Abstract
Background
Syndrome X in women is thought to be caused by coronary microvascular dysfunction, the exact site of which is unknown. The aim of this study was to characterize the microvascular site of dysfunction in these patients using myocardial contrast echocardiography (MCE).
Methods
Women with exertional angina, a positive test on stress imaging, but no coronary artery disease (study group, n=18), and age-matched control women also with no coronary artery disease (n=17) were enrolled. MCE was performed at rest and during dipyridamole-induced hyperemia. Mean microbubble velocity (β) and myocardial blood volume (A) were measured, and myocardial blood flow (A β) was computed. In addition, plasma concentrations of eicosanoids, female sex hormones, and C-reactive protein were measured.
Results
Rest β and myocardial blood flow (A·β) were higher in the study compared to the control women (1.61±0.68 vs. 0.74±0.44, P=0.0001, and 157±121 vs. 54±54, P=0.0001, respectively) despite similar heart rate and systolic blood pressure. After administration of dipyridamole, whereas the changes in A, and A·β were not significantly different between the 2 groups, β reserve (the ratio of stress/rest β) was markedly lower in the study group (1.48±0.62 vs. 2.78±0.94, P=0.0001). Blood hematocrit, eicosanoids, female sex hormones, glucose, and C-reactive protein were not different between the 2 groups.
Conclusion
Coronary autoregulation is abnormal in patients with Syndrome X (higher resting β and myocardial blood flow and lower β reserve), which suggests that the coronary resistance vessels are the site of microvascular abnormality.
Keywords: syndrome X, myocardial contrast echocardiography, coronary autoregulation
Introduction
Syndrome X has been defined as classical angina pectoris associated with a positive stress test but a normal coronary angiogram in mostly peri-menopausal women1. Two independent mechanisms have been implicated in this syndrome: endothelial dysfunction2,3 and reduced coronary blood flow (CBF) reserve4–6. The former is an endothelial dependent paradoxical vasoconstriction of epicardial coronary arteries during intracoronary administration of acetylcholine, and the second is the inability to increase CBF with an endothelial independent vasodilator such as adenosine or Dipyridamole. Abnormal CBF reserve can result from abnormalities in coronary resistance vessels, capillaries7–9, or abnormal rheology10–12.
We hypothesized that the site of the microvascular dysfunction in syndrome-X is the resistance coronary vessels. In order to test this hypothesis we used myocardial contrast echocardiography (MCE), which with its ability to quantify both myocardial blood flow (MBF) velocity and myocardial blood volume (MBV), can locate the site of microvascular dysfunction8–11,13. We also tested whether eicasonoids (potent coronary vasodilators and constrictors14,15) have a role in the microvascular dysfunction in patients with syndrome-X.
Methods
Study Population
We studied 35 women. The study group consisted of 18 women suspected to have syndrome-X based on classical angina pectoris, positive stress test on imaging (either stress perfusion nuclear scan or stress echocardiography), and normal coronary arteries on either coronary angiography or on computed x-ray tomography. The control group consisted of 17 age-matched women with no cardiac symptoms, who had normal coronary arteries on computed x-ray tomography. All subjects were 45 to 75 years of age. The exclusion criteria were: pregnancy; allergy to the drugs: Definity, Dipyridamole, Aminophylline or Iodine; known significant valve disease, left ventricular systolic dysfunction, congenital heart disease, or cardiac shunt, pulmonary hypertension, moderate or severe asthma, active infection, malignancy, uncontrolled hypertension, smoking and chronic kidney disease
Study Protocol
The study protocol was approved by Institutional Review Board at the Oregon Health & Science University. Subjects identified as potential candidates for the study provided written informed consent. Medication use and duration were recorded. Baseline demographics, clinical data, and a urine sample to check for pregnancy in women of childbearing age were collected. Blood was collected for measurement of eicosanoids, lipids, sex hormones, C-reactive protein, glucose and hematocrit.
For the 15 subjects with normal coronary arteries on coronary angiography performed within the past two years no further tests were done. For the 3 subjects with no coronary angiography and all 17 controls angiography was performed using computed x-ray tomography. Only subjects with normal coronary arteries by either technique were included in the study. All subjects also underwent echocardiography to rule out any significant cardiac abnormality.
Computed x-ray Tomography
All subjects with a heart rate >60 beats·min−1 received metoprolol intravenously (5–25 mg, in incremental doses of 5 mg) unless systolic blood pressure was <100 mm Hg or other contraindications were present. All subjects also received 0.6 mg nitroglycerin sublingually 3–5 min prior to image acquisition, which was performed with a 128-slice multi-detector computed tomography scanner (Philips Medical Systems, Best, The Netherlands). Image acquisition was performed during a single breath hold in inspiration with prospective electrocardiographic (ECG) triggering if the heart rate was regular and ≤65 beats·min−1 or retrospective gating (with or without tube dose modulation) if it was higher. Imaging parameters included a slice collimation of 128×0.625 mm, a gantry rotation time of 420-ms, a tube voltage of 120-kV, and an effective tube current of 200–250 mA for prospectively ECG triggered exams or 750–850 mA for retrospectively ECG gated exams. Contrast agent (Visipaque, GE Healthcare, Princeton, USA) was injected intravenously at a rate of 5 mL·min−1 for opacification of the coronary artery lumen.
