Abstract
Early epithelial injury after lung transplantation may contribute to development of bronchiolitis obliterans syndrome (BOS). We evaluated the relationship between early post-operative soluble receptor for advanced glycation end product (sRAGE) levels, a marker of type I alveolar cell injury, and BOS. We performed a cohort study of 106 lung transplant recipients between 2002 and 2006 at the University of Pennsylvania with follow-up through 2010. Plasma sRAGE was measured 6 and 24 hours after transplantation. Cox proportional hazards models were used to evaluate the association between sRAGE and time to BOS, defined according to ISHLT guidelines. Sixty (57%) subjects developed BOS. The average time to BOS was 3.4 years. sRAGE levels measured at 6 hours (HR per SD of sRAGE: 1.69, 95% CI: 1.11, 2.57, p=0.02) and 24 hours (HR per SD of sRAGE: 1.74, 95% CI: 1.14, 2.65, p=0.01) were associated with an increased hazard of BOS. Multivariable Cox regression indicated this relationship was independent of potential confounders. Elevated plasma sRAGE levels measured in the immediate post-operative period are associated with the development of BOS. Early epithelial injury after transplantation may contribute to the development of fibrosis in BOS.
Keywords: Bronchiolitis Obliterans Syndrome, Lung transplantation, Biomarkers, RAGE
Introduction
Bronchiolitis obliterans syndrome (BOS) following lung transplantation is a form of chronic lung allograft dysfunction characterized by progressive airflow obstruction (1). BOS is common, affecting almost 50% of recipients at 5 years (2). It is the leading cause of death after the first year of lung transplantation and it is a major contributor to the morbidity of lung transplant recipients (2). Several risk factors for BOS have been identified, including CMV infection (3), acute cellular rejection (ACR) (4), and primary graft dysfunction (PGD), a form of acute lung injury occurring within 72 hours of transplantation (5).
While mechanisms underlying the development of BOS have not been completely elucidated, BOS may be related to either repeated episodes of epithelial injury or aberrant repair mechanisms (6). One hypothesis to explain the relationship between PGD and BOS is that the initial injury from PGD triggers an exuberant but dysregulated epithelial repair process. Persistence of the aberrant repair process in response to additional insults to the graft, such as viral infections and episodes of ACR, may eventually lead to BOS. This hypothesis is supported by recent work demonstrating aberrant epithelial cellular composition after an external injury in an animal model of BOS (7).
Prior studies of BOS have primarily focused on later risk factors, either months after transplantation or at the time of diagnosis of BOS (8). However, it is not clear when the epithelial injury leading to BOS begins, and the period immediately after transplantation may be important due to the inflammatory response related to ischemia reperfusion injury (IRI) (9). Although IRI is thought to be a major contributor to PGD (10), it may also be associated with sub-clinical epithelial injury independent of clinically significant PGD, triggering an abnormal epithelial repair process that leads to BOS.
The Receptor for Advanced Glycation End products (RAGE) is a multi-ligand pattern recognition receptor implicated in acute and chronic inflammation (11). We have previously demonstrated an association with increased levels of soluble receptor for advanced glycation end products (sRAGE), the extracellular ligand-binding domain, with PGD after lung transplantation (12). sRAGE may have roles in endothelial injury and innate immune signaling. However, RAGE is highly expressed in epithelial cells in the lung (13) and is a recognized marker of type I alveolar epithelial injury in both animal models of acute lung injury (ALI) and human studies of ALI (14, 15). In physiologic states, sRAGE is important in modulating adhesion of alveolar epithelial cells to the basement membrane (16). A role for sRAGE in fibrosis, during which this relationship is disrupted, has been postulated (18). Early changes in sRAGE may be a signal of early epithelial damage, as well as a marker of later lung injury, manifesting as BOS.
In this study we aimed to evaluate the association between plasma sRAGE measured 6 and 24 hours after transplantation with subsequent development of BOS. We hypothesized that plasma sRAGE levels would be associated with the development of BOS, independent of potentially confounding variables.
