Abstract
Thymic stromal lymphopoietin (TSLP) is an epithelial cell derived cytokine important for the initiation and development of T helper (Th2) cell-mediated allergic inflammation. In this study, we identified a positive association between interleukin-9 (IL-9) and TSLP concentration in the serum of infants with atopic dermatitis. In primary cell cultures, the addition of TSLP led to an increase in IL-9 production from human and mouse Th9 cells, and induced an increase in Signal Transducer and Activator of Transcription 5 (STAT5) activation and binding to the Il9 promoter. In vivo, use of an adoptive transfer model demonstrated that TSLP promoted IL-9-dependent, Th9 cell-induced allergic inflammation by acting directly on T cells. Moreover, transgenic expression of TSLP in the lung stimulated IL-9 production in vivo, and anti-IL-9 treatment attenuated TSLP-induced airway inflammation. Together, our results demonstrate that TSLP promotes Th9 cell differentiation and function, and define a requirement for IL-9 in TSLP-induced allergic inflammation.
Introduction
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine critical for regulating Th2 immunity and allergic inflammation (Al-Shami et al., 2005; Soumelis et al., 2002; Yoo et al., 2005; Zhou et al., 2005). TSLP is highly expressed in keratinocytes of atopic dermatitis (AD) skin lesions and in asthmatic bronchial epithelial cells (Soumelis et al., 2002; Ying et al., 2005). TSLP transgenic expression in keratinocytes or lung epithelial cells results in AD-like skin inflammation or severe airway inflammation and hyperactivity, respectively (Li et al., 2005; Yoo et al., 2005; Zhou et al., 2005). Moreover, TSLP receptor-deficient (Tslpr, or cytokine receptor-like factor 2 (Crlf2)) mice are protected from developing allergic skin and lung inflammation (Al-Shami et al., 2005; He et al., 2008; Zhou et al., 2005).
TSLP exerts its biological activities by binding to a heterodimeric receptor complex consisting of the IL-7 receptor α-chain (IL-7Rα) and the TSLP receptor chain (TSLPR) (Pandey et al., 2000; Park et al., 2000). This binding results in the activation of Signal Transducer and Activator of Transcription 5 (STAT5) (Isaksen et al., 1999; Pandey et al., 2000; Rochman et al., 2007; Rochman et al., 2010). TSLP induced pathogenesis of asthma-like airway inflammation is eliminated by genetic targeting or inhibition of IL-4 (Zhou et al., 2008), suggesting that TSLP is acting upstream of Th2 effector cells. Indeed, TSLP induces allergic inflammation by directly promoting Th2 cell proliferation and Th2 cytokine production (He et al., 2008; Omori and Ziegler, 2007). TSLP also promotes dendritic cell maturation and migration to draining lymph nodes, where DC support the differentiation of allergen-specific naive CD4+ T cells into Th2 cells (Soumelis et al., 2002).
IL-9 promotes allergic inflammation and is associated with various Th2 cell-mediated responses, including mucus production from lung epithelial cells and pulmonary mastocytosis (Goswami and Kaplan, 2011). Consistent with increased expression of IL-9 in lungs of asthmatic patients (Erpenbeck et al., 2003; Ying et al., 2002), over-expression of IL-9 results in asthma-like airway inflammation and bronchial hyper-responsiveness (Temann et al., 1998). In support of a pathogenic role for IL-9, anti-IL-9 treatment inhibits airway inflammation, hyperreactivity and airway remodeling in mouse models of asthma (Chang et al., 2010; Cheng et al., 2002; Kearley et al., 2011; Kung et al., 2001).
A T helper cell subset specialized for the production of IL-9, termed Th9 cells, is generated in the presence of TGF-β1 and IL-4 (Dardalhon et al., 2008; Veldhoen et al., 2008). Th9 cells are related to Th2 cells in that they require STAT6, GATA3 and IRF4 for development, though distinct from Th2 cells in their requirement for PU.1 (Chang et al., 2010; Dardalhon et al., 2008; Goswami et al., 2012; Staudt et al., 2010; Veldhoen et al., 2008). Th9 cells, as sources of IL-9, contribute to allergic inflammation (Chang et al., 2010; Staudt et al., 2010). Th9 cells differentiated from atopic patients secrete higher amounts of IL-9 than those from non-atopic patients (Yao et al., 2011). Furthermore, allergic donors have substantially greater numbers of circulating Th9 cells than non-allergic donors, and circulating numbers of Th9 cells correlate with levels of serum IgE (Jones et al., 2012).
Although an important role for TSLP in Th2 cell differentiation and function has been established, whether TSLP also contributes to Th9 cell-mediated inflammation, and whether IL-9 is required for TSLP-mediated inflammation, has not been defined. In this report, we demonstrate that TSLP functions directly on human and mouse Th9 cells to increase IL-9 production and enhances the ability of Th9 cells to promote airway inflammation.
Results
Correlation of serum IL-9 and TSLP concentration in atopic infants
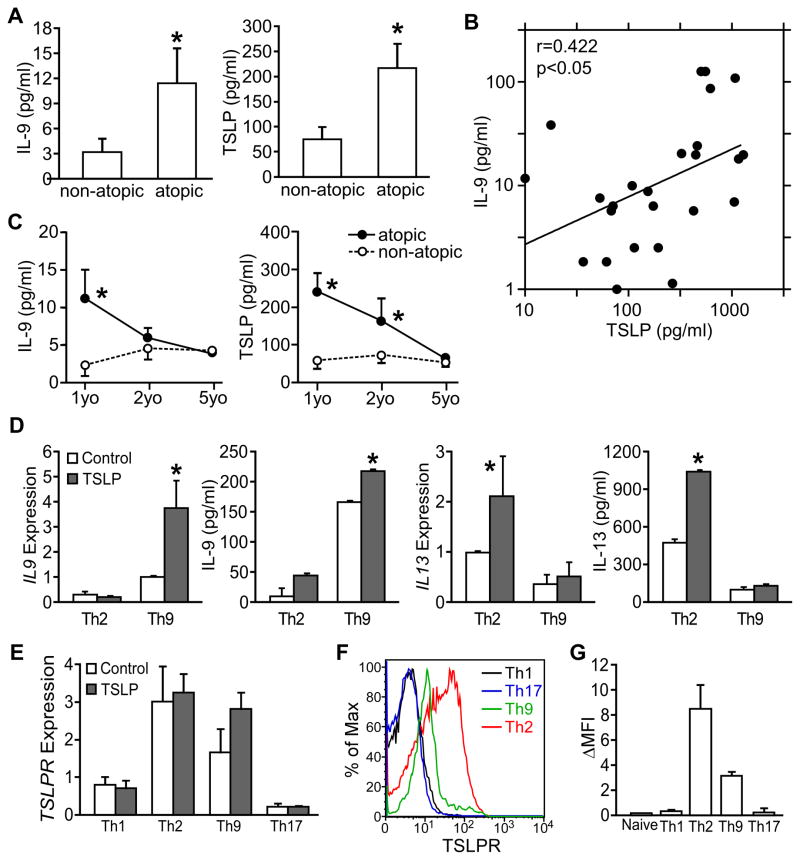
In a recent study of infants with atopic or non-atopic dermatitis, we reported that polyclonal- or allergen-stimulated PBMCs isolated from atopic infants (18 to 30 months old) produced significantly higher IL-9 than non-atopic controls, although there was no difference in the production of Th1, Th2 or Th17 cytokines (Yao et al., 2011). To explore this further, we measured serum cytokine concentrations in the same children at 6–18 months of age, and one and four years later. Among the cytokines analyzed, our data showed that atopic patients (n=54) showed significantly higher serum IL-9 and TSLP than non-atopic patients (n=25, p<0.05) (Fig. 1A). Moreover, the serum TSLP concentration positively correlated with IL-9 concentration (r=0.422, p<0.05) (Fig. 1B). However, both serum IL-9 and TSLP concentrations declined with age, and decreased to low levels when the cohort reached 5 years old showing no significant differences between atopic and non-atopic dermatitis patients (Fig. 1C). Low concentrations of serum IL-9 and TSLP were also observed in a separate population of severe asthmatic adults (Fig. S1A), suggesting that meaningful concentrations of these cytokines are most easily observed in young atopic infants. These results suggest a link between TSLP and IL-9 early in atopic disease.
