Abstract
Functional relationships (from systematic manipulation of critical variables) are advocated for revealing fundamental processes of (comparative) cognition—through examples from my work in psychophysics, learning, and memory. Functional relationships for pigeon wavelength (hue) discrimination revealed best discrimination at the spectral points of hue transition for pigeons—a correspondence (i.e., functional relationship) similar to that for humans. Functional relationships for learning revealed: Item-specific or relational learning in matching to sample as a function of the pigeons’ sample-response requirement, and same/different abstract-concept learning as a function of the training set size for rhesus monkeys, capuchin monkeys, and pigeons. Functional relationships for visual memory revealed serial position functions (a 1st order functional relationship) that changed systematically with retention delay (a 2nd order relationship) for pigeons, capuchin monkeys, rhesus monkeys, and humans. Functional relationships for rhesus-monkey auditory memory also revealed systematic changes in serial position functions with delay, but these changes were opposite to those for visual memory. Functional relationships for proactive interference revealed interference that varied as a function of a ratio of delay times. Functional relationships for change detection memory revealed (qualitative) similarities and (quantitative) differences in human and monkey visual short term memory as a function of the number of memory items. It is concluded that these findings were made possible by varying critical variables over a substantial portion of the manipulable range to generate functions and derive relationships.
Keywords: comparative cognition, psychophysics, learning, memory, pigeons, monkeys, humans
This article was inspired by suggestions from the nominating committee for a research-award presentation (2012 Comparative Cognition Society meeting). These suggestions included: Organize your presentation (and this article) around a theme that has guided your work (hence the title), include earlier work that some (younger) members of the audience may be unfamiliar, and mention individuals that have influenced your work. Therefore, I begin by mentioning individuals that shaped my interests and thinking, followed by: A psychophysical study of pigeon color vision; two concept learning studies with pigeons and monkeys; visual list memory studies with pigeons, monkeys and humans; auditory list memory studies with monkeys; a proactive-interference memory study with pigeons; and a visual short-term memory study with monkeys and humans in change detection.
As an undergraduate at Stanford University, I was greatly influenced by Gordon H. Bower (see Figure 1). I became interested in experimental psychology and comparative cognition from an introductory psychology course, taught by Richard C. Atkinson with the introductory text book written by Stanford professor Ernest R. Hilgard. Hilgard and Atkinson were deeply involved in learning and emphasized animal learning and comparative studies of learning. My laboratory introduction was an animal learning course taught by Gordon Bower who recently had arrived from Yale University where he conducted his graduate work with Neal Miller (see Bower, 2011). Bower’s learning course was a revelation for me. I and the other students in the course conducted a series of experiments with our own rat and were introduced to learning issues, many of which are still actively pursued today. Following the learning course, I conducted a research project with Professor Bower related to his influential learning model (Bower, 1962), involving different item types, conditions, and interference (a topic revisited later in this article). When it came time for me to apply for graduate studies, Gordon advised me to go to Columbia University which had strong programs in experimental psychology.
Figure 1.
Gordon Bower: undergraduate mentor at Stanford University.
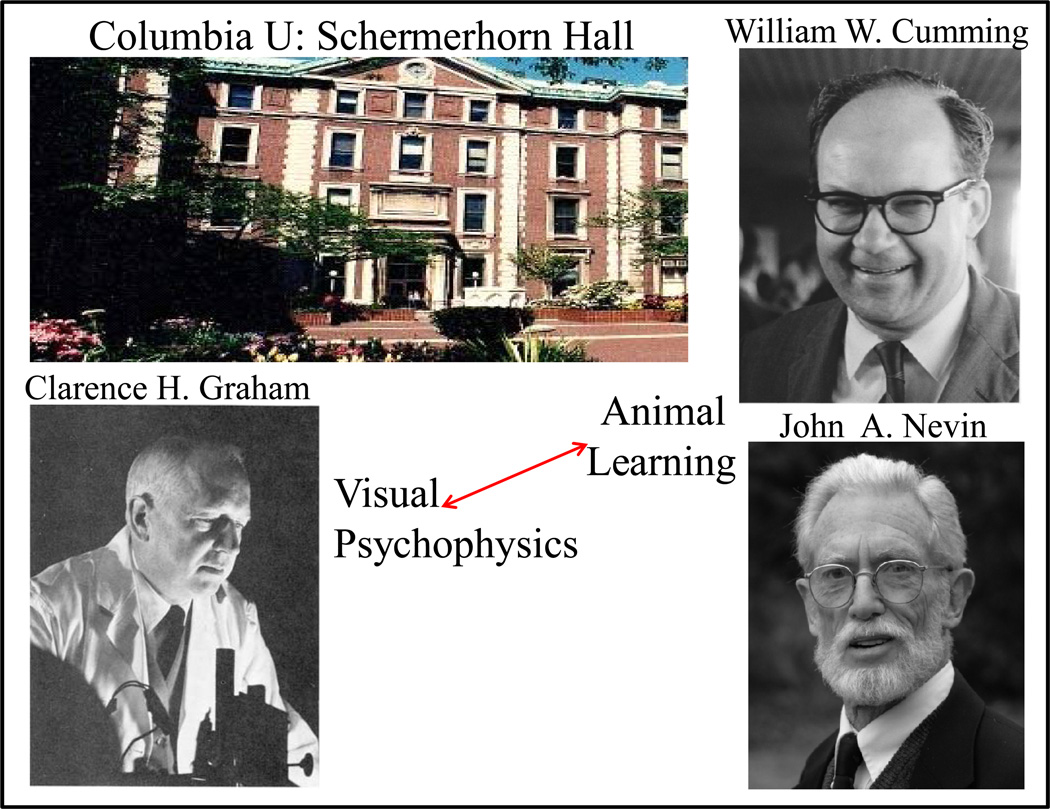
I arrived at Columbia University in 1965. The psychology department was located in Schermerhorn Hall (since its inception in 1898, and still is, see Figure 2). The inscription above the doorway reads "for the advancement of NATURAL SCIENCE ‘speak to the earth and it shall teach thee’". Research at Columbia was concentrated in two areas of experimental psychology (legacies of James McKeen Catell, the first Chair of Psychology, and Robert Sessions Woodworth, Catell’s student). One area was visual psychophysics—headed by Professor Clarence H. Graham. The other area was animal learning which included William (Bill) W. Cumming (with whom I began my studies), William N. (Nat) Schoenfeld, and Herbert (Herb) S. Terrace (who recently had arrived from Harvard where he worked with B. F. (Fred) Skinner). Two years later John A. (Tony) Nevin arrived (with whom I completed by studies, following the unexpected death of Professor Cumming). Since I was interested in visual psychophysics as well as animal learning, much of my graduate work combined these fields in animal psychophysical studies with encouragement and support from these professors at Columbia.
Figure 2.
Professor Graham and graduate sponsors Bill Cumming and Tony Nevin at Columbia University. Arrow indicates combining these fields for animal psychophysical studies.
Functional Relationships for Investigating Psychophysical Processes
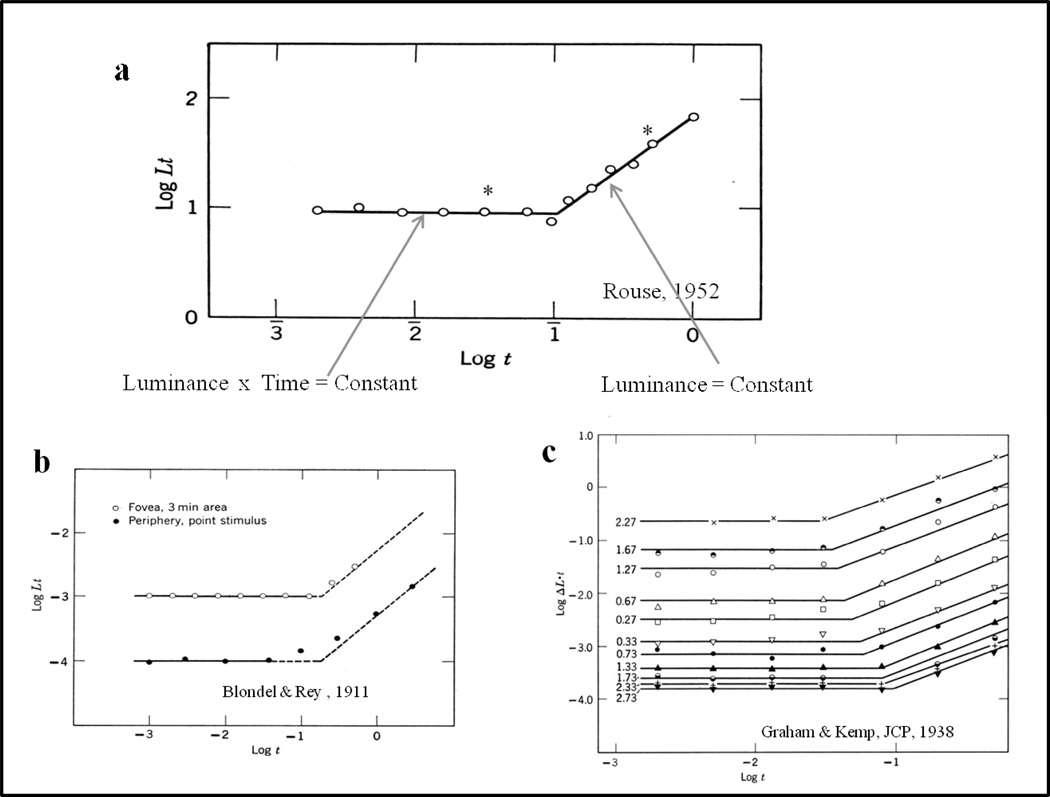
At Columbia I took courses in visual psychophysics from Professor Graham who had just published his seminal book: Vision and Visual Psychophysics. Psychophysics was to me the embodiment of systematically varying critical variables to reveal functional relationships and identify lawful relationships. An example of the type of functional relationships I am referring to is shown in Figure 3. This example serves as a prototype for the approach that, I as a second year graduate student, wanted to bring to the investigation of comparative cognitive processes. Figure 3a shows the required intensity of a visual stimulus presented for a fixed amount of time to the fovea for seeing. The left-hand (horizontal) limb of the function shows the lawful relationship: log luminance multiplied by time is constant—up to the ‘knee’ of the function. “In a word” (as Professor Graham was fond of saying) photoreceptors integrate light intensity that falls upon them up to a certain time (the ‘knee’ in the function) and is known as Bloch’s law (or Bunsen-Roscoe law). After the ‘knee’ there is a second limb to the function; this second limb shows log luminance being constant.
Figure 3.
(a) Functional relationships for an example from human psychophysical detection of light in the fovea, (b) the periphery, (c) and contrast changes from different backgrounds. These functional relationships show that the product of luminance and time was constant for the left-hand ‘limb’ and luminance was constant for the right-hand ‘limb’ in each condition.
Among aspects that impressed me about those functional relationships shown in Figure 3 was my realization that if researchers had studied only one or two points (e.g., the two points with an asterisk in Figure 3a), it is unlikely that these functional relationships would have been discovered. These functional relationships for the fovea extend to peripheral vision (Figure 3b) and to different levels of adaptation (Figure 3c), showing that they are basic relationships for seeing and determining vision and how photoreceptors work.
Pigeon Color-Vision Psychophysics
As an enthusiastic graduate student, I wanted to generate functional relationships for color discrimination (wavelength or hue) of a nonhuman animal, the pigeon (the primary laboratory animal at Columbia), over a substantial range of the pigeon’s visible spectrum. And…I wanted to compare these discrimination results to the pigeon’s category hue boundaries to see if similar relationships would be shown as had been shown for humans. At that time, all psychophysical experiments were conducted with hardware optical systems (e.g., lenses, mirrors etc.). I took an optical engineering graduate course at Columbia, designed my optical apparatus, came up with an inexpensive method to produce controllable narrow-band wavelengths of light (Wright, 1972a), and machined the hardware to mount the lenses, mirrors, light source, and interference filters to control wavelengths (Figure 4a).
Figure 4.
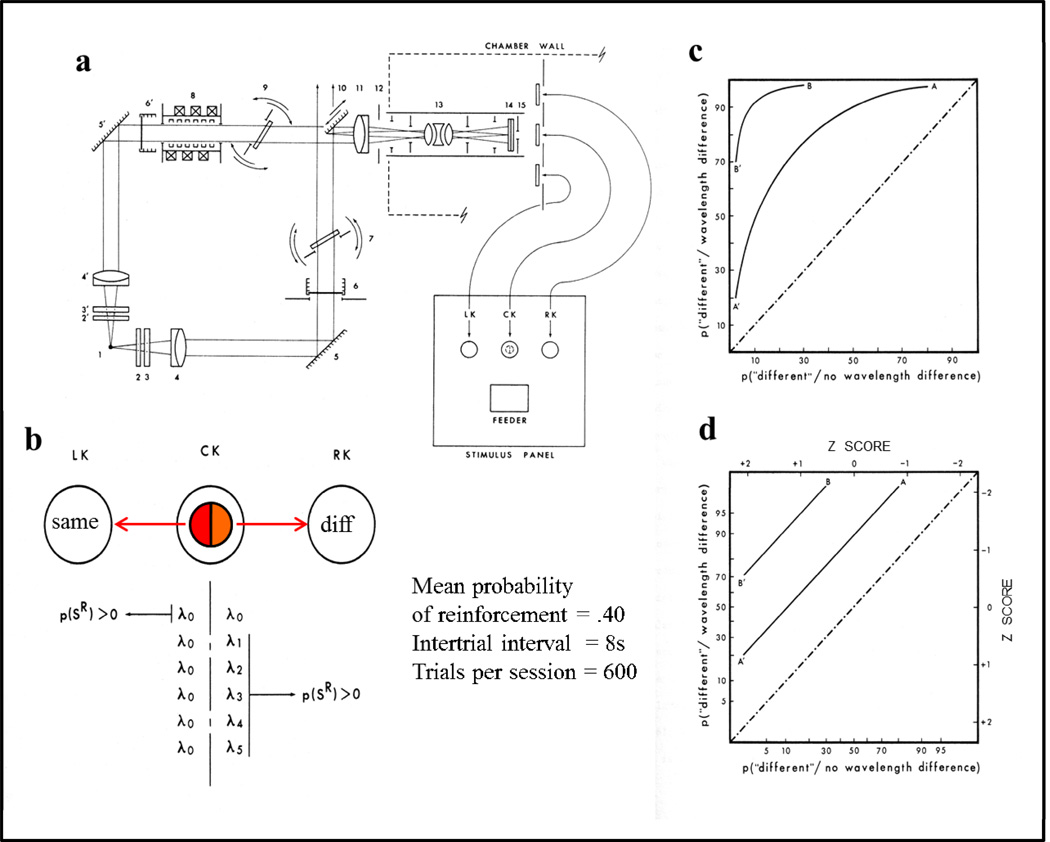
a) Top view of apparatus and (b) procedure for studying pigeon color (hue) discrimination including a split field of two wavelengths of light, same/different ‘report’ pecking keys, and variable reinforcement probability (for correct responses) to generate receiver operating characteristics (ROCs) on (c) linear and (d) z-score axes.
The procedure was a same/different task. The pigeons viewed a split field as shown in Figure 4b. A standard or reference wavelength was presented on the left half of the field. A comparison wavelength was presented on the right half of the split field. On trials where the comparison wavelength was equal to the standard wavelength (16.7% of the trials), the correct response was to peck the left (“same”) response key. On trials where the comparison wavelength was different from the standard wavelength, the correct response was to peck the right (“different”) key. Five wavelength differences and a same-wavelength condition were intermixed within each 600-trial session. The probability of reinforcement (mixed grain access) was set to 0.40 for correct responses, but varied systematically across sessions to generate receiver operating characteristics (ROCs).
Two hypothetical ROC functions are shown in Figure 4c plotted on the unit square (linear % correct) with Hits being correct identifications of wavelength (color) differences and False Alarms being incorrect identifications (different) when there was no wavelength difference (same). Each session produced one point on each of five ROCs, one point for each of the five wavelength differences tested. By plotting the ROCs on z-score (standard deviation) scales, they become linear (as they should according to signal detection theory) shown in Figure 4d.
