Abstract
The study of the regulatory signaling hierarchies of human heart development is limited by a lack of model systems that can reproduce the precise developmental events that occur during human embryogenesis. The advent of human pluripotent stem cell (hPSC) technology and robust cardiac differentiation methods affords a unique opportunity to monitor the full course of cardiac induction in vitro. Here we show that stage-specific activation of insulin signaling strongly inhibited cardiac differentiation during a monolayer-based differentiation protocol that used TGFβ superfamily ligands to generate cardiomyocytes. However, insulin did not repress cardiomyocyte differentiation in a defined protocol that employed small molecule regulators of canonical Wnt signaling. By examining the context of insulin inhibition of cardiomyocyte differentiation, we determined that the inhibitory effects by insulin required Wnt/β-catenin signaling and that the cardiomyocyte differentiation defect resulting from insulin exposure was rescued by inhibition of Wnt/β-catenin during the cardiac mesoderm (Nkx2.5+) stage. Thus, insulin and Wnt/β-catenin signaling pathways, as a network, coordinate to influence hPSC differentiation to cardiomyocytes, with the Wnt/β-catenin pathway dominant to the insulin pathway. Our study contributes to the understanding of the regulatory hierarchies of human cardiomyocyte differentiation and has implications for modeling human heart development.
Keywords: canonical Wnt signaling, insulin signaling, human pluripotent stem cells, mesendoderm, cardiac mesoderm
INTRODUCTION
Human pluripotent stem cells (hPSCs) provide unprecedented opportunities for studying and understanding human embryology in vitro, including establishing a comprehensive model system to identify and characterize the regulatory signaling hierarchies of human heart commitment. It has been demonstrated that cell fate specification and maturation from hPSCs follows a sequence and time course highly reminiscent of early human development [1–3]. Thus hPSC-based developmental model systems, which have the capacity to recapitulate the normal maturation sequence present in the embryo in a controlled, stepwise fashion, should also provide the opportunity to test the effects of unidentified developmental stimuli and complex cross-talk between cell signaling pathways, thereby enabling an improved understanding of human heart development.
Insulin and insulin-like growth factor (IGF) signaling direct cardiomyocyte proliferation in vivo in E10.5 mouse embryos [4, 5]. McDevitt et al. identified that insulin/IGF signaling also mediates proliferation of cardiomyocytes differentiated from human embryonic stem cells (hESCs) in vitro [6]. The important roles of insulin/IGF signaling during the earliest stages of cardiac development in vivo, including differentiation of cardiac progenitors, are largely unknown, however. The robust cardiac differentiation potential of hPSCs provides a unique way to study insulin regulation of cardiac differentiation in vitro.
Insulin mediates its effects through the structurally similar insulin receptor (IR), insulin-like growth factor 1 receptor (IGF1R), and insulin-like growth factor 2 receptor (IGF2R). Downstream signaling involves activation of phosphatidylinositol 3-kinase (PI3K) and Akt pathways [7]. Self-renewal of hPSCs requires insulin/IGF signaling [8]. The insulin signaling pathway has also emerged as one of the key regulators of cardiac differentiation of pluripotent cells in vitro. Naito et al. reported that insulin/Akt signaling plays a critical positive role in early cardiomyogenesis of mouse embryonal carcinoma (P19CL6) cells [9]. Insulin exerts its positive effects on cardiac differentiation in P19CL6 cells by regulating canonical Wnt signaling [9]. Studies of cardiac differentiation in hPSCs using co-culture systems and embryoid bodies (EBs), however, indicated that the insulin/IGF pathway negatively regulates cardiac differentiation during early differentiation stages [10]. For example, mouse visceral endoderm-like (END-2) cell-conditioned medium has been shown to enhance cardiac differentiation in hPSC-derived embryoid bodies (EBs) [11]. It has been suggested that removal of insulin by END-2 cells partially mediates enhancement of cardiac differentiation in END-2 conditioned medium [10]. Freud et al. demonstrated that insulin, acting primarily via IGF1R and PI3K/Akt, has an inhibitory effect during early cardiac differentiation stages by redirecting mesendoderm derivatives, including cardiogenic mesoderm and endoderm, to neuroectoderm [7]. The mechanisms by which insulin/Akt signaling mediate these inhibitory effects on cardiac differentiation of hPSC are unknown.
Abnormalities in heart developmental can result in congenital heart diseases, the most common types of birth defects in humans [12]. Cell signaling pathways that mediate cardiogenesis are of particular interest because of their potential use for cardiac regeneration [13]. Understanding the detailed signaling networks that dictate cardiac commitment will undoubtedly contribute to future treatment and regenerative strategies. Several extrinsic signaling pathways have been implicated in cardiac differentiation of hPSCs, including the transforming growth factor β (TGFβ) superfamily, broblast growth factor (FGF), insulin and insulin-like growth factor, and Wnt signaling [14–17]. However, these signaling pathways are often studied in isolated and in undefined conditions. Thus, how these signaling pathways coordinate as a network to influence hPSC fate is unclear.
Over the past decade, substantial advances have been made in generating cardiomyocytes from hPSCs by providing defined developmental cues at the appropriate temporal differentiation stage. The addition of specific factors in defined medium can provide insights into mechanisms of insulin regulation of cardiac differentiation. Sequential addition of Activin A and BMP4 to defined RPMI/B27 medium has been reported to generate approximately 30% cardiomyocytes in the H7 hESC line [18]. However, the efficiency of this Activin A and BMP4 monolayer directed differentiation protocol can be highly variable between cell lines and experimental repeats [19]. Optimization of stage-specific concentrations of insulin, which is present in B27 supplement, might increase the robustness of this protocol. Here, we adopted and revised this monolayer directed differentiation protocol by using insulin-free medium to enhance cardiac differentiation. We first applied various concentrations of insulin at different points of cardiac differentiation and showed that insulin signaling has stage-specific roles on cardiac differentiation. During early differentiation, insulin signaling exerts strong inhibitory effects on cardiac commitment, resulting in few cardiomyocytes. When we tested the temporal effects of insulin on cardiac differentiation using a differentiation protocol that uses small molecule modulators of Wnt signaling [17] rather than TGFβ superfamily ligands to drive cardiomyocyte commitment, insulin’s strong inhibitory effects on cardiomyocyte differentiation were not observed. We then illustrate that appropriate temporal regulation of Wnt signaling is sufficient to rescue the inhibitory effects of insulin on cardiac differentiation. Thus, we employed a chemically defined system to examine the combinatorial effects of signaling pathways that govern hPSC cardiac fate determination and showed that Wnt/β-catenin signaling is dominant to insulin signaling for cardiac differentiation of hPSCs.
