Abstract
Bronchiolitis obliterans syndrome (BOS), a condition of irreversible small airway fibrosis, is the principal factor limiting long-term survival after lung transplantation. Bronchoscopy and bronchoalveolar lavage (BAL), techniques central to lung transplant clinical practice, provide a unique opportunity to interrogate the lung allograft during BOS development and identify potential disease mechanisms or biomarkers. Over the past twenty years, numerous studies have evaluated the BAL cellular composition, cytokine profiles, and protein constituents in lung transplant recipients with BOS. To date, however, no summative evaluation of this literature has been reported. We developed and applied objective criteria to qualitatively rank the strength of associations between BAL parameters and BOS in order to provide a comprehensive and systematic assessment of the literature. Our analysis indicates that several BAL parameters, including neutrophil count, interleukin-8, alpha defensins, and MMP-9, demonstrate highly replicable associations with BOS. Additionally, we suggest that considerable opportunity exists to increase the knowledge gained from BAL analyses in BOS through increased sample sizes, covariant adjustment, and standardization of BAL technique. Further efforts to leverage analysis of BAL constituents in BOS may offer great potential to provide additional in-depth and mechanistic insights into the pathogenesis of this complex disease.
Keywords: Bronchoalveolar Lavage, Bronchiolitis Obliterans Syndrome, Lung Transplantation
Introduction
Lung transplantation is a viable short-term therapy for many advanced lung diseases. Long-term outcomes, however, remain limited with a median 5-year survival rate of only 50% (1). Bronchiolitis obliterans syndrome (BOS), a condition of progressive small airways fibrosis manifested by increasing airflow limitation, is the most widely described form of chronic lung allograft dysfunction (CLAD) and is a principal factor contributing to poor long-term survival (2). Despite its clinical significance, the mechanisms leading to BOS remain poorly understood (3).
Because lung transplant recipients regularly undergo bronchoscopy as part of their clinical care, examination of the cellular composition, cytokine profile, and protein constituents in bronchoalveolar lavage (BAL) fluid provides a unique window into the microenvironment of the lung allograft. Since the initial description of BOS, numerous studies have been published in which BAL fluid was examined from lung transplant recipients with this condition. To date, however, no summative evaluation of this body of literature has been reported. The purpose of this article is to apply objective criteria to evaluate the methodological quality of each study linking BAL parameters to BOS in order to provide an overall assessment of the strength of correlation between any specific BAL parameter and BOS. We then comment specifically on the most replicable associations with BOS and how they have impacted our understanding of disease pathogenesis. Finally, we highlight opportunities to enhance future studies and gain further insights into BOS and emerging CLAD phenotypes.
Study Identification and Appraisal
On June 30, 2012, a PubMed search was conducted using the search terms “Lung Transplant” AND “Bronchoalveolar Lavage,” and results were subsequently limited to articles published between 1991 and 2012. Using this technique, 1,176 articles were identified. The study title and abstract were manually reviewed and articles not relevant to the study of BAL in BOS were excluded based on the following hierarchy: not focused on lung transplant (n=419), non-human study (n=157), not focused on BOS (n = 449), not utilizing BAL (n = 46), and full text article not available for review (n=1). We also chose to exclude articles that focused exclusively on analysis of BAL parameters associated with gastroesophageal reflux disease (e.g., bile acids, pepsin), given that this topic was recently reviewed elsewhere (4) [n = 4]. The remaining 100 articles were read and categorized with respect to study design, number of patients included, follow-up time, and BAL parameter(s) assessed.
In order to determine which BAL parameters were the most reliable, each parameter was qualitatively ranked based on its replicability across independent studies and on the individual trial designs. Parameters fell into one of three broad categories largely based on sample size and inter-study agreement. A parameter was considered highly replicable if at least three studies found it to be significant, in which each study featured greater than 30 subjects (at least 15 with BOS). A parameter was considered moderately replicable if it was found to be significant in two studies following the criteria described above, or in three or more studies including >= 20 subjects (at least 10 with BOS). A parameter was considered weakly or non-replicable if it was assessed in only one study, assessed in studies providing disparate results, or assessed in studies not meeting the aforementioned methodological criteria. The majority of studies describing high and moderately replicable parameters accounted for or omitted concurrent infection and acute rejection. Notably, although follow-up time is an important consideration in evaluating study strength, heterogeneity in reporting precluded its inclusion in these objective criteria.
Overview of Studies
Applying the above criteria, we identified 100 articles describing 176 distinct parameters in the BAL of lung transplant recipients with BOS. Commonly identified BAL parameters were subclassified into the following major categories: cellular composition, cytokine and chemokine profiles, innate immune components, matrix metalloproteinases, and markers of oxidative stress. Considering all available studies, the overall median sample size was 39.5, and the median number of patients with BOS was 13. Table 1 outlines the median sample size and other characteristics with respect to each individual BAL parameter category. While the majority of studies featured strictly cross-sectional designs, 36% included a longitudinal component which examined the trajectory of parameters over time. Of the 176 parameters described, our analysis identified 7 as highly replicable, 13 as moderately replicable, and 156 as weakly or non-replicable. A summary of high and moderately replicable BAL constituents in BOS is outlined in Table 2, and the remaining weakly or non-replicable parameters are summarized in Supplementary Tables 1 and 2.
Table 1.
Overview of Studies Examining BAL from Lung Transplant Recipients with BOS.
| BAL Parameter* | Number of Published Studies | Sample Size, median (min-max) | Subjects with BOS, median (min-max) | Longitudinal Component | Explicit adjustment for acute rejection and infection |
|---|---|---|---|---|---|
| Cell Composition | 50 | 53.1 (14 – 194) | 15.8 (3 – 61) | 16 (32%) | 31 (62%) |
| Cytokines and Chemokines | 43 | 53 (9 – 194) | 16.5 (3– 54) | 22 (51%) | 25 (58%) |
| Innate Immune Components | 23 | 48.2 (16 – 121) | 19.8 (6 – 56) | 7 (30%) | 12 (52%) |
| Matrix Metalloproteinases | 9 | 28 (20 –72) | 12.5 (7 – 34) | 3 (30%) | 7 (70%) |
| Markers of Oxidative Stress | 11 | 39.2 (22 – 86) | 14.6 (5 – 35) | 2 (18%) | 6 (55%) |
Among the 100 total BAL studies, some examined multiple biomarkers and are thus included in more than one category.
Table 2.
