed] [Google Scholar]
- Leube DT, Weis S, Freymann K, Erb M, Jessen F, Heun R, Grodd W, Kircher TT. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease--A VBM study. International Journal of Geriatric Psychiatry. 2008;23:1114–1118. doi: 10.1002/gps.2036. [DOI] [PubMed] [Google Scholar]
- Liscic RM, Storandt M, Cairns NJ, Morris JC. Clinical and psychometric distinction of frontotemporal and Alzheimer dementias. Archives Of Neurology. 2007;64:535–540. doi: 10.1001/archneur.64.4.535. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI. Task interference from prospective memories covaries with contextual associations of fulfilling them. Memory & Cognition. 2006;34:1037–1045. doi: 10.3758/bf03193250. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Landau JD. An investigation of everyday prospective memory. Memory & Cognition. 1998;26:633–643. doi: 10.3758/bf03211383. [DOI] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proceedings of the National Academy of Sciences. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer CA, Sohlberg MM, Crinean J. Focus on clinical research: Perceptions of memory function in individuals with closed-head injury. The Journal of Head Trauma Rehabilitation. 1987;2:74–84. [Google Scholar]
- McCauley SR, McDaniel MA, Pedroza C, Chapman SB, Levin HS. Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology. 2009;23:201–209. doi: 10.1037/a0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage Publications; 2007. [Google Scholar]
- Mulcahy NJ, Call J. Apes Save Tools for Future Use. Science. 2006;312:1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- Naqshbandi M, Feeney MC, McKenzie TLB, Roberts WA. Testing for episodic-like memory in rats in the absence of time of day cues: Replication of Babb and Crystal. Behavioural Processes. 2007;74:217–225. doi: 10.1016/j.beproc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Naqshbandi M, Roberts WA. Anticipation of Future Events in Squirrel Monkeys (Saimiri sciureus) and Rats (Rattus norvegicus): Tests of the Bischof-Kohler Hypothesis. Journal of Comparative Psychology. 2006;120:345–357. doi: 10.1037/0735-7036.120.4.34. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Sutherland RJ. Evidence for episodic memory in a pavlovian conditioning procedure in rats. Hippocampus. 2007;17:1149–1152. doi: 10.1002/hipo.20346. [DOI] [PubMed] [Google Scholar]
- Packard MG. Glutamate Infused Posttraining into the Hippocampus or Caudate-Putamen Differentially Strengthens Place and Response Learning. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of Hippocampus or Caudate Nucleus with Lidocaine Differentially Affects Expression of Place and Response Learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pizzo MJ, Crystal JD. Time-place learning in the eight-arm radial maze. Learning & Behavior. 2004;32:240–255. doi: 10.3758/bf03196025. [DOI] [PubMed] [Google Scholar]
- Pizzo MJ, Crystal JD. The influence of temporal spacing on time-place discrimination. Learning & Behavior. 2006;34:131–143. doi: 10.3758/bf03193189. [DOI] [PubMed] [Google Scholar]
- Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- Raby CR, Clayton NS. Prospective cognition in animals. Behavioural Processes. 2009;80:314–324. doi: 10.1016/j.beproc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Woods SP, Poquette AJ, McTaggart AB, Sethna J, Williams RC, Tröster AI. A differential deficit in time- versus event-based prospective memory in Parkinson's disease. Neuropsychology. 2011;25:201–209. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA. Evidence for future cognition in animals. Learning and Motivation. 2012;43:169–180. [Google Scholar]
- Roberts WA, Feeney MC. The comparative study of mental time travel. Trends in Cognitive Sciences. 2009;13:271–277. doi: 10.1016/j.tics.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, MacPherson K, Petter M, McMillan N, Musolino E. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320:113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- Roitblat H. Codes and coding processes in pigeon short-term memory. Learning & Behavior. 1980;8:341–351. [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic Simulation of Future Events. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23:168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Singer R, Zentall T. Pigeons learn to answer the question “where did you just peck?” and can report peck location when unexpectedly asked. Learning & Behavior. 2007;35:184–189. doi: 10.3758/bf03193054. [DOI] [PubMed] [Google Scholar]
- Singer RA, Zentall TR. Pigeons learn to answer the question 'where did you just peck?' and can report peck location when unexpectedly asked. Learning & Behavior. 2007;35:184–189. doi: 10.3758/bf03193054. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology-Learning Memory and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE, Hunt RR, McVay JC, McConnell MD. The cost of event-based prospective memory: Salient target events. Journal of Experimental Psychology-Learning Memory and Cognition. 2007;33:734–746. doi: 10.1037/0278-7393.33.4.734. [DOI] [PubMed] [Google Scholar]
- Storandt M. Cognitive deficits in the early stages of Alzheimer's disease. Current Directions in Psychological Science. 2008;17:198–202. [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genetic, Social, & General Psychology Monographs. 1997;123:133–167. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Turek FW, Moore RY. Handbook of Behavioral Neurobiology: Circadian Clocks. New York: Plenum; 2001. [Google Scholar]
- Timberlake W, White W. Winning isn't everything: Rats need only food deprivation and not food reward to efficiently traverse a radial arm maze. Learning and Motivation. 1990;21:153–163. [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time- and event-based prospective memory. Journal of the International Neuropsychological Society. 2007;13:365–369. doi: 10.1017/S1355617707070452. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic Press; 1972. [Google Scholar]
- Tulving E. What is episodic memory? Current Directions in Psychological Science. 1993;2:67–70. [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem FGDG. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wilson AG, Crystal JD. Prospective memory in the rat. Animal Cognition. 2012;15:349–358. doi: 10.1007/s10071-011-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL Group, T.H.N.R.C. Markers of Macrophage Activation and Axonal Injury are Associated With Prospective Memory in HIV-1 Disease. Cognitive and Behavioral Neurology. 2006;19:217–221. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of Striatal Subregions to Place and Response Learning. Learning & Memory. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR. Animals may not be stuck in time. Learning & Motivation. 2005;36:208–225. [Google Scholar]
- Zentall TR. Mental time travel in animals: A challenging question. Behavioural Processes. 2006;72:173–183. doi: 10.1016/j.beproc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zentall TR. Coding of stimuli by animals: Retrospection, prospection, episodic memory and future planning. Learning and Motivation. 2010;41:225–240. doi: 10.1016/j.lmot.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR, Clement T, Bhatt R, Allen J. Episodic-like memory in pigeons. Psychonomic Bulletin & Review. 2001;8:685–690. doi: 10.3758/bf03196204. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Clement TS, Bhatt RS, Allen J. Episodic-like memory in pigeons. Psychonomic Bulletin & Review. 2001;8:685–690. doi: 10.3758/bf03196204. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Singer RA, Stagner JP. Episodic-like memory: Pigeons can report location pecked when unexpectedly asked. Behavioural Processes. 2008;79:93–98. doi: 10.1016/j.beproc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9525–9529. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Crystal JD. Validation of a rodent model of episodic memory. Animal Cognition. 2011;14:325–340. doi: 10.1007/s10071-010-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hohmann, Andrea G, Crystal JD. Rats answer an unexpected question after incidental encoding. Current Biology. 2012;22:1149–1153. doi: 10.1016/j.cub.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]