Abstract
Fear and avoidance of activity may play a role in fostering disability in whiplash-associated disorders (WAD). This study examined the role of fear following WAD and assessed the effectiveness of three treatments targeting fear. People still symptomatic from WAD Grade I–II injuries approximately 3 months previously (n = 191) completed questionnaires (e.g., Neck Disability Index [NDI]) and were randomized to one of the treatments: (1) informational booklet (IB) describing WAD and the importance of resuming activities; (2) IB + didactic discussions (DD) with clinicians reinforcing the booklet; and (3) IB + imaginal and direct exposure desensitization (ET) to feared activities. DD and ET participants received three 2-hour treatment sessions. Absolute improvements in NDI were in predicted direction (ET = 14.7, DD = 11.9, IB = 9.9). ETs reported significantly less post-treatment pain severity, compared to the IB (M = 1.5 vs. 2.3, p < 0.001, d = 0.6) and DD (M = 1.5 vs. 2.0, p = 0.039, d = 0.6) groups. Reduction in fear was the most important predictor of improvement in NDI (β = 0.30, p < 0.001), followed by reductions in pain (β = 0.20, p = 0.003) and depression (β = 0.18, p = 0.004). The mediational analysis confirmed that fear reduction significantly mediated the effect of treatment group on outcome. Results highlight the importance of fear in individuals with subacute WAD, and suggest the importance of addressing fear, via exposure therapy and/or educational interventions, to improve function.
1.0 Introduction
Between 10 and 42% of people who experience a whiplash-associated disorder (WAD) develop chronic pain [2] and may experience other symptoms (post-traumatic stress, depression, and anxiety [18]). The Quebec Task Force on WADs developed a 4-category classification system [34]. The majority of WAD patients have the least severe injuries; namely, Grade I (neck symptoms, but no physical signs) or Grade II (neck symptoms plus musculoskeletal signs). The mechanisms underlying persistence of symptoms involve interactions among demographic (sex, age [23]), psychological (depression [6;15;21;29;43]), genetic [20], and accident-related [11] factors.
Studies have reported that WAD patients tend to avoid activities they fear will exacerbate their pain, produce further injury, or both [24]. The Fear-Avoidance Model (FAM) [41] postulates a downward spiral in which fear of pain/reinjury (also called kinesiophobia) leads to activity avoidance, resulting in physical deconditioning, loss of confidence, and delayed recovery. Research applying the FAM to WAD patients has, with some exceptions [4;5], generally supported the role of fear in symptom severity and chronicity [22;24;35;37;38],
Exposure therapy (ET) has been advocated as a treatment for low back pain patients who are fearful [40] and research has supported the efficacy of ET for these patients [12;17;44]. The efficacy of ET for patients with WAD has been supported in preliminary research [9], however the approach has not been studied systematically.
Educational programs, often combined with physical therapy (PT), have demonstrated efficacy in the treatment of some musculoskeletal disorders [30;42], but the efficacy of such programs for WAD patients is uncertain [10;12;32;33]. No research has been conducted to compare the effectiveness of ET and educational interventions to treat WAD or to examine the possibility that benefits from educational interventions are mediated by fear-reduction.
The emphasis of the present paper is the meditational role of fear in perpetrating WAD symptoms. The study assessed the role of ET and two kinds of educational interventions in promoting fear-reduction and clinical improvement among subacute WAD patients who: (1) had WAD symptoms for three months, (2) sustained Grade I or Grade II WAD; and (3) indicated significant fear of pain and/or reinjury. A six week randomized controlled trial was conducted comparing three groups: (1) informational booklet (IB) describing WAD symptoms and the importance of resuming normal activities; (2) IB + didactic discussions (DD) with a physician that amplified the IB; and (3) IB + desensitization to feared and avoided activities using imaginal and direct exposure (ET). We postulated that both intensive education (DD) and exposure therapy (ET) would benefit participants more than an informational booklet (IB), and that the effectiveness of the treatments would be mediated by fear reduction. We postulated that ET would be more effective than an educational program. Three primary hypotheses were tested: (1) improvement in neck disability from pre-to-post treatment will be: ET > DD > IB, (2) after collapsing across treatment groups, participants showing the greatest reductions in fear after treatment will demonstrate the most improvement in neck disability, and (3) reduction in fear mediates the association between treatment type and functional improvement.
2.0. Methods
2.1. Participants
Study participants were recruited through referrals from community physicians and by community advertisements. Inclusion criteria for treatment participation included: (1) significant neck pain attributed to a motor vehicle collision ([MVC] defined as maximal neck pain = four or greater on an 11-point scale, where 0 = no pain and 10 = worst pain possible, during the preceding week) approximately 2 months earlier (M months = 2.0 ± 0.8); (2) fulfilled the Quebec Task Force classification of Whiplash Associated Disorders (WAD) Grades I or II [34]; (3) no related hospitalization following the MVC; (4) no indication of loss of consciousness following the MVC; (5) symptoms associated with injuries to areas other than the neck were either absent or relatively minor, (based on the examining physician’s judgment and participants’ pain severity ratings of these injuries compared to ratings of their neck pain); (6) no current substance abuse; and (7) significant fear of neck-specific movements (defined as fear ratings of at least 4/10 on three or more of the Pictorial Fear of Activities Scale [PFActS-C] [35]). As shown in Figure 1, a total of 326 people were assessed for eligibility for the treatment program. Of this total, 100 were excluded because their WAD symptoms had subsided substantially or resolved completely by the time of initial assessment (i.e., in less than 3 months). Of the remaining 226 people, three were excluded because they had evidence of Grade III or Grade IV WAD, 30 were excluded because they did not demonstrate significant fear of neck-specific movements, and two refused to participate in treatment. The remaining 191 individuals were randomly assigned to three treatment groups: ET, DD, or IB. Specifically, when a block of six participants was found to be eligible, a computer-generated list of random numbers was used for allocation of the participants to one of the three treatment groups. This process was repeated for each newly eligible block of six participants. However, the enrollment rates varied somewhat leading to unequal numbers within groups (see Figure 1).
Figure 1.