MATERIALS AND METHODS
Maintenance of hPSCs
Transgene free human iPSCs (6-9-9 and 19-9-11) [20], lentiviral integrated human iPSC (IMR90C4) [21] and hESCs (H9, H13, H14) [22] were maintained on Matrigel (BD Biosciences) or Synthemax plates (Corning) in mTeSR1 medium (STEMCELL Technologies).
Cardiac-directed differentiation using Gsk3 inhibitor, Activin A and BMP4
hPSCs maintained on Matrigel in mTeSR1 were dissociated into single cells with Accutase (Invitrogen) at 37°C for 5 min and then seeded onto a Matrigel-coated cell culture dish at 100,000 – 200,000 cell/cm2 in mTeSR1 supplemented with 5 μM ROCK inhibitor (Y-27632, Stemgent)(day −5). Cells were cultured in mTeSR1, changed daily until day −3. Cells were then cultured in mTeSR1 containing either 1 μM BIO or 1 μM CHIR99021 (CH) from day −3 to day 0. At day 0, cells were treated with 100 ng/ml Activin A (R&D) and 1% serum replacement (Invitrogen) in RPMI/B27-insulin. After 24 hr, the medium was changed to RPMI/B27-insulin supplemented with 5 ng/ml BMP4 (R&D) for another 4 days. At day 5 and every 3 days later, the medium was changed with fresh RPMI/B27.
Cardiac-directed differentiation via small molecule modulators of Wnt signaling
Cells were dissociated and plated as described in the Activin/BMP4 protocol. When hPSCs maintained on Matrigel or Synthemax plates achieved confluence, cells were treated with 12 μM CH in RPMI/B27-insulin for 24 hr (day 0 to day 1). The medium was changed to RPMI/B27-insulin, followed by 2 μg/ml dox treatment at 36 hours post addition of CH for transgenic cell lines. For genetically unmodified lines, 5 μM Inhibitor of either Wnt Production-2 (IWP2) (Tocris) or IWP4 (Stemgent), which prevent palmitylation of Wnt ligands by Porcupine, thereby blocking secretion of active Wnt ligand [23], was added at day 3 to inhibit Wnt signaling and removed during the day 5 medium change. Cells were maintained in the RPMI/B27 starting from day 5, with the medium changed every 3 days.
Lentiviral production and infection of hPSCs
The inducible β-catenin knockdown vector ishcat-1 (Addgene plasmid 36297) and Wnt reporter vector 7TGP (Addgene plasmid 24305) were used for lentivirus particle production. These vectors were cotransfected with the helper plasmids psPAX2 and pMD2.G (Addgene plasmids 12260 and 12259) into HEK-293TN cells (System Biosciences) for virus production. Virus-containing media were collected at 48 and 72 hr after transfection and used for hPSC infection in the presence of 6 μg/ml polybrene (Sigma). Transduced cells were selected and clonally isolated based on resistance to 1 μg/ml puromycin.
RT-PCR and Quantitative RT-PCR
Total RNA was prepared with the RNeasy mini kit (QIAGEN) and treated with DNase (QIAGEN). 1 μg RNA was reverse transcribed into cDNA via Oligo (dT) with Superscript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was done in triplicate with iQ SYBR Green SuperMix (Bio-Rad). RT-PCR was done with Gotaq Master Mix (Promega) and then subjected to 2% agarose gel electrophoresis. ACTB was used as an endogenous control. The primer sequences are listed in Supplementary Table S1.
Flow cytometry
Cells were dissociated into single cells and then fixed with 1% paraformaldehyde for 20 min at room temperature and stained with primary and secondary antibodies in PBS plus 0.1% Triton X-100 and 0.5% BSA. Data were collected on a FACSCaliber flow cytometer (Beckton Dickinson) and analyzed using FlowJo. Antibodies are listed in Supplementary Table S2.
Western Blot Analysis
Cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce) in the presence of Halt Protease and Phosphatase Inhibitor Cocktail (Pierce). Proteins were separated by 10% Tris-Glycine SDS/PAGE (Invitrogen) under denaturing conditions and transferred to a nitrocellulose membrane. After blocking with 5% milk in TBST, the membrane was incubated with primary antibody overnight at 4°C. The membrane was then washed, incubated with an anti-mouse/rabbit peroxidase-conjugated secondary antibody (Cell Signaling) at room temperature for 1 hr, and developed by SuperSignal chemiluminescence (Pierce). Antibodies are listed in Supplementary Table S2.
Intracellular Ca2+ transient assay in hPSC derived cardiomyocytes
hPSC-derived cardiomyocytes were treated with 10 μM Fluo-4 AM (Life technologies, F14217) in RPMI/B27 medium for 15 min at 37°C in a 5% CO2 incubator. After 15 min incubation, cells were washed with PBS two times and then fed with RPMI/B27 medium. Cells were then incubated in 37°C, 5% CO2 for 30 min before imaging. Calcium transients of single cardiomyocytes were recorded with a temporal resolution of 10 frames per second. The data were then quantified as the background-subtracted fluorescence intensity changes normalized to the background-subtracted baseline fluorescence using Image J.
Statistics
Data are presented as mean ± standard error of the mean (SEM). Statistical significance was determined by Student’s t-test (two-tail) for two groups or one-way ANOVA for multiple groups with post hoc testing using Tukey method using Microcal Origin, v8.0. P < 0.05 was considered statistically significant.