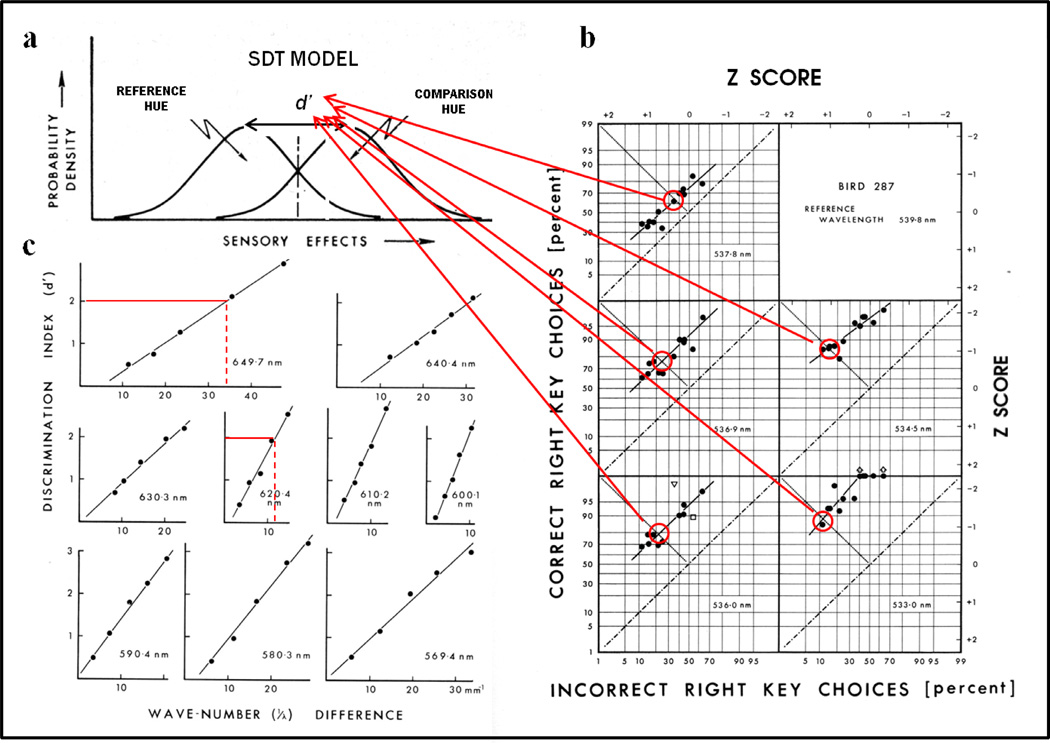
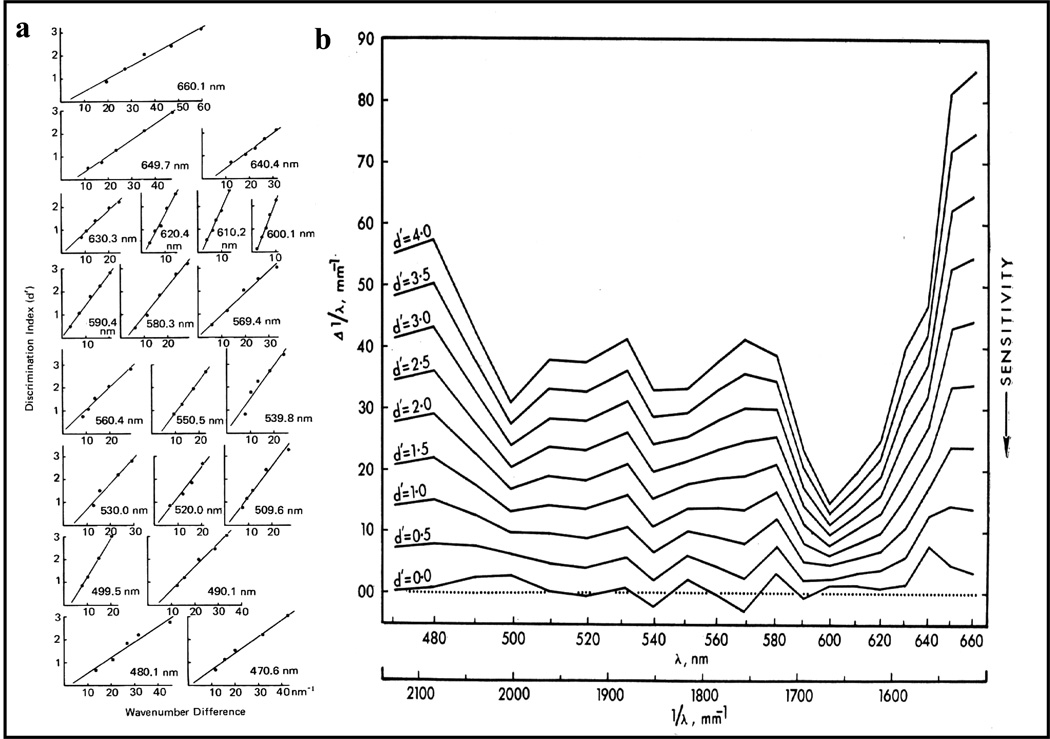
Figure 5a shows an example from one (out of 4) pigeon, at one (out of 20) reference wavelength (539.8 nm). Only a few nanometers separated the comparison wavelengths from this standard wavelength, demonstrating that hue discrimination was excellent at this spectral point. Linear ROCs of unit slope have the same value of discriminability (d’) at all points along the ROC function, but any difference from unit slope will yield different measures of d’. Therefore, d’ was computed at the point where the ROC crossed the negative diagonal, a point of neutral bias as shown in Figure 5b where the proportion of Hits and Correct Rejections are equal (Green & Swets, 1966). These d’ values for equal bias (sometimes referred to as d’s) were computed for the 5 wavelength differences, at each of the 20 reference wavelengths, for the 4 pigeons in this study (Wright, 1972b).
Figure 5.
(a) Computation of d’ values according to signal detection theory for examples (b) of equal-bias points (circled) from bird 287’s linear ROCs on z-score axes. (c) Psychometric functions of d’ as a function of wave number (reciprocal of wavelength) difference for 9 reference wavelengths and examples (reference wavelengths of 649.7 & 620.4 nm) for calculating the wave-number differences for the performance criterion of d’=2.0.
The resulting d’ values were then plotted as a function of wavelength difference between the standard and comparison wavelengths as shown for the 9 standard-wavelength examples in Figure 5c. The psychometric functions shown in Figure 5c are approximately linear with only small to negligible intercepts and the slopes of these functions change systematically with the standard wavelength. These psychometric functions shown in Figure 5c can be thought of as second-order functional relationships because they were derived from the ROCs (first-order functional relationships, Figure 5a). Moreover, from these psychometric functions, psychophysical functions were derived in order to more directly compare the pigeon’s hue discrimination. For example, notice the steep slope (i.e., sharply rising accuracy) as the wavelength difference increases for the function on the middle right (600.1 nm), compared to the shallow rise in accuracy for the upper most function (649.7 nm). The psychometric functions were intersected at fixed performance values (criteria) to compare hue discrimination across the pigeon’s visible spectrum. Two examples are shown in Figure 5c for a performance criterion of d’= 2.0. For reference wavelengths of 649.7 nm and 620.4 nm, horizontal lines are drawn at d’= 2.0. At the point where they intersect the psychometric functions specifies the wave-number difference (wave-number is the reciprocal of wavelength) for this performance criterion. By repeating this computation at all 20 of the standard (reference) wavelengths tested resulted in a psychophysical function showing how hue discrimination changes across (that portion of) the visible spectrum. And, of course, by using multiple d’ criteria—a matrix of psychophysical functions was produced.
The entire set of 20 psychometric functions for pigeon 287 is shown in Figure 6a. Those from the other three pigeons were similar (Wright, 1972b, 1978). Mean wave-number differences for the 4 subjects at nine different performance (d’) criteria are shown as a matrix of psychophysical functions in Figure 6b (Wright, 1974). Dips in the psychophysical functions show points of good hue discrimination. These psychophysical functions can be thought of as 3rd order functional relationships because they are derived from the 2nd order psychometric functional relationships (e.g., Figure 6a). Notice the pronounced dip at 600 nm. At this spectral point, the pigeon’s hue discrimination is much better than the human’s. (Indeed, I could not see hue differences at this spectral point that the pigeons were regularly discriminating and therefore had to check the dials to make sure the apparatus was working properly before each test session.)
Figure 6.
(a) All 20 psychometric functions for bird 287. (b) Mean psychophysical hue discrimination functions for the group of 4 pigeons at 9 d’ criteria values showing best discrimination at spectral points of 600, 540, and 500 nm.
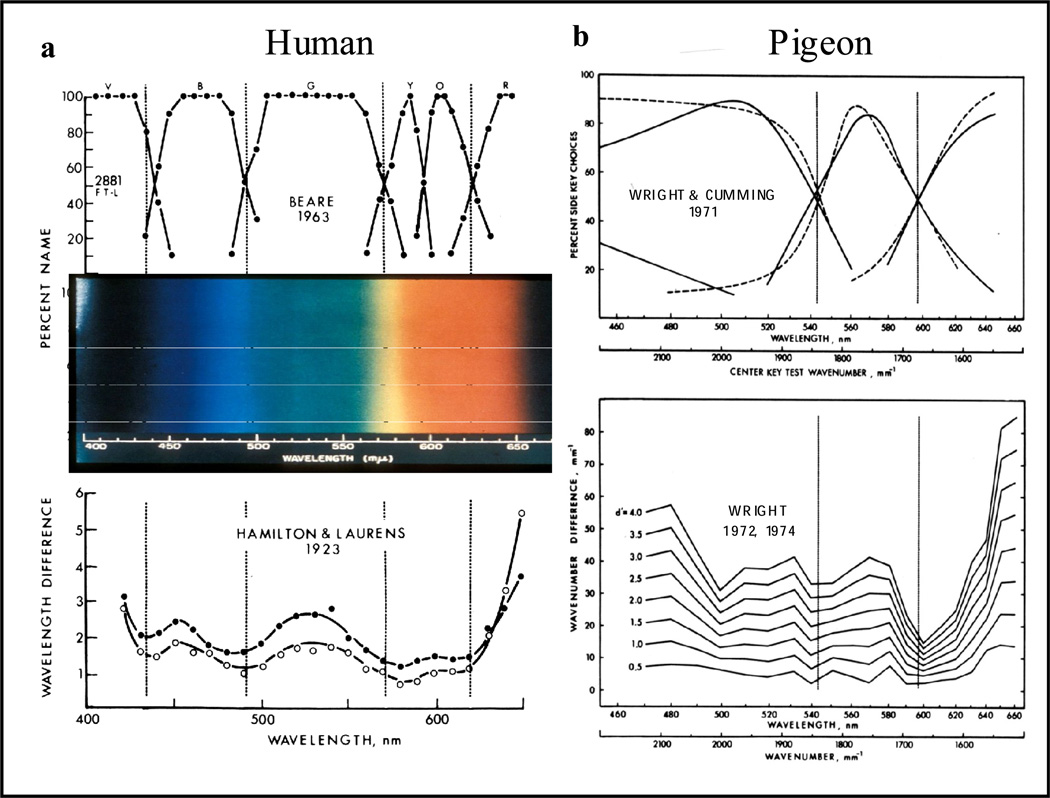
Points of good hue discrimination occur at wavelengths where there is a transition between hues (e.g., blue to green for humans). Figure 7a (top left) shows transitions between human hues, identified by human color naming. These points of human hue transition line up with the dips in the human wavelength discrimination function (Figure 7a, bottom left). The overlaid wavelength spectrum, as seen by humans, emphasizes that it is the points of hue transition that produce the best hue discrimination. Using these relationships from human color vision, I wanted to test for similar functional relationships with pigeons. I and my sponsor Bill Cumming had developed a color-naming procedure for pigeons using a matching-to-sample procedure with 3 horizontally aligned pecking keys (Wright & Cumming, 1971). Pigeons learned matching to sample with three different wavelength stimuli and then were transfer tested with intermediate test wavelengths as samples on the center pecking key. The idea was to have the pigeons identify which of two (side key) training wavelengths/colors was more like the test color (center key). At test wavelengths where choices switched from one training wavelength to the other were spectral points of hue transition for the pigeon. These hue-switch points remained invariant over changes in the three training wavelengths revealing two spectral points (542 and 598 nm) of pigeon hue transition (Figure 7b). These points of hue transition line up with two of the minima of the pigeon’s hue discrimination function, a similar relationship as shown for humans. This comparison of color naming and hue discrimination for pigeons can be thought of as 4th order functional relationship (1st order—ROCs; 2nd order—psychometric functions of d’ vs. wave-number difference; 3rd order—psychophysical functions of wave-number difference for different d’ criteria; 4th order—hue-discrimination and color-naming comparisons).
Figure 7.
(a) Human color naming showing transitions between hues corresponding to points (dips) of best human hue discrimination. (b) Pigeon color naming from a matching-to-sample wavelength generalization experiment showing the correspondence of transitions between three of the pigeon hues to two of the points of best pigeon hue discrimination.
Taken together these functional relationships show qualitative similarity between color naming and hue discrimination for both pigeons and humans. But there are quantitative differences; the spectral points of hue transition for pigeons are different than those for humans reflecting differences in photopigments and photoreceptors between these two species.
Functional Relationships for Investigating Learning
These functional relationships for pigeon color vision were a prototype for my studies in learning. The logic is similar. But how to generate functional relationships for learning, seemed somewhat less obvious than for psychophysics. Take the issue of animal concept learning. Researchers for years had trained pigeons in matching-to-sample tasks and then tested them for transfer to novel colors. But these studies yielded little or no evidence for abstract-concept learning, prompting comparative-cognition theorists to conclude that pigeons did not have the cognitive ability to learn abstract concepts (D’Amato et al., 1985, Premack, 1983; Thomas, 1980, 1996; Thompson, 1995). None of these “failure-to-find” studies, however, had generated functional relationships. From the advantage point of hindsight, this is an issue not unlike those encountered in the early stages of visual psychophysics. One first has to identify some critical variable (or variables) before mapping out a functional relationship (cf., Kamil, 1988)—which brings us to cognitive processes and the topic of functional relationships for concept learning (and memory processing to be discussed later).
