Abstract
Background
We hypothesize that intrathecal (IT) granulomas arising from the IT infusion of several opiates may result from the degranulation of meningeal mast cells (MC). Given functional covariance between cutaneous and meningeal mast cells, we propose that opioids that do not degranulate cutaneous mast cells will not produce a granuloma. An opioid meeting this criteria is the phenylpiperadine Alfentanil HCl.
Methods
Three experiments were accomplished in dogs. 1) Cutaneous MC degranulation. Flare areas on the dog abdomen were measured after intradermal (ID) alfentanil, morphine or compound 48–80. 2) Dose ranging of analgesic effects of IT alfentanil infusion. Dogs with lumbar IT catheters received continuous infusion for 24 hrs of different concentrations (1–20 mg/mL/d) of alfentanil and analgesic effects were assessed. iii) Granuloma inducing effects. Dogs received IT Alfentanil (20 mg/ml/d; N = 5; 22–28 days)) or morphine (12 mg/mL/d; N=3; 22–30 days) and spinal cord harvested for histopathology after 22–30d of infusion.
Results
1) ID Morphine (10 mg/mL) and Compound 48–80 (1mg/mL) but not alfentanil at concentrations up to 20mg/mL produced a cutaneous flare. Intrathecal Alfentanil infusion produced increases in thermal escape latency at concentrations as low as 2 mg/mL/d). A significant depression of arousal was noted in the dogs receiving 20 mg/mL. Over the 22–30 day infusion period, morphine (12 mg/mL/d) resulted in granulomas in all three animals examined whereas intrathecal alfentanil at 20 mg/mL/d failed to initiate a granuloma in any animal.
Conclusions
These results support the hypothesis linking mast cell degranulation and intrathecal granulomas.
INTRODUCTION
The intrathecal infusion for 2–4 weeks of several opioids, including morphine, hydromorphone and methadone, but not fentanyl results in the concentration- dependent formation of intrathecal space-occupying masses (granulomas) in the dog.1–4 The characteristics of these granulomas parallel closely the phenomena observed in humans receiving such intrathecal infusions. 5–7,8 The mechanisms underlying this phenomenon are not understood. The failure of fentanyl, a potent opiate agonist to produce granulomas in the canine model suggests the phenomena is not mediated by a classic opiate receptor. 9 An interesting consideration that we entertained was that the opioid effects on mast cells paralleled this profile. It has been long appreciated that agents such as morphine, but not fentanyl, degranulate mast cells 10,11–14; and this effect is not considered to be reversed by naloxone.15,16 We note that degranulation of mast cells by some but not all opiates and the absence of antagonist sensitivity provides an intriguing link. Mast cells are readily observed in spinal and supraspinal meninges. 17–19 Further, there is an extensive literature indicating that activation of dural mast cells leads to the local release of factors that evoke vasodilatation of the meningeal vessel and enhance the transmigration of inflammatory cells into the local milieu. 20,21 These linkages lead to the organizing hypothesis that high local concentrations in the lumbar CSF of opiates that are able to degranulate mast cells (e.g. morphine) will lead to dural mast cell degranulation and the migration of inflammatory cells into the local milieu, leading to a space-occupying granuloma. This very speculative hypothesis raises an interesting prediction that agents that evoke mast cell degranulation will yield granulomas when infused spinally, while those that do not initiate degranulation will not lead to a granuloma. While opiates readily degranulate cutaneous mast cells, it has been reported that opiates do not degranulate mast cells in other tissues (e.g. including lung, intestinal, heart or blood basophils) 22,23. This difference may reflect the common embryology of skin and meninges.24,25 Accordingly, we would predict that the ability of an opiate to degranulate cutaneous mast cells would provide an indicator as to whether that agent will similarly produce an intrathecal granuloma. As noted above, fentanyl has little propensity to produce degranulation and in the dog does not yield a granuloma at the highest concentrations examined.9 To extend this speculation we decided to examine the effects of alfentanil. This molecule is an anilinoperadine, like fentanyl, with a slightly lower log P (3.8 vs. 2.1) and has been shown to evoke a potent, naloxone-reversible analgesia after intrathecal bolus and infusion delivery in rodents 26–30, bolus delivery in dogs 31 and in humans.32 Previous reports have suggested that like fentanyl, this molecule also does not produce a significant cutaneous mast cell degranulation.14 Accordingly, we hypothesized that alfentanil, in contrast to morphine, would not produce cutaneous flare in the dog after intradermal delivery and that it would not produce an intrathecal granuloma after infusion at elevated concentrations.
MATERIALS AND METHODS
All studies described here were accomplished under protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Beagle dogs (Ridglan Farms Inc. Mt. Horeb, WI, or equivalent), 12 to 16 months in age and weighing 9–16 kilograms, were individually housed in runs with wood shavings and given ad libitum access to food and water. There are three sets of studies: the effects of intradermal drugs on cutaneous flare, the effects of intrathecal alfentanil on thermal escape latency, and the effects of intrathecal agents on granuloma formation.
Intradermal drugs to study flare formation
Anesthesia was induced and maintained with IV propofol (5 μg/kg/min). Animals were intubated, body temperature maintained with an underbody heating pad and continuously monitored for oxygen saturation, end tidal CO2 and heart and respiratory rates. The chest and abdomen were shaven and surgically prepped. Intradermal injections of drug solutions were delivered randomly in volumes of 50μL at up to 12 –14 sites (6–7/side). The time of injection was designated as T = 0. The injection sites were marked with a dot of ink. The diameter of the flare (redness) around each injection site was measured across the longest and shortest axis and recorded without reference to drug treatment at T = 0, 10, 30 and 60 min. Flare area immediately after and at intervals were calculated in mm2 as an oval (3.14 × a × b where a = half-length of long axis and b = half-length of short axis). Animals were used at 5-day intervals. This protocol is similar to that previously described to assess cutaneous flare in dogs.33,34
Continuous intrathecal drug delivery
To characterize the effects of intrathecal drugs on granuloma formation, animals were implanted with a chronic lumbar intrathecal catheter. Animals were adapted for a minimum of 10 days prior to surgery. A nylon vest was placed on each dog at 48 hours prior to intrathecal catheter placement for acclimation.
