Abstract
Strong evidence has accumulated over the last several years, showing that low sleep quantity and/or quality plays an important role in the elevation of blood pressure. We hypothesized that increasing sleep duration serves as an effective behavioral strategy to reduce blood pressure in pre-hypertension or type 1 hypertension.
Twenty-two participants with pre-hypertension or stage 1 hypertension and habitual sleep durations of 7 hours or less, participated in a 6 week intervention study. Subjects were randomized to a sleep extension group (48±12yrs,N=13) aiming to increase bedtime by one hour daily over a 6 week intervention period, or to a sleep maintenance group (47±12yrs,N=9) aiming to maintain habitual bedtimes. Both groups received sleep hygiene instructions. Beat-to-beat blood pressure was monitored over 24 hours and 24h-urine and a fasting blood sample were collected pre- and post-intervention.
Subjects in the sleep extension group increased their actigraphy-assessed daily sleep duration by 35±9min, while subjects in the sleep maintenance condition increased slightly by 4±9min (p=0.03 for group effect). Systolic and diastolic beat-to-beat BP averaged across the 24-hour recording period significantly decreased from pre- to post-intervention visit in the sleep extension group by 14±3 and 8±3 mmHg, respectively (p<0.05), Though the reduction of 7±5 and 3±4 mmHg in the sleep maintenance group was not significant, it did not differ from the BP reduction in the sleep extension group (p=0.15 for interaction effect). These changes were not paralleled by pre- to post intervention changes in inflammatory or sympatho-adrenal markers, nor by changes in caloric intake.
While these preliminary findings have to be interpreted with caution due to the small sample size, they encourage future investigations to test whether behavioral interventions designed to increase sleep duration serve as an effective strategy in the treatment of hypertension.
INTRODUCTION
High blood pressure, or hypertension, is a public health challenge worldwide, with an overall prevalence of more than 25% in the adult population (Kearney et al. 2004). There is strong evidence accumulated over the past years that blood pressure is affected by sleep quantity and quality. Epidemiological and clinical evidence suggest that individuals with shorter sleep durations have higher blood pressure (Gottlieb et al. 2006;Stranges et al. 2010;Vgontzas et al. 2009). In addition, experimental studies showed that when sleep is reduced or eliminated for one or more days, systolic and diastolic blood pressure (BP) increase in healthy controls (Meier-Ewert et al. 2004;Ogawa et al. 2003). Furthermore, Lusardi and colleagues (Lusardi et al. 1999) reported that hypertensive individuals respond to only a single night of sleep restriction to 4 hours with an increase in night time as well as day time BP. This finding suggests that hypertensive individuals may be at particular risk for the blood-pressure increasing effects of insufficient sleep.
As there is ample evidence that sleep loss may play a contributing role in the elevation of BP, this raises the question whether this effect is reversible. Several clinical trials have shown the effectiveness of behavioral modification strategies in the treatment of elevated blood pressure, in particular physical exercise, dietary changes, and weight loss (Dickinson et al. 2006). In this context, increasing the amount of sleep may serve as another behavioral strategy in the treatment of hypertension.
With respect to mechanistic pathways that may underlie the relationship between sleep duration and blood pressure, experimental studies have shown that shortening the duration of sleep leads to an increase in sympatho-adrenal (catecholamines), inflammatory, as well as metabolic markers (for review, see Mullington et al. 2009). Elevations of those markers are related to elevations of blood pressure (Bautista 2003);Currie et al. 2012), and therefore may serve as potential mediators of the association between sleep duration and blood pressure.
This study was conducted to test the hypothesis that increasing the duration of sleep lowers blood pressure in pre-hypertensive and hypertensive individuals. In addition, we studied changes of inflammatory, sympatho-adrenal, and metabolic markers that may act as potential mediators of the proposed blood pressure lowering effect of sleep extension.
METHODS
The study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center.
Subjects
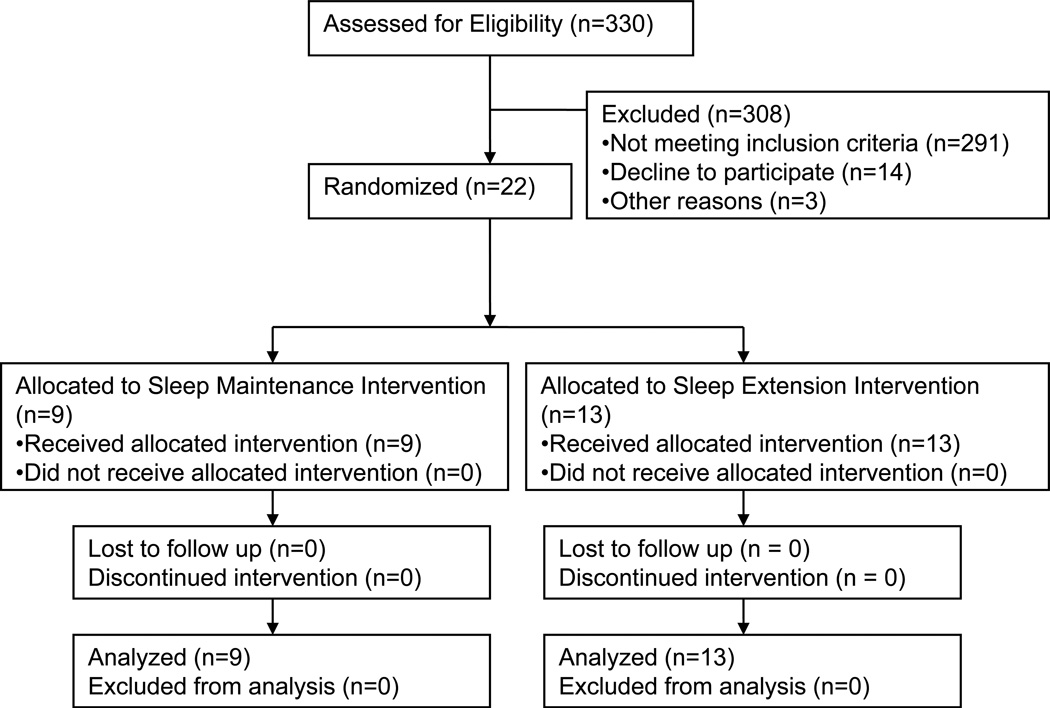
Twenty-two hyper- or prehypertensive subjects between the ages of 25 to 65 years completed the study (see Table 1 for subject characteristics and figure 1 for subject flow during the study). Subjects were recruited through online advertisements and paper fliers posted at various locations in the Boston area. Interested subjects were invited for an initial screening visit into the Clinical Research Center to have the study explained and to obtain written informed consent. Subjects were screened by a general health questionnaire and interview to collect detailed information about sleep and health status, and had their blood pressure assessed by a nurse (average of 5 measures at 3 minute intervals, using an automated oscillometric device; GE Medical SIT Inc., Milwaukee, WI).