Significant BAL Parameters with Highly or Moderately Replicable Associations with BOS.
| Parameter | Direction of Association | HIGH | MOD. |
|---|---|---|---|
| Cell Composition | |||
| Neutrophils (5–42) | ↑ | ||
| T-regulatory cells (45, 46) | ↓ | ||
| Mesenchymal Stromal Cells (51–53) | ↑ | ||
| Cytokines, Chemokines, Growth Factors | |||
| IL-6 (17, 27–29, 31, 57, 59, 88) | ↑ | ||
| IL-8 (5, 10–12, 18, 19, 23, 25–31, 35, 55–59). | ↑ | ||
| ET-1 (52, 64) | ↑ | ||
| CCL2/MCP-1 (23, 27, 56, 59, 62, 63) | ↑ | ||
| CCL5/RANTES (18, 23, 56, 59) | ↑ | ||
| MIG/CXCL9 (63, 65) | ↑ | ||
| IP-10/CXCL10 (44, 65) | ↑ | ||
| Innate Immune Components | |||
| SP-A (74–76) | ↓ | ||
| SLPI (5, 14, 19, 73) | ↓ | ||
| CCSP (20, 68, 69) | ↓ | ||
| Neutrophil Elastase (14, 21, 73, 77, 89) | ↑ | ||
| Alpha Defensins/HNPs (5, 66–69) | ↑ | ||
| Matrix Metalloproteinases | |||
| MMP-9 (6, 15, 55, 57–59, 73, 76, 82) | ↑ | ||
| Oxidative Stress | |||
| Myeloperoxidase (7, 10, 14, 25, 26, 59) | ↑ | ||
| Oxidized/Reduced Glutathione (7, 26, 84) | ↑ | ||
| Methionine Sulfoxide (7, 14) | ↑ | ||
BAL Cell Composition
By far the neutrophil is the most predominant cell type in the BAL fluid of patients with BOS, whether described as a percentage of total cells or in absolute cell count. Thirty-six out of the 38 studies examining neutrophils found them to be elevated in association with BOS (5–42). More convincingly, the degree of BAL neutrophilia increases in correlation with increasing BOS stage (9). Going beyond a simple association, several studies have attested the value of elevated BAL neutrophil counts prior to the onset of BOS as a factor useful in predicting its future development. For example, Neurohr et al. followed 63 patients for three years post-transplant and demonstrated that neutrophil proportions greater than 20% predicted BOS onset a median of 232 days post-bronchoscopy (19). Similarly, Slebos et al. showed that a percentage of neutrophils of at least 16% predicted future BOS, while Reynaud-Gauber, et al. reported a threshold of 24% (23, 24, 28).
These data suggest that neutrophils play a central role in the development or progression of BOS. More recently, it has also been proposed that BAL neutrophilia can be used to identify a subphenotype of CLAD, neutrophilic reversible airway dysfunction (NRAD), which may be responsive to treatment with azithromycin (43). While further studies are necessary to better understand the role of neutrophils in promoting epithelial injury and subsequent fibrosis, the concept of using a BAL parameter to identify disease subtypes that respond differentially to treatment is intriguing and represents a priority for further research.
Beyond neutrophils, a variety of other cell types have been evaluated for their association with BOS. Given the alloimmune basis for acute lung allograft rejection, several studies have examined lymphocyte subpopulations in BOS BAL (28, 37, 44–50). While the levels of total lymphocytes, CD4+ cells, and CD8+ cells were noted to have only weakly or non-replicable associations with BOS, there is growing interest in the protective role of FOXP3+CD4+ T-regulatory (T-reg) cells and their ability to control the immune response and reduce local inflammation. Indeed, T-regs were significantly reduced in the BAL from lung transplant recipients with BOS (45, 46). The proportion of T-regs has also been shown to be predictive of BOS susceptibility, with levels less than 3.2% significantly predicting the development of BOS within two years post-transplant (45). Furthermore, Gregson et al. demonstrated that CCR7+ T-regs, which have central memory functions, were decreased to an even greater extent in BOS BAL(46).
More recently, significant attention has been paid to multipotent donor-derived resident lung mesenchymal stromal cells (MSCs), defined as cells that express stem cell markers CD73, CD90, and CD105 in the absence of hematopoietic markers, and their potential role in promoting the small airway fibrosis typical of bronchiolitis obliterans. Badri et al. demonstrated a two-fold increase in MSCs in the BAL from lung transplant recipients with BOS, and this important finding has since been replicated in two additional studies (51–53). Moreover, MSCs isolated from the BAL of BOS patients have been shown to have a more proliferative and profibrotic phenotype than those isolated from stable lung transplant recipients (52–54). These findings not only highlight the importance of MSCs in promoting or sustaining a fibrogenic milieu, but also represent a unique translational approach to leverage cells from the BAL for ex vivo culture to provide additional mechanistic insights into disease pathogenesis.
BAL Cytokines, Chemokines, and Growth Factors
While BAL levels of a variety of cytokines and chemokines have been evaluated, interleukin-8 (IL-8) has been the most striking and consistent parameter associated with BOS. IL-8 is a pro-inflammatory cytokine and potent neutrophil chemotractant released primarily by alveolar macrophages and epithelial cells. Eighteen out of the 20 studies examining IL-8 have shown it to be elevated in the BAL of lung allograft recipients with BOS (5, 10–12, 18, 19, 23, 25–31, 35, 55–59). Additionally, multiple longitudinal studies have demonstrated that IL-8 begins to increase up to one year prior to clinical BOS diagnosis, suggesting that IL-8 plays a role in BOS development (19, 23, 27).
Building upon the previous results that considered cellular components in BOS, eight studies have shown IL-8 levels to correlate strongly with BAL neutrophil counts (10, 12, 18, 19, 23, 26, 28, 35). In a well-designed cohort of 86 patients, DiGiovine et al. demonstrated that IL-8 levels were positively correlated with percent neutrophils with an overall correlation coefficient of 0.85. Furthermore, the authors performed chemotaxis experiments and confirmed that IL-8 accounts for at least a portion of neutrophil chemotactic activity (10). These observations provide compelling evidence that IL-8 is a driving force for neutrophil influx into the lung allograft.
The CC chemokines, also involved in inflammation, have received considerable attention as well. Of these chemokines, CCL2, also known as monocyte chemotractant protein-1 (MCP-1), has provided the most striking and highly replicable association with BOS. Along with recruiting monocytes, MCP-1 attracts memory T-cells and dendritic cells, and may contribute to the differentiation of monocytes into fibrogenic macrophages (60, 61). All six of the studies examining MCP-1 found it to be elevated in the BAL of lung transplant recipients with BOS (23, 27, 56, 59, 62, 63). In addition, Reynaud-Gaubert et al. demonstrated elevated MCP-1 prior to clinical BOS diagnosis (23).
Also noteworthy are moderately replicable cytokines and chemokines, including interleukin-6 (IL-6), endothelin-1 (ET-1), monokine induced by gamma interferon (MIG, also known as CXCL9), interferon gamma induced protein 10 (IP-10, also known as CXCL10), and Regulated Upon Activation Normal T-Cell Expressed and Secreted (RANTES, also known as CCL5). Although studies involving these cytokines have not been reported as consistently, each has been described as elevated in cross-sectional studies (23, 27, 44, 52, 59, 64, 65) and several were demonstrated to be elevated prior to BOS onset in longitudinal studies (23, 27, 63, 64).