i) Surgical preparation
Dogs were prepared with chronic intrathecal catheters by the surgical placement of the catheter approximately 72 hours prior to start of dosing. Antibiotic sulfamethoxazole-trimethoprim (240mg tablet, 15–25mg/kg, oral, twice daily) was given 48 hours before and after surgery. Dogs received atropine (0.04mg/kg, IM) prior to sedation with xylazine (1.5mg/kg, IM). After intubation, anesthesia was maintained under spontaneous ventilation with 1.0–2.0% isoflurane and 60% N2O/40% O2. Intraoperatively, animals were monitored as described above. Surgical areas were shaved and prepared with chlorhexadine scrub and solution. Using sterile technique, the cisterna magna was exposed by combined blunt and sharp dissection. The dura was exposed; and, through a small incision (1–2mm) the intrathecal catheter (fabricated of polyurethane tubing (0.61 mm OD) and sterilized by E-beam irradiation) was inserted and passed caudally a distance of approximately 40–42 cm to a level corresponding to the L2–3segment. Dexamethasone sodium phosphate (0.25mg/kg, IM) was administered just after catheter placement. The external catheter was tunneled to exit at the left scapular region. Upon closure of the incision, isoflurane was removed and the animal recovered. Butorphanol tartrate (Torbugesic®; 0.04mg/kg, IM; Fort Dodge Animal Health, 235 E. 42nd St, NY, NY, 10017) was administered upon recovery and as necessary to relieve post-operative discomfort. Following recovery, a nylon vest was placed on the animal and an infusion pump (Minimed 507, Medtronic, 18000 Devonshire St., Northridge, CA 91325) was secured in the vest pocket where it was connected to the externalized end of the intrathecal catheter.
ii) Initiation of continuous intrathecal infusion
In the present studies, animals were started with low doses that were increased stepwise over time. Thermal escape testing was performed at intervals over 24 hrs as previously described, with 2–3 days between periods of infusion to allow washout. This allowed development of analgesic dose response curves. Further, as we have previously reported, the initiation of infusion of high concentrations of opiates in the canine model can result in behavioral depression, agitation and motor signs 3. These effects can be tolerated by a stepwise ramping of the infusion as employed here. Time of continuous infusion was counted from the date of initiation of the 12 mg/d infusion of morphine and 20mg/mL of alfentanil.
iii) Behavioral observations
On a daily basis, specific indices of arousal (depression) (0 to −3) and loss of coordinated ambulation (0 to−3) and hind limb weakness (0 to −3) were assessed. These indices have been previously validated.35,36 For purposes of data presentation, the overall motor impairment in the safety study was presented as the cumulation of the absolute value of the observed muscle tone and coordination score at (e.g. 0–6 where 0 is no detectable change and 6 represents profound motor impairment).
For analysis, the arousal (depression) score in the dose response analysis reports the cumulative score made at the five assessment intervals over the 24 hr infusion intervals (e.g. with a total score of 5 observations × 3 = 15, indicating severe depression throughout the infusion interval). For the motor score, the same cumulative analysis was performed with maximum impairment being 5 × 6 = 30, indicating a profound motor impairment throughout the infusion interval).
iv) Analgesic assessment
To assess the analgesic effects of intrathecal alfentanil during the initial dose ranging, a hind paw thermal escape model was employed in which the animal stood with its hind paws on a glass surface through which a focused projection bulb was targeted on the anterior center of the metatarsal pad of the left and right hind paws, and the escape latency from lamp actuation to paw withdrawal was recorded twice for each hind paw. 37 The mean response latency of either hind paw was recorded. Antinociception data was collected before and at 1,2,4, 8 and at 24 hrs after the initiation of a given infusion.
iv) Necropsy
At the completion of the continuous infusion cycle, dogs are deeply anesthetized with an intravenous dose of propofol (5 μg/kg/min, IV) and exsanguinated by perfusion with saline followed by 10% formalin. The spinal column is exposed by laminectomy. The condition of the spinal cord and overlying dura is noted and the location of the catheter tip in situ. The spinal cord is cut (taking care to keep the dura intact) and removed in four blocks approximating the cervical, thoracic, lumbar (catheter tip region) and lower lumbar (below catheter tip). These blocks are placed in fixative (10% neutral buffered formalin) and maintained at 4 °C until submitted for histopathologic analysis. Spinal blocks are embedded in paraffin, sectioned at approximately 3–5 μm and stained with hematoxylin-eosin. To estimate cross-sectional area, H&E sections are taken at the level of the largest girth of the mass (typically at or near the lumbar catheter tip) and the cross-sectional area of the granuloma and the spinal cord was manually outlined using Image-Pro Plus 5.1 software to measure area in outlined pixels and pixel count was converted into mm2).
Drugs
Preservative-free, Food and Drug Administration–approved formulations of morphine sulfate (Infumorph; Abbott, Abbott Park, IL) and saline vehicle (0.9% NaCl; Abbott) were used. Alfentanil HCl was obtained as a powder from the National Institute of Drug Abuse. Solutions were prepared following rigid aseptic precautions and sterilized by passing through a sterile 0.22 μ filter. The mast cell degranulating compound 48/80 was obtained from Sigma Chemical (St. Louis, MO). All drugs were prepared in saline (0.9%) unless otherwise stated. Doses and concentrations employed are indicated in the text. We limited the maximum alfentanil concentration to 20 mg/mL, because at higher concentrations (40mg/mL) evidence of incomplete solubility was noted (data not shown).
Statistics
For end points such as the cutaneous flare and arousal scores, comparisons were made across treatment using 1 way ANOVA with post hoc comparison to the saline groups treatment group using Bonferroni corrections for multiple comparison. Critical values associated with p<0.05 level were considered to be statistically significant. All analyses employed the Prism software (v.4.0c for MAC OS X).