RESULTS
Insulin inhibits cardiac differentiation induced by Activin A and BMP4
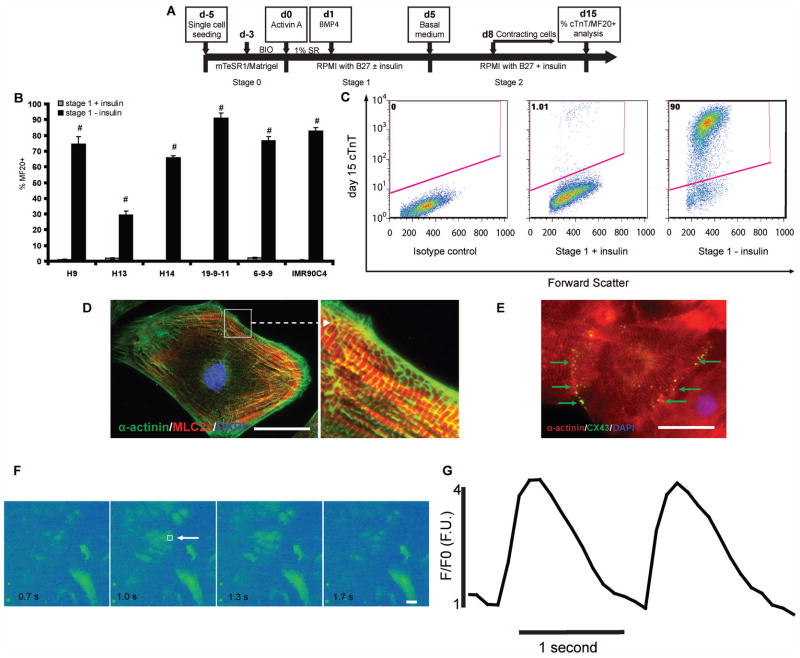
Insulin/Akt signaling is required for generating cardiomyocytes from mouse pluripotent P19CL6 cells [9]. Insulin also has been shown to inhibit cardiac differentiation when present during the early stages of hPSC differentiation in an END-2 cell co-culture system [10] and in EBs using differentiation medium containing serum [24]. The context-dependent effects of insulin signaling on mouse and human pluripotent cells inspire further study under more defined conditions to identify insulin’s specific role in cardiac commitment. In order to assess the stage-specific effects of insulin during hPSC differentiation to cardiomyocytes, we employed a defined differentiation protocol that sequentially presents a Gsk3 inhibitor, Activin A, and BMP4 to an hPSC monolayer (GiAB protocol) [17]. This GiAB protocol is a modified version of the monolayer directed differentiation protocol reported by Laflamme et al. [18], with Gsk3 inhibitor pre-treatment of undifferentiated cells to provide more robust cardiac differentiation in multiple hPSC lines [17]. Fig. 1a shows a schematic of the GiAB protocol. Starting from day −5, singularized hESCs and iPSCs were expanded on Matrigel in mTeSR1 for 2 days, followed by another 3 days in mTeSR1 supplemented with a Gsk3 inhibitor, either 1 μM BIO or CHIR 99021. Then, a medium consisting of RPMI supplemented with B27 was used to initiate differentiation. Two compositions of B27 were used in this study, B27 which contains a proprietary concentration of insulin and B27 without insulin. To stimulate differentiation at day 0, the RPMI/B27 medium, with or without insulin, was supplemented with 100 ng/ml Activin A and 1% serum replacement. At day 1, we changed the medium to RPMI/B27, with or without insulin, supplemented with 5 ng/ml BMP4. Medium was not changed between days 1 and 5. At day 5, RPMI/B27 containing insulin was used, and this medium was replaced every 3 days (Fig. 1A). Three hESC lines (H9, H13, H14) and three iPSC lines (19-9-11, 6-9-9 and IMR90C4) were tested for when and how insulin exerts inhibitory effects on cardiomyocyte differentiation in this defined differentiation system. At day 15, flow cytometric analysis of sarcomere myosin heavy chain expression (MF20 antibody) was performed. The GiAB protocol produced cardiomyocytes in all lines tested (30–90% MF20+ cells) when RPMI/B27 lacking insulin was used as a base medium (Fig. 1B). When RPMI/B27 containing insulin was used throughout the differentiation process, very few (<5%) MF20+ cells were obtained from all six lines tested (Fig. 1B). These results have been verified by anti-cardiac troponin T (cTnT) flow cytometric analysis at day 15 (Fig. 1C). Flow cytometric analysis of day 30 cells differentiated via the GiAB protocol with or without insulin revealed that insulin administration also blocked generation of cTnT+ cardiomyocytes at day 30 (Fig. S1). Coimmunolabeling for α-actinin (sarcomeric Z-line) and MLC-2a (sarcomeric A-band) of cardiomyocytes generated by the GiAB protocol in the absence of insulin showed clear alternating Z-line (green) and A-band staining (red), suggestive of cardiac myofilament formation (Fig. 1D). Coimmunolabeling for α-actinin (red) and connexin 43 (green) revealed that the gap junction protein connexin 43 (green) localized at the points of cardiomyocyte–cardiomyocyte (red) contact (Fig. 1E), similar to prior observations of connexin 43 co-localization in human iPSC-derived cardiomyocytes [25]. In order to assess the functional integrity of the differentiating cardiomyocytes, we performed dynamic Ca2+ transient analysis. The normalized Ca2+ transient intensity (F/F0) (Fig. 1F,G) observed cardiomyocyte produced via the GiAB protocol is similar to results previously reported in human iPSC-derived cardiomyocytes [26]. These results demonstrate that the amount of insulin present in B27 is sufficient to block the cardiac differentiation potential of hPSCs induced by Activin A and BMP4.
Figure 1.
(A) Schematic of protocol for hPSC differentiation to cardiomyocytes via Gsk3 inhibition, Activin A, and BMP4. The differentiation process is divided into three stages, d-5 to d0 (stage 0); d0 to d5 (stage 1); d5 and after (stage 2). (B–C) H9, H13, and H14 hESC lines and 19-9-11, 6-9-9, and IMR90C4 iPSC lines were cultured on Matrigel in mTeSR1 and then differentiated into cardiomyocytes according to (A). 15 days after initiation of differentiation, cells were analyzed by flow cytometry using the MF20 antibody. Error bars represent the s.e.m. of three independent experiments. # p<0.005, each pair of time points with insulin versus without insulin; t test. (C) 15 days after initiation of differentiation of 19-9-11 cells using the protocol shown in (A), cells differentiated in the presence and absence of insulin during stage 1 were analyzed for cTnT expression by flow cytometry. (D–G) Cardiomyocytes were generated from H9 cells using the protocol described in (A) without insulin during stage 1. At day 30, cells were individualized and replated on 0.1% gelatin coated coverslips. Immunostaining for (D) α-actinin (green) and MLC2a (red) shows sarcomere organization and (E) connexin 43 (green) and α-actinin (red) immunostaining shows gap junction formation between neighboring cardiomyocytes. Scale bar = 50 μm. (F–G) Cells were treated with 10 μM Fluo-4 AM for 15 min and then Ca2+ transients were recorded with a temporal resolution at 10 frames per second. Box (white arrowhead) in panel (F) denotes the site of analysis of absolute fluorescence normalized to initial fluorescence (F/F0) shown in panel G. Scale bar = 50 μm.