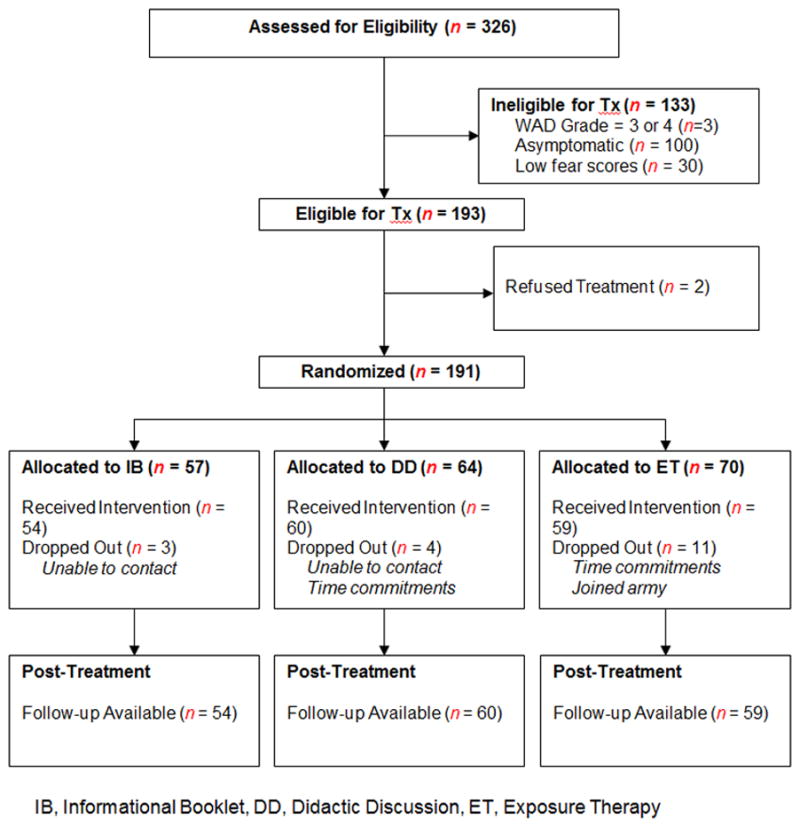
CONSORT Diagram of the study.
2.2. Measures
Participants provided demographic information (i.e., age, gender, and race), as well as information related to their symptoms and characteristics of the MVC (e.g., type of collision, perceived severity of collision, wearing seatbelt). Self-reported disability and psychosocial measures included: (1) neck disability as the primary outcome (Neck Disability Index [NDI] [39]; (2) pain severity on a 0 – 6 scale (subscale of the Multidimensional Pain Inventory [MPI] [16]); (3) pain interference (subscale of the MPI [16]); (4) depressed mood (Center for Epidemiological Studies Depression Scale [CES-D] [27]); (5) self-efficacy (Chronic Pain Self-Efficacy Scale [CPSS]) [1]; (6) pain-related anxiety (Pain Anxiety Symptoms Scale [PASS] [19]); (7) post-traumatic stress disorder (PTSD Checklist [PCLC] [28]); and (8) fear of specific neck movements (PFActS-C [36]). The PFActS-C asks participants to view 72 color photographs of a model engaged in various cervical movements and to rate (on a scale from 0 – 10) how much fear they would experience if they attempted the movement depicted in each picture. The PFActS-C has been shown to have high internal consistency (Cronbach’s alpha = 0.98), satisfactory test-retest reliability (ICC = 0.86), and convergent and discriminant validity as shown by correlations with the Tampa Scale of Kinesiophobia, PASS, Pain Catastrophizing Scale, and NDI [24;36].
2.2.1. Initial Evaluation
All study procedures were approved by the University of Washington Institutional Review Board. All participants were provided a comprehensive, three-hour initial evaluation consisting of physical and psychological assessments, including all measures described above. Following evaluation, each participant was randomly assigned to one of the three treatment conditions: IB (n = 57), DD (n = 64), or ET (n = 70).
2.3. Interventions
Information Booklet (IB)
Participants in the IB group received a booklet containing basic information about MVCs, whiplash injuries, and associated pain problems. The booklet described the structures that might be injured in WAD, distinguished between serious conditions (fracture or neurologic injury) and “soft tissue” injuries, and pointed out that physicians are usually unable to make specific diagnoses for patients with soft tissue injuries. It emphasized that once a serious condition has been ruled out and a reasonable amount of time (up to 3 weeks) has been allowed to promote initial healing, WAD patients can help themselves by gradually increasing their activity. Further emphasis was made that continued inactivity and excessive protection of the neck can impede recovery. The booklet mentioned that fear of pain/reinjury is understandable for WAD patients, but encouraged readers to recognize that fear is often excessive, and to make sure that they do not let this fear prevent them from gradually increasing their activity levels. Thus, the booklet discussed fear of pain/reinjury, but did not emphasize it or say anything about exposure as a way of addressing this fear [A copy of the booklet is available upon request from the authors]. Participants in the IB group continued their present care and no additional therapist contact was provided by the research team. Any improvement over time in this group was due to conventional care received, spontaneous remission, or due to information provided in the IB. This group served as the baseline against which the efficacy of the other treatments (described below) was compared.
Didactic Discussion + Information Booklet (DD)
Those in the DD condition received the educational booklet + three biweekly educational presentations in a one-on-one format. The first session included a physician, psychologist, and PT reviewing and expanding on information contained in the IB. The second and third sessions were conducted by a psychologist and a PT. During the first session, each of the three speakers delivered a 30-minute didactic presentation allowing 10–15 minutes for questions. During the second and third sessions, the PT and psychologist each delivered a 60-minute presentation, allowing 10–15 minutes for discussion. Topics covered by the physician, PT, and psychologist extended general information contained in the booklet. The physician specifically focused on: anatomical and neurophysiological aspects of whiplash injury and pain, changes over time in the factors involved in neck pain (distinguishing between initial factors such as inflammation, and later factors such as deconditioning or altered nervous system processing), and medications that might be used for neck pain. Topics covered by the psychologist included: stress and pain as consequences of MVCs, stress vs. relaxation responses, mechanisms of anxiety, and strategies for coping with stress. Topics covered by the PT addressed: pain and body mechanics, hurt vs. harm, sleep hygiene, activity regulation and pacing, the importance of gradual increases in activity in WAD, and flare-up management.