Methods
Study Population
The study cohort consisted of 106 subjects who received a lung transplant at the University of Pennsylvania between October 2002 and March 2006 and had a plasma sRAGE measurement 6 or 24 hours after transplantation. Some of the subjects in the present study were enrolled in a prior multi-center cohort study of plasma sRAGE concentration with PGD (12). Demographic and operative data were collected prospectively for all subjects as described in detail elsewhere (17). Longitudinal variables beyond the early transplant period, including spirometric data for the evaluation of BOS, were collected via chart review. Longitudinal follow-up data were collected until December 31, 2010. The Institutional Review Board at the University of Pennsylvania approved this study. Informed consent was obtained from each subject enrolled in the cohort.
Clinical Outcomes and Covariates
The primary outcome was the development of BOS stage 1 or greater, defined by a decrease in FEV1 by 20% or more from baseline (defined as the average of the 2 highest FEV1 measurements after transplant), persistent over at least 3 weeks (1). We used a statistical algorithm to define BOS with subsequent manual review of the spirometry to ensure there was a sustained and irreversible drop in FEV1. We excluded subjects who met the criteria for BOS within 180 days of transplantation to avoid misclassification from early airway complications and those who died within 180 days of transplant (n=17) to avoid biases due to competing risks. We took into account other factors that may have contributed to the development of BOS in our analysis, including ACR and GERD. ACR was defined according to the International Society for Heart and Lung Transplantation criteria as perivascular or peribronchial mononuclear inflammation on clinical pathology reading (18). For the purposes of this study, we analyzed ACR (grades 0–4) as a time-varying covariate, including all biopsies obtained after transplantation. Gastro-esophageal reflux (GERD) was defined by esophagram with barium swallow indicating reflux, visual or biopsy proven changes on esophagogastroduodenoscopy consistent with reflux, or having undergone a Nissen fundoplication after transplantation. GERD evaluations were performed after transplantation at the discretion of the treating physician. If subjects did not have any diagnostic evaluation, they were considered not to have GERD for the purposes of this study. We adjusted for peak FEV1 based on evidence indicating those with a supranormal peak FEV1 after transplantation have a lower risk of BOS (19). Grade 3 PGD at 72 hours, ACR, peak FEV1, cardiopulmonary bypass use (CPB), recipient race, diagnosis, gender, and age, donor race, gender, and age, volume of blood transfusion during the first 24 hours after transplantation (PRBC), transplant type (bilateral vs. single), pulmonary artery systolic pressure (PASP) at the time of transplantation, and GERD were evaluated as possible confounders based on a hypothesized association with sRAGE and BOS. We generated a multivariable model including grade 3 PGD at 72 hours, ACR, peak FEV1, diagnosis, and transplant type, regardless of whether they met the statistical definition for confounding, as these variables might be important in the relationship between sRAGE and BOS.
Measurement of sRAGE
Plasma samples were obtained in citrated tubes 6 and 24 hours after reperfusion of the lung allograft(s). Samples were centrifuged within 60 minutes of collection and stored at −80°C. Plasma levels of sRAGE were measured by sandwich ELISA (R&D, Minneapolis, MN). The intra-assay coefficient of variation was 6%. We created a standardized sRAGE variable by dividing each plasma sRAGE measurement by the standard deviation of sRAGE from the cohort and used this in the analyses.
Statistical Analysis
Descriptive statistics were generated using chi square tests for categorical variables and two-tailed Student t-tests for continuous variables. Analysis of the relationship between sRAGE levels and time to BOS was performed using Cox regression models. In secondary analysis, we accounted for death as a competing risk to ensure there was no change in the association between sRAGE and BOS when considering subjects who died before development of BOS (20). We evaluated sRAGE both as a continuous variable and as a categorical variable, using quartiles. We used a bivariate confounder selection strategy, and confounding was defined as a greater than 20% change in hazards ratio between sRAGE and BOS upon addition of a covariate to the model (21). Longitudinal analyses using generalized estimating equations were performed to explore a relationship in trend of FEV1 over time with quartiles of sRAGE. In sensitivity analyses, we analyzed the multivariable model without peak FEV1, as this variable is encompassed in the definition of BOS (1). For all of the statistical analyses, a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA 12.0 (STATA Corp., College Station, TX).
Results
There were 106 subjects in the cohort, 60 (57%, 95% CI: 47%, 66%) developed BOS. All subjects had a 24-hour sRAGE measurement and 94 subjects had a 6-hour sRAGE measurement. The average time to development of BOS was 3.4 ± 1.8 years. Subjects with BOS were older; more frequently had COPD as their pre-transplant diagnosis, had a higher frequency of GERD, and higher mean levels of sRAGE (Table 1).
