Abstract
Type 2 diabetes is now a worldwide epidemic, strongly correlated with an elevated incidence of obesity. Obesity-associated adipose tissue inflammation is a major cause of the decreased insulin sensitivity seen in type 2 diabetes. Recent studies have shed light on the crosstalk between the immune system and organismal metabolism. This review discusses the connection between inflammation in adipose tissue and systemic insulin resistance, focusing on the roles of innate and adaptive immune cell subsets in the pathogenesis of this metabolic disease.
Keywords: Obesity, Type 2 diabetes, Inflammation, Adipose tissue, Insulin resistance
1. Introduction
Type 2 diabetes is a global health problem characterized by a defect in insulin secretion and/or a decrease in sensitivity to insulin, also termed insulin resistance. The result is an increase in blood glucose levels as adipocytes and muscle cells are compromised in their glucose uptake, while hepatocytes continue to produce glucose. Type 2 diabetes is strongly associated with obesity, currently a worldwide epidemic [1, 2]. The association between these conditions is thought to reflect chronic, low-grade inflammation driven by long-term nutrient excess. Hypothesized mechanisms promoting inflammation include: oxidative stress, endoplasmic reticulum stress, hypoxia, amyloid and lipid deposition, lipotoxicity and glucotoxicity.
We will summarize what is currently known about the role of the immune system in the pathogenesis of obesity-induced metabolic disease. We will start by providing evidence for a connection between inflammation in adipose tissue and systemic insulin resistance. Then, we will explore the role of innate immune system cells (e.g. macrophages, mast cells, eosinophils) in insulin resistance, addressing whether there might be an external stimulus by which these cells sense the local environment. We will end by investigating the role of different adaptive immune system cells (e.g. CD8+ T cells, CD4+ TH1 cells, CD4+Treg cells, B cells, NKT cells) in insulin resistance and the possible interplay between them.
2. Obesity induces chronic inflammation in adipose tissue
Adipose tissue was traditionally considered to be a long-term energy-storage organ, but now it is known to also have a key role in the integration of systemic metabolism. Systemic inflammation and obesity-induced insulin resistance are initiated largely in the adipose tissue, which releases cytokines, adipokines and fatty acids; downstream effects on the liver and muscle ensue. Mice with adipocyte-specific knockout or transgenesis of certain genes have given insight into the role adipocytes play in systemic insulin resistance. JUN N-terminal kinase 1 (JNK1) deficiency in adipocytes suppressed high-fat diet (HFD)-induced insulin resistance in the liver due to JNK1-dependent secretion of interleukin-6 (IL-6) [3]. Transgenic mice expressing a dominant-negative cAMP Response Element Binding protein (CREB) in adipocytes displayed increased whole-body insulin sensitivity upon diet-induced and genetic obesity due to increased adiponectin secretion and glucose transporter 4 (GLUT4) expression [4]. Similarly, adipocyte-specific deletion of GLUT4 or overexpression of monocyte chemoattractant protein-1 (MCP-1) resulted in systemic insulin resistance. Adipose tissue is a key site for engendering systemic insulin resistance as analogous genetic manipulations specifically targeting the liver [5] or muscle [6] showed tissue-autonomous phenotypes.
Adipose tissue is composed of different types of fat. Visceral adipose tissue (VAT, e.g. omental or epididymal fat) was found to have a greater effect on insulin sensitivity, higher percentage of large adipocytes, and greater numbers of inflammatory cells when compared with subcutaneous adipose tissue (SAT) [7, 8]. HFD-feeding increased the number of adipocyte precursor cells in both VAT and SAT by as much as 270% [9]. Adipocyte precursor cells isolated from the VAT and SAT had distinct patterns of gene expression, differentiation potential, and response to environmental and genetic influences. Another difference between depots following HFD was that the number of non-adipocyte precursor cells in VAT increased dramatically after 2–4 weeks on HFD; in contrast this number remained constant in SAT [9]. Therefore, not all fat is functionally equivalent; most of the studies referenced in this review have conducted their experiments using VAT.