Table 1.
Sample Characteristics
| Measure/Unit | Sleep maintenance group |
Sleep extension group |
P-Value | |
|---|---|---|---|---|
| N | 9 | 13 | ||
| Sex | Female/Male | 7/2 | 6/7 | 0.15 |
| Race | White/Black/ Asian/Other |
8/0/1/0 | 10/1/2/0 | 0.54 |
| Ethnicity | Non-Hispanic/ Hispanic |
9/0 | 12/1 | 0.59 |
| Age (yrs) | Mean±SEM Range |
48.4±12.3 31–64 |
46.9±12.3 26–64 |
0.78 |
| Screening BMI (kg/m2) | Mean±SEM Range |
26.0±1.2 22.5–34.2 |
26.5±1.2 21.8–38.0 |
0.77 |
| Antihypertensive medication* |
No/yes | 5/4 | 6/7 | 0.50 |
| Other medication** | No/yes | 7/2 | 11/2 | 0.55 |
| BP classification | Prehypertension/ Hypertension |
3/6 | 5/8 | 0.58 |
| Beat-to-beat SBP at baseline (mmHg) |
146±7 | 142±4 | 0.67 | |
| Beat-to-beat DBP at baseline (mmHg) |
84±3 | 82±3 | 0.75 | |
| Actigraphy-based sleep duration at baseline (hrs)*** |
Mean±SEM Range |
6.2±0.3 5.3–7.3 |
6.3±0.2 4.8–7.4 |
0.80 |
Antihypertensive medication: Sleep maintenance group: captoril (N=1), lisipronil (N=2), metapronol (N=1). Sleep extension group: atenolol (N= 2), diltiazem (N=1), diovan (N=1), hydrochlorothiazide (N=1), lisipronil (N=2).
Other medication: Sleep maintenance group: Levotyroxine (N=1), prilosec (N=1). Sleep extension group. Celexa (N=1), lovastatin/aspirin (N=1).
Actigraphy-based nighttime sleep duration averaged across a 2-week recording period.
P-value based on Fisher’s exact test for categorical variables and F test for continuous variables.
Figure 1.
Subject flow during the study.
Subjects met criteria for pre-hypertension, defined as systolic BP of 120–139 mmHg and diastolic BP of 80–89 mmHg (Chobanian et al. 2003), or hypertension type 1 defined as systolic BP of 140–159 mmHg and diastolic BP of 90–99 mmHg. The subject's primary care physician was also asked to verify a history of prehypertension or hypertension and an absence of other significant medical conditions. Eligible subjects had no current history of any severe medical disorder, including sleep disorders (verified with screening polysomnography during baseline assessment), psychiatric, neurological, endocrinological, or cardiovascular disorder, except prehypertension or hypertension.
Subjects were either on no medication or stable medication from 6 weeks prior to the study until the end of participation. Subjects were informed that the study will be discontinued and rescheduled in the case of a change in medication or medication dosage while in the study.
In addition, eligible subjects either had to have a habitual sleep duration of <7 hours, as determined with daily 2-week sleep log recordings or had to have a habitual sleep duration that was >1 hour shorter than their self-estimated sleep need (e.g., a self-estimated sleep need of 8.5 hours and a sleep diary-based duration of 7.0 hours).
Study Protocol
The duration of the study for each subject was approximately eight weeks, including:
2-week baseline period to assess habitual sleep duration via sleep diary/actigraphy
Baseline CRC visit to carry out 24 hours of continuous BP recording, polysomnography, and a fasting morning blood draw.
6-week intervention period consisting of either extending or maintaining habitual sleep duration as assessed in (a)
Follow-up CRC visit identical to the baseline visit
During the baseline and follow-up CRC visits, BP was recorded continuously throughout a 24h period, starting at 12pm. Sleep was recorded during the overnight bedtime period, in order to screen participants for breathing-related and limb movement disorders. Table 2 shows sleep parameters of the baseline night separately for the two intervention groups. Bedtime during the baseline CRC visit was scheduled based on the subject’s habitual lights out and lights on times, while bedtime at the follow-up CRC visit was based on the extended/unchanged habitual bedtimes (depending on randomized condition). The following morning, 1.5 hours after the end of the bedtime period, a fasting blood sample was taken after the subject was in a seated position for 15min. Twenty-four hour baseline and follow-up visits were scheduled during the week days as well as weekend days, depending on the time availability and preference of the subject. Before completion of the baseline visit, subjects were randomly assigned to one of the following groups:
Extension of habitual sleep duration: Habitual bedtimes, calculated from sleep log data, were extended by prolonging time in bed by 60 min daily for the following 6 weeks. Subjects were given bedtimes that started 30 min earlier and ended 30 min later than their usual lights out/lights on times.