RESULTS
Cutaneous flare produced by intradermal agents
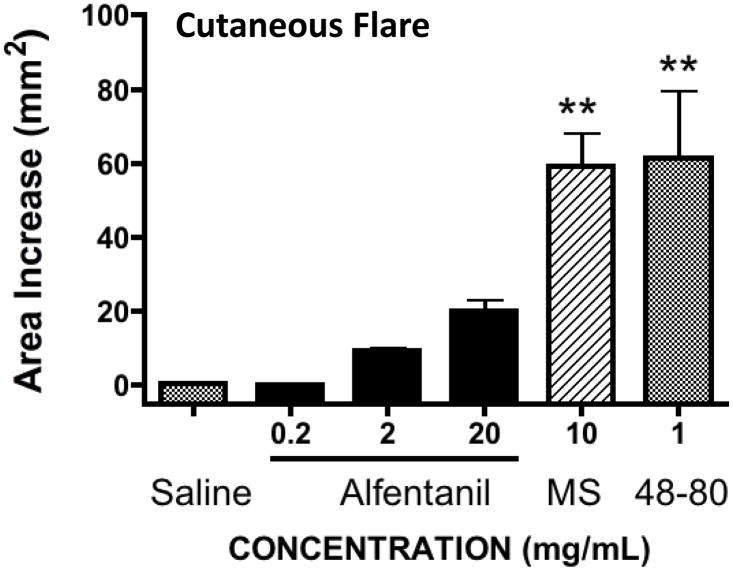
Intradermal injection of saline (50μL) resulted in a transient bleb that resolved within 10 min with no observed flare. Intradermal Compound 48/80 (1 mg/mL) and morphine (10 mg/mL) resulted in a very strong flare response that disappeared by 2 hrs. In contrast, as shown in Figure 1, a 1 way ANOVA revealed a statistically significant main effect across treatments (p<0.0001) and post hoc comparisons indicated that alfentanil at the highest concentration (20 mg/mL) resulted in a small numerical increase in flare that was not statistically different from control, (p>0.05). In contrast the flare produced by Compound 48–80 and morphine statistically greater than vehicle. (p<0.05) .
FIGURE 1. Intradermal flare dose response.
Area of cutaneous flare (mm2) observed after the intradermal injection (50μL) of saline (vehicle), alfentanil (0.2, 2 or 20 mg/mL), morphine (MS:10mg/mL) or compound 48–80 (1 mg/mL). 1 Way ANOVA, post hoc **: p<0.01 as compared to vehicle (N= 5–6 sites/treatment)
Intrathecal infusion of alfentanil and analgesia
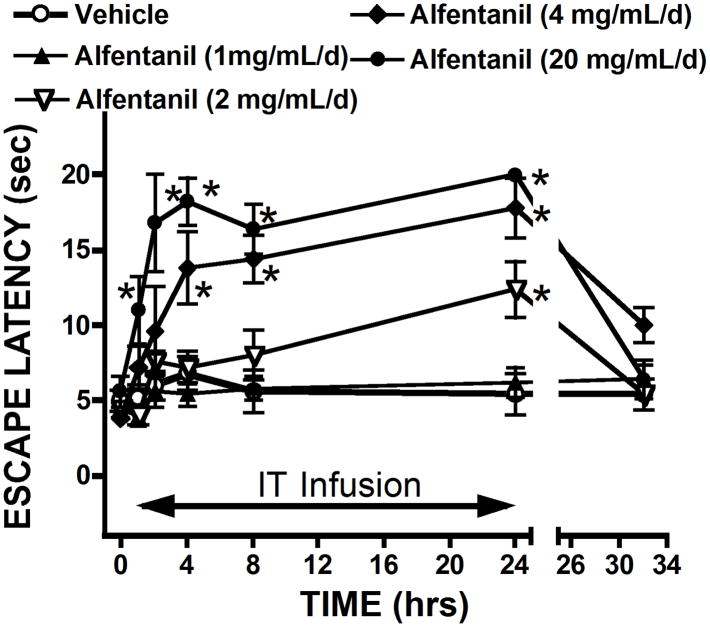
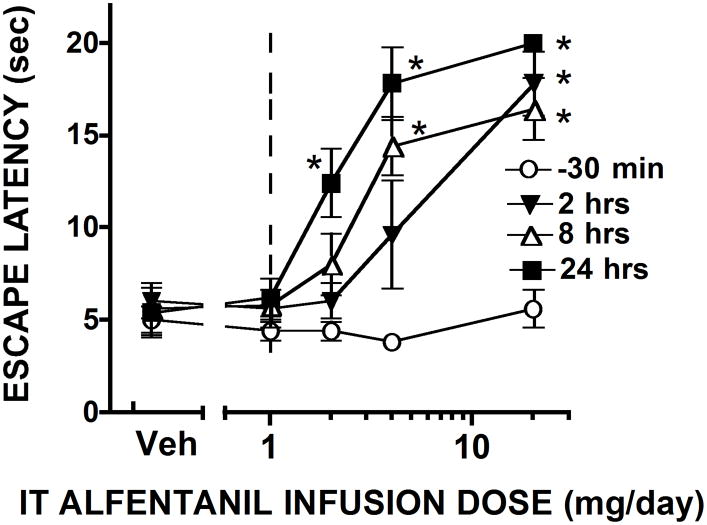
Figure 2 displays the time effect curves for changes in the thermal escape latency over the 24 hr time course after the initiation of the infusion with several doses of alfentanil. As indicated, the time after which significant changes in threshold (as compared to pre-infusion baseline) was concentration-dependent with 20 mg/d showing a significant increase by 2 hrs, while 2 mg/d showed a significant increase above baseline only by 24 hrs. These effects were all reversible by termination of the infusion with significant declines to near pre-infusion baselines noted by 8 hrs. By 2–3 days, all baselines were at normal as indicated by the pre-infusion thresholds for each of the infusion sequences (see Figures 2 and 3).
FIGURE 2. Time effect curve for alfentanil.
Thermal escape latency measured before and at intervals after the initiation of the infusion of vehicle or alfentanil in concentrations of 1mg/mL/d; 2mg/mL/d, 4 mg/mL/d or 20 mg/mL/d) for 24 hrs and at 8 hr after the termination of infusion. N = 5 dogs/infusate dose). 2 way repeated measures ANOVA, *: p<0.05 vs. time 0 (baseline).
FIGURE 3. DR curve for alfentanil.
Dose response curves (derived from data presented in Figure 2), plotting thermal escape latencies assessed for each treatment group pre-injection n(−30) and at 2, 8 and 24 hrs. 2 ways ANOVA; post hoc * p<0.05 vs. vehicle.