Table 1.
Demographic characteristics of the study population. P values were calculated using t-test or chi square test. BOS: bronchiolitis obliterans syndrome. GERD: Gastro-esophageal reflux disease.
| BOS (n=60) | No BOS (n=46) | p value | |
|---|---|---|---|
| Time to BOS (yrs) | 3.4 ± 1.8 | ||
| Age | 57 ± 7 | 54 ± 11 | 0.06 |
| Bilateral transplant | 22 (51%) | 21 (49%) | 0.14 |
| Race | |||
| Caucasian | 53 (88%) | 41 (89%) | 0.63 |
| African American | 6 (10%) | 3 (7%) | |
| Hispanic | 1 (2%) | 1 (2%) | |
| Other | 0 (0%) | 1 (2%) | |
| Female Gender | 25 (42%) | 13 (28%) | 0.12 |
| Native Disease | 0.19 | ||
| COPD | 42 (70%) | 23 (50%) | |
| CF | 2 (3%) | 4 (9%) | |
| IPF | 12 (20%) | 16 (34%) | |
| PPH | 0 (0%) | 1 (2%) | |
| Sarcoid | 2 (3%) | 0 (0%) | |
| CHD | 1 (2%) | 2 (4%) | |
| Other | 1 (1%) | 0 (0%) | |
| Grade 3 PGD at 72 hrs | 4 (7%) | 3 (6%) | 0.98 |
| AR grade 2 or higher (n=104) | 39 (65%) | 23 (53%) | 0.19 |
| GERD (999((n=104) | 19 (32%) | 4 (9%) | <0.01 |
| sRAGE at 6 hours | 9112 ± 11405 | 6484 ± 6954 | 0.20 |
| sRAGE at 24 hours | 5249 ± 7213 | 2897 ± 2576 | 0.04 |
Plasma sRAGE levels at 6 hours and 24 hours were associated with an increased hazard of BOS (HR per SD of sRAGE at 6 hours: 1.71, 95% CI: 1.12, 2.62, p=0.02; HR per SD of sRAGE at 24 hours: 1.75, 95% CI: 1.14, 2.68. p=0.01). When analyzed by quartile, sRAGE at 6 hours was not associated with time to BOS (log rank p=0.76), however, sRAGE at 24 hours was (log rank p=0.01) (Figure 1). Measured at 24 hours, the highest quartile (quartile 4) of sRAGE had the greatest hazard of BOS (HR 3.09, 95% CI: 1.49, 6.43, p<0.01) when compared to the lowest quartile (quartile 1). We were unable to detect a difference between quartile 2 (HR: 1.59, 95% CI: 0.75, 3.34, p=0.23) or quartile 3 (HR: 1.27, 95% CI: 0.59, 2.76, p=0.54) when compared to quartile 1.
Figure 1.
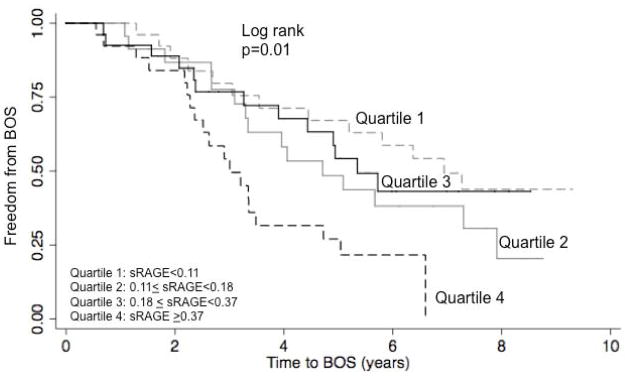
Kaplan Meier plot describing the association of quartiles of standardized sRAGE values with BOS. Quartile 1 is the lowest quartile of sRAGE and quartile 4 is the highest. p value generated from log rank test.
Multivariable model
The association between sRAGE and time to BOS remained significant after bivariable adjustment for all evaluated potential confounders, including cardiopulmonary bypass use and volume of blood transfusion during the first 24 hours (Table 2). Our multivariable model, after adjusting for hypothesized confounders, demonstrated a HR for time to BOS of 1.67 (95% CI: 1.51, 1.84, p<0.01) for each SD of SRAGE measured at 6 hours. Based on the results of our bivariable model, we added PASP and volume of blood transfusion to the 24-hour analysis. The HR for time to BOS of 2.80 (95% CI: 2.39, 3.28, p<0.01) for each SD of sRAGE measured at 24 hours (Table 2). Exclusion of peak FEV1 from the model did not significantly change the analysis (6 hr HR: 1.73, 95% CI: 1.57, 1.91, p<0.01), 24 hr HR: 2.70 (95% CI: 2.34, 3.12, p<0.01).