Maintenance of habitual sleep duration: Subjects were instructed to stay on their habitual bedtimes for the following 6 weeks by providing bedtimes that matched their lights out/lights on times.
Table 2.
Sleep screening parameters.
| Parameter* | Sleep Maintenance | Sleep Extension | p-value |
|---|---|---|---|
| TRT (Total recording time, min) | 461±13 | 473±14 | 0.54 |
| TST (total sleep time, min) | 358±25 | 398±19 | 0.21 |
| WASO (wake after sleep onset, min) | 68.3±17.2 | 56.3±13.7 | 0.59 |
| SOL (sleep onset latency, min) | 21.0±8.2 | 16.8±3.8 | 0.61 |
| SE (sleep efficiency, %) | 77.3±4.2 | 84.0±2.9 | 0.19 |
| N1 (sleep stage N1, %) | 7.1±0.9 | 7.8±0.9 | 0.58 |
| N2 (sleep stage N2, %) | 39.5±4.3 | 47.8±2.4 | 0.10 |
| N3 (sleep stage N3, %) | 19.7±2.2 | 12.6±1.7 | 0.02 |
| R (sleep stage R, %) | 14.7±2.4 | 19.2±2.7 | 0.25 |
| AHI (apnea-hypopnea index, events/hour) | 3.9±1.7 | 2.9±1.2 | 0.61 |
| Limb movements (events/hour) | 4.6±1.4 | 3.5±0.7 | 0.48 |
| PLMS (periodic limb movements in sleep, series/hour) | 1.0±0.9 | 0.4±0.2 | 0.44 |
Note that sleep recordings were performed in the presence of 24h beat-to-beat BP recordings, which lead to a reduction of sleep efficiency of about 10% (unpublished observation).
In order to evoke similar study expectations and compliance in both groups, subjects in both groups were provided with, and asked to follow, a set of sleep hygiene recommendations designed to improve overall sleep habits. These instructions included information on optimal timing of exercise, fluid intake, alcohol consumption, eating habits; establishing an undisturbed and comfortable sleeping environment; and emphasized the importance of a regular sleep/wake schedule. Assigned sleep schedules and the sleep hygiene recommendations were discussed with subjects; this information was also provided in written form. For subjects in the sleep extension condition who could not increase their time in bed in the morning due to work- or family related requirements, their bed period was moved earlier by 60 minutes instead of 30min earlier (N=3).
Throughout the intervention phase, subjects were contacted by the PI on a weekly basis to discuss any problems with the protocol and enhance compliance. Following the 6-week intervention phase, subjects returned to the CRC for a follow up visit that was identical to the baseline visit.
Blood pressure recording and analysis
Ambulatory beat-to-beat blood pressure: The primary blood pressure outcome was beat-to-beat blood pressure recorded over the two 24-hour periods (pre/post intervention) by digital photoplethysmography (Portapres system, Finapres Medical Systems, Amsterdam, The Netherlands). Beat-to-beat blood pressure was measured with use of two small finger cuffs applied to the third and fourth fingers of the non-dominant hand, which inflate and deflate continuously (alternating between cuffs at 30-minute intervals). This methodology allows for continuous assessment of blood pressure over longer (hours, days) periods and consequently for more accurate assessment of blood pressure variations over the course of a 24-hour period. Previous data have demonstrated that Portapres values are highly correlated with intra-brachial measures of blood pressure over 24-hour periods (Castiglioni et al. 1999).
BP recordings were processed blinded with respect to study condition using Beatscope 1.1a and Matlab R2010a. Beats classified by the Beatscope software as artifact (physiocal beats [Finapres calibration mechanism], beats that did not have a pulse of 5 sec, beats with spiked waveforms, incomplete beats, beats with large oscillations, and beats that appeared damped) were automatically removed, as were beats that were greater than 3 standard deviations outside a 30-minute moving average window. Cleaned data were averaged into 15-minute bins and graphed for visual inspection. Two subjects in the sleep extension group were missing their follow-up portapres recording due to a mechanical failure in recording. One subject in the sleep maintenance group had baseline SBP values continuously above 200 mmHg; as this was likely due to a damaged finger cuff, this recording was excluded from further processing. Thus, three subjects entered mixed model analysis with data from one rather than two 24h-BP recordings. Outcome variables were systolic and diastolic BP, and heart rate (HR) estimated from beat-to beat BP values.
Automated oscillometric blood pressure: Standard oscillometric blood pressure measurements were collected every 2 hours during the waking period, and BP collected 1.5 hour after waking up were used to provide a standard clinical correlate for the continuous blood pressure measurements. To minimize variability in single time point assessment of blood pressure, subjects were seated quietly for 15 minutes prior to the collection of five blood pressure recordings using the automated Dinamap system (GE Medical SIT Inc., Milwaukee, WI). These measurements were averaged to create a single systolic, diastolic, and HR measurement for each measurement session.
Sleep recording
Sleep recordings (Embla® systems, Medcare US, Buffalo) and scoring were performed according to standard criteria (American Academy of Sleep Medicine 2007). Subjects with an apnea-hypopnea index of ≥15 or leg movements greater than 10/hour were excluded from participation, as these are indicative of obstructive sleep apnea and restless legs syndrome, respectively.