No major changes in arousal (sedation) were noted as compared to vehicle for the 1, 2, 4 mg/d doses though the 24 hr infusion. However, by 2 hrs after initiation of the infusion of 20 mg/mL, a reduction in spontaneous activity was noted. Animals could be readily aroused, but displayed evident lethargy. Thus, 1way ANOVA showed statistical significance across treatment (p = 0.0104) and a post hoc revealed that only the 20 mg/mL/d infusion was different from vehicle (p<0.05) (Figure 4). Assessment of motor scores revealed no loss of motor function, up through the alfentanil 20 mg/d dose, though as noted the high dose animals were evidently lethargic and showed impaired ambulation though they could bear weight. Thus, 1way ANOVA showed statistical significance across treatment (p<0.0001) and a post hoc revealed that only the 20 mg/mL/d infusion was different from vehicle (p<0.05) (Figure 4). All animals recovered baseline arousal and motor scores at the 8 hr time point (after termination of the infusion at 24 hrs).
FIGURE 4. IT alfentanil infusion effects on arousal/motor.
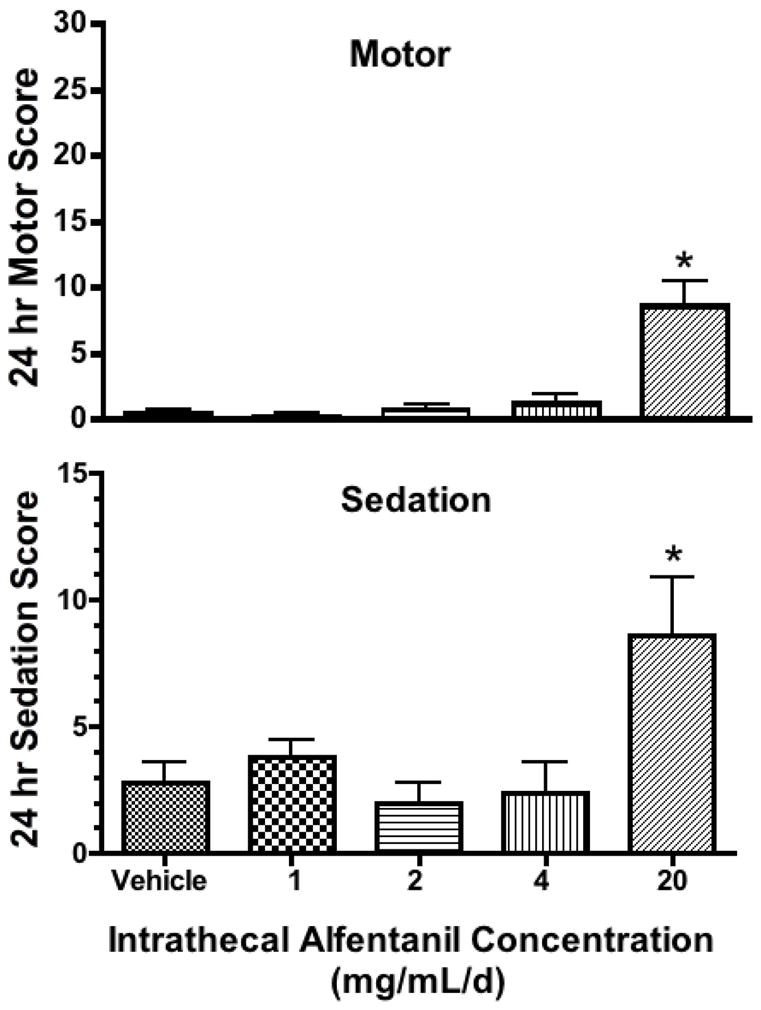
Cumulative sedation scores (A) and motor function (B) scores over the 24 hr interval of infusion of intrathecal vehicle, 1,2,4,or 20 mg/mL. Maximum motor and sedation scores indicating maximum impairment would be 30 and 15, respectively. (N = 5 dogs/infusate dose). 1 Way ANOVA, post hoc *: p<0.05 as compared to vehicle.
The 20 mg/d dose in the 5 dogs was the equivalent of approximately 1.8 mg/kg/d. In a single dog, a subcutaneous catheter was placed in the upper back, 20mg/ml/d was delivered (e.g. equivalent to approximately 0.075 mg/kg/hr) and similar testing was performed. There was a modest reduction in spontaneous activity, no change in motor function and there was no significant increase in thermal escape latency over the 24 hr infusion (data not shown).
Intrathecal infusion of alfentanil and granuloma formation
As indicated in Table 1, three animals received 12 mg/mL morphine for 22–30 days. Five animals received alfentanil infusion of 20mg/mL for 22 to 28 days. Over the period of infusion, morphine animals developed increasing neurological signs that included hind limb spasticity, altered gait and an increased sensitivity to touch applied to the hindquarters (see Table 1). Bowel and bladder function were not detectably impaired. Alfentanil animals displayed evident lethargy during the first 3–4 days of high dose infusion, but displayed no neurological indices of motor impairment. Behavioral lethargy was absent after the first week and animals showed normal food and fluid consumption.
TABLE 1.
Spinal cord and granuloma cross sectional area and motor deficits at sacrifice in dogs receiving intrathecal infusion of Morphine or Alfentanil
| Animal ID | ID | Infusate | Survival (days) | Spinal cord Cross sectional area (mm2) | Granuloma cross sectional) area (mm2) | % granuloma | Motor Deficit** |
|---|---|---|---|---|---|---|---|
| 133 7476 | M1 | Mor 12 mg/d | 22 | 26.61 | 19.69 | 74.2 | 4 |
| 134 5967 | M2 | Mor 12 mg/d | 22 | 28.57 | 18.26 | 63.9 | 3 |
| 136 2918 | M3 | Mor 12 mg/d | 30 | 25.97 | 2.21 | 8.5 | 3 |
| 135 5024 | A1 | Alf 20 mg/d | 28 | 34.65 | - | - | 0 |
| 134 6921 | A2 | Alf 20 mg/d | 22 | 19.96 | - | - | 0 |
| 136 1083 | A3 | Alf 20 mg/d | 27 | 30.64 | - | - | 0 |
| 133 9100 | A4 | Alf 20 mg/d | 27 | 30.14 | - | - | 0 |
| 134 0175 | A5 | Alf 20 mg/d | 22 | 33.94 | - | - | 0 |
Motor deficit determined at end of infusion interval: O (none) though 6 (severe). See text for additional commentary.
At necropsy, animals receiving alfentanil showed no evident defects or meningeal discoloration. Sections of the lumbar, thoracic and cervical cord stained for hematoxylin-eosin revealed no signs of a meningeal mass or abnormality. Figure 5 represents lumbar sections taken just at the catheter tip of three of the five alfentanil animals. As indicated, there were no abnormalities as defined by meningeal thickening, inflammatory cells or any evident mass. In contrast, the three animals receiving morphine displayed evident compressive masses with two exceeding 60% of the cross-sectional area of the lumbar section (Table 1).