Matching-to-Sample Learning: Pigeons
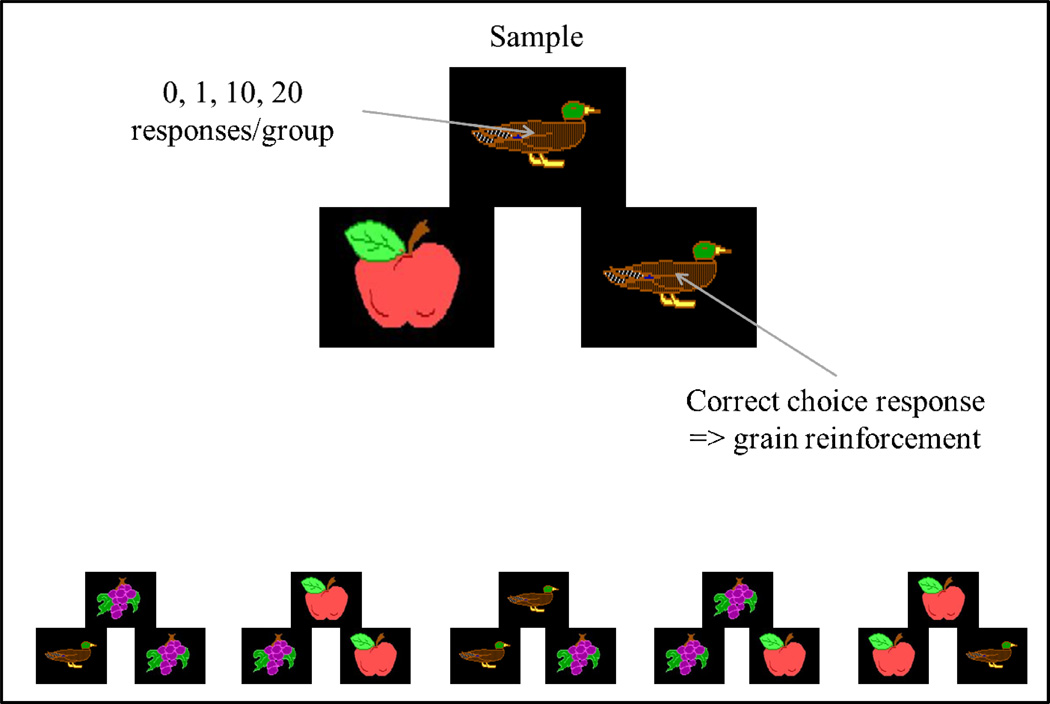
A matching-to-sample study serves as an example for what a functional relationship may tell us about learning (Wright, 1997). Four groups of pigeons made either 0, 1, 10, or 20 responses to the sample stimulus (cartoon) prior to being presented with two comparison/choice cartoons (see Figure 8). Other training displays are shown at the bottom of Figure 8 for one of the two subgroups of each response group. (The other subgroup had right and left positions of comparison cartoons reversed.) A peck to the picture that matched the sample was followed by mixed grain being placed on top of the chosen picture (Figure 9a). The cartoon pictures were presented on the floor of the test chamber by tipping the video monitor on its back so that the screen pointed up, the rationale being that placing grain reinforcement on top of the correct picture might enhance learning.
Figure 8.
Matching-to-sample procedure for 4 groups (4 pigeons/group) with different sample-response requirements to test item-specific and relational learning with combinations of duck, apple, and grape cartoons. Other training displays for one of the two subgroups are shown at the bottom of the figure.
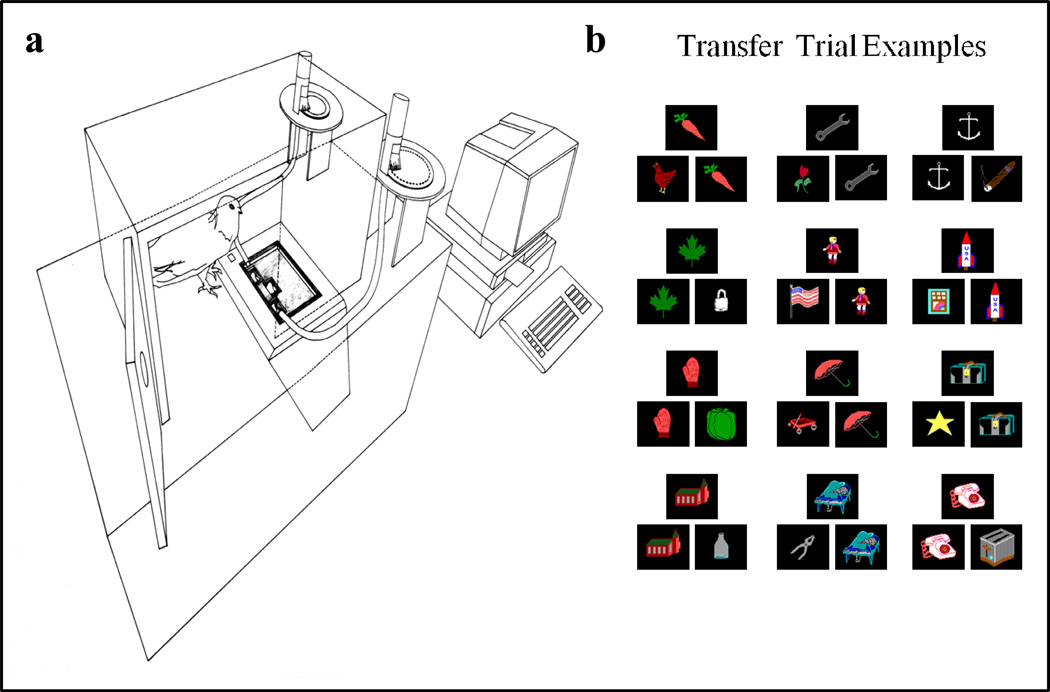
Figure 9.
Left: Apparatus for the matching-to-sample experiment (Figure 8) with the stimuli projected from the floor and mechanical systems to deliver reinforcement grain on top of correct choice stimuli. Right: Examples of novel-stimulus transfer trials.
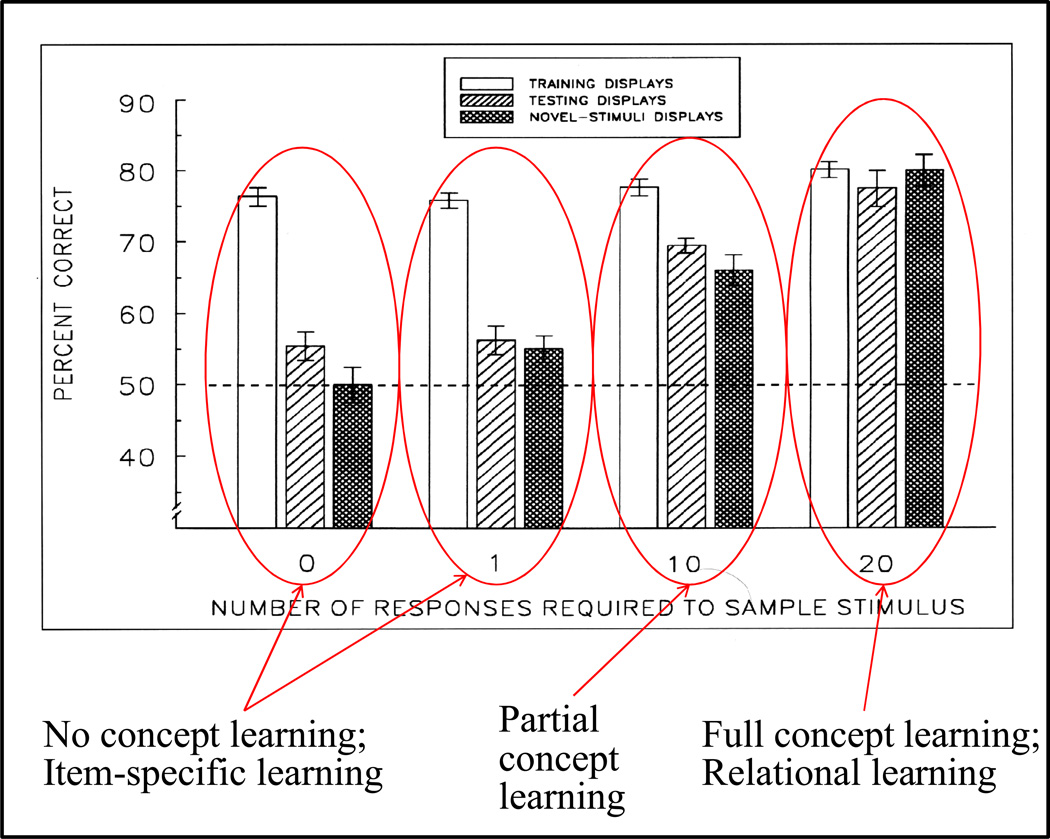
All groups learned the task to a high accuracy levels shown in each left-hand (unfilled) histogram for the four groups in Figure 10. The critical difference between groups was the transfer to novel stimuli shown by the right-hand (dark filled) histograms. (There were five transfer sessions, each contained 84 training trials and 10 transfer trials; transfer-trial examples are shown in Figure 9b.) The two groups on the left side of Figure 10 (0-, 1-response groups) showed little or no novel-stimulus transfer. Therefore, if this experiment had been conducted only with these two groups (one required sample response had been typical for most pigeon matching-to-sample experiments), then the conclusion might have been that pigeons do not learn the concept of matching and instead learn the matching task item specifically. (Configural learning, a type of item-specific learning, was shown by the lack of transfer to training displays of the other subgroup, see Wright, 1997 for further details).
Figure 10.
Results from the four different groups of pigeons trained with either 0, 1, 10, or 20 sample pecks prior to the presentation of the choice cartoons. The first bar (unfilled) for each group shows the group baseline training accuracy during transfer testing. The most important transfer result is the right-hand bar for each group which shows novel transfer accuracy and conclusions that can be drawn from the results of each group.
Nevertheless, by including a 10-response group, partial novel-stimulus transfer was shown for pigeons (Figure 10). Had this group been the only group trained and tested, then the conclusion might have been that pigeons can only partially learn the abstract concept of matching. Yet, by adding still another group, the 20-response group, it became clear that pigeons do indeed have the cognitive ability to fully learn the abstract concept of matching. For the 20-response group there was no statistical difference between transfer and baseline performance, showing that they had fully learned the concept. Needless to say, if this had been the only group tested then the conclusion would have been that pigeons learn abstract concepts fully, just like nonhuman primates.
Together, this continuum of sample response effects shows a functional relationship that determines the type of learning—from item-specific learning (i.e., no concept learning) to relational learning and eventually to full concept learning. The partial concept learning of the 10-reponse group is a transition group, likely a blend of these two types of learning (individual subjects showed partial transfer, not the result of a group average of some with complete transfer and others with no transfer).
Same/Different Learning: Pigeons, Rhesus, Capuchins
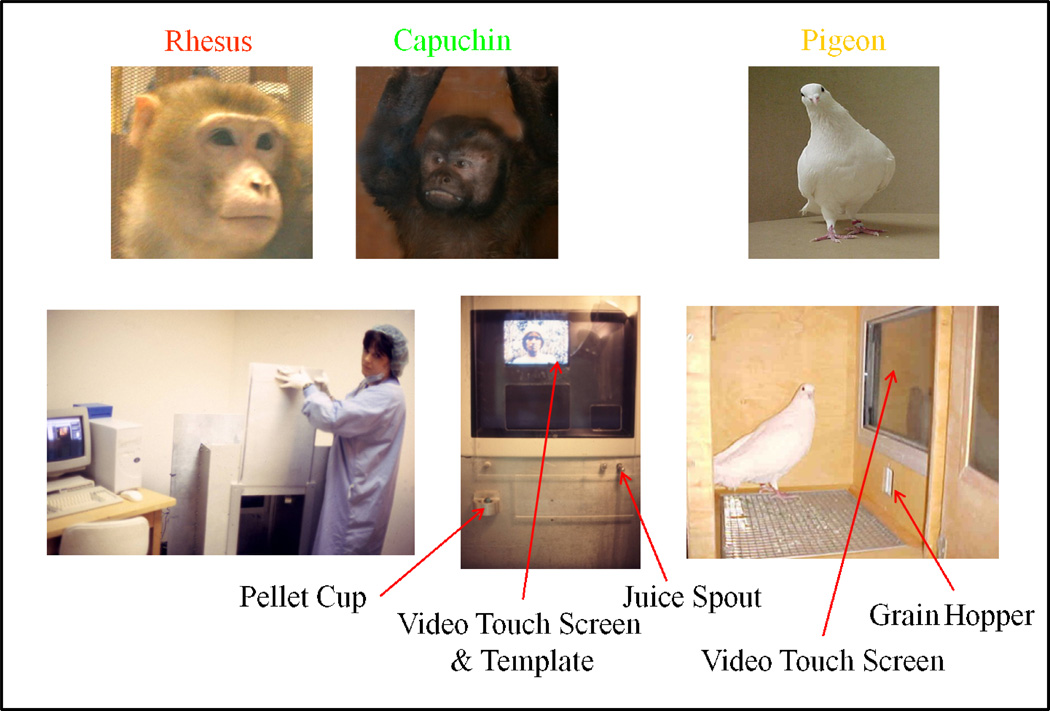
This learning task was conducted with three species tested with very similar procedures, including the same stimuli and set-size manipulations to produce functional relationships and leading to direct species comparisons (Katz et al., 2002; Katz & Wright, 2006; Wright et al., 2003; Wright & Katz, 2006). The monkeys and pigeons were tested in apparatus appropriate for the species shown in Figure 11, but they all had the same video monitors, touch screens, and stimuli (equated for visual angle). Pigeons received grain reinforcement for correct responses, whereas monkeys received banana pellets or Tang orange drink. Monkeys, but not pigeons, had a Plexiglas template to guide responses.
Figure 11.
Rhesus and capuchin monkeys tested in custom aluminum chambers with a juice spout, pellet cup, and template to guide responses. Pigeons tested in custom wooden chamber with a grain hopper and a similar video monitor and touch screen as used with the monkeys.