Figure 1. Relationship between TSLP and IL-9 expression in humans.
(A–C) Serum IL-9 and TSLP in children with dermatitis. Venous blood (2 ml) was collected by venipuncture and serum was isolated. Serum IL-9 and TSLP were measured by multiplex bead assay.
(A) Serum IL-9 and TSLP concentration of atopic dermatitis infants and non-atopic dermatitis infants (6 to 18 months old). Concentrations of IL-9 and TSLP below detection limit were counted as zero. Atopic: N=54; non-atopic: N=25
(B) Positive correlation between serum IL-9 and TSLP concentration presented in (A). Samples with IL-9 concentration below detection limit were omitted in correlation analysis.
(C) Changes of serum IL-9 and TSLP concentration of the same cohort of patients from first visit (1yo, 6 to 18 months old), one-year follow-up (2yo) to 5 years of age (5yo).
(D and E) TSLP enhances human Th9 cell generation. Naïve CD4+ T cells were isolated from PBMC taken from 5 healthy donors and differentiated to Th9 and Th2 cells in the presence or absence of 10 ng/ml human TSLP. (D) IL-9 and IL-13 expression in Th9 and Th2 cell cultures determined by qPCR and multiplex assay.
(E) TSLPR expression in different T helper subsets cultured in the presence or absence of 10 ng/ml human TSLP.
(F–G) TSLPR expression was determined on naïve CD4 T cells and T helper cell subsets. Results are shown from one donor (F) or presented as the average mean fluorescence intensity (background subtracted) of 4 donors (G).
All data are presented as the mean ± SEM. *, p<0.05. See Figure S1 for additional information.
TSLP promotes Th9 cell differentiation in vitro
To define a functional relationship between TSLP and IL-9, naïve CD4+ T cells from PBMC of healthy donors were differentiated to Th9 and Th2 cells in the presence or absence of 10 ng/ml human TSLP. Significantly increased mRNA and protein expression (p<0.05) of IL-9 and IL-13 were only detected in the respective Th9 and Th2 cell cultures in the presence of TSLP (Fig. 1D). However, TSLP did not have significant effects on cytokine production from Th1 or Th17 cell cultures (data not shown). Importantly, we found no significant contamination of DCs in our purified naïve CD4+ T cells (Fig. S1B), further supporting the conclusion that TSLP was acting directly on T cells. TSLPR expression was greater in Th2 and Th9 cell cultures than in Th1 and Th17 cell cultures, and TSLPR was expressed on the surface of Th2 and Th9 cells, but was at background levels on Th1, Th17 and naïve CD4 T cells (Fig. 1E–G).
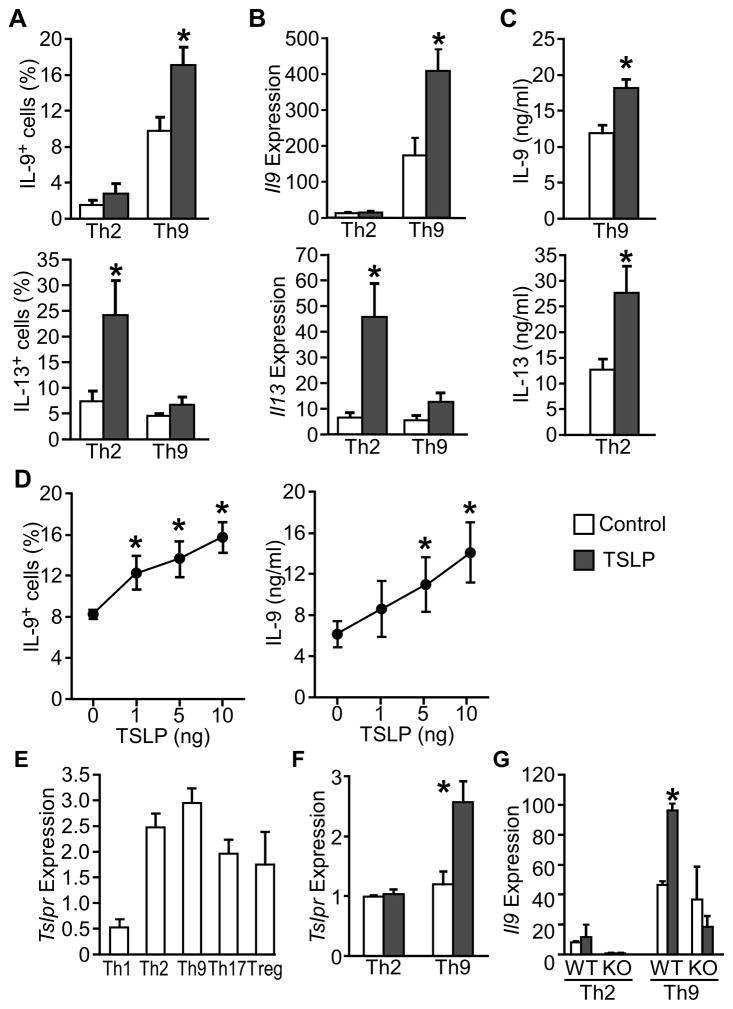
Next, we took advantage of mouse models to further define the role of TSLP on Th9 cell differentiation and function in vitro and in vivo. We found mouse TSLP had similar effects on Th9 and Th2 cell differentiation, increasing the IL-9+ population, Il9 expression and IL-9 production in Th9 cell cultures, and the IL-13+ population, Il13 expression and IL-13 protein production in Th2 cell cultures (Fig. 2A–C). Similar results were observed in Th2 cultures for IL-4 (data not shown). Furthermore, TSLP enhanced IL-9 production in a dose-dependent manner (Fig. 2D).
Figure 2. TSLP enhances murine Th9 cell generation.
Mouse splenic naïve CD4+ T cells were isolated and differentiated to Th9 and Th2 cells in the presence or absence of 10 ng/ml TSLP.
(A) Intracellular staining of IL-9-positive and IL-13-positive population in Th9 and Th2 cell cultures assessed by flow cytometry.
(B) Il9 and Il13 expression in Th9 and Th2 cell cultures was determined by qPCR.
(C) ELISA analysis of IL-9 and IL-13 secreted by Th9 and Th2 cells, respectively.
(D) Intracellular staining (left) or ELISA analysis (right) of Th9 cell cultures incubated with increasing doses of TSLP.
(E) Tslpr expression in Th subsets was determined by qPCR.
(F) Tslpr expression in Th9 cells and Th2 cells incubated with or without TSLP.
(G) Il9 expression in Th2 or Th9 cell cultures, incubated with or without TSLP. Data are representative of 3–4 experiments and are presented as the mean ± SEM of at least 3 replicate samples. *, p<0.05. WT, wild type BALB/c cells; KO, BALB/c TSLPR-deficient cells.
Mouse Th2 cells express higher TSLPR than Th1 and Th17 cells (Kitajima et al., 2011). Consistent with our human data (Fig. 1E), we found that mouse Th9 cells expressed equivalent amounts of Tslpr expression to Th2 cells (Fig. 2E). Moreover, TSLP treatment further induced Tslpr expression in Th9 but not Th2 cells (Fig. 2F). TSLP-induced Il9 expression was not observed in TSLPR-deficient Th9 cell cultures (Fig. 2G).
Taken together, our results show that both human and mouse Th9 cells express TSLP receptor, and that TSLP acted directly on CD4+ T cells to promote Th9 cell differentiation and IL-9 production in vitro.