A region-of-interest was placed in the descending thoracic aorta, and image acquisition was automatically initiated once a selected threshold (150 Hounsfield units) had been reached with bolus tracking. Images were initially reconstructed at mid-diastolic phase (75% of R-R interval) of the cardiac cycle. Additional reconstructions were performed if motion artifacts were present.
All images were analyzed independently by an experienced observer who was blinded to the clinical information with a 3-dimensional workstation (Brilliance, Philips Medical Systems, Best, The Netherlands). For detection of significant coronary artery stenosis, a segment model based on the American Heart Association classification with the addition of the posterior left ventricular branch as segment 16 and the intermediate branch as segment 17 was used.16 Coronary segments were identified relative to the origin of side branches. All data sets were assessed qualitatively for the presence of luminal obstruction within all coronary segments, including side branches. Assessment was performed on original axial source images, thin-slice multi-planar reconstructions, and 5-mm maximal intensity projections.
Myocardial Contrast Echocardiography
MCE was performed using a SONOS 7500 system (Philips Healthcare, Andover, MA). Intermittent ultraharmonic imaging was performed with a phased-array transducer transmitting at a frequency of 1.3 MHz and receiving at a frequency of 2.6 MHz gated to transmit at end-systole (peak of the T-wave on the ECG). The gain, compression, depth, transmit focus and mechanical index (>1.0) were optimized at the beginning of each study and were held constant throughout.
For contrast, 1.5 mL of Definity® (Lantheus Medical Imaging, North Billerica, MA) was diluted in 28.5 mL of saline solution (0.09%) for a total volume of 30 mL17. This solution was administered as a continuous intravenous infusion at a rate of 90 mL·hr−1 using a model AS40A (Baxter International, Inc., Deerfield, IL) syringe infusion pump. The infusion rate was assessed by the presence of homogeneous opacification of the left ventricular cavity. It was adjusted to achieve minimal attenuation at the level of the mitral valve in the apical 4-chamber view.
Prior to data acquisition, adequate opacification of the myocardium was assessed by gating at every 8th cardiac cycle during steady state microbubble concentration (approximately 2 min after initiation of infusion). For baseline images, continuous imaging of several cardiac cycles was digitally acquired in Ultraharmonics. Digital acquisition of intermittent imaging was then performed using AutoBeat Sequencing programmed to acquire 4 frames of contrast-enhanced images at pulsing intervals of 1, 2, 3, 4, 5 and 8 cardiac cycles. Digital images were written directly to magneto-optical disk.
For vasodilator stress, Dipyridamole (0.56 mg·kg−1) was administered over a period of 4 min. At 3 min into the administration, Definity infusion was restarted. As steady state was being achieved, assessment of wall motion was performed using Power Modulation, low mechanical index (0.10) imaging. Once steady state was achieved, MCE was repeated using Intermittent/Ultrahamonic imaging modality. During vasodilator stress, continuous monitoring of ECG, blood pressure, and pulse oximetry was performed. Image acquisition was completed within 10 minutes of Dipyridamole administration. Aminophylline (up to 240 mg intravenously over 3 min) was readily available to reverse any adverse effects of Dipyridamole.
Digitally acquired images were transferred from magneto-optical disk to computer with custom-designed software18. Baseline continuous images were appended with end-systole images using custom-designed software to create a single image clip. For background subtraction, 2 frames at end-systole were selected from the continuous imaging portion of the image clip. All subsequent frames from each PI that had minimal shift within the ultrasound sector were selected. These were aligned with background images using cross-correlation.
A single observer analyzed all the MCE data and was blinded to all patient information. Although 2-, 3-, and 4-chamber views were obtained at both rest and stress, only the 4-chamber view was used for generating time versus acoustic intensity plots. A large region-of-interest was placed over the mid inter-ventricular septum, with care taken to exclude the endocardial and epicardial targets. AI was measured in the region-of-interest automatically from each of the aligned frames, from which plots of time versus background-subtracted acoustic intensity were generated and fitted to an function: y = A (1 - e −βt), where y is acoustic intensity at pulsing interval of t, A is the plateau acoustic intensity representing myocardial or capillary blood volume (MBV), β is the rate constant that represents the myocardial blood flow velocity13. β reserve was calculated as Stress β /Rest β.