Hotamisligil et al made an early connection between obesity, inflammation and insulin resistance [10, 11], reporting an induction of tumor necrosis factor (TNF)-α, a proinflammatory cytokine, in adipose tissue from four rodent models of obesity and diabetes, and demonstrating that neutralization of TNF-α in obese fa/fa rats ameliorated insulin resistance. Furthermore, mice deficient in TNF-α had significantly improved insulin sensitivity in both diet-induced obesity and the Lepob/ob model of obesity [12]. Since this finding, follow-up studies have shown that inflammatory signals disrupt insulin action and mediate insulin resistance in obesity. Insulin stimulates tyrosine phosphorylation of insulin receptor substrate (IRS) proteins. IRS-modifying enzymes, such as inflammation- and stress-induced kinases Iκβ kinase-β (IKKβ) and JNK, phosphorylate IRS-1 at serine residues, resulting in the inability of IRS-1 to engage in insulin-receptor signaling. Bone-marrow chimeras and selective genetic ablation procedures showed that the IKKβ-NF-κB and JNK signaling pathways account for most of the local production of pro-inflammatory cytokines in the adipose tissue, liver and muscle [3, 5, 13–16]. Similar to the case of rodents, in obese individuals excess adipose mass has been associated with increased levels of the pro-inflammatory marker C-reactive protein (CRP) in the blood [17]. Increased levels of CRP, its inducer IL-6, and IL-1β were predictive of the development of type 2 diabetes in various populations [17]. In both murine models and humans, it is evident that obesity triggers the accumulation of immune cells, particularly macrophages, in VAT, resulting in local inflammation and ultimately systemic insulin resistance.
3. Innate immune system cells in obesity-induced insulin resistance
3.1 Adipose-tissue macrophages
A key function of macrophages is to remove apoptotic cells in an immunologically silent manner to prevent the release of noxious substances. Metabolic dysfunction in obese individuals has been correlated with the presence of histological features in inflamed adipose tissue called crown-like structures, which represent an accumulation of macrophages around dead adipocytes [18, 19]. These adipose-tissue macrophages (ATMs) can comprise nearly 40% of the cells in obese adipose tissue [20, 21]. As macrophages are also potent producers of inflammatory mediators, they drive the heightened inflammatory responses seen in obesity.
Macrophages have been segregated into two broad groups: M1s induced in vitro with granulocyte-macrophage colony-stimulating factor (GM-CSF) secrete proinflammatory cytokines; M2s induced in vitro with macrophage colony-stimulating factor (M-CSF) and IL-4 secrete anti-inflammatory cytokines. M2 macrophages have been associated with the repair of injured tissues and the resolution of inflammation [22]. In lean mice, M2-like macrophages are the predominant resident ATMs. In the expanding adipose tissue of obesity, the local population shifts from M2-like macrophages to M1-like ATMs that express CD11c and are characterized by the accumulation of lipids. Greater than 90% of these M1-like cells originate from recruited monocytes that become CD11c+ [23, 24]. Conditional ablation of CD11c+ cells in mice with diet-induced obesity significantly reduced adipose tissue and skeletal muscle macrophages, decreased proinflammatory cytokine levels, and increased insulin sensitivity [25]. However, it was not clear from this study whether the resulting increase in insulin sensitivity was due to the loss of ATMs or the loss of muscle CD11c+ macrophages.
Lumeng et al. have proposed a “phenotype switch” model in which obesity promotes the recruitment of M1-like ATMs that shift the noninflammatory mileau maintained by M2-like resident ATMs toward a proinflammatory state [26–28]. On the contrary, human ATMs display mixed M1/M2 remodeling phenotypes [29, 30]. In line with the human data, a recent study transcriptionally characterized CD11c+ ATMs in obese mice and concluded that insulin resistance is coincident with a mixed M1/M2 phenotype [31]. ATMs respond to the local environment by polarizing accordingly; thus, in vivo the macrophage populations are much more dynamic and varied and do not fit into a stringent M1 or M2 classification.
Lipodystrophy, a condition with reduced adipose tissue mass was accompanied by metabolic outcomes similar to those seen in obesity: overt adipose tissue inflammation, insulin resistance, hepatic steatosis, and dyslipidemia. Similar to ATMs in obesity, ATMs in lipodystrophy also had a mixed M1/M2 phenotype [32]. However, the ATMs isolated from obese adipose tissue and those from lipodystrophic adipose tissue had different surface marker expression, gene expression, and responded differently to potent stimuli. It is important to keep in mind that controversy remains over the ‘promiscuous’ markers (e.g. MHC class II, CD11c, F4/80) used to distinguish between macrophages and classical dendritic cells (cDCs). Recently, a molecular signature shared by lymphoid-tissue CD8− cDCs and CD8+ cDCs and nonlymphoid-tissue CD103+ cDCs and absent from tissue macrophages was identified [33]. Gene expression profiling of adipose-tissue dendritic cells and macrophages will enable us to better define the two populations. It remains possible that what we have been calling ATMs might just as well be dendritic cells.