Interestingly, despite a growing interest in interleukin-17 (IL-17) in the development of lung transplant rejection, only weakly replicable evidence supports its role as a BAL biomarker of BOS (29, 59, 63). It is possible that assessment of different IL-17 subtypes may have contributed to disparate findings among these studies. Additionally, as is the case with many cytokines and chemokines, IL-17 levels may be near the lower limit of detection by traditional assays, and alternative approaches that consider tissue staining or flow cytometry-based methods to examine intracellular cytokine production might ultimately prove more valuable.
BAL Innate Immune Components
Increasingly, the innate immune system is recognized to play a key role in instructing host adaptive immunity and contributing to the development of allograft rejection. For this reason, several components important to innate host defense, including antimicrobial peptides (alpha defensins and secretory leukoprotease inhibitor [SLPI]), surfactant proteins, and neutrophil elastase have been assessed in the BAL from lung allograft recipients with and without BOS and these results have substantiated a role for the innate immune system in this complex process.
To date, alpha defensins, primarily neutrophil-derived antimicrobial peptides, are the innate immune elements holding the most highly replicable BOS association, with multiple studies reporting an increase in their levels in the BAL of patients with established BOS (5, 66–69). Nelsestuen et al, demonstrated alpha defensin levels 10 to 100 times greater in allograft recipients with BOS compared to stable post-transplant patients. This increase was substantially higher than the corresponding increase in neutrophil counts, suggesting that increased alpha defensins are more than simply a reflection of concomitant neutrophilia. Furthermore, the authors showed that alpha defensin levels begin to increase in the BAL up to 15 months prior to BOS diagnosis. Since alpha defensins have been shown to induce epithelial and fibroblast proliferation and increase collagen expression, they may play a plausible role in initiating a fibrotic response to allograft infection or other exogenous insults (66, 70–72).
Beyond alpha defensins, the associations of SLPI, surfactant protein A (SP-A), and neutrophil elastase with BOS has been evaluated with moderately replicable results. Out of the four studies assessing SLPI, three found diminished levels in the BAL of lung transplant recipients with BOS. In addition, Neurohr et al. described decreased SLPI in stable patients who subsequently developed BOS (5, 14, 19, 73). The surfactant proteins are similarly reduced in association with BOS, and a reduction in SP-A in particular may predict future BOS onset (74–76). In contrast to these results, neutrophil elastase has been noted to be increased in the BAL. These findings are, however, pathophysiologically compatible, as neutrophil elastase is known to facilitate SLPI degradation (14, 21, 73, 77).
Matrix Metalloproteinases
The matrix metalloproteinases (MMPs) are a family of zinc-containing enzymes, some of which are thought to contribute to extracellular matrix remodeling. MMPs also display proteolytic activity, and are able to break down several of the lung’s structural proteins, including gelatins, collagens, fibronectin, and elastin (78, 79). While several MMPs have been evaluated in the BAL of lung transplant recipients, the most striking is MMP-9.
MMP-9 is a gelatinase, and contributes to the migration of inflammatory cells through the endothelial layer (80, 81). Nine studies clearly showed MMP-9 to be elevated in the BAL of patients with BOS using multiple methodologies (protein array analysis, specific ELISA, gelatin zymography, and immunocytochemical staining) in well-constructed cohorts absent of concurrent infection or acute rejection (6, 15, 55, 57–59, 73, 76, 82). In addition, Ramirez et al. demonstrated MMP-9 elevation an average of 140 days prior to BOS onset, indicating that MMP-9 plays a relatively early role in matrix remodeling (57). Notably, multiple studies have also shown MMP-9 levels to correlate strongly with neutrophils, suggesting that neutrophil influx during BOS could contribute to MMP-9 elevation (15, 73). Other MMPs and their counterparts, the tissue inhibitors of metalloproteinases, have also been evaluated, but their associations with BOS have not been as striking or consistent.
Oxidative Stress
In addition to defensins and MMPs, activated neutrophils release reactive oxygen species (ROS). ROS can invoke epithelial injury, deplete antioxidant defenses, and stimulate the release of pro-inflammatory cytokines (83). One of the most prominent ROS is myeloperoxidase, shown in six distinct studies to be elevated in the BAL of lung allograft recipients with BOS (7, 10, 14, 25, 26, 59). In addition, Riise et al. described elevated myeloperoxidase up to five months prior to BOS diagnosis, indicating that oxidative stress may contribute to early epithelial injury and cytokine release (25).
Along with specific ROS, several studies have focused on general indicators of oxidative stress, including levels of glutathione, lipid peroxidation, and oxidized metathione. One of the most compelling oxidative stress measures is glutathione, a prominent antioxidant. The reduced form of glutathione has been shown to be decreased in association with BOS, while the oxidized form is significantly increased (7, 26, 84). Similarly, Madil et al. showed increased lipid peroxidation in a well-defined cohort of 58 patients, 35 of which had BOS. The authors also demonstrated lipid peroxidation and oxidized glutathione to be even higher in the BAL of patients with severe BOS compared to mild BOS (84). Oxidized metathione was described as increased in two studies, and was also shown to correlate significantly with increased myeloperoxidase, further indicating that neutrophil-derived ROS contributes to oxidative stress (7, 14). Together, these consistent results suggest a net increase in oxidative stress in association with BOS.
BAL Parameters in the Context of Emerging CLAD Phenoytpes
In general, it remains uncertain how the BAL parameters evaluated to date relate to emerging subphenotypes of CLAD, for example NRAD and restrictive allograft syndrome (RAS). Additional complexity is imposed given the variable approaches to identify these novel phenotypes as well as ambiguity regarding the exact relationship of NRAD or RAS to BOS. For example, NRAD is typically described to precede BOS, while in some cases RAS may develop after BOS onset (43, 85). Although the majority of studies included in this review predate the description of NRAD and RAS, both of these new disease subtypes are heralded by airflow limitation and thus are likely represented to some extent within these prior patient cohorts. In fact, such phenotypic heterogeneity may have contributed to variability in observed results, and this is of particular interest with respect to BAL neutrophilia.
Only two of the 100 articles identified for this review specifically stratified patients according to one of these emerging CLAD phenotypes (59, 76). In the first, Verleden et al demonstrated that BAL from patients with NRAD had greater cytokine and growth factor variability than BAL from patients with non-neutrophilic BOS (59). This lends pathophysiologic credibility to the concept that NRAD may be an important antecedent inflammatory state central to the establishment of airways fibrosis and BOS. In the second, Kosanam et al performed proteomic analysis comparing three patients with RAS to four stable lung recipient controls (76). Interestingly, several of the BAL parameters differentially expressed in the RAS group paralleled those found to be highly or moderately replicable within the current review, including an increase in MMP-9 and myeloperoxidase and a decrease in SP-A, suggesting that some similarities may exist among all forms of CLAD. Moving forward, longitudinal studies with rigorous CLAD phenotyping will be necessary to fully elucidate the BAL profiles and important mechanistic overlaps or distinctions between BOS, NRAD, and RAS.