Eicosanoid Measurements
Arachidonic acid derivatives epoxyeicosatrienic acids (EETs) and their metabolites dihydroxyeicosatrienoic acids (DHETs) as well as hydroxyeicosatetraenoic acids (HETEs) were analyzed using the 4000 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems) with electrospray ionization (ESI) in negative mode, as previously described19. The amount of DHETs, EETs or HETEs compounds in the sample were calculated by comparison of the area ratio of the compound with the appropriate standard, then compared to a standard curve generated from plasma spiked with known amounts of DHETs, EETs and HETEs.
Statistical analysis
Data are expressed as either mean ±1 SD or percent. Comparisons between the 2 groups were performed using t-test or Fisher’s exact test. Differences were considered significant at p <0.05.
Results
Patient characteristics
The baseline characteristics of the study and control groups are listed in Table 1. Women in the study group had a higher BMI and were more likely to have a history of hypertension and bilateral oophorectomy. There were no differences between race, other risk factors, or medications including hormone replacement therapy between the two groups. LV size, wall thickness, and parameters of systolic and diastolic function on echocardiography were normal and similar between the two groups.
Table 1.
Baseline Subject Characteristics
| Variable | Study Subjects (n=18) | Control Subjects (n=17) | P-value |
|---|---|---|---|
| Age (years) | 58±6 | 58±8 | 0.83 |
| Caucasian (%) | 15 (79) | 15 (94) | 0.35 |
| Body Mass Index (Kg·m−2) | 29±6 | 24±3 | 0.007 |
| History of Hypertension (%) | 9 (50) | 2 (13) | 0.03 |
| History of Diabetes Mellitus (%) | 1 (6) | 1 (6) | 1.0 |
| Family History of CAD (%) | 13 (68) | 9 (56) | 0.50 |
| History of Bilateral Oophorectomy (%) | 6 (32) | 1 (6) | 0.09 |
| History of Smoking (%) | 4 (21) | 3 (19%) | 1.0 |
| Medication Use | |||
| Aspirin (%) | 5 (26) | 2 (13) | 0.41 |
| Diuretic (%) | 3 (16) | 0 (0) | 0.23 |
| ACE inhibitor (%) | 2 (11) | 0 (0) | 0.49 |
| Calcium channel Blocker (%) | 1 (6) | 0 (0) | 1.0 |
| β-blocker (%) | 3 (16) | 1 (6) | 0.61 |
| HRT (%) | 10 (53) | 10 (63) | 0.73 |
CAD=coronary artery disease; ACE=angiotensin converting enzyme; HRT=hormone replacement therapy
Although the inclusion criterion for interval between angiography and study was 2 years, the mean interval for the 15 subjects who underwent angiography was 4.8±3.6 months. Three subjects and all 17 controls underwent computed tomography to visualize the coronary arteries within 0.34±7.5 months of the study.
Myocardial Contrast Echocardiography Results
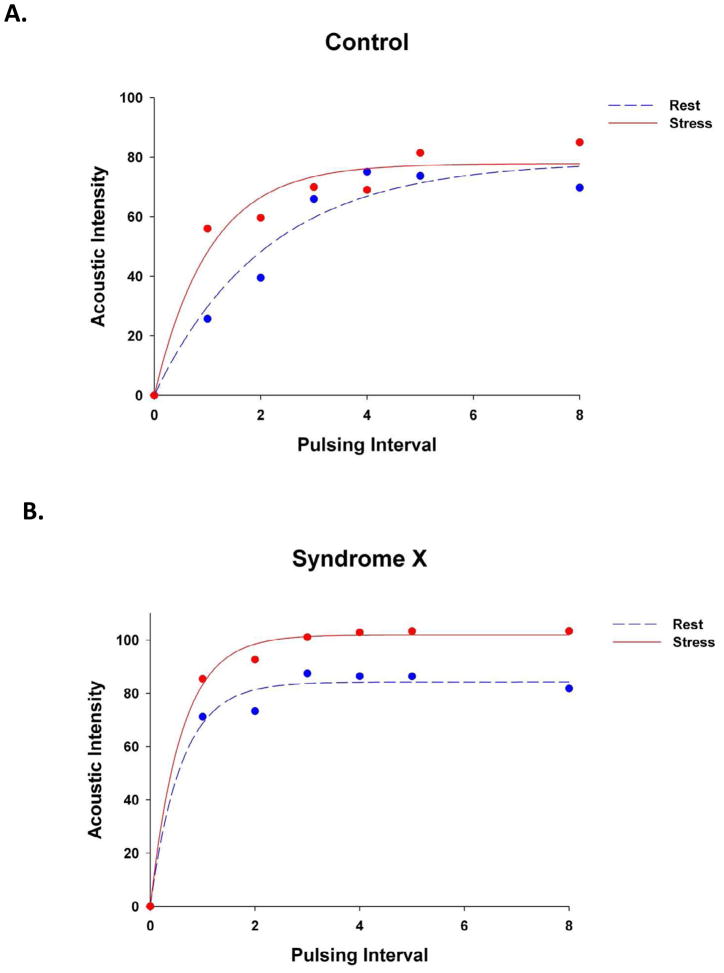