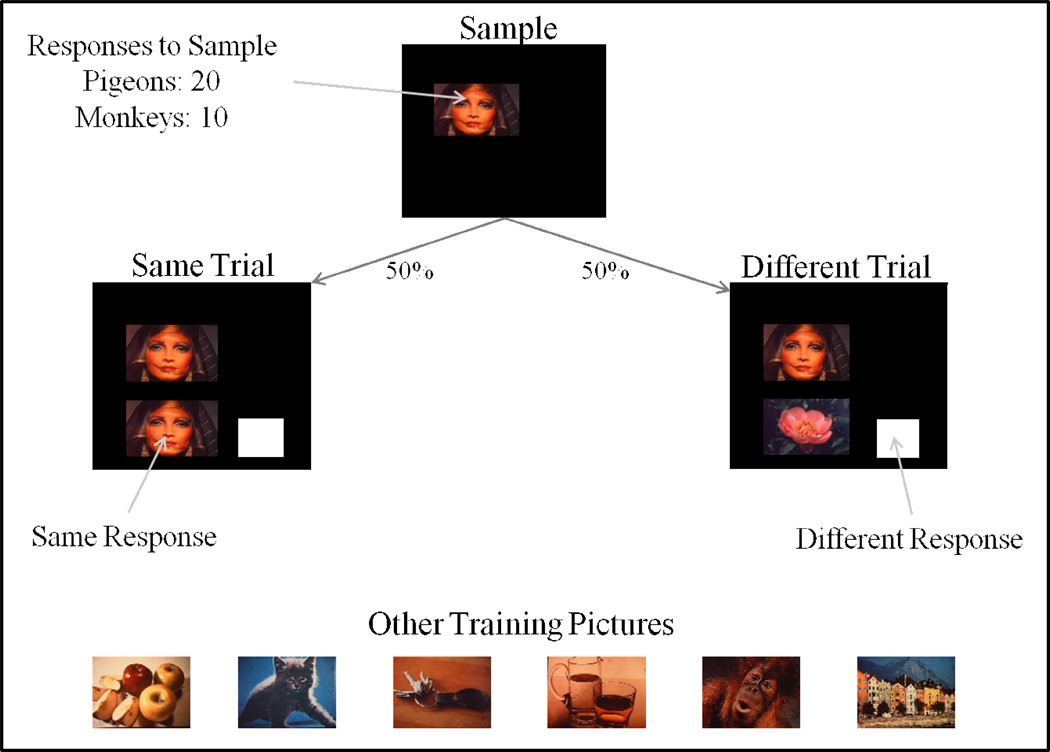
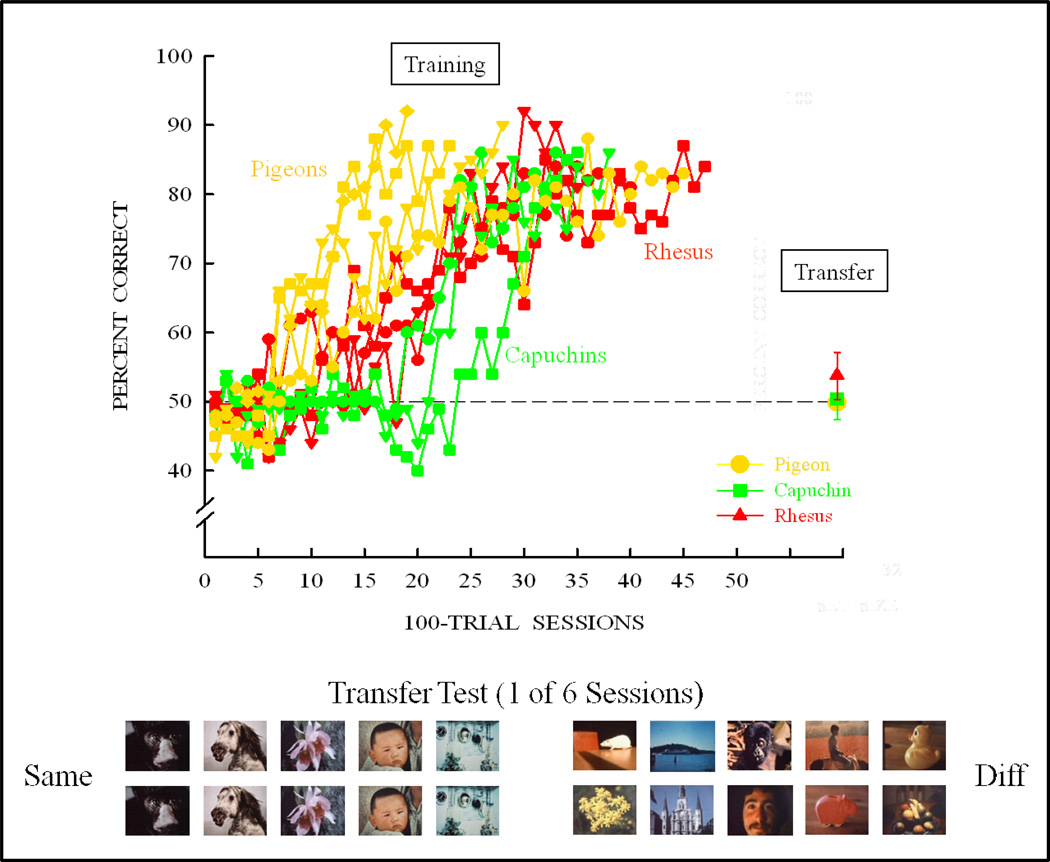
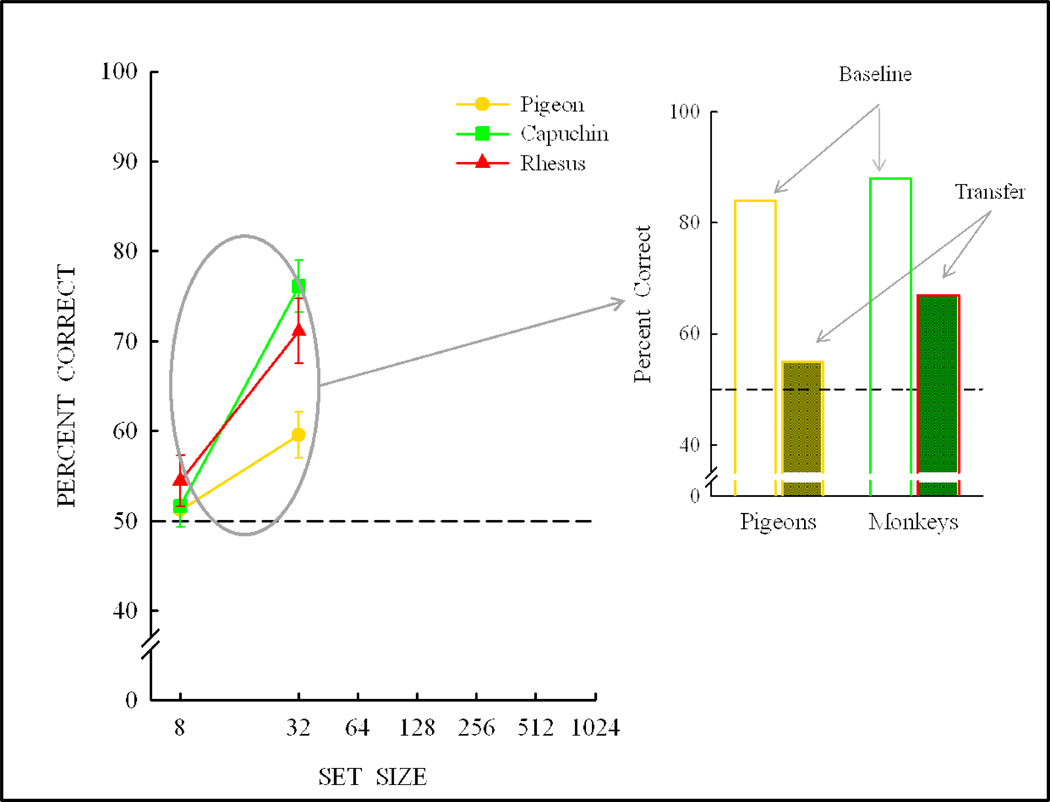
Figure 12 shows the same/different task. Pigeons pecked the sample 20 times (the training condition from the previous experiment that produced concept learning). Half the trials were same and half different. A “same” response was to the lower picture, and a “different” response was to the white rectangle. Other training pictures are shown at the bottom of Figure 12, for the initial training set of 8 pictures. The different species learned the task at similar rates as shown in Figure 13, notwithstanding an early advantage for many of the pigeons. Following learning (80% criterion), the species were tested for their transfer to novel stimuli. Ten novel stimulus trials were randomly intermixed with 90 training trials (some examples from the first transfer test are shown at the bottom of Figure 13). None of the species showed any significant transfer or concept learning. Therefore, the training set was expanded to 32 pictures followed by a novel-stimulus transfer test. Following learning with the 32-item training set, some of the species showed some transfer (Figure 14). If this experiment had been conducted with a training set from the circled region of Figure 14, the likely conclusion would have been that pigeons do not (possibly cannot) learn a same/different concept, but monkeys do partially learn (transfer less than baseline) this same/different concept.
Figure 12.
Same/Different testing procedure with sample touch/peck requirements, “same”/”different” choice responses and examples of the initial 8 training pictures.
Figure 13.
Training and transfer for monkeys and pigeons with the initial set of 8 pictures of Figure 12 and the 5 same and 5 different novel transfer trials that were used in the first transfer test session following learning.
Figure 14.
Transfer performance with the training set expanded from 8 to 32 pictures. For pigeons, transfer following training with set sizes expanded from the initial 8-item set but less than 32 items would likely show little or no transfer, whereas monkeys would likely show partial transfer and partial abstract-concept learning.
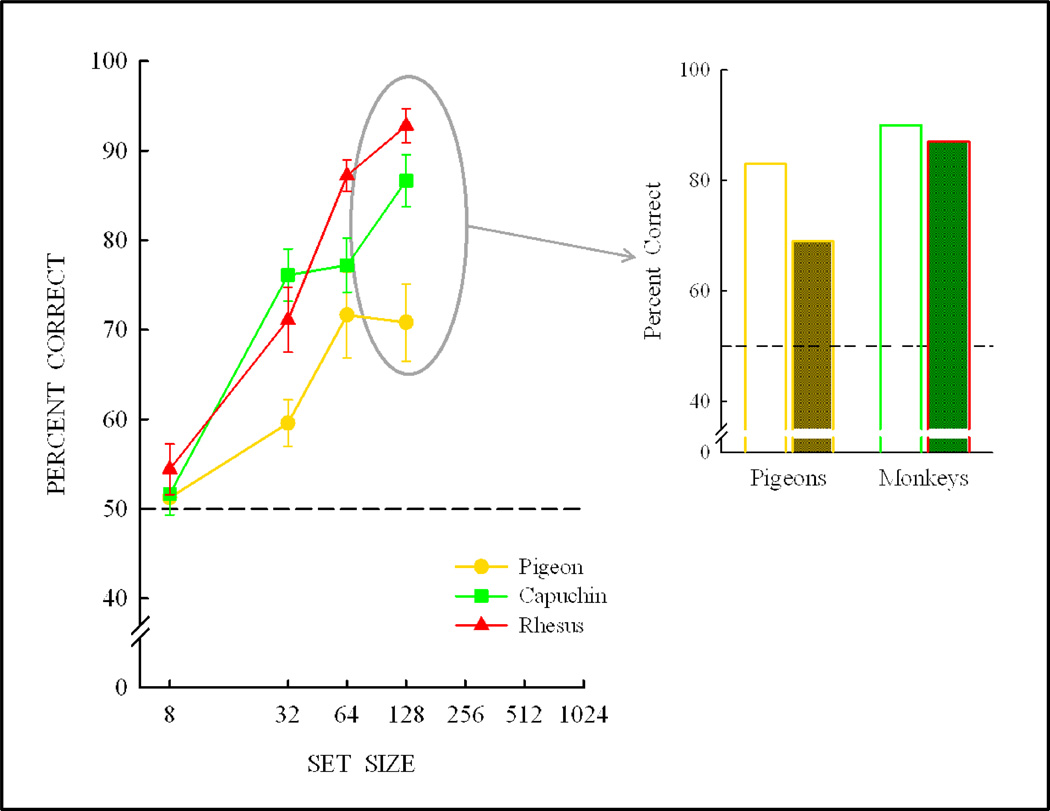
The training set was then expanded to 64 items followed by learning and testing and then to 128 items. At the 128-item training set, transfer had improved considerably as shown in Figure 15. If the experiment had been conducted with only this 128-item set size, then the likely conclusion would have been that pigeons can partially learn a same/different concept and that monkeys can fully learning a same/different concept.
Figure 15.
Transfer with further expansion of the training set to 64 items and then to 128 item. Pigeons now show partial transfer (and partial concept learning) following training on 128-item set relative to their baseline performance, whereas at this same set size monkeys show transfer equivalent to their baseline performance—and therefore full abstract-concept learning.
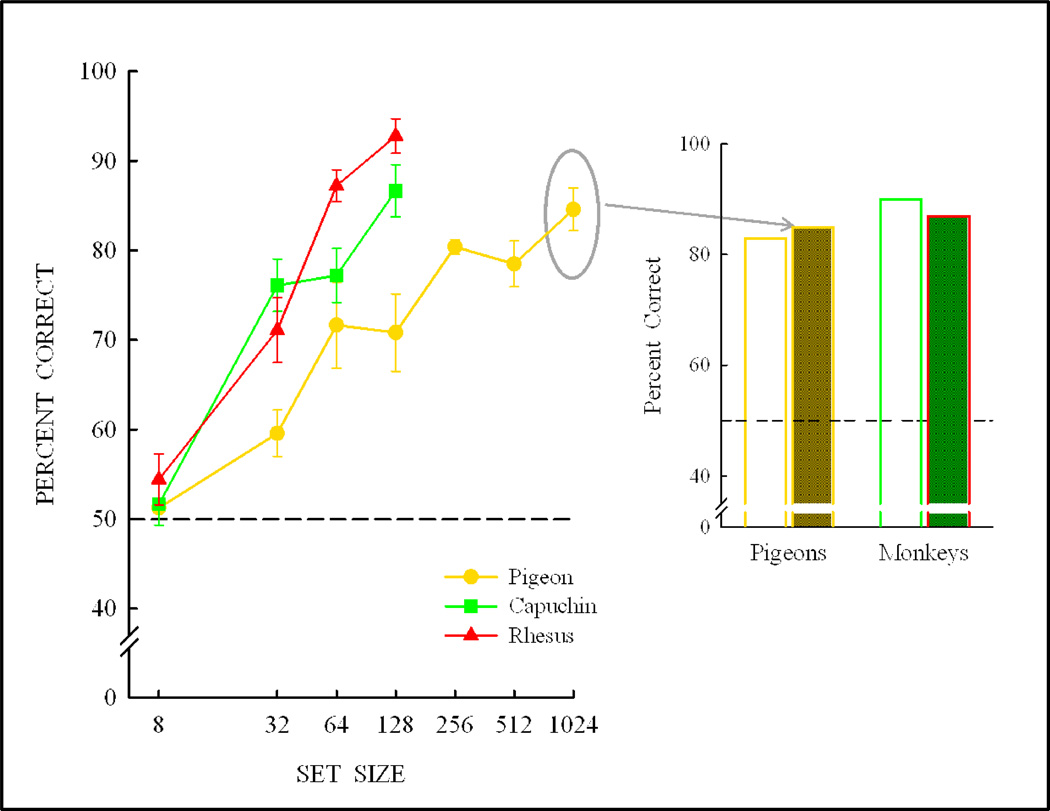
Encouraged by these trends, we further expanded the pigeons’ training set to 256, 512, and 1024 items as shown in Figure 16. Following training with these stimulus sets, pigeons also transferred their performance to novel items at a level equivalent to their baseline performance. If the experiment had been conducted only with a 256 (or 512, or 1024)) item training set, then the likely conclusion would have been that pigeons, like monkeys, can fully learn a same/different concept and can transfer their performance to novel stimuli equivalent to their baseline performance.
Figure 16.
Pigeon transfer with further expansion of the training set to 1024 pictures, resulting in transfer equivalent to their baseline performance and full concept learning—like the monkeys did following training on the 128-item set.
Together these functional relationships show that pigeons, as well as monkeys, have the cognitive ability to learn a same/different abstract concept—a qualitative similarity in learning ability. Nevertheless, it appeared that pigeons required more exemplars of the rule (i.e., more training pairs) to learn the same/different abstract concept than monkeys—a result that would have pointed to a quantitative difference among these species.
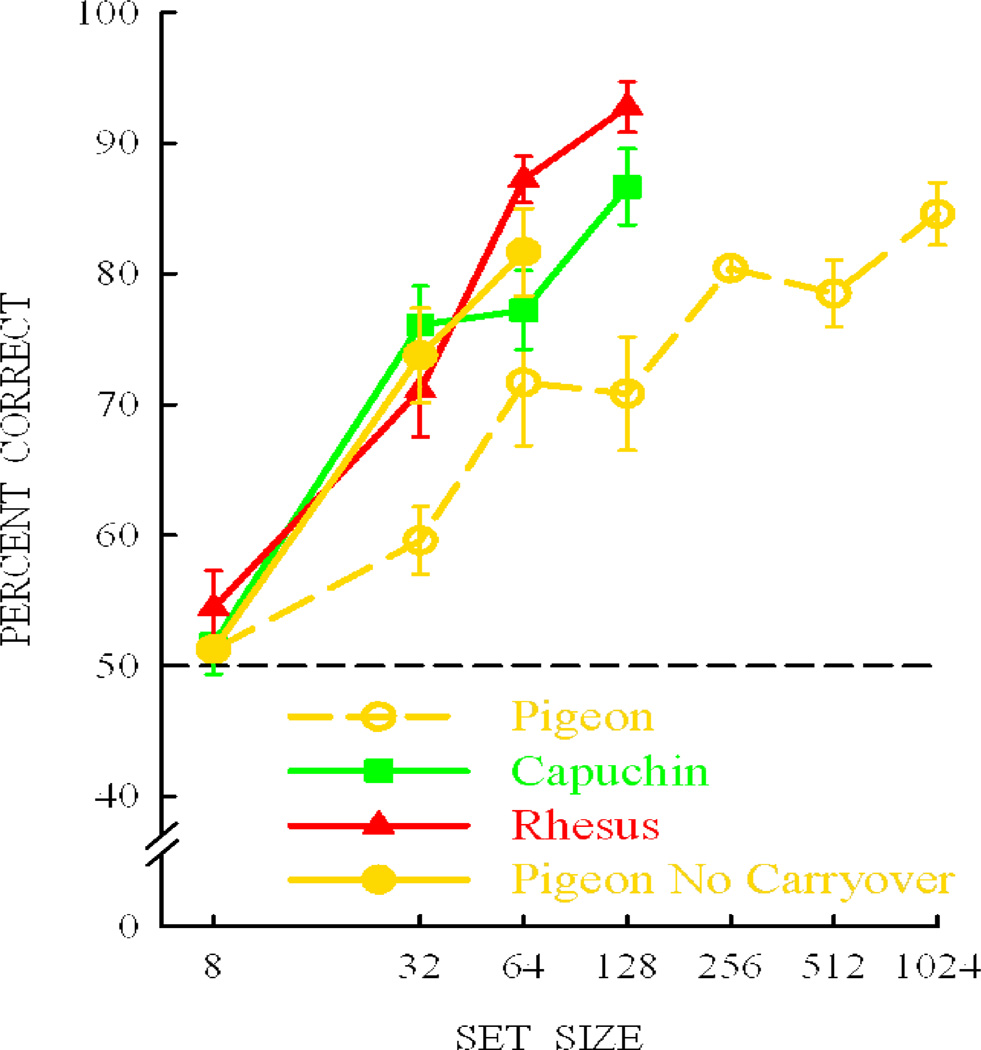
Further research, however, showed that altering the pigeon’s training resulted in levels of transfer comparable to that shown by monkeys. Groups of experimentally-naïve pigeons were trained initially on either the 32-item set or the 64-item set (Nakamura & Wright et al., 2009). The training stimuli were the same stimuli used to train the pigeons and monkeys at 32- and 64-item set sizes in the prior experiment. The level of transfer following initial training on either the 32- or 64-item sets was equivalent to that for monkeys and was midway between that for capuchin and rhesus monkeys at those same set sizes, as shown in Figure 17. Despite differences in training history, these results suggest that under some conditions pigeons do transfer at the same level as monkeys and do not need more exemplars of the rule to transfer at these levels.