Exposure Therapy + Information Booklet (ET)
Those in the ET condition received the informational booklet + three biweekly skills training and exposure therapy (imaginal and in vivo desensitization) sessions in a one-on-one format. Prior to the first session, the psychologist selected PFActS-C photographs that a participant rated as fear-provoking during baseline assessment. Pictures that received the highest ratings were designated as “High Fear” pictures; pictures that had received the lowest ratings (though greater than zero) were designated as “Low Fear” pictures; pictures that had received intermediate ratings were designated as “Moderate Fear” pictures. Each participant had a unique hierarchy of movements based on their ratings of photographs from low-to-high fear; this was used to sequence stimuli during imaginal desensitization by imaginal or actual exposure to the activities.
The first session included the physician lecture as in the DD group, in addition to relaxation training by the psychologist. Also, during this session the psychologist asked each participant to imagine performing the movements depicted in 2–3 Low Fear PFActS-C photographs. The PT then showed the participant the same 2–3 pictures, and asked him or her to actually engage in the specific depicted movements. Participants were asked to relax before confronting pictures, and to attempt to remain relaxed while imagining or actually performing the movements depicted in the photograph. Exposure to a picture was repeated until a participant was able to report they were able to remain calm while imagining or actually carrying out the depicted movement (participants were asked to rate their level of concern or worry about performing the activity depicted on a 1–10 scale with 0 = No Concern or Worry, 10 = Very High Concern or Worry, and a reduction of 30% was used to classify that the participant was able to remain calm). During the second and third sessions, the psychologist and PT again had participants confront 2–3 photographs – Moderate Fear pictures in session two, and High Fear pictures in session three. The psychologist and PT conferred prior to each treatment session, and coordinated their activities so that a participant would receive imaginal and in vivo exposure to the identical photographs or activities depicted in the photographs.
2.4. Post-treatment Evaluation
Within 10 days after the third session (matched for the IB subjects), all participants received a post-treatment evaluation consisting of physical and psychological assessments. The psychological and self-report assessments were identical to those utilized in the initial assessment and evaluation.
2.5. Data Analysis
All analyses were performed using SPSS version 20.0. Descriptive statistics were calculated as means and standard deviations for continuous variables and percentages for categorical variables. One-way Analysis of Variance (ANOVA) or 2 analyses were performed (for continuous and categorical variables respectively), to determine whether participants in the three treatment groups differed with respect to baseline characteristics (e.g., age, race, severity of the collision).
To assess the effect of treatment on the primary outcome variable, NDI, the Analysis of Covariance (ANCOVA) method was utilized, using baseline NDI, age, and attorney involvement related to the MVC as covariates, and treatment group as the fixed factor. Similarly, ANCOVA was used to assess the effect of treatment for all secondary outcomes separately, with each of the respective baseline measures used as a covariate in each model, in addition to age and attorney involvement. Effect-size statistics in the form of partial eta2 were reported for all significant p-values. To identify significant pairwise differences among the three treatment groups, all significant p-values for all ANOVA and ANCOVA tests were followed-up with post-hoc tests using the Bonferroni procedure. Effect size statistics in the form of Cohen’s d [8] were reported for all significant pairwise differences. Following the analysis for the effect of treatment, a sequential multiple regression model (collapsing over treatment group) was used to test the predictive effect of fear-reduction on improvements in functional scores, after adjusting for pre-to-post treatment changes on all other physical and psychosocial measures.
Finally, a mediation model was tested to assess the meditational effect of pre-to-post changes in fear (i.e., PFActS-C) on the relationship between treatment group and pre-to-post changes in NDI (Figure 3), controlling for age and attorney involvement. Thus, we wanted to assess if any changes in our primary outcome measure were associated with changes in fear resultant from treatment, although we recognize that causality cannot be definitely established with cross-sectional data. In meditational analyses, two estimated effects are of central importance: the indirect effect and the direct effect of a predictor X on an outcome Y, through the mediator M. Specifically, the direct effect explicitly quantifies the change in the outcome Y that is due to a one-unit change in predictor X, when the mediating variable is held constant (c path). An estimate of the indirect or mediated effect quantifies the change in Y with a one-unit change in X, resulting from the effect of X on M, which in turn affects Y. This indirect effect is calculated through the product of the path coefficients from X to M (a path) by the path coefficient from M to Y (b path), estimating if the mediator accounts for some or all of the demonstrated relationship between X and Y [26].
Figure 3.
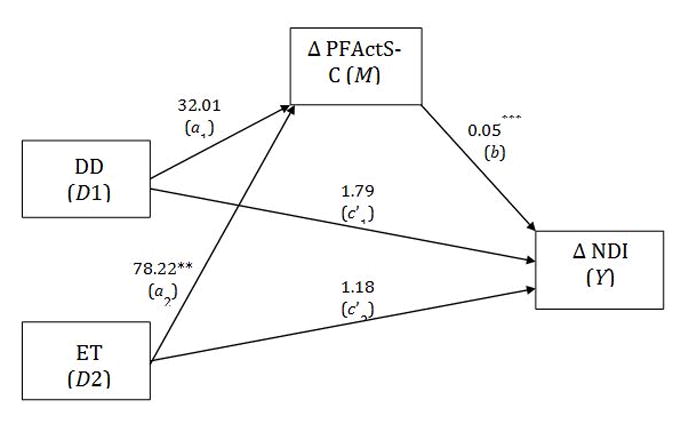
Mediational analysis for the relationship between treatment and functional improvement, through changes in fear.
DD = Didactic Discussions; ET = Exposure Therapy; PFActS-C = Fear; NDI = Neck Disability Index. *** = p < 0.001.
In the current study, the predictor variable X was treatment group, the mediator M was fear, and the outcome variable Y was change in NDI. A complicating issue was that instead of being a quantitative variable, the predictor variable was a qualitative variable with three categories. Therefore, the mediational analysis was modified using Hayes and Preacher’s path analytic technique for multicategorical predictor variables (Hayes AF, Preacher KJ. Indirect and Direct Effects of a Multicategorical Causal Agent in Statistical Mediation Analysis, under review). Using this approach, direct and indirect effects are calculated as discussed above, but are referred to as relative indirect and direct effects because they are estimated for the ET and the DD groups separately, compared to the IB group (the reference group). Thus, we simultaneously estimated the relative direct (c′i) and relative indirect (aib) effects of a three-category predictor variable X (i.e., treatment group) separately on changes in Y (i.e., functional improvement), through changes in M (i.e., fear). Similar to a direct effect using a continuous predictor, the relative direct effect estimates the relationship between treatment group and functional improvement, but separately for the DD (c′1 path) and ET (c′2 path) groups in comparison to the IB group (Figure 3).
