Abstract
Among 12 billion injections administered annually, unsafe delivery leads to >20 million infections and >100 million reactions. In an emerging new concept, freeze-dried plant cells (lettuce) expressing vaccine antigens/biopharmaceuticals are protected in the stomach from acids/enzymes but are released to the immune or blood circulatory system when plant cell walls are digested by microbes that colonize the gut. Vaccine antigens bioencapsulated in plant cells upon oral delivery after priming, conferred both mucosal and systemic immunity and protection against bacterial, viral or protozoan pathogens or toxin challenge. Oral delivery of autoantigens was effective against complications of type 1diabetes and hemophilia, by developing tolerance. Oral delivery of proinsulin or exendin-4 expressed in plant cells regulated blood glucose levels similar to injections. Therefore, this new platform offers a low cost alternative to deliver different therapeutic proteins to combat infectious or inherited diseases by eliminating inactivated pathogens, expensive purification, cold storage/transportation and sterile injections.
Keywords: Autoantigens, bioencapsulation, diabetes, hemophilia, infectious diseases, lyophilization, molecular farming, oral vaccines
1. Introduction
Infectious diseases pose the greatest threat for human lives. Production of cost effective, safe and easily deliverable vaccines against current and emerging infectious diseases is needed. The morbidity and mortality rate due to infectious diseases are very high in developing countries and every year > 9.5 million people die [1], despite development of vaccines. This is because of several limitations of current vaccines including their safety, complex production methods that require prohibitively expensive fermentation systems, purification systems, cold storage, cold transportation and sterile delivery [2]. Although vaccination is considered to be the most efficient means for health intervention to combat infectious diseases, the high cost of vaccination makes it unaffordable for developing countries [2]. Injected therapeutics requires administration by health care professionals and imposes risks of contamination. There is also reduced patient compliance due to the physical discomfort.
Similarly, conventional delivery of therapeutic proteins utilizes sterile needle and syringe. Compliance is one of the most important factors, especially, among diabetic patients who may take more than 60,000 injections during their life time [3]. Although new technologies for the parenteral delivery of insulin, such as insulin injection port, pump, jet injector, and implantable pumps provide new alternatives to daily injections, there have been several disadvantages, including skin irritation, erythema, abscess formation, scarring, and site infections [4]. Currently, the commercial production of therapeutic proteins/vaccines is mainly dependent on bacterial, yeast or mammalian cell expression systems. The production also requires expensive purification steps, cold storage and additional delivery cost to patients, contributing to higher cost of drugs.
Parenteral injections via intramuscular, intravenous, intradermal, and other routes are widely used for delivery of therapeutic proteins and vaccine antigens. The WHO estimates that 12 billion injections are administered worldwide annually, of which 600 million are vaccinations and 11.4 billion for other treatments [5]. Unsafe medical injections have led to 15 million HBV infections, 1 million HCV infections, 340,000 HIV infections, 3 million bacterial infections and 850,000 injection site abscesses [6]. The site of virus and bacterial infection is the mucosal surface, parenteral vaccination has another immense disadvantage because the immune response produced is mainly systemic and little or no mucosal immunity is elicited [7]. So the development of more convenient and cost-effective delivery system has long been desired. There have been a great number of investigations on efficient delivery of therapeutic proteins/vaccines to patients who are dependent on long-term treatments. In this article, we review the literature on various delivery methods currently used or in development for administration of biopharmaceuticals, autoantigens and vaccines or vaccine antigens. The advantages and disadvantages of each method are discussed using specific examples.
Most importantly, we describe a new concept for oral delivery of therapeutic proteins, autoantigens and vaccine antigens expressed and bioencapsulated in plant cells. Freeze-dried plant cells (lettuce) expressing vaccine antigens or biopharmaceuticals are protected in the stomach from acids and enzymes but are released to the immune or blood circulatory system when plant cell walls are digested by microbes that colonize the gut. Oral boosters of vaccine antigens after priming confer both mucosal and systemic immunity and protection against pathogen or toxin challenge. Oral delivery of autoantigens confers tolerance and relief against allergic immune responses. Oral delivery of biopharmaceutical proteins confer desired functions (e.g. regulation of blood glucose levels).
This technology facilitates long-term storage of vaccines at room temperature and eliminates: cold storage, transportation, inactivated or attenuated pathogens and expensive fermentation, purification and sterile injections. Plant based expression system offers several unique advantages including low cost of manufacturing, easy scale up, minimal risk of contamination with human pathogens or toxins that are encountered in bacterial, yeast or mammalian cell culture systems. Three types of plant expression systems are currently used: transient plant viral system and stable expression via the plant nuclear or chloroplast genomes [2]. Among these, the plant chloroplast system is particularly suitable for oral delivery because of high levels of expression (up to 70% of the total leaf protein) of therapeutic proteins [8];, thereby facilitating large scale production (up to 300 million doses per acre) or delivery of appropriate doses for the human body [9,10]. In addition, harvest of leaves before emergence of reproductive structures and maternal inheritance of transformed chloroplast genomes containing genes coding for therapeutic proteins offer several layers of biological containment of transgenes [11,12]. Such containment addresses one of the major environmental concerns in using genetically modified plants. In addition, multigene engineering is feasible in a single step [13–15], facilitating parallel expression of several subunits of vaccine antigens [16–18] or biopharmaceuticals [8,19,20]. Therapeutic proteins expressed in chloroplasts form disulfide bonds and are properly folded and fully functional [20–23].
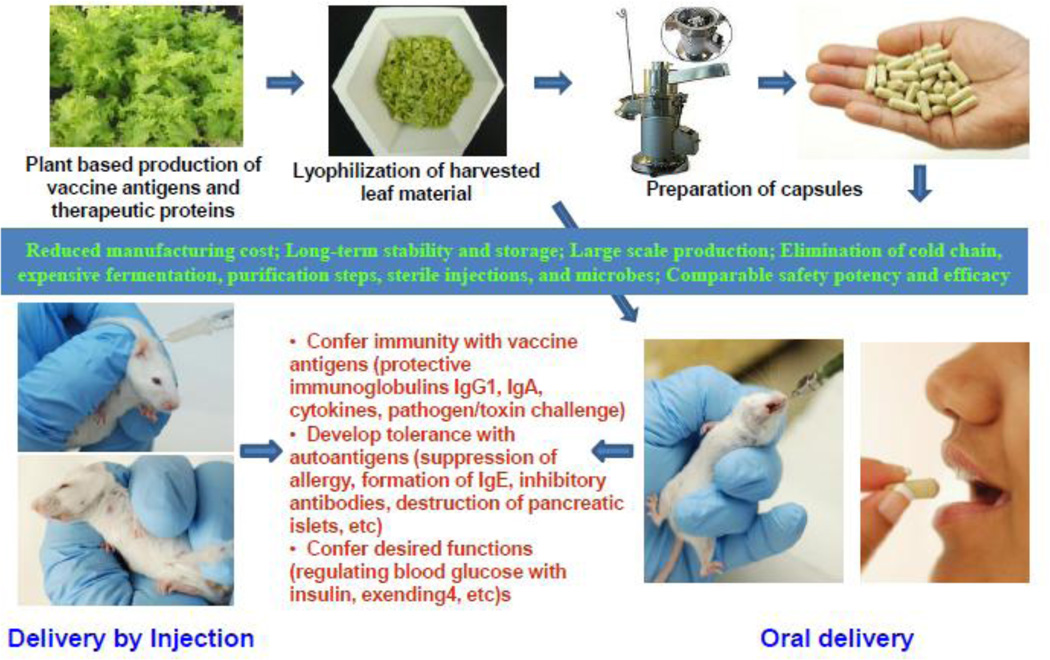
The general process for expression, bioencapsulation, lyophilization, preparation of capsules and evaluation of functionality of biopharmaceutical proteins, vaccine antigens or autoantigens, delivered by injections or oral gavage is described in figure 1. Foreign genes are first expressed in lettuce chloroplasts by bombardment of leaves with chloroplast vectors using the gene gun. After confirmation of stable integration of foreign genes into all of the chloroplast genomes in each plant cell -up to 10,000 copies of transformed genomes per cell [24] and absence of any native chloroplast genome and expression of the correct size fully functional protein, genetically modified lines are transferred to the greenhouse for increasing biomass. Harvested leaves are lyophilized, powdered and stored in moisture free environment at room temperature. Machines are commercially available for processing lyophilized leaf materials into desired particle size and packaging into capsules. Lyophilization process also eliminates microbes that are usually present within intercellular spaces in fresh leaves. Therapeutic proteins maintain their integrity, folding (with disulfide bonds, pentameric or multimeric structures) for several months or years when stored at room temperature [25] and functionality (by conferring immunity with vaccine antigens or developing tolerance with autoantigens or regulating blood glucose with insulin, exendin-4, etc). In this review, we give selected examples of oral delivery of biopharmaceutical proteins (insulin, exendin-4), autoantigens (diabetes, hemophilia) or vaccine antigens (cholera, malaria, plague) bioencapsulated in plant cells and evaluation of their efficacy when compared with the injectable delivery system.
Fig. 1.
An outline of the process of oral delivery of plant-derived vaccine antigens and biopharmaceuticals. Foreign genes are first expressed in lettuce chloroplasts by bombardment of leaves with chloroplast vectors using the gene gun. After confirmation of stable integration of foreign genes into all of the chloroplast genomes in each plant cell and expression of the correct size protein and functionality, genetically modified lines are transferred to the greenhouse for increasing biomass. Harvested leaves are lyophilized, powdered and stored in moisture free environment. Machines are commercially available for processing lyophilized leaf materials into desired particle size and packaging into capsules. Evaluation process includes microbial count in lyophilized materials, integrity of therapeutic proteins after prolonged storage (folding with disulfide bonds, pentameric or multimeric structures) and functionality by conferring immunity with vaccine antigens (protective immunoglobulins IgG1, IgA, cytokines, pathogen/toxin challenge) or developing tolerance with autoantigens (suppression of allergy, formation of IgE, inhibitory antibodies, destruction of pancreatic islets, etc) or conferring desired functions (regulating blood glucose with insulin, exendin-4, etc).
2. Current delivery methods for biopharmaceutical proteins and vaccines
2.1 Parenteral delivery
The commonly used parenteral methods for vaccine delivery are subcutaneous, intramuscular, intradermal, intraperitoneal and intravenous injections. The type and quality of immune response depends on the route of parenteral administration as immune response elicited is based on the type of antigen presenting cells (APCs) encountered [26]. For example intraperitoneal is more often associated with uptake by macrophages, whereas intradermal is associated with uptake by dendritic cells (DCs) [27]. The current licensed vaccines such as measles, mumps and rubella are delivered thorough subcutaneous (s.c.). However, subcutaneous method of vaccine delivery is often associated with systemic and not mucosal immunity. Centers for Disease Control and Prevention (CDC) recommended intramuscular method for the delivery of vaccines containing adjuvant to avoid local irritation, inflammation, granuloma formation, or necrosis [28]. Based on the immunogenicity, influenza vaccines are recommended to deliver via intramuscular method [29]. Influenza vaccine has less reactogenity if administered intramuscularly than subcutaneously or intradermally [30,31].
In intradermal method, small amount of vaccine antigen is introduced into the dermal layers of skin using needle. Intradermal injection was first used for diagnosis of tuberculosis disease [32]. The dermis and epidermis are particularly known to be rich in DCs, a professional APC which can stimulate antigen-specific immune responses. Currently, intradermal delivery (i.d.) is used for Tuberculosis (BCG) and rabies vaccination. The efficiency of immune response in intradermal delivery method depends on the type of vaccine. In case of inactivated poliovirus vaccine (IPV), immune response of infants after two or three 0.1 ml doses by i.d. was equivalent to two 0.5 ml intramuscular doses [33].
2.2 Mucosal delivery
Mucus covers the surface of digestive, respiratory and urogenital tracts of human body. The viscous, elastic and highly adhesive mucus is the first contact point for foreign particulates entering the body. Due to the nature of the mucus, particulates are trapped and then rapidly cleared by the mucus clearance mechanism. Moreover, the mucus is constantly regenerated to protect the body. There have been lots of efforts to develop more efficient ways to deliver medications through the mucosal layers. Among several mucosal systems, oral and nasal routes for drug delivery have been extensively studied. Currently, to improve efficiency of drug delivery, other mucosal approaches including buccal, pulmonary, ocular, and rectal routes are also explored [34].
2.2.1 Mucosal delivery methods for treatment of diabetes
Oral route through gastrointestinal tract (GIT) is an ideal choice for administration of most drugs for its simplicity and convenience. For diabetes patients, this delivery route is very attractive because it can avoid invasive administration of insulin and peripheral hyperinsulinemia. However, enzymatic breakdown and low permeability of insulin in GIT still remain as major obstacles. To overcome these obstacles, several approaches including absorption enhancers, enzyme inhibitors, mucoadhesive polymers, and formulation vehicles (emulsions, liposomes, microspheres, and nanoparticles) have been explored. However, there are several side effects that should be addressed, such as increased possibility for passage of unwanted molecules into serum caused by absorption enhancers, disturbance of normal metabolism of other proteins caused by protease inhibitors, and detailed evaluation on toxicity of polymers used as vehicles [35, 36].
Nasal insulin route has been considered as an alternative to parenteral injection for the treatment of diabetes because of several advantages: large area and high vascularization for insulin absorption due to microvilli, enabling rapid absorption and fast onset; easy access to systemic circulation with minimal loss of insulin; bypass of harsh environmental conditions in the GIT and of first-pass metabolism in liver; and direct delivery of drug to the central nervous system via the olfactory region [37]. Like other routes, this delivery system also has some drawbacks. Bioavailability of high-molecular weight and polar drugs is very low, and mucociliary clearance reduces membrane transport of drug. Enzymatic degradation is another limitation [36]. Among several approaches for nasal delivery of insulin, chitosan-based mucoadhesive systems showed the ability to slow mucociliary clearance rate, in which blood glucose levels were reduced once insulin was administered with the mucoadhesive agents [38,39]. Therefore, there are some limitations in use of nasal insulin administration such as intra- and inter-individual differences in bioavailability of insulin, and an administrable dose of insulin at one time [36].
Buccal route for insulin delivery has several advantages: direct access to the systemic circulation through the internal jugular vein that allows insulin to avoid hepatic first-pass metabolism; low enzymatic activity; bypass of destructive environment of stomach; painless administration, easy drug withdrawal; and supply of constant, predictable drug concentration to the blood [40–42]. Detergents as absorption enhancers are mostly used to increase the permeation of insulin. But bitter taste discouraged acceptance, especially for long-term administration. Buccal delivery of insulin was successful when deformable phospholipid vesicles or transferosomes were used as carriers [43]. Mucoadhesive nanoparticles [44], mucoadhesive tablet system [45], buccoadhesive patches [46], mucobuccal sponges [47] and insulin spray systems [48] were developed to enhance bioavailability of insulin buccally. Bilaminated film for buccal insulin delivery was also developed in which the film was designed to release insulin unidirectionally towards the buccal mucosa and to avoid loss of drugs due to salivary wash out [49]. Ora-lyn™ is the only buccal insulin formulation available on the market until now. However, there are some limitations for using buccal delivery. Salivary scavenging and small area available for drug absorption are common disadvantages [42].
Unique anatomical features of alveoli make lung an efficient delivery route for drugs because the alveoli have a large surface area available for an absorption up to 100 m2, a very short diffusion path of 0.5 µm to systemic circulation, and a dense capillary network up to 5 L of blood per minute [50]. Lower proteolytic activity in the lung is another advantage when compared to other administration routes [51]. All of the features allow quick access of drug into systemic circulation. So it has been considered that inhaled insulin could be an ideal alternative to parenteral injection of insulin. Various dry powder formulations of insulin have been developed for delivery via pulmonary route. Exubera is the first inhaled insulin to be delivered into lung, which appeared on the market in 2006 (Pfizer). This powder form of fast-acting human insulin was devised to treat type I and II patients, but it was withdrawn from the market within one year for several reasons. Insulin consumed 10 times higher doses in amount when compared to subcutaneous injections. Therapy costs were increased 3 to 4 times higher when compared to the standard therapy, due to complex inhalation equipment. Security concerns were raised after FDA warned possible risk of lung cancer in ones who had smoking background [51]. So Exubera lost its popularity in the market from physicians and patients. After Exubera’s discontinuation, as of January 2012, MannKind’s inhaled insulin product (Afrezza) is still waiting for FDA approval [52].