Methodological Limitations of Current Studies
In evaluating the current studies that consider BAL parameters in BOS, several common limitations emerge, as summarized in Table 3. Perhaps most notable are small sample sizes, lack of control for potential confounders, and absence of detailed methods related to BAL collection and handling. Several aspects of BAL technique, such as the anatomic site of lavage, the amount of fluid and number of aliquots instilled, return volume, and timeliness of transport and suspension in appropriate culture media have been shown to affect the recovery of cellular and acellular alveolar constituents(86).
Table 3.
Common Methodological Concerns in Studies Examining BAL in BOS.
| Methodological Concerns |
|---|
| Small sample size |
| Cross-sectional design |
| Inconsistent time-matching post-transplant in cases and controls |
| Insufficient follow-up post-transplant |
| Inconsistent adjustment for concurrent infection or acute rejection |
| Variation in BAL collection, processing, and normalization |
Recently, an American Thoracic Society committee performed an extensive literature review related to BAL standardization and provided key recommendations to limit BAL variability; including targeting a total instilled volume of 100 to 300mL in 3 to 5 aliquots at a consistent anatomic site. Furthermore, they offer a detailed report on sample processing methods that can by employed to decrease inconsistency in the detection of cellular elements in particular(86). Efforts to adhere to these newly developed recommendations and improve on the other methodological limitations highlighted here may lead to more reproducible observations in future studies examining BAL parameters in BOS.
Conclusions and Future Directions
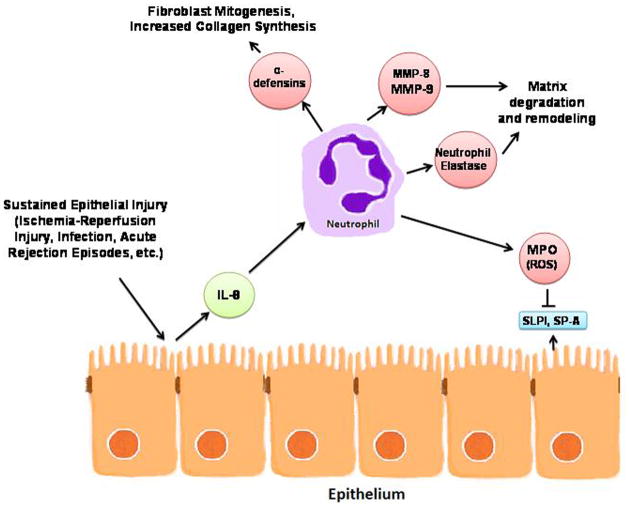
Examination of the cellular composition, cytokine profile, and protein constituents in bronchoalveolar lavage (BAL) fluid has not only provided a unique window into the microenvironment of the lung allograft, but has also suggested plausible pathobiologic mechanisms that contribute to the development and progression of BOS. Interestingly, by considering the collective core of most replicable parameters and their relationships to each other, a plausible scenario develops which highlights a central role for neutrophils and their inflammatory products in BOS (Figure 1).
Figure 1.
Proposed central pathobiologic role of neutrophils in BOS as supported by BAL studies
It is likely that sustained epithelial injury from a myriad of post-transplant insults (ischemia-reperfusion injury, pulmonary infection, cellular or humoral acute rejection) leads to release of IL-8 and other pro-inflammatory cytokines, which serve to activate the airway epithelium and promote the influx of inflammatory cells, including neutrophils. These activated neutrophils then release MMPs, defensins, and ROS that result in matrix degradation, fibroblast proliferation, depletion of antioxidant defense, and, ultimately, airway remodeling and fibrosis.
While this is one plausible mechanism suggested by the most reproducible BAL observations; BAL data are best interpreted within the context of information derived from non-BAL related clinical studies of BOS and experimental models of lung allograft rejection. Collectively, these lines of research have established that humoral immunity, innate immunity and autoimmunity in addition to cellular immunity contribute to BOS development (3). Within this framework, it is interesting that several reproducible BAL changes identified in our review support such diverse mechanisms. For example, changes in RANTES and IP-10 could affect auto as well as alloreactive T-cells and alterations in clara cell secretory protein or SP-A could influence monocyte and macrophage function. It is also important to note that although many of the most replicable BAL parameters can be mechanistically related to neutrophils, they can also function in other ways. IL-8, for instance, is recognized as a potent neutrophil chemotractant, but also promotes angiogenic activity that may be important during the fibroproliferative phase of BOS (87).
Although these studies of BAL analysis have provided interesting insights into the mechanisms underlying BOS, there are many opportunities to enhance the quality of the data being generated. Future research examining BAL from lung transplant recipients with BOS should include large sample sizes, rigorous clinical phenotyping, post-transplant time matching, standardized BAL collection and handling techniques, and control for confounding factors such as acute rejection and infection. Studies should also consider how parameters vary with newly identified CLAD subphenotypes (e.g. NRAD and RAS), and how they change across longitudinal prospective patient cohorts. Furthermore, positive associations should be validated in independent populations, and this will likely require collaboration between multiple transplant centers.
In conclusion, over the last twenty years numerous studies have analyzed BAL as a tool to predict, diagnose, and understand BOS. Methodological concerns notwithstanding, these studies have provided important insights into disease pathogenesis. As the quality of studies continues to improve, BAL analysis can not only be leveraged to gain further insight into BOS pathogenesis, but can also serve to generate biomarkers that may be incorporated into models to predict disease development or treatment response.
Supplementary Material
Acknowledgments
Funding statement: SMP acknowledges current funding from the National Heart Lung and Blood Institute P50 HL107180, R34 HL105422-01, and K24-091140-01. In addition, SMP receives funding from the Biomarker Factory, Roche Organ Transplant Foundation, and the Lung Transplant Foundation.