Relative indirect effects are similarly modeled for each treatment group by first estimating the relationship between each of the DD and ET treatment groups and fear, relative to the IB group (a path). The coefficient reflecting the strength of the relationship is then multiplied by the coefficient for the relationship between fear and functional improvement (b path), after controlling for treatment group. Thus, two distinct indirect effects (a1b and a2b) were calculated to estimate if fear significantly mediated the effect of treatment on functional improvement, with one effect estimated for the ET group in comparison to the IB group, and the other effect for the DD group in comparison to the IB group. These effects were tested for significance using a non-parametric bootstrapping procedure that a) overcomes the non-normality of the cross-product of a and b, and b) provides bias-corrected, 95% confidence intervals based on 1000 bootstrapped samples. Statistical significance was interpreted using an alpha of 0.05.
3.0. Results
3.1. Demographic, Accident, and Baseline Characteristics
As indicated in the CONSORT Diagram (Figure 1), 87% (196/226) of the people with persistent WAD symptoms reported significant fear of some cervical movements. Tables 1 and 2 compare participants in the three treatment groups with respect to demographic variables, accident characteristics, PFActS-C scores, physical performance measures, and psychometric measures at baseline. A small and marginally significant effect was observed for differences in age among the groups. Otherwise, groups were equivalent on all the baseline variables. Although a slightly greater number of participants dropped out of the ET group (n = 11) compared to the DD (n = 4) and IB (n = 3) groups, differences in treatment completion rates among the groups were not statistically significant (p = 0.076) (See Table 1).
Table 1.
Demographic and Accident Characteristics (N = 191).
| Variables | IB N = 57 |
DD N = 64 |
ET N = 70 |
p-value | partial eta2 |
|---|---|---|---|---|---|
| Age (SD) | 35.1 (12.0) | 40.4 (12.4) | 36.4 (12.2) | 0.046† | 0.03 |
| Gender - % Female (N) | 70% (40) | 70% (45) | 71% (50) | 0.985 | |
| Race - % Caucasian (N) | 75% (43) | 75% (48) | 74% (52) | 0.631 | |
| Hit by other vehicle - % (N) | 87% (48) | 88% (52) | 91% (62) | 0.763 | |
| Rear-end collision - % (N) | 73% (35) | 74% (40) | 84% (52) | 0.596 | |
| Perceived severity of collision | |||||
| Moderately Severe or worse - % (N) | 86% (49) | 79% (50) | 77% (54) | 0.440 | |
| Wearing seatbelt - % (N) | 96% (55) | 91% (58) | 94% (65) | 0.406 | |
| Reduction in activities since collision - % (N) | 86% (49) | 85% (53) | 85% (58) | 0.994 | |
| Consulted Attorney - % (N) | 28% (16) | 25% (16) | 26% (118) | 0.928 | |
| Planning/Involved with Lawsuit - % (N) | 28% (15) | 16% (10) | 19% (13) | 0.259 | |
| Treatment Completers - % (N) | 95% (54) | 94% (60) | 84% (59) | 0.076 |
Note. Effect Size Magnitudes: Small (0.01); Medium (0.09); Large (0.25)
IB, Informational Booklet; DD, Didactic Discussion; ET, Exposure Therapy
Post-hoc tests did not reveal any significant differences between pairwise comparisons.
Table 2.
Mean Values for Baseline Physical, Functional, and Psychosocial Measures
| Variables | IB n = 57 | DD n = 64 | ET n = 70 | p-value |
|---|---|---|---|---|
| Neck-related Disability, NDI | 34.3 (15.4) | 32.2 (14.1) | 33.3 (14.1) | 0.737 |
| Neck-related Fear of Movement, PFActS-C | 253.3 (146.0) | 232.2 (168.0) | 245.4 (154.0) | 0.754 |
| Pain Severity, MPI | 3.2 (1.3) | 2.9 (1.1) | 3.1 (1.2) | 0.221 |
| Pain Interference, MPI | 2.9 (1.4) | 2.4 (1.2) | 2.8 (1.4) | 0.140 |
| Self-Efficacy, CPSS | 202.6 (55.2) | 210.7 (55.2) | 201.4 (58.7) | 0.593 |
| PTSD Symptom Checklist, PCLC | 36.6 (13.3) | 38.7 (15.9) | 40.7 (14.0) | 0.281 |
| Anxiety, PASS | 72.3 (26.4) | 66.4 (30.3) | 68.8 (27.0) | 0.512 |
| Depressive Symptoms, CESD | 19.3 (10.3) | 20.1 (9.3) | 20.6 (10.7) | 0.779 |
IB, Informational Booklet; DD, Didactic Discussion; ET, Exposure Therapy; NDI, Neck Disability Index; PFActS-C, Pictorial Fear of Activity Scale – Cervical; MPI, Multidimensional Pain Inventory; CPSS, Chronic Pain Self-efficacy Scale; PTSD, Post-traumatic Stress Disorder; PCLC, Post Traumatic Stress Disorder Checklist; PASS, Pain Anxiety Symptom Scale; CES-D, Center for Epidemiological Studies Depression scale. Standard deviations are listed in parentheses.
3.2. Treatment Outcomes
At post-treatment, all primary and secondary outcomes were analyzed using their respective baseline measure, age, and attorney involvement as covariates (Table 3). For the primary outcome of functional status as measured by the NDI, a moderate effect size among the groups was observed after adjusting for baseline NDI. Post-hoc tests indicated only the ET fared significantly better on the NDI at post-treatment compared to the IB group (M NDI = 18.9 vs. 24.4; p = 0.019; d = 0.4) (Figure 2).
Table 3.