Transdermal route could increase patient compliance by providing painless delivery methods. Additionally, there is no chance of drug degradation attributed by gastrointestinal enzymes and of first-pass liver effect that can prematurely metabolize drugs [53]. However, human skin is well equipped with an effective barrier in which stratum corneum, outermost layer of skin, provides low impermeability against molecules, particularly large hydrophilic molecules [54]. Besides, another transport barrier is the tight junctions in viable epidermis [55]. Therefore, various approaches have been made to overcome these barriers for insulin delivery transdermally. Iontophoresis has been used in combination of absorption enhancers to carry insulin across skin [56,57]. Ultrasound [58], thermal ablation [59], microneedles [60,61], and microdermabrasion [62] were also carried out to administer insulin through skin. Recently, Phosphagenics completed human trial with its patented TPM/insulin formulation safely delivered insulin to patients with type 1 diabetes [63].
Taken together, mucosal delivery routes for insulin have been explored extensively to help millions of diabetic patients who depend on multiple daily insulin injections. To make mucosal delivery of insulin clinically successful, insulin should be delivered fast and accurately to exert its glucose lowering effect and the process should be reproducible each time of insulin delivery. Only Oral-lyn is still on the market as an oral delivery product for insulin administration after the failure of Exubera. Various other mucosal delivery systems of insulin [35] are in development as described in this section.
2.2.2 Mucosal delivery of autoantigens
Gut-associated lymphoid tissue (GALT) is largest immune organ in human body (~ 300 m2) comprising of large number of lymphoid cells distributed throughout the intestine (~1012 cells/m). It is responsible for mucosal immune response against foreign antigens and plays a primary role in creating tolerogenic environment by which tolerance induction occurs as a default immune mechanism. Depending on the dose of antigen administered, tolerance types are changed from regulatory T-cell induction (low dose) to anergy/deletion (high dose). In addition, microbiota living in intestine provides an additional major source of natural antigenic stimulation. Thus, the tolerogenic environment can be created by complex interplay between commensals, T cells, and DCs [64].
In order to be successfully delivered via oral route, autoantigen should pass through the gastrointestinal tract and reach the mucosal surface. Oral delivery of autoantigens as purified proteins packaged in microspheres, expressed in hemolymph of silk worms, bioencapsulated in plant cells or engineered in Lactococcus lactis conferred protection against autoimmune diseases. However, non-uniformity of antigen content in microsphere and variable release rate are major disadvantages of this system. The CTB-insulin fusion protein expressed in hemolymph of silkworm larvae when fed orally to non-obese diabetic mice delayed diabetes symptoms [65]. At 35 weeks of age, all the mice receiving wild-type virus-infected hemolymph developed diabetes whereas in the CTB–insulin hemolymph receiving group, only 54% (8/15) of mice developed diabetes [65]. Oral inoculation of recombinant vaccinia virus (rVV) harboring the CTB fused to proinsulin gene (CTB-INS) and C-terminal peptide from glutamate decarboxylase (CTB-GAD) in NOD mice minimized hyperglycemia when compared to control mice with fully developed hyperglycemia by 25 weeks of age [66]. Only 60% of orally gavaged mice with rVV-CTB-INS and rVV-CTB-GAD developed hyperglycemia at the age of 31 weeks. In addition, insulitis was decreased in mice with oral inoculation of vaccinia virus with CTB proinsulin fusion gene expression cassette along with increased IgG1 titers indicating activation of Th2 response. However, it is not easy to expand the production of recombinant vaccinia virus, the inserted gene is occasionally deleted from the vaccinia virus vector and virus components are presented to antigen presenting cells instead of the autoantigen. Purified protein has been used for oral delivery studies in several investigations for therapy of autoimmune disorders. The CTB fused to three copies of peptide 531–545 (3p531) from GAD65 when fed orally to NOD mice showed less pancreatic inflammation and delayed diabetes development. The incidence of diabetes was 39% (7/18) in CTB-3p531 fusion protein administered in 35 weeks old NOD mice [67]. Although upon oral administration of purified protein or peptide disease symptoms were improved, they are degraded and hydrolyzed before reaching the absorption site and therefore is not a reproducible option for oral delivery of therapeutic proteins.
Plant-platform production of autoantigens has been also studied as an alternative method for oral delivery. Progression of diabetes was suppressed in NOD mice after oral administration of murine autoantigen glutamic acid decarboxylase 67 (GAD67) expressed in plant cells [68]. Further, combined immunotherapy with murine IL-4 and human GAD65 expressed in plant tissue increased IgG1 anti-GAD antibodies levels, generated T – regulatory cells and induced oral tolerance [69]. Allergen-specific induction of oral tolerance and improvement in symptoms against allergies triggered by pollen or mite has been shown when powdered rice seeds expressing corresponding T-cell epitopes were fed orally [70,71]. Moreover, the aberrant immune response was more effectively suppressed by fusing CTB with the T-cell epitope than the epitope alone [72]. Oral administration of potato tubers expressing CTB-insulin fusion protein (0.1% of total soluble protein) to NOD mice has been shown to reduce insulitis and improve diabetic symptoms [73]. At 30 weeks of age, 50% of mice were diabetic in the group fed with CTB–INS when compared with the 100% diabetic mice in the control CTB only group [73].
The nasal drug delivery system has been used due to abundant vascular plexus, easy accessibility, enhanced bioavailability by evading gastrointestinal damage and hepatic first pass metabolism and potential delivery to the cerebrospinal fluid by-passing the blood brain barrier via nose-brain pathway [37,74]. Immunotherapy of several autoimmune disorders has been explored using relevant autoantigens delivered via intranasal route for nasal tolerization. The perception of mucosal tolerance in experimental autoimmune glomerulonephritis (EAG, an animal model of Goodpasture’s disease) was examined by nasal administration of different doses (25, 100 and 250 µg/rat) of NC1 domain of alpha3 chain of type IV collagen (alpha3IVNC1) for 3 consecutive days in Wistar Kyoto (WKY) rats [75]. A dose-dependent outcome was seen with 250 µg dose leading to significant reduction in antibodies, proliferative response of splenocytes and intensity of crescentic glomerulonephritis. In Sjögren’s syndrome, alpha-fodrin has been identified as an autoantigen. The mice immunized intranasally with two different doses (1 and 10 µg) of alpha-fodrin had late development of antibodies with no substantial variation between the two doses and successfully hampered the progression of experimental Sjögren’s syndrome [76]. Further, the alpha-fodrin treated mice had significantly less lymphocytic infiltration in salivary glands and higher number of Foxp3+CD4+CD25+ regulatory T cells when compared to control groups with intranasal administration of phosphate buffered saline (PBS) or glutathione S transferase (GST).
The intranasal administration of altered collagen type II 263–272 peptide to tolerate rheumatoid arthritis (RA) generated in Lewis rats arrested the histologic lesion of the joints, improved body weight, lowered the arthritis scores and prevented the expansion of collagen induced arthritis [77]. Nasal tolerance has also been investigated to prevent antibody responses against therapeutic proteins used in replacement therapies for genetic disease. For example, antibody formation against coagulation factors was suppressed in murine models of hemophilia A and B following nasal peptide administration [78,79]. A suppressive immune response was observed, characterized by induction of Treg and upregulation of IL-10 and TGF-β. However, it is unclear whether this approach can robustly control the pathogenic antibody response in factor replacement therapy.
Immunotherapy of autoimmune type 1 diabetes has been investigated and suppression of diabetes was induced after intranasal treatment with altered B:9–23 peptide or truncated peptide (B:9–21) along with a mucosal adjuvant cholera toxin [80]. Further it was established that elimination of cytotoxic T cell epitope from the B:9–23 peptide by amino acid substitution was crucial for diabetes prevention using mucosal tolerance. The three tandem peptides (p217-236, p524-538 and p290-306) obtained from glutamic acid decarboxylase 65 fused to CTB has also been demonstrated to prevent the diabetes development in nasally immunized non-obese diabetic mice [81]. Although the nasal route of delivery has been demonstrated to be successful in several autoimmune models, the bioavailability of the delivered drug impacts the success of this route of delivery.
2.2.3 Mucosal delivery of vaccine antigens
Mucosal method of vaccine delivery has many advantages including easier administration, reduced adverse effects and potential for frequent boosting in addition to induction of mucosal immunity, which are local sites for pathogens infection [82]. Delivery of vaccine antigens via mucosal surface activates mucosal B and T-lymphocytes and this pathway stimulates S-IgA antibody response [83]. Thus, the mucosal method of vaccine delivery is often immunologically superior in protecting mucosal sites than needle-based delivery although parenteral delivery method generates protective systemic immune response [84,85]. The gastrointestinal tracts also develop an adaptive immune response, which distinguishes between dietary or commensal antigen and pathogenic invasions [26]. Moreover, the Peyer’s patches are distributed along the length of the gastrointestinal tract [86], and include specialized follicular-associated epithelial cells, APCs, and effector B and T cells [87] generating high immune response within the mucosa. So, mucosal tissues are heavily populated with cells of the immune system and intestinal lining contains more lymphoid cells and produces more antibodies than any other organ in the body [88]. Intestinal epithelial cells also express various cytokines, cytokine receptors and molecules involved in T-cell binding and potentially costimulation [89–91]. Generally mucosal vaccination is used to prevent initial stages of disease as well as to block its development [92]. Different immunization routes, such as oral, rectal, and intranasal, can induce generalized mucosal immune responses. The commonly used mucosal vaccine delivery routes are oral, nasal and rarely rectal.
Immunogenicity and protection of orally immunized animals with plant-derived vaccine antigens have been investigated. Although parenteral immunization is effective to control many infectious diseases, oral immunization is more effective against enteric diseases. It has been shown that mice immunized orally with CTB expressed in potato induced both mucosal and systemic immune response, which is sufficient to protect cholera infection [73]. In another report, orally immunized with CTB produced in rice induced CTB-specific IgG as well as IgA while no or low level of IgA was found in mice immunized with purified rCTB [93].
Recently, immunogenicity of a plant-derived chimeric EspA, Intimin and Tir (EIT) of E. coli O157:H7 have been studied in mice immunized either subcutaneously or orally with recombinant purified protein or soluble rEIT protein from transgenic canola seed. Highest level of serum anti-EIT IgG titers was observed when a single primary subcutaneous immunization was followed by four oral boosters. Fecal IgA titer was produced in mice groups with oral boosters while no IgA was induced in parenteral group [94]. This report shows that oral delivery method is more effective by inducing both mucosal and systemic immune response. Orally delivered plant-derived vaccine antigens have shown serum and mucosal immune responses with minimal safety concerns in pre-clinical trials [95]. Prime-boost vaccination strategies have also shown great potential for stimulation of long-term immune responses [96–98]. Subcutaneous prime/oral boost strategy has been successfully employed with chloroplast-derived antigens, such as plague F1-V fusion protein and CTB-malaria antigen fusions [16,18]. Moreover, orally immunized mice offered greater protection and immunity than subcutaneous mice when challenged with lethal dose of aerosolized Y. pestis [18].
Intranasal immunizations are simple and administered by drops or sprays [99]. In intranasal delivery system, lower doses are required to obtain comparable antibody titers when compared to oral immunization. Nasal administration enables antigens to access very specialized mechanisms for antigen sampling including antigen uptake by M cells [100]. Moreover, the human nasal mucosa is extremely rich in various DCs both within and beneath the epithelium [101] and vaccine uptake into the blood circulatory system by absorption through mucosa can be relatively fast [102]. Intranasal immunization of mice with influenza A/PR8 (H1N1) VLPs enhanced systemic and mucosal antibody responses and survived when challenged with lethal dose of homologous virus as well as a high dose of heterosubtypic virus challenge [103]. Humoral and cellular immune responses of plant-derived influenza H5N1 (A/Anhui/1/05) antigen alone or with adjuvant, bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) have been studied in intranasally or intramuscularly immunized mice. While intramuscular immunized mice with c-di-GMP adjuvant did not enhance the immune response to plant-derived influenza H5 antigen, intranasal c-di-GMP-adjuvanted vaccine induced strong mucosal and systemic humoral immune responses. Moreover, intranasal immunized mice stimulated Th1/Th2 profile with high frequencies of multifunctional Th1 CD4+ cells [104]. Th1 cytokines are known to stimulate cell-mediated immunity which will benefit in eliminating intracellular pathogens [105,106].
In spite of many attractive advantages of mucosal immunization over traditional injection routes, only few vaccines are approved for human use. An appropriate adjuvant is often required for effectiveness of vaccines delivered orally via mucosal route, which can initiate and support the transition from innate to adaptive immunity [107]. Mucosal adjuvants are required not only to boost mucosal and systemic immunity, but also to prevent the induction of mucosally induced tolerance [108]. Many cytokines have been evaluated as adjuvants and have shown the induction of antigen specific immune responses, mucosal immune response, cell-mediated immunity and protection against toxin or pathogen challenge following nasal delivery [109].
3. Oral delivery of pharmaceutical proteins encapsulated in plant cells
Plant-based production of pharmaceutical proteins for oral delivery has emerged as an excellent way to protect the therapeutic protein from harsh environment of gastrointestinal tract via encapsulation within the plant cell [2,110]. In addition, plant based expression system offers low cost of manufacturing, easy scale up, good manufacturing practice, ability to perform post translational modifications, no human pathogen risks accompanying animal-derived elements in manufacture and free from toxins that could contaminate bacteria or mammalian cell culture preparations. Moreover, expression of therapeutic proteins in chloroplasts of plants has added advantage of high levels of expression, transgene stacking and biological containment [12,111].
3.1 Mechanism of oral delivery of pharmaceutical proteins encapsulated in plant cells
Oral delivery of bioencapsulated CTB fused therapeutic proteins/peptides facilitates receptor-mediated delivery via the intestinal epithelium. In addition, introduction of furin cleavage site between the fusion protein and CTB facilitates the release of fused protein/peptide. The CTB domain expressed in chloroplasts folds properly into pentameric structure and binds to the GM1 receptors present on enterocytes and then enter the intestinal cells via endocytosis. GM1 receptors on lipid raft serves to concentrate CTB fused pharmaceutical proteins followed by retrograde trafficking of the receptor-ligand complex into the lumen of the endoplasmic reticulum through the Golgi cisternae and/or TGN. The therapeutic proteins fused to CTB are then cleaved by furin within the TGN and released into extracellular fluid and submucosal vessels, including the lymphatic system via exocytosis. Besides the receptor-mediated endocytosis, CTB fusion proteins could be directly taken up by GALT through M cells on Peyer’s patch [110]. The GFP protein was detected in ileum, liver, spleen tissue as well as within macrophages and dendritic cells after oral delivery of CTB-GFP fusion protein expressing leaf material to mice [110]. Immunostaining with cell specific marker antibodies showed that orally administered leaf material expressing CTB fused coagulation factor IX (CTB-FIX) was delivered to the ileum, especially to the space between M cells and CD11c+ dendritic cells in Peyer’s patches. Further, FIX was detected in plasma within two hours [112]. When CTB-EX4 was orally gavaged to mice, glucose lowering effect was observed within 30 min [25]. Therefore, more efficient oral delivery of therapeutic proteins could be achieved using chloroplast based expression of CTB-fused therapeutic proteins and addresses the current obstacles of low permeability of large molecules and rapid enzymatic degradation of proteins in GIT.