Abbreviations
- BOS
Bronchiolitis obliterans syndrome
- CLAD
Chronic lung allograft dysfunction
- BAL
Bronchoalveolar lavage
- NRAD
Neutrophilic reversible airway dysfunction
- T-reg
T-regulatory cell
- MSC
Mesenchymal stromal cell
- IL-8
Interleukin-8
- MCP-1
Monocyte chemotractant protein-1
- IL-6
Interleukin-6
- IL-13
Interleukin-13
- IL-17
Interleukin-17
- ET-1
Endothelin-1
- MIG
Monocyte induced by gamma interferon
- IP-10
IFN-inducible protein 10
- RANTES
Regulated upon activation normal T-cell expressed and secreted
- SLPI
Secretory leukoprotease inhibitor
- SP-A
Surfactant protein A
- MMP
Matrix metalloproteinase
- ROS
Reactive oxygen species
- RAS
Restrictive allograft syndrome
Footnotes
Conflict of interest: The authors have no relevant conflicts of interest to disclose.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27(9):957–69. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 3.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140(2):502–8. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 4.Davis CS, Gagermeier J, Dilling D, Alex C, Lowery E, Kovacs EJ, et al. A review of the potential applications and controversies of non-invasive testing for biomarkers of aspiration in the lung transplant population. Clin Transplant. 2010;24(3):E54–61. doi: 10.1111/j.1399-0012.2010.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RL, Hiemstra PS, Ward C, Forrest IA, Murphy D, Proud D, et al. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Eur Respir J. 2008;32(3):670–7. doi: 10.1183/09031936.00110807. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee B, Ling KM, Sutanto EN, Musk M, Yerkovich ST, Hopkins PM, et al. The airway epithelium is a direct source of matrix degrading enzymes in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2011;30(10):1175–85. doi: 10.1016/j.healun.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Behr J, Maier K, Braun B, Schwaiblmair M, Vogelmeier C. Evidence for oxidative stress in bronchiolitis obliterans syndrome after lung and heart-lung transplantation. Transplantation. 2000;69(9):1856–60. doi: 10.1097/00007890-200005150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Belperio JA, DiGiovine B, Keane MP, Burdick MD, Ying Xue Y, Ross DJ, et al. Interleukin-1 receptor antagonist as a biomarker for bronchiolitis obliterans syndrome in lung transplant recipients. Transplantation. 2002;73(4):591–9. doi: 10.1097/00007890-200202270-00020. [DOI] [PubMed] [Google Scholar]
- 9.Devouassoux G, Drouet C, Pin I, Brambilla C, Brambilla E, Colle PE, et al. Alveolar neutrophilia is a predictor for the bronchiolitis obliterans syndrome, and increases with degree of severity. Transpl Immunol. 2002;10(4):303–10. doi: 10.1016/s0966-3274(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 10.DiGiovine B, Lynch JP, 3rd, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157(9):4194–202. [PubMed] [Google Scholar]
- 11.D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, et al. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Transplantation. 2000;70(2):362–7. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hertz MI, Henke CA, Nakhleh RE, Harmon KR, Marinelli WA, Fox JM, et al. Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992;89(21):10385–9. doi: 10.1073/pnas.89.21.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch J, Elssner A, Mazur G, Maier KL, Bittmann I, Behr J, et al. Bronchiolitis obliterans syndrome after (heart-)lung transplantation. Impaired antiprotease defense and increased oxidant activity. Am J Respir Crit Care Med. 1999;160:1640–6. doi: 10.1164/ajrccm.160.5.9902012. [DOI] [PubMed] [Google Scholar]
- 15.Hubner RH, Meffert S, Mundt U, Bottcher H, Freitag S, El Mokhtari NE, et al. Matrix metalloproteinase-9 in bronchiolitis obliterans syndrome after lung transplantation. Eur Respir J. 2005;25(3):494–501. doi: 10.1183/09031936.05.00091804. [DOI] [PubMed] [Google Scholar]
- 16.Laan M, Linden A, Riise GC. IL-16 in the airways of lung allograft recipients with acute rejection or obliterative bronchiolitis. Clin Exp Immunol. 2003;133(2):290–6. doi: 10.1046/j.1365-2249.2003.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnan A, Mege JL, Escallier JC, Brisse J, Capo C, Reynaud M, et al. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1996;153:1431–6. doi: 10.1164/ajrccm.153.4.8616577. [DOI] [PubMed] [Google Scholar]
- 18.Mamessier E, Milhe F, Badier M, Thomas P, Magnan A, Reynaud-Gaubert M. Comparison of induced sputum and bronchoalveolar lavage in lung transplant recipients. J Heart Lung Transplant. 2006;25(5):523–32. doi: 10.1016/j.healun.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Neurohr C, Huppmann P, Samweber B, Leuschner S, Zimmermann G, Leuchte H, et al. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28(5):468–74. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Nord M, Schubert K, Cassel TN, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73(8):1264–9. doi: 10.1097/00007890-200204270-00013. [DOI] [PubMed] [Google Scholar]
- 21.Nunley D, Dauber J, Iacono A, Keenan R, Zeevi A, Cornwell R, et al. Unopposed neutrophil elastase in bronchoalveolar lavage from transplant recipients with cystic fibrosis. Am J Respir Crit Care Med. 1999;159(1):258–61. doi: 10.1164/ajrccm.159.1.9712068. [DOI] [PubMed] [Google Scholar]
- 22.Reid D, Snell G, Ward C, Krishnaswamy R, Ward R, Zheng L, et al. Iron overload and nitric oxide-derived oxidative stress following lung transplantation. J Heart Lung Transplant. 2001;20(8):840–9. doi: 10.1016/s1053-2498(01)00282-0. [DOI] [PubMed] [Google Scholar]
- 23.Reynaud-Gaubert M, Marin V, Thirion X, Farnarier C, Thomas P, Badier M, et al. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant. 2002;21(7):721–30. doi: 10.1016/s1053-2498(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 24.Reynaud-Gaubert M, Thomas P, Badier M, Cau P, Giudicelli R, Fuentes P. Early detection of airway involvement in obliterative bronchiolitis after lung transplantation. Functional and bronchoalveolar lavage cell findings. Am J Respir Crit Care Med. 2000;161(6):1924–9. doi: 10.1164/ajrccm.161.6.9905060. [DOI] [PubMed] [Google Scholar]
- 25.Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, et al. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J. 1999;14(5):1123–30. doi: 10.1183/09031936.99.14511239. [DOI] [PubMed] [Google Scholar]
- 26.Riise GC, Williams A, Kjellstrom C, Schersten H, Andersson BA, Kelly FJ. Bronchiolitis obliterans syndrome in lung transplant recipients is associated with increased neutrophil activity and decreased antioxidant status in the lung. Eur Respir J. 1998;12(1):82–8. doi: 10.1183/09031936.98.12010082. [DOI] [PubMed] [Google Scholar]
- 27.Scholma J, Slebos DJ, Boezen HM, van den Berg JW, van der Bij W, de Boer WJ, et al. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med. 2000;162(6):2221–5. doi: 10.1164/ajrccm.162.6.9911104. [DOI] [PubMed] [Google Scholar]