Post-Treatment Outcomes Adjusted for Baseline Measures (N = 173)
| Variables | IB n = 54 | DD n = 60 | ET n = 59 | p-value | partial eta2 |
|---|---|---|---|---|---|
|
| |||||
| Primary Outcome | |||||
| Neck-related Disability, NDI | 24.4 (13.1) | 20.3 (13.7) | 18.6 (14.0)* | 0.017 | 0.05 |
|
| |||||
| Secondary Outcomes | |||||
| Pain Severity, MPI | 2.3 (1.4) | 2.0 (1.3) | 1.5 (1.3)† | < 0.001 | 0.09 |
| Neck-related Fear of Movement, PFActS-C | 158.1 (143.2) | 105.7 (139.2)* | 77.0 (117.0)* | 0.001 | 0.09 |
| Self-Efficacy, CPSS | 240.0 (48.2) | 244.7 (58.0) | 261.9 (44.0)* | 0.019 | 0.05 |
| PTSD Symptom Checklist, PCLC | 32.3 (9.4) | 28.9 (9.2) | 28.0 (11.2)* | 0.015 | 0.05 |
| Anxiety, PASS | 60.3 (24.0) | 49.1 (25.0) | 54.7 (25.7) | 0.006 | 0.06 |
| Pain Interference, MPI | 1.9 (1.3) | 1.6 (1.2) | 1.5 (1.5) | 0.060 | |
| Depressive Symptoms, CESD | 14.2 (10.2) | 11.9 (8.5) | 10.8 (9.1) | 0.060 | |
Note. Effect Size Magnitudes: Small (0.01); Medium (0.09); Large (0.25)
Significantly different compared to IB group only, in post-hoc tests.
Significantly different compared to both IB and DD groups, in post-hoc tests.
IB, Informational Booklet; DD, Didactic Discussion; ET, Exposure Therapy; NDI, Neck Disability Index; PFActS-C, Pictorial Fear of Activity Scale – Cervical; MPI, Multidimensional Pain Inventory; CPSS, Chronic Pain Self-efficacy Scale; PTSD, Post-traumatic Stress Disorder; PASS, Pain Anxiety Symptom Scale; CES-D, Center for Epidemiological Studies Depression scale. Standard deviations are listed in parentheses.
Figure 2.
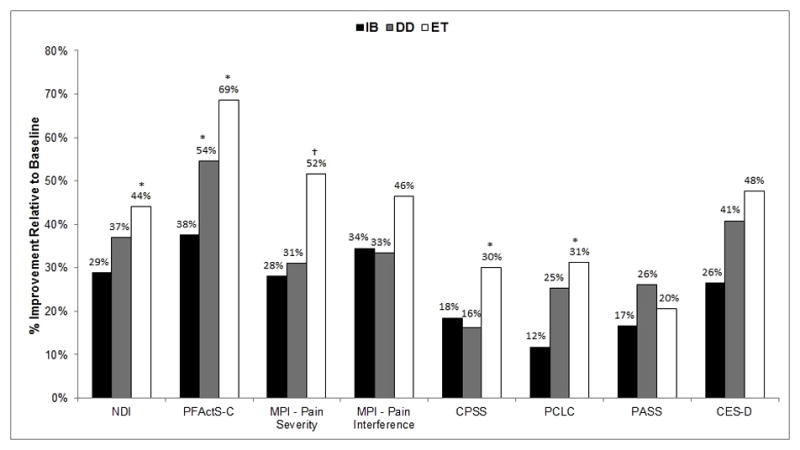
Overall improvement in post-treatment outcomes relative to baseline. IB, Informational Booklet; DD, Didactic Discussion; ET, Exposure Therapy NDI, Neck Disability Index; PFActS-C, Pictorial Fear of Activity Scale – Cervical; MPI, Multidimensional Pain Inventory; CPSS, Chronic Pain Self-efficacy Scale; PCLC, Post-traumatic Stress Disorder Checklist; PASS, Pain Anxiety Symptom Scale; CES-D, Center for Epidemiological Studies Depression Scale.
*Significantly different compared to IB group only, in post-hoc tests.
†Significantly different compared to both IB and DD groups, in post-hoc tests.
Secondary outcomes that were significantly different among the groups included measures of neck-related fear of movement (PFActS-C), pain severity, self-efficacy (CPSS), PTSD symptoms (PCLC), and anxiety levels (PASS) (Table 3). For the PFActS-C, post-hoc tests indicated the ET evidenced significantly lower fear levels, compared to the IB group (M PFActS-C = 77.0 vs. 158.1; p < 0.001; d = 0.6). Lower pain severity was also reported by the ET group, compared to both the IB group (M pain severity = 1.5 vs. 2.3, p < 0.001, d = 0.6) and the DD group (M pain severity = 1.5 vs. 2.0, p = 0.039, d = 0.4). Post-hoc tests on the CPSS revealed that the ET group demonstrated greater self-efficacy at post-treatment, relative to the IB group only (M CPSS = 261.9 vs. 240.0; p = 0.024; d = 0.5). In terms of PTSD symptoms at post-treatment, post-hoc tests only indicated significantly lower scores for the ET group, compared to the IB group (M PCLC = 28.0 vs. 32.3; p = 0.017; d = 0.4).
3.3. Changes within Treatment Groups
Within each of the three treatment groups, t-tests revealed statistically significant reductions in NDI from pre- to post-treatment (p < 0.001 for all comparisons). Summed over treatment groups, NDI scores dropped 35% - from a mean of 32.1 to a mean of 21.0. t-tests also revealed significant drops in PFActS-C scores from pre- to post- treatment within each group (p < 0.001 for all comparisons). Summed over treatment groups, PFActS-C scores dropped 52% - from a mean of 232.2 to 111.8.
3.4. Predictors of Improvement in NDI
Sequential multiple regression was conducted to determine the relative importance of the effect of neck-related fear of movement on improvement in functional status (NDI), after adjusting for pre-to-post changes on several other variables that might be expected to be associated with improvement in NDI (Table 4). The first step in the sequential analysis revealed that changes in pain severity, self-efficacy (CPSS), depression (CES-D), PTSD symptoms (PCLC), Pain Anxiety Symptoms Scale (PASS), and Pain Interference accounted for 50% of the variance in NDI change. The second step revealed that change in fear was a significant (p < .001) additional predictor of NDI change, accounting for an additional 7% of the variance. Examination of weights revealed that reduction in fear was the most important predictor of improvement in NDI (β = 0.30, p < 0.001), followed by reductions in pain (β = 0.20, p = 0.003) and depression (β = 0.18, p = 0.004).