3.2 Do ATMs play a role in obesity-induced systemic insulin resistance?
Genetic studies using knockout, transgenic, and bone marrow chimera techniques to disable or enhance inflammatory pathways have provided insight on the role of ATMs in obesity-induced systemic insulin resistance. The most common system used is based on the LysM-Cre mice. The Cre recombinase is expressed under the control of a myeloid-cell-specific promoter (LysM). The recombinase catalyzes the recombination, usually deletion, of the gene flanked by LoxP sites. Roughly 80–90% of macrophages and neutrophils have the desired deletion. Knockout of IKKβ [5], the insulin receptor [34], or fatty acid-binding protein 4 and 5 (FABP4, FABP5) [35] in LysM-expressing cells protected mice from HFD-induced glucose intolerance, hyperinsulinemia, and insulin resistance. Decreases in inflammatory mediators (IL-1, IL-6, TNF-α) were observed, as well as a reduction in ATMs. These results suggested a role for ATMs in insulin resistance; however, a role for other LysM-expressing cell populations (e.g. neutrophils) or a role for another cell population affected by the genetically altered ATMs was not ruled out.
Bone-marrow chimera strategies have provided strong evidence supporting the involvement of bone-marrow-derived cells in insulin sensitivity. Saturated fatty acids such as oleate and palmitate can induce adipose-tissue inflammation through the induction of cells expressing Toll-like receptor 4 (TLR4). Mice lacking TLR4 were substantially protected from inflammation and insulin resistance induced by lipid infusion [36]. Similarly, bone-marrow-chimera studies using Tlr4−/− donors demonstrated that TLR4 signaling in hematopoietic cells is important for the development of hepatic and adipose-tissue insulin resistance in obese mice [37]. Protection from obesity-induced insulin resistance was seen when hematopoietic cells with the genetic manipulation of molecules involved in macrophage migration, CXCR2 [38], or an alpha4 integrin (Y991A) mutation [39] were transplanted. A decrease in macrophage infiltration into adipose tissue was observed. The molecules mentioned above play a role in influencing ATMs to instigate systemic insulin resistance; however, it is also possible that these molecules are required to bring in other critical players.
Bone-marrow-specific deletion of the Cap gene (which encodes a member of the PI3K-independent pathway linked to GLUT4 translocation) protected against HFD-induced insulin resistance [40]. A decrease in ATMs and lymphocytes was noted. Add-back experiments where respective wild-type cell types were transferred would have provided more insight into the sequence of inflammatory events. Other studies using adoptive transfer have found the perturbed gene to exacerbate insulin resistance (e.g. kruppel-like factor 4 [41], G protein-coupled-receptor 120 [42]), to have no effect on insulin resistance (IL10 [43]), or to have a function in adipocytes rather than in hematopoietic cells (PKC-ζ [44]). Bone-marrow-chimera experiments have the advantage of narrowing down the pathways involved in insulin resistance to the hematopoietic compartment.
Although most studies have investigated obesity-induced insulin resistance by focusing on either hematopoietic cells or adipocytes, some recent papers have taken these observations one step further by demonstrating that there is important cross-talk between ATMs and adipocytes. Deletion of FABP4 and FABP5 in adipocytes results in reduced expression of inflammatory cytokines in macrophages as well as improved insulin sensitivity [35]. Neither macrophages alone nor adipocytes alone could account for the entire impact of FABPs on systemic insulin resistance, so it is likely that interactions between the two cell types drive the local inflammation.
Calorie restriction in HFD-fed mice resulted in adipose lipolysis, which was associated with rapid accumulation of ATMs [44]. It appears that these macrophages were recruited to take up lipids to control local lipid concentrations. On a different note, the apoptosis inhibitor of macrophage (AIM) protein produced by macrophages was incorporated into adipocytes, thereby stimulating lipolysis [45, 46]. Obese AIM−/− mice had diminished macrophage infiltration and were protected from insulin resistance [47]. In conclusion, this crosstalk between macrophages and adipocytes accelerated inflammation, as saturated fatty acids from lipolysis activate TLR4, which induces TNF-α and downstream cytokines, adipokines and chemokines.