- 28.Slebos DJ, Postma DS, Koeter GH, Van Der Bij W, Boezen M, Kauffman HF. Bronchoalveolar lavage fluid characteristics in acute and chronic lung transplant rejection. J Heart Lung Transplant. 2004;23(5):532–40. doi: 10.1016/j.healun.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911–20. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 30.Vanaudenaerde BM, Wuyts WA, Geudens N, Nawrot TS, Vos R, Dupont LJ, et al. Broncho-alveolar lavage fluid recovery correlates with airway neutrophilia in lung transplant patients. Respir Med. 2008;102(3):339–47. doi: 10.1016/j.rmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Willems-Widyastuti A, Scheers H, Van Raemdonck DE, et al. Circulating and intrapulmonary C-reactive protein: a predictor of bronchiolitis obliterans syndrome and pulmonary allograft outcome. J Heart Lung Transplant. 2009;28(8):799–807. doi: 10.1016/j.healun.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Ward C, Snell GI, Zheng L, Orsida B, Whitford H, Williams TJ, et al. Endobronchial biopsy and bronchoalveolar lavage in stable lung transplant recipients and chronic rejection. Am J Respir Crit Care Med. 1998;158(1):84–91. doi: 10.1164/ajrccm.158.1.9707117. [DOI] [PubMed] [Google Scholar]
- 33.Ward C, Whitford H, Snell G, Bao H, Zheng L, Reid D, et al. Bronchoalveolar lavage macrophage and lymphocyte phenotypes in lung transplant recipients. J Heart Lung Transplant. 2001;20(10):1064–74. doi: 10.1016/s1053-2498(01)00319-9. [DOI] [PubMed] [Google Scholar]
- 34.Whitford HM, Orsida B, Pais M, Levvey B, Ward C, Williams TJ, et al. Features of bronchoalveolar lavage (BAL) in lung transplant recipients (LTR) who later develop bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2001;20(2):176. doi: 10.1016/s1053-2498(00)00355-7. [DOI] [PubMed] [Google Scholar]
- 35.Zheng L, Walters EH, Ward C, Wang N, Orsida B, Whitford H, et al. Airway neutrophilia in stable and bronchiolitis obliterans syndrome patients following lung transplantation. Thorax. 2000;55(1):53–9. doi: 10.1136/thorax.55.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng L, Whitford HM, Orsida B, Levvey BJ, Bailey M, Walters EH, et al. The dynamics and associations of airway neutrophilia post lung transplantation. Am J Transplant. 2006;6(3):599–608. doi: 10.1111/j.1600-6143.2006.01222.x. [DOI] [PubMed] [Google Scholar]
- 37.Reynaud-Gaubert M, Thomas P, Gregoire R, Badier M, Cau P, Sampol J, et al. Clinical utility of bronchoalveolar lavage cell phenotype analyses in the postoperative monitoring of lung transplant recipients. Eur J Cardiothorac Surg. 2002;21(1):60–6. doi: 10.1016/s1010-7940(01)01068-5. [DOI] [PubMed] [Google Scholar]
- 38.Elssner A, Vogelmeier C. The role of neutrophils in the pathogenesis of obliterative bronchiolitis after lung transplantation. Transpl Infect Dis. 2001;3(3):168–76. doi: 10.1034/j.1399-3062.2001.003003168.x. [DOI] [PubMed] [Google Scholar]
- 39.Mertens V, Blondeau K, Van Oudenhove L, Vanaudenaerde B, Vos R, Farre R, et al. Bile acids aspiration reduces survival in lung transplant recipients with BOS despite azithromycin. Am J Transplant. 2011;11(2):329–35. doi: 10.1111/j.1600-6143.2010.03380.x. [DOI] [PubMed] [Google Scholar]
- 40.Ohshimo S, Bonella F, Sommerwerck U, Teschler H, Kamler M, Jakob HG, et al. Comparison of serum KL-6 versus bronchoalveolar lavage neutrophilia for the diagnosis of bronchiolitis obliterans in lung transplantation. J Heart Lung Transplant. 2011;30(12):1374–80. doi: 10.1016/j.healun.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 42.Stovold R, Forrest IA, Corris PA, Murphy DM, Smith JA, Decalmer S, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175(12):1298–303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 43.Vanaudenaerde BM, Meyts I, Vos R, Geudens N, De Wever W, Verbeken EK, et al. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. Eur Respir J. 2008;32(4):832–43. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]
- 44.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703–11. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhorade SM, Chen H, Molinero L, Liao C, Garrity ER, Vigneswaran WT, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation. 2010;90(5):540–6. doi: 10.1097/TP.0b013e3181e8dabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregson AL, Hoji A, Palchevskiy V, Hu S, Weigt SS, Liao E, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA- T regulatory cells. PLoS One. 2010;5(6):e11354. doi: 10.1371/journal.pone.0011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodge G, Hodge S, Chambers D, Reynolds PN, Holmes M. Bronchiolitis obliterans syndrome is associated with absence of suppression of peripheral blood Th1 proinflammatory cytokines. Transplantation. 2009;88(2):211–8. doi: 10.1097/TP.0b013e3181ac170f. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead BF, Stoehr C, Finkle C, Patterson G, Theodore J, Clayberger C, et al. Analysis of bronchoalveolar lavage from human lung transplant recipients by flow cytometry. Respir Med. 1995;89(1):27–34. doi: 10.1016/0954-6111(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 49.Whitford H, Walters EH, Levvey B, Kotsimbos T, Orsida B, Ward C, et al. Addition of inhaled corticosteroids to systemic immunosuppression after lung transplantation: a double-blind, placebo-controlled trial. Transplantation. 2002;73(11):1793–9. doi: 10.1097/00007890-200206150-00016. [DOI] [PubMed] [Google Scholar]
- 50.Zheng L, Orsida B, Whitford H, Levvey B, Ward C, Walters EH, et al. Longitudinal comparisons of lymphocytes and subtypes between airway wall and bronchoalveolar lavage after human lung transplantation. Transplantation. 2005;80(2):185–92. doi: 10.1097/01.tp.0000165091.31541.23. [DOI] [PubMed] [Google Scholar]
- 51.Badri L, Murray S, Liu LX, Walker NM, Flint A, Wadhwa A, et al. Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2011;183(8):1062–70. doi: 10.1164/rccm.201005-0742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salama M, Andrukhova O, Jaksch P, Taghavi S, Kelpetko W, Dekan G, et al. Endothelin-1 governs proliferation and migration of bronchoalveolar lavage-derived lung mesenchymal stem cells in bronchiolitis obliterans syndrome. Transplantation. 2011;92(2):155–62. doi: 10.1097/TP.0b013e318222c9ea. [DOI] [PubMed] [Google Scholar]