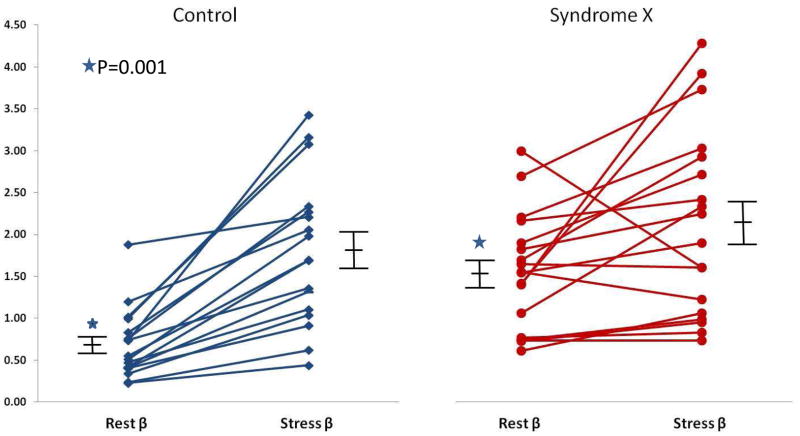
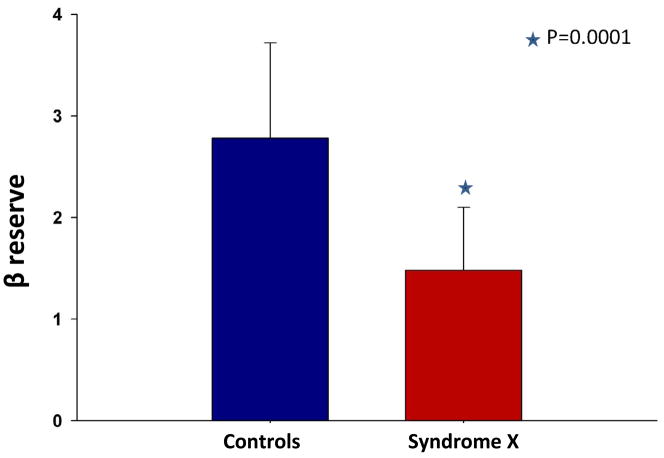
Figure 1 illustrates MCE time versus AI plots at rest (blue circles and line) and stress (red circles and line) in a subject from the control group (A) and a patient from the study group (B). The mean microbubble velocity (β) was higher at rest in the study patient but did not increase appreciably after dipyridamole administration, while in the control subject β increased 4 fold. When data from all subjects were considered, rest β and myocardial blood flow (A·β) were higher in the study compared to the control group despite similar heart rate, systolic blood pressure, and double product in the 2 groups (Table 2). After administration of dipyridamole, the changes in A, and A·β were not significantly different between the 2 groups. β increased more in the control (Figure 2A) compared to the study (Figure 2B) group but the difference did not achieve statistical significance. β reserve (the ratio of stress/rest β), however, was markedly lower in the study group (Table 2, Figure 3). We have previously defined a β reserve of 2.0 as normal based on subjects with <50% coronary diameter stenosis20. Three of the syndrome-X patients had a β reserve of >2.0 while 3 of the control subjects has a β reserve of <2.0
Figure 1.
MCE time versus acoustic intensity plots at rest (blue circles and dashed lines) and stress (red circles and solid lines) in a subject from the control group (A) and a patient from the study group (B). β is higher at rest in the study patient but does not increase after dipyridamole, while in the control subject β increases 4 fold. See text for details.
Table 2.
MCE Results
| Variable | Study Subjects (n=18) | Control Subjects (n=17) | P-value |
|---|---|---|---|
| Rest Heart rate (beats·min−1) | 66±9 | 67±8 | 0.75 |
| Rest Systolic BP (mmHg) | 124±19 | 118±8 | 0.19 |
| Rest Diastolic BP (mmHg) | 71±9 | 66±16 | 0.33 |
| Rest Double Product (·−100) | 84±15 | 80±13 | 0.40 |
| Rest A | 99±30 | 88±35 | 0.32 |
| Rest β | 1.61±0.68 | 0.74±0.44 | 0.0001 |
| Rest A·β | 157±121 | 54±54 | 0.0001 |
| Stress Heart rate (beats·min−1) | 84±14 | 81±10 | 0.48 |
| Stress Systolic BP (mmHg) | 120±16 | 118±9 | 0.75 |
| Stress Diastolic BP (mmHg) | 62±8 | 67±11 | 0.12 |
| Stress Double Product (·−100) | 101±22 | 96±14 | 0.43 |
| Stress A | 101±27 | 76±22 | 0.02 |
| Stress β | 2.29±1.10 | 1.86±0.85 | 0.21 |
| Stress A·β | 236±143 | 162±111 | 0.14 |
| Change in A during stress | −1±20 | −3±17 | 0.70 |
| Change in β during stress | 0.63±0.99 | 1.13±0.72 | 0.09 |
| Change in A·β during stress | 68±113 | 93±78 | 0.45 |
| β Reserve | 1.48±0.62 | 2.78±0.94 | 0.0001 |
BP=blood pressure
Figure 2.