Peroxisome proliferator–activated receptor (PPAR)-γ and -δ are ligand-dependent transcription factors that suppress pro-inflammatory genes in macrophages and induce M2s [48]. Mice with a deletion of PPAR-γ in LysM-expressing cells have impaired differentiation of M2 macrophages and derepression of proinflammatory macrophage pathways, thus displaying more severe insulin resistance compared with that of controls [28, 49]. These results established that M2s had a protective role in obesity-induced insulin resistance; however they did not clearly show that M1-like ATMs were the cause of insulin resistance as it is possible that M2s may have been suppressing other cell types. Similarly, deletion of PPAR-δ in LysM-expressing cells exacerbated obesity-induced insulin resistance. These mice had increased body mass, but no difference was observed in ATM numbers [50]. This study demonstrated the complexity of obesity-induced insulin resistance, challenging the simple view of ATMs being the source of inflammatory mediators causing systemic insulin resistance.
A cohesive picture of the functional consequences of macrophage infiltration into adipose tissue during obesity has not yet emerged as there are still gaps and variations in the different genetic models. Furthermore, there appears to be an intricate crosstalk between macrophages and adipocytes that warrants additional studies in order for us to begin to understand the impact of this interaction on systemic insulin resistance. As type 2 diabetes is a multi-organ disease, some of these genetic modifications have impacted hepatic insulin resistance [51] or muscle insulin resistance [25], so it is important to keep in mind not only what occurs in the adipose tissue but also effects in the liver and muscle.
3.3 Role of innate immunity in the sensing of metabolic imbalance
As discussed, there is evidence suggesting that macrophages are involved in obesity-induced insulin resistance, but the fundamental question of the mechanism of their recruitment to the adipose tissue remains. Pattern recognition receptors are well known metabolic sensors on innate immune cells. The Nod-like receptor family (NLR) has been shown to sense obesity-induced signals. They are classically thought to be activated by danger signals from dying and stressed cells. In macrophages, NLR recognition of these nonmicrobe-originated ‘danger signals’ stimulates the cryptopyrin/NLRP3 inflammasome, leads to caspase-1 activation and results in subsequent IL-1β and IL-18 production. Nlrp3−/− animals were protected from insulin resistance, and had more metabolically active fat cells and a higher fat oxidation rate compared with those of wild-type animals [52]. Similarly, caspase-1-deficient mice displayed enhanced insulin sensitivity [53]. In ATMs, the NLRP3 inflammasomes sensed lipotoxicity-associated increases in intracellular ceramide from fatty acids to induce caspase-1 autoactivation and IL-1β and IL-18 production [53]. Palmitate is one of the most abundant saturated fatty acids in plasma and was substantially elevated following HFD [54]. Palmitate induced the activation of the NLRP3-adaptor apoptotic speck protein (ASC) inflammasome, resulting in caspase-1, IL-1β and IL-18 production in hematopoietic cells, which impaired insulin signaling and led to insulin resistance [55]. Another obesity-related factor, islet amyloid polypeptide (IAPP), also triggered NLRP3 inflammasome activation and potentiated type 2 diabetes [56]. IAPP is secreted by beta-cells at the same time as insulin, and forms amyloid deposits in the pancreas, which is speculated to exacerbate disease severity. Leading candidate activators of the NLRP3 inflammasome in adipose tissue include: oxidized low-density lipoprotein and cholesterol crystals [57, 58], monosodium urate [59], or products from hypoxia [60] and adipocyte death [61].
Another family of sensors is the TLR family, molecules expressed by innate immune system cells and adipocytes. TLR4 is activated by free fatty acids to generate proinflammatory signals and NF-κB activation, which happens in both leukocytes and non-hematopoietic cells. Knock-out mice were protected from insulin resistance induced by diet and lipid infusion [36, 37]. Besides responding to fatty acids, TLRs sense gut microbes in a way that contributes to metabolism, as TLR2-deficient mice were protected from diet-induced insulin resistance under germ-free conditions, and succumbed to subclinical inflammation, insulin resistance, glucose intolerance and obesity in a non-germ-free facility. Furthermore, this sequence of events could be induced in wild-type mice by microbiota transplantation which, in turn, was reversible when these individuals were treated with antibiotics. The authors hypothesized that the microbiota in TLR2-deficient mice induced changes in gut permeability, leading to an increase in serum lipopolysaccharide (LPS) levels, which correlated with insulin resistance [62, 63]. In these whole-body gene knock-out models, it is difficult to determine which cell type and at what location the critical process was occurring. These papers introduced not only a new site of action (the gut) but also new players (gut microbiota) to increase the level of complexity of obesity-induced systemic insulin resistance.