Insulin inhibits cardiac mesoderm but not mesendoderm formation
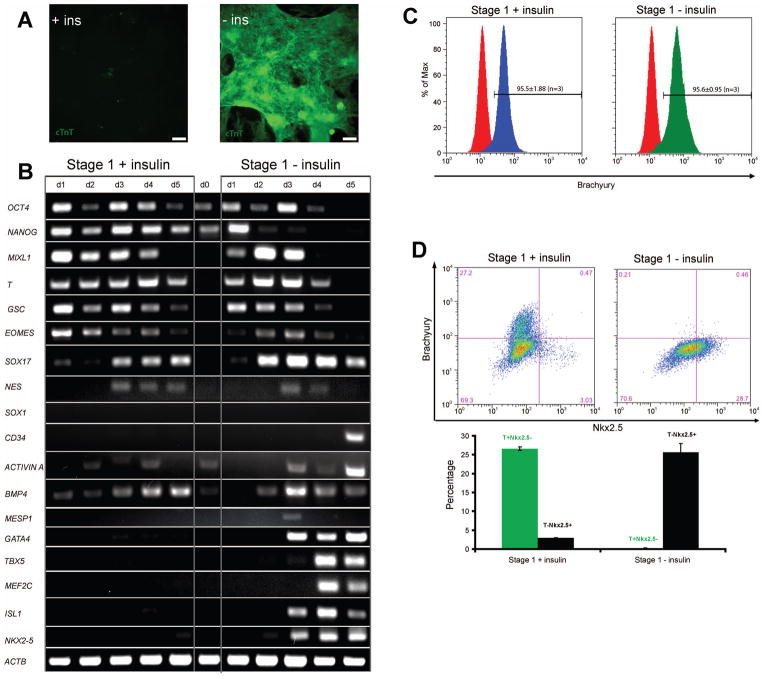
To understand the potential mechanism underlying the inhibitory effect of insulin on hPSC differentiation to cardiomyocytes, we analyzed expression of several key developmental regulators by RT-PCR during differentiation using the GiAB protocol with or without insulin. As expected, cells cultured in RPMI/B27 lacking insulin produced a monolayer of spontaneously contracting cardiomyocytes (Movie S1). At day 15, cells in this confluent monolayer exhibited cTnT immunostaining (Fig. 2A). Gene expression analysis showed the sequential up-regulation of mesendoderm genes T, MIXL1, GSC, and EOMES followed by the cardiac mesoderm transcription factors GATA4, TBX5, MEF2C, ISL1 and NKX2-5 (Fig. 2B). When RPMI/B27 medium containing insulin was used in the GiAB protocol, however, few cTnT+ cardiomyocytes were formed at day 15 and spontaneous contraction was not observed in the cell monolayers (Fig. 2A and Movie S2). Whereas repression of brachyury expression by insulin has been reported during cardiac differentiation of hESCs [7], interestingly, we found that cells exposed to RPMI/B27 containing insulin expressed mesendoderm markers MIXL1 and T to a similar extent as cells differentiated in RPMI/B27 without insulin (Fig. 2B). Flow cytometry revealed that the GiAB protocol produced highly enriched populations of ~95% Brachyury+ cells using RPMI/B27 with or without insulin (Fig. 2C). While a previous report indicated that insulin inhibits mesendoderm differentiation of hESCs by END-2 cell co-culture and EB formation [7], our observations suggest that insulin does not inhibit mesendoderm differentiation induced by Activin A/BMP4 under defined conditions.
Figure 2.
(A) H9 cells were differentiated as described in Fig. 1(A). At day 15 hPSCs differentiated in the presence and absence of insulin during stage 1 (days 0–5) were analyzed for cTnT expression by immunofluorescence. Scale bar = 200 μm. (B) At different time points, mRNA was collected and RT-PCR analysis of pluripotent (OCT4, NANOG), mesendoderm (MIXL1, T, GSC, EOMES), ectodermal (NES, SOX1), endodermal (SOX17), hematopoietic progenitor (CD34), growth factors (ACTIVIN A, BMP4) and cardiac gene expression (MESP1, GATA4, TBX5, MEF2C, ISL1, NKX2-5) was performed. (C–D) After initiation of differentiation, cells were analyzed for (C) brachyury expression at day 1 and (D) brachyury and Nkx2.5 expression at day 5 by flow cytometry. Error bars represent the s.e.m. of three independent experiments.
In the presence of RPMI/B27 containing insulin, expression of the mesendoderm genes MIXL1, T, and EOMES was sustained longer than in RPMI/B27 without insulin (Fig. 2B). Cells differentiated in RPMI/B27 lacking insulin downregulated mesendoderm genes starting at day 3 and subsequently upregulated expression of the cardiac mesoderm genes TBX5 [27], GATA4 [28], MEF2C [29], ISL1 [30, 31] and NKX2.5 [32] (Fig. 2B). EOMES has been suggested as a mesendoderm marker during hPSC differentiation [33] while MESP1 is generally considered as a cardiac mesoderm marker [34] that can be induced by EOMES [35, 36]. The observation that insulin does not inhibit EOMES expression but does block MESP1 expression further demonstrates that insulin inhibits cardiac mesoderm differentiation of hPSCs. Insulin was also found to inhibit the hematopoietic progenitor gene CD34 [37] expression, but did not affect ectodermal (NES and SOX1) [38] or endodermal (SOX17) [38] gene expression. In addition, endogenous ACTIVIN A and BMP4 were expressed during differentiation in the presence and absence of insulin, suggesting that insulin’s inhibition effects were not related to endogenous Activin A/BMP4 expression.
Flow cytometric analysis of brachyury and Nkx2.5 expression revealed that at day 5, 26.6 ± 0.4% of cells retained brachyury expression but only 2.97 ± 0.04% of cells expressed Nkx2.5 when the cells were differentiated in RPMI/B27 containing insulin (Fig. 2D). However, when cells were differentiated in RPMI/B27 lacking insulin, at day 5 few cells (0.21 ± 0.06%) expressed brachyury but 25.7 ± 2.3% expressed Nkx2.5+ (Fig. 2D). At day 7, the percentage of cells expressing brachyury in the population differentiated in the presence of insulin decreased to 2.57 ± 0.52%; however, few (2.60 ± 0.24%) cells were Nkx2.5+ at this stage (Fig. S2). These results suggest that insulin does not merely change the kinetics of differentiation. Instead insulin may change the trajectory of differentiation by exerting its effects on fate choice during developmental transitions.