3.2 Oral delivery of functional peptides bioencapsulated in plant cells
Insulin is the key medication to treat diabetes and used for treatment of about 40% of diabetes patients. Type I diabetes patients are totally dependent on insulin injections. One third of patients of type 2 diabetes, which accounts for 90% of diabetes cases worldwide, require insulin therapy to control blood glucose level [113]. According to research report “Insulin Delivery Systems Market Analysis Forecast to 2014” by RNCOS, it is estimated that global diabetes population will reach around 552 million by 2030. Thereby, significant growth is expected in global insulin delivery systems at a CAGR (compound annual growth rate) of ~ 8% during 2012–2014. However, insulin is an unstable protein and has relatively short shelf life. Moreover, overdose of insulin could threaten patient’s life. So this necessitates diabetic patients to be more cautious to inject insulin with accurate doses, leading to serious noncompliance [113]. In addition, current insulin therapy does not include C-peptide, which is released during the maturation process of proinsulin into insulin. The C-peptide has already been known to ameliorate diabetes-induced complications [114].
For treatment of type 2 diabetes, a number of medications have been developed and marketed. Recently, three injectable forms of incretin-based peptides received approval by FDA, exenatide in 2005, liraglutide in 2010, and Bydureon (long acting form of exenatide) in 2012. These glucose-dependent insulin secretagogues have much longer half-life in the circulation when compared to their analogue, glucagon-like peptide-1 (GLP-1), which has a half-life less than 2 min. Among the insulin delivery systems (insulin syringes, pens, pumps, and jet injectors), insulin syringes and pens dominate the global insulin delivery systems [115].
Peptides have broad range of usefulness for clinical approaches. As therapeutic agents, various peptides were developed to treat human diseases such as endocrine disorders, infectious diseases, and cancers by acting as agonists or antagonists. Few such examples are, enfuvirtide (Fuzeon ®), inhibitor HIV entry into cells; EX4 (Byetta ®), agonist of insulin secretion; and Leuprolide (Lupron), anti-prostate cancer drug. In addition to pharmaceutical purposes, they can be also used as a carrier to shuttle chemotherapeuticals or a tracer to the tumor for imaging purposes or targeted therapy [116]. Functionality of these peptides is attributed to their small size, which provides high affinity and specificity towards target, non-immunogenic, and ability to disrupt protein-protein interactions and penetrate further into tissues [117]. Traditionally, these functional peptides were synthesized based on solid-phase chemistry, but now many of them are produced using recombinant technology. Currently, about 60 of peptides were approved as drugs, and about 140 peptides are under clinical trials [118]. However, these peptide drugs have several challenges. They are highly prone to enzymatic degradation, have very short life in the circulatory system, and no accessibility to intracellular targets. So there have been a lot of efforts to address these issues. To increase in vivo stability, Polyethylene Glycol (PEG) [119], antibody Fc domain [120], and human serum albumin [121,122] are fused to peptide drugs. Furthermore, various methods are used to protect functional peptides from serum proteases and peptidases, which include N-terminal acetylation, C-terminal amidation, the use of non-natural amino acids, and cyclization via disulfide bonds [123]. Despite such tremendous efforts, there is still no oral availability of peptide drugs. In addition, peptide drugs need to be stored at low temperature due to short shelf life after purification or chemical synthesis. Therefore, we describe recent progress in plant-based oral delivery of two functional peptides for the treatment of diabetes.
3.2.1 Proinsulin for treatment of type I and type II diabetes
Recently, Boyhan and Daniell [20] developed a strategy to treat diabetes in which transgene construct was designed to enable oral delivery of functional insulin. Three furin cleavage sites were introduced at CTB/B-chain, the B-chain/C-peptide, and the C-peptide/A-chain. These insertions of protease-recognizable sequence made the chimeric protein cleavable into mature insulin and a C-peptide, once administered orally. Proinsulin is processed via prohormone convertases PC2/PC3 into mature form in pancreatic β-cell. Therefore, maturation of proinsulin to insulin is specific for neuroendocrine cells. However, furin is an ubiquitous endopeptidase, which is located in trans-Golgi network (TCN), endosomal system, and cell surface [124]. So this newly designed construct of CTB-Proinsulin facilitates release of the functional insulin efficiently in the gut after oral delivery. Another feature of this system is that C-peptide can be released along with insulin due to the furin cleavage sites. There have been lots of clinical and experimental data to support the fact that C-peptide is a biologically active peptide. Administration of C-peptide to type I diabetes patient, who lack the peptide, showed the amelioration of diabetes-induced renal and nerve dysfunction [114]. For example, the C-peptide decreased glomerular hyperfiltration, reduced urinary albumin excretion and reversed renal structural abnormalities in type I diabetes. In addition, the peptide stimulated nerve Na+/K+-ATPase activity, increased endoneurial blood flow, increased nerve conduction velocity, and prevented nerve structural changes. Moreover, C-peptide is able to bind to insulin and induce disaggregation of its hexameric form, thereby increase the availability of biologically active, monomeric insulin [114].
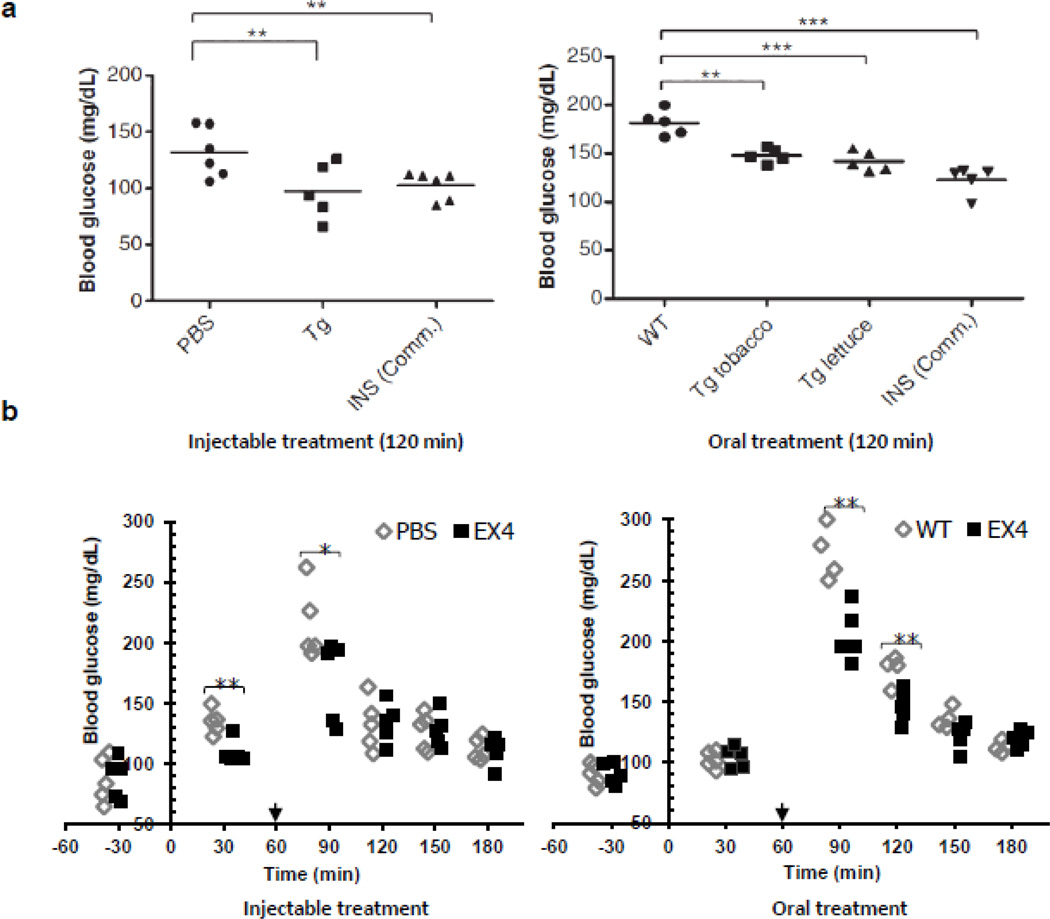
For bioencapsulated oral delivery of the functional insulin/C-peptide, both tobacco and lettuce were used to create transplastomic plants [20]. Each of the plant species showed stable expression of CTB-PFx3 (CTB-proinsulin containing 3 furin cleavage sites) and the functional pentameric structure of the fusion protein using GM1 ELISA assay. The expression level of the protein reached up to 53% and 47% of total leaf protein (TLP) in tobacco and lettuce, respectively. In animal model experiment, glucose lowering effect of purified CTB-PFx3 was comparable to that of commercial insulin, when administered intraperitoneally (Fig. 2a). The hypoglycemic effect was confirmed again in vivo. When mice were fed with CTB-PFx3-expressing tobacco and lettuce plant materials, blood glucose level was reduced 2 hr after oral gavage (Fig. 2a). It is noteworthy that CTB-PFx3 expressing lettuce also showed hypoglycemic effect comparable to the commercial insulin. This should facilitate further preclinical and clinical studies for low cost oral delivery of insulin and C-peptide.
Fig. 2.
Functional evaluation of CTB-proinsulin and CTB-exendin-4 delivered by injection or oral gavage in mice. (a) Blood glucose level after intraperitoneal (IP) injection of purified CTBP-Fx3, commercial insulin (INS Comm.) and oral delivery of control (WT), plant cells expressing CTB-PFx3 (Tg tobacco, Tg lettuce). © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2010) 9: 585–598 [20]. (b) Blood glucose level after intraperitoneal (IP) injection of purified commercial EX4 and oral administration of untransformed tobacco (WT), tobacco expressing CTB-EX4 (EX4). PBS was also injected as negative control. Arrows indicate the time point of glucose spike. *P < 0.05, **P < 0.01, ***P<0.001. © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2012): In Press [25].
3.2.2 Exendin-4 peptide for treatment of type II diabetes
Next, we investigated oral delivery of EX4 for the treatment of type II diabetes. EX4 was first isolated from salivary gland of lizard Heloderma suspectum. This 39-amino acid peptide hormone has 53% similarity to mammalian GLP-1 in amino acid sequence. EX4 has higher affinity to GLP-1receptor and longer half-life (3.3–4 h) than GLP-1 (< 2 min). Most importantly, EX4 acts in a glucose dependent fashion and reduce risk of hypoglycemia which is often associated with the use of insulin [125]. In addition, EX4 suppresses glucagon secretion, lowers the blood glucose level, increases β-cell mass via stimulation of β-cell neogenesis, stimulates β-cell proliferation, and inhibit β-cell apoptosis. Furthermore, EX4 helps delay gastric emptying so that the glucose derived from meal appears late in the bloodstream, and is involved in reduced food intake and weight loss [126]. Exenatide, a synthetic exendin-4, was approved by FDA in 2005 for treating patients with type II diabetes, who are non-responsive to metformin and/or sulfonylurea therapy and is now marketed as Byetta® [127].
CTB-EX4 expressing transplastomic tobacco plant showed relatively high expression level of the fusion protein, up to 14.3% of total leaf protein. The EX4 was fused to CTB, and a hinge domain and a furin cleavage site were inserted in between them to minimize steric hindrance of the fused EX4 and to facilitate the release of EX4 into bloodstream after passage across intestinal cells in the gut. Another feature of this study was the use of the lyophilized CTB-EX4 transplastomic leaves to maximize oral delivery of functional peptide. In in vitro assay with β-TC6 pancreatic cell line, purified CTB-EX4 stimulated the secretion of insulin similar to commercial EX4 [25]. To investigate in vivo hypoglycemic effect triggered by orally administered EX4, the freeze-dried CTB-EX4 leaves were powdered and used for oral delivery into mice. Glucose lowering effect of the plant-derived EX4 reached peak at 90 min after oral gavage (Fig. 2b) and was comparable to that of commercially available EX4 (Fig. 2b) [25]. Current expensive systems (daily injection of Exenatide) for the treatment of type II diabetes are not available for a large population in developing countries. So the next step is to produce Exenatide in the lettuce chloroplasts to lower the cost of incretin treatment for type 2 diabetes.
3.3 Oral delivery of autoantigens bioencapsulated in plant cells
There have been many efforts to use plant cells as a method for oral delivery of autoantigens. However, as seen in previous study in which CTB-insulin fusion protein was expressed in potato up to 0.1% of total soluble protein [73], low expression levels is one of hurdles to be overcome because low expression levels might not be sufficient to induce oral tolerance in human large absorptive surface area in intestinal mucosa and would require higher amount of plant materials. The problem of lower expression levels was addressed by plastid transformation technology with higher expression levels (up to 70% total leaf protein) of CTB-proinsulin fusion protein in tobacco and edible crop lettuce [8]. Oral gavage of leaf material to NOD mice protected beta cells of pancreatic islets from lymphocytic infiltration and induced Th2 mediated oral tolerance. Oral administration of leaf material expressing anticoagulation factor IX fused with CTB prevented inhibitor development and deadly anaphylaxis in hemophilia B mice [112]. Further, the plant-based oral delivery offers low cost of delivery of therapeutic proteins [2]
3.3.1 Proinsulin to delay the onset of type I diabetes
Type 1 diabetes is an autoimmune disorder stemming from the damage of insulin-producing pancreatic β-cells in the islets of Langerhans. This damage is caused by storming autoreactive CD4 and CD8 T cells [128]. Amongst several proteins recognized by T-cell repertoire as autoantigens, insulin appeared to be a major autoantigenic target of the T cell autoimmune attack in the non-obese diabetic (NOD) mouse model of diabetes and in type 1 diabetes (T1D) in humans [128,129]. The NOD mice serve as an established animal model of human T1D, characterized by insulitis as a result of lymphocytic infiltration of the pancreatic islets, drop in insulin levels and higher plasma glucose levels [130]. Further, progression of diabetes in NOD mice can be predicted by monitoring insulin autoantibody levels as onset of diabetes is strongly associated with development of insulin antibodies. Insulin along with its immunogenic epitopes administered via mucosal route including oral or intranasal exposure have been evaluated for diabetes development using the NOD mouse model [129,131]. Tolerance induction through mucosal exposure to autoantigens is largely related to induction of regulatory T-cell (Treg) in several autoimmune disease models [64,131].
Plant-based synthesis allows cost-effective and large scale production of autoantigens as opposed to current cell culture systems [2]. Production of insulin in plant cells for tolerance induction has been investigated in two studies [19,73]. Further, mucosal adjuvants including CTB and ricin toxin B subunit (RTB) have been utilized to enhance efficacy of insulin therapy for tolerance induction. Administration of CTB fused to antigens via oral, nasal or sublingual route have been shown to enhance tolerance when compared to antigens alone [132]. In addition to stimulation of TGF-β and IL-10 production, the CTB ability to bind GM1 ganglioside facilitates mucosal antigen uptake and their presentation by different APCs. Potato plants synthesizing insulin alone or conjugated to CTB (CTB-INS) with expression levels of 0.05% and 0.1% respectively of total soluble protein were generated by Agrobacterium mediated transformation [73]. The CTB-INS expressing transgenic potato tuber fed to NOD mice generated serum and intestinal anti-CTB antibodies along with rise in anti-insulin IgG1 levels indicating an insulin specific Th2 lymphocyte response. Also CTB-INS fed NOD mice showed significant reduction in insulitis and incidence of diabetes when compared to NOD mice fed with untransformed potatoes.