3.4 Other innate immune cells
Other innate immune system cells have also been suggested to participate in obesity-induced insulin resistance. Mast cells are present in higher numbers in the adipose tissue of obese mice and humans compared with their lean counterparts. Mast cells have been suggested to contribute to glucose intolerance in adipose tissue and muscle in experiments using mast cell-deficient Kit mutant mice or pharmacological stabilization of mast cells [64]. It is important to note that mast cell-deficient mice on HFD gained significantly less body and visceral-fat weight than wild-type controls, which raises the question of whether enhanced insulin sensitivity was a result of reduced adiposity and, thereby, reduced inflammation only secondarily. In addition, there continues to be controversy over the fidelity of the mast cell-deficient models used in these studies [65].
Eosinophils in VAT produce IL-4 and IL-13, cytokines that promote differentiation of ATM preferentially into M2 cells. Mice genetically deficient for eosinophils had increased adiposity and insulin resistance when placed on a HFD [66]. Wu et al suggested that in lean mice eosinophils control adipose tissue inflammation and promote insulin sensitivity. Similar to the argument for mast cells, the fact that eosinophil-deficient mice gained more weight on HFD than controls clouds the issue of whether increased adiposity or inflammation was the primary drive of enhanced insulin resistance.
4. Adaptive immune system cells in obesity induced T2D
The involvement of the innate system, particularly macrophages, in obesity-induced inflammation has been the central focus of the field for many years. Recently, however, several reports implicating the adaptive immune system have emerged. One of the early clues came from data showing a correlation between body mass index (BMI) and CD3 transcript levels in human VAT [67]. BMI also correlated with transcripts encoding the chemokine RANTES, known to recruit leukocytes to sites of inflammation [67, 68] A set of recent papers has shown that T cells in adipose tissues play a role in the regulation of ATM numbers and activation state. In general, among the T lymphocytes, it appears that CD8+ T cells [69, 70] and CD4+ T helper 1 (TH1) cells [71] promote insulin resistance; to the contrary, CD4+ regulatory T (Treg) cells counter it. A role for TH2 cells has been suggested [71], but remains in question [72].
CD8+ T cells in obese VAT have an activated phenotype, as the frequency of CD44+CD62L− effector-memory cells was significantly higher, while naïve CD44−CD62L+CD8+ T cells was decreased in obese compared with lean VAT [73]. CD8+ effector T cells were shown to be involved in the recruitment and activation of ATMs and promoted a pro-inflammatory cascade associated with insulin resistance using genetic modifications and transfer experiments[69]. Depletion of CD8+ T cells resulted in reduced macrophage infiltration into VAT, decreased production of pro-inflammatory mediators, and increased insulin sensitivity. Contrary to studies stating that macrophages are the first cells to infiltrate the VAT, this report argued that infiltration of CD8+ T cells at 4 weeks of HFD preceded the accumulation of macrophages. These findings suggested that obesity promoted the activation of CD8+ T cells, which led to the recruitment, differentiation and activation of ATMs. Another group performed similar kinetic studies but did not find significant T-cell enrichment in VAT during the first 4 months of HFD feeding [74]. The temporal pattern of accumulation of different lymphoid cell populations in adipose tissue during the development of obesity is still not fully understood. Contradictory results may reflect: (1) methods of quantification of T cells by flow cytometry, (2) the age of mice at initiation of HFD-feeding, (3) the length of HFD-feeding, and (4) the precise composition of the HFD and normal chow used.
CD4+ T cells can be divided into several distinct populations, including TH1 and TH17 cells, which produce proinflammatory cytokines, and TH2 cells and regulatory T cells (Tregs), which produce anti-inflammatory cytokines. An increase in IFNγ-producing adipose-tissue-resident effector CD4+ T cells in obese mice [75] and humans [76] has been reported. In mice, an increase in IFNγ enhanced the accumulation of M1 macrophages in obese fat, accompanied by elevated expression of TNFα and MCP-1 [75]. No role for TH17 cells or their major product, IL-17, has so far been clearly demonstrated.