Figure 5.
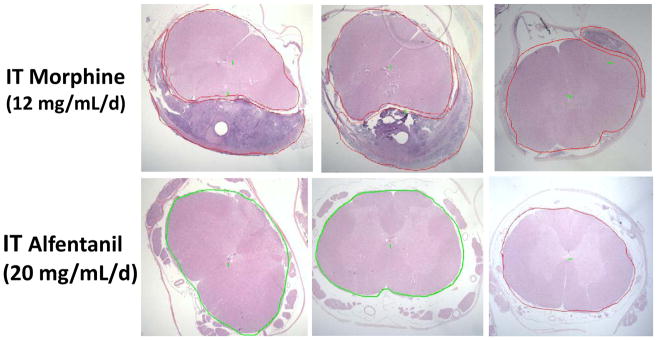
Representative lumbar histological sections of dog spinal cord taken proximal to the catheter tip in (top) 3 dogs receiving intrathecal infusion of morphine (12 mg/mL/d) or alfentanil (bottom) 20mg/mL in 3 of 5 dogs. None of the alfentanil dogs displayed a granuloma (see Table 1).
DISCUSSION
The present study examined the effects of intrathecally infused alfentanil in the canine model. The essential observations were that alfentanil produced a dose dependent antinociception and that at the maximum tolerable dose, a 22–28 day intrathecal exposure failed, unlike morphine, to initiate a granuloma. These results coincided with the absence of an effect of intradermal alfentanil on cutaneous flare, a marker of mast cell degranulation. The following sections will discuss issues relevant to these findings.
Intrathecal alfentanil and analgesic activity
Intrathecal mu opioids produce a potent antinociception. As with other anilinopiperadines, such as fentanyl and sufentanil, alfentanil produces a potent analgesia after intrathecal delivery in rodent,26–30 bolus delivery in dogs 31 and in humans. 32. In the present studies we first undertook to determine the minimal analgesic dose for continuously infused intrathecal alfentanil. To simplify the process, a group of dogs were exposed sequentially to 24 hr infusion intervals of alfentanil in several concentrations. As anticipated there was a concentration-dependent increase in thermal escape latency with the minimum dose of 2 mg/d producing a just significant increase in the thermal escape latency and only after 24 hrs, This repeated exposure strategy was employed to efficiently define an effective dose. We recognize that multiple exposures may lead to possible confounds related to effects of repeated testing, drug accumulation and/or the development of a degree of tolerance. However, by separating the individual exposures by 2–3 days and demonstrating that the thresholds were essentially unaltered over the several series of exposures, we believe the observations provide an estimate of the apparent potency of the intrathecally infused alfentanil. Importantly, in these studies, we showed that the minimum effective doses, e.g. that which produced a just significant increase in the threshold (by 24 hrs) as compared to control, was 2 mg/d. We note that the increase in latency was not due to motor impairment at doses up to 20mg/d or to sedation at doses up through 4 mg/d. We note that at the highest dose there were clearly robust non-spinal effects as evidenced by the sedation observed during the 1d day infusion. It is evident that such non-spinal actions may have contributed to the observed changes in thresholds. We would, however, note in a single animal that while the SQ delivery of this dose produced sedation, it did not produce strong corresponding analgesic effects, suggesting that the intrathecal analgesia in this model was primarily spinal in origin. Previous work with systemic non-analgesic, sedating agents, such as acepromazine, failed to show a change in thermal nociceptive threshold in this model. 37
In comparison to intrathecal alfentanil infusion, previous work examining the effects of intrathecal infusions of morphine indicated that significant increases could be achieved with 1 mg/mL morphine. While alfentanil is considered to be slightly more potent than morphine, 29,30,31 the assessment of potency of a continuous intrathecally infused agent is heavily impacted by the PK of the compound. While morphine may be less potent as an opioid ligand than alfentanil, the fact that it is cleared more slowly than lipid soluble agents such as alfentanil 38 means that it will tend to accumulate and appear to be more potent than a similar infusion of alfentanil, e.g., a PK dependent potency.
Intrathecal granulomas
In humans, as in dogs and sheep, the intrathecal infusion of high concentrations of several opiates, including morphine and hydromorphone, results in a space occupying accumulation of inflammatory cells in the intrathecal space.5–8 Consistent with the role of local concentration, this granuloma typically shows its largest expanse proximal to the levels of the catheter tip, 3 where the local drug concentrations are highest and diminish as a function of the distribution gradient as one progresses distal to the infusion site.39
These intrathecal masses, arising from the meninges have been shown to be constituted of organized collections of polymorphonuclear cells as well as mononuclear cells, macrophages and lymphocytes, suggesting a mixed acute/chronic inflammatory response. At a more advanced stage these intrathecal masses may also display necrotic centers. These cellular collections have been variously referred to as inflammatory masses, pyogranuloma or simply granulomas.3,40
Origin of granuloma
While the origin of the granuloma from the meninges is clear, its mechanisms of initiation are not. While local infections can give rise to granulomas,41 many granulomas are not associated with markers of infection.3 Nor does the granuloma represent a general incompatibility of the excipients or reaction to infused agents.3,4,40,42 Thus, a variety of continuous intrathecally infused agents in the canine model fail to produce a granuloma (see for example36,43–45). Thus, the single consistent component is morphine itself. While several opiates produce the granuloma, one very potent opiate (fentanyl) does not, suggesting that this effect is not a simple opioid receptor mediated effect.9 In recent work, we have indeed shown that the granuloma producing effects of morphine are not blocked by concurrent delivery of an opiate antagonist.46
Role of meningeal mast cells
As reviewed, the novel structure activity relationship of the granuloma and it’s parallels with mast cells, along with work showing that mast cells are expressed in the meninges and that dural mast cell activation leads to plasma extravasation and inflammatory cell migration, raise this as a potential linkage. 21,47–52 An important link in this speculative hypothesis is that an agent that does not degranulate mast cells will have a lower propensity to produce a granuloma. The present study extended this reasoning to consider whether a second opiate (other than fentanyl) that was said to have a low propensity to produce mast cell degranulation, alfentanil, would yield a granuloma with chronic intrathecal infusion.