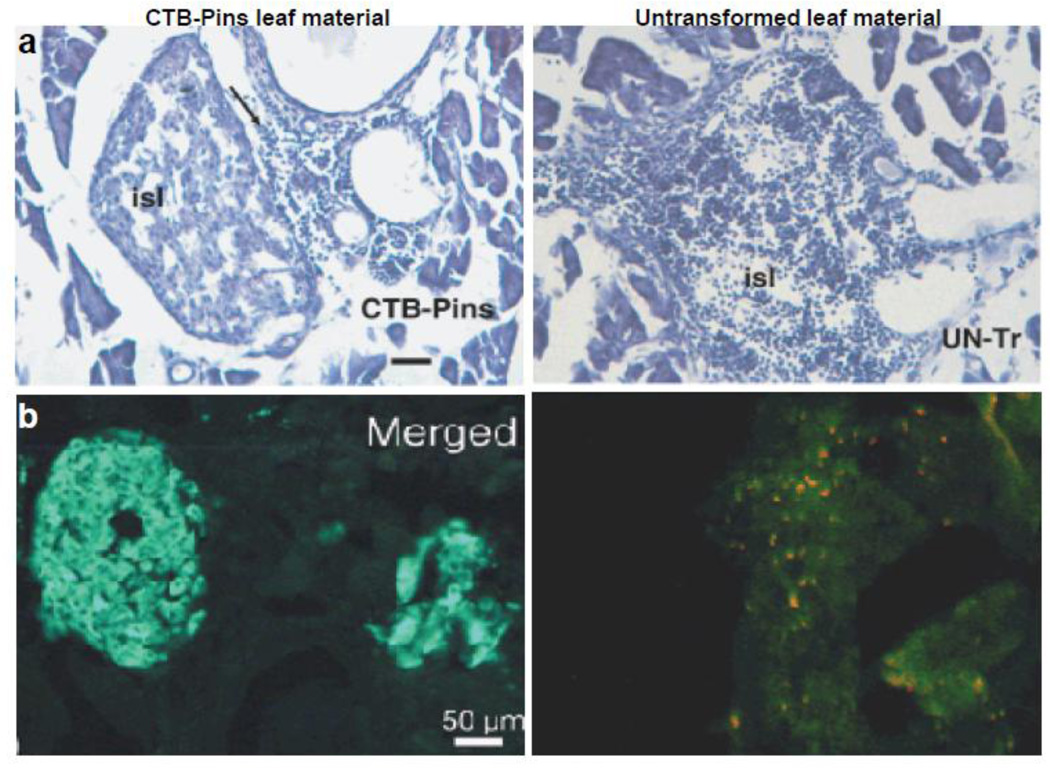
The limitation of lower expression levels of proinsulin was addressed by plastid transformation technology [8]. In addition, edible crop lettuce was used for expression which not only requires minimal processing but also offers an ideal oral delivery system. The cholera toxin B subunit -human proinsulin (CTB-Pins) fusion protein was expressed in lettuce and tobacco chloroplast transgenic lines with a GPGP hinge region in between. CTB-Pins expression levels in tobacco leaves reached up to ~16% of total soluble protein (TSP) or 72% of total leaf protein (TLP) whereas in lettuce accumulation was ~2.5% TSP or 25% TLP [8,19]. These expression levels were several hundred folds higher than achieved in any other study and should offer sufficient dose of proinsulin for oral tolerance in human clinical studies. The proinsulin gains entry into the intestinal cells via pentameric arrangement of CTB, which is responsible for binding to the GM1 receptors. Chloroplast-derived CTB-Pins fusion protein was found to assemble into pentameric form as confirmed by GM1 binding assay and demonstrate chloroplast’s ability to carry out precise folding and formation of disulfide bonds. Efficacy of CTB-Pins expressing tobacco leaf material was tested by orally feeding 8 mg of powdered leaf material to 5-week old NOD female mice weekly along with negative controls CTB-green fluorescent protein (CTB-GFP), interferon-GFP (IFN-GFP) or untransformed leaf material [19]. After 7 weeks of feeding, pancreas from CTB-Pins fed NOD mice showed mostly intact β-cells with minimal cellular infiltration. The lymphocytes were located at the periphery but not within the islets whereas untransformed or other control groups showed extensive lymphocyte invasion into the islets leading to insulitis (Fig. 3a). This insulitis induced apoptotic destruction of β-cells as shown by expression of activated caspase-3 (a typical hallmark of apoptosis) whereas CTB-Pins fed mice lowered caspase-3 expression (Fig. 3b) [19]. Further, immunohistochemistry revealed proximity of IL-4 and IL-10 producing cells to the pancreatic islets in CTB-Pins fed NOD mice as a result of perivascular migration of these cells from adjoining blood vessels. Hence, suppression of insulitis in CTB-Pins NOD mice is facilitated through regulatory Th2 cells [19]. The CTB-Pins fed mice also showed high serum IgG1, low serum IgG2a and low mucosal IgA levels against CTB as compared to other groups. In addition, blood and urine glucose values were lower in CTB-Pins mice when compared to that of control groups. The protection against development of insulitis in NOD mice conferred by oral administration of CTB-Pins bioencapsulated within plant cells has great potential for translation of oral tolerance in human clinical studies.
Fig. 3.
Pancreatic lymphocyte infiltration and insulin production in non-obese diabetic mice. (a) Haematoxylin and eosin staining of a cryosections of the pancreas (showing an islet, isl) of a NOD mouse receiving cholera toxin B subunit–human proinsulin (CTB-Pins) for 7 weeks with lymphocytes seen outside the islet (arrow) compared to a large islet with severe lymphocytic infiltration in a mouse receiving untransformed plant leaf material (UN-Tr). Fifty sections per animal were analyzed and CTB-Pins showed significantly less cellular infiltration than untreated group. Insulitis score was ~1 in CTB-pins treated group and ~5 in untreated control group (*P < 0.05). Scale bar, 50 µm. (b) Merged image of insulin and caspase-3 double immunostaining in Langerhans islets of a NOD mouse receiving CTB-Pins and untransformed plant leaf material. © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2007) 5: 495–510 [19].
3.3.2 Coagulation factor IX for suppression of immune response during treatment of hemophilia B
Deficiency of blood coagulation factor IX (FIX) results in a hereditary bleeding disorder hemophilia B (Christmas disease; heritable factor IX deficiency). The incidence of hemophilia B is estimated 1 in 25,000 to 30,000 male births worldwide [133]. Patients with hemophilia B suffer from frequent and recurring bleeding episodes depending upon the severity of the condition. Current treatment of hemophilia B involves replacement of missing blood coagulation factor IX with plasma-derived FIX (Konyne-80; Bebulin®; AlphaNine® SD; Mononine®; Nonafactor®) or recombinant FIX (Benefix®) administered by intravenous infusion [134]. Most serious complication of replacement therapy is the formation of high affinity neutralizing IgG antibodies called inhibitors directed against FIX rendering the therapy ineffective [135]. The disease condition also has far reaching consequence on patient’s mobility, functional ability, quality of life, morbidity and mortality [134]. The probability of inhibitor development is rare (1.5%–3%) in hemophilia B and can be determined by genetic characteristics, age at substitution, treatment schedule, treatment modalities, family history of inhibitors and environmental factors [135–137]. To circumvent inhibitor formation with FIX replacement therapy, treatment with bypassing agents such as recombinant activated FVII or plasma-derived activated prothrombin complex (pd-aPCC) concentrate can also be utilized [138]. However, both bypassing agents are expensive and pose risk of development of thrombosis and thromboembolism because of their procoagulant activity. The pd-aPCC also imparts other harmful effects including anamnestic response and anaphylaxis [138]. Immune tolerance induction (ITI) protocols may be used to eliminate inhibitors, which involve repeated infusion of FIX concentrate to induce tolerance and reinstate typical response to replacement therapy. However, the success rate is very low (13–31%) and the treatment can cost up to several hundred thousand dollars [138]. Further, the profound risk of associated life threatening side effects including anaphylaxis and nephrotic syndrome cannot be overlooked.
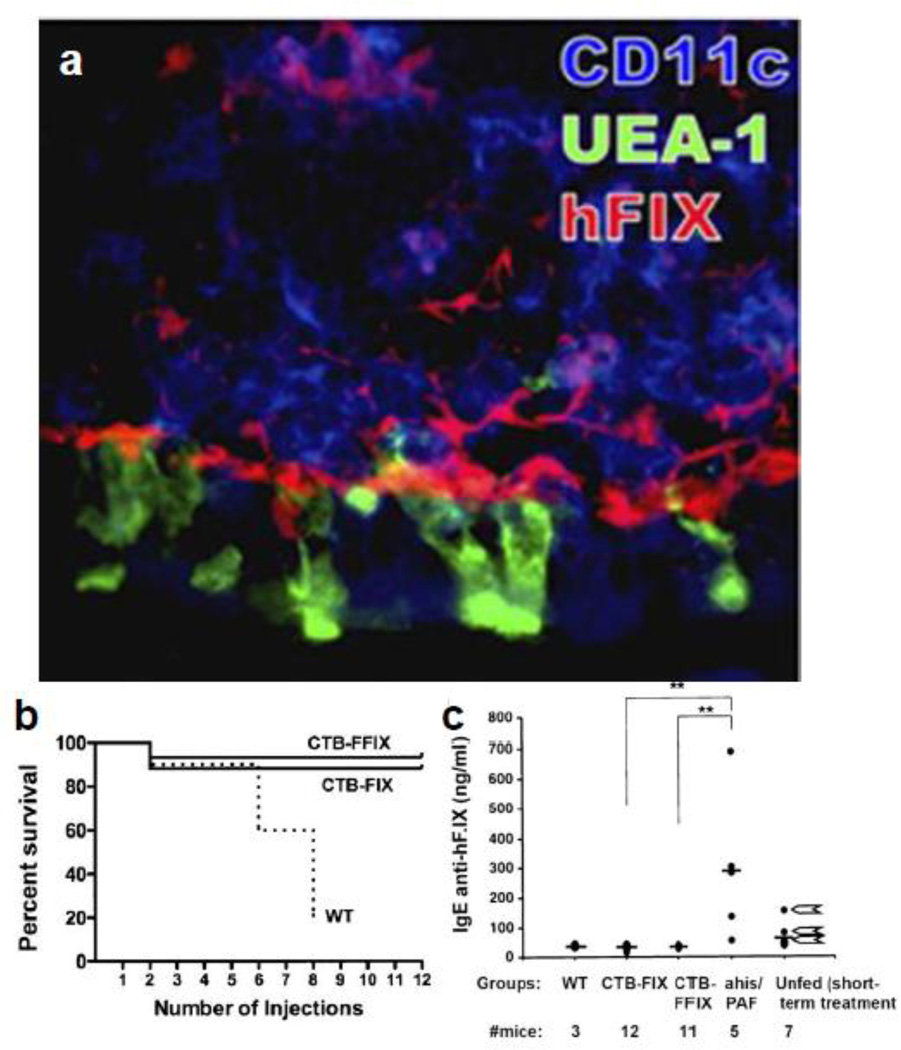
As an alternative to ITI protocols and bypassing agents, a prophylactic protocol was developed using oral delivery of plant leaf material expressing functionally inactive FIX polypeptide fused with CTB (CTB-FIX) to prevent inhibitor formation and life threatening anaphylactic reactions during FIX replacement therapy [112]. Tobacco plants were generated expressing CTB-FIX (0.19% of TSP) and CTB-FFIX (3.8% of TSP) in chloroplasts. Immunohistochemistry analysis of tissue from mice fed with CTB-FFIX material twice per day for 2 days revealed that FIX antigen was delivered to epithelial M cells and CD11c+ dendritic cells in Peyer's patches that forms interface between the gut associated lymph tissue and the luminal microenvironment (Fig. 4a). The hF.IX antigen was also observed in liver and plasma within 2 hours of feeding, illustrating systemic delivery of antigen [112]. Three experimental groups of hemophilia B mice with targeted factor IX gene deletion were biweekly oral gavaged frozen powdered leaf material (250 mg/dose) from untransformed (WT), CTB-FIX or CTB-FFIX transplastomic plants for 2 months [112]. A weekly therapeutic dose of recombinant FIX protein (hF.IX) was infused intravenously a month after and continued for 2 months. Acute allergic response to therapeutic dose was noticed in WT-fed or unfed mice starting from fourth week of therapy and 75–80% of mice passed away within 6–8 weeks except mice with intravenous infusion of antianaphylactic drugs (antihistamine tripolidine and antiplatelet-activating factor). On the contrary, 90–93% of FIX leaf material fed mice stayed alive without any indication of allergic or anaphylactic response even after twelve infusion doses (Fig. 4b). In addition, inhibitor titers were very high in surviving WT-fed and unfed mice whereas inhibitor titers were absolutely suppressed in FIX-fed mice and were similar to background inhibitor titers of naiive hemophilia B mice (no feeding and no treatment) [112]. An IgE-dependent mechanism was linked with allergic reactions in hemophilia B mice as high titer IgE were found in control mice with subsequent anaphylaxis whereas IgE titers were low in CTB-FIX/CTB-FFIX with no anaphylactic responses (Fig. 4c) and thus represents a new animal model to investigate anaphylaxis. Further, IgG2a/complement- dependent mechanism of anaphylaxis was ruled out in hemophilia B mice as IgG2a against hF.IX was not detected in WT-fed or unfed mice. The CTB-FIX/CTB-FFIX mice also showed IgG1 titers, which did not cause enhanced clearance of infused hF.IX and thus were non-inhibitory antibodies. Low titers of TGF-β dependent antibodies (IgG2b and IgA) were also detected probably derived from Th3 a cell, which is a typical feature of mucosal immunity. In conclusion, oral delivery protocol of CTB-FIX involved an atypical immune response classified by suppression of IgE and production of TGF-β dependent antibodies as well as non-inhibitory IgG1 and thus provided long term protection to hemophilia B mice from inhibitor and allergic reactions [112]. The oral administration protocol involving transplastomic plant leaves expressing antigen opens a new platform and should be effective in inducing oral tolerance in various genetic diseases, where antibodies develop during treatment.
Fig. 4.
Suppression of fatal anaphylaxis and IgE immunoglobulin response in Hemophilia B mice through induction of oral tolerance. (a) hF.IX antigen delivery to the GALT. Peyer's patch and villi of ileum of CTB-FFIX-fed mouse stained for hF.IX (red), M cells (UEA-1, green), and CD11c (blue) are shown. (b) Mice survival in orally fed wild-type (WT, n= 10 mice at the onset of protein therapy), CTB-FIX (n=17), or CTB-FFIX (n=15) plant material after 0–12 intravenous injections of hF.IX protein. (c) IgE titers in WT-, CTB-, CTB-FFIX-fed mice, unfed mice that received antihistamine/anti-PAF before a sixth injection of hF.IX, and mice that received four hF.IX administrations. Arrows next to data points from unfed mice with four administrations of hF.IX protein indicate animals that died after a subsequent fifth injection. i.e., after 8 weekly i.v. injections of hF.IX. Copyright © by National Academy of Sciences of the United States of America, Proc. Natl. Acad. Sci. U.S.A. (2010) 107: 7101–7106 [112].
3.4 Oral delivery of vaccine antigens bioencapsulated in plant cells
Because the first site of pathogen infection is the mucosal surface, orally delivered vaccines should offer greater protection by inducing both mucosal and systemic immunity. The orally delivered plant-based vaccines have shown immunogenicity and protection against bacterial, viral and protozoan pathogens [2]. Here we discuss the current status on expression and oral delivery of plant-derived vaccine antigens against infectious diseases using a few examples.
3.4.1 Plague vaccine
Plague caused by Yersinia pestis is one of the most deadly infectious diseases of mankind. It is transmitted to human by the bite of infected fleas [139]. Millions of people have died because of outbreaks of plague pandemics in human history [140]. The 1347–1351 Black Death plague killed 66% of European population and over 100 million people globally. Y. pestis is listed as one of the six category-A pathogen due to its potential use as a biological weapon [141]. Currently there is no commercially available human vaccine against plague in the United States and manufacture of this vaccine was ceased in 1998 due to multiple side effects and short-term effectiveness against pneumonic plague [142]. Recently in February 2011, plague reappeared in Ambilobe in the northern region of Madagascar which caused many deaths [143]. Therefore, plague has the potential for outbreak anytime and World Health Organization (WHO) has categorized plague as a re-emerging infectious disease [144].
Among the several subunit vaccines in development, the fraction 1 (F1) outer capsular and low-calcium-response V (LcrV or V) proteins are the most effective against Y. pestis and immunogenic when use individually or in combination [144]. In addition, it has been shown that F1 and LcrV antigens activate dendritic cells to induce primary T-cell responses thereby developing protective immunity against plague [145]. The plague vaccine antigens F1, V and the F1–V fusion were engineered and expressed in plants using all three possible strategies: nuclear transformation, chloroplast transformation and plant-virus based expression vectors [2,144]. Plant-derived purified recombinant plague antigens are immunogenic and provide protection in vaccinated guinea pigs [146] and non-human primate Cynomolgus Macaques against Y. pestis aerosol challenge [147,148]. However, there are requirements for expensive purification steps and cold chain for injectable vaccines.