The roles of CD4+ and CD8+ T cells in obesity-induced insulin resistance was addressed by transfer into recombination-activating genes (RAG)-null mice, which lack all lymphocytes. CD4+, but not CD8+, T cells dampened the weight gain, VAT mass, high glucose, and elevated TNFα and IL-6 induced by HFD [71]. Winer et al proposed that the improved metabolic phenotype was primarily due to the activities of TH2 cells. However, the reduction in weight gain could have been at least partially due to the initiation of colitis, as there are well-established animal models of colitis where disease is induced by the transfer of CD4+ T cell depleted of Foxp3+ Treg cells [77, 78]. An alternative interpretation of Winer et al’s observation is that improved insulin resistance may merely be a result of decreased body weight caused by colitis. The authors also suggested that the protective effect of CD4+ T cells reflected polarization toward the TH2 phenotype as defined by the production of IL-4 and IL-13, and a decreased frequency of Gata3+ T cells in the VAT of obese mice. However, VAT Tregs express enhanced levels of Gata3 [79], so Gata3+CD4+ T cells cannot be equated to TH2 cells.
Treg cells have an important role in the maintenance of self-tolerance and the suppression of autoreactive T cells, thus the prevention of autoimmunity [80]. VAT Treg cells were abundant (>50% of the CD4+ T cell compartment) in lean mice, and protected them from insulin resistance by inhibiting inflammatory macrophages and probably also by dampening production of proinflammatory cytokines by adipocytes [70]. VAT Treg cell number decreased dramatically upon development of obesity by an as-yet unknown mechanism. Induction of Treg cells in obese mice improved insulin sensitivity, and reduced macrophage numbers and TNFα levels [70, 81]. Gene expression profiling of fat Treg cells from lean mice revealed that this Foxp3+CD4+ population had only about 60% of the signature of Foxp3+CD4+ Treg cells typical of lymphoid organs [70]. A number of the genes that were over- or under-expressed coded for molecules involved in leukocyte migration and extravasation. Moreover, the TCR repertoire of fat Treg cells from lean mice was distinct from that of VAT T-conventional (Tconv) cells, as well as that of both T cell subsets in lymphoid tissues. Strikingly, there were several examples of VAT Treg cells with the same protein sequence and different nucleotide sequences in the CDR3 region important for recognizing MHC-peptide complexes. These data suggest that (1) fat Treg cells are unlikely to have converted from Tconv cells, and (2) there may be a selective pressure favoring certain TCRs with a particular antigenic specificity. TCR repertoire bias has also been observed for CD8+ T cells and CD4+ TH1 cells isolated from VAT of obese mice [69, 70, 73].
VAT Foxp3+ Tregs specifically express PPARγ, which appears to interact with Foxp3. Recently, Cipolletta et al. showed that mice lacking PPARγ specifically in Treg cells displayed reduced Treg numbers and increased macrophage and monocyte numbers in VAT [72]. Interestingly, PPARγ stimulation by the thiazolidinedione drug pioglitazone (Pio) increased Treg cell numbers in VAT of HFD obese mice, leading to improved insulin sensitivity. Thiazolidinediones play a role in suppressing VAT inflammation by increasing PPARγ-expressing Tregs in VAT.
B cells accumulated in the visceral fat of obese mice after 3 weeks of feeding on HFD [82]. Feeding B-cell-deficient mice on HFD or treating wild-type HFD-fed mice with B-cell-depleting anti-CD20 antibody protected them from insulin resistance and glucose intolerance. B cell production of IgG antibodies activated proinflammatory macrophages and T cells. The transfer of B cells or serum IgG from HFD-fed mice into B-cell-deficient recipients resulted in the transfer of insulin resistance. However, it remains possible that these IgGs induced adipocyte death or inhibited insulin signaling molecules, thus having nothing to do with initiation of the inflammatory cascade of events in the VAT, as it has been documented that there are T-cell aberrancies in B-cell deficient mice [83–85].