Intrathecal alfentanil and the granuloma
In the present study, the infusion of three dogs with morphine 12 mg/mL/d) resulted in evident intrathecal granulomas in all three animals. These observations recapitulated previous data that this exposure would reliably yield an identifiable mass in the dog. 2–4 Previous work has shown that with morphine an incidence of granulomas was observed at doses as low as 1–3 mg/d.3 This was in the dose range wherein intrathecally infused morphine produced a potent analgesia2,9. In contrast, intrathecal treatment with alfentanil for over 3 weeks with 20 mg/mL/d, a dose that was at least 10x the minimum dose producing a significant analgesia in the canine model, resulted in no identifiable mass in five animals. Whether higher doses of alfentanil might have had an effect is not known. These results distinguish alfentanil from morphine in terms of their respective ability to generate a space occupying granuloma at a greater than analgesic dose. An important issue relates to the existing human experience. While there is extensive use of intrathecal morphine in humans, there is less with anilinopiperadines. This lower use relates to their relatively rapid clearance and correspondingly high plasma levels leading to non spinal effects., Nevertheless, we are aware in the published literature of two case reports raising the possibility that fentanyl or sufentanil both can initiate spinal granulomas. In one case report, intrathecal infusion of fentanyl (2.7 mg/d) was associated with an intrathecal mass.53 In a second report, intrathecal infusion of sufentanil (17μg/d) in a patient developed a granuloma.54 In both cases, it appears that the patients had prior exposure to a variety of agents and drug history, concentration and duration of exposure were not clearly outlined. These two examples, however, suggest the possible role of other mechanisms in addition to the potential contribution of mast cells.
Cutaneous mast cells as predictor of granuloma inducing activity
The present studies examined the effects of alfentanil on cutaneous mast cell degranulation as assessed by the flare produced by intradermal delivery. As shown here and elsewhere, intradermal agents such as compound 48–80, and opiates such as morphine yield a cutaneous flare reflecting a local vasodilation that is blocked by mast cell stabilizers.14–16 We note that all mast cells are not comparable. Thus, while opiates degranulate cutaneous mast cells, they do not activate mast cells present in the lung, intestinal, heart or blood basophils). 22,23 While speculative, we note that dura and skin arise embryologically from the somitic mesoderm and neural crest 24,25, raising the hypothesis that cutaneous and dural mast cells may share certain pharmacologies including their response to opiates. In recent work, we indeed demonstrated that in ex vivo dural mast cells as well as in vivo (evidenced by cutaneous flare evoked by intradermal injection) were readily degranulated by several opioids, but not by fentanyl.46 Accordingly, in the present work, we showed that concentrations up to 20 mg/mL of alfentanil had no significant effect upon cutaneous flare, permitting us to address the hypothesis that an opiate that does not degranulate a mast cell will not produce a granuloma. Indeed, as shown, a granuloma was not observed in five dogs receiving 20mg/mL/d). We suggest that this might be a facile way to screen drugs for their propensity to produce a granuloma. There are several considerations accompanying this hypothesis. First, specific examination of local skin concentration response curves might be illuminating to define precise local drug concentrations in vivo that lead to flare formation. It is likely that if the spinal drug target relevant to the analgesic actions of the intrathecal agent requires a very low CSF concentration relative to that which produces flare (mast cell degranulation), then the therapeutic use of the agent might not be accompanied by granuloma formation. Thus, ziconotide can produce histamine release (leading to a prominent hypotension).55 However, intrathecal ziconotide in neither preclinical canine safety evaluations56,57 or in humans56,58 has been reported to be associated with intrathecal granulomas. This may reflect upon the extremely low concentrations required of this agent for intrathecal analgesia. Secondly, the use of the flare presupposes that the vasodilation reflects upon mast cell degranulation and not some direct effect upon the vasculature. This can be assessed by demonstrating that evoked flares are prevented by mast cell stabilizers such as cromolyn.14–16,46
Concluding comment
The present studies provide support for the hypothesis that an opiate that does not produce a flare (and therefore does not degranulate some populations of mast cells) does not produce a granuloma after intrathecal infusion in the dog. Alfentanil now joins fentanyl as an opiate, which, to date, does not produce flare and which does not yield a granuloma in the canine model. Importantly, these data support the hypothesized possible role of mast cells, but the work does not support the use of continuous intrathecal alfentanil infusion in humans. Several issues mitigate against its use. First, while its lipid solubility is less than fentanyl, this agent has been shown to have a high clearance from the spinal cord into plasma38 and is accompanied by side effects as observed in the present studies. Second and most importantly, while there is a report of bolus delivery of intrathecal alfentanil in humans 32, initiation of the use of this agent in humans would require systematic preclinical evidence of pharmacokinetics and safety.59,60 Those data do not currently exist. The present studies were designed to assess the propensity of the anilinopiperadines to produce granulomas, not its specific formulation stability or safety. In any case, further studies are required to confirm the role of mast cells in the formation of an intrathecal granuloma.
Summary statement.
Alfentanil does not degranulate cutaneous mast cells and its intrathecal infusion at the highest concentrations employed, unlike morphine, does not produce intrathecal granulomas in dog.
Acknowledgments
Funding:
This project was supported by NIDA-15353 (TLY).
We would like to thank Nicole Tozier and Mary Ceccolini for their expert technical assistance in the performance of the initial phases of these studies. We thank the Alfred Mann Foundation for providing a number of the Mini-med pumps used in these investigations. We would like to particularly acknowledge our debt to Dr. Chris Bernards, who pointed us to the similarity of the meningeal phenomena to the events that occur in migraine, the aberrant effects of opiates in degranulating mast cells and the potential role of mast cells. Dr. Bernards sadly passed away on January 12, 2012.
Footnotes
Authorship:
Dr. Tony Yaksh was responsible for the organization of the study, animal surgical preparation, necropsy and paper writing.
Joanne Steinauer was responsible for histological procedures, tissue preparation, histopathology and histology data analysis.
Samantha Williams was responsible for day to day management of animal health and welfare, and assisted with surgery and necropsy.
Shelle Malkmus was responsible for day to day management of animal health and welfare and assisted with the surgery and necropsy.
Conflict of Interest: The authors declared no conflicts of interest.