- 53.Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach PH, et al. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol. 2011;178(6):2461–9. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, Lama VN. Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med. 2012;185(1):77–84. doi: 10.1164/rccm.201105-0834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, et al. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182(7):4423–31. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meloni F, Vitulo P, Cascina A, Oggionni T, Bulgheroni A, Paschetto E, et al. Bronchoalveolar lavage cytokine profile in a cohort of lung transplant recipients: a predictive role of interleukin-12 with respect to onset of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2004;23(9):1053–60. doi: 10.1016/j.healun.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez AM, Nunley DR, Rojas M, Roman J. Activation of Tissue Remodeling Precedes Obliterative Bronchiolitis in Lung Transplant Recipients. Biomark Insights. 2008;3:351–9. doi: 10.4137/bmi.s686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verleden SE, Vandooren J, Vos R, Willems S, Dupont LJ, Verleden GM, et al. Azithromycin decreases MMP-9 expression in the airways of lung transplant recipients. Transpl Immunol. 2011;25(2–3):159–62. doi: 10.1016/j.trim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Verleden SE, Vos R, Mertens V, Willems-Widyastuti A, De Vleeschauwer SI, Dupont LJ, et al. Heterogeneity of chronic lung allograft dysfunction: insights from protein expression in broncho alveolar lavage. J Heart Lung Transplant. 2011;30(6):667–73. doi: 10.1016/j.healun.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91(9):3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, van Damme J, et al. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95(3):1370–6. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Berlin A, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108(4):547–56. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neujahr DC, Perez SD, Mohammed A, Ulukpo O, Lawrence EC, Fernandez F, et al. Cumulative exposure to gamma interferon-dependent chemokines CXCL9 and CXCL10 correlates with worse outcome after lung transplant. Am J Transplant. 2012;12(2):438–46. doi: 10.1111/j.1600-6143.2011.03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salama M, Jaksch P, Andrukhova O, Taghavi S, Klepetko W, Aharinejad S. Endothelin-1 is a useful biomarker for early detection of bronchiolitis obliterans in lung transplant recipients. J Thorac Cardiovasc Surg. 2010;140(6):1422–7. doi: 10.1016/j.jtcvs.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 65.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169(2):1037–49. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 66.Nelsestuen GL, Martinez MB, Hertz MI, Savik K, Wendt CH. Proteomic identification of human neutrophil alpha-defensins in chronic lung allograft rejection. Proteomics. 2005;5(6):1705–13. doi: 10.1002/pmic.200401036. [DOI] [PubMed] [Google Scholar]
- 67.Saini D, Angaswamy N, Tiriveedhi V, Fukami N, Ramachandran S, Hachem R, et al. Synergistic effect of antibodies to human leukocyte antigens and defensins in pathogenesis of bronchiolitis obliterans syndrome after human lung transplantation. J Heart Lung Transplant. 2010;29(12):1330–6. doi: 10.1016/j.healun.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf T, Oumeraci T, Gottlieb J, Pich A, Brors B, Eils R, et al. Proteomic bronchiolitis obliterans syndrome risk monitoring in lung transplant recipients. Transplantation. 2011;92(4):477–85. doi: 10.1097/TP.0b013e318224c109. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics. 2006;6(3):1001–10. doi: 10.1002/pmic.200500105. [DOI] [PubMed] [Google Scholar]
- 70.Murphy CJ, Foster BA, Mannis MJ, Selsted ME, Reid TW. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155(2):408–13. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Raghunath M, Tan D, Lareu RR, Chen Z, Beuerman RW. Defensins HNP1 and HBD2 stimulation of wound-associated responses in human conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2006;47(9):3811–9. doi: 10.1167/iovs.05-1360. [DOI] [PubMed] [Google Scholar]
- 72.Oono T, Shirafuji Y, Huh WK, Akiyama H, Iwatsuki K. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch Dermatol Res. 2002;294(4):185–9. doi: 10.1007/s00403-002-0310-6. [DOI] [PubMed] [Google Scholar]
- 73.Riise GC, Ericson P, Bozinovski S, Yoshihara S, Anderson GP, Linden A. Increased net gelatinase but not serine protease activity in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2010;29(7):800–7. doi: 10.1016/j.healun.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 74.D’Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930–8. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 75.Meloni F, Salvini R, Bardoni AM, Passadore I, Solari N, Vitulo P, et al. Bronchoalveolar lavage fluid proteome in bronchiolitis obliterans syndrome: possible role for surfactant protein A in disease onset. J Heart Lung Transplant. 2007;26(11):1135–43. doi: 10.1016/j.healun.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Kosanam H, Sato M, Batruch I, Smith C, Keshavjee S, Liu M, et al. Differential proteomic analysis of bronchoalveolar lavage fluid from lung transplant patients with and without chronic graft dysfunction. Clin Biochem. 2012;45(3):223–30. doi: 10.1016/j.clinbiochem.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Scott JP, Holt DW, Wallwork J. Neutrophil elastase and obliterative bronchiolitis. Transpl Int. 1994;7:S402–3. doi: 10.1111/j.1432-2277.1994.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 78.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 79.Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2(1):10–9. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 81.Parks WC. Matrix metalloproteinases in lung repair. Eur Respir J. 2003;44:36s–8s. doi: 10.1183/09031936.03.00001203. [DOI] [PubMed] [Google Scholar]
- 82.Taghavi S, Krenn K, Jaksch P, Klepetko W, Aharinejad S. Broncho-alveolar lavage matrix metalloproteases as a sensitive measure of bronchiolitis obliterans. Am J Transplant. 2005;5(6):1548–52. doi: 10.1111/j.1600-6143.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- 83.Madill J, Aghdassi E, Arendt B, Hartman-Craven B, Gutierrez C, Chow CW, et al. Lung transplantation: does oxidative stress contribute to the development of bronchiolitis obliterans syndrome? Transplant Rev. 2009;23(2):103–10. doi: 10.1016/j.trre.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Madill J, Aghdassi E, Arendt BM, Gutierrez C, Singer L, Chow CW, et al. Oxidative stress and nutritional intakes in lung patients with bronchiolitis obliterans syndrome. Transplant Proc. 2009;41(9):3838–44. doi: 10.1016/j.transproceed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30(7):735–42. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 86.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–14. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 87.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115(5):1150–62. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magnan A, Mege JL, Reynaud M, Thomas P, Capo C, Garbe L, et al. Monitoring of alveolar macrophage production of tumor necrosis factor-alpha and interleukin-6 in lung transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1994;150(3):684–9. doi: 10.1164/ajrccm.150.3.8087338. [DOI] [PubMed] [Google Scholar]