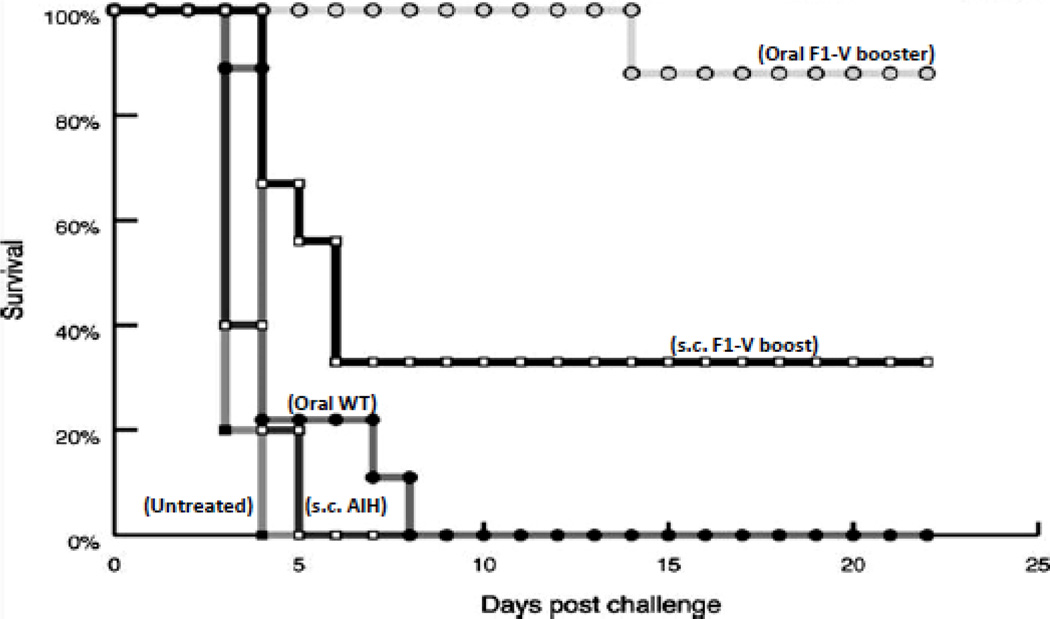
Therefore, the fusion protein F1-V was expressed in chloroplasts with levels up to 14.8% TSP [18]. The functionality of chloroplast-derived F1-V antigen was studied. Mice were primed subcutaneously (s.c.) with doses of adjuvant containing F1-V and then boosted with either adjuvanted s.c. doses (s.c. F1-V) or unadjuvanted oral doses (oral F1-V). Mice immunized with F1-V (s.c.) and boosted orally with F1-V bioencapsulated in plant cells showed higher level of serum F1-V specific IgG1than with s.c. boosters. Following challenge with aerosolized Y. pestis, 88% of the oral F1-V mice and 33% of the F1-V given s.c. (with adjuvant) were protected while all the control mice died within 3 days (Fig. 5). Mice with the highest IgG1 titers survived when challenged with live Y. pestis. This is consistent with other studies that demonstrated F1 and V specific IgG1 titers are significantly correlated with protection in mice against Y. pestis. For an effective protection of pneumonic plague, vaccine antigen must have the ability to stimulate both mucosal and systemic immune responses.
Fig. 5.
Protection against Yersinia pestis challege after immunization. Mice receiving oral boosts of chloroplast-derived F1-V survived longer than mice receiving subcutaneous boosts Animals were challenged with 15 LD50 of Y. pestis CO92 (whole-body LD50, 6.8 × 104 CFU) While control mice had up to 1010 Y. pestis counts in their spleen, survivors had no detectable Y. pestis, confirming 10-log reduction in the mean bacterial burden among survivors. Copyright © American Society for Microbiology, Infection and Immunity (2008) 76: 3640–3650 doi:10.1128/IAI.00050-08 [18].
3.4.2 Cholera vaccine
Cholera is an acute, diarrheal disease caused by infection of the intestine with the bacterium Vibrio cholerae. Primary transmission of this disease is contaminated water [149]. The infection is caused by cholera toxin (CT) secreted by Vibrio cholera after colonizing in the human intestine. World Health Organization (WHO) listed cholera as one the top three diseases and it affects an estimated 3–5 million people worldwide, and mortality rate is estimated to be 100,000–130,000 deaths annually [149] and remains the most devastating diarrheal disease, especially under severe weather conditions that increase water pollution. Dukoral, an orally administered, inactivated whole cell vaccine is available in over 60 countries, which has an overall efficacy of about 52% during the first year and 62% in the second year [150,151]. However, it is not currently recommended by the CDC for most people traveling from the United States to endemic countries [152]. Moreover, with the current vaccine immunity is lost in children within three years and adults are not fully protected [153] and remain prohibitively expensive for routine use in cholera-endemic areas in developing countries [154], especially at times of outbreak. Therefore development of safe, low cost and effective cholera vaccine candidate is highly recommended.
The CTB which has binding site for the plasma membrane receptor of the intestinal epithelial cells (GM1) [155,156] is an ideal oral vaccine antigen for cholera. Moreover, oral vaccine has been known to generate both mucosal and systemic immunity [26,157]. However, production of CTB in bacterial system or other systems which involved purification will not be a cost effective technology due to expensive downstream processing. Vaccine antigens expressed in plant chloroplasts are protected from acids and enzymes present in stomach due to bio-encapsulation [18,110]. A plant-derived oral vaccine was created and its effectiveness was investigated in a clinical trial involving human volunteers. In such clinical trials, serum and/or mucosal immune response was induced in human subjects, who consumed potato expressing heat labile enterotoxin B subunit (LT-B) of Escherichia coli. Subjects who were immunized orally with LT-B expressed in a potato cell showed a similar degree of mucosal B-cell priming to that of LT derived from E.coli cells [158]. In an effort to produce CTB for long-term storage, CTB antigen was expressed in maize and was shown that orally immunized mice were protected from CT challenge [159]. The stability of CTB in rice seeds stored at room temperature for long time and their protection from digestive enzymes have been studied [93]. They have been shown that 75% of the CTB accumulated in rice seeds remained intact after pepsin treatment, one of the proteolytic enzyme found in the digestive system and immunogenicity was maintained even after storage at room temperature for 1.5 years. Moreover, mice immunized with rice-expressed CTB induced CTB-specific IgG as well as IgA while no or low level of IgA was found in mice immunized with purified rCTB and showed protection against CT [93]. Further, this study was extended to nonhuman primates and orally immunized macaque induced only high level of serum IgG but not IgA [160]. Out of three immunized macaque, only one maintained CTB-specific IgG titers more than 6 months and gradually decreased in other two which was recovered after additional oral booster [160]. Considering the life span of macaques (30 years), long term retention of immunity should be increased. Moreover, production of large quantity of CTB vaccine with minimum processing and maximum public compliance is necessary in order to develop an effective vaccine against cholera and easily available at the time of outbreak.
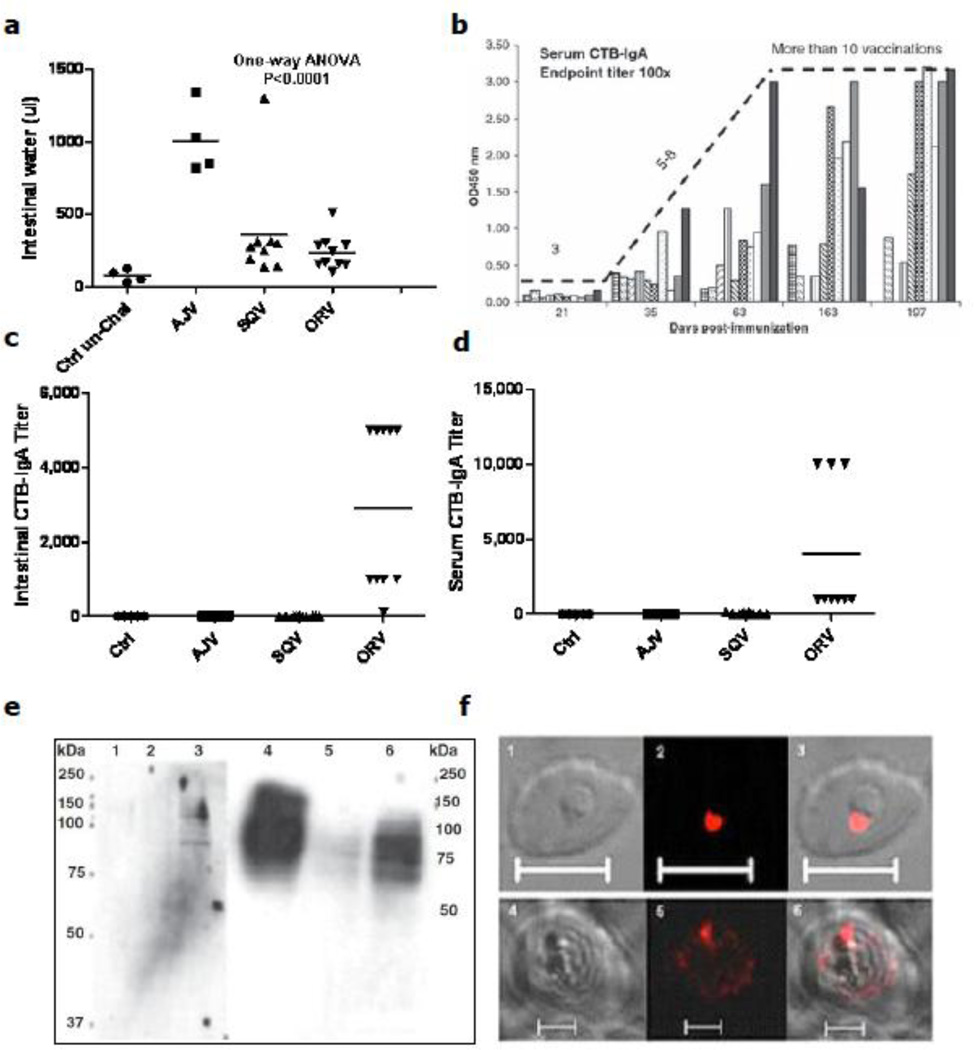
Chloroplast transformation technology has many advantages over conventional transgenic plants [2,161]. Thus, CTB was the first expressed vaccine antigen in chloroplasts and it showed proper folding when evaluated with GM1 binding assay [162]. Further, CTB fused with two malarial vaccine antigens apical membrane antigen-1 (AMA1) and merozoite surface protein-1 (MSP1) has been expressed in tobacco and lettuce chloroplasts at high level [16]. Proper folding and binding of chloroplast-derived CTB-AMA1 and CTB-MSP1 to GM1-ganglioside was shown by GM1 binding assays. Mice immunized subcutaneously (SQV) or orally (ORV) with purified antigens or transplastomic leaves were protected against cholera toxin challenge. Protection was significantly correlated with volume of intestinal water retention (Fig. 6a). Orally immunized mice induced CTB specific IgA in both sera and intestinal content while no IgA titers was observed in SQV mice (Fig. 6c, d). Orally immunized mice also induced CTB specific IgG1. Although no IgA titer was observed in injected mice, they were protected from CT challenge. Thus, protection of mice in orally immunized mice is correlated with CTB-specific titers of intestinal/serum IgA and IgG1 whereas only IgG1 was observed in injected mice (Fig. 6c, d). Moreover, the resulting chloroplast-derived antigens conferred long term immunity ((Fig. 6b, >300 days), almost half life span of the mice, which would be a great advantage for human use.
Fig. 6.
Evaluation of cholera toxin (CT) challenge and cross-reactivity of antisera generated in mice immunized with cholera and malarial vaccine antigens. (a) CT challenge in control and vaccinated mice. (b) Determination of effectiveness of numbers of boosters to generate CTB-specific serum IgA in orally gavaged mice with plant cells expressing CTB. Ten-week-old mice were boosted subcutaneously (until 189 days) or orally (until 219 days). Sera were collected until 197 days post-immunization.(c & d) CTB-antigen-specific serum and intestinal IgA in different groups of mice. Control (Ctrl), adjuvant (ADJ), subcutaneous vaccinated (SQV) and orally vaccinated (ORV) mice (e) Immunoblot analysis: Sera collected from immunized mice recognized the native 83-kDa apical membraneantigen-1 (AMA1) protein (lanes 1–3) and the native 190 kDa MSP-1protein (lanes 4–6) respectively. (f) Immunofluorescence analysis: The AMA1 antibodies recognized the apical end of the parasite in the ring developmental stage of intraerythrocytic growth (1, 2 and 3). The MSP-1 sera from immunized mice (bottom row) detected the developing merozoites at the schizont stage of the parasitic growth (4, 5 and 6). Bar size = 10 um. © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2010) 8: 223–242 [16].
3.4.3 Malaria vaccine
Despite significant progress in vaccine development against several bacterial and viral pathogens, development of vaccines against protozoan pathogens has been slow. There are no vaccines against protozoan parasites Plasmodium and Entamoeba. Entamoeba histolytica infection causes amoebic dysentery and abscesses in liver. Among protozoans, amoebiasis is the second leading cause of death after malaria resulting in ~100,000 deaths every year. LecA, a potential vaccine candidate against amoebiasis, comprising of a cysteine-rich fragment of lectin harboring carbohydrate recognition domain of Gal/GalNAc lectin was expressed in plants via chloroplast genetic engineering [163]. In mice, subcutaneous vaccination with crude extracts of transplastomic leaves generated higher immunoglobulin G titres, up to 10,000 [163]. Further, oral administration of lecA plant material should generate systemic as well as mucosal immune response and represents a suitable vaccination strategy for protection against amoebiasis.
Malaria is still a global challenge with an estimated 216 million cases in 2010 worldwide, in which majority of cases (81%) were in African region followed by South-East Asia (13%) and Eastern Mediterranean Regions (5%) [164]. Current report estimated 655,000 malaria deaths globally in 2010, with about 86% of deaths among young children [164]. In an effort to develop vaccine candidate for malaria, a number of potential malarial proteins have been studied and are currently in various stages of pre-clinical and clinical trials [165–167].
Currently there is no licensed vaccine for prevention of malaria. Thus, the development of malaria vaccine would reduce the burden of this deadly transmitted disease. But many challenges hamper in developing a robust and efficient vaccine against malaria because of antigens complexity, high polymorphism among parasitic proteins, lack of appropriate animal model, high cost of vaccine development and delivery [168]. To this end, researchers have tried to identify many vaccine candidates for malaria and current clinical trials are under way investigating several blood stage candidates such as AMA1, MSP1, and erythrocyte surface antigen [169,170]. The AMA1 is a leading asexual blood-stage vaccine candidate [171], a type I integral membrane protein [172]. Animal and in vitro studies support a crucial role for AMA1 during invasion of RBCs [173,174] and conferred immunity [175]. The C-terminal region of this protein is highly conserved and can be blocked by specific antibodies. AMA1 is not only found in the asexual blood stage merozoites but is also expressed by sporozoites and in liver of merozoites stage [172]. Another leading asexual blood stage vaccine candidate MSP1 plays a critical role in parasite invasion of RBCs [176] and several vaccination studies have shown protection in both mice and monkeys from malaria infection [177–180]. However, successful mass vaccination of malaria will critically depend on low cost and scalable methods of vaccine production, distribution and delivery since most of the malaria-endemic areas have poor infrastructure and poverty [181].
Therefore, in an attempt to develop oral malaria vaccines AMA1 and MSP1 vaccine antigens have been expressed in tobacco and lettuce chloroplasts [16]. Malarial antigens exhibit poor immunogenicity even when used with adjuvant [182]. In order to increase its mucosal immunogenicity, AMA1 and MSP1 antigens were fused with CTB which has high mucosa-binding property. Immunized mice with chloroplast-derived CTB-AMA1 and CTB-MSP1antigens induced anti-MSP1 antibody titers, which is correlated with number of doses. Subcutaneously immunized mice (SQV) showed higher anti-MSP1 antibody (IgG1) than orally immunized mice (ORV). However, ORV mice induced both systemic and mucosal immune response, whereas SQV mice did not induce IgA. Immunoblots showed that anti-AMA1 antibody developed in the immunized mice sera hybridized and recognized 83 kDa schizont protein of Plasmodium (Fig. 6e). Similarly, anti-MSP1 antibodies also hybridized and recognized both 190 kDa ring and schizont proteins (Fig. 6e). Specificity of antibody generated by chloroplast-derived vaccine antigens was further confirmed by immunofluorescence assay. Both AMA1 and MSP1 antibodies recognized ring stage and shizont stage of malarial parasite, respectively (Fig. 6f) Further, functionality of antigen specific antibodies was confirmed by parasite inhibition assays and both ORV and SQV conferred more than 100% inhibition [16]. Thus, production of plant-derived vaccine offers a new platform for advancing this field towards human clinical studies, which would confer protection through systemic and/or mucosal immunity with minimal processing.