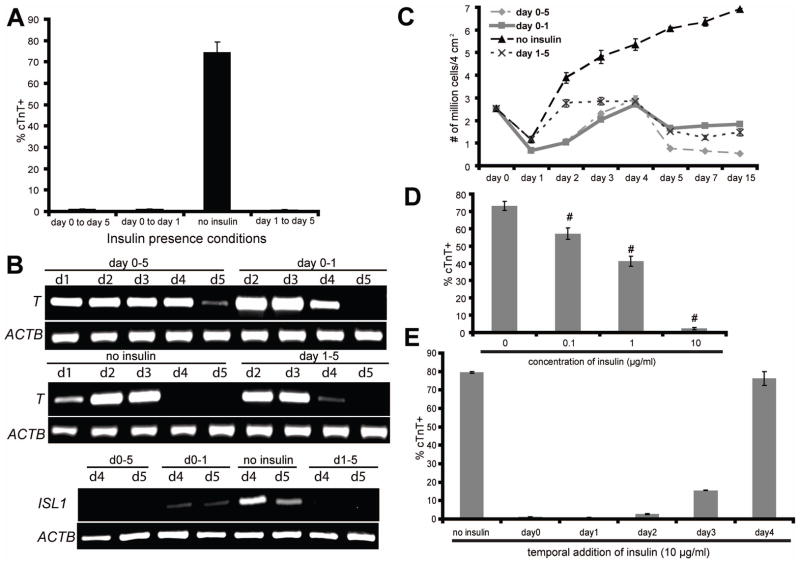
To identify the differentiation stage at which insulin exerts its inhibitory effects on cardiac differentiation, we differentiated hPSCs in RPMI/B27 with or without insulin from day 0–1 and/or days 1–5 using the GiAB protocol. RPMI/B27 lacking insulin from days 0–5 produced 74.5 ± 4.8% cTnT+ cardiomyocytes at day 15, while RPMI/B27 with insulin at either day 0–1 or days 1–5 eliminated cardiomyocyte differentiation (Fig. 3A). Insulin abrogation of cardiomyocyte differentiation during these same time frames was also observed in the Matrix Sandwich Protocol which applies a Matrigel overlay and Activin A/BMP4 to stimulate efficient cardiac differentiation [39] (Fig. S3). Consistent with the cardiomyocyte differentiation results, the presence of insulin during either day 0–1 or days 1–5 in the GiAB protocol inhibited downregulation of T and also reduced expression of the cardiac progenitor marker ISL1 as compared to differentiation in the absence of insulin (Fig. 3B). Quantification of the number of cells in culture at different stages of differentiation revealed that differentiation in RPMI/B27 containing insulin from day 0–1 and/or days 1–5 reduced cell viability or expansion during the GiAB protocol (Fig. 3C).
Figure 3.
(A–D) H9 cells were differentiated as described in Fig. 1(A), with insulin present or absent during the indicated stages of differentiation. (A) 15 days after initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry. (B) At different time points, mRNA was collected and RT-PCR analysis of mesendoderm (T) and cardiac gene expression (ISL1) was performed. (C) At different time points, single cells were prepared by Accutase treatment and counted. (D–E) Cardiomyocytes were generated from H9 cells using the protocol described in Fig. 1(A), with RPMI/B27-insulin medium used from day 0 to day 5. (D) At day 1, indicated concentrations of insulin were added into the culture medium and flow cytometry for cTnT expression was performed at day 15. Error bars represent the s.e.m. of eight independent experiments. # p<0.005, each sample with insulin versus without insulin; t test. (E) 10 μg/ml insulin was added to the culture medium at the indicated time points of differentiation. 15 days after initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry.
Error bars represent the s.e.m. of three independent experiments.
To identify the concentration dependence of the inhibition of cardiomyocyte differentiation by insulin, we added known concentrations of insulin to RPMI/B27 lacking insulin. When added on day 1, as little as 0.1 μg/ml insulin decreased differentiation efficiency, as determined by the percentage of cells expressing cTnT at day 15, and 10 μg/ml insulin fully blocked cardiomyocyte differentiation (Fig. 3D and Movies S3–5). To more deeply explore the kinetics of insulin inhibition of cardiomyocyte differentiation, 10 μg/ml insulin was added to the GiAB differentiation system at different time points (days 0–4) and the percentage cTnT+ cells at day 15 was quantified by flow cytometry. Insulin strongly inhibited the formation of cTnT+ cells when added early in differentiation (day 0–2), and resulted in a partial inhibition of cardiomyocyte differentiation when added on day 3. However, insulin addition at day 4 had little inhibitory effect on cardiomyocyte differentiation, indicating that insulin inhibits cardiomyocyte differentiation only at early stages (Fig. 3E).
Insulin does not inhibit cardiomyocyte differentiation induced by temporal regulation of canonical Wnt signaling
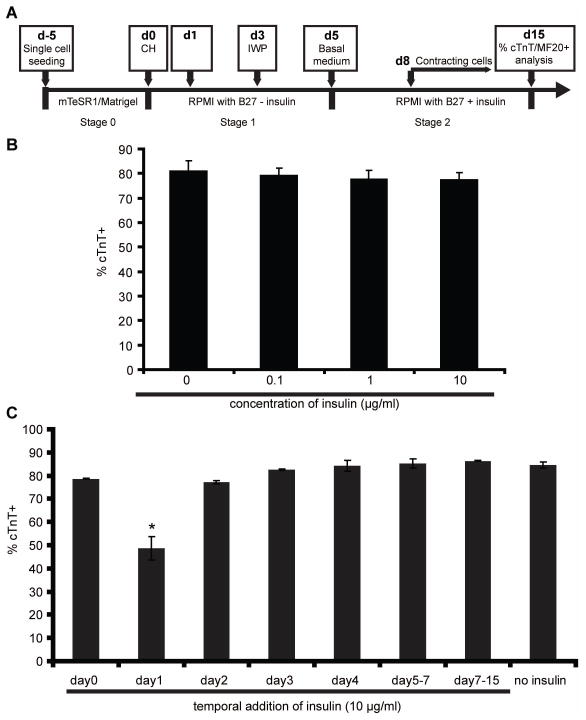
We then asked whether the inhibitory effect of insulin on cardiomyocyte differentiation depends upon the mechanism of cardiomyocyte induction. In order to test this, we used a cardiac differentiation protocol that employs small molecule inhibitors to modulate canonical Wnt signaling at appropriate temporal stages of differentiation in the absence of growth factors (Fig. 4A). This small molecule protocol (sequential treatment of Gsk3 inhibitor and Wnt signaling inhibitor, GiWi) induces robust cardiomyocyte differentiation in multiple hPSC lines [17]. We varied the concentration of insulin (from 0.1 to 10 μg/ml) added on day 0 and quantified the purity of cTnT-expressing cells at day 15. As expected, no exogenous insulin addition resulted in more than 80% cTnT+ cardiomyocytes. Interestingly, we did not observe a substantial inhibitory effect of 0.1 to 10 μg/ml insulin on cardiac differentiation using this GiWi differentiation protocol (Fig. 4B). Unlike the GiAB protocol in which addition of 10 μg/ml insulin at day 0 fully blocked cardiomyocyte formation in response to the growth factors Activin A and BMP4, the same concentration of insulin addition at day 0 had no statistically significant effect on cTnT+ cell generation during the GiWi protocol. We then tested the time dependence of potential inhibitory effects of insulin on cardiac differentiation by adding 10 μg/ml insulin at different time points during the GiWi cardiomyocyte differentiation protocol. We found that, except at day 1 when insulin addition decreased cardiomyocyte differentiation by approximately 40%, insulin had little effect on cTnT+ cardiomyocyte differentiation when presented during the GiWi differentiation protocol (Fig. 4C).