Sleep diary
Subjects kept a sleep diary for the duration of the study that was completed both before going to bed and after waking up. Information obtained was bedtime (the time the subject got into bed), lights out (the time of first attempted sleep), estimated time to fall asleep (sleep latency), number and length of awakenings during the night (wake after sleep onset or WASO), time of awakening in the morning, the total bed period (bedtime to time out of bed in the morning), time and length of daytime naps, and sleep quality upon awakening (assessed by 100mm visual analog scale with the anchor points of poorly [0] – excellent [100]). Sleep duration was calculated as time between sleep onset and final awakening in the morning, minus time spent awake during the night. Sleep diary-based bedtime (excluding from time into bed to lights out) was used to set the analysis period for the actigraphy recordings, and diary-based sleep duration was used as a validity check of acitgraphy-based sleep duration.
Actigraphy
An actigraph was worn concurrently with the collection of sleep diary data (Mini Mitter Actiwatch® 64, Respironics, Bend, Oregon). Data were sampled at an epoch length of 5min. Sleep indices were calculated using Actiware-Sleep 3.4 algorithms (Mini-Motionlogger, Ambulatory Monitoring, Inc Ardsley, NY). If actigraphy-based sleep duration and sleep diary-based sleep duration were discrepant by more than 2 hours, the day was excluded from further processing. This equated to 5.2% out of the 1574 recording days; this procedure has been previously used by (Mezick et al. 2009). Daily actigraphy data were averaged across the 2-week baseline period as well as weeks 1–2, 3–4, and 5–6 of the intervention period. Actigraphy data were not available in one subject due to a technical recording failure, reducing the sample size of the sleep extension group to N=12. Two other subjects (one in each group), had only a baseline actigraphy recording due to technical problems.
Food records were obtained in order to control for changes in nutrient intake during study participation. Once per week during both the baseline and intervention periods, subject were asked to list all items consumed at the time the foods were eaten, including portion sizes based on standard household measurements or dimensions. Records were analyzed using the Nutritionist Pro computer program (Nutritionist Pro, Axxya Systems version 4.6.0, Stafford, TX). Daily caloric and sodium intakes were averaged across study days in order to create averages for the 2-week baseline period and the 6-week intervention period.
Body weight and body fat measures were obtained at the baseline and the follow-up visit by the nutritional core at the Clinical Research Center. Percent body fat mass was measured using bioelectric impedance (Model Quantum II, Lean Body software, RJL Systems, Clinton Township, Michigan), and body mass index (BMI) was calculated as kg/m2.
Blood/urine collection and assays
An overnight fasting blood sample was taken 1.5 hours after lights on (end of bedtime period) at the baseline and the follow-up CRC visit. Prior to each blood draw, subjects spent 15 minutes resting comfortably in a seated position. Blood was collected on ice in K3-EDTA or Corvac tubes and immediately centrifuged. Urine was collected over a 24-h time period. Plasma/serum and urine was stored at −80°C until assayed.
White blood cells (WBC) were counted at the BIDMC lab using an Advia 2120 system (Siemens AG, Germany). Interleukin-6 (IL-6) was measured in plasma using a high sensitivity enzyme immunosorbent assay (ELISA, Quantikine ® HS, R&D Systems, Minneapolis, MN). The detection limit for this assay is 0.094 pg/ml. Our average intra-assay coefficient of variation was 5.2%; the manufacturer’s inter-assay coefficient of variation is 7.2%. High sensitivity C reactive protein (CRP) was measured in serum using an immunochemiluminometric assay (Labcorp.com). Norepinephrine was measured in urine by the Harvard Catalyst Central Laboratory using a radioimmunoassay (IBL America, Minneapolis, MN), and concentrations were calculated as per mg urinary creatinine (Labcorp.com).
Statistics
Linear mixed models were used to estimate the effects of sleep extension on the various outcome variables (sleep duration derived from actigraphy, blood pressure variables, inflammatory and metabolic-related markers). For blood pressure analysis, pre-post intervention visit, time of day (hourly from 12pm to 12pm the following day), protocol (sleep maintenance vs. extension), and the interaction between pre-post intervention visit by protocol were treated as fixed factors, whereas subject and the interaction between subject and pre-post intervention visit were treated as random effects. Simple contrasts were calculated to estimate at which time points values differed between pre- and post-intervention within groups or between groups. IL-6 and CRP values were log-transformed before statistical processing in order reach a normal distribution. PAWS 18.0 was used for statistical analysis (IBM SPSS, Chicago, IL). The alpha value was set to p<0.05 (2-tailed) for main effects, and p<0.10 (2-tailed) for interaction effects. Text and graphs report mean +/− SEM.
RESULTS
Table 1 presents subjects characteristics in the sleep extension and sleep maintenance group. Medication and medication dosage did not change throughout the protocol for any of the subjects, as captured by daily diary recordings.