4. Lyophilization of plant cells confers stability, retains folding and eliminates microbes
Plant-derived vaccine antigens are protected from acids and enzymes present in stomach due to bio-encapsulation [18,110]. Recently, there have been some studies to use freeze-dried plant leaves, containing pharmaceutical proteins or peptides, for oral delivery. Lyophilized leaves have various advantages over fresh materials in regard to long-term storage, increase of therapeutic protein content, antigen stability and decrease of microbial contamination. Rapid-frozen leaf materials in liquid nitrogen were crumbled to obtain relatively equal surface-area to volume ratios and then placed into containers, sealed with porous lid. Then lyophilization proceeded in low temperature (−50°C) under vacuum (~0.036 mBar) to protect proteins. This process usually can be completed within 1 ~ 3 days depending on the amount of leaf materials to be freeze-dried. After lyophilization, dried leaves are stored in moisture-free condition at room temperature. For oral delivery, the dried leaves are ground and filtered using mesh to obtain uniformly fine particles. This powder can be packaged in capsules or resuspended in saline for oral gavage. Machines are commercially available for such processing and packaging of capsules from lyophilized leaves (Fig. 1).
Our recent oral delivery studies with freeze-dried CTB-EX4 expressing leaves showed glucose regulating effect when delivered into mice orally, similar to injections. Moreover, the lyophilized CTB-EX4 was stable up to 10 months at room temperature [25]. Further studies about the long term storage of therapeutic proteins were carried out with transplastomic lettuces expressing soluble antigen (PA; protective antigen from Bacillus anthracis) and CTB fused proinsulin (CTB-Pins). The lyophilized PA did not show any degradation products after 6-month storage at room temperature and any cleaved products after various durations of lyophilization (24, 48, and 72 hr) (Fig. 7a, b). Furthermore, PA was stable in lyophilized lettuce store at room temperature for more than 15 months (Fig. 7e). The antigen contents of both lyophilized leaf materials increased up to ~ 24 fold higher than fresh leaves (Fig. 7c, d). In addition, lyophilization offers additional advantages including removal of microbial contamination. There were no detectable microbes from the lyophilized lettuce when plated on various nutrient media (Fig. 7f).
Fig. 7.
Evaluation of stability of antigens in lyophilized lettuce after prolonged storage. (a) Western blot analysis to evaluate stability of lyophilized anthrax protective antigen (PA) expressed in lettuce leaves after storage at room temperature for 2 (1), 4 (2), and 6 (3) months. PA, standard (100 ng), WT, untransformed lettuce. (b) PA stability after 3 months of storage, and lyophilization for different durations: (1) 24, (2) 48 and (3) 72 hrs. (c) Western blot analysis of fresh (F) and lyophilized (L) leaves expressing PA. Total soluble protein (10 µl) was loaded on an equal quantity basis (50 mg from each sample were extracted in 300 ul of extraction buffer) to examine the increase of PA content after lyophilization. (d) Fold increase of specific antigen after lyophilization. PA, lettuce transplastomic plant expressing PA; CTB-Pins, lettuce transplastomic plant expressing CTB-Proinsulin; white bar, fresh material; grey bar, lyophilized material. (e) Evaluation of long-term stability of PA in lyophilized lettuce leaves after storage at room temperature for 15 (1), 13 (2), 3 months (3), and 1 week (4). F, fresh leaf; total soluble protein (20 ng) was loaded in each lane for immunoblot assay. (f) Microbial burden of leaves expressing PA. 1, fresh leaf; 2, lyophilized leaf; 3, commercially available freeze-dried alfalfa capsules. © Plant Biotechnology Journal, Society for Experimental Biology, Association of Applied Biologists and Blackwell Publishing Ltd, Plant Biotechnology Journal (2012): In Press [25].
5. Conclusions and future perspectives
The first plant derived therapeutic protein was recently approved by FDA. This is the injectable glucocerebrosidase enzyme replacement therapy made in carrot cells to treat Gaucher’s disease (a lysosomal storage disorder). Pfizer is the first big pharma to market a plant cell made biopharmaceutical [183]. However, injectable products still face challenges of prohibitively expensive purification, cold storage/transportation and sterile delivery. Therefore, FDA approval of the first plant made therapeutic protein opens the door for developing orally delivered vaccines made in plant cells for infectious and inherited diseases. The low hanging fruit in advancing this concept is the use of vaccine antigens bioencapsulated in plant cells as boosters for currently approved injectable vaccines that require several booster injections. For example, the anthrax vaccine requires 8 boosters, costing the military hundreds of millions of dollars. Immunity and protection is lost for several other vaccines within one or two years (e.g. BCG vaccine for tuberculosis) and pathogens develop resistance against drugs (tuberculosis or malaria). As pointed out above with several examples, oral boosters using vaccine antigens bioencapsulated in plant cells offer greater immunity and protection than injectable boosters. Therefore, developing boosters for currently approved vaccines should offer the very first opportunity to advance this platform in the clinic and provide a significant reduction in cost by elimination of prohibitively expensive purification, cold storage/transportation and sterile delivery.
Proper storage of vaccines at required temperatures is essential to maintain their efficacy. The Centers for Disease Control and Prevention (CDC) Vaccines for children (VFC) program administered vaccines to ~40 million children at a cost of $3.6 billion, last year. A recent report published by the office of inspector general [184] states that among the selected providers inspected, 76% were exposed to inappropriate temperatures that can reduce vaccine potency and efficacy, increasing the risk that children are not provided with maximum protection against preventable diseases. While providers in the US rarely face power failures, frequent power shortages are of common occurrence in developing countries and rural primary health centers often lack power or proper storage facilities. Therefore, developing technologies to increase shelf life, eliminate cold storage and transportation is of paramount importance for effective delivery of vaccines around the globe.
The oral polio vaccine (OPV) has enormous impact on eradication of polio in the world because of lower cost than injectable IPV and ability to induce intestinal immune response [185]. Although attenuated bacterial strains are used as oral vaccines, there are many challenges regarding safety and immunity. Current concerns of OPV include poor vaccine efficacy, instability in reversion to neuro-virulence and shedding of circulating vaccine derived polioviruses. There are reports of oral polio vaccine-associated paralytic polio and the development of vaccine-derived polioviruses in a small number of individuals [186]. Therefore, there are efforts to discontinue OPV after eradication of wild polio strains. Although IPV induces serum antibodies, it offers very low levels of mucosal immunity. Thus, there is a risk of continued wild poliovirus circulation due to multiplication in intestine and fecal shedding of poliovirus [187]. In addition, IPV is five times more expensive than OPV and requires sterile injections. Orally delivered plant-derived vaccine antigens are protected because of bio-encapsulation within plant cells and several studies have shown the effectiveness of plant-derived vaccines when delivered orally [2]. Elimination of pathogen strains (killed or attenuated) is highly desired to avoid future outbreaks.
While non-invasive oral delivery of functional therapeutic proteins such as insulin or cytokines bioencapsulated in plant cells can provide therapy in some cases, this technology platform is also highly attractive for tolerogenic delivery of autoantigens. Autoimmune diseases develop as a result of failure of self-tolerance mechanism and improper immune response to one’s own proteins. In developed countries, autoimmune disorders are third leading reason for illness and death trailing behind heart disease and cancer [188]. There are > 80 diverse autoimmune diseases caused by production of autoreactive T-cells or autoantibodies targeted against autoantigens. Several autoantigens of major autoimmune diseases capable of inducing tolerance have been identified and investigated using different routes of delivery [189]. Similarly, therapeutic proteins used to treat genetic diseases (i.e. inherited protein deficiencies) such as storage disorders or hemophilia, are often rejected by the immune system. Antibody formation renders replacement therapies ineffective, and antibody-antigen complexes can cause immunotoxicities [190–192]. T cell responses against therapeutic gene products are an additional concern for emerging gene therapies [193]. However, we found that oral delivery of even non-functional protein antigens can effectively prevent pathogenic antibody responses in treatment of hemophilia, a concept that should be applicable to a multitude of genetic diseases [112].
Use of this concept for oral delivery of functional therapeutic proteins several times every day would face several technical challenges. Delivery of the correct dose will be the first major challenge. However, proteins like EX4 act based on glucose concentration in the blood and therefore, oral delivery of even 5,000-fold excess dose didn’t cause any hypoglycemia [25]. Since the patients with diabetes require multiple doses of daily injection, repeated oral delivery of bioencapsulated CTB-PFx3 or -EX4 might raise concerns about safety. Our current oral delivery system uses CTB as carrier for therapeutic peptides. However, CTB was known for its immunogenic property by provoking mucosal immune response against a protein that fused to CTB [194,195]. However, there have been numerous studies showing that CTB promote immunological tolerance against certain types of mucosally co-administered antigens [196]. For examples, CTB-proinsulin fusion protein suppressed the activation of dendritic cell (DC) by up-regulating Toll like receptor 2 (TLR-2) [197]. Likewise, dendritic cell maturation was severely retarded when autoantigen glutamic acid decarboxylase was fused with CTB [198]. This means that immune suppression of T lymphocytes was enhanced when proteins were fused to CTB. Thus, immune responses did develop against the tethered proteins. Immune tolerance induction using CTB-linked autoantigens was successful in several other pre-clinical studies on allergies or autoimmune diseases [132]. Moreover, CTB-based immunotherapy shows promise in human clinical studies. Behcet’s disease, an autoimmune eye disease caused by abnormal T cell reactivity to a specific peptide (BD peptide), was suppressed in some patients after CTB-BD peptide was orally delivered 3 times weekly for 12~16 weeks [199].
Contrary to autoantigens, more worries about the development of immune response against heterologous therapeutic peptide, EX4, could be raised although it has 53% amino acid similarity to human GLP-1. In the clinical trial, EX4 was injected twice daily for 30 weeks and immunogenicity test showed the formation of anti-EX4 antibody [200], however, any adverse immune response from patients have not been reported since its first use in clinic in 2005. According to the latest FDA safety update for Exenatide (FDA drug safety information, 2009), more than 6.6 million people were prescribed with no reports about adverse immune response in type II patients routinely receiving EX4. However, immune response test against Exenatide conducted by Eli Lilly [201] showed that antibody positive patients decreased over time and remained low through 82 weeks in most patients who developed antibodies. Moreover, as pointed out above, CTB conjugated form of autoantigens can induce immune system to tolerate the autoantigens. Therefore, it is expected that CTB conjugated EX4 should help develop tolerance rather than stimulate immune response.
The relevance of the repeated oral delivery of bioencapsulated CTB-therapeutic peptides as a treatment method can be further supported by several factors: large mucosal area (approximately 1.8 m2 ~ 2.7 m2 against body weight) [202]; numerous GM1 receptors on epithelial cell (15,000 binding sites per cell) [203]; and rapid turnover rate of intestinal endothelial cells [204]. As an alternative to CTB-based receptor mediated delivery, cell-penetrating peptides (CPPs)/protein transduction domains (PTDs) would be ideal carriers for therapeutic protein or peptides [205,206] if the oral delivery system based on CPPs/PTDs is successful in human clinical trials in the future.
Acknowledgements
Studies reported here are supported in part by the NIH R01 GM 63879, NIH R01 HL 109442 and NIH R01 HL 107904, Bill and Melinda Gates Foundation Global Health grant OPP 1031406 and the Juvenile Diabetes Research Foundation grant 17-2011-286 to Dr. Henry Daniell.