Figure 17.
Groups of experimentally naïve pigeons trained initially with either 32 or 64 item sets showing improved transfer which is now equivalent to monkeys trained at these same set sizes with these same items. The results suggest that the previous pigeon groups had detrimental carryover effects from their training with smaller set sizes prior to their transfer at these set sizes.
We suggest that whatever pigeons learn with small training sets, carry over and interfere with learning and transfer with the next larger training set. Transfer can be thought of as a measure of the size of the stimulus domain within which performance is accurate. The stimulus domain for accurate performance seems to grow in some proportion to the training set size. Apparently, the pigeons’ domain becomes resistant to growth following learning with small training sets. We have referred to this effect as restricted-domain relational learning (Elmore et al., 2009; Katz & Wright, 2009; Wright, 2010; Wright & Katz, 2009; Wright & Lickteig, 2010). Restricted-domain carryover effects may be a common property (to a greater or lesser degree) of transfer and generalization—a reluctance to perform outside of one’s comfort zone—once something has been learned.
Functional Relationships for Investigating Memory Processing
Visual List Memory
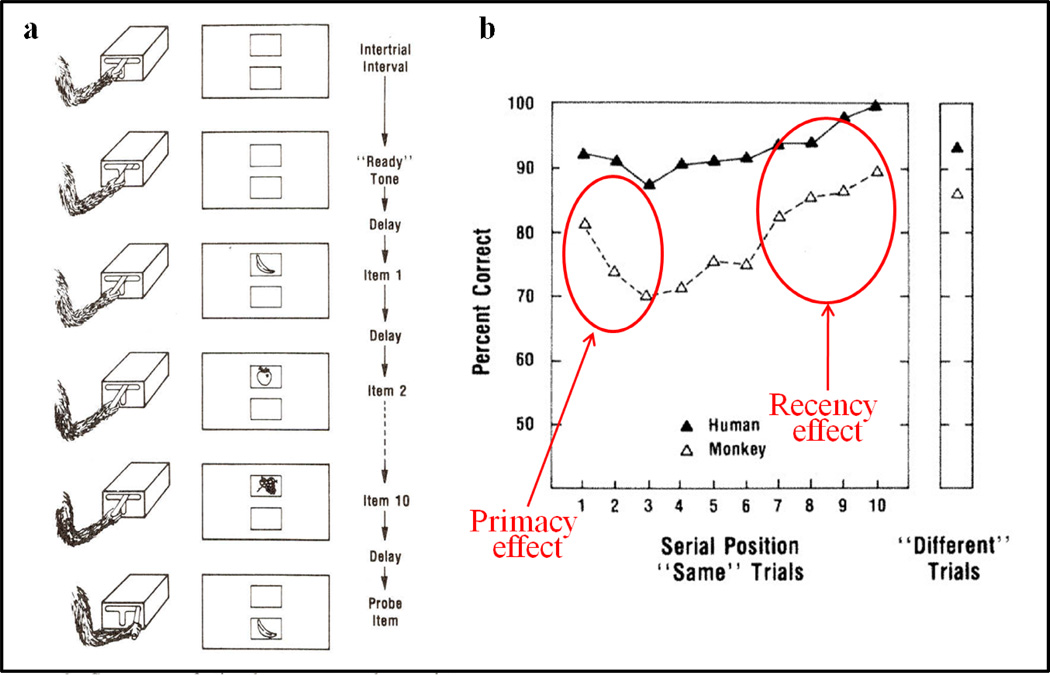
We began animal list-memory studies after I and my students read (in 1977) Robert Crowder’s 1976 seminal book “Principles of Learning and Memory.” We conducted the first nonhuman primate visual list memory study (Sands & Wright, 1980a,b; 1982). In this task shown in Figure 18a, a rhesus monkey, Oscar, worked in a primate chair facing two vertically-aligned back-projection screens. Oscar pushed down on a 3-position level to start a trial. A Carousel projector projected 10 pictures (1-s on, 1-s off) on the upper of two screens. After a 1-s delay a single (test) picture appeared on the lower screen. If the test picture matched one of the list items (as it did in the example in Figure 18a), then a lever movement to the right was correct and Oscar was reinforced with Tang orange drink. If the test picture did not match any of the list pictures (50% of the time), then a lever movement to the left was correct and was reinforced accordingly. By testing Oscar’s memory over many days we were able to determine Oscar’s accuracy for different positions in 10-item lists—the serial position function. The serial position function is among the most important cognitive functional relationships and continues to be a test bed for theories of memory (Ebbinghaus, 1902; Glenberg, 1983). As shown in Figure 18b, good memory for the first list items is the primacy effect and good memory for the last list items is the recency effect. This was the first primacy effect for a nonhuman animal. Primacy effects, at the time, were considered to be unique to humans because animals had not shown primacy effects and primacy effects were thought to depend upon rehearsal—a verbally mediated memory strategy instrumental to long-term memory. The recency effect, by contrast, was thought to represent short-term memory. According to the Modal model of memory, items come into short-term memory. If they were sufficiently rehearsed, then they were transferred to long-term memory—resulting in a primacy effect (Atkinson & Shiffrin, 1968). If they were not rehearsed sufficiently, then (according to the model) they were forgotten. Since nonhuman animals were thought incapable of strategic rehearsal control, this monkey primacy effect was unexpected. This monkey’s primacy effect raised issues about the meaning of primacy and recency serial position effects and the role of rehearsal in memory processing.
Figure 18.
(a) Schematic of a 10-item list-memory testing procedure. A monkey hand and arm is shown starting a trial by pressing downward on a lever. List pictures are then sequentially presented on an upper screen. Following a delay, a single test picture is presented on a lower screen. The subject moves the lever to the right, a correct response (“same”), indicating that the test picture was in the list. (Left lever movements would indicate that the test picture was not in the list.) (b) Serial position functions for a monkey (Oscar) and a human tested with the same procedure including stimuli, presentation rates, delays, and response lever. Good memory for the first list items (circled) show primacy effects and good memory for the last list items (circled) show recency effects for the monkey and human.
Further evidence that rehearsal was not necessary for the primacy effect of the serial position function was provided by a series of experiments with the interstimulus interval (ISI) procedure. Increasing ISI had been shown to increase human memory performance (e.g., Intraub, 1980; Proctor, 1983). As the interval between stimuli was progressively increased (e.g., 80 ms to 5000 ms) memory performance increased—a remarkable result because more time will have elapsed as the ISI increased which should have resulted in greater decay and forgetting. But just the opposite occurred, suggesting that better memory performance was the result of progressively more rehearsal. We tested list memory of humans and monkeys on the ISI procedure with travel-slide pictures (Cook et al., 1991). Humans (as expected) showed an ISI effect, but monkeys did not, suggesting that the monkeys did not rehearse these pictures in these list-memory tasks. In other human memory tests, we showed that, unlike for travel slides, there was no ISI effect for kaleidoscope pictures—a result suggesting that humans do not spontaneously code or rehearse kaleidoscope pictures. Moreover, by teaching names (codes) for 40 kaleidoscope pictures, these same participants then showed ISI effects, and the magnitude of the ISI effect was related to their rehearsal strategy as shown by overt rehearsals and from posttest interviews (Wright et al., 1990). Also noteworthy was that rehearsal did not affect the magnitude of the primacy effect, but instead improved memory performance for the middle items (the dip in the serial position function). Together these results and findings suggest that many of the same processes that produce serial position effects found in humans can be found in rhesus monkeys—a non-verbal, non-rehearsing animal.
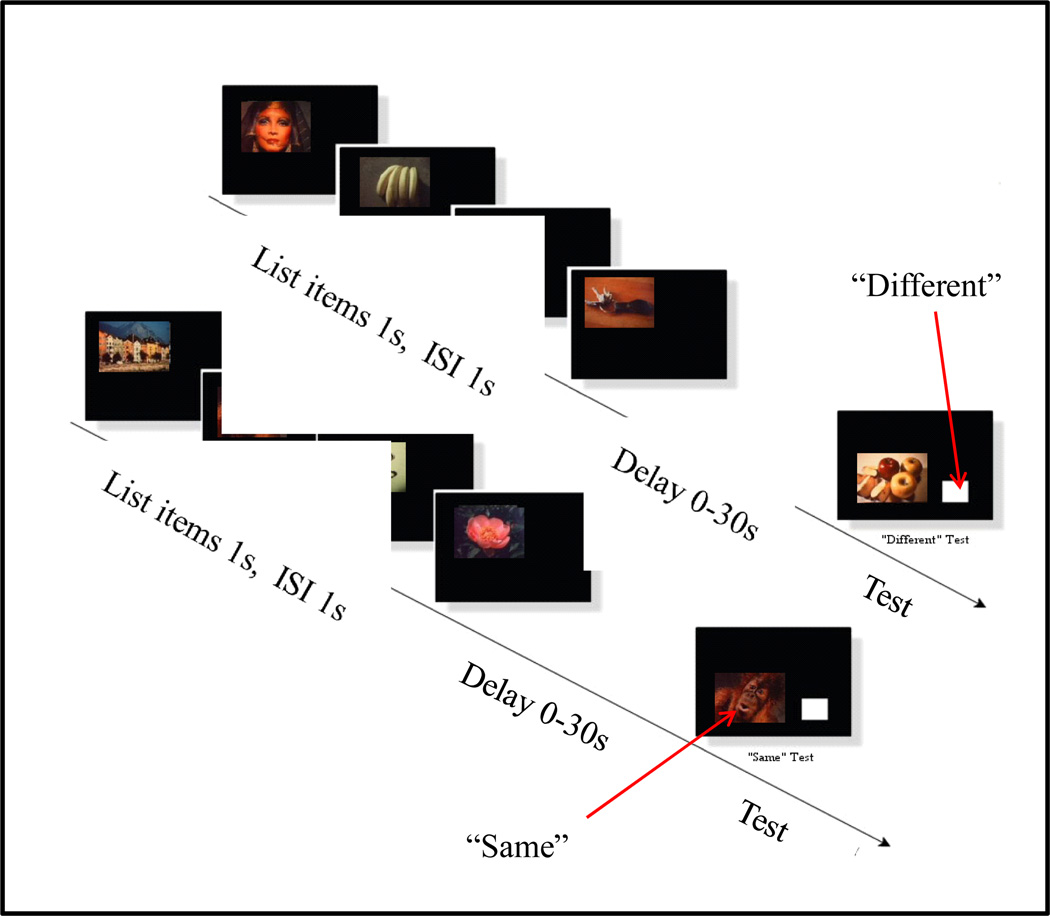
To further explore primacy and recency effects, we used short 4-item memory lists to explore how primacy and recency effects changed with retention delay as well as to accommodate pigeons which had difficulty with list longer than 4 items (e.g., Santiago & Wright, 1984; Wright, 1989, 1998b, 1999a, 2007; Wright et al., 1984, 1985). Lists of four “travel slide” pictures were used to test rhesus monkeys, capuchin monkeys and pigeons (see Figure 19). Lists of four kaleidoscope pictures were used to test humans to avoid ceiling effects (and also somewhat leveled the “playing” field to the animals).
Figure 19.
Examples of two, 4-item list-memory trials with travel slides for testing animal list memory.
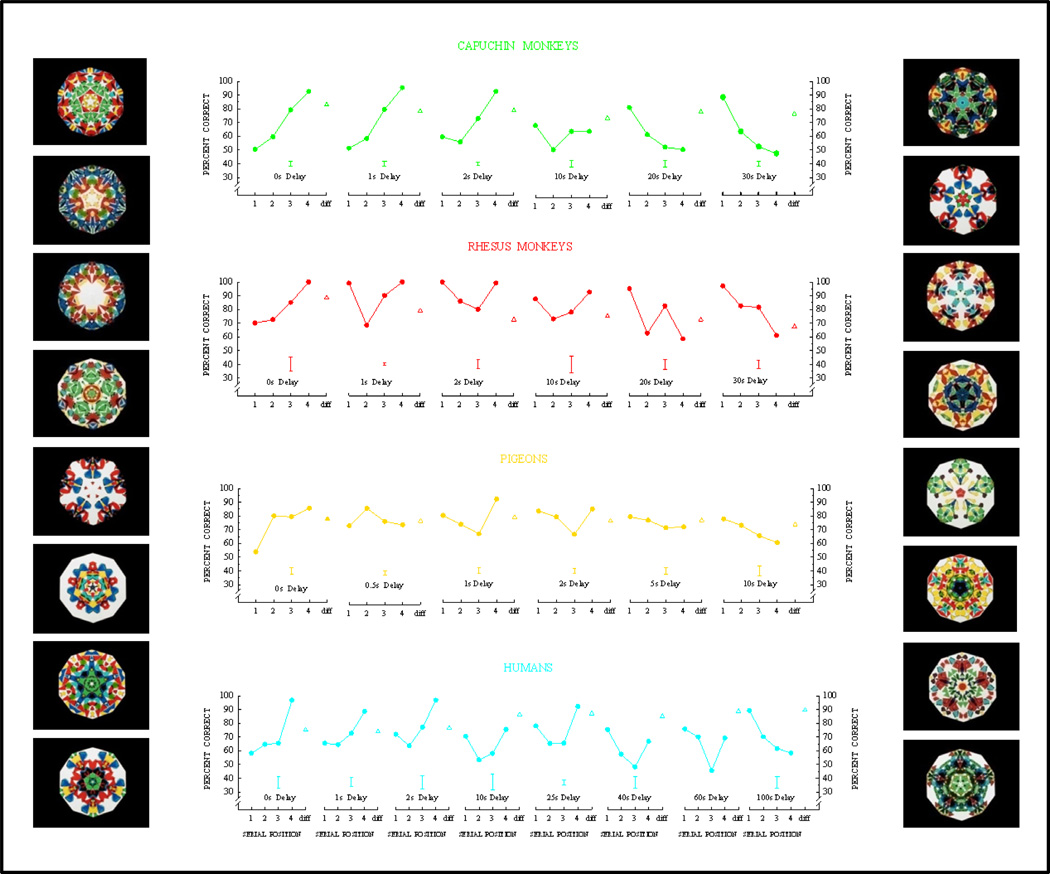
The serial position functions for the four species are shown in Figure 20. The form of the serial position function changed systematically with retention interval. At the shortest delay, the serial position function was upward sloping, showing virtually pure recency performance. As the delay was increased, a primacy effect appeared, giving the function its characteristic U-shape. At the longest delays, the recency effect had dropped out, and the serial position function was downward sloping, showing virtually pure primacy performance. These time-course changes for each species can be thought of as 2nd order functional relationships (with the serial position function being the 1st order functional relationship). All four species showed similar changes and a trend toward a primacy effect as time increased. Indeed, Endel Tulving, labeled this trend “The law of Primacy” in a festschrift book honoring Gordon Bower (Gluck, Anderson, & Kosslyn, 2008). The same pattern of changes for the four species reveals a qualitative similarity and can be thought of as a 3rd order functional relationship (a comparison among 2nd order functional relationships). But there was a time-course difference among the species. The changes took about 30 s for rhesus and capuchin monkeys, 10 s for pigeons, and 100 s for humans. Different time courses may represent a quantitative difference in memory processing among species.