TSLP increases STAT5 activation and binding to target genes
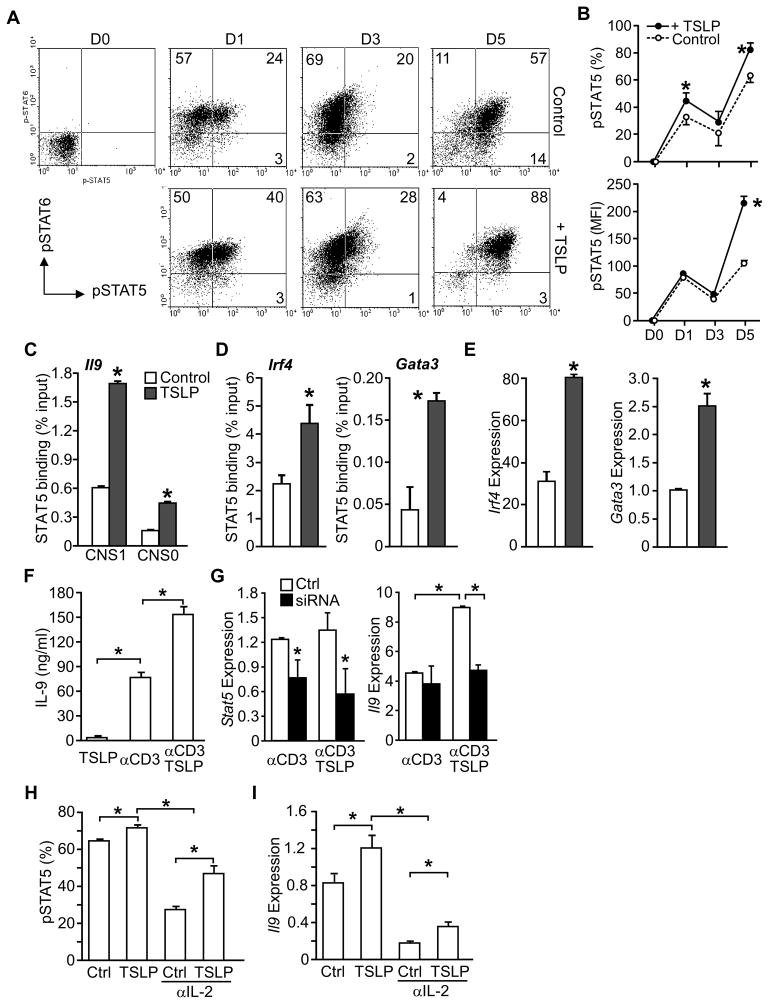
Although STAT6 activation is critical in Th9 cell differentiation, the role of STAT5 in Th9 cell development has not been reported. In both human and murine cells, TSLP activates STAT5 in CD4+ T cells (Omori and Ziegler, 2007; Rochman et al., 2007; Rochman et al., 2010). To explore TSLP-mediated STAT5 activation in Th9 cell differentiation, CD4+ T cells were cultured under Th9 conditions in the presence or absence of TSLP, and assessed for intracellular phospho-STAT5 (pSTAT5) and pSTAT6. The majority of cells during Th9 differentiation are pSTAT6-positive, and TSLP slightly enhanced the percentage of pSTAT6+ cells (Fig. 3A, Fig. S2A) (Goswami et al., 2012). TSLP had a more dramatic effect on pSTAT5, increasing the percentage of pSTAT5-positive early in differentiation, and increasing both the percentage of pSTAT5-positive cells and the intensity of pSTAT5 staining later in differentiation (Fig. 3A and B).
Figure 3. Enhanced STAT5 function in TSLP-treated Th9 cells.
(A and B) Naive CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured in IL-4, TGF-β, and anti-IFN-γ(Th9 cell conditions) with or without TSLP. On day 0, 1, 3, and 5 during differentiation, cells were stained for intracellular phospho-STAT6 (pSTAT6)and phospho -STAT5 (pSTAT5).
(A) Dot plots of intracellular staining. Numbers in ow cytometry dot plots indicate the percentages of cells in each quadrant. (B) The percentages of pSTAT5+ cells, and the intensity (mean fluorescence intensity, MFI) of pSTAT5 staining is represented as the mean ± SEM.
(C–E) Naive CD4+ T cells were cultured in Th9 conditions with or without TSLP for 24 hours.
(C) STAT5 binding to conserved noncoding sequences (CNS) of Il9 was examined by chromatin immunoprecipitation (ChIP) and qPCR.
(D) STAT5 binding to the promoters of the indicated genes was evaluated by ChIP and qPCR.
(E) Gene expression of Irf4, and Gata3 was determined using qPCR.
(F and G) Naïve CD4+ T cells were cultured in Th9 conditions without TSLP.
(F) Th9 cells were restimulated with anti-CD3 in the presence or absence of TSLP and IL-9 production was evaluated by ELISA.
(G) Th9 cells were transfected with Stat5 siRNA or control siRNA (Ctrl) and rested for 24 hr. Cells were restimulated with anti-CD3 and relative Stat5 and Il9 expression were determined by qRCR.
(H and I) Naïve CD4+ T cells were cultured in Th9 conditions with TSLP or without TSLP (Ctrl), and in the presence or absence of IL-2 neutralizing antibody.
(H) The percentage of pSTAT5+ cells were determined by intracellular staining and flow cytometry.
(I) Relative Il9 expression was evaluated by qPCR. Data are representative of 2–4 experiments and are presented as the mean ± SEM of at least 3 replicate samples. *, p<0.05. See Figure S2 and Table S1 for additional information.
To determine how STAT5 impacts IL-9 production, binding of STAT5 to the Il9 locus was tested by chromatin immunoprecipitation (ChIP). Addition of TSLP to Th9 cell cultures resulted in an increase in STAT5 binding at two Il9 conserved noncoding sequences (CNS1 and CNS0), compared to cultures without TSLP (Fig. 3C). Although TSLP treatment did not lead to increases in total acetylation of histones H3 or H4, or a significant change in H3K27 tri-methylation at either conserved region, there was an increase in acetylation of H3K9/18 at the Il9 promoter (Fig. S2B). Moreover, addition of TSLP in Th9 cell cultures induced greater STAT5 binding to the Irf4 and Gata3 promoters (Fig. 3D), two factors required for Th9 cell development, but not the Maf promoter (Fig. S2C). Concomitantly, TSLP enhanced Irf4 and Gata3 expression (Fig. 3E) but had no effect on Maf expression (Fig. S2C). Similarly, there was no change in expression of the PU.1-encoding Sfpi1 gene (data not shown), consistent with the induction of this gene by TGFβ signaling pathways (Goswami et al., 2012).
TSLP also enhanced the production of IL-9 from Th9 effector cells, following five days of differentiation (Fig. 3F). Transient transfection of differentiated Th9 cells with Stat5-specific siRNA for 24 h significantly (p<0.05) diminished Stat5 expression, and eliminated the co-stimulatory effect of TSLP on Il9 expression in Th9 cells re-stimulated with anti-CD3 (Fig. 3G). However, TSLP had only modest effects on the expression of Irf4 and Gata3 in already differentiated Th9 cells and STAT5 siRNA during restimulation had only modest effects on Irf4 and Gata3 expression (Fig. S2D).
IL-2 is essential for IL-9 production by T cells (Houssiau et al., 1995; Schmitt et al., 1994). Since both IL-2 and TSLP can signal through STAT5, we tested whether TSLP enhanced IL-9 production by inducing IL-2. Blocking IL-2 signaling with IL-2 neutralization antibody during Th9 cell differentiation resulted in a decrease in STAT5 phosphorylation (Fig. 3H) and Il9 expression (Fig. 3I). TSLP was able to recover partial STAT5 activation and Il9 expression, although not to the levels observed when IL-2 signaling was present in the cultures. Anti-IL-2 had a modest effect on STAT6 phosphorylation and exogenous TSLP did not affect pSTAT6 (Fig. S2E). Together, these results suggest that STAT5 is a downstream effector in TSLP-induced Th9 cell differentiation. TSLP can induce STAT5 phosphorylation and IL-9 expression independently of, though it clearly cooperates with, IL-2.