Table 2.
Association of plasma sRAGE levels at 6 and 24 hours with time to BOS. PGD: Primary graft dysfunction, ACR: acute cellular rejection, GERD: Gastro-esophageal reflux disease, PASP: Pulmonary artery systolic pressure
| Variable | Hazard ratio (95% CI) sRAGE at 6 hours (n=94) | p value | Hazard Ratio (95% CI) sRAGE at 24 hours (n=106) | p value |
|---|---|---|---|---|
| sRAGE per 1 SD | 1.71 (1.12, 2.62) | 0.02 | 1.75 (1.14, 2.68) | 0.01 |
| Adjusted for | ||||
| Grade 3 PGD at 72 hours | 1.69 (1.11, 2.57) | 0.01 | 2.23 (1.24, 4.01) | 0.01 |
| ACR (per increase in grade by 1) | 1.76 (1.61, 1.93) | <0.01 | 1.68 (1.54, 1.84) | <0.01 |
| Peak FEV1 | 1.57 (1.03, 2.38) | 0.03 | 1.89 (1.24, 2.90) | <0.01 |
| CBP | 1.69 (1.11, 2.58) | 0.02 | 2.08 (1.31, 3.32) | <0.01 |
| Recipient Race | 1.70 (1.11, 2.60) | 0.01 | 1.81 (1.14, 2.88) | 0.01 |
| Recipient Diagnosis | 1.65 (1.08, 2.53) | 0.02 | 1.78 (1.16, 2.73) | 0.01 |
| Recipient Sex | 1.76 (1.15, 2.67) | 0.01 | 1.88 (1.22, 2.92) | 0.01 |
| Recipient Age | 1.70 (1.11, 2.61) | 0.01 | 1.73 (1.14, 2.63) | 0.01 |
| Donor Sex | 1.66 (1.09, 2.54) | 0.02 | 1.71 (1.12, 2.61) | 0.01 |
| Donor Age | 1.72 (1.12, 2.64) | 0.01 | 1.75 (1.14, 2.67) | 0.01 |
| Donor Race | 1.60 (0.98, 2.62) | 0.06 | 1.78 (1.15, 2.75) | 0.01 |
| Blood (ml) during first 24 hrs | 2.02 (1.23, 3.34) | 0.01 | 2.35 (1.41, 3.90) | <0.01 |
| Transplant type | 1.66 (1.09, 2.54) | 0.02 | 2.15 (1.36, 3.41) | <0.01 |
| PASP at transplantation | 1.73 (1.13, 2.64) | 0.01 | 2.16 (1.29, 3.60) | <0.01 |
| GERD | 1.74 (1.13, 2.67) | 0.01 | 1.61 (1.04. 2.49) | 0.03 |
| Multivariable model* | 1.67 (1.51, 1.84) | <0.01 | 2.80 (2.39, 3.28) | <0.01 |
adjusted for grade 3 PGD at 72 hrs, diagnosis, ACR, transplant type, peak FEV1. 24 hour model also includes PASP and blood transfusion.
Consistent with our previous findings, plasma sRAGE measured at 24 hours was also associated with grade 3 PGD at 72 hours (OR for each SD of sRAGE at 24hrs: 5.56, 95% CI: 1.82, 16.98 p<0.01). Subjects in the highest quartile of sRAGE at 24 hours had a greater frequency of grade 3 PGD at 72 hours compared to subjects in all other quartiles (15% vs. 4%, p=0.04). Higher sRAGE levels were associated with increasing grade of PGD (p for trend at 6 hours=0.01, p for trend at 24 hours<0.01) (Supplemental Figure 1). We evaluated the relationship of sRAGE at 24 hours and BOS while adjusting for grade 3 PGD at 72 hours; and the association between sRAGE and BOS remained significant on adjustment (HR 2.23, 95% CI: 1.24, 4.01, p=0.01) (Table 2).