Figure 20.
Serial position functions showing primacy and recency effect changes as a function of retention delay for monkeys, pigeons, and humans. (The fourth item is the last list item.) Mean group error bars are shown below each serial position function. Different-trial performance is shown to the right of each serial position function. Animals were tested with “travel pictures” (Figure 19), and humans were tested with kaleidoscope patterns and kaleidoscope examples are shown on the sides of the figure.
Together these systematic serial position function changes constrain possible explanations. Consider the consistent result that memory for the first list item improves with retention delay. This is just opposite to the typical notion that memory is supposed to decay with time.
Auditory List Memory
Changes in the visual serial position functions with delay raised the issue as to whether memory in other modalities would show similar changes. For example, would auditory memory reveal similar changes with delay? We therefore embarked upon training rhesus monkeys in an auditory list memory task, despite other researchers having been unsuccessful in this endeavor. We too were unsuccessful—for more than two years—but eventually succeeded with a new procedure that required the monkeys to touch the sound source—the speaker. Copper screens were placed in front of the speakers to record touches (via a low impedance CMOS circuit). By touching the stimulus, they learned rapidly. We even took advantage of a ‘built in’ fading procedure, where initially the test sound was played from the correct side speaker only. In just a few trials, the monkeys were touching the speaker from which the sound came. We then gradually increased the sound coming from the incorrect speaker. To our surprise, the monkeys learned the basic task in a matter of several weeks. They also showed full transfer to novel sounds (equivalent to baseline performance) and abstract-concept learning (Wright, Shyan, & Jitsumori, 1990)—a feat that was judged to be beyond the cognitive capabilities of rhesus monkeys (e.g., D'Amato, Salmon, & Colombo, 1985; Premack, 1983; Thomas, 1980).
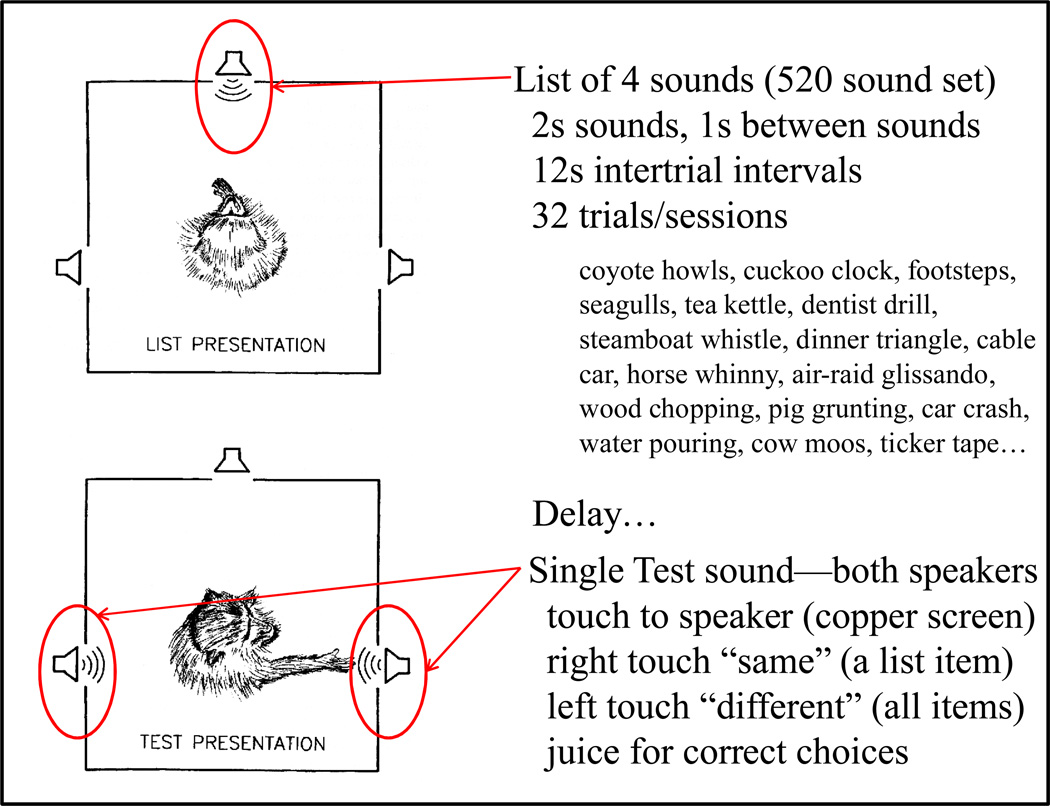
The auditory list memory task is shown in Figure 21. Monkeys touched the center speaker producing a list of four sounds. Following a delay, a test sound was played simultaneously from both side speakers. If the test sound matched one of the list sounds, then the correct touch response was to the right-side speaker (same response). If the test sound was different from all the list sounds, then the correct touch response was a touch to the left-side speaker (different response). Natural and environmental sounds were used, selected from a 520-item set (Wright & Rivera, 1997).
Figure 21.
Top-view schematic of the monkey auditory list-memory procedure. Auditory lists were presented from the front speaker. Following presentation of the list and a delay, a single test sound was played (simultaneously) from both side speakers. A right speaker touch was a “same” response, indicating that the test sound was one of the list sounds. (A left speaker touch would indicate that the test sound was not one of the list sounds.) Other procedure details and names of some of the sounds are shown.
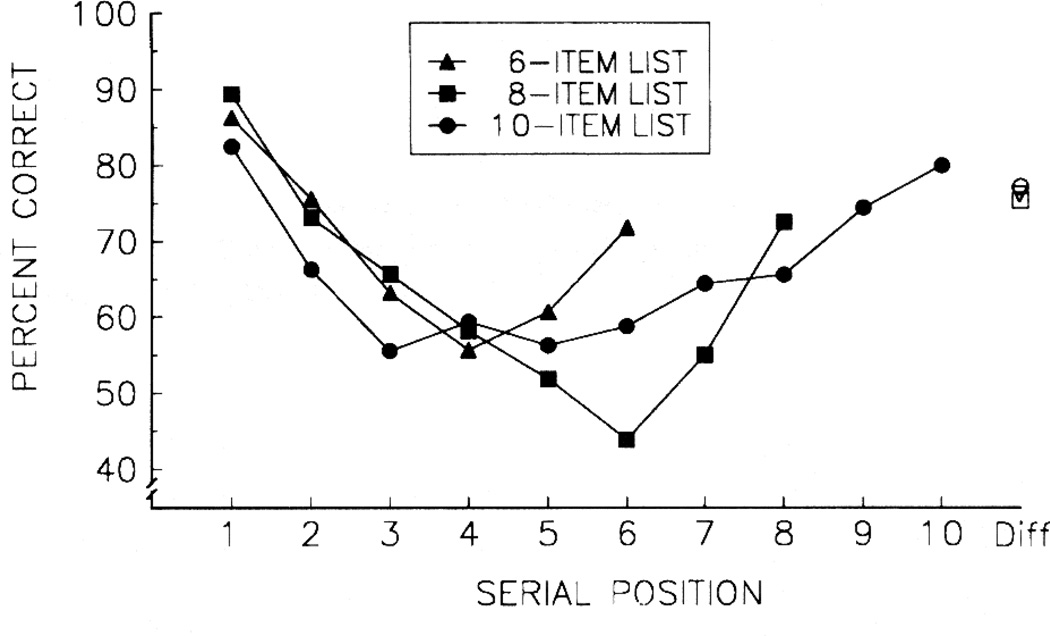
Auditory serial position functions for 6, 8, and 10 sound lists are shown in Figure 22. These auditory serial position functions are “U” shaped, not unlike Oscar’s visual 10-item function (Figure 18). One difference is that these auditory serial position functions show an emphasized primacy effect compared to the previously shown visual serial position function. This auditory emphasis on primacy effects will be somewhat more apparent in the next study with shorter auditory lists and delay manipulations similar to those used with visual 4-item lists.
Figure 22.
Mean auditory serial position functions for two monkeys with lists of 6, 8, or 10 sounds.
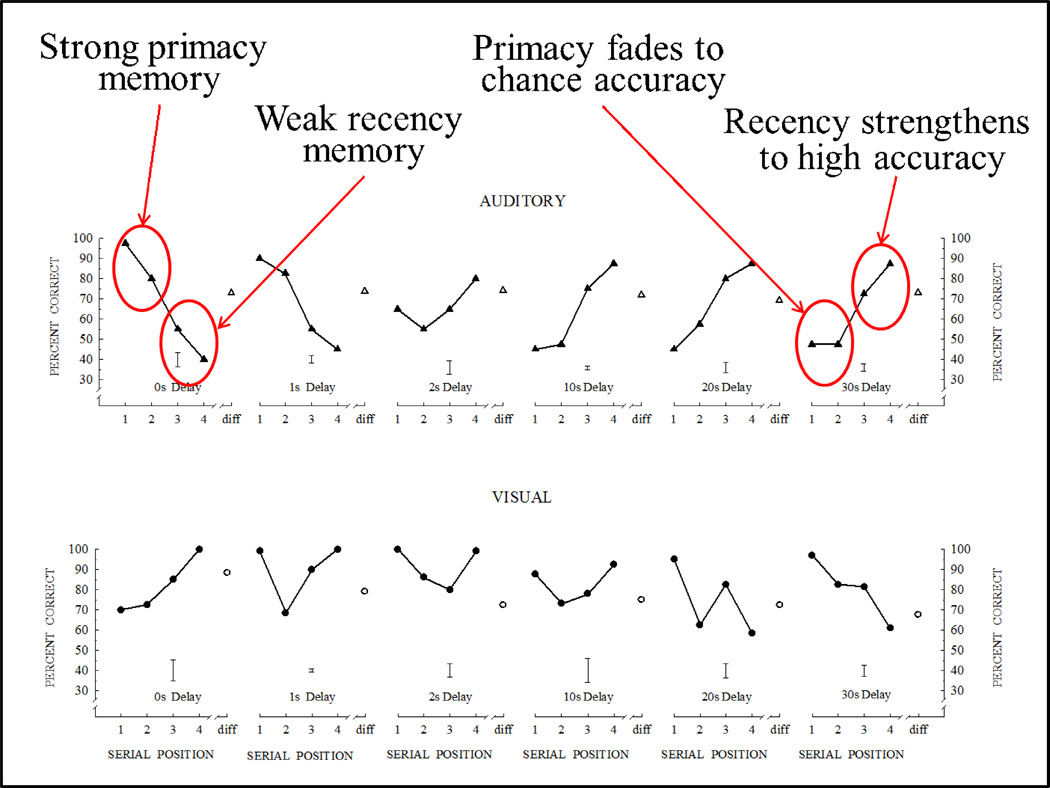
Figure 23 shows the mean performance of two rhesus monkeys in the auditory 4-item list memory task with delays of 0, 1, 2, 10, 20, & 30s, along with the rhesus visual 4-item list memory tested at the same delays for comparison. At short delays, the serial position functions were downward sloping, showing virtually pure primacy performance. As the delay increased, a recency effect appeared and grew in strength, giving the function its characteristic U-shape. At longer delays, the primacy effect dropped out, and the serial position function was upward sloping showing virtually pure recency performance. These opposite shaped serial position functions for auditory memory were a complete surprise to us and were replicated (at the insistence of the Editor) in five additional experiments (Wright, 1998a). Moreover, six further experiments showed same general form of the results under somewhat different conditions, including with intermixed and blocked delays and after a layoff of four years (Wright, 1998b, 1999b, 2002, 2007).
Figure 23.
Comparison of auditory and visual 4-item serial position functions for rhesus monkeys tested at the same retention delays. The auditory and visual serial position functions are opposite in form and are shown to change in opposite ways with changes in delay.
Only by comparing sets of functional relationships for auditory and visual memory were these modality differences apparent. One can think of auditory and visual serial-position-function comparisons as a 3rd order functional relationship (with the 1st order being individual serial position functions, and the 2nd order being changes in the serial position functions with delay).
The importance of different shaped serial position functions for visual and auditory memory is unclear, but may be related to associative-learning evidence showing that visual stimuli are more easily associated with food getting and auditory stimuli are more easily associated with danger avoidance (e.g., Shapiro, Jacobs, & LoLordo, 1980). How visual memory might be adapted to food getting, is that if an animal has had success foraging for food in distinctive patch, then it should remember to feed in a similar patch (visual recency, short delay). On the other hand, if it feeds in a depleting patch (e.g., berries that ripen in the morning and are depleted as the day wears on), then it will need to remember to go to this patch first thing in the morning after an overnight delay (visual primacy, long delay). For auditory memory, if an animal hears a danger sound, then it will need to remember the starting point of the sound (auditory primacy, short delay) to determine whether the sound (e.g., one made by a predator) is coming toward it and in which direction to escape. On the other hand, if an animal hears a danger sound and the sound stops, then it will need to remember where the sound was last heard (auditory recency, long delay) in order to avoid the spot where a potential predator might be hiding in wait.