Sleep duration
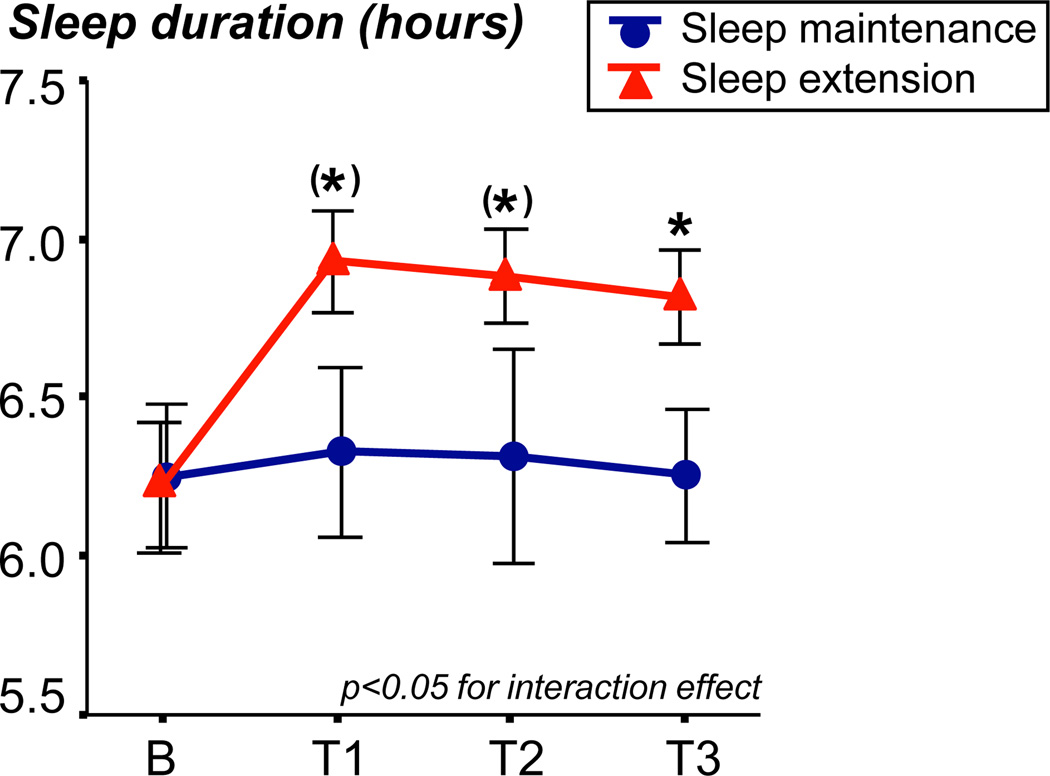
Figure 2 shows the average sleep duration as assessed by actigraphy averaged across the 2-week baseline phase, and weeks 1–2, 3–4, and 5–6 of the intervention phase in the sleep extension and sleep maintenance groups. A significant interaction effect between time and group (p=0.03) indicated the hypothesized increase of sleep duration in the sleep extension group, while sleep duration in the sleep maintenance group did not change. Compared to baseline, subjects in the sleep extension group increased their daily sleep duration during the intervention period by 35±9min, while subjects in the sleep maintenance condition increased slightly by 4±9min (p=0.03 for group effect). In addition, participants with a shorter sleep duration at baseline showed a greater increase in sleep duration during the extension phase (r=-0.71, p<0.01).
Figure 2.
Sleep duration based on actigraphy recordings in the sleep extension group (N=13) and sleep maintenance group (N=8). Data were averaged across 2 weeks of baseline recording (B), and across intervention weeks 1–2 (T1), 3–4 (T2), and 5–6 (T3). *p<0.05, (*)p<0.10.
Blood pressure
Beat-to-beat blood pressure (Portapres system)
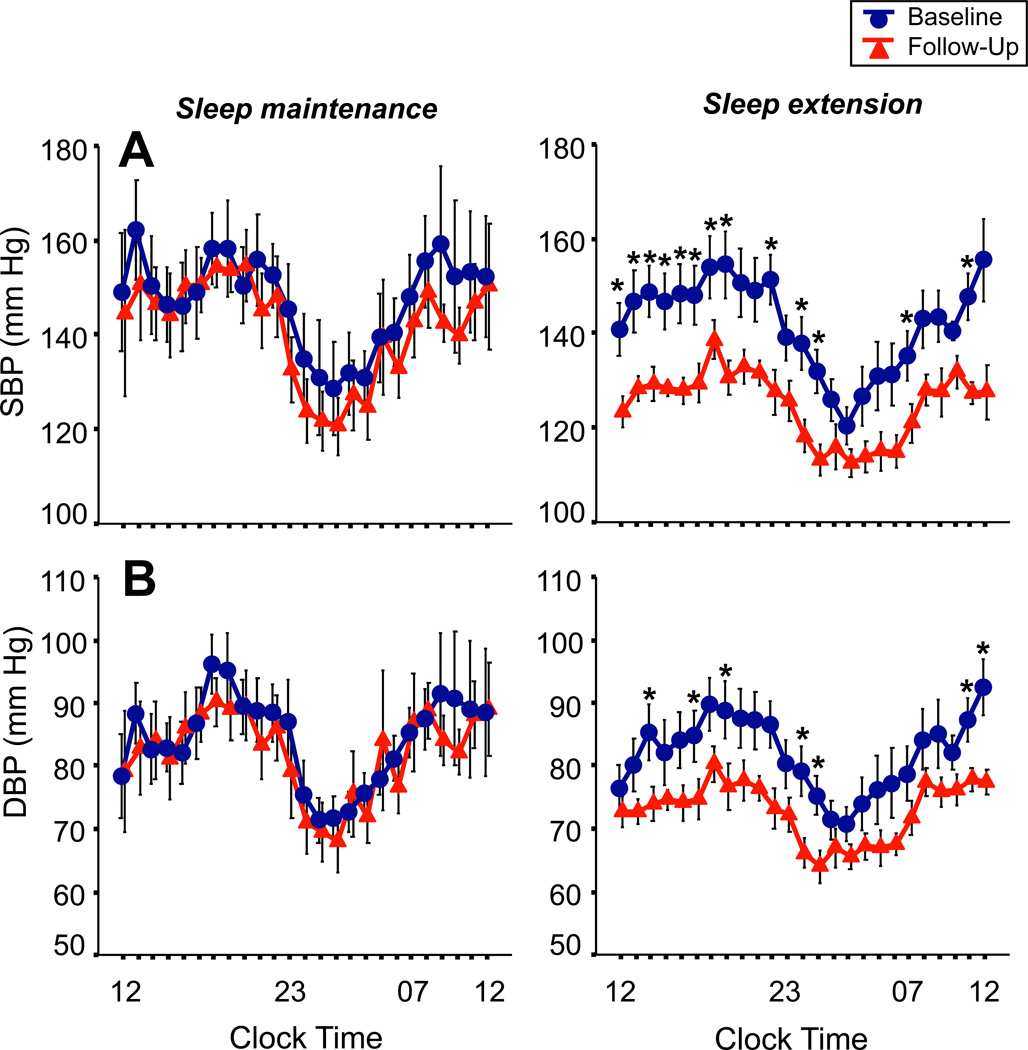
Figure 3A shows the beat-to beat systolic blood pressure (SBP) averaged in hourly intervals across the 24h recording period before and after the 6-week intervention period in the sleep maintenance and sleep extension groups. There was a significant pre- to post-intervention effect in the groups combined (p<0.01), while the interaction effect between group and pre-post intervention visit failed to reach significance (p=0.15). Analyzing the pre- to post-intervention effect for groups separately, there was a significant reduction of SBP values in the sleep extension group from pre- to post-intervention visit (p<0.001), while SBP values did not significantly differ in the sleep maintenance group between the pre- and post-intervention visit (p=0.45). Averaging across the 24h recording period, SBP decreased from pre- to post intervention by 7±5 mmHg in the sleep maintenance group, and by 14±3 mmHg in the sleep extension group.