β values at rest and during Dipyridamole stress in individual patients along with mean ±1 standard error of the mean in the control group (A) and the study group (B). Resting β is higher in the study compared to control groups (marked by asterisk) and change in β during stress is less in the study compared to control group (not achieving statistical significance). See text for details.
Figure 3.
β reserve in the control versus syndrome-X patients with mean±1 standard deviation. See text for details.
Laboratory Results
Table 3 lists the results of blood tests performed in the two groups. The study subjects had lower total and HDL cholesterol and higher triglycerides, VLDL cholesterol and C-reactive protein compared to the control group. However, the mean values were within the normal range in the study group. There were no differences between the two groups in terms of glucose, hematocrit, sex hormones and eicosanoids (the latter measured in all controls and 17 of the 18 subjects).
Table 3.
Laboratory Results
| Variable | Study Subjects (n=18) | Control Subjects (n=17) | P-value |
|---|---|---|---|
| Total Cholesterol (mg·dL−1) | 186±41 | 212±35 | 0.05 |
| HDL Cholesterol (mg·dL−1) | 54±11 | 68±23 | 0.03 |
| LDL Cholesterol (mg·dL−1) | 108±39 | 129±23 | 0.09 |
| VLDL Cholesterol (mg·dL−1) | 24±13 | 15±6 | 0.02 |
| Triglycerides (mg·dL−1) | 120±64 | 77±32 | 0.02 |
| C-reactive Protein (mg·dL−1) | 0.50±0.20 | 0.10±0.40 | 0.0001 |
| Glucose (mg·dL−1) | 86±14 | 89±13 | 0.48 |
| Hematocrit (%) | 39±4 | 37±3 | 0.18 |
| FSH (mIU·mL−1) | 62±35 | 66±47 | 0.78 |
| LH (IU·L−1) | 24±10 | 26±18 | 0.66 |
| Estrone (pg·mL−1) | 38±37 | 26±11 | 0.15 |
| Progesterone (ng·mL−1) | 0.35±0.40 | 0.60±0.06 | 0.17 |
| All EET’s¥ | 551±228 | 508±258 | 0.65 |
| All HETE’s§ | 5307±2921 | 4417±3063 | 0.44 |
FSH=follicular stimulation hormone; LH=luteinizing hormone
Includes the sum of 8,9- EET and DHET, 11,12- EET and DHET, and 14,15- EET and DHET
Includes the sum of 5-,11-,12-,15- and 20 HETE. These were measured in all controls and 17 of the 18 study subjects.
Discussion
There is controversy regarding syndrome-X, beginning with its very existence. One argument put forth against it even being an entity is based on Bayesian probability. The pre-test probability of coronary artery disease in peri-menopausal women with chest pain is low. Exercise ECG has only a modest sensitivity and specificity for obstructive coronary artery disease. Thus, it can be argued that peri-menopausal women with chest pain are very likely to have a false positive exercise ECG, and it is therefore expected that many of the women with positive exercise ECG will have normal coronary angiograms21. This argument is probably true for women with atypical chest pain.
Notwithstanding, women with classical angina, abnormal stress test, and normal angiograms have been demonstrated to have abnormal CBF reserve using multiple approaches4–6,22–24. Unlike most patients with normal coronary angiograms who have excellent long-term outcome25–27, a significant minority of syndrome-X women have adverse outcomes28, which has been attributed to microvascular disease. Based on this putative mechanism for angina and adverse outcomes, it has been suggested that the term syndrome-X be replaced by ‘microvascular disease’.
But the coronary microvasculature is extensive with a complex structure consisting of many components29–31. Abnormal CBF reserve can result from abnormalities in resistance vessels (150–300μm in diameter) or capillaries7–9, or could be related to abnormal rheology10–12. In this study we used MCE to define the specific site of microvascular dysfunction. Our results indicate that the dysfunction exists at the level of the resistance vessels which are responsible for coronary autoregulation. We found that despite normal heart rate, blood pressure, rate-pressure product, and left ventricular size and thickness indicating normal oxygen demand, the resting MBF was significantly increased in patients with syndrome-X. This finding implies that either coronary autoregulation is abnormal or myocardial oxygen consumption exceeds demand, requiring a higher level of MBF. The latter is very unlikely. There is only one prior study that found resting MBF to be high in syndrome-X patients using positron emission tomography24. In all other studies the resting flow was either similar to controls or was not reported4–6,22,23. The advantage of MCE and positron emission tomography is that they measure actual nutrient blood flow, while the other methods like intracoronary Doppler and flow wire measure total CBF which depends on the size of the vascular bed the artery supplies.