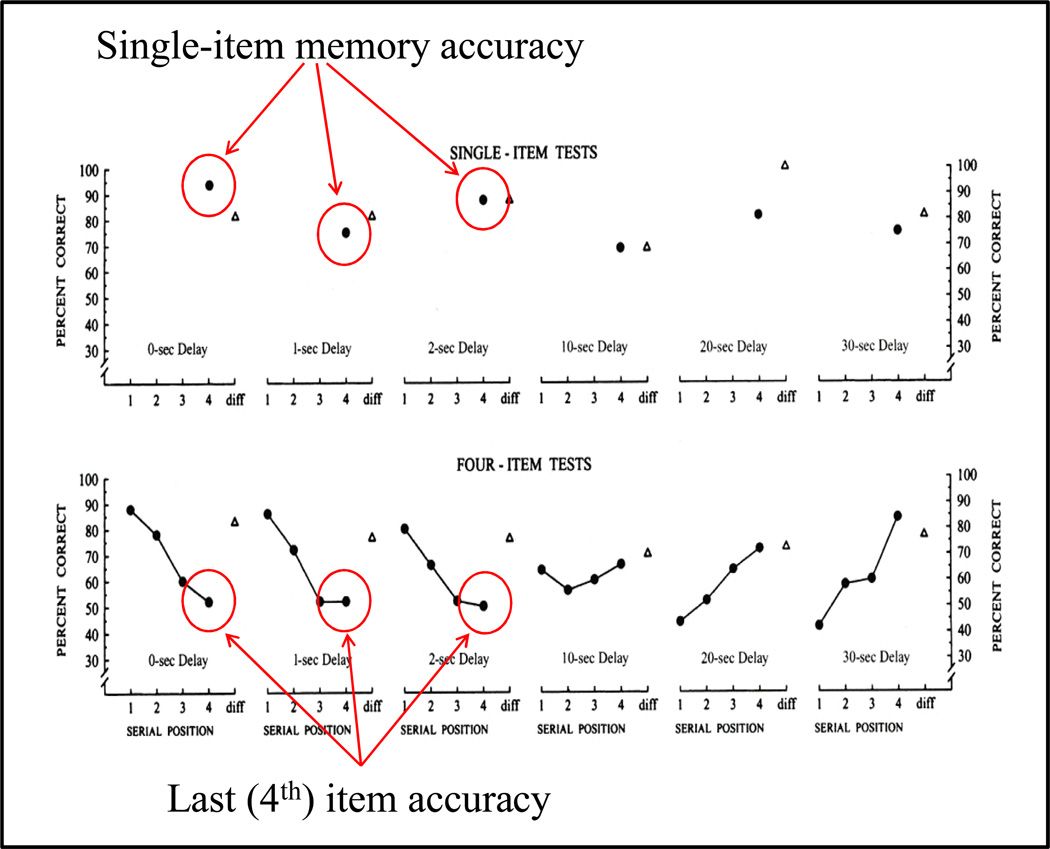
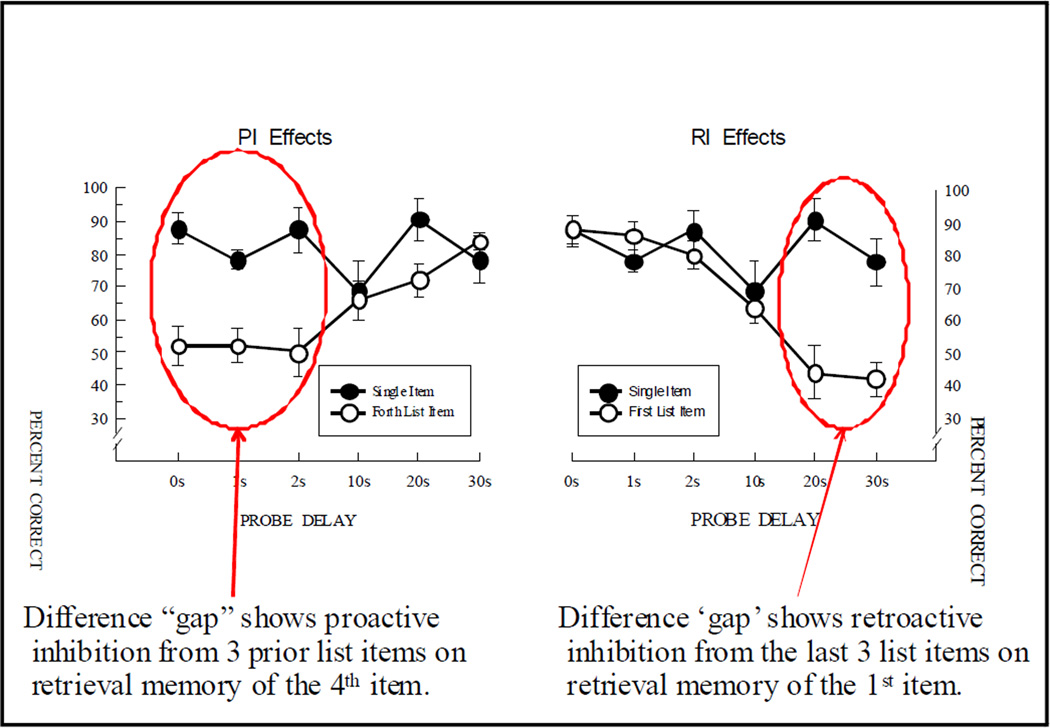
To further explore processes that might be responsible for these time dependent auditory serial position function changes, we focused on retrieval inhibition of the subject’s memory for the list items at test. In one test, we tested single item memory and compared this performance to memory performance of the fourth list item (Wright & Roediger, 2003). The logic of this experiment was that performance with a single item ought to be just like memory performance for the fourth list item, but with the first three items removed. That is, the memory-item presentation, delay time, and test presentation would be identical for the fourth list item and a single item. Figure 24 shows that at the three shortest delays there is good memory for single items, but relatively poor memory for the 4th list item. Figure 25 shows this performance “gap” which is the result of the first three list items inhibiting (proactively) retrieval of memory of the fourth item at these short delays. At the two longest delays, there is also a performance “gap” between single item performance and first item list memory performance (although the comparison is less direct than for the fourth list item). This gap is likely the result of the last three list items retroactively inhibiting retrieval memory of the first item at these long delays. As was true in the prior experiments, only by comparing functional relationships across a substantial portion of the manipulable range was it possible to observe the proactive and retroactive inhibitory effects among auditory list items on rhesus monkey auditory memory.
Figure 24.
Single-item auditory memory and 4-item auditory list memory with performance for single items and 4th (last) list items circled at 0-, 1-, and 2-s delays to emphasize differences, in spite of the similar events (delay, test) following these items.
Figure 25.
Left: Single-item auditory memory compared to 4th (last) item auditory list memory showing the ‘gap’ in accuracy produced by proactive interference from the previous 3 list items on. Right: Single-item auditory memory compared to first-item of a 4-item auditory list showing the ‘gap’ in accuracy likely produced by retroactive interference retrieval memory of the 1st list item from the last 3 list items at longer delays.
Proactive Interference in Visual Memory
In same/different tasks, proactive interference (PI) occurs when previously seen sample pictures are later re-presented as test pictures on trials with nonmatching sample pictures (i.e., different trials). Having seen the test picture before, maybe in just in the previous trial, tends to create confusion as to whether this picture was the sample picture in the current trial or in some previous trial. Proactive interference is endemic to all tasks where stimuli are repeated (Keppel & Underwood, 1962). If small sets of stimuli are used, then by necessity, the stimuli will be repeated from trial to trial with PI growing and eventually saturating. Therefore, investigations of proactive interference need to be conducted with trial-unique stimuli to minimize repetition and interference, thereby allowing effects of specifically placed interfering stimuli to be evaluated.
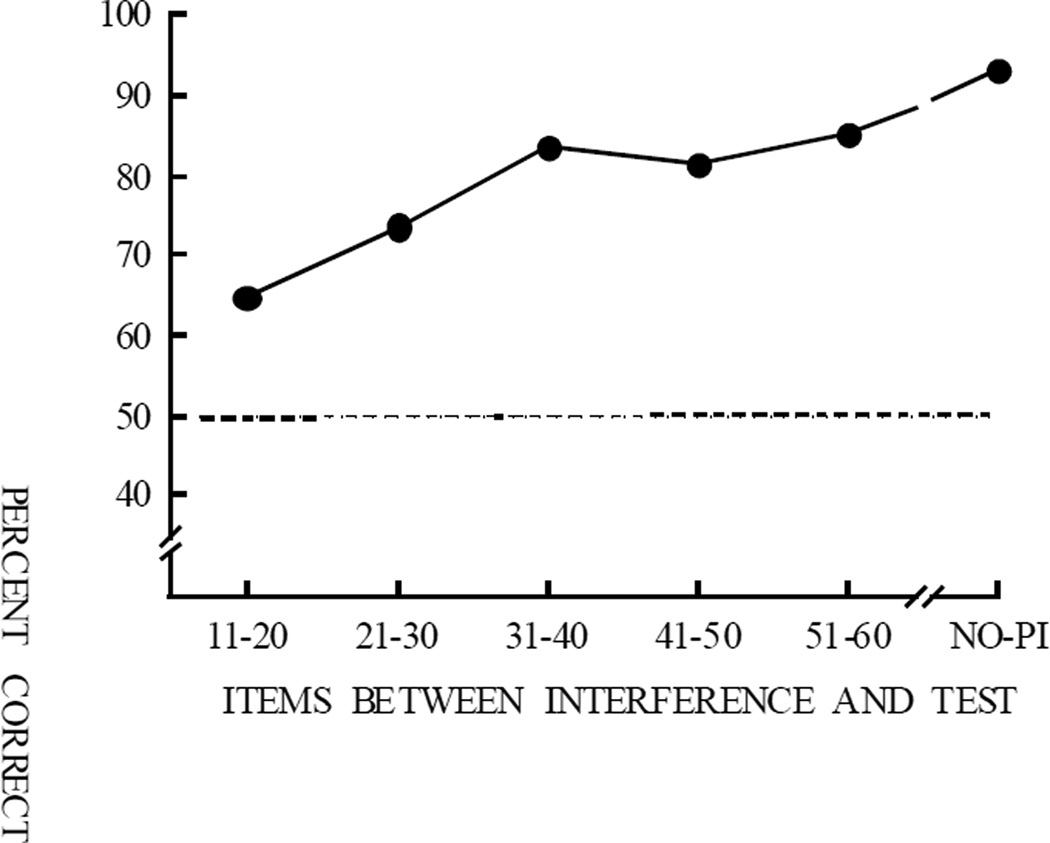
We conducted a PI test with a rhesus monkey, Oscar, performing the previously described 10-item visual list memory task (Figure 18). Interfering stimuli were placed 1, 2, 3, 4, 5, or 6 trials prior to the test (Figure 26). On the PI test, the test item was different from the 10 items in the “current” list (i.e., the correct response was “different”). Having seen the test item before, however, tended to create confusion and increase the chances that Oscar would report “same”—that the test item was in the list on the test trial. When the interfering item was in the immediately preceding trial, there was a large 36% interference effect. Even when the interfering stimulus was 6 trials prior (as many as 60 items before the test), there was still a substantial 10% interference effect (Wright et al., 1986). This PI functional relationship shows how far back (e.g., 60 items) items can interfere with memory performance and consequently how far back they are remembered. If an item is not remembered, then it cannot interfere with later performance.
Figure 26.
Proactive interference for a monkey accurately performing a 10-item list memory task (see Figure 18). On interference test trials, the test picture matched a list picture from a trial seen 1–6 trials previous but differed from all pictures in the current trial. Proactive interference decreases with trial separation showing a proactive interference function.
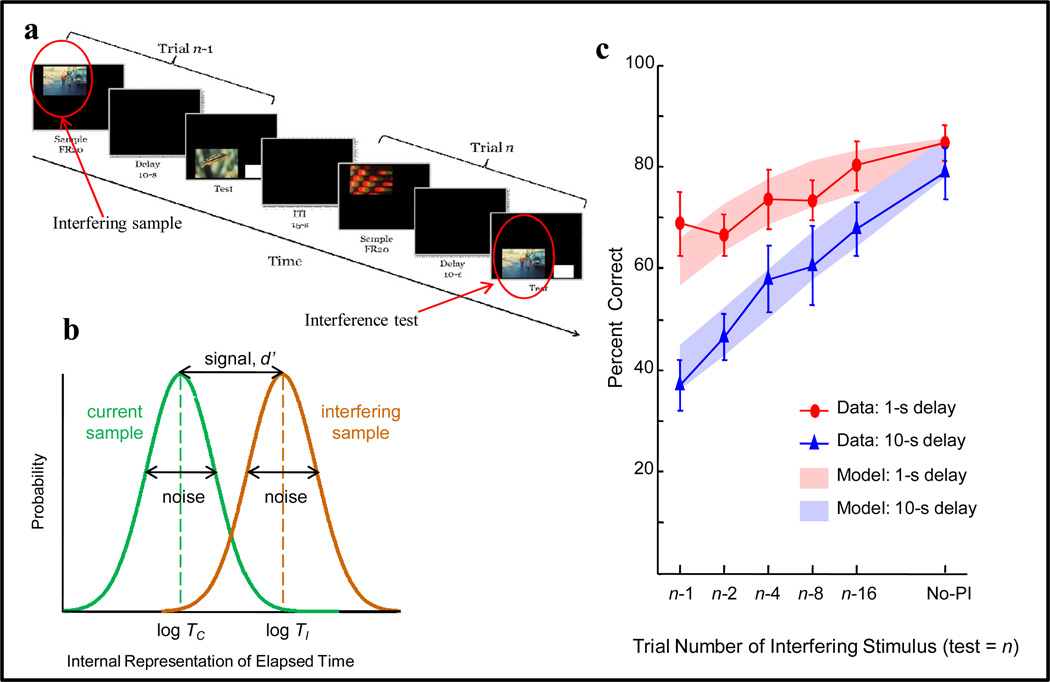
A single-item memory task, offers a somewhat simpler test of PI. We tested pigeons in a single-item memory task shown in Figure 27a for interfering stimuli presented 1 to 16 trials previous to the test (n). The task was same/different, similar to the one described previously (Figure 12). Pigeons pecked the sample stimulus 20 times, followed by a delay (1s or 10s, in a block design), a test stimulus and white rectangle, choice response, and a 15-s intertrial interval. Baseline-trial stimuli were selected (without replacement) from a 1024 stimulus set (Wright et al., 2012). The results show that even with a 1-s delay, there was considerable PI and that this PI dissipated as a function of increased trial separation. Notice that with a 10-s delay, there is a larger 47% PI effect when the interfering stimulus in the immediately preceding trial. Here too, the PI effect dissipates with increasing trial separation, but interference remains substantially greater than with the 1-s delay.
Figure 27.
(a) Example of two trials from a proactive interference test with pigeons where the interfering stimulus was presented on the preceding trial (n-1) and repeated as the test stimulus on trial n. (b) Signal detection theory model of elapsed time: Log time to the sample on the current (test) trial (log TC) and log time to the interfering sample (log TI). (c) Percentage correct performance for 1-s and 10-s delays, and model fits (1 sigma bands) based on the time ratio log (TC /TI)—see text for further explanation.
Greater interference at a longer 10-s delay than at the shorter 1-s delay is counterintuitive. With the 10-s delay, interfering stimuli were encountered more distantly in the past than with the 1-s delay (! 200 s more distantly for n-16). More distantly in the past should, according to models of decay or limited capacity, translate to more forgetting and therefore less interference. But just the opposite occurred. We explained this counterintuitive finding using a signal detection theory model showing that interference depended on time ratios: Log time to the current trial sample divided by log time to the interfering sample (Figure 27b). The model was fit simultaneously to both PI functions using the same parameters (bias and maximum accuracy) for each pigeon. The model fit shown in Figure 27c accounts for 95% of the variance (including the no-PI condition).
One implication of this critical time ratio is that the data cannot be explained by “familiarity” models, in which the subject simply reports whether the test stimulus was or was not seen before, including models based on decaying familiarity. According to such models, performance would depend only on the absolute time to the interfering stimulus, not the critical time ratio shown here. Another implication is that time-outs following incorrect responses (popular in the training of animal subjects) should hasten learning by reducing proactive interference, in addition to any effect of delaying the next opportunity for reinforcement—a popular explanation in animal learning.
Of course, none of these findings or conclusions would have been possible without producing functional relationships (i.e., PI functions) for these different delays and showing that a model based upon time ratios could account for the results.
Functional Relationships for Short-Term Memory Processes: Multiple Item Displays
Change detection is a popular procedure to study visual short-term memory (VSTM). Change detection is well suited to investigating animal as well as human short-term memory because many memory objects can be presented within the time period of VSTM and change detection appears to be independent of verbal strategies, labeling, or rehearsal.