Table 4.
Relative contribution of PFActS-C towards Change in NDI from Baseline to Post-Treatment, adjusting for other measures (N = 173).
| Model | Beta | p-value | ||
|---|---|---|---|---|
|
| ||||
| 1 | R2 = 0.50 | (Constant) | 0.916 | |
| p < 0.001 | Δ Pain Severity | 0.23 | 0.001 | |
| Δ CPSS | −0.25 | 0.001 | ||
| Δ CES-D | 0.15 | 0.024 | ||
| Δ PTSD Checklist | 0.21 | 0.002 | ||
| Δ Pain Interference | 0.10 | 0.238 | ||
| Δ PASS | 0.07 | 0.283 | ||
|
| ||||
| 2 | R2 = 0.57 | (Constant) | 0.340 | |
| p < 0.001 | Δ PFActS-C | 0.30 | < 0.001 | |
| Δ Pain Severity | 0.20 | 0.003 | ||
| Δ CES-D | 0.18 | 0.004 | ||
| Δ CPSS | −0.18 | 0.005 | ||
| Δ PTSD Checklist | 0.17 | 0.008 | ||
| Δ Pain Interference | 0.05 | 0.506 | ||
| Δ PASS | 0.04 | 0.475 | ||
Note. NDI, Neck Disability Index; ROM, Range of Motion; PFActS-C, Pictorial Fear of Activity Scale – Cervical; MPI, Multidimensional Pain Inventory; CPSS, Chronic Pain Self-efficacy Scale; PTSD, Post-traumatic Stress Disorder; PASS, Pain Anxiety Symptom Scale; CES-D, Center for Epidemiological Studies Depression scale
3.5. Mediational Fear Analysis
Pre-to-post changes in fear were assessed as a potential mediator for the relationship between treatment group and pre-to-post changes in NDI, controlling for age and attorney involvement. As shown in Figure 3, being in the ET group was significantly associated with reductions in fear relative to the IB group, while this relationship was not significant for the DD group, demonstrating the efficacy of our ET. Further, when controlling for treatment, reductions in fear were significantly associated with improvement in function. The relative direct effects for both the DD and ET groups compared to the IB were not statistically significant. Traditional goodness of fit statistics are not appropriate to this modeling procedure because ordinary least squares regression is used to estimate the paths (rather than structural equation modeling), thus R and R2 values are instead used to assess model fit. The percentage of variance in functional improvement accounted for by our model was acceptable (R = 0.54, R2 = 0.30). In support of our hypothesis, the indirect effect for the ET group relative to the IB group was significantly associated with improvement in function (a2b = 3.82; SE = 1.20, 95% CI = 1.70, 6.34), but was non-significant for the DD group relative to the IB group (a1b = 1.56; SE = 1.23, 95% CI = −0.73, 4.14).
4.0. Discussion
The results of this study indicate that fear of pain/reinjury is common among people with subacute WAD, and that recovery from WAD among fearful people can be facilitated by treatment that reduces their fear. Specifically, the results confirmed our hypothesis that after collapsing across treatment groups, reduction in fear would be the most important predictor of improvement in function. Moreover, the mediational analysis confirmed our hypothesis that the effect of type of treatment on improvement in function was mediated by reductions in fear. This pattern of results supports the FAM, since it implies that fear of pain/reinjury contributes to delayed recovery among people with subacute WAD from MVCs. The effect of treatment group on pre-to-post treatment changes in neck function was statistically significant, and in the predicted order of ET > DD > IB. Post-hoc tests revealed that while the difference between the ET and IB groups was statistically significant, the difference between the ET and DD groups was not. The same pattern was found for changes in fear as a function of treatment group. The ET group did realize significantly greater improvements in pain severity and perceived self-efficacy than the DD and IB groups. One possible explanation of the failure of the ET group to show more definite superiority over the DD group is that the ET program was not optimally designed. The ET treatment consisted of only 3 exposure sessions, with participants receiving both in vitro (imaginal) and in vivo (actual) exposure to feared physical movements. Future research is needed to determine the relative efficacy of in vitro vs. in vivo vs. combination exposure treatment, and the optimum number of treatment sessions.
Fear reduction over the course of treatment, as measured by the PFActS-C, was substantial – 52% for the cohort as a whole. The amount of functional improvement observed in the three treatment groups was also substantial enough to be clinically as well as statistically significant. The minimally clinically important difference (MCID) on a scale such as the NDI is defined as “the smallest change that is important to patients” [21]. The MCID for the NDI has been found by different investigators to range from 3.5 [25] to 9.5 [7]. Mean improvement scores in all three treatment groups in this study met even the highest criterion (9.5), and in the predicted order: ET (M change = 14.7) > DD (M change = 11.9) > IB (M change = 9.9).
The above pattern of results highlights the distinction between two issues: (1) the importance of fear reduction as a determinant of clinical improvement in WAD patients with fear of movement/re-injury, and (2) the determination of what interventions are most effective in promoting fear reduction in order to cause clinical improvement. Our results strongly support the conclusion that fear reduction is associated with clinical improvement, but they are ambiguous with respect to the relative efficacy with which different interventions foster functional improvement via reductions in fear. They suggest that an educational intervention that emphasizes a rehabilitative approach to WAD and addresses common concerns of WAD patients is as effective, or almost as effective, as an intervention (ET) that historically has been viewed as the gold standard approach to treating fears. In fact, even a brief and inexpensive educational intervention – an educational booklet – was effective in promoting fear reduction and functional improvement in those with subacute WAD.
The effectiveness of the DD treatment runs counter to the body of research suggesting that educational interventions alone are not effective in treating WAD [13]. It is difficult to compare our results with those of the 15 studies analyzed in a recent Cochrane collection review of educational interventions for neck pain [13] because of substantial differences in the WAD patients studied, the specific educational interventions, and the outcome measures used. For example, only one of the studies in the review used the NDI [31], and baseline NDI scores for participants in that study (M = 14.2) differed markedly from baseline NDI scores for our cohort (M = 33.2). Our data support the conclusion that educational interventions can promote functional improvement in WAD patients if they focus on the need for patients with subacute WAD to take concrete steps to increase their activity levels, and address the barriers patients may encounter as they try to implement this strategy.