Figure 4.
(A) Schematic of GiWi protocol for fully defined, growth factor-free differentiation of hPSCs to cardiomyocytes via treatment with small molecule modulators of Wnt signaling. (B–C) Cardiomyocytes were generated from 19-9-11 cells using the cardiac directed differentiation protocol described in (A), with exposure to 12 μM CHIR99021 at day 0 and 5 μM IWP4 at day 3. (B) At day 0, the indicated concentration of insulin was added into the culture medium. 15 days after differentiation, cTnT expression was determined by flow cytometry. Error bars represent the s.e.m. of three independent experiments. No statistically significant difference between samples were identified using one-way ANOVA and Tukey post tests (p>0.05). (C) 10 μg/ml insulin was added to the culture medium at different time points of differentiation. 15 days after initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments. * p<0.05, data were compared using one-way ANOVA and Tukey post tests, with the asterisk indicating day 1 is significantly different from other samples.
β-catenin is required for inhibition of cardiomyocyte differentiation by insulin
The observation that insulin does not exert a strong inhibitory effect on cardiomyocyte generation in the GiWi protocol is intriguing since early inhibitory effects of insulin have been observed in numerous different cardiac differentiation systems of hPSCs, including END-2 cell co-culture [10], EB differentiation [7], Matrigel overlay/Activin A/BMP4-mediated differentiation [39, 40], and Gsk3 inhibitor/Activin A/BMP4-mediated differentiation (Fig. 1B). We speculate that downregulation of Wnt signaling by IWPs during the GiWi protocol might mitigate insulin’s inhibitory effect on cardiomyocyte differentiation. Indeed, the interaction of insulin/Akt signaling and Wnt signaling has been demonstrated in early stages of cardiac differentiation of the mouse P19CL6 line [9].
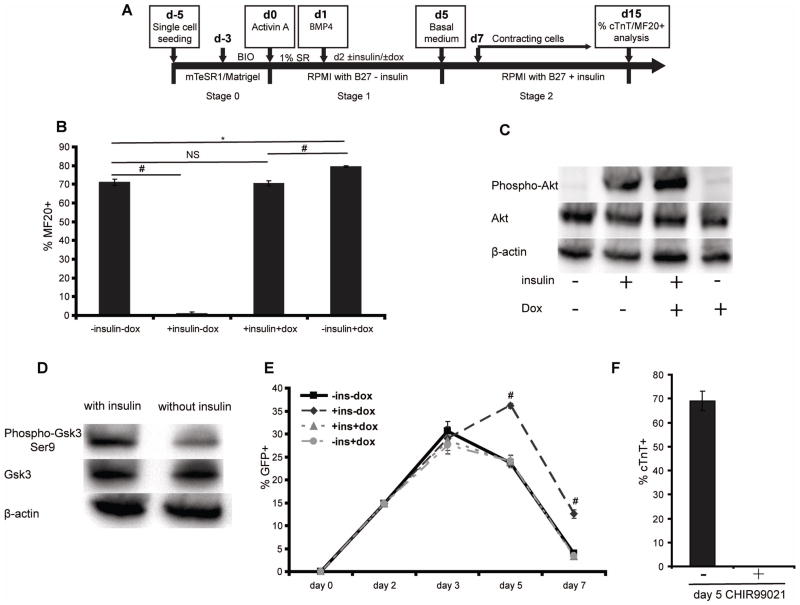
To investigate the hypothesis that downregulation of canonical Wnt signaling abrogates insulin’s inhibitory effects on cardiomyocyte differentiation of hPSCs, we used the hESC H9 ishcat-1 transgenic line to express doxycycline-inducible β-catenin shRNA at different time points during the GiAB differentiation protocol (Fig. 5A). In the absence of insulin and doxycycline addition, this transgenic line generated 71.15 ± 1.50% MF20+ cardiomyocytes, similar to non-transduced H9 cells (Fig. 5B, 1B). Addition of insulin at day 2 completely blocked cardiac differentiation. However, β-catenin knockdown at day 2 fully rescued the inhibition of cardiac differentiation induced by insulin addition, generating 70.63 ± 1.39% MF20+ cardiomyocytes (Fig. 5B). Furthermore, western blot analysis of Akt activation showed that when H9 ishcat-1 cells were differentiated into cardiomyocytes by β-catenin knockdown, the insulin/Akt signaling pathway activated independently of canonical Wnt signaling (Fig. 5C). Gsk3β phosphorylates and promotes degradation of β-catenin and thus downregulates canonical Wnt signaling. Gsk3β activity is suppressed by phosphorylation at the Ser9 site [41]. Treatment of differentiating cells with insulin resulted in increased phosphorylation of Gsk3β at Ser9 (Fig. 5D), suggesting that that insulin might to activate canonical Wnt signaling through downregulation of Gsk3β.
Figure 5.
(A) Schematic of the GiAB differentiation protocol with insulin and doxycycline modulation. (B) H9 ishcat-1 cells were differentiated as described in (A). 10 μg/ml insulin and 2 μg/ml dox were added or not, as indicated, into the culture medium at day 2. 15 days after initiation of differentiation, cells were analyzed using the MF20 antibody by flow cytometry. Error bars represent the s.e.m. of three independent experiments. # p<0.005, * p<0.05, NS p>0.05, each group comparison indicated by the line; t test. (C) H9 ishcat-1 cells were differentiated as shown in (A) and western blot analysis of Akt phosphorylation was performed on day 3. (D) H9 cells were differentiated as shown in Figure 1(A) with or without insulin and western blot analysis of Gsk3β phosphorylation was performed on day 3. (E) H9-7TGP-ishcat-1 cells were differentiated as described in (A). At different time points, single cells were prepared with Accutase treatment and GFP+ cells were quantified with flow cytometry. # p<0.005, one-way ANOVA of the same day samples. (F) H9-7TGP-ishcat-1 cells were differentiated as described in (A). 3 μM CH was added or not into culture medium at day 5. 15 days after initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments.
In order to study the dependence of Wnt signaling activation on insulin/Akt signaling in the context of cardiac differentiation, we generated another transgenic line, H9-7TGP-ishcat-1, for both monitoring Wnt signaling activation via GFP expression and temporally modulating Wnt signaling pathway via dox addition. Canonical Wnt signaling was activated transiently, peaking at day 3, during cardiac differentiation induced by Activin A and BMP4 in the absence of insulin and dox addition. Addition of insulin without dox, however, produced higher and more sustained Wnt signaling activity on day 5 compared to the condition without insulin addition (Fig. 5E) and abolished cTnT+ cell generation on day 15 (Fig. S4). This result indicates that insulin signaling modulates Wnt signaling during the early stage of cardiac differentiation of hPSCs, blocking cardiomyocyte generation at a later stage.