- 89.Meyer KC, Nunley DR, Dauber JH, Iacono AT, Keenan RJ, Cornwell RD, et al. Neutrophils, unopposed neutrophil elastase, and alpha1-antiprotease defenses following human lung transplantation. Am J Respir Crit Care Med. 2001;164(1):97–102. doi: 10.1164/ajrccm.164.1.2006096. [DOI] [PubMed] [Google Scholar]
- 90.Langenbach SY, Zheng L, McWilliams T, Levvey B, Orsida B, Bailey M, et al. Airway vascular changes after lung transplant: potential contribution to the pathophysiology of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2005;24(10):1550–6. doi: 10.1016/j.healun.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Ross DJ, Cole AM, Yoshioka D, Park AK, Belperio JA, Laks H, et al. Increased bronchoalveolar lavage human beta-defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;78(8):1222–4. doi: 10.1097/01.tp.0000137265.18491.75. [DOI] [PubMed] [Google Scholar]
- 92.Reinsmoen NL, Bolman RM, Savik K, Butters K, Hertz MI. Are multiple immunopathogenetic events occurring during the development of obliterative bronchiolitis and acute rejection? Transplantation. 1993;55(5):1040–4. doi: 10.1097/00007890-199305000-00017. [DOI] [PubMed] [Google Scholar]
- 93.Bargagli E, Madioni C, Prasse A, Fossi A, Filippi R, Bianchi N, et al. Eosinophilic cationic protein in bronchoalveolar lavage fluid of lung transplant patients. Clin Chem Lab Med. 2008;46(4):563–4. doi: 10.1515/CCLM.2008.097. [DOI] [PubMed] [Google Scholar]
- 94.O’Connell PJ, Mba-Jonas A, Leverson GE, Heisey DM, Meyer KC, Love RB, et al. Stable lung allograft outcome correlates with the presence of intragraft donor-derived leukocytes. Transplantation. 1998;66(9):1167–74. doi: 10.1097/00007890-199811150-00010. [DOI] [PubMed] [Google Scholar]
- 95.Reinsmoen NL, Bolman RM, Savik K, Butters K, Hertz M. Differentiation of class I- and class II-directed donor-specific alloreactivity in bronchoalveolar lavage lymphocytes from lung transplant recipients. Transplantation. 1992;53(1):181–9. doi: 10.1097/00007890-199201000-00036. [DOI] [PubMed] [Google Scholar]
- 96.Carroll KE, Dean MM, Heatley SL, Meehan AC, Mifsud NA, Kotsimbos TC, et al. High levels of mannose-binding lectin are associated with poor outcomes after lung transplantation. Transplantation. 2011;91(9):1044–9. doi: 10.1097/TP.0b013e318212c7d6. [DOI] [PubMed] [Google Scholar]
- 97.Hodge S, Dean M, Hodge G, Holmes M, Reynolds PN. Decreased efferocytosis and mannose binding lectin in the airway in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2011;30(5):589–95. doi: 10.1016/j.healun.2011.01.710. [DOI] [PubMed] [Google Scholar]
- 98.Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Willems-Widyastuti A, Dupont LJ, Van Raemdonck DE, et al. C-reactive protein in bronchoalveolar lavage fluid is associated with markers of airway inflammation after lung transplantation. Transplant Proc. 2009;41(8):3409–13. doi: 10.1016/j.transproceed.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 99.Sundaresan S, Alevy YG, Steward N, Tucker J, Trulock EP, Cooper JD, et al. Cytokine gene transcripts for tumor necrosis factor-alpha, interleukin-2, and interferon-gamma in human pulmonary allografts. J Heart Lung Transplant. 1995;14(3):512–8. [PubMed] [Google Scholar]
- 100.Keane MP, Gomperts BN, Weigt S, Xue YY, Burdick MD, Nakamura H, et al. IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J Immunol. 2007;178(1):511–9. doi: 10.4049/jimmunol.178.1.511. [DOI] [PubMed] [Google Scholar]
- 101.Sandmeier P, Speich R, Grebski E, Vogt P, Russi EW, Weder W, et al. Iron accumulation in lung allografts is associated with acute rejection but not with adverse outcome. Chest. 2005;128(3):1379–84. doi: 10.1378/chest.128.3.1379. [DOI] [PubMed] [Google Scholar]
- 102.Charpin JM, Valcke J, Kettaneh L, Epardeau B, Stern M, Israel-Biet D. Peaks of transforming growth factor-beta mRNA in alveolar cells of lung transplant recipients as an early marker of chronic rejection. Transplantation. 1998;65(5):752–5. doi: 10.1097/00007890-199803150-00027. [DOI] [PubMed] [Google Scholar]
- 103.Bergmann M, Tiroke A, Schafer H, Barth J, Haverich A. Gene expression of profibrotic mediators in bronchiolitis obliterans syndrome after lung transplantation. Scand Cardiovasc J. 1998;32(2):97–103. doi: 10.1080/14017439850140247. [DOI] [PubMed] [Google Scholar]
- 104.Hodge S, Holmes M, Banerjee B, Musk M, Kicic A, Waterer G, et al. Posttransplant bronchiolitis obliterans syndrome is associated with bronchial epithelial to mesenchymal transition. Am J Transplant. 2009 Apr;9(4):727–33. doi: 10.1111/j.1600-6143.2009.02558.x. [DOI] [PubMed] [Google Scholar]
- 105.Jonosono M, Fang KC, Keith FM, Turck CW, Blanc PD, Hall TS, et al. Measurement of fibroblast proliferative activity in bronchoalveolar lavage fluid in the analysis of obliterative bronchiolitis among lung transplant recipients. J Heart Lung Transplant. 1999;18(10):972–85. doi: 10.1016/s1053-2498(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 106.Meyer KC, Cardoni AL, Xiang Z, Cornwell RD, Love RB. Vascular endothelial growth factor in human lung transplantation. Chest. 2001;119(1):137–43. doi: 10.1378/chest.119.1.137. [DOI] [PubMed] [Google Scholar]
- 107.Meloni F, Solari N, Miserere S, Morosini M, Cascina A, Klersy C, et al. Chemokine redundancy in BOS pathogenesis. A possible role also for the CC chemokines: MIP3-beta, MIP3-alpha, MDC and their specific receptors. Transpl Immunol. 2008;18(3):275–80. doi: 10.1016/j.trim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Wood KL, Nunley DR, Moffatt-Bruce S, Pope-Harman A, Huang Q, Shamo EN, et al. The role of heat shock protein 27 in bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(7):786–91. doi: 10.1016/j.healun.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Charpin JM, Stern M, Grenet D, Israel-Biet D. Insulinlike growth factor-1 in lung transplants with obliterative bronchiolitis. Am J Respir Crit Care Med. 2000;161(6):1991–8. doi: 10.1164/ajrccm.161.6.9905049. [DOI] [PubMed] [Google Scholar]
- 110.Hodge S, Hodge G, Ahern J, Liew CL, Hopkins P, Chambers DC, et al. Increased levels of T cell granzyme b in bronchiolitis obliterans syndrome are not suppressed adequately by current immunosuppressive regimens. Clin Exp Immunol. 2009;158(2):230–6. doi: 10.1111/j.1365-2249.2009.04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Andrade JA, Christie JD, Alexander CB, Young KR, McGiffin DC, Zorn GL, et al. Association of reactive nitrogen species metabolites, myeloperoxidase, and airway inflammation in lung transplants. J Investig Med. 2001;49(2):166–72. doi: 10.2310/6650.2001.34043. [DOI] [PubMed] [Google Scholar]
- 112.Courtade M, Carrera G, Paternain JL, Martel S, Carre PC, Folch J, et al. Metallothionein expression in human lung and its varying levels after lung transplantation. Toulouse Lung Transplantation Group. Chest. 1998;113(2):371–8. doi: 10.1378/chest.113.2.371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.