Like others before us, we found that MBF reserve was also significantly reduced in these patients, which can be explained either on the basis of increased resting MBF or inability of the resistance vessels to dilate during stress, or both. In our study the abnormal MBF reserve resulted from both an increase in resting MBF (which was significantly higher in the patients with syndrome-X) and an inability to adequately increase MBF with Dipyridamole (which although more pronounced in syndrome-X patients, was not statistically different from controls). The inability of the resistance vessels to dilate during an increase in myocardial oxygen demand is another feature of abnormal coronary regulation and can explain the angina and abnormal stress test in these women. To our knowledge this is the first study to show that coronary autoregulation is impaired in syndrome-X women.
While coronary autoregulation has been well studied, the signals for coronary vasodilation and vasoconstriction during alterations in coronary perfusion pressure are not known. We recently reported that reducing coronary perfusion pressure while maintaining normal coronary blood flow is associated with an incremental decrease in coronary vein and plasma levels of HETEs,32 indicating that coronary autoregulation may be controlled by the eicosanoids HETEs and EETs, which are derived from arachidonic acid by cytochrome P-450 ω-hydroxylases or epoxygenases, respectively. HETEs are potent vasoconstrictors and EETs are potent vasodilators that act on the coronary resistance vessels14,15,33.
Since patients with syndrome-X have abnormal coronary autoregulation, we hypothesized that they could have abnormal levels of EETs and/or HETEs or their metabolites. Whereas our preliminary results (with half as many patients) indicated that these patients had lower levels of DHETs (stable metabolite of EETs)34, results from the entire cohort reported here did not demonstrate any significant differences between syndrome-X patients and controls in any of the specific EETs, DHETs or HETEs or all of them combined. We measured eicosanoid levels at rest and not during stress. There were also no differences between patients with syndrome-X and controls in terms of sex hormones. This latter association has also been extensively studied and the role of sex hormones in the pathogenesis of syndrome-X is inconclusive35.
When the coronary arterial and venous systems are maximally vasodilated, the bottleneck to hyperemic flow is capillary resistance (which does not change with a coronary vasodilator)7. Therefore, reduced MBF reserve can also occur either because of reduced capillary number or diameter, or both, as can occur in hypertension, post-infarction, and in dilated cardiomyopathy8,9. MCE provides an accurate assessment of myocardial capillary density or MBV7,13,20. In this study, we did not see any difference in MBV at rest or stress between syndrome-X patients and controls, excluding the capillaries as the site of reduced MBF reserve in syndrome-X patients despite the fact that 50% of the syndrome-X patients had a history of hypertension. The hypertension was well treated and none of the patients had left ventricular hypertrophy.
MBF reserve can be attenuated from rheological factors independent of coronary vascular anatomy, such as elevated hematocrit and other variables that affect whole blood viscosity10–12. There was no difference in blood hematocrit between the two groups. Although we did not measure whole blood viscosity in these patients, factors that may affect it such as elevated serum triglycerides10 or glucose12 were not present in either group. Although the triglyceride and VLDL levels were higher in the syndrome-X women compared to controls they were still within the normal range and not high enough to cause any changes in rheology. Similarly, there is no reason to suspect that erythrocyte charge, deformability, or mobility would be different in the two groups.
Strengths and Weaknesses of the Study
Our study sample is small because it is very difficult to find patients who have classical effort angina and yet have a normal coronary angiogram. Most peri-menopausal women with chest pain do not have effort angina-the chest pain is atypical and can also occur at rest. In this study we intentionally selected patients who had an abnormal stress imaging test than simply a stress ECG for two reasons: one, the negative predictive value for coronary artery disease with this form of stress testing is relatively high compared to ECG, and two, because it provides a more definite evidence of myocardial ischemia that an ECG. Although these patients exhibited regional myocardial abnormalities during stress, we generated MCE data only from the mid-interventricular septum from all patients and controls. Our reasoning was that since syndrome-X involves the entire myocardium, we should be consistent in terms of placement of the region-of-interest. This region is also least likely to be affected by artifacts on MCE.
We did not assess endothelial function in these patients because an abnormal response to acetylcholine is not specific for syndrome-X and can be associated with atherosclerosis, which is common in women with syndrome-X as well as in men. Similarly we were unable to assess whether the abnormality of resistance vessels in syndrome-X women is structural or functional, or both. A study that examined retinal arteriolar dimensions in a large number of men and women at risk for atherosclerosis found that arteriolar narrowing was seen only in women36. Whether the same findings are present in the heart is unknown. Furthermore structural problems in the heart at the level of the resistance vessels is difficult to determine because of difficulties in sampling enough vessels during cardiac biopsy as well as fixing the heart on autopsy37,38.