TSLP enhances Th9 cell-mediated allergic airway inflammation
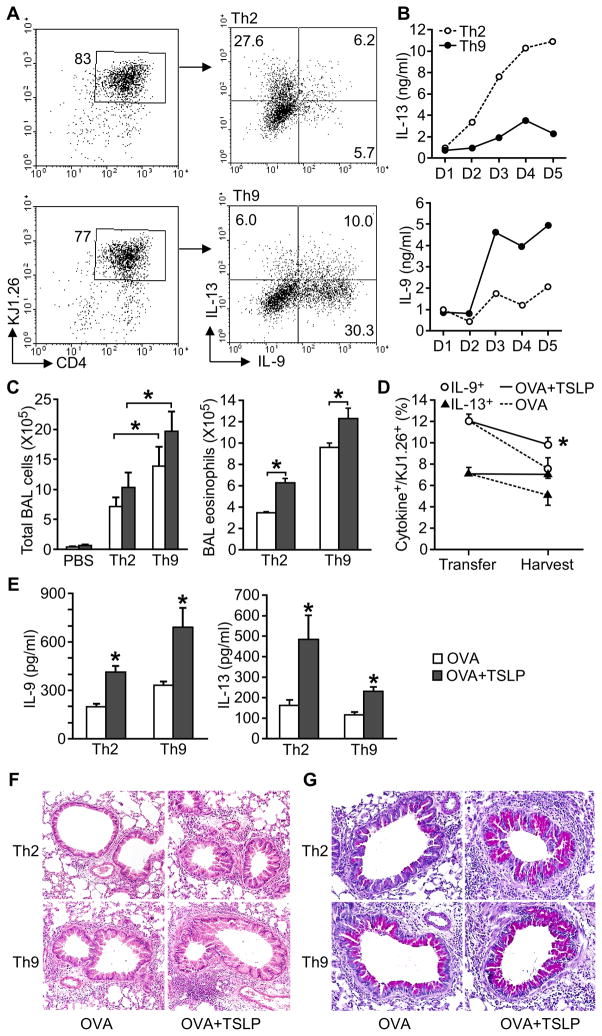
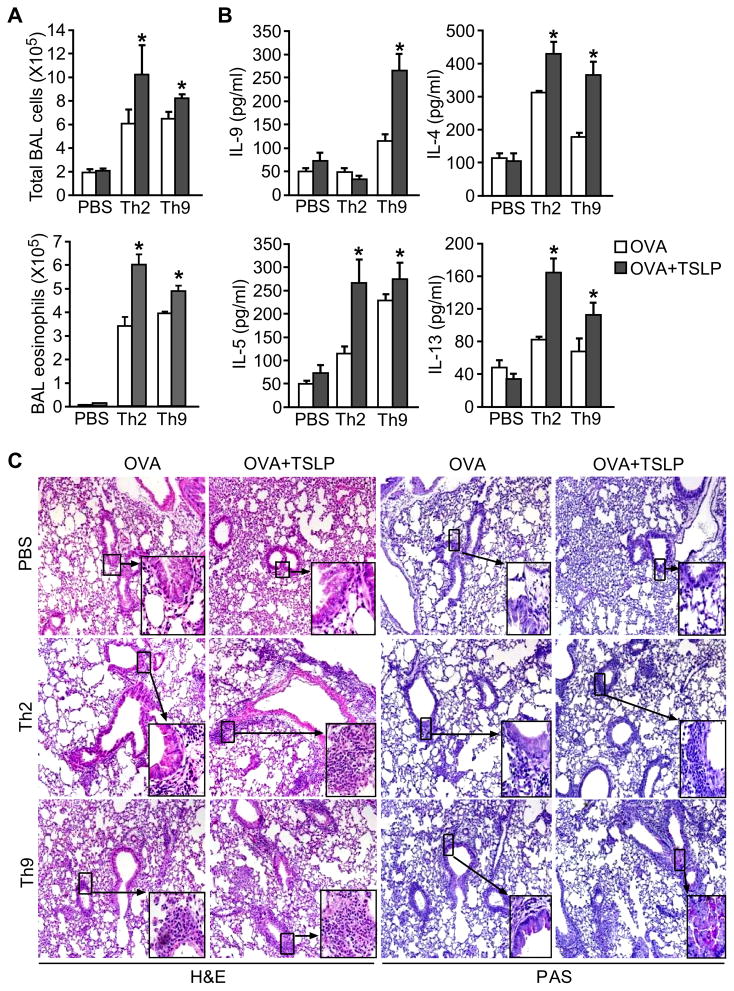
To determine the ability of TSLP to promote Th9 cell-mediated allergic lung inflammation in vivo, we adoptively transferred Th2 or Th9 cells differentiated from DO11.10 mice to BALB/c mice and intranasally (i.n.) challenged the recipient mice with OVA or OVA+TSLP for 5 days. Intracellular staining and ELISA analyses confirmed the differentiated cells had the characteristics of Th2 (high IL-13, low IL-9) or Th9 cells (high IL-9, low IL-13) (Fig. 4A and B). After challenge, recipients of either Th2 or Th9 cells developed airway inflammation, and TSLP further increased inflammation (Fig. 4C). TSLP was particularly effective at enhancing Th9 cell-mediated eosinophilia in the BAL (Fig. 4C). To evaluate the stability of Th9 cells after transfer and challenge, we analyzed cytokine expression by DO11.10 CD4+ T cells in the BAL from Th9 cell recipient mice. We found that the KJ1.26+ cells maintained a higher percentage of IL-9+ cells than IL-13+ cells, and TSLP attenuated the loss of cytokine expression observed between transfer and harvest (Fig. 4D). OVA+TSLP challenge induced more IL-13 and IL-9 in BAL from recipients of Th2 or Th9 cells, compared with OVA challenged mice. However, TSLP had greater effects on the induction of IL-13 in Th2 cells and IL-9 in Th9 cells, than in mice receiving the reciprocal cells (Fig. 4E). Histological analysis of lungs demonstrated that TSLP had a modest effect on peribronchial infiltration in Th2 cell recipients, but greatly enhanced airway inflammation in Th9 cell recipients (Fig. 4F). Mucus production in both recipients of Th2 and Th9 cells was increased by TSLP (Fig. 4G). Thus, TSLP effectively promotes Th9 cell-mediated allergic airway inflammation, with some parameters of inflammation showing greater TSLP-mediated effects than on Th2 cells.
Figure 4. TSLP exacerbates Th9 cell-mediated allergic inflammation.
(A) Naïve CD4+ T cells from DO11.10 mice were differentiated under Th2 and Th9 conditions with OVA323–339 peptide and APC. Before transfer, IL-13+ and IL-9+ cells were measured in DO11.10 Th2 and Th9 cell cultures by intracellular cytokine staining following stimulation with PMA/Ionomycin.
(B) Concentration of accumulating IL-13 and IL-9 during Th2 and Th9 cell differentiation of DO11.10 T cells was detected using ELISA.
(C – G) Differentiated Th cells were injected to wild type recipients via tail vein, with PBS injection as control. Mice were analyzed after OVA or OVA+TSLP challenge for 5 days. After 5 days, recipient mice were analyzed for (C) BAL total cell and eosinophil counts, (D) percentage of IL-9+ and IL-13+ Th9 cells before transfer and in the BAL of recipient mice of Th9 cells, and (E) BAL cytokine concentration by ELISA. Lung pathology was examined by staining with (F) hematoxylin and eosin (H&E) and (G) Periodic acid-Schiff (PAS).
All data represent mean ± SEM (n=6–7 mice) and are representative of at least 2 experiments. *, p < 0.05.