Our results are consistent with the FAM, and are especially congruent with a modification of the FAM recently proposed by Buitenhuis and de Jong [3]. This modified model, named the Causal Beliefs-Anxiety Model (CBAM), incorporates major elements of the FAM, including an emphasis of kinesiophobia as an important factor in delayed recovery from WAD. It also emphasizes the importance of dysfunctional belief systems in the development of chronic WAD symptoms. An important implication of the CBAM is that WAD patients with high levels of fear should benefit not only from ET, but also from information that counters catastrophic beliefs about WAD.
Two different types of fear were assessed in this study. The PFActS-C asked participants hypothetical questions – how afraid would they be to carry out actions depicted in photographs. The PCLC assessed PTSD symptoms attributable to their MVCs. Several studies have suggested the potential role of post-traumatic stress in instigating and maintaining symptoms following MVCs [e.g., 4;21;35]. Our data showed significant correlations between PCLC and PFActS-C scores, both at pre-treatment (r = 0.42), and post-treatment (r = 0.61). Also, participants in all three groups showed significant reductions in PCLC scores from pre- to post-treatment, and changes in PCLC scores correlated significantly with changes in PFActS-C scores (r = 0.32, p < 0.01). Thus, although PTSD symptoms from an MVC are logically distinguishable from fear of pain/reinjury, the two kinds of fear appear to overlap, to have similar effects on recovery, and respond to the same interventions.
The present study has several limitations. One is that although our analysis supported the hypothesis that changes in fear mediated the effects of treatment on NDI change, we did not perform serial assessments of fear and NDI that would have permitted more sophisticated longitudinal analytic schemes to elucidate the causal connections among treatment inputs, changes in fear, and functional recovery. Additionally, we did not have follow-up data to determine whether the treatment benefits we observed were sustained and by which groups. Another limitation is that our results are relevant only to people with subacute Quebec Task Force [19] Grade I and II WADs who indicate significant fear of movement. We do not consider this constraint to be serious, since an overwhelming majority of WAD patients have Grade I or Grade II WADs [14], and since fear is common among these patients. Indeed, in our cohort 87% (196/226) of the people with persistent WAD symptoms reported significant fear of some cervical movements. Still another limitation is that some of the improvement demonstrated by participants could have reflected spontaneous recovery resulting from the passage of time, rather than responses to the study interventions. A final limitation is that the participants were volunteers who responded to media announcements; they may not be representative of all individuals who have sustained WAD during the initial three months post MVC.
Despite these limitations, our data highlight the importance of persistent fear in delayed recovery among patients with subacute WAD Grades I and II who indicate at least moderate levels of fear about performing some physical activities, and support the hypothesis that treatment that reduces fear is likely to promote functional recovery for this population. The study did not clearly identify the treatment strategy that is most likely to foster functional recovery via fear reduction, but there was suggestive evidence favoring ET. Since both the ET and DD required comparable amounts of effort and therefore cost, the most reasonable strategy would seem to favor providing the ET. However, the improvement demonstrated by the DD participants suggests that well designed educational programs can also be helpful fearful WAD patients.
The results of this study have important implications. They suggest that, for the roughly 85% of WAD patients who indicate fear about performance of physical activity, it is important to identify, understand, and address their individual fears about pain as these add to the burden of pain, pain-related activity limitations, and potentially contribute to the development and maintenance of disability. It is reasonable to expect that addressing these fears in the early stages would facilitate WAD recovery.
Acknowledgments
Support for this study was provided by the National Institute of Arthritis and Muscularskeletal and Skin Diseases grant #R01 AR 47298, ClinTrials.gov: NCT00021476.
Footnotes
Exposure therapy treatment targeting fear of movement in individuals with whiplash-associated disorder is demonstrated as an effective means for functional improvement and pain reduction.
Conflict of Interest: There is no conflict of interest associated with any of the co-authors of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 2.Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58(3):283–307. doi: 10.1016/0304-3959(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 3.Buitenhuis J, de Jong PJ. Fear avoidance and illness beliefs in post-traumatic neck pain. Spine (Phila Pa 1976) 2011;36(25 Suppl):S238–243. doi: 10.1097/BRS.0b013e3182388400. [DOI] [PubMed] [Google Scholar]
- 4.Buitenhuis J, de Jong PJ, Jaspers JPC, Groothoff JW. Relationship between posttraumatic stress disorder symptoms and the course of whiplash complaints. J Psychosom Res. 2006;61(5):681–689. doi: 10.1016/j.jpsychores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Buitenhuis J, Jaspers JPC, Fidler V. Can kinesiophobia predict the duration of neck symptoms in acute whiplash? Clin J Pain. 2006;22(3):272–277. doi: 10.1097/01.ajp.0000173180.54261.0a. [DOI] [PubMed] [Google Scholar]
- 6.Carroll LJ, Liu Y, Holm LW, Cassidy JD, Cote P. Pain-related emotions in early stages of recovery in whiplash-associated disorders: their presence, intensity, and association with pain recovery. Psychosom Med. 2011;73(8):708–715. doi: 10.1097/PSY.0b013e31822f991a. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89(1):69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J: Lawrence Erlbaum; 1988. [Google Scholar]
- 9.de Jong JR, Vangronsveld K, Peters ML, Goossens ME, Onghena P, Bulte I, Vlaeyen JW. Reduction of pain-related fear and disability in post-traumatic neck pain: a replicated single-case experimental study of exposure in vivo. J Pain. 2008;9(12):1123–1134. doi: 10.1016/j.jpain.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Derebery J, Giang GM, Gatchel RJ, Erickson K, Fogarty TW. Efficacy of a patient-educational booklet for neck-pain patients with workers’ compensation: a randomized controlled trial. Spine (Phila Pa 1976) 2009;34(2):206–213. doi: 10.1097/BRS.0b013e318193c9eb. [DOI] [PubMed] [Google Scholar]