We used high mechanical index intermittent harmonic imaging because it is the most robust form of MCE for generating quantitative date. The destruction of microbubbles that occurs with high mechanical index pulses produces excellent signal with relatively few micro-bubbles in the myocardium. This allows use of low microbubble concentrations that do not result in myocardial attenuation from left ventricular contrast39 and also permit myocardial data to be obtained where the relationship between myocardial microbubble concentration and signal intensity is in the normal range40. Finally this form of imaging has been validated using several independent approaches varying from radiolabeled microspheres13 to positron emission tomomography41.
Finally it has been reported that myocardial bridging and excessive coronary tortuosity can result in stress-induced myocardial perfusion abnormalities42. We carefully evaluated the coronary angiograms and computed tomograms of our subjects and did not find either of these abnormalities. In fact the coronary arteries in all our subjects were pristine and as normal as it can get on angiography. It is also possible that patients with mild atherosclerosis can also have microvascular disease in addition to coronary disease. We, however, did not include this group in our study.
Conclusions
Coronary autoregulation is abnormal in patients with Syndrome X (higher resting β and MBF and lower β reserve and stress MBF), which implicates the coronary resistance vessels as the site of microvascular abnormality. Capillary density (A) is normal in these patients as are factors that affect rheology. We were unable to find any associations between the abnormal MCE findings and vasoactive eicosanoids, female sex hormones, and C-reactive protein.
Acknowledgments
Supported in part by grants from the Bechen Family and the Alpha Phi Foundations, Portland, Oregon (for Dr Rinkevich) and from the National Institutes of Health, Bethesda, Maryland (R01 NS44313 and NS070837 for Dr. Alkayed and PO1-HD034430 for Dr. Kaul).
We would like to thank Dennis Koop, PhD for analysis of the eicosanoids in the OHSU metabolomics core laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kemp HG, Elliott WC, Gorlin R. The angina syndrome with normal coronary arteriography. Trans Assoc Am Physicians. 1967;80:59–70. [PubMed] [Google Scholar]
- 2.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 3.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 4.Cannon RO, 3rd, Epstein SE. “icrovascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RO, 3rd, Watson RM, Rosing DR, Epstein SE. Angina caused by reduced vasodilator reserve of the small coronary arteries. J Am Coll Cardiol. 1983;1:1359–1373. doi: 10.1016/s0735-1097(83)80037-0. [DOI] [PubMed] [Google Scholar]
- 6.Reis SE, Holubkov R, Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 7.Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C, Kaul S. Role of capillaries in determining coronary blood flow reserve: New insights using myocardial contrast echocardiography. Am J Physiol. 1999;277:H2363–H2372. doi: 10.1152/ajpheart.1999.277.6.H2363. [DOI] [PubMed] [Google Scholar]
- 8.Kaul S, Jayaweera AR. Myocardial capillaries and coronary flow reserve. J Am Coll Cardiol. 2008;52:1399–1401. doi: 10.1016/j.jacc.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Kaul S. What is coronary blood flow reserve? Insights using myocardial contrast echocardiography. J Echocardiogr. 2012;10:1–7. doi: 10.1007/s12574-011-0100-2. [DOI] [PubMed] [Google Scholar]
- 10.Rim S-J, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. The Decrease in Coronary Blood Flow Reserve During Hyperlipidemia Is Secondary to an Increase in Blood Viscosity. Circulation. 2001;104:2704–2709. doi: 10.1161/hc4701.099580. [DOI] [PubMed] [Google Scholar]
- 11.Bin JP, Doctor A, Lindner JR, Leong-Poi H, Fisher NG, Le ED, et al. Effects of Nitroglycerin on Erythrocyte Rheology and Oxygen Unloading: Novel Role of S-Nitrosohemoglobin in Relieving Myocardial Ischemia. Circulation. 2006;113:2502–2508. doi: 10.1161/CIRCULATIONAHA.106.627091. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan M, Herrero P, McGill JB, Bennik J, Heere B, Lesniak D, et al. The Effects of Plasma Insulin and Glucose on Myocardial Blood Flow in Patients with Type 1 Diabetes Mellitus. J Am Coll Cardiol. 2005;46:42–48. doi: 10.1016/j.jacc.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 13.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 14.Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 16.Trabold T, Buchgeister M, Kuttner A, Heuschmind M, Kopp AF, Schroder S, et al. Estimation of radiation exposure in 16-detector row computed tomography of the heart with retrospective ECG-gating. Rofo. 2003;175:1051–1055. doi: 10.1055/s-2003-40926. [DOI] [PubMed] [Google Scholar]