References
- 1.North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine: Case report and laboratory experience. Neurosurgery. 1991;29:778–784. doi: 10.1097/00006123-199111000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Allen JW, Horais KA, Tozier NA, Wegner K, Corbeil JA, Mattrey RF, Rossi SS, Yaksh TL. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: A combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581–589. doi: 10.1097/00000542-200609000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–187. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Michael A, Buffen E, Rauck R, Anderson W, McGirt M, Mendenhall HV. An in vivo canine study to assess granulomatous responses in the MedStream Programmable Infusion System (TM) and the SynchroMed II Infusion System(R) Pain Med. 2012;13:175–184. doi: 10.1111/j.1526-4637.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 5.Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ. Inflammatory masses associated with intrathecal drug infusion: A review of preclinical evidence and human data. Pain Med. 2002;3:300–312. doi: 10.1046/j.1526-4637.2002.02048.x. [DOI] [PubMed] [Google Scholar]
- 6.Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: Report and observations on 41 patients. Neurosurgery. 2002;50:78–86. doi: 10.1097/00006123-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Deer TR, Prager J, Levy R, Rathmell J, Buchser E, Burton A, Caraway D, Cousins M, De Andres J, Diwan S, Erdek M, Grigsby E, Huntoon M, Jacobs M, Kim P, Kumar K, Leong M, Liem L, McDowell G, Panchal S, Rauck R, Saulino M, Sitzman BT, Staats P, Stanton-Hicks M, Stearns L, Wallace M, Willis KD, Witt W, Yaksh T, Mekhail N. Neuromodulation; Polyanalgesic Consensus Conference-2012: Consensus on Diagnosis, Detection, and Treatment of Catheter-Tip Granulomas (Inflammatory Masses); 2012. [DOI] [PubMed] [Google Scholar]
- 8.Aprili D, Bandschapp O, Rochlitz C, Urwyler A, Ruppen W. Serious complications associated with external intrathecal catheters used in cancer pain patients: A systematic review and meta-analysis. Anesthesiology. 2009;111:1346–1355. doi: 10.1097/ALN.0b013e3181bfab9a. [DOI] [PubMed] [Google Scholar]
- 9.Allen JW, Horais KA, Tozier NA, Yaksh TL. Opiate pharmacology of intrathecal granulomas. Anesthesiology. 2006;105:590–598. doi: 10.1097/00000542-200609000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Feldberg W, Paton WD. Release of histamine from skin and muscle in the cat by opium alkaloids and other histamine liberators. J Physiol. 1951;114:490–509. doi: 10.1113/jphysiol.1951.sp004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosman N, Jensen SM, Johansen FF. Histamine release from isolated rat mast cells induced by opiates: Effect of sterical configuration and calcium. Agents Actions. 1982;12:417–424. doi: 10.1007/BF01965920. [DOI] [PubMed] [Google Scholar]
- 12.Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: Evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol. 1984;73:775–781. doi: 10.1016/0091-6749(84)90447-0. [DOI] [PubMed] [Google Scholar]
- 13.Levy JH, Brister NW, Shearin A, Ziegler J, Hug CC, Jr, Adelson DM, Walker BF. Wheal and flare responses to opioids in humans. Anesthesiology. 1989;70:756–760. doi: 10.1097/00000542-198905000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: An in vivo microdialysis study in human skin. Anesth Analg. 2004;98:364–370. doi: 10.1213/01.ANE.0000097168.32472.0D. table of contents. [DOI] [PubMed] [Google Scholar]
- 15.Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci. 1993;53:1391–1399. doi: 10.1016/0024-3205(93)90581-m. [DOI] [PubMed] [Google Scholar]
- 16.Baldo BA, Pham NH. Histamine-releasing and allergenic properties of opioid analgesic drugs: Resolving the two. Anaesth Intensive Care. 2012;40:216–235. doi: 10.1177/0310057X1204000204. [DOI] [PubMed] [Google Scholar]
- 17.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–220. doi: 10.1002/jemt.1085. [DOI] [PubMed] [Google Scholar]
- 19.Michaloudi H, Batzios C, Chiotelli M, Grivas I, Papadopoulos GC. Mast cells populations fluctuate along the spinal dura mater of the developing rat. Brain Res. 2008;1226:8–17. doi: 10.1016/j.brainres.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;105:375–390. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118:5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebertz JM, Hermens JM, McMillan JC, Uno H, Hirshman C, Hanifin JM. Functional differences between human cutaneous mast cells and basophils: a comparison of morphine-induced histamine release. Agents Actions. 1986;18:455–462. doi: 10.1007/BF01964946. [DOI] [PubMed] [Google Scholar]
- 23.Tharp MD, Kagey-Sobotka A, Fox CC, Marone G, Lichtenstein LM, Sullivan TJ. Functional heterogeneity of human mast cells from different anatomic sites: In vitro responses to morphine sulfate. J Allergy Clin Immunol. 1987;79:646–653. doi: 10.1016/s0091-6749(87)80162-8. [DOI] [PubMed] [Google Scholar]
- 24.Catala M. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: The ventricular system, meninges and choroid plexuses. Arch Anat Cytol Pathol. 1998;46:153–169. [PubMed] [Google Scholar]
- 25.O’Rahilly R, Muller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- 26.Bernards CM, Luger TJ, Malmberg AB, Hill HF, Yaksh TL. Liposome encapsulation prolongs alfentanil spinal analgesia and alters systemic redistribution in the rat. Anesthesiology. 1992;77:529–535. doi: 10.1097/00000542-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Wallace MS, Yanez AM, Ho RJ, Shen DD, Yaksh TL. Antinociception and side effects of liposome-encapsulated alfentanil after spinal delivery in rats. Anesth Analg. 1994;79:778–786. doi: 10.1213/00000539-199410000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Isackson J, Wallace MS, Ho RJ, Shen DD, Yaksh TL. Antinociception and side effects of L- and D-dipalmitoylphosphatidyl choline liposome-encapsulated alfentanil after spinal delivery in rats. Pharmacol Toxicol. 1995;77:333–340. doi: 10.1111/j.1600-0773.1995.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 29.Buerkle H, Yaksh TL. Comparison of the spinal actions of the mu-opioid remifentanil with alfentanil and morphine in the rat. Anesthesiology. 