TSLP-enhanced allergic airway inflammation requires IL-9
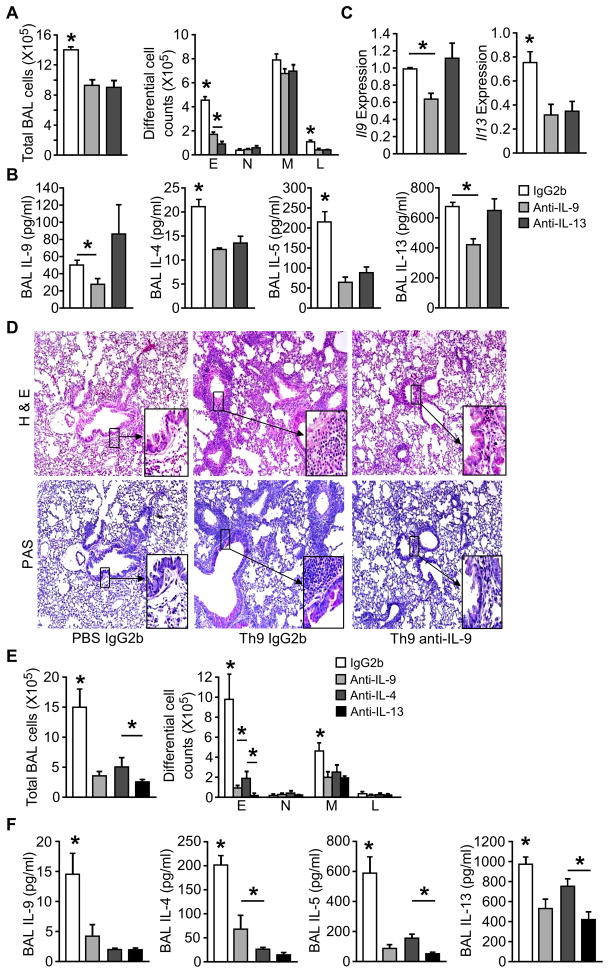
Since OVA+TSLP-challenged Th9 cell recipient mice also had increased concentrations of IL-13 in BALF (Fig. 4E), we next examined whether TSLP enhanced airway inflammation by promoting Th2 cytokine or IL-9 expression. We transferred OVA-specific Th9 cells into BALB/c mice, and challenged the mice i.n. with OVA+TSLP for 5 days. On days 1, 3 and 5, mice received i.v. injection of 10 μg anti-IL-9 or anti-IL-13 before OVA+TSLP intranasal challenge. Neutralizing IL-9 decreased total cell and eosinophil counts in BAL, compared to IgG2b-injected mice (Fig. 5A). Amounts of IL-9 and Th2 cytokines were decreased in the BAL fluid from anti-IL-9 treated mice (Fig. 5B). Consistent with a previous report that IL-9-induced airway inflammation was dependent on IL-13 (Temann et al., 2007), blocking IL-13 in Th9 cell recipient mice reduced airway inflammation and Th2 cytokines, but had little effect on IL-9 protein and gene expression in the lung (Fig. 5A–C). While OVA+TSLP challenged recipients treated with control antibody showed severe inflammatory cell infiltration (H&E staining) and mucus production (PAS staining), anti-IL-9 treatment greatly attenuated airway inflammation and goblet metaplasia (Fig. 5D). These data demonstrated a requirement for IL-9 in the TSLP-mediated enhancement of Th9-mediated allergic airway inflammation.
Figure 5. IL -9 is required for TSLP augmented allergic airway inflammation in recipients of Th9 and Th2 cells.
(A–D) Differentiated DO11.10 Th9 cells were transferred to recipient mice as described in Figure 5. During challenge (OVA + TSLP), 10 μg/mouse anti-IL-9, anti-IL-13 or IgG2b control mAb was administrated via tail vain injection on days 1, 3 and 5. Mice were analyzed 24 h after the last treatment for (A) total cell and differential cell counts in BAL, (B) BAL cytokine concentration by multiplex bead assay, (C) Il9 and Il13 expression in lungs by qPCR, and (D) tissue pathology by Hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) staining of paraffin-embedded lung sections.
(E and F) Differentiated DO11.10 Th2 cells were transferred to recipient mice. During challenge (OVA + TSLP), 10 μg/mouse anti-IL-9, anti-IL-13, or 50 μg/mouse anti-IL-4 mAb was administrated via tail vain injection on days 1, 3 and 5. Control mice received IgG2b control mAb. Mice were analyzed 24 h after the last treatment for (E) total cell and differential cell counts in BAL, and (F) BAL cytokine concentration by multiplex bead assay.
All data represent mean ± SEM (n=5 mice), and are representative of at least 2 experiments. *, p < 0.05
Because we also detected IL-9 in recipients of Th2 cells, we tested the requirement for individual cytokines in Th2 cell recipients treated with anti-IL-9, anti-IL-4, anti-IL-13, or control antibody. All three neutralizing antibodies reduced airway inflammation (Fig. 5E) and BAL cytokines (Fig. 5F) in recipient mice. However, Th2 cytokines concentrations were greater, and IL-9 amounts were less in BAL from Th2 cell recipient mice than from recipients of Th9 cells. The fact that anti-IL-9 can diminish airway inflammation in Th2 cell recipient mice suggests that even the low amounts of IL-9 found in these mice are important for inflammation.
TSLP acts directly on Th9 cells to enhance their function in vivo
The ability of TSLP to enhance Th9-mediated allergic lung inflammation in the transfer model could be through direct effects of TSLP on Th9 cells, or on other TSLP-responsive cells in the lung environment. TSLP can have direct effects on Th9 development (Fig. 2) and by enhancing TCR-stimulated IL-9 production from Th9 effector cells (Fig. 3F). To evaluate whether TSLP acted directly on Th9 cells in vivo, we transferred OVA-specific Th2 or Th9 cells into Tslpr−/− recipient mice followed by OVA or OVA+TSLP challenge for 5 days. Compared to PBS injected control mice, both Th2 and Th9 cell recipient mice showed increased inflammatory cellular infiltrates in BAL, and the presence of TSLP further elevated total cell and eosinophil counts in BAL (Fig. 6A). Further analysis of cytokines in BAL fluid demonstrated that increased Th2 cytokines were seen in recipients of either Th2 or Th9 cells after OVA+TSLP challenge, whereas enhanced IL-9 expression was only seen in mice that received Th9 cells (Fig. 6B). Similar to wild type BALB/c recipient mice (Figure 4), OVA+TSLP challenge resulted in exacerbated lung inflammatory cell infiltration and mucus production in both Th2 and Th9 cell recipient mice, even though recipients were deficient in TSLPR (Fig. 6C). These data indicate that TSLP increased cytokine expression in Th2 and Th9 cells by acting directly on T cells.
Figure 6. TSLP acts directly on transferred Th9 cells to enhance effector function.
(A – C) TSLP enhanced Th9 effector function in vivo. Naïve CD4+ T cells from DO11.10 mice were differentiated under Th2 and Th9 skewed conditions with OVA323–339 peptide and APC. Differentiated Th cells were injected to Tslpr−/− recipients via tail vein, with PBS injection as control. Mice were analyzed after challenge with OVA or OVA+TSLP for 5 days for (A) BAL total cell and eosinophil counts, (B) BAL cytokine concentration using ELISA, and (C) tissue pathology using hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) staining of paraffin-embedded lung sections.
All data represent mean ± SEM (n=5), and are representative of 2 experiments. *, p < 0.05.