Abbreviations
- APC
antigen presenting cell
- AMA
apical membrane antigen-1
- BCG
Bacille Calmette Guerin
- CDC
Centers for Disease Control and Prevention
- CTB
cholera toxin B subunit
- DC
dendritic cell
- EX4
exendin-4
- FIX
blood coagulation factor IX
- GAD
glutamate decarboxylase
- GIT
gastrointestinal tract
- GLP-1
glucagon-like peptide-1
- ID
intradermal injection
- IPV
inactivated poliovirus vaccine
- LT-B
heat labile enterotoxin B subunit
- MSP1
merozoite surface protein-1
- NOD
non-obese diabetic
- ORV
orally immunized mice
- PA
protective antigen from Bacillus anthracis
- SC
subcutaneous injection
- SQV
subcutaneously immunized mice
- TLP
total leaf protein
- TSP
total soluble protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engaging Communities to Improve Global health: Reducing Disease Burden through Collaborative Approaches; Johannesburg and Durban; 2010. http://conferences.thehillgroup.com/obssr/engagingcommunities/about.html. [Google Scholar]
- 2.Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitesh SC, Sanjeev C, Vandana H, Alka A, Vijender S. Recent advances in insulin delivery systems: an update. World Applied Sciences Journal. 2010;11:1552–1556. [Google Scholar]
- 4.Akhrass F, Skinner N, Boswell K, Travis LB. Evolving trends in insulin delivery: in pursuit of improvements in diabetes management. AHDB. 2010;3:117–122. [PMC free article] [PubMed] [Google Scholar]
- 5.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 6.Report of the SIGN 2010 Meeting: annual meeting of the safe injection global network; Dubai. 2010. http://www.who.int/injection_safety/toolbox/sign2010_meeting.pdf. [Google Scholar]
- 7.Lebre F, Borchard G, Conceição Pedroso de Lima M, Borges O. Progress Towards a Needle-Free Hepatitis B Vaccine. Pharm. Res. 2011;28:986–1012. doi: 10.1007/s11095-010-0314-4. [DOI] [PubMed] [Google Scholar]
- 8.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson J, Koya V, Leppla SH, Daniell H. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine. 2004;22:4374–4384. doi: 10.1016/j.vaccine.2004.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniell H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002;20:581–586. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. U.S.A. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quesada-Vargas T, Ruiz ON, Daniell H. Characterization of heterologous multigene operons in transgenic chloroplasts: transcription, processing, and translation. Plant Physiol. 2005;138:1746–1762. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Hahn FM, Baidoo E, Kahlon TS, Wood DF, McMahan CM, Cornish K, Keasling JD, Daniell H, Whalen MC. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab. Eng. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol. J. 2010;8:223–242. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanagaraj AP, Verma D, Daniell H. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol. 2011;76:323–333. doi: 10.1007/s11103-011-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts-oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol. J. 2011;9:585–598. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SB, Li B, Jin S, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol. J. 2011;9:100–115. doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz ON, Alvarez D, Torres C, Roman L, Daniell H. Metallothionein expression in chloroplasts enhances mercury accumulation and phytoremediation capability. Plant Biotechnol. J. 2011;9:609–617. doi: 10.1111/j.1467-7652.2011.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol. J. 2012 doi: 10.1111/pbi.12008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelosi A, Shepherd R, Walmsley AM. Delivery of plant-made vaccines and therapeutics. Biotechnol. Adv. 2012;30:440–448. doi: 10.1016/j.biotechadv.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Newman KD, Elamanchili P, Kwon GS, Samuel J. Uptake of poly (D, L-lactic-co-glycolic acid) microspheres by antigen-presenting cells in vivo. J. Biomed. Mater. Res. 2002;60:480–486. doi: 10.1002/jbm.10019. [DOI] [PubMed] [Google Scholar]
- 28.Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization: recommendations of the advisory committee on immunization practices (ACIP) 2011 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. [PubMed]
- 29.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2005;54:1–40. [PubMed] [Google Scholar]
- 30.Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J. Infect. Dis. 1972;125:656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- 31.Brown H, Kasel JA, Freeman DM, Moise LD, Grose NP, Couch RB. The immunizing effect of influenza A/New Jersey/76 (Hsw1N1) virus vaccine administered intradermally and intramuscularly to adults. J. Infect. Dis. 1977;136:S466–S471. doi: 10.1093/infdis/136.supplement_3.s466. [DOI] [PubMed] [Google Scholar]
- 32.Mantoux C. L’intradermo-reaction a la tuberculine et son interpretation clinique. Presse Med. 1910;18:10–13. [Google Scholar]
- 33.Nirmal S, Cherian T, Samuel BU, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine. 1998;16:928–931. doi: 10.1016/s0264-410x(97)00293-4. [DOI] [PubMed] [Google Scholar]
- 34.Cone RA. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Park K, Kwon IC, Park K. Oral protein delivery: Current status and future prospect. React Funct. Polym. 2011;71:344–349. [Google Scholar]
- 36.Khafagy ES, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug. Deliv. Rev. 2007;59:1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Jadhav KR, Gambhire MN, Shaikh IM, Kadam VJ, Pisal SS. Nasal drug delivery system-factors affecting and applications. Curr. Drug Ther. 2007;2:27–38. [Google Scholar]
- 38.Varshosaz J, Sadrai H, Alinagari R. Nasal delivery of insulin using chitosan microspheres. J. Microencapsul. 2004;21:761–774. doi: 10.1080/02652040400015403. [DOI] [PubMed] [Google Scholar]
- 39.Varshosaz J, Sadrai H, Heidari A. Nasal delivery of insulin using bioadhesive chitosan gels. Drug Deliv. 2006;13:31–38. doi: 10.1080/10717540500309040. [DOI] [PubMed] [Google Scholar]
- 40.Veuillez F, Kalia YN, Jacques Y, Deshusses J, Buri P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001;51:93–109. doi: 10.1016/s0939-6411(00)00144-2. [DOI] [PubMed] [Google Scholar]
- 41.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Bandyopadhyay, Buccal bioadhesive drug delivery – A promising option for orally less efficient drugs. J. Control. Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Kumria R, Goomber G. Emerging trends in insulin delivery: Buccal route. J. Diabetol. 2011;2:1. [Google Scholar]
- 43.Yang TZ, Wang XT, Yan XY, Zhang Q. Phospholipid deformable vesicles for buccal delivery of insulin. Chem. Pharm. Bull. 2002;50:749–753. doi: 10.1248/cpb.50.749. [DOI] [PubMed] [Google Scholar]
- 44.Luo Y, Xu H, Huang K, Gao J, Peng H, Sheng X. Study on nanoparticle system for buccal delivery of insulin. Conf. Proc. IEEE Eng. Med. Biol. 2005;5:4842–4845. doi: 10.1109/IEMBS.2005.1615556. [DOI] [PubMed] [Google Scholar]
- 45.Hosny EA, Elkheshen SA, Saleh SI. Buccoadhesive tablets for insulin delivery: In vitro and in vivo studies. Boll. Chim. Farm. 2002;141:210–217. [PubMed] [Google Scholar]
- 46.Sahni J, Raj S, Ahmad FJ, Khar RK. Design and in vitro characterization of buccoadhesive drug delivery system of insulin. Indian J. Pharm. Sci. 2008;70:61–65. doi: 10.4103/0250-474X.40333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portero A, Teijeiro-Osorio T, Alonso MJ, Remuñán-López C. Development of chitosan sponges for buccal administration of insulin. Carbohydr. Polym. 2007;68:617–625. [Google Scholar]
- 48.Xu BH, Huang KX, Zhu YS, Gao QH, Wu QZ, Tian WQ, Sheng XQ, Chen ZX, Gao ZH. Hypoglycaemic effect of a novel insulin buccal formulation on rabbits. Pharmacol. Res. 2002;46:459–467. doi: 10.1016/s1043661802002049. [DOI] [PubMed] [Google Scholar]
- 49.Cui F, He C, He M, Tang C, Yin L, Qian F, Yin C. Preparation and evaluation of chitosan-ethylenediaminetetraacetic acid hydrogel films for the mucoadhesive transbuccal delivery of insulin. J. Biomed. Mater. Res. A. 2009;89:1063–1071. doi: 10.1002/jbm.a.32071. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Zhu J, Tang Y, Chen X, Yang Y. The preparation and application of pulmonary surfactant nanoparticles as absorption enhancers in insulin dry powder delivery. Drug Dev. Ind. Pharm. 2009;9:1059–1065. doi: 10.1080/03639040902769628. [DOI] [PubMed] [Google Scholar]
- 51.Klingler C, Müller BW, Steckel H. Insulin-micro- and nanoparticels for pulmonary delivery. Int. J. Pharm. 2009;377:173–179. doi: 10.1016/j.ijpharm.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 52. http://www.gurufocus.com.
- 53.Prausnitz MR, Langer R. Transdermal drug delivery. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prausnitz MR. Overcoming skin’s barrier: the search for effective and user-friendly drug delivery. Diabetes Technol. Ther. 2001;3:233–236. doi: 10.1089/152091501300209606. [DOI] [PubMed] [Google Scholar]
- 55.Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM. Tight junctions form a barrier in human epidermis. Eur J. Cell Biol. 2010;89:839–842. doi: 10.1016/j.ejcb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Pillai O, Nair V, Panchagnula R. Transdermal iontophoresis of insulin: IV. Influence of chemical enhancers. Int. J. Pharm. 2004;269:109–120. doi: 10.1016/j.ijpharm.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 57.Rastogi R, Anand S, Dinda AK, Koul V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev. Ind. Pharm. 2010;36:993–1004. doi: 10.3109/03639041003682012. [DOI] [PubMed] [Google Scholar]
- 58.Boucaud A, Garrgue MA, Machet L, Vaillant L, Patat F. Effect of sonication parameters on transdermal of insulin to hairless rats. J. Control. Release. 2002;81:113–119. doi: 10.1016/s0168-3659(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 59.Levin G. Advances in radio-frequency transdermal drug delivery. Pharm. Technol. 2008;32:s12–s19. [Google Scholar]
- 60.Lee K, Lee HC, Lee DS, Jung H. Drawing lithography: three dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010;22:483–486. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- 61.Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type I diabetes using hollow microneedles. Diabetes Technol. Ther. 2009;11:329–337. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews S, Lee JW, Prausnitz M. Recovery of skin barrier after stratum corneum removal by microdermabrasion. AAPS Pharm. Sci. Tech. 2011;12:1393–1400. doi: 10.1208/s12249-011-9715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. http://www.Phosphagenics.com.
- 64.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol. Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong Z, Jin Y, Zhang Y. Oral administration of a cholera toxin B subunit-insulin fusion protein produced in silkworm protects against autoimmune diabetes. J. Biotechnol. 2005;119:93–105. doi: 10.1016/j.jbiotec.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 66.Denes B, Krausova V, Fodor N, Timiryasova T, Henderson D, Hough J, Yu J, Fodor I, Langridge WH. Protection of NOD mice from type 1 diabetes after oral inoculation with vaccinia viruses expressing adjuvanted islet autoantigens. J. Immunother. 2005;28:438–448. doi: 10.1097/01.cji.0000171315.82997.9a. [DOI] [PubMed] [Google Scholar]
- 67.Gong Z, Pan L, Le Y, Liu Q, Zhou M, Xing W, Zhuo R, Wang S, Guo J. Glutamic acid decarboxylase epitope protects against autoimmune diabetes through activation of Th2 immune response and induction of possible regulatory mechanism. Vaccine. 2010;28:4052–4058. doi: 10.1016/j.vaccine.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Ma SW, Zhao DL, Yin ZQ, Mukherjee R, Singh B, Qin HY, Stiller CR, Jevnikar AM. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat. Med. 1997;3:793–796. doi: 10.1038/nm0797-793. [DOI] [PubMed] [Google Scholar]
- 69.Ma S, Huang Y, Yin Z, Menassa R, Brandle JE, Jevnikar AM. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5680–5685. doi: 10.1073/pnas.0307420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F. A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17525–17530. doi: 10.1073/pnas.0503428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, Ohtomo T, Ohmachi Y, Noda Y, Hirose S, Okumura K, Ogawa H, Takada K, Hirasawa M, Hiroi T, Takaiwa F. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol. J. 2011;9:982–990. doi: 10.1111/j.1467-7652.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- 72.Takagi H, Hiroi T, Yang L, Takamura K, Ishimitsu R, Kawauchi H, Takaiwa F. Efficient induction of oral tolerance by fusing cholera toxin B subunit with allergen-specific T-cell epitopes accumulated in rice seed. Vaccine. 2008;26:6027–6030. doi: 10.1016/j.vaccine.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 73.Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 74.Upadhyay S, Parikh A, Joshi P, Upadhyay UM, Chotai NP. Intranasal drug delivery system - A glimpse to become maestro. J. Appl. Pharm. Sci. 2011;1:34–44. [Google Scholar]
- 75.Reynolds J, Prodromidi EI, Juggapah JK, Abbott DS, Holthaus KA, Kalluri R, Pusey CD. Nasal administration of recombinant rat alpha3(IV)NC1 prevents the development of experimental autoimmune glomerulonephritis in the WKY rat. J. Am. Soc. Nephrol. 2005;16:1350–1359. doi: 10.1681/ASN.2004121026. [DOI] [PubMed] [Google Scholar]
- 76.He J, Zhao J, Li Z. Mucosal administration of alpha-fodrin inhibits experimental Sjögren's syndrome autoimmunity. Arthritis Res. Ther. 2008;10:R44. doi: 10.1186/ar2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao J, Li R, He J, Shi J, Long L, Li Z. Mucosal administration of an altered CII263-272 peptide inhibits collagen-induced arthritis by suppression of Th1/Th17 cells and expansion of regulatory T cells. Rheumatol. Int. 2008;29:9–16. doi: 10.1007/s00296-008-0634-4. [DOI] [PubMed] [Google Scholar]
- 78.Cao O, Armstrong E, Schlachterman A, Wang L, Okita DK, Conti-Fine B, High KA, Herzog RW. Immune deviation by mucosal antigen administration suppresses gene-transfer- induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rawle FE, Pratt KP, Labelle A, Weiner HL, Hough C, Lillicrap D. Induction of partial immune tolerance to factor VIII through prior mucosal exposure to the factor VIII C2 domain. J. Thromb. Haemost. 2006;4:2172–2179. doi: 10.1111/j.1538-7836.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi M, Abiru N, Arakawa T, Fukushima K, Zhou H, Kawasaki E, Yamasaki H, Liu E, Miao D, Wong FS, Eisenbarth GS, Eguchi K. Altered B:9–23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J. Immunol. 2007;179:2082–2088. doi: 10.4049/jimmunol.179.4.2082. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Yang J, Jin L, Feng J, Lu Y, Sun Y, Li T, Cao R, Wu J, Fan H, Liu J. Immunotherapy of autoimmune diabetes by nasal administration of tandem glutamic acid decarboxylase 65 peptides. Immunol. Invest. 2009;38:690–703. doi: 10.3109/08820130903124770. [DOI] [PubMed] [Google Scholar]
- 82.O'Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharm Res. 2004;21(9):1519–1530. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGhee JR, Lamm ME, Strober W. Mucosal immune responses: an overview. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. San Diego: Academic Press; 1999. pp. 485–506. [Google Scholar]
- 84.MacPherson A, McCoy K, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 85.Neutra M, Kozlowski P. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 86.Dukes C, Bussey HJR. The number of lymphoid follicles of the human large intestine. J. Pathol. 1926;29:111–116. [Google Scholar]
- 87.Neutra M, Mantis N, Kraehenbuhl J. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 88.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 89.Martin-Villa JM, Ferre-Lopez S, Lopez-Suarez JC, Corell A, Perez-Blas M, Arnaiz-Villena A. Cell surface phenotype and ultramicroscopic analysis of purified human enterocytes: a possible antigen-presenting cell in the intestine. Tissue Antigens. 1997;50:586–592. doi: 10.1111/j.1399-0039.1997.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez-Juan C, Perez-Blas M, Suarez-Garcia E, Lopez-Suarez JC, Muzquiz M, Cuadrado C, Martín-Villa JM. Lens culinaris, Phaseolus vulgaris and Vicia faba lectins specifically trigger IL-8 production by the human colon carcinoma cell line CACO-2. Cytokine. 2000;12:1284–1287. doi: 10.1006/cyto.1999.0731. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Juan C, Perez-Blas M, Valeri AP, Aguilera N, Arnaiz-Villena A, Pacheco-Castro A, Martin-Villa JM. Cell surface phenotype and cytokine secretion in Caco-2 cell cultures: increased RANTES production and IL-2 transcription upon stimulation with IL-1beta. Tissue Cell. 2001;33:570–579. doi: 10.1054/tice.2001.0212. [DOI] [PubMed] [Google Scholar]
- 92.Medina E, Guzman CA. Modulation of immune responses following antigen administration by mucosal route. FEMS Immunol. Med. Microbiol. 2000;27:305–311. doi: 10.1111/j.1574-695X.2000.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 93.Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10986–10991. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amani J, Mousavi SL, Rafati S, Salmanian AH. Immunogenicity of a plant-derived edible chimeric EspA, Intimin and Tir of Escherichia coli O157:H7 in mice. Plant Sci. 2011;180:620–627. doi: 10.1016/j.plantsci.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Streatfield S. Mucosal immunization using recombinant plant-based oral vaccines. Methods. 2006;38:150–157. doi: 10.1016/j.ymeth.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 96.Haglund K, Leiner I, Kerksiek K, Buonocore L, Pamer E, Rose JK. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing Human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 2002;76:7506–7517. doi: 10.1128/JVI.76.15.7506-7517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hayden CA, Streatfield SJ, Lamphear BJ, Fake GM, Keener TK, Walker JH, Clements JD, Turner DD, Tizard IR, Howard JA. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine. 2012;30:2937–2942. doi: 10.1016/j.vaccine.2012.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Cristillo A, Mboudjeka I, Shen S, Wu-Chou T, Montefiori D, Mascola J, Lu S, Markham P. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J. Med. Primatol. 2005;34:226–236. doi: 10.1111/j.1600-0684.2005.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Almeida AJ, Alpar HO. Nasal delivery of vaccines. J. Drug Target. 1996;3:455–467. doi: 10.3109/10611869609015965. [DOI] [PubMed] [Google Scholar]