In analyses accounting for death as a competing risk, the association between sRAGE and BOS was unchanged at 24 hours (HR: 1.82, 95% CI: 1.18, 2.79, p=0.01), however, the association between sRAGE at 6 hours and BOS was slightly attenuated (HR: 1.67, 95% CI: 0.95, 3.00, p=0.08). In GEE analyses focusing on the relationship of sRAGE and changes in FEV1, the average FEV1 using all measurements after transplantation in quartile 4 of sRAGE measured at 6hours was 177cc smaller (p<0.01) than quartile 1, and 5cc smaller in quartile 4 of sRAGE measured at 24 hours (p=0.90).
Discussion
In this study, we demonstrated an association between plasma sRAGE levels measured at both 6 and 24 hours post-operatively with long-term risk for BOS. These findings suggest that early epithelial injury (within 24 hours) after transplantation may be important in later development of BOS. The relationship between sRAGE and time to BOS remained significant despite adjustment for multiple potential confounders including ACR, cardiopulmonary bypass, and blood transfusion.
RAGE is a pattern recognition receptor that is highly expressed in the lung during physiologic states (13). It is thought to be a biologic marker of type I alveolar cell injury, and is important in lung homeostasis through regulation of lung adhesion of alveolar epithelial cells to the basement membrane (13). Prior research has demonstrated a role for RAGE in chronic fibrotic lung diseases, such as IPF (22, 23). Over-expression of RAGE has been demonstrated in lungs from subjects with IPF, particularly in the fibroblastic foci (24). Although there is also evidence indicating RAGE may have a protective effect against fibrosis (25), it may be that circulating plasma sRAGE is indicative of decreased RAGE in the epithelium in fibrotic disease. In this study, we found subjects with BOS had higher levels of sRAGE than those without, and higher levels than reported levels in healthy controls (26), adding to the literature supporting the role of sRAGE as a marker for the development of lung fibrosis and demonstrating the importance of early injury in long-term outcomes.
As we have previously published, sRAGE levels are associated with PGD (12), and others have demonstrated an association between RAGE in donor BAL fluid with PGD (27). In this study, we have demonstrated an association between sRAGE levels early after transplantation and BOS. Although the association between sRAGE and BOS was independent of grade 3 PGD, sRAGE may be a more sensitive marker of early epithelial injury than the clinical measures currently used to detect PGD. Therefore, the association between sRAGE and BOS may be useful in further clarifying the association between early sub-clinical epithelial injury and clinically apparent PGD with BOS.
If sRAGE is a mediator of the development of BOS, the mechanism may be via epigenetic changes occurring during ischemia reperfusion injury or clinically apparent PGD after lung transplantation leading to persistent changes in the RAGE pathway. Epigenetic changes have been demonstrated in a model of kidney transplant where DNA methylation after ischemia reperfusion injury persisted for up to 6 months (28). This type of change in regulation of RAGE isoforms may lead to up-regulation of the innate immune pathway leading to chronic inflammation and fibrosis (29). Further studies examining RAGE in bronchoalveolar lavage fluid at the time of transplant, genetic modifications of RAGE pre and post-transplant, and longitudinal measurements of sRAGE at later timepoints will be important in clarifying whether the association between sRAGE is a mediator of the development of BOS and important in the link between PGD and BOS, or a marker of another underlying process.
The association between sRAGE at 6 hours and BOS was slightly attenuated when accounting for death as a competing risk. This may be because we excluded all early deaths, had limited power because we had fewer subjects at the 6-hour timepoint, or that the relationship between sRAGE and BOS was truly confounded by death. Validation of this timepoint in a large sample size is necessary.
There are several limitations to this study. We did not measure sRAGE in plasma at later timepoints, therefore we cannot comment on changes in sRAGE levels over time. Not every subject in our cohort was uniformly screened for GERD, however, if they were not evaluated, it seems unlikely they had symptomatic GERD. This is a single-center study and may not be generalizable to other centers. As no one in this cohort had documented CMV pneumonitis; we were unable to evaluate the effect of CMV in mediating or confounding the relationship between sRAGE and BOS. This study focused only on the BOS phenotype and did not account for other types of chronic allograft dysfunction, such as restrictive allograft syndrome (RAS) (30). Finally, while we did not have a committee to adjudicate BOS, we took into account factors, including ACR and GERD that may have contributed to the development of BOS.