IL-9 is required for TSLP-induced airway inflammation in vivo
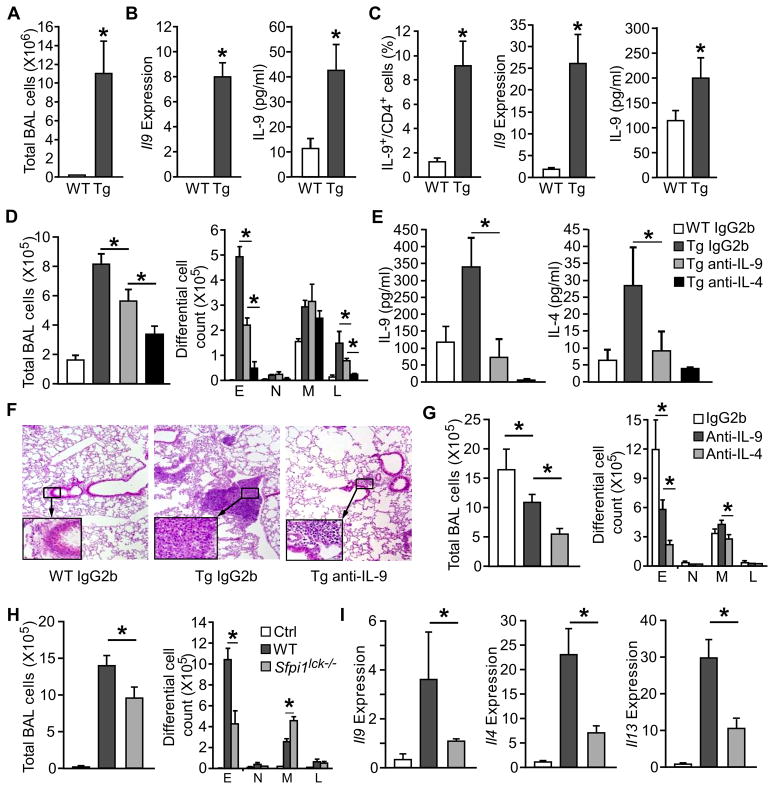
Lung-specific TSLP transgenic mice (SPC-TSLP mice) develop spontaneous allergic airway inflammation with increased Th2 cytokines, serum IgE and airway eosinophilia (Zhou et al., 2005). Considering the ability of TSLP to promote Th9 differentiation and function, we next wanted to determine if IL-9 is required for TSLP induced airway inflammation. In addition to airway leukocyte infiltration (Fig. 7A) and increased Th2 cytokines (Zhou et al., 2005), the SPC-TSLP mice showed increased Il9 expression by BAL cells and IL-9 concentration in BAL fluid (Fig. 7B), compared to littermate control mice. Similarly, anti-CD3-stimulated lung-draining mediastinal lymph node cells from SPC-TSLP mice had greater numbers of Th9 cells and expression of IL-9, compared to non-transgenic controls (Fig. 7C).
Figure 7. IL-9 is required for maximal TSLP-induced airway inflammation in TSLP-induced chronic and acute airway inflammation.
(A – C) BAL fluid, lung tissue, and lung-draining mediastinal lymph nodes were collected from 6 month old SPC-TSLP transgenic mice (Tg) and littermate controls (WT) and analyzed for, (A) cell number in the bronchoalveolar lavage (BAL) of transgenic and littermate control mice (B) Il9 gene expression in BAL cells and IL-9 present in the BAL fluid, and (C) IL-9 production by mDLN cells following stimulation with PMA/ionomycin (intracellular staining, left), or anti-CD3 stimulation (qPCR and ELISA, meddle and right).
(D – F) Neutralization of IL-9 in SPC-TSLP transgenic mice. Anti-IL-9 (10 μg/mouse), anti-IL-13 (10 μg/mouse), anti-IL-4 (50μg/mouse), or IgG2b isotype control (10 μg/mouse) were injected to SPC-TSLP and littermate control mice via tail vein twice a week for 4 weeks starting at 6 weeks of age. At 10 weeks of age, mice were analyzed for (D) total and differential cell counts in the BAL, (E) cytokine concentration in the BAL, and (F) lung histology by hematoxylin and eosin (H&E). WT, littermate controls; Tg: SPC-TSLP transgenic mice.
(G) Neutralization of IL-9 in TSLP-induced acute airway inflammation. BALB/c mice were treated intranasally every other day with 50 μg OVA and 500 ng TSLP for two weeks. Anti-IL-9 (10 μg/mouse), anti-IL-4 (50μg/mouse), or IgG2b isotype control were injected i.v. 30 min. before OVA+TSLP treatment. Total and differential cell counts in the BAL were analyzed 24 h after the last treatment.
(H – I) Role of Th9 cells in TSLP-induced acute airway inflammation. C57BL/6 and Sfpi1lck−/− mice were treated with OVA+TSLP as described in (G). Control group were C57BL/6 mice receiving OVA alone. 24 h after the last treatment, mice were analyzed for (H) total and differential cell counts in the BAL, and (I) cytokine gene expression in the lung by qPCR.
All data represent mean ± SEM (n=4–6 mice) and are representative of at least two separate experiments. *, p < 0.05.
To examine whether the increased IL-9 production contributed to airway inflammation in SPC-TSLP mice, transgenic and littermate control mice were treated with 10 μg/mouse anti-IL-9, 50 μg/mouse anti-IL-4, or control antibodies intravenously twice a week for 4 weeks beginning at 6 weeks of age, when the transgenic mice began to develop airway inflammation. We observed that pulmonary inflammation, assessed by total cell number and by number of eosinophils and lymphocytes in the BAL, was decreased in the anti-IL-9 treated SPC-TSLP mice, compared to IgG2b control antibody treated mice (Fig. 7D). Amounts of IL-9 and IL-4 were decreased in the BAL fluid after anti-IL-9 treatment in SPC-TSLP mice (Fig. 7E). Blocking IL-4 signaling not only reduced airway inflammation as we have previous shown (Zhou et al., 2008), but also dramatically decreased BAL IL-9 concentration as would be expected from the role of IL-4 in Th9 generation (Fig. 7E). Histopathological analysis confirmed that anti-IL-9 treatment attenuated lung inflammation with decreased peribronchial and perivascular accumulation of eosinophils and lymphocytes (Fig. 7F). Taken together, lung-specific TSLP overexpression induced IL-9 production, and IL-9 contributed to the induction of airway inflammation.
The transcription factor PU.1, encoded by Sfpi1 gene, is required for the development of Th9 cells but not Th2 cells (Chang et al., 2010). To delineate the contribution of Th9 cells in TSLP-dependent airway inflammation, we adapted a model where mice were treated intranasally with OVA and TSLP every other day for two weeks (Headley et al., 2009; Lei et al., 2011). In this TSLP-induced acute model, neutralization of IL-9 and IL-4 reduced airway inflammation and eosinophilia (Fig. 7G). The co-administration of OVA and TSLP, but not OVA alone, stimulates airway inflammation and cytokine expression (Fig. 7H). However, in mice with a conditional deletion of PU.1 in T cells (Sfpi1lck−/− mice) airway inflammation (Fig. 7H) and cytokine expression in the lung were diminished (Fig. 7I). Thus, Th9 cells are required for TSLP-induced allergic inflammation.
Discussion
TSLP plays important roles in regulating Th2 cell-mediated immunity and allergic diseases (Zhang and Zhou, 2012). TSLP functions by stimulating multiple cell types including dendritic cells, epithelial cells and mast cells (Allakhverdi et al., 2007; Fontenot et al., 2009; Ito et al., 2005; Semlali et al., 2010; Soumelis et al., 2002; Wang et al., 2006). In addition, TSLP, in conjunction with TCR stimulation, can act directly on naïve CD4+ T cells to promote cell proliferation and Th2 differentiation (Omori and Ziegler, 2007; Rochman et al., 2007; Sokol et al., 2008) while suppressing Treg differentiation (Lei et al., 2011). In this study, we report that TSLP acts on both human and mouse CD4+ T cells to promote Th9 differentiation and function. Based on data correlating TSLP and IL-9 serum levels in atopic infants, we tested the requirement for IL-9 in models of allergic inflammation. Neutralizing IL-9 attenuated SPC-TSLP transgene- and OVA+TSLP-induced allergic airway inflammation. TSLP and IL-9 have already been shown to be required in several models of allergic inflammation, including the OVA/alum and papain models (Al-Shami et al., 2005; Chang et al., 2010; Cheng et al., 2002; He et al., 2008; Kung et al., 2001; Wilhelm et al., 2011; Zhou et al., 2005). Our data now establishes that Th9 cells are an indispensable component of TSLP-mediated allergic inflammation.