Figure 3.
Systolic blood pressure (SBP, panel A) and diastolic blood pressure (DBP, panel B) across the 24h baseline (circles) and follow-up (triangles) period in the sleep maintenance group (N=9) and sleep extension group (N=13). *p<0.05 compared to follow-up measures.
Figure 3B shows the beat-to-beat diastolic blood pressure (DBP). Like SBP, there was a significant pre- to post- intervention effect in the groups combined (p<0.04), while the interaction effect between group by pre-post visit failed to reach significance (p=0.21). When analyzing the pre- to post-intervention effect for groups separately, there was a significant reduction of DBP values in the sleep extension group (p<0.02), while DBP values in the sleep maintenance group did not change significantly (p=0.64). Averaging across the 24h period, DBP values decreased by 3±4 mmHg from pre- to post-intervention in the sleep maintenance group, and by 8±3 mmHg from pre- to post-intervention in the sleep extension group.
Beat-to-beat heart rate (HR) did not show a significant pre- to-post intervention effect for the groups combined (p=0.19), nor a significant interaction effect between pre-post intervention visit and group (p=0.87). Averaging across the 24h period, HR values decreased by 1±1 beats/min from pre- to post-intervention in the sleep maintenance group, and by 2±2 beats/min from pre- to post-intervention in the sleep extension group.
While the sample size is small for correlational analysis, there was a trend towards a significant association between a decrease of beat-to-beat SBP across the 24h period and an increase in diary-based sleep duration in the sleep extension group (r=-0.53, p=0.09). Correlations between other sleep and BP measures did not reach or trended towards significance.
Oscillometric blood pressure (Dinamap system)
Oscillometric SBP measured 1.5 hours after waking up showed a significant pre- to post-intervention effect (p<0.05), but no interaction effect (p=0.82), due to a trend towards a significant decrease in SBP in both groups (sleep maintenance group: decrease of 8±4 mmHg, p=0.08; sleep extension group: decrease of 7±2 mmHg, p=0.07). Oscillometric DBP did not show a significant pre- to-post intervention effect in the groups combined (p=0.22), nor an interaction effect (p=0.83). Oscillometric DBP decreased slightly from pre- to post-intervention by 3±3 mmHg in the sleep maintenance group and 4±2 mmHg in the sleep extension group. Oscillometric HR did not show a significant pre- to-post intervention effect (p=0.10), nor a significant interaction effect between pre-post intervention visit and group (p=0.58). Oscillometric HR decreased slightly from pre- to post-intervention by 3±4 beats/min in the sleep maintenance group and 2±2 mmHg in the sleep extension group.
Inflammatory and sympatho-adrenal markers
As shown in table 3, WBC, IL-6, and CRP slightly decreased in both the sleep maintenance and sleep extension group from pre- to post-intervention visit, but there was no significant interaction effect between group and pre-post intervention visit (p>0.10). A slight decrease in both groups was also observed for urinary norepinephrine concentration from pre- to post-intervention visit, but the interaction term was not significant (p=0.12). When analyzing the pre- to post-intervention effect for groups separately, the slight reduction seen for WBC, CRP, IL-6, and norepinephrine was not significant for either the sleep maintenance group (p>0.10) or the sleep extension group (p>0.10).
Table 3.
Changes of inflammatory and sympatho-adrenal markers, body weight- and diet-related indices from pre- to post- intervention visit.
| Measure/Unit | Sleep maintenance group | Sleep extension group | Interaction | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | P-value | |
| WBC (K/uL) | 5.8±0.5 | 5.6±0.5 | 5.7±0.3 | 5.4±0.3 | 0.88 |
| IL-6 (pg/ml) | 0.93±0.23 | 0.89±0.19 | 1.12±0.18 | 1.09±0.22 | 0.61 |
| CRP (mg/L) | 0.67±0.21 | 0.48±0.12 | 0.71±0.21 | 0.59±0.12 | 0.12 |
| Norepinephrine (ng/mg Creatinine) | 38.1±5.1 | 34.4±4.3 | 32.0±4.0 | 27.9±3.9 | 0.92 |
| BMI (kg/m2) | 26.1±1.2 | 26.0±1.3 | 26.5±1.2 | 26.8±1.2 | 0.14 |
| Body fat (%) | 30.7±2.5 | 30.2±1.6 | 25.3±2.2 | 24.0±1.5 | 0.74 |
| Daily caloric intake (kcal) | 1914±256 | 1993±235 | 1895±235 | 1814±195 | 0.56 |
| Daily sodium intake (mg) | 2218±312 | 2621±332 | 2701±265 | 2610±302 | 0.39 |
Body weight and diet indices
As can be seen in table 3, BMI and body fat did not change from pre- to post intervention visit in either group (p>0.10 for interaction effect). In addition, no pre-to-post intervention effect was observed when analyzed for groups separately (p>0.10 for sleep maintenance group, p>0.10 for sleep extension group). Daily total caloric intake averaged across the 2-week baseline phase and 6-week intervention phase, respectively, did not differ significantly between groups (p=0.56 for interaction effect). In addition, intake of protein, carbohydrates, fat, or sodium did not differ between groups (p>0.10 for interaction effect). None of the dietary measures reached significance when analyzed for groups separately (p>0.10 for sleep maintenance group, p>0.10 for sleep extension group).