In conclusion, early plasma sRAGE levels are associated with increased hazard of BOS. Early epithelial injury, either sub-clinical or manifesting as concurrent PGD, may be a risk factor for later BOS. Our study prioritizes a focus on epithelial injury and RAGE for longitudinal studies aimed at understanding the link between early lung injury and BOS.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health (Grants: HL081619, HL087115, HL096845, HL088263, HL103836, K24HL103844)
Abbreviations
- BOS
Bronchiolitis Obliterans Syndrome
- PGD
Primary Graft Dysfunction
- sRAGE
Soluble Receptor for Advanced Glycation End-Products
- IPF
Idiopathic Pulmonary Fibrosis
- IRI
Ischemia Reperfusion injury
- ACR
Acute Cellular Rejection
Footnotes
Disclosures:
Drs. Shah, Bellamy, Lee, Diamond, Cantu, Mangalmurti, Kawut, Ware and Christie have nothing to disclose.
Author Contributions:
Dr. Shah conducted the statistical analysis and primarily wrote the manuscript. Dr. Bellamy assisted with the statistical analysis. Drs. Lee, Diamond, Cantu, Mangalmurti, and Kawut were involved in subject recruitment, analysis of the data, and edited the manuscript. Dr. Ware performed plasma sRAGE measurements. Dr. Christie supervised the data collection and the statistical analyses, assisted with interpretation of results, provided funding and edited the manuscript.
References
- 1.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002 Mar;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 2.Hertz MI, Aurora P, Benden C, Christie JD, Dobbels F, Edwards LB, et al. Scientific Registry of the International Society forHeart and Lung Transplantation: introduction to the 2011 annual reports. J Heart Lung Transplant. 2011 Oct;30(10):1071–7. doi: 10.1016/j.healun.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010 Jun 15;181(12):1391–6. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton CM, Iversen M, Carlsen J, Mortensen J, Andersen CB, Steinbruchel D, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant. 2009 Sep;28(9):888–93. doi: 10.1016/j.healun.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007 Mar 1;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 6.Knoop C, Estenne M. Chronic allograft dysfunction. Clin Chest Med. 2011 Jun;32(2):311–26. doi: 10.1016/j.ccm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, et al. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6(3):e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 2010 Apr;31(2):189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 9.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003 Feb 15;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 10.Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short-and long-term outcomes. Semin Respir Crit Care Med. 2010 Apr;31(2):161–71. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 11.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004 Nov;6(13):1219–25. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009 Nov 15;180(10):1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006 May 1;173(9):1008–15. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008 Dec;63(12):1083–9. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley ST, Medina C, Kasper M, Ehrhardt C. Interplay between RAGE, CD44, and focal adhesion molecules in epithelial- mesenchymal transition of alveolar epithelial cells. Am J Physiol LungCell Mol Physiol. 2011 Apr;300(4):L548–59. doi: 10.1152/ajplung.00230.2010. [DOI] [PubMed] [Google Scholar]
- 17.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003 Oct;124(4):1232–41. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 18.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996 Jan;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 19.Eberlein M, Permutt S, Brown RH, Brooker A, Chahla MF, Bolukbas S, et al. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011 Jan 1;183(1):79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 20.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995 Jun;51(2):524–32. [PubMed] [Google Scholar]
- 21.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 1;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 22.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007 Dec;293(6):L1427–36. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 23.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008 Mar;172(3):583–91. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006 Nov;19(11):1437–45. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 25.Queisser MA, Kouri FM, Konigshoff M, Wygrecka M, Schubert U, Eickelberg O, et al. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol. 2008 Sep;39(3):337–45. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 26.Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7(4):R817–24. doi: 10.1186/ar1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelaez A, Force SD, Gal AA, Neujahr DC, Ramirez AM, Naik PM, et al. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant. 2010 Apr;10(4):900–7. doi: 10.1111/j.1600-6143.2009.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker MD, Chambers PA, Lodge JP, Pratt JR. Ischemia-reperfusion injury and its influence on the epigenetic modification of the donor kidney genome. Transplantation. 2008 Dec 27;86(12):1818–23. doi: 10.1097/TP.0b013e31818fe8f9. [DOI] [PubMed] [Google Scholar]
- 29.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004 Jun;113(11):1641–50. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011 Jul;30(7):735–42. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