- 100.Neutra RM, Pringault E, Kraehenbuhl JP. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 101.Jahnsen FL, Gran E, Haye R, Brandtzaeg P. Human nasal mucosa contains antigen-presenting cells of strikingly different functional phenotypes. Am. J. Respir. Cell Mol. Biol. 2004;30:31–37. doi: 10.1165/rcmb.2002-0230OC. [DOI] [PubMed] [Google Scholar]
- 102.Suman JD. Nasal drug delivery. Exp. Opin. Biolog. Therapy. 2003;3:519–523. doi: 10.1517/14712598.3.3.519. [DOI] [PubMed] [Google Scholar]
- 103.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One. 2010;5:e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madhun AS, Haaheim LR, Nostbakken JK, Ebensen T, Chichester J, Yusibov V, Guzman CA, Cox RJ. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine. 2011;29:4973–4982. doi: 10.1016/j.vaccine.2011.04.094. [DOI] [PubMed] [Google Scholar]
- 105.Gor DO, Rose NR, Greenspan NS. TH1–TH2: a procrustean paradigm. Nat. Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 106.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 108.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxinbased adjuvants. Vaccine. 2002;20:2431–2438. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 109.Thompson AL, Staats HF. Cytokines: The Future of Intranasal Vaccine Adjuvants. Clin. Dev. Immunol. 2011;2011 doi: 10.1155/2011/289597. Article ID 289597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–961. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verma D, Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145:1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patel YR. Drug delivery in diabetes: making effective treatment tolerable. ONdrugDelivery. 2006:4–7. http://www.ondrugdelivery.com/publications/diabetes.pdf. [Google Scholar]
- 114.Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50:503–509. doi: 10.1007/s00125-006-0559-y. [DOI] [PubMed] [Google Scholar]
- 115.Insulin Delivery Systems Market Forecast to 2014. 2012 http://www.rncos.com/Report/IM369.htm. [Google Scholar]
- 116.Bellmann-Sickert K, Beck-Sickinger AG. Peptide drugs to target G protein-coupled receptors. Trends Pharmacol. Sci. 2010;31:434–441. doi: 10.1016/j.tips.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 117.McGregor DP. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharmacol. 2008;8:616–619. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Lax R. The future of peptide development in the pharmaceutical industry. PharManufacturing. 2012 http://www.polypeptide.com/assets/002/5188.pdf. [Google Scholar]
- 119.Diago M, Crespo J, Olveira A, Pérez R, Bárcena R, Sánchez-Tapias JM, Muñoz-Sánchez M, Romero-Gómez M. Clinical trial: pharmacodynamics and pharmacokinetics of re-treatment with fixed-dose induction of peginterferon alpha-2a in hepatitis C virus genotype 1 true non-responder patients. Aliment Pharmacol. Ther. 2007;26:1131–1138. doi: 10.1111/j.1365-2036.2007.03470.x. [DOI] [PubMed] [Google Scholar]
- 120.Kumar TR, Soppimath K, Nachaegari SK. Novel delivery technologies for protein and peptide therapeutics. Curr. Pharm. Biotechnol. 2006;7:261–276. doi: 10.2174/138920106777950852. [DOI] [PubMed] [Google Scholar]
- 121.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 122.Subramanian GM, Fiscella M, Lamouse´-Smith A, Zeuzem S, McHutchison JG. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic Hepatitis C. Nat. Biotechnol. 2007;25:1411–1419. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 123.Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- 124.Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 125.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul. Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 127.Davidson MB, Bate G, Kirkpatrick P. Exenatide. Nat. Rev. Drug Discov. 2005;4:713–714. doi: 10.1038/nrd1828. [DOI] [PubMed] [Google Scholar]
- 128.Rowe PA, Campbell-Thompson ML, Schatz DA, Atkinson MA. The pancrease in human type 1 diabetes. Semin. Immunopathol. 2011;33:29–43. doi: 10.1007/s00281-010-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 131.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun JB, Czerkinsky C, Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 2010;71:1–11. doi: 10.1111/j.1365-3083.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 133.Jenkins PV, Keenan C, Keeney S, Cumming T, O’Donnell JS. Clinical utility gene card for: haemophilia B. Eur. J. Hum. Genet. 2012;20 doi: 10.1038/ejhg.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gater A, Thomson TA, Strandberg-Larsen M. Haemophilia B: impact on patients and economic burden of disease. Thromb. Haemost. 2011;106:398–404. doi: 10.1160/TH11-03-0193. [DOI] [PubMed] [Google Scholar]
- 135.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br. J. Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 136.Ghosh K, Shetty S. Immune response to FVIII in hemophilia A: an overview of risk factors. Clin. Rev. Allergy Immunol. 2009;37:58–66. doi: 10.1007/s12016-009-8118-1. [DOI] [PubMed] [Google Scholar]
- 137.Serban M, Mihailov D, Pop L, Ionita H, Ursu E, Talpos-Niculescu S, Ritli L, Baghiu D, Uscatescu V, Petrovanu C, Stancu P, Savescu D, Cucuianu A, Schramm W. Development of inhibitors in haemophilia. Ongoing epidemiological study. Hamostaseologie. 2011;31(Suppl 1):S20–S23. [PubMed] [Google Scholar]
- 138.Benson G, Auerswald G, Elezović I, Lambert T, Ljung R, Morfini M, Remor E, Salek SZ. Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice. Eur. J. Haematol. 2012;19 doi: 10.1111/j.1600-0609.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 139.Russell P, Eley SM, Hibbs SE, Manchee RJ, Stagg AJ, Titball RW. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine. 1995;13:1551–1556. doi: 10.1016/0264-410x(95)00090-n. [DOI] [PubMed] [Google Scholar]
- 140.Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K. Plague as a biological weapon: medical and public health management, Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 142.Titball RW, Williamson ED. Vaccination against bubonic and pneumonic plague. Vaccine. 2001;19:4175–4184. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 143. http://www.promedmail.org. [Google Scholar]
- 144.Alvarez ML, Cardineau GA. Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol. Adv. 2010;28:184–196. doi: 10.1016/j.biotechadv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 145.Kingston R, Burke F, Robinson JH, Bedford PA, Jones SM, Knight SC, Williamson ED. The fraction 1 and V protein antigens of Yersinia pestis activate dendritic cells to induce primary T cell responses. Clin. Exp. Immunol. 2007;149:561–569. doi: 10.1111/j.1365-2249.2007.03452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santi L, Giritch A, Roy CJ, Marillonnet S, Klimyuk V, Gleba Y, Webb R, Arntzen CJ, Mason HS. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc. Natl. Acad. Sci. U. S. A. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mett V, Lyons J, Musiychuk K, Chichester JA, Brasil T, Couch R, Sherwood R, Palmer GA, Streatfield SJ, Yusibov V. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine. 2007;25:3014–3017. doi: 10.1016/j.vaccine.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 148.Chichester JA, Musiychuk K, Mett V, Lyons J, Yusibov V. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine. 2009;27:3471–3474. doi: 10.1016/j.vaccine.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 149.Cholera vaccines. A brief summary of the March 2010 position paper. http://www.who.int/immunization/Cholera_PP_Accomp_letter_Mar_10_2010.pdf. [Google Scholar]
- 150.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. Oral vaccines for preventing cholera. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lopez AL, Clemens JD, Deen J, Jodar L. Cholera vaccines for the developing world. Hum. Vaccin. 2008;4:165–169. doi: 10.4161/hv.4.2.5122. [DOI] [PubMed] [Google Scholar]
- 152.Is a vaccine available to prevent cholera? 2011 http://www.cdc.gov/cholera/general/#vaccine.
- 153.Olsson L, Parment PA. Present and future cholera vaccines. Expert Rev. Vaccines. 2006;5:751–752. doi: 10.1586/14760584.5.6.751. [DOI] [PubMed] [Google Scholar]
- 154.Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, von Seidlein L, Carbis R, Han SH, Shin SH, Attridge S, Rao R, Holmgren J, Clemens J, Bhattacharya SK. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS ONE. 2008;3:e2323. doi: 10.1371/journal.pone.0002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Haan L, Verweij W, Agsteribbe E, Wilschut J. The role of ADP-ribosylation and GM1-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin. Immunol. Cell Biol. 1998;76:270–279. doi: 10.1046/j.1440-1711.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 156.Tsuji T, Watanabe K, Miyama A. Monomer of the B subunit of heat-labile enterotoxin from enterotoxigenic Escherichia coli has little ability to bind to GM1 ganglioside compared to its coligenoid. Microbiol. Immunol. 1995;39:817–819. doi: 10.1111/j.1348-0421.1995.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 157.Mastroeni P, Bowe F, Cahill R, Simmons C, Dougan G. Vaccines against gut pathogens. Gut. 1999;45:633–635. doi: 10.1136/gut.45.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 159.Karaman S, Cunnick J, Wang K. Expression of the cholera toxin B subunit (CT-B) in maize seeds and a combined mucosal treatment against cholera and traveler’s diarrhea. Plant Cell Rep. 2012;31:527–537. doi: 10.1007/s00299-011-1146-3. [DOI] [PubMed] [Google Scholar]
- 160.Nochi T, Yuki Y, Katakai Y, Shibata H, Tokuhara D, Mejima M, Kurokawa S, Takahashi Y, Nakanishi U, Ono F, Mimuro H, Sasakawa C, Takaiwa F, Terao K, Kiyono H. A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J. Immunol. 2009;183:6538–6544. doi: 10.4049/jimmunol.0901480. [DOI] [PubMed] [Google Scholar]
- 161.Daniell H, Chebolu S, Kumar S, Singleton M, Falconer R. Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine. 2005;23:1779–1783. doi: 10.1016/j.vaccine.2004.11.004. (2005) [DOI] [PubMed] [Google Scholar]
- 162.Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chebolu S, Daniell H. Stable expression of Gal/GalNAc lectin of Entamoeba histolytica in transgenic chloroplasts and immunogenicity in mice towards vaccine development for amoebiasis. Plant Biotechnol. J. 2007;5:230–239. doi: 10.1111/j.1467-7652.2006.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. http://www.who.int/malaria/world_malaria_report_2011/en/
- 165.Angov E, Aufiero BM, Turgeon AM, Handenhove MV, Ockenhouse CF, Kester KE, Walsh DS, McBride JS, Dubois MC, Cohen J, Haynes JD, Eckels KH, Hepper DG, Ballou WR, Diggs CL, Lyon JA. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-142 malaria vaccine. Mol. Biochem. Parasitol. 2003;128:195–204. doi: 10.1016/s0166-6851(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 166.Malkin E, Dubovsky F, Moree M. Progress towards the development of malaria vaccines. Trends Parasitol. 2006;22:292–295. doi: 10.1016/j.pt.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 167.Malkin E, Hu J, Li Z, Chen Z, Bi X, Reed Z, Dubovsky F, Liu J, Wang Q, Pan X, Chen T, Giersing B, Xu Y, Kang X, Gu J, Shen Q, Tucker K, Tierney E, Pan W, Long C, Cao Z. A phase 1 trial of PfCP2.9: an AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine. 2008;26:6864–6873. doi: 10.1016/j.vaccine.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 168.Aide P, Bassat Q, Alonso PL. Towards an effective malaria vaccine. Arch. Dis. Child. 2007;92:476–479. doi: 10.1136/adc.2005.092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–1498. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 170.Maher B. Malaria: the end of the beginning. Nature. 2008;451:1042–1046. doi: 10.1038/4511042a. [DOI] [PubMed] [Google Scholar]
- 171.Good MF, Kaslow DC, Miller LM. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 1998;16:57–87. doi: 10.1146/annurev.immunol.16.1.57. [DOI] [PubMed] [Google Scholar]
- 172.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 173.Dutta S, Haynes JD, Moch JK, Barbosa A, Lanar DE. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12295–12300. doi: 10.1073/pnas.2032858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hodder AN, Crewther PE, Anders RF. Specificity of the Protective Antibody Response to Apical Membrane Antigen 1. Infect. Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Narum DL, Haynes JD, Fuhrmann S, Moch K, Liang H, Hoffman SL, Sim BK. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acid. Infect. Immun. 2000;68:1964–1966. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria meroxoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chang SP, Case SE, Gosnell WL, Hashimoto A, Kramer KJ, Tam LQ, Hashiro CQ, Nikaido CM, Gibson HL, Lee-Ng CT, Barr PJ, Yokota BT, Hut GS. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Darko CA, Angov E, Collins WE, Bergmann-Leitner ES, Girouard AS, Hitt SL, McBride JS, Diggs CL, Holder AA, Long CA, Barnwell JW, Lyon JA. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 2005;73:287–297. doi: 10.1128/IAI.73.1.287-297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Egan AE, Blackman MJ, Kaslow DC. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed and/or -rechallenged Aotus vociferons monkeys. Infect. Immun. 2000;68:1418–1427. doi: 10.1128/iai.68.3.1418-1427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tian JH, Kumar S, Kaslow DC, Miller LH. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect. Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang L, Webster DE, Campbell AE, Dry IB, Wesselingh SL, Coppel RL. Immunogenicity of Plasmodium yoelii merozoite surface protein 4/5 produced in transgenic plants. Int. J. Parasitol. 2008;38:103–110. doi: 10.1016/j.ijpara.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 182.Sachdeva S, Mohmmed A, Dasaradhi PV, Crabb BS, Katyal A, Malhotra P, Chauhan VS. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine. 2006;24:2007–2016. doi: 10.1016/j.vaccine.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 183.Ratner M. Pfizer stakes a claim in plant cell-made biopharmaceuticals. Nat. Biotechnol. 2010;28:107–108. doi: 10.1038/nbt0210-107. [DOI] [PubMed] [Google Scholar]
- 184. http://oig.hhs.gov/oei/reports/oei-04-10-00430.asp.
- 185.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 186.Thompson KM, Tebbens RJ. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev. Vaccines. 2012;11:449–459. doi: 10.1586/erv.11.195. [DOI] [PubMed] [Google Scholar]
- 187.Hird TR, Grassly NC. Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002599. e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity – friends or foes? Trends Immunol. 2009;30:409–414. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 189.Steinman L. Immune therapy for autoimmune diseases. Science. 2004;305:212–216. doi: 10.1126/science.1099896. [DOI] [PubMed] [Google Scholar]
- 190.Wang J, Lozie J, Johnson G, Kirshner S, Verthelyi D, Pariser A, Shores E, Rosenberg A. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat. Biotechnol. 2008;26:901–908. doi: 10.1038/nbt.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nayak S, Sivakumar R, Cao O, Daniell H, Byrne BJ, Herzog RW. Mapping the T helper cell response to acid α-glucosidase in Pompe mice. Mol. Genet. Metabol. 2012;106:189–195. doi: 10.1016/j.ymgme.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jayandharan GR, Srivastava A. Hemophilia: Disease, Diagnosis and Treatment. J. Genet. Syndr. Gene Ther. 2011;(S1):005. doi: 10.4172/2157-7412.S1-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, Ertl HCJ, High KA, Herzog RW. Impact of the Underlying Mutation and the Route of Vector Administration on Immune Responses to Factor IX in Gene Therapy for Hemophilia B. Mol. Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McKenzie SJ, Halsey JF. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J. Immunol. 1984;133:1818–1824. [PubMed] [Google Scholar]
- 195.Van der Heijden PJ, Bianchi AT, Dol M, Pals JW, Stok W, Bokhout BA. Maninpulation of intestinal immune responses against ovalbumin by cholera toxin and its B subunit in mice. Immunology. 1991;72:89–93. [PMC free article] [PubMed] [Google Scholar]
- 196.Sánchez J, Holmgren J. Cholera toxin – A foe & a friend. Indian J. Med. Res. 2011;133:153–163. [PMC free article] [PubMed] [Google Scholar]
- 197.Odumosu O, Payne K, Baez I, Jutzy J, Wall N, Langridge W. Suppression of dendritic cell activation by diabetes autoantigens linked to the cholera toxin B subunit. Immunobiology. 2011;216:447–456. doi: 10.1016/j.imbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Odumosu O, Nicholas D, Payne K, Langridge W. Cholera toxin B subunit linked to glutamic acid decarboxylase suppresses dendritic cell maturation and function. Vaccine. 2011;29:8451–8458. doi: 10.1016/j.vaccine.2011.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, Mizushima Y, Holmgren J, Lehner T. Oral tolerization with peptide 336–351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet's disease. Clin. Exp. Immunol. 2004;137:201–208. doi: 10.1111/j.1365-2249.2004.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 201.Eli Lilly . Electronic Medicines Compendium. Leatherhead: Datapharm; 2011. [accessed May 2012]. Byetta (exenatide) Summary of Product Characteristics. (Netherlands). http://www.medicines.org.uk/EMC/medicine/19257/SPC/Byetta+5+micrograms+solution+for+injection%2c+prefilled+pen.++Byetta+10+micrograms+solution+for+injection%2c+prefilled+pen./ [Google Scholar]
- 202.Wilson JP. Surface area of the small intestine in man. Gut. 1967;8:618–621. doi: 10.1136/gut.8.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Holmgren J, Lönnroth I, Månsson J, Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc. Natl. Acad. Sci. U.S.A. 1975;72:2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fishman PH, Bradley RM, Hom BE, Moss J. Uptake and metabolism of exogenous gangliosides by cultured cells: effect of choleragen on the turnover of GM1. J. Lipid Res. 1983;24:1002–1011. [PubMed] [Google Scholar]
- 205.Wadia JS, Dowdy SF. Protein transduction technology. Curr. Opin. Biotechnol. 2002;13:52–56. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 206.Khafagy ES, Morishita M. Oral biodrug delivery using cell-penetrating peptide. Adv. Drug Deliv. Rev. 2012;64:531–539. doi: 10.1016/j.addr.2011.12.014. [DOI] [PubMed] [Google Scholar]