DISCUSSION
The current preliminary study demonstrates that increasing average nightly sleep duration by approximately 30 minutes over a 6-week period leads to a significant reduction of beat-to-beat systolic and diastolic blood pressure (BP) in hypertensive and pre-hypertensive subjects, while the control group who maintained their habitual sleep duration did not show a significant reduction in BP. Though the sample size is too small for a definitive test (i.e. interaction effect), findings suggest that behavioral interventions designed to increase sleep duration may serve as an effective strategy in the treatment of high blood pressure, and justify further investigations with larger sample size.
To date, findings on the effects of manipulating sleep duration on blood pressure come almost exclusively from studies in which sleep duration was experimentally reduced, rather than extended. For example, total sleep deprivation over 3 days in healthy volunteers showed an average increase of 7 and 4 mm Hg for systolic and diastolic BP, respectively (Meier-Ewert et al. 2004). Under experimental conditions mimicking more ecologically valid forms of short sleep durations, subjects restricted to 4 hours of sleep/night for 10 consecutive days had mean arterial pressures 6–8 mmHg above the control group who had 8 hours/night (Broussard et al. 2004;Mullington et al. 2009). In a clinical population of hypertensive and pre-hypertensive subjects, even half a night of sleep restriction has been shown to increase mean 24h systolic BP from 145 to 150 mm Hg (Lusardi et al. 1999). Further, in a clinical insomnia population, the highest risk of hypertension was found in those who also had polysomnographic sleep duration of less than 6 hours per night (Vgontzas et al. 2009).
Our preliminary findings suggest that the blood pressure-elevating effect of short sleep duration is reversible by increasing habitual sleep duration. This BP-lowering effect of sleep extension was evident after an intervention period of 6 weeks. As the study was designed with only a single follow-up visit, the time course of this effect is unknown (e.g., whether the change in BP was a function of successive days of sleep extension). A recent study failed to find a BP-lowering effect of sleep extension of 2 hours per weekend day over a period of one week in workers with habitual sleep durations of less than 6 hours (Kubo et al. 2011). However, blood pressure was assessed by only a single daytime measurement.
While beat-to-beat systolic BP decreased on average by 14 mmHg in the sleep extension group, we also saw a decrease by 7 mmHg in the sleep maintenance group. This reduction of BP in the sleep maintenance group may reflect habituation to the CRC environment, but may also reflect the fact that the sleep maintenance group was an active control (i.e., subjects in this group, as well as in the sleep extension group, were asked to follow a set of sleep hygiene instructions, such as maintaining regularity of bedtimes). Future studies may include a passive control group, in order to evaluate the effects of sleep hygiene alone on blood pressure.
Using the traditional oscillometric blood pressure measurement system (Dinamap), there was a significant overall reduction of SBP, with both groups showing a trend towards a significant reduction in SBP. DBP and HR did not differ from pre-to post intervention in either group. Thus, the marked reduction of beat-to-beat (Portapres) SBP and DBP in the sleep extension group was not captured by oscillometric BP monitoring (Dinamap) 1.5 hours after wake-up. This is likely due to the fact that beat-to-beat BP assessment reflects a more fine-grained measurement of BP, as it captures about 85,000 signals within a 24h-recording period, compared to much fewer signals by using the oscillometric BP system. In addition, previous work has demonstrated that BP recordings based on the Portapres system are more sensitive to intra-arterial BP changes than oscillometric BP recordings (Hirschl et al. 1996;Manios et al. 2007).
Compared to standard behavioural intervention strategies, such as exercise and dietary interventions, the BP reduction that we saw in response to increased sleep duration appears to be of comparable magnitude. For example, endurance training over a 6-month period has been reported to reduce intra-arterial SBP and DBP by 10 and 7 mmHg, respectively (Somers et al. 1991). Comprehensive lifestyle interventions over a 9-week period, including dietary and exercise interventions, reduced 24-hour ambulatory SBP and DBP by 10 and 5 mmHg, respectively (Blumenthal et al. 2010). Compared to these standard behavioural interventions, it appears that the sleep extension approach may serve as an effective strategy in the treatment of high blood pressure, either alone or in combination with standard lifestyle modification.
The current study also examined a number of potential mediators of the blood pressure lowering effect of sleep extension, such as changes in body composition and diet, as well as inflammatory and sympatho-adrenal markers. BMI and body fat, as well as total caloric intake and sodium intake did not change between the pre- to post- intervention visit in either the sleep extension or the sleep maintenance group. As such, it is unlikely that changes in body composition or diet may have contributed to the blood pressure lowering effect of sleep extension. We also did not see differences in white blood cell (WBC) counts, the inflammatory markers IL-6 and CRP, or the sympatho-adrenal marker norepinephrine, within or between groups from the pre to post intervention period. These markers have been shown to increase with experimental sleep loss in most studies(Dimitrov et al. 2007;Haack et al. 2007;Haack et al. 2009;Irwin et al. 1999;van Leeuwen et al. 2009;Vgontzas et al. 1999), and epidemiological studies have reported an association between high IL-6 and CRP levels with short sleep durations (for review, see (Mullington et al. 2009). In addition, high blood pressure has been associated with elevated inflammatory markers (Gillum and Mussolino 1994;Niskanen et al. 2004). While the current study findings did not show a change of inflammatory markers within 6 weeks of sleep extension, it is an open question whether a single measurement 6 weeks apart is sufficient to detect a meaningful change in these systems.