STAT proteins are critical promoters of T helper cell differentiation, with individual or combinations of STAT proteins required for specific lineages (Stritesky and Kaplan, 2011). Th2 and Th9 cells require STAT6, although STAT3 is required for Th2 but not Th9 cell development (Dardalhon et al., 2008; Goswami et al., 2012; Stritesky et al., 2011; Veldhoen et al., 2008). STAT5 activation also plays an important role in Th2 cell differentiation and a constitutively active STAT5 can induce Th2 cell differentiation independent of IL-4 and STAT6 (Zhu et al., 2003). STAT5 binds to multiple cytokine genes including Th2 cytokines and Il9 (Cote-Sierra et al., 2004; Fung et al., 2005; Liao et al., 2008). This is consistent with our observations that STAT5 binds directly to the Il9 locus in Th9 cells. Moreover, reducing Stat5 expression eliminated the ability of TSLP to enhance anti-CD3-induced Il9 expression. STAT5 may additionally promote Th9 development by binding to genes encoding other transcription factors required for Th9 development, including Irf4 and Gata3. Th9 development is also dependent upon IL-2 (Houssiau et al., 1995; Schmitt et al., 1994), and IL-2 is clearly responsible for a proportion of pSTAT5 in developing Th9 cells. TSLP was capable of activating STAT5 and inducing IL-9 when IL-2 was blocked, suggesting that TSLP can function independently of IL-2. However, there is clearly an additive effect when cells are exposed to both cytokines. Thus, our data suggest that STAT5 contributes to Th9 differentiation and that it serves as an important conduit between TSLP stimulation and IL-9 production.
TSLP enhances the differentiation of Th2 and Th9 cells derived from either human or mouse naïve CD4 T cells. Recent studies reported that Th2 cells expressed more TSLPR than Th1 or Th17 cells (Kitajima et al., 2011), and TSLP was critical for antigen-induced Th2 cytokine secretion by skin-infiltrating Th2 effectors in an atopic dermatitis model (He et al., 2008). We found that both human and mouse Th9 cells expressed TSLP receptor chain equal to Th2 cells, and in mouse cells expression was further amplified by TSLP stimulation. Thus, Th9 effectors might be a critical TSLP receptor cell type. Indeed, TSLP doubled IL-9 production from anti-CD3 stimulated Th9 cells and from in vivo effectors. Thus, TSLP likely contributes to both differentiation and effector function of Th9 cells.
The development of allergic inflammation in the lung requires the cooperation of Th2 and Th9 cells. The division of labor between these subsets is still unclear. Previous experiments have suggested that IL-9 promotes type 2 cytokine production by innate lymphoid cells in the lung (Wilhelm et al., 2011) and IL-13-deficiency inhibits the allergic inflammation initiated by an IL-9 transgene (Temann et al., 2007). This was recapitulated in our studies showing that blockade of IL-13 diminished inflammation in Th9 cell recipient mice. Conversely however, blockade of IL-9 inhibited the development of inflammation in recipients of Th2 cells. This is consistent with the requirement for Th9 cells, established using mice with PU.1-deficient T cells, in models of allergic airway inflammation, even when Th2 cell development is normal (Chang et al., 2010). Thus, it is difficult to develop an ordered model of Th9 and Th2 cell function, and each subset likely contributes parallel but overlapping functionality in the development of allergic inflammation.
In summary, we have elucidated a mechanism of TSLP-mediated allergic airway inflammation through enhancing Th9 cell differentiation and IL-9 production. This function of TSLP is conserved between human and murine T helper cells. Together with the action of TSLP on Th2 cell differentiation, our data provide insight into the ability of TSLP to promote allergic disease. Given that both TSLP and Th9 cells are required for other types of inflammation (Jabeen and Kaplan, 2012; Zhang and Zhou, 2012), it will be important to establish if the link between TSLP and IL-9 is important for additional human diseases.
Experimental Procedures
Human Subjects
Children with chronic dermatitis (n=116) recruited for this study have previously been described (Tepper et al., 2008). Subjects were excluded for a history of prior wheezing, lower respiratory tract illness, treatment with asthma medications, or congenital heart disease. Patient sample collection and analysis were approved by the Institutional Review Board of Indiana University and required parental consent for samples from infants.
Mice
BALB/c, C57BL/6 and DO11.10 TCR transgenic mice were purchased from Jackson Laboratories. Spfi1-conditional mutant mice, Tslpr mutant (Tslpr−/−) mice and SPC-TSLP transgenic mice were described previously (Dakic et al., 2005; Zhou et al., 2005). All mice were housed in specific pathogen-free conditions and all experiments were performed as approved by the IACUC.
Multiplex analysis of patient serum cytokines
Venous blood (2 ml) was collected by venipuncture and serum was isolated. Patients were defined as atopic on the basis of positive allergen-specific serum IgE as described previously (Tepper et al., 2008; Yao et al., 2010; Yao et al., 2011). The concentration of IL-9 and TSLP in the serum was measured with the Milliplex MAP Kit according to the manufacturer’s protocol (Millipore) using a Luminex 200 system (Luminex) for data acquisition and analysis. Cytokine concentrations were calculated with Bio-Plex Manager 2.3 software with a five-parameter curve-fitting algorithm applied for standard-curve calculations.
Quantitative RT-PCR
Total RNA was isolated from anti-CD3 (2 μg/ml)-restimulated cells with Trizol and reverse transcribed according to manufacturer’s instructions (Invitrogen Life Technologies, Carlsbad, CA). Quantitative PCR was performed with Taqman Fast Universal PCR Master Mix and commercially available primers for genes with the 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). RNA was normalized to expression levels of β2-microglobulin and relative expression was calculated by the change-in-threshold (−ΔΔCT) method.
Helper T cell differentiation
Human and mouse Th cell differentiation was performed as described (Chang et al., 2010; Yao et al., 2011). After 5–6 d of culture, differentiated cells were restimulated for 24 h with plate-bound anti-CD3 (2 μg/ml), and cell-free supernatants collected after centrifugation were stored at -20 °C for measuring cytokine production. In some experiments, cell pellets were collected after 6 h of restimulation for RNA extraction. TSLPR expression was examined by flow cytometry (Biolegend).
Naïve OVA-specific CD4+ T cells from DO11.10 mice were purified from spleens and lymph nodes as described above and were stimulated with 10 μg/ml OVA323–329 peptide and mitomycin-treated, CD4+ T cell-depleted APCs (1:5) in the presence of anti-CD28 (0.5 μg/ml), anti-IFN-γ (10 μg/ml), IL-4 (10 ng/ml), without (Th2 cell differentiation) or with 2 ng/ml TGF- β1 (Th9 cell differentiation). On day 3 of culture, cells were expanded by adding half the dose of the original cytokines in fresh medium. After 5 days of culture, differentiated cells were analyzed by intracellular staining before adoptive transfer.
Intracellular staining
After re-stimulation with anti-CD3 (4 μg/ml) for 5 h, monensin was added to the cells for the final 2 h of stimulation. Cells were xed with 4% neutral buffered paraformaldehyde and permeabilized with saponin. Cells were then stained with uorochrome-conjugated anti-mouse IL-9, IL-13, CD4 and KJ1.26 (eBioscience). For intracellular phospho-STAT staining, T cells were collected and xed with 1.5% formaldehyde for 10 min at room temperature. After xation, cells were permeabilized for 15 min at 4 °C with 100% methanol. Cells were then stained for phospho-STAT5 or phospho-STAT6 (BD PharMingen) for 30 min at room temperature.