Natural killer T (NKT) cells recognize lipid antigens in the context of CD1d molecules on antigen presenting cells [86]. Among the different types of NKT cells, type-1 or invariant NKT are the most abundant and best characterized [87]. Type-1 NKT cells have been extensively studied using a prototypical lipid antigen: the marine sponge-derived α-galactosylceramide (α-GalCer), which is not found in mammals [88]. NKT cells were reported to infiltrate into the VAT in association with ATMs during the development of HFD-induced insulin resistance [89]. The activation of NKT cells with α-GalCer exacerbated glucose intolerance and VAT inflammation. Furthermore, CD1d-deficient mice had less inflammation in VAT and liver coupled with ameliorated insulin resistance [90]. However, in this study the CD1d-deficint mice gained less weight, which may be attributed to the metabolic improvements instead of the lack of NKT cells. In contrast, two other studies reported no effect on HFD-induced glucose intolerance and insulin resistance in CD1d-deficient mice [91, 92]. Following up on the reports using CD1d-deficient mice, a recent study involved injecting α-GalCer and found that activation of NKT cells enhanced M2-like macrophage polarization, which enhanced insulin seinsitivity [93]. VAT-resident type 1 NKT cell numbers were negatively correlated with BMI, insulin resistance, and fasting glucose levels in humans. In conclusion, this study corroborated the previous two reports where no metabolic effect was seen in CD1d-deficint mice, which indicated that NKT cells in the absence of stimulation are dispensible for glucose homeostasis upon chronic obesity. In the gain-of-function experiments where a strong agonist was provided, NKT cells significantly impacted the inflammatory responses in VAT of obese mice. It is possible there is a mammalian α-GalCer-like agonist at steady-state in lean VAT that promotes insulin sensitivity by activating NKT cells, which in turn promotes M2-like cells. However, the evidence provided suggests that the role of NKT cells in obesity-induced insulin resistance is minimal as overt obesity was able to trump the protective role of these NKT cells.
5. Concluding remarks and future prospects
To this date, the most convincing studies shedding light on the etiology of obesity-induced insulin resistance appear to be those on ATMs, CD8+ T cells and Treg cells. The combination of reduced Treg cells in VAT coupled with an increase in CD8+ T cells to promote macrophage recruitment during obesity-induced inflammation might provide a positive feedback loop that ultimately progresses to insulin resistance. Alternatively, the macrophages could be recruiting the T cells. As the field uncovers answers, more questions arise. What is the order in which the different immune cell populations infiltrate the VAT? What chemokines and chemokine receptors are necessary for the homing of cells into the VAT? Is there crosstalk between different cell populations (including adipocytes), and what is the mechanism? What is the function of these cell populations that reside in or infiltrate the VAT? What instigates the shift from an anti-inflammatory environment in VAT to a pro-inflammatory environment? Do CD8+ T and Treg cells respond to an antigen(s); if so, what is it?
Therapeutic possibilities with our current knowledge can be directed to either suppress the culprits causing VAT inflammation or enhance the inhibitory capability of the anti-inflammatory arm (e.g. Tregs). Specific approaches to shift the local population to more M2-like ATMs can be devised as we begin to understand the processes that shape the polarization of proinflammatory ATMs. Alternatively, selectivity can be achieved by inhibiting monocyte chemotaxis, as these are the predominant cell types that migrate into VAT and polarize into proinflammatory ATMs. The other culprit, CD8+ T cells can be curbed by inhibiting mechanisms that CD8+ T cells use to recruit and promote differentiation of ATMs in VAT. Vaccination strategies can also be devised if research shows that there is an endogenous stimulus that activates CD8+ T cells causing it to be pathogenic. Another therapeutic path is the enhancement of Treg cell numbers and function using cell-specific drugs (similar to Pio). Furthermore, finding Treg effector molecules and utilizing it for therapy is a promising approach. Proper manipulation of immune cell subsets present in adipose tissue can decrease local inflammation and obesity-induced insulin resistance.
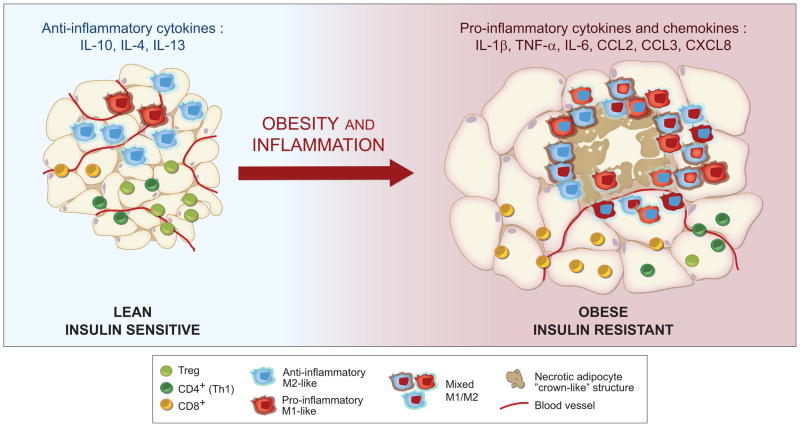
Figure 1. Obesity results in inflammation and changes in immune system cells in adipose tissue.