To test whether ectopic activation of Wnt signaling on day 5 abolished cardiac differentiation, we differentiated the H9-7TGP-ishcat-1 line using the GiAB protocol with or without CH addition at day 5 to activate Wnt signaling. Addition of CH completely abolished cardiac differentiation of H9-7TGP-ishcat-1 cells via Activin A and BMP4 (Fig. 5F). These results indicate that abnormal activation of Wnt signaling on day 5 contributes to the low yield of cardiomyocytes on day 15. Remarkably, dox addition at day 2 downregulated Wnt signaling on day 5 (Fig. 5E) in conjunction with rescue of the inhibitory effects of insulin on cardiomyocyte differentiation (Fig. S4).
Our experiments demonstrated that insulin blocks cardiomyocyte differentiation during the GiAB protocol but not during the GiWi protocol. In order to understand whether insulin also affects endothelial cell and smooth muscle cell differentiation, we used flow cytometry to characterize PECAM-1 and smooth muscle actin (SMA) expression in cell populations produced by the GiAB and GiWi protocols in the presence and absence of insulin [42]. No PECAM-1 positive endothelial cells were detected in either protocol with insulin present or absent. We also found that the small SMA+ population, about 5–6% of the cells, was not affected by the presence of insulin in either protocol (Fig. S5).
DISCUSSION
Human pluripotent stem cells are a useful in vitro tool to identify and understand mechanisms of human development. hPSCs can complement animal models to enhance our understanding of human development. In this study, we have identified interactions between the insulin pathway and Wnt/β-catenin signaling in controlling hPSC cardiac fate determination.
Insulin, a commonly used component of mammalian cell culture, is important for cell survival and proliferation. Insulin/IGF signaling also plays key roles in human stem cell self-renewal [8, 43, 44] and endoderm differentiation [45]. Insulin/Akt signaling is required for cardiac differentiation of mouse pluripotent P19CL6 cells [9]. In addition, cardiac differentiation of hPSCs has been shown to be negatively influenced by insulin signaling in numerous differentiation protocols [7, 10, 24, 46]. However, the downstream mechanisms underlying insulin’s inhibitory effects on cardiac differentiation are less clear. Insulin has been reported to inhibit formation of mesendoderm (brachyury+) cells and their derivatives including endoderm, and cardiac mesoderm from hPSCs using co-culture and EBs differentiation systems [7]. With the recent development of fully-defined cardiomyocyte differentiation protocols, it is important to evaluate the stage and mechanism by which insulin inhibits cardiac differentiation, limiting production of cardiomyocytes under fully defined conditions.
Using the defined GiAB protocol, we showed that 10 μg/ml insulin is sufficient to completely ablate cardiac differentiation of all 6 hPSC lines that we tested. The effects of insulin on cardiac differentiation using this defined system are consistent with previously reported results that insulin inhibits cardiac differentiation induced by serum and/or TGFβ superfamily ligands [7, 10, 24].
Using a cardiac differentiation protocol employing small molecule modulators of Wnt signaling, however, we found that insulin does not exert a strong inhibitory effect on human cardiomyocyte generation. Using chemical and genetic approaches to characterize the context-dependent effects of insulin in different differentiation protocols, we revealed a previously unappreciated but essential cell signaling interplay between insulin signaling and Wnt signaling as being required for cardiac development of hPSCs. Our study exposes a central and direct role for the canonical Wnt signaling on cardiac differentiation of hPSCs and demonstrates that insulin’s inhibitory effects can be rescued by inhibition of canonical Wnt signaling. Insulin/Akt signaling has been demonstrated to activate Rac1 [47], which controls nuclear localization of β-catenin and activation of canonical Wnt signaling [48]. Indeed insulin/Akt has been shown to activate canonical Wnt signaling in mouse pluripotent P19CL6 lines in early stages of cardiac differentiation [9].
Previous studies have guided successful efforts to differentiate hPSCs to specific cardiac cell types, including cardiomyocytes [17, 18], endothelial cells [49–51], and smooth muscle cells [52, 53]. However, the generation of complex three-dimensional organ tissues in vitro remains a major challenge for translational studies. One example showed that directed differentiation of hPSCs into intestinal tissue in vitro is possible [54]. Directed differentiation of hPSCs into complex three-dimensional organ tissue requires deep understanding of cell signaling molecular cross-talk. Our study suggests that insulin signaling exerts its inhibitory effects on cardiomyocyte differentiation in hPSCs through activation of canonical Wnt signaling. The essential cell signaling interplay described here should contribute to our understanding of human heart development and provide cues for generating complex three-dimensional cardiac organ tissue from hPSCs in vitro which would facilitate unprecedented studies of human heart development and disease.
Supplementary Material
H9 cells were differentiated according to the GiAB protocol shown in Fig. 1(A). RPMI/B27 without insulin was used for differentiation. Movie S1 shows day 15 cardiomyocytes.
H9 cells were differentiated according to the GiAB protocol shown in Fig. 1(A). RPMI/B27 containing insulin was used for differentiation. Movie S2 shows day 15 differentiated cells.
H9 cells were differentiated as described in Movie S1 with RPMI/B27-insulin as basal medium. At day 1, 0 μg/ml (Movie S3), 1 μg/ml (Movie S4) or 10 μg/ml (Movie S5) insulin was added into the cell culture. Movies show day 15 differentiated cells.
Figure S1. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin. 30 days post-initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry.
Figure S2. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin. 7 days post-initiation of differentiation, cells were analyzed for brachyury and Nkx2.5 expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments.
Figure S3. 19-9-11 cells were cultured on Matrigel in mTeSR1 and when the monolayer of cells reached 80–90% confluence, a thin layer of Matrigel (0.5 mg per 6-well plate) was overlaid. Cells were then cultured in mTeSR1 for another 1–2 days until the cells were 100% confluent. At this point, day 0, the cells were exposed to 100 ng/ml Activin A and Matrigel (0.5 mg/6-well plate) in RPMI/B27 with or without insulin. After 24 hours, the medium was changed and cells were exposed to 10 ng/ml BMP4 and 10 ng/ml bFGF in RPMI/B27 with or without insulin. 15 days post-initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry. Error bars represent the s.e.m for at least two independent experiments.
Figure S4. H9-7TGP-ishcat-1 cells were differentiated as described in Fig. 5(A). 10 μg/ml insulin and 2 μg/ml dox was added or not, as indicated, into the culture medium at day 2. 15 days after initiation of differentiation, cells were analyzed by flow cytometry using the MF20 antibody. Error bars represent the s.e.m. of three independent experiments.