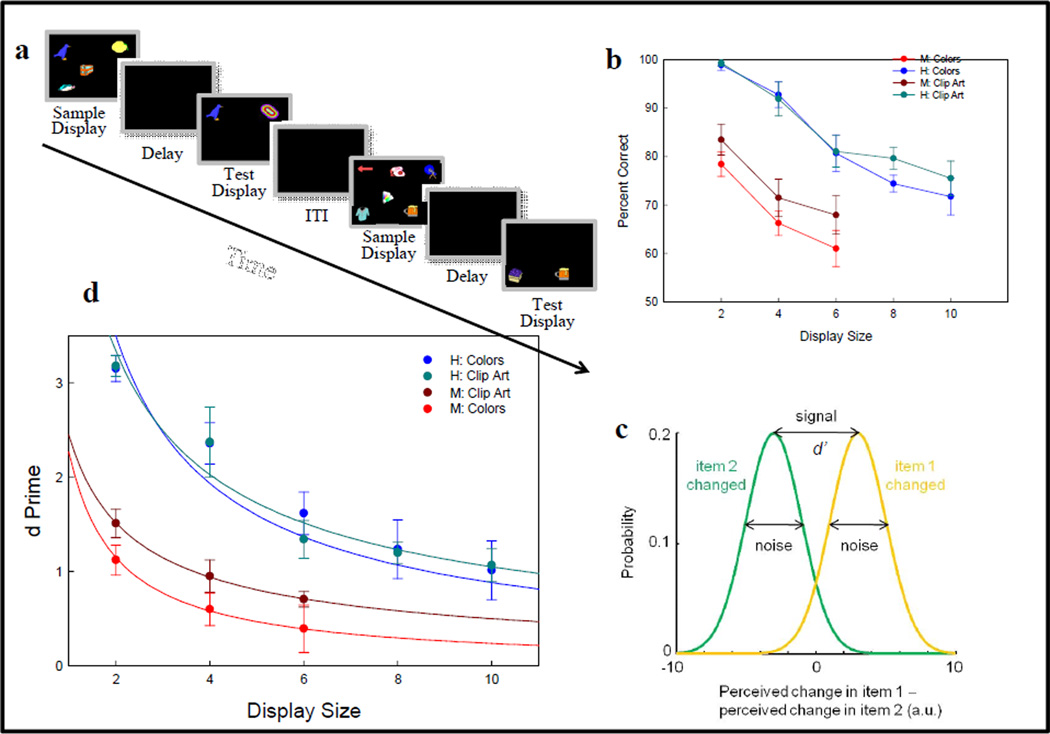
We compared VSTM of humans and monkeys in similar change detection tasks (Elmore et al., 2011). Two examples trials are shown in Figure 28a where clip-art objects (from a set of 976) were presented simultaneously in a sample array. Following a retention delay, one of two test objects was changed and the subjects had to touch the changed object. (Monkeys received juice or pellet reward for correct choices and humans received a tone and green light in the testing room.) The number of memory items varied from trial to trial (monkeys: 2–6 items, humans, 2–10 items). Percent correct performance is shown in Figure 28b and varies as a function of display size for colors (shown in blue and red) and clip art (shown in green and brown). These functional relationships (Figure 28b) are descriptive accounts which do not specify how memory should vary with display size or how the brain might produce these memory results. By contrast, we proposed a continuous-resource account that stipulates a noisy VSTM (Figure 28c) and discrimination (d' from signal detection theory) that is predicted to vary as the inverse power law of display size, as confirmed by the good fits (R2s>0.87) shown in Figure 28d (Bays & Husain, 2008; Elmore et al., 2011; Wilken & Ma, 2004). This continuous-resource model accounts for VSTM of both species better than traditional fixed-capacity models (e.g., human capacity: 4 ± 1: Cowan, 2001, 2005). Fixed capacity models do not have noisy memory (storage is all or none—a likely invalid assumption given the noisy, probabilistic nature of the nervous system), and predict that capacity will be the same at all display sizes, except for display sizes less than the capacity limit where performance should be perfect (100% correct). Neither requirement was met by humans in this study, (nor in some other studies e.g., Alvarez & Cavanagh, 2004; Eng, Chen, & Jiang, 2005). Moreover, the computed fixed capacity for monkeys was less than 1 item—unlikely to be a valid conclusion given the species’ survival and accurate 10- & 20-item list memory performance by monkey Oscar (see Figure 18 for 10-item performance and Sands & Wright, 1980a, b). None of these comparisons would have been possible without testing functional relationships for humans and monkeys across a substantial range of their short-term memory for multiple objects.
Figure 28.
(a) Examples of two change-detection trials, one with 4 and the other with 6 different clip-art objects, used to test human and monkey short-term memory. (b) Changes in percent correct as a function of display size (number of memory items) for colored objects and clip art objects. (c) Signal detection theory model for detecting (d’) which of two test objects was changed. (d) Good model fits to d’ plotted as a function of the inverse power law of display size—as predicted from signal detection theory.
Concluding Remarks
This article is organized around a common theme that early on became an integral part of the research—the generation of functional relationships—which my collaborators and I have conducted over the past four decades. More than 17 different studies are presented and discussed showing that functional relationships—generated by varying critical parameters over a substantial portion of their manipulable range—are essential for determining how cognition works. Moreover, the tricky endeavor of comparing cognitive processes across species—comparative cognition—perhaps is most dependent upon functional relationships. Different species need to be tested with the same stimuli and parameters (e.g., viewing time, ISI, delay, visual angle etc.) in order to make direct species comparisons and be effective in claiming cognitive similarities and/or differences among those species. But some conditions often have to vary (e.g., reinforcement type: juice vs. grain, response type: touch vs. peck, testing chamber: large vs. small, etc.). Most important, is whether the task and experimental arrangement are conducive to accurate performance by each of the species. The rub is that seldom will the conditions be equally amenable to accurate performance by the different species, producing uncertainty whether simple accuracy-level differences represent functional cognitive differences or reactions to arbitrary parameter choices. This is where functional relationships come into play. By systematically varying critical parameters (e.g., number of display items to-be-remembered), functional relationships provide a means to effectively compare species despite accuracy level differences (e.g., Figure 28). Some functional relationships compared in this article suggest qualitative similarities in visual processing, learning, and memory, while at the same time pointing to quantitative differences. Comparing functional relationships (like those shown in this article) in conjunction with studies of the neural basis of this behavior should, in my opinion, be able to provide definitive evidence about cognitive mechanisms and strong implications about their evolution.
Of course, none of what is presented here would have been possible without the many talented collaborators I have been privileged to work with and continuing support from my sponsors. I would be remiss if I did not mention the continuing support from my graduate sponsor, Tony Nevin, graduate committee member, Herb Terrace, and my undergraduate sponsor, Gordon Bower. Several years ago at a celebration for Gordon receiving the 2007 National Medal of Science Award (Figure 29a), Gordon expressed his support in the fly leaf of my copy of his then-just-published festschrift book (Figure 29b).
Figure 29.
(a) Gordon Bower receiving the National Medal of Science from President Bush 2007. (b) Note by Gordon Bower on the flyleaf of my copy of his festschrift book “Memory and Mind” (notice the reference to “The Law of Primacy”).
Highlights.
Functions and their relationships are shown to reveal psychophysical laws.
Functional relationships are shown to reveal different types of learning and memory.
Functional relationships are shown to reveal species similarities and differences.
Acknowledgement
Research and preparation of this article was supported by NIH grants R01MH072616 and R01MH091038. I am grateful for the helpful comments and suggestions by Jeff Katz and Jon Crystal (Action Editor).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez GA, Cavanagh P. The Capacity of Visual Short-Term Memory is Set Both by Visual Information Load and by Number of Objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. Vol. 2. New York: Academic Press; 1968. pp. 89–105. [Google Scholar]
- Bays PM, Husain M. Dynamic shifts of limited working memory resources in human vision. Science. 2008;321:851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH. An association model for response and training variables in paired-associate learning. Psychological Review. 1962;69:34–53. doi: 10.1037/h0039023. [DOI] [PubMed] [Google Scholar]
- Bower GH. Neal E. Miller. Proceedings of the American Philosophical Society. 2011;155:358–365. [Google Scholar]
- Cook RG, Wright AA, Sands SF. Interstimulus interval and viewing time effects in monkey list memory. Animal Learning & Behavior. 1991;19:153–163. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N. Working memory capacity. New York: Psychology Press; 2005. [Google Scholar]
- Crowder RG. Principles of learning and memory. Hillsdale, NJ: Erlbaum; 1976. [Google Scholar]
- D’Amato MR, Salmon DP, Colombo M. Extent and limits of the matching concept in monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:35–51. doi: 10.1037//0097-7403.11.1.35. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus HE. Grundzuge der Psychologie. [Basic psychology] Leipzig: Von Veit; 1902. [Google Scholar]
- Elmore LC, Ma WJ, Magnotti JF, Leising KK, Passaro AD, Katz JS, Wright AA. Visual Short-Term Memory Compared in Rhesus Monkeys and Humans. Current Biology. 2011;21:975–979. doi: 10.1016/j.cub.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore LC, Wright AA, Rivera JJ, Katz JS. Individual differences: Either relational learning or item-specific learning in a Same/Different Task. Learning & Behavior. 2009;37:204–213. doi: 10.3758/LB.37.2.204. [DOI] [PubMed] [Google Scholar]
- Eng HY, Chen D, Jiang Y. Visual working memory for simple and complex visual stimuli. Psychonomic Bulletin & Review. 2005;12:1127–1133. doi: 10.3758/bf03206454. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Bradley MM, Kraus TA, Renzaglia GJ. Studies of the long-term recency effect: Support for a contextually guided retrieval hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1983;9:231–255. [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Gluck MA, Anderson JR, Kosslyn SM. A Festschrift for Gordon H. Bower. New York: Erlbaum; 2008. Memory and Mind. [Google Scholar]
- Intraub H. Presentation rate and the representation of briefly glimpsed pictures in memory. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:1–12. [PubMed] [Google Scholar]
- Kamil AC. A synthetic approach to the study of animal intelligence. In: Leger DW, editor. Comparative Perspective in Modern Psychology: Nebraska Symposium on Motivation. vol. 35. Lincoln, Nebraska: University of Nebraska Press; 1988. pp. 230–257. [PubMed] [Google Scholar]
- Katz JS, Wright AA, Bachevalier J. Mechanisms of same/different abstract-concept learning by rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(4):358–368. [PubMed] [Google Scholar]
- Katz JS, Wright AA. Same/different concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:80–86. doi: 10.1037/0097-7403.32.1.80. [DOI] [PubMed] [Google Scholar]
- Katz JS, Sturz BR, Wright AA. Domain is a moving target for relational learning. Behavioural Processes. 2010;83:172–175. doi: 10.1016/j.beproc.2009.12.006. PMCID: PMC282002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Underwood BJ. Proactive inhibition in short-term retention of single items. Journal of Verbal Learning & Verbal Behavior. 1962;1:153–161. [Google Scholar]
- Nakamura T, Wright AA, Katz JS, Bodily KD, Sturz BR. Abstract-concept learning carryover effects from the initial training set in pigeons (Columba livia) Journal of Comparative Psychology. 2009;123:79–89. doi: 10.1037/a0013126. PMID: 19196044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D. Animal cognition. Annual Review of Psychology. 1983;34:351–362. [Google Scholar]
- Proctor RW. Recognition memory for pictures as a function of poststimulus interval: An empirical clarification of existing literature. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1983;9:256–262. doi: 10.1037//0278-7393.9.2.256. [DOI] [PubMed] [Google Scholar]
- Sands SF, Wright AA. Primate memory: retention of serial list items by a rhesus monkey. Science. 1980a;209:938–940. doi: 10.1126/science.6773143. [DOI] [PubMed] [Google Scholar]
- Sands SF, Wright AA. Serial probe recognition performance by a rhesus monkey and a human with 10- and 20-item lists. Journal of Experimental Psychology: Animal Behavior Processes. 1980b;6:386–396. [PubMed] [Google Scholar]
- Sands SF, Wright AA. Monkey and human pictorial memory scanning. Science. 1982;216:1333–1334. doi: 10.1126/science.7079768. [DOI] [PubMed] [Google Scholar]
- Santiago HC, Wright AA. Pigeon memory: Same/Different concept learning, serial probe recognition acquisition and probe delay effects in the serial position function. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:498–512. [PubMed] [Google Scholar]
- Thomas RK. Evolution of Intelligence: an approach to its assessment. Brain, Behavior and Evolution. 1980;17:454–472. doi: 10.1159/000121814. [DOI] [PubMed] [Google Scholar]
- Thomas RK. Investigating cognitive abilities in animals: unrealized potential. Cognitive Brain Research. 1996;3:157–166. doi: 10.1016/0926-6410(96)00003-1. [DOI] [PubMed] [Google Scholar]
- Thompson RKR. Natural and relational concepts in animals. In: Roitblat HL, Meyer J-A, editors. Comparative Approaches to Cognitive Science. Cambridge, MA: MIT Press; 1995. pp. 175–224. [Google Scholar]
- Tulving E. On the law of Primacy. In: Gluck MA, Anderson JR, Kosslyn SM, editors. Memory and Mind. NY, NY: Erlbaum; 2008. pp. 31–48. [Google Scholar]
- Wilken P, Ma WJ. A detection theory account of change detection. Journal of Vision. 2004;4:1120–1135. doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- Wright AA. Construct a monochromator from a single interference filter. Journal of the Experimental Analysis of Behavior. 1972a;18:61–63. doi: 10.1901/jeab.1972.18-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA. Psychometric and psychophysical hue discrimination functions for the pigeon. Vision Research. 1972b;12:1447–1464. doi: 10.1016/0042-6989(72)90171-x. [DOI] [PubMed] [Google Scholar]
- Wright AA. Psychometric and Psychophysical theory within a framework of response bias. Psychological Review. 1974;81:322–347. [Google Scholar]
- Wright AA. Construction of equal-hue discriminability scales for the pigeon. Journal of the Experimental Analysis of Behavior. 1978;29:261–266. doi: 10.1901/jeab.1978.29-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA. Memory processing by pigeons, monkeys, and people. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 23. New York: Academic press; 1989. pp. 25–70. [Google Scholar]
- Wright AA. Concept learning and learning strategies. Psychological Science. 1997;8:119–123. [Google Scholar]
- Wright AA. Auditory list memory in rhesus monkeys. Psychological Science. 1998a;9:91–98. [Google Scholar]
- Wright AA. Auditory and visual serial position functions obey different laws. Psychonomic Bulletin and Review. 1998b;5:564–584. [Google Scholar]
- Wright AA. Visual list memory in capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1999a;113:74–80. doi: 10.1037/0735-7036.113.1.74. [DOI] [PubMed] [Google Scholar]
- Wright AA. Auditory list memory and interference in monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 1999b;25:284–296. [PubMed] [Google Scholar]
- Wright AA. Monkey auditory list memory: Tests with mixed and blocked retention delays. Animal Learning & Behavior. 2002;30:158–164. doi: 10.3758/bf03192917. [DOI] [PubMed] [Google Scholar]
- Wright AA. An Experimental Analysis of memory processing. Journal of the Experimental Analysis of Behavior. 2007;88:405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA. Functional relationships for determining similarities and differences in comparative cognition. Behavioural Processes. 2010;85:246–251. doi: 10.1016/j.beproc.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Wright AA, Cook RG, Rivera JJ, Shyan MR, Neiworth JJ, Jitsumori M. Naming, Rehearsal, and Interstimulus Interval Effects in Memory Processing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:1043–1059. doi: 10.1037//0278-7393.16.6.1043. [DOI] [PubMed] [Google Scholar]
- Wright AA, Cumming WW. Color naming functions for the pigeon. Journal of the Experimental Analysis of Behavior. 1971;15:7–17. doi: 10.1901/jeab.1971.15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA, Katz JS. Mechanisms of same/different concept learning in primates and avians. Behavioural Processes. 2006;72:234–254. doi: 10.1016/j.beproc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wright AA, Katz JS. A case for restricted-domain relational learning. Psychonomic Bulletin & Review. 2009;16:907–913. doi: 10.3758/PBR.16.5.907. PMID: 19815797. [DOI] [PubMed] [Google Scholar]
- Wright AA, Katz JS, Ma W. How to be proactive about interference: Lessons from animal memory. Psychological Science. 2012;23:453–458. doi: 10.1177/0956797611430096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA, Lickteig MT. What is learned when concept learning fails: A theory of restricted-domain relational learning. Learning & Motivation. 2010;4(4):273–286. [Google Scholar]
- Wright AA, Rivera JJ. Memory of auditory lists by rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:441–449. doi: 10.1037//0097-7403.23.4.441. [DOI] [PubMed] [Google Scholar]
- Wright AA, Rivera JJ, Katz JS, Bachevalier J. Abstract-concept learning and list-memory processing by capuchin and rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29(4):184–198. doi: 10.1037/0097-7403.29.3.184. [DOI] [PubMed] [Google Scholar]
- Wright AA, Roediger HL., III Interference processes in monkey auditory list memory. Psychonomic Bulletin & Review. 2003;10(3):696–702. doi: 10.3758/bf03196534. [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF. Monkey Memory: Same/Different concept learning, serial probe acquisition, and probe delay effects. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:513–529. [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory Processing of serial lists by pigeons, monkeys, and people. Science. 1985;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- Wright AA, Shyan MR, Jitsumori M. Auditory same/different concept learning by monkeys. Animal Learning & Behavior. 1990;18:287–294. [Google Scholar]
- Wright AA, Urcuioli PJ, Sands SF. Proactive interference in animal memory research. In: Kendrick DF, Rilling M, Denny R, editors. Theories of Animal Memory. Hillsdale N.J: Erlbaum; 1986. pp. 101–125. [Google Scholar]