Lean adipose tissue has elevated fractions of anti-inflammatory M2-like ATMs and Tregs, the local environment is dominated by anti-inflammatory cytokines (IL-10, IL-4, IL-13). Long-term nutrient excess leads to apoptotic and necrotic death of adipocytes, as well as decreased vascularity. Upon obesity, the adipose tissue has a mixed M1/M2 phenotype of ATMs, more CD8+ T cells than CD4+ Th1 cells, and fewer Tregs. The effector cells promote chronic inflammation through the production of pro-inflammatory cytokines and chemokines (IL-1β, TNF-α, IL-6, CCL2, CCL3, CXCL8). Graphics by Catherine Laplace.
Highlights.
Role of immune system in the pathogenesis of obesity-induced metabolic disease.
Connection between inflammation in adipose tissue and systemic insulin resistance.
The role of innate immune system cells in insulin resistance.
The role of different adaptive immune system cells in insulin resistance.
Acknowledgments
The lab’s work on immunometabolism has been supported by the NIH (RO1 DK092541) and the Ellison Foundation. CJS was funded on a Cancer Immunology Training Grant (T32 CA70083).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi L, Saberi M, Zmuda E, Wang Y, Altarejos J, Zhang X, et al. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 2009;9:277–286. doi: 10.1016/j.cmet.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 6.Sabio G, Kennedy NJ, Cavanagh-Kyros J, Jung DY, Ko HJ, Ong H, et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 9.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 13.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 24.Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 30.Bourlier V, Zakaroff-Girard A, Miranville A, De BS, Maumus M, Sengenes C, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 31.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012 doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saberi M, Woods NB, de LC, Schenk S, Lu JC, Bandyopadhyay G, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neels JG, Badeanlou L, Hester KD, Samad F. Keratinocyte-derived chemokine in obesity: expression, regulation, and role in adipose macrophage infiltration and glucose homeostasis. J Biol Chem. 2009;284:20692–20698. doi: 10.1074/jbc.M109.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feral CC, Neels JG, Kummer C, Slepak M, Olefsky JM, Ginsberg MH. Blockade of alpha4 integrin signaling ameliorates the metabolic consequences of high-fat diet-induced obesity. Diabetes. 2008;57:1842–1851. doi: 10.2337/db07-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesniewski LA, Hosch SE, Neels JG, de LC, Pashmforoush M, Lumeng CN, et al. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med. 2007;13:455–462. doi: 10.1038/nm1550. [DOI] [PubMed] [Google Scholar]
- 41.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, et al. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 2011;54:888–899. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Kim JY, Nogueiras R, Linares JF, Perez-Tilve D, Jung DY, et al. PKCzeta-regulated inflammation in the nonhematopoietic compartment is critical for obesity-induced glucose intolerance. Cell Metab. 2010;12:65–77. doi: 10.1016/j.cmet.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–422. doi: 10.1084/jem.189.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11:479–492. doi: 10.1016/j.cmet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108:12072–12077. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 49.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odegaard JI, Ricardo-Gonzalez RR, Red EA, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sachithanandan N, Graham KL, Galic S, Honeyman JE, Fynch SL, Hewitt KA, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60:2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stienstra R, Joosten LA, Koenen T, van TB, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002;5:545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–1799. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strissel KJ, Stancheva Z, MIYOSHI H, Perfield JW, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 62.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 63.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 68.Madani R, Karastergiou K, Ogston NC, Miheisi N, Bhome R, Haloob N, et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am J Physiol Endocrinol Metab. 2009;296:E1262–E1268. doi: 10.1152/ajpendo.90511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 70.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23:431–437. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pacifico L, Di RL, Anania C, Osborn JF, Ippoliti F, Schiavo E, et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 77.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 78.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185:5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 81.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baumgarth N, Jager GC, Herman OC, Herzenberg LA. CD4+ T cells derived from B cell-deficient mice inhibit the establishment of peripheral B cell pools. Proc Natl Acad Sci U S A. 2000;97:4766–4771. doi: 10.1073/pnas.97.9.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 86.Van KL. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 88.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 89.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 90.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS ONE. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, et al. Impact of CD1d deficiency on metabolism. PLoS ONE. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS ONE. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]