Chromatin immunoprecipitation
ChIP assay was performed as previously described (Yu et al., 2007). Immunoprecipitations were performed with rabbit polyclonal antibodies (rabbit control IgG (Santa Cruz), AcH3, AcH4, AcH3K9–18, and H3K27me3 (Upstate)) or mouse monoclonal antibodies (mouse IgG and anti-Stat5a/b (Upstate)). Quanti cation of binding DNA was performed with SYBR Green Fast PCR Master Mix (See Table S1 for primer sequences) via ABI 7500 Fast Real-time PCR System. To quantify immunoprecipitated DNA, a standard curve was generated from serial dilutions of input DNA. To calculate ChIP results as a percentage of input, the amount of the immunoprecipitated DNA from the IgG control was subtracted from the amount of the immunoprecipitated DNA from the specic antibody ChIP followed by normalizing against the amount of the input DNA.
siRNA silencing of STAT5
Th9 cells were transfected with control or Stat5-specific siRNA (Santa Cruz Biotechnology) with a Mouse T Cell Nucleofector kit according to the manufacturer’s instructions (Amaxa Biosystems). T cells (5 × 106) were suspended in Mouse T cell Nucleofector Solution and were transfected with 1 μg siRNA usingthe X -001 transfection program (Amaxa). Transfected cells were plated in pre-warmed culture media at 37 °C. After restingfor 24 h, cells were stimulated and RNA was collected for quantitative PCR.
Adoptive transfer experiments and cytokine neutralization
Differentiated OVA-specific Th2 or Th9 cells (2–3 × 106T cells in 200 μl PBS) were adoptively transferred into wild-type or Tslpr-deficient recipient mice via tail vein injection. Twenty-four hours after cell transfer, mice were challengedintranasally (i.n.) with 100 μg OVA or 100 μg OVA plus 500 ng TSLP for 5 days. Mice were then sacrificed 24 h after last challenge for further analysis.
To neutralize cytokine inrecipient sof Th2 or Th9 cells, mice were injected via tail vein with anti-IL-9 (10 μg/dose), anti-IL-4 (50 μg/dose), anti-IL-13 (10 μg/dose), or IgG2b control Ab (10 μg/dose, R&D Systems)on days 1, 3 and 5. To neutralize cytokines in SPC-TSLP transgenic mice, anti-IL-9 (10 μg/dose), anti-IL-4 (50 μg/dose), or IgG2b control Ab (10 μg/dose) were injected via tail vein twice a week for 4 weeks from 6 weeks of age.Mice were sacrificed 24 h after last injection for further analysis.
Bronchoalveolar lavage, tissue fixation, and staining
Mice were euthanized, and bronchoalveolar lavage (BAL) was performed as described previously (Lei et al., 2011). Briefly, lungs were washed three times with cold PBS. BAL fluid fractions were centrifuged at 1400 × g for 5 min at 4 °C. Pellets were resuspended and counted. Cytospin preparations were stained with modified Wright-Giemsa stain, and differential cell counts were evaluated by counting at least 200 cells for determination of relative percentage of each cell type in the BAL. The cytokine levels in BAL fluid were assessed by ELISA or Multiplex assay as indicated. After lavage, lungs were excised from thorax cavity, inflated with 4% neutral buffered formaldehyde, and fixed overnight at room temperature. Tissues were embedded in paraffin, sectioned, and stained with H&E or periodic acid-Schiff (PAS) stain.
Isolation and restimulation of cells from lung draining lymph nodes
Mediastinal draining lymph nodes were dissected from sensitized and challenged mice, and single-cell suspensions were prepared before stimulation in vitro with plate-bound 2 μg/ml anti-CD3 (17A2; Biolegend) for 72 h. Cytokine levels in cell-free supernatants were measured using ELISA. The amount of IL-9 produced was determined by ELISA with anti-IL-9 (D8402E8; BD Biosciences) as the capture antibody and biotin-labeled anti-IL-9 (D9302C12; BioLegend) as the secondary antibody.
Data and statistical analysis
Data are presented as mean ± SEM. ANOVA with Bonferroni posttests and Student t test were performed with Prism version 4.00 (GraphPad). Spearman’s correlation was performed on serum cytokine values, excluding samples where either cytokine was undetectable in multiplex assays.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AI057459 to MHK and R01 AI085046 to BZ. Support provided by the HB Wells Center was in part from the Riley Children’s Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Arima M, Honda K, Hirata H, Eda F, Yoshida N, Fukushima F, Ishii Y, Fukuda T. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002;166:409–416. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201:1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, Krug N. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J Allergy Clin Immunol. 2003;111:1319–1327. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- Fontenot D, He H, Hanabuchi S, Nehete PN, Zhang M, Chang M, Nehete B, Wang YH, Ma ZM, Lee HC, et al. TSLP production by epithelial cells exposed to immunodeficiency virus triggers DC-mediated mucosal infection of CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:16776–16781. doi: 10.1073/pnas.0907347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MM, Chu YL, Fink JL, Wallace A, McGuire KL. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene. 2005;24:4624–4633. doi: 10.1038/sj.onc.1208507. [DOI] [PubMed] [Google Scholar]
- Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, Kaplan MH. STAT6-Dependent Regulation of Th9 Development. J Immunol. 2012;188:968–975. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiau FA, Schandene L, Stevens M, Cambiaso C, Goldman M, van Snick J, Renauld JC. A cascade of cytokines is responsible for IL-9 expression in human T cells. Involvement of IL-2, IL-4, and IL-10. J Immunol. 1995;154:2624–2630. [PubMed] [Google Scholar]
- Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol. 2012;24:303–307. doi: 10.1016/j.coi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-β promote TH9 cell–mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129:1000–1010. e1003. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41:1862–1871. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung TT, Luo B, Crawley Y, Garlisi CG, Devito K, Minnicozzi M, Egan RW, Kreutner W, Chapman RW. Effect of anti-mIL-9 antibody on the development of pulmonary inflammation and airway hyperresponsiveness in allergic mice. Am J Respir Cell Mol Biol. 2001;25:600–605. doi: 10.1165/ajrcmb.25.5.4533. [DOI] [PubMed] [Google Scholar]
- Lei L, Zhang Y, Yao W, Kaplan MH, Zhou B. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186:2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting Edge: Direct Action of Thymic Stromal Lymphopoietin on Activated Human CD4+ T Cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- Semlali A, Jacques E, Koussih L, Gounni AS, Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125:844–850. doi: 10.1016/j.jaci.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Kaplan MH. Changing the STATus quo in T helper cells. Transcription. 2011;2:179–182. doi: 10.4161/trns.2.4.16614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- Tepper RS, Llapur CJ, Jones MH, Tiller C, Coates C, Kimmel R, Kisling J, Katz B, Ding Y, Swigonski N. Expired nitric oxide and airway reactivity in infants at risk for asthma. J Allergy Clin Immunol. 2008;122:760–765. doi: 10.1016/j.jaci.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Barbe-Tuana FM, Llapur CJ, Jones MH, Tiller C, Kimmel R, Kisling J, Nguyen ET, Nguyen J, Yu Z, et al. Evaluation of airway reactivity and immune characteristics as risk factors for wheezing early in life. J Allergy Clin Immunol. 2010;126:483–488. e481. doi: 10.1016/j.jaci.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011;128:1357–1360. e1355. doi: 10.1016/j.jaci.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy. 2002;32:866–871. doi: 10.1046/j.1365-2222.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Thieu VT, Kaplan MH. Stat4 limits DNA methyltransferase recruitment and DNA methylation of the IL-18Ralpha gene during Th1 differentiation. The EMBO journal. 2007;26:2052–2060. doi: 10.1038/sj.emboj.7601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunologic research. 2012;52:211–223. doi: 10.1007/s12026-012-8264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, Smedt TD, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of Thymic Stromal Lymphopoietin-Induced Airway Inflammation through Inhibition of Th2 Responses. J Immunol. 2008;181:6557–6562. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 Activation Plays a Critical Role in Th2 Differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.