1996;84:94–102. doi: 10.1097/00000542-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Buerkle H, Yaksh TL. Continuous intrathecal administration of shortlasting mu opioids remifentanil and alfentanil in the rat. Anesthesiology. 1996;84:926–935. doi: 10.1097/00000542-199604000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Sabbe MB, Grafe MR, Mjanger E, Tiseo PJ, Hill HF, Yaksh TL. Spinal delivery of sufentanil, alfentanil, and morphine in dogs. Physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920. doi: 10.1097/00000542-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Hughes DA, Hill DA. Intrathecal alfentanil with and without bupivacaine for analgesia in labour. Anaesthesia. 2000;55:1116–1121. doi: 10.1046/j.1365-2044.2000.01547-3.x. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein I, Nadel JA, Graf PD, Caughey GH. Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J Clin Invest. 1990;86:555–559. doi: 10.1172/JCI114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker AB, Chung KF, McDonald DM, Lazarus SC, Frick OL, Gold WM. Mast cell heterogeneity in dog skin. Anat Rec. 1985;213:477–480. 530–471. doi: 10.1002/ar.1092130402. [DOI] [PubMed] [Google Scholar]
- 35.Yaksh TL, Provencher JC, Rathbun ML, Myers RR, Powell H, Richter P, Kohn FR. Safety assessment of encapsulated morphine delivered epidurally in a sustained-release multivesicular liposome preparation in dogs. Drug Deliv. 2000;7:27–36. doi: 10.1080/107175400266768. [DOI] [PubMed] [Google Scholar]
- 36.Yaksh TL, Rathbun ML, Dragani JC, Malkmus S, Bourdeau AR, Richter P, Powell H, Myers RR, Lebel CP. Kinetic and safety studies on intrathecally infused recombinant-methionyl human brain-derived neurotrophic factor in dogs. Fundam Appl Toxicol. 1997;38:89–100. doi: 10.1006/faat.1997.2314. [DOI] [PubMed] [Google Scholar]
- 37.Wegner K, Horais KA, Tozier NA, Rathbun ML, Shtaerman Y, Yaksh TL. Development of a canine nociceptive thermal escape model. J Neurosci Methods. 2008;168:88–97. doi: 10.1016/j.jneumeth.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Flack SH, Bernards CM. Cerebrospinal fluid and spinal cord distribution of hyperbaric bupivacaine and baclofen during slow intrathecal infusion in pigs. Anesthesiology. 2010;112:165–173. doi: 10.1097/ALN.0b013e3181c38da5. [DOI] [PubMed] [Google Scholar]
- 40.Butt MT. Morphologic changes associated with intrathecal catheters for direct delivery to the central nervous system in preclinical studies. Toxicol Pathol. 2011;39:213–219. doi: 10.1177/0192623310391679. [DOI] [PubMed] [Google Scholar]
- 41.Lehmberg J, Scheiwe C, Spreer J, van Velthoven V. Late bacterial granuloma at an intrathecal drug delivery catheter. Acta neurochirurgica. 2006;148:899–901. doi: 10.1007/s00701-006-0810-9. discussion 901. [DOI] [PubMed] [Google Scholar]
- 42.Gradert TL, Baze WB, Satterfield WC, Hildebrand KR, Johansen MJ, Hassenbusch SJ. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology. 2003;99:188–198. doi: 10.1097/00000542-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 43.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–427. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Yaksh TL, Horais KA, Tozier N, Rathbun M, Richter P, Rossi S, Grafe M, Tong C, Meschter C, Cline JM, Eisenach J. Intrathecal ketorolac in dogs and rats. Toxicol Sci. 2004;80:322–334. doi: 10.1093/toxsci/kfh168. [DOI] [PubMed] [Google Scholar]
- 45.Chiari A, Yaksh TL, Myers RR, Provencher J, Moore L, Lee CS, Eisenach JC. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology. 1999;91:824–832. doi: 10.1097/00000542-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 46.Yaksh T, Allen J, Williams S, Horais K, Malkmus S, Scadeng M, Steinauer J, Rossi SR. Meningeal mast cells and intrathecal morphine evoked granuloma formation. Anesthesiology. 2012 doi: 10.1097/ALN.0b013e31828351aa. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 48.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 49.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- 50.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 51.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 52.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhag. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zacest AC, Carlson JD, Nemecek A, Burchiel KJ. Surgical management of spinal catheter granulomas: operative nuances and review of the surgical literature. Neurosurgery. 2009;65:1161–1164. doi: 10.1227/01.NEU.0000359223.11215.D9. discussion 1164–1165. [DOI] [PubMed] [Google Scholar]
- 54.Gupta A, Martindale T, Christo PJ. Intrathecal catheter granuloma associated with continuous sufentanil infusion. Pain Med. 2010;11:847–852. doi: 10.1111/j.1526-4637.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 55.Bowersox SS, Singh T, Nadasdi L, Zukowska-Grojec Z, Valentino K, Hoffman BB. Cardiovascular effects of omega-conopeptides in conscious rats: Mechanisms of action. J Cardiovasc Pharmacol. 1992;20:756–764. [PubMed] [Google Scholar]
- 56.Skov MJ, Beck JC, de Kater AW, Shopp GM. Nonclinical safety of ziconotide: An intrathecal analgesic of a new pharmaceutical class. Int J Toxicol. 2007;26:411–421. doi: 10.1080/10915810701582970. [DOI] [PubMed] [Google Scholar]
- 57.Yaksh TL, de Kater A, Dean R, Best BM, Miljanich GP. Pharmacokinetic Analysis of Ziconotide (SNX-111), an Intrathecal N-Type Calcium Channel Blocking Analgesic, Delivered by Bolus and Infusion in the Dog. Neuromodulation. 2012 doi: 10.1111/j.1525-1403.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoederath P, Gautschi OP, Land M, Hildebrandt G, Fournier JY. Formation of two consecutive intrathecal catheter tip granulomas within nine months. Cent Eur Neurosurg. 2010;71:39–42. doi: 10.1055/s-0029-1202359. [DOI] [PubMed] [Google Scholar]
- 59.Yaksh TL. Spinal Delivery and Assessment of Drug Safety, Fundamental Neuropathology for Pathologists and Toxicologists. In: Bolon B, Butt M, editors. Principles and Techniques. Hoboken, John Wiley & Sons, Inc; 2011. pp. 452–462. [Google Scholar]
- 60.Walker SM, Yaksh TL. Review article: neuraxial analgesia in neonates and infants: A review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–662. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]