Figure S5. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin (GiAB protocol). H9 cells were also differentiated as described in Fig. 4(A) (GiWi protocol). 30 days post-initiation of differentiation, cells were analyzed for PECAM-1 and smooth muscle actin (SMA) expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments.
Acknowledgments
This work was supported by NIH grant R01 EB007534 and NSF grant EFRI 0735903.
Footnotes
Author contributions:
Xiaojun Lian: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Jianhua Zhang: conception and design, collection and/or assembly of data, data analysis and interpretation; Kexian Zhu: collection and/or assembly of data, data analysis and interpretation; Timothy J. Kamp: conception and design, manuscript writing, final approval of manuscript; Sean P. Palecek: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing. All the other authors declare no competing financial interests.
References
- 1.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 2.Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. doi: 10.1242/dev.01451. [DOI] [PubMed] [Google Scholar]
- 3.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Cavallero S, Gu Y, et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xin M, Kim Y, Sutherland LB, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund C, Ward-van Oostwaard D, Monshouwer-Kloots J, et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells. 2008;26:724–733. doi: 10.1634/stemcells.2007-0617. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Schulz TC, Sherrer ES, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito AT, Akazawa H, Takano H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 10.Xu XQ, Graichen R, Soo SY, et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958–970. doi: 10.1111/j.1432-0436.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 11.Graichen R, Xu X, Braam SR, et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–370. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 12.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 13.Zhu W, Shiojima I, Ito Y, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 15.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Azarin SM, Lian X, Larson EA, et al. Modulation of Wnt/beta-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33:2041–2049. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 19.Paige SL, Osugi T, Afanasiev OK, et al. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 22.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P, Klos M, Bollensdorff C, et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res. 2012;110:1556–1563. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruneau BG, Logan M, Davis N, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211:100–108. doi: 10.1006/dbio.1999.9298. [DOI] [PubMed] [Google Scholar]
- 28.Kuo CT, Morrisey EE, Anandappa R, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Gene Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 29.Edmondson DG, Lyons GE, Martin JF, et al. Mef2 Gene-Expression Marks the Cardiac and Skeletal-Muscle Lineages during Mouse Embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 30.Bu L, Jiang X, Martin-Puig S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 31.Qyang Y, Martin-Puig S, Chiravuri M, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Elliott DA, Braam SR, Koutsis K, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 33.Vallier L, Touboul T, Chng Z, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima S, Takagi A, Inoue T, et al. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development. 2000;127:3215–3226. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 35.Costello I, Pimeisl IM, Drager S, et al. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13:1084–1091. doi: 10.1038/ncb2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Ameele J, Tiberi L, Bondue A, et al. Eomesodermin induces Mesp1 expression and cardiac differentiation from embryonic stem cells in the absence of Activin. EMBO Rep. 2012;13:355–362. doi: 10.1038/embor.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Klos M, Wilson GF, et al. Extracellular Matrix Promotes Highly Efficient Cardiac Differentiation of Human Pluripotent Stem Cells: The Matrix Sandwich Method. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Klos M, Wilson GF, et al. Extracellular Matrix Promotes Highly Efficient Cardiac Differentiation of Human Pluripotent Stem Cells: The Matrix Sandwich Method. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 42.Paige SL, Thomas S, Stoick-Cooper CL, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–232. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bendall SC, Stewart MH, Menendez P, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 45.McLean AB, D’Amour KA, Jones KL, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 46.Tran TH, Wang X, Browne C, et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27:1869–1878. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 47.Gunduz D, Thom J, Hussain I, et al. Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3-kinase/Akt-mediated Rac1 activation. Arterioscler Thromb Vasc Biol. 2010;30:1237–1245. doi: 10.1161/ATVBAHA.110.203901. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Tu X, Joeng KS, et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 50.James D, Nam HS, Seandel M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie CQ, Zhang J, Villacorta L, et al. A highly efficient method to differentiate smooth muscle cells from human embryonic stem cells. Arterioscler Thromb Vasc Biol. 2007;27:e311–312. doi: 10.1161/ATVBAHA.107.154260. [DOI] [PubMed] [Google Scholar]
- 53.Cheung C, Bernardo AS, Trotter MW, et al. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H9 cells were differentiated according to the GiAB protocol shown in Fig. 1(A). RPMI/B27 without insulin was used for differentiation. Movie S1 shows day 15 cardiomyocytes.
H9 cells were differentiated according to the GiAB protocol shown in Fig. 1(A). RPMI/B27 containing insulin was used for differentiation. Movie S2 shows day 15 differentiated cells.
H9 cells were differentiated as described in Movie S1 with RPMI/B27-insulin as basal medium. At day 1, 0 μg/ml (Movie S3), 1 μg/ml (Movie S4) or 10 μg/ml (Movie S5) insulin was added into the cell culture. Movies show day 15 differentiated cells.
Figure S1. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin. 30 days post-initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry.
Figure S2. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin. 7 days post-initiation of differentiation, cells were analyzed for brachyury and Nkx2.5 expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments.
Figure S3. 19-9-11 cells were cultured on Matrigel in mTeSR1 and when the monolayer of cells reached 80–90% confluence, a thin layer of Matrigel (0.5 mg per 6-well plate) was overlaid. Cells were then cultured in mTeSR1 for another 1–2 days until the cells were 100% confluent. At this point, day 0, the cells were exposed to 100 ng/ml Activin A and Matrigel (0.5 mg/6-well plate) in RPMI/B27 with or without insulin. After 24 hours, the medium was changed and cells were exposed to 10 ng/ml BMP4 and 10 ng/ml bFGF in RPMI/B27 with or without insulin. 15 days post-initiation of differentiation, cells were analyzed for cTnT expression by flow cytometry. Error bars represent the s.e.m for at least two independent experiments.
Figure S4. H9-7TGP-ishcat-1 cells were differentiated as described in Fig. 5(A). 10 μg/ml insulin and 2 μg/ml dox was added or not, as indicated, into the culture medium at day 2. 15 days after initiation of differentiation, cells were analyzed by flow cytometry using the MF20 antibody. Error bars represent the s.e.m. of three independent experiments.
Figure S5. H9 cells were cultured on Matrigel and treated with 1 μM BIO for 3 days before exposure to 100 ng/ml Activin A at day 0 and 5 ng/ml BMP4 at day 1 in RPMI/B27 with or without insulin (GiAB protocol). H9 cells were also differentiated as described in Fig. 4(A) (GiWi protocol). 30 days post-initiation of differentiation, cells were analyzed for PECAM-1 and smooth muscle actin (SMA) expression by flow cytometry. Error bars represent the s.e.m. of three independent experiments.