Several limitations apply to the current study. This preliminary study cannot address whether the BP-reducing effect of sleep extension is based on an actual increase in sleep duration, or is simply a result of an extension of time spent in bed. Though we found an association between the increase of sleep duration and decrease in blood pressure from pre- to post-intervention, this association is based on a small sample size. Future studies will need to control the effect of bedtime alone, by introducing an additional control group with increased bedtimes, but without an increase in sleep duration. Additionally, this is an exploratory study and given the small sample size, assessment of changes in sleep variables as predictors of the blood-pressure lowering effect of sleep extension was limited. For the same reason, we also did not investigate BP response differences between pre-hypertensive vs. hypertensive subjects, between subjects who are or are not on anti-hypertensive medication, or between women and men. As can be seen in table 1, groups are not identical with respect to sex ratio, and differential effects of sex on the BP-lowering effect may exist. This work will need to be accomplished in future studies.
Though current study findings have to be treated with caution due to the small sample size, they are supportive of the hypothesis that increasing habitual sleep duration can reduce blood pressure in pre-hypertensive and hypertensive adults. In light of the worldwide high prevalence of hypertension (Kearney et al. 2004) and its concomitant risks for cardiovascular disease (Wang et al. 2006), as well as the growing population characterized by short habitual sleep durations (National Sleep Foundation 2005), current study findings encourage investigations with greater sample size to test whether sleep extension can serve as a behavioural intervention strategy in the treatment of hypertension.
ACKNOWLEDEMENT
We thank Dr. James Ware, Harvard School of Public Health, Department of Biostatistics, and Dr. Shiva Gautam, Harvard Medical School, CTSC Biostatistics Program at BIDMC, for developing the optimal statistical approach.
This study was funded by grant number 0535241N from the American Heart Association to MH, R01 AG28324 from the National Institute of Aging to JMM, and grant number UL1 RR025758 and M01-RR-01032 from the National Center for Research Resources to the Harvard Clinical and Translational Science Center.
Footnotes
CONTRIBUTORS
MH and JMM developed the design of the study and were involved in data collection, analysis, and interpretation. JS and DC contributed to data collection and interpretation. NS and HME contributed to data interpretation. All authors participated in manuscript writing.
CONFLICT OF INTEREST STATEMENT
None.
References
- American Academy of Sleep Medicine. Rules, terminology and technical specifications. Westchester, IL, USA: 2007. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J. Hum. Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH Diet Alone and in Combination With Exercise and Weight Loss on Blood Pressure and Cardiovascular Biomarkers in Men and Women With High Blood Pressure The ENCORE Study. Arch. Intern. Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard J, Haack M, Serrador J, Mullington J. Effects of sleep restriction on blood pressure. Sleep. 2004;27:A171. [Google Scholar]
- Castiglioni P, Parati G, Omboni S, et al. Broad-band spectral analysis of 24 h continuous finger blood pressure: comparison with intra-arterial recordings. Clin. Sci. 1999;97:129–139. [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Currie G, Freel EM, Perry CG, Dominiczak AF. Disorders of Blood Pressure Regulation-Role of Catecholamine Biosynthesis, Release, and Metabolism. Curr. Hypertens. Reports. 2012;14:38–45. doi: 10.1007/s11906-011-0239-2. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J. Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30:401–411. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- Gillum RF, Mussolino ME. White blood-cell counts and hypertension incidence - The NHANES-I epidemiologic follow-up-study. J. Clin. Epidemiol. 1994;47:911–919. doi: 10.1016/0895-4356(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: The sleep heart health study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Haack M, Lee E, Cohen D, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: Potential mediator of increased spontaneous pain. Pain. 2009;145:136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschl MM, Binder M, Herkner H, et al. Accuracy and reliability of noninvasive continuous finger blood pressure measurement in critically ill patients. Crit. Care. Med. 1996;24:1684–1689. doi: 10.1097/00003246-199610000-00014. [DOI] [PubMed] [Google Scholar]
- Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J. Clin. Endocrinol. Metab. 1999;84:1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2004;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kubo T, Takahashi M, Sato T, Sasaki T, Oka T, Iwasaki K. Weekend sleep intervention for workers with habitually short sleep periods. Scand. J. Work Environ. Health. 2011;37:418–426. doi: 10.5271/sjweh.3162. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am. J. Hypertens. 1999;12:63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- Manios E, Vemmosa K, Tsivgoulis G, Barlas G, Eleni K, Spengos K. Comparison of noninvasive oscillometric and intra-arterial blood pressure measurements in hyperacute stroke. Blood Pressure Monitor. 2007;12:149–156. doi: 10.1097/MBP.0b013e3280b083e2. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinol. 2009;34:1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador J, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog. Cardiovasc. Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation Sleep in America Poll 2005 - Adult sleep habits and styles. Washington, DC 20005: 2005. [Google Scholar]
- Niskanen L, Laaksonen DE, Nyyssonen K, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–865. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26:986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- Somers VK, Conway J, Johnston J, Sleight P. Effects of endurance training on baroreflex sensitivity and blood-pressure in borderline hypertension. Lancet. 1991;337:1363–1368. doi: 10.1016/0140-6736(91)93056-f. [DOI] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Cappuccio FP, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- van Leeuwen WMA, Lehto M, Karisola P, et al. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. Plos One. 2009:4. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao DP, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with Objective Short Sleep Duration is Associated with a High Risk for Hypertension. Sleep. 2009;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Wang WY, Lee ET, Fabsitz RR, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease - The Strong Heart Study. Hypertension. 2006;47:403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]