- 17.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 18.Linka AZ, Sklenar J, Wei K, Jayaweera AR, Skyba DM, Kaul S. Assessment of transmural distribution of myocardial perfusion with contrast echocardiography. Circulation. 1998;98:1912–1920. doi: 10.1161/01.cir.98.18.1912. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation. 2001;103:2560–2565. doi: 10.1161/01.cir.103.21.2560. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher RH, Fletcher SW, Wagner EH. The Essentials. 2. 1988. Clinical Epidemiology. [Google Scholar]
- 22.Panting JR, Gatehouse PD, Yang G-Z, Grotheus F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 23.Sestito A, Lanza GA, Di Monaco A, Lamendola P, Careri G, Tarzia P, et al. Relation between cardiovascular risk factors and coronary microvascular dysfunction in cardiac syndrome X. J Cardiovasc Med. 2011;12:322–327. doi: 10.2459/JCM.0b013e3283406479. [DOI] [PubMed] [Google Scholar]
- 24.Galassi AR, Crea F, Araujo LI, Lammertsma AA, Pupita G, Yamamoto Y, et al. Comparison of regional myocardial blood flow in syndrome X and one-vessel coronary disease. Am J Cardiol. 1993;72:134–139. doi: 10.1016/0002-9149(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 25.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 26.Papanicolaou MN, Califf RM, Hlatky MA, McKinnis RA, Harrell FE, Jr, Mark DB, et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol. 1986;58:1181–1187. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 27.Pitts WR, Lange RA, Cigarroa JE, Hillis LD. Repeat coronary angiography in patients with chest pain and previously normal coronary angiogram. Am J Cardiol. 1997;80:1086–1087. doi: 10.1016/s0002-9149(97)00610-3. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 29.Kassab GS, Lin DH, Fung YB. Morphometry of pig coronary arterial trees. Am J Physiol. 1993;265:H350–H365. doi: 10.1152/ajpheart.1993.265.1.H350. [DOI] [PubMed] [Google Scholar]
- 30.Kassab GS, Lin DH, Fung YB. Morphometry of pig coronary venous system. Am J Physiol. 1994;267:H2100–H2113. doi: 10.1152/ajpheart.1994.267.6.H2100. [DOI] [PubMed] [Google Scholar]
- 31.Kassab GS, Lin DH, Fung YB. Topology and dimensions of pig coronary capillary network. Am J Physiol. 1994;267:H319–H325. doi: 10.1152/ajpheart.1994.267.1.H319. [DOI] [PubMed] [Google Scholar]
- 32.Le DE, Nugent M, Zhao Y, Bragadeesh T, Alkayed N, Kaul S. Mechanism of Myogenic Response in Coronary Autoregulation. J Am Coll Cardiol. 2012;59:A103. [Google Scholar]
- 33.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 34.Gupta NC, Rinkevich D, Belcik T, Alkayed N, Coop D, Kaul S. Role of Epoxyeicosatrienoic Acids in Syndrome X in Women. Circulation. 2011;124:A16236. [Google Scholar]
- 35.Kaski JC. Overview of gender aspects of cardiac syndrome X. Cardiovasc Res. 2002;53:620–626. doi: 10.1016/s0008-6363(01)00460-6. [DOI] [PubMed] [Google Scholar]
- 36.Wong TY, Klein R, Sharrett AC, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The atherosclerosis risk in communities study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 37.Opherk D, Zebe H, Wethe E, Mall G, Durr C, Gravert B, et al. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981;63:817–825. doi: 10.1161/01.cir.63.4.817. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PJ, Livesly B, Oram S, Olsen ECJ, Armstrong P. Angina pectoris with normal coronary arteries: Transvenous myocardial biopsy in diagnosis. Lancet. 1974;2:677–680. doi: 10.1016/s0140-6736(74)93260-7. [DOI] [PubMed] [Google Scholar]
- 39.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Basis for stenosis detection using venous administration of microbubbles during myocar¬dial con¬trast echocardiography: bolus or continuous infusion? J Am Coll Cardiol. 1998;32:252–260. doi: 10.1016/s0735-1097(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 40.Le DE, Bin JP, Coggins M, Linder J, Wei K, Kaul S. Relation Between Myocardial Oxygen Consumption and Myocardial Blood Volume: A Study Using Myocardial Contrast Echocardiography. J Am Soc Echocardiogr. 2002;15:857–863. doi: 10.1067/mje.2002.121275. [DOI] [PubMed] [Google Scholar]
- 41.Vogel RMD, Indermühle A, Reinhardt J, Meier P, Siegrist PT, Namdar MMD, Kaufmann PA, Seiler C. The Quantification of Absolute Myocardial Perfusion in Humans by Contrast Echocardiography. Algorithm and Validation. J Am Coll Cardiol. 2005;45:754–62. doi: 10.1016/j.jacc.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 42.Gaibazzi N, Rigo F, Reverberi C. Severe coronary tortuosity or myocardial bridging in patients with chest pain, normal coronary arteries, and reversible myocardial perfusion defects. Am J Cardiol. 2011;108:973–978. doi: 10.1016/j.amjcard.2011.05.030. [DOI] [PubMed] [Google Scholar]