- 11.Dolinis A. Risk factors for ‘whiplash’ in drivers: a cohort study of rear-end traffic crashes. Injury. 1997;28:173–179. doi: 10.1016/s0020-1383(96)00186-6. [DOI] [PubMed] [Google Scholar]
- 12.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40(11):694–704. doi: 10.2519/jospt.2010.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross A, Forget M, St George K, Fraser MM, Graham N, Perry L, Burnie SJ, Goldsmith CH, Haines T, Brunarski D. Patient education for neck pain. Cochrane Database Syst Rev. 2012;3:CD005106. doi: 10.1002/14651858.CD005106.pub4. [DOI] [PubMed] [Google Scholar]
- 14.Hartling L, Brison RJ, Ardern C, Pickett W. Prognostic value of the Quebec Classification of Whiplash-Associated Disorders. Spine (Phila Pa 1976) 2001;26(1):36–41. doi: 10.1097/00007632-200101010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Kamper SJ, Rebbeck TJ, Maher CG, McAuley JH, Sterling M. Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain. 2008;138(3):617–629. doi: 10.1016/j.pain.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 17.Leeuw M, Goossens ME, van Breukelen GJ, de Jong JR, Heuts PH, Smeets RJ, Köke AJ, Vlaeyen JW. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138(1):192–207. doi: 10.1016/j.pain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Mayou R, Bryant B, Duthie R. Psychiatric consequences of road traffic accidents. BMJ. 1993;307(6905):647–651. doi: 10.1136/bmj.307.6905.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50(1):67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- 20.McLean SA. The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine (Phila Pa 1976) 2011;36(25 Suppl):S226–232. doi: 10.1097/BRS.0b013e3182387fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67(5):783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 22.Nieto R, Miro J, Huguet A. The fear-avoidance model in whiplash injuries. Eur J Pain. 2009;13(5):518–523. doi: 10.1016/j.ejpain.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Ozegovic D, Carroll LJ, Cassidy JD. What influences positive return to work expectation? Examining associated factors in a population-based cohort of whiplash-associated disorders. Spine (Phila Pa 1976) 2010;35(15):E708–E713. doi: 10.1097/BRS.0b013e3181d12432. [DOI] [PubMed] [Google Scholar]
- 24.Pedler A, Sterling M. Assessing fear-avoidance beliefs in patients with whiplash-associated disorders: a comparison of 2 measures. Clin J Pain. 2011;27(6):502–507. doi: 10.1097/AJP.0b013e31820d97b0. [DOI] [PubMed] [Google Scholar]
- 25.Pool JJ, Ostelo RW, Hoving JL, Bouter LM, de Vet HC. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine (Phila Pa 1976) 2007;32(26):3047–3051. doi: 10.1097/BRS.0b013e31815cf75b. [DOI] [PubMed] [Google Scholar]
- 26.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: A self-report despression scale for research in the general population. Appl Psych Meas. 1977;3:385–401. [Google Scholar]
- 28.Saunders BE, Arata CM, Kilpatrick DG. Development of a crime-related post-traumatic stress disorder scale for women within the symptom checklist-90-revised. J Trauma Stress. 1990;3(3):439–448. [PubMed] [Google Scholar]
- 29.Schmitt MA, van Meeteren NL, de Wijer A, van Genderen FR, van der Graaf Y, Helders PJ. Patients with chronic whiplash-associated disorders: relationship between clinical and psychological factors and functional health status. Am J Phys Med Rehabil. 2009;88(3):231–238. doi: 10.1097/PHM.0b013e318198b684. [DOI] [PubMed] [Google Scholar]
- 30.Senlof P, Denison E, Lindberg P. Long-term follow-up of tailored behavioural treatment and exercise based physical therapy in persistent musculoskeletal pain: A randomized controlled trial in primary care. Eur J Pain. 2009;13(10):1080–1088. doi: 10.1016/j.ejpain.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Sherman KJ, Cherkin DC, Hawkes RJ, Miglioretti DL, Deyo RA. Randomized trial of therapeutic massage for chronic neck pain. Clin J Pain. 2009;25(3):233–238. doi: 10.1097/AJP.0b013e31818b7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soderlund A. The role of educational and learning approaches in rehabilitation of whiplash-associated disorders in lessening the transition to chronicity. Spine (Phila Pa 1976) 2011;36:S280–S285. doi: 10.1097/BRS.0b013e3182388220. [DOI] [PubMed] [Google Scholar]
- 33.Soderlund A, Lindberg P. An integrated physiotherapy/cognitive-behavioural approach to the analysis and treatment of chronic whiplash associated disorders, WAD. Disabil Rehabil. 2001;23(10):436–447. doi: 10.1080/09638280010008870. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E. Scientific monograph of the Quebec Task Force on whiplash-associated disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20(8 Suppl):1S–73S. [PubMed] [Google Scholar]
- 35.Sterling M, Chadwick BJ. Psychologic processes in daily life with chronic whiplash: relations of posttraumatic stress symptoms and fear-of-pain to hourly pain and uptime. Clin J Pain. 2010;26(7):573–582. doi: 10.1097/AJP.0b013e3181e5c25e. [DOI] [PubMed] [Google Scholar]
- 36.Turk DC, Robinson JP, Sherman JJ, Burwinkle T, Swanson K. Assessing fear in patients with cervical pain: development and validation of the Pictorial Fear of Activity Scale-Cervical (PFActS-C) Pain. 2008;139(1):55–62. doi: 10.1016/j.pain.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vangronsveld K, Peters M, Goossens M, Linton S, Vlaeyen J. Applying the fear-avoidance model to the chronic whiplash syndrome. Pain. 2007;131(3):258–261. doi: 10.1016/j.pain.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Vernon H, Guerriero R, Soave D, Kavanaugh S, Puhl A, Reinhart C. The relationship between self-rated disability, fear-avoidance beliefs, and nonorganic signs in patients with chronic whiplash-associated disorder. J Manip Physiol Ther. 2011;34:506–513. doi: 10.1016/j.jmpt.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manip Physiol Ther. 1991;14(7):409–415. [PubMed] [Google Scholar]
- 40.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther. 2001;39(2):151–66. doi: 10.1016/s0005-7967(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 41.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62(3):363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M, Balderson BH, Saunders K, Miglioretti DL, Lin EH, Berry S, Moore JE, Turner JA. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain. 2005;113(3):323–330. doi: 10.1016/j.pain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Williamson E, Williams M, Gates S, Lamb SE. A systematic literature review of psychological factors and the development of late whiplash syndrome. Pain. 2008;135(1–2):20–30. doi: 10.1016/j.pain.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 44.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136(3):271–80. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]


