Abstract
Introduction
Obesity is a major risk factor for several musculoskeletal conditions that are characterized by an imbalance of tissue remodeling. Adult stem cells are closely associated with the remodeling and potential repair of several mesodermally derived tissues such as fat, bone, and cartilage. We hypothesized that obesity would alter the frequency, proliferation, multipotency, and immunophenotype of adult stem cells from a variety of tissues.
Materials and Methods
Bone marrow-derived mesenchymal stem cells (MSCs), subcutaneous adipose-derived stem cells (sqASCs), and infrapatellar fat pad-derived stem cells (IFP cells) were isolated from lean and high-fat diet induced obese mice, and their cellular properties were examined. To test the hypothesis that changes in stem cell properties were due to the increased systemic levels of free fatty acids (FFAs), we further investigated the effects of FFAs on lean stem cells in vitro.
Results
Obese mice showed a trend toward increased prevalence of MSCs and sqASCs in the stromal tissues. While no significant differences in cell proliferation were observed in vitro, the differentiation potential of all types of stem cells was altered by obesity. MSCs from obese mice demonstrated decreased adipogenic, osteogenic, and chondrogenic potential. Obese sqASCs and IFP cells showed increased adipogenic and osteogenic differentiation, but decreased chondrogenic ability. Obese MSCs also showed decreased CD105 and increased PDGFRα expression, consistent with decreased chondrogenic potential. FFA treatment of lean stem cells significantly altered their multipotency but did not completely recapitulate the properties of obese stem cells.
Conclusions
These findings support the hypothesis that obesity alters the properties of adult stem cells in a manner that depends on the cell source. These effects may be regulated in part by increased levels of FFAs, but may involve other obesity-associated cytokines. These findings contribute to our understanding of mesenchymal tissue remodeling with obesity, as well as the development of autologous stem cell therapies for obese patients.
Keywords: Mesenchymal Stem Cells, MSC, ASC, Infrapatellar fat pad, Osteoarthritis, Obesity, High-fat diet, Free fatty acids, Cell therapy, Regeneration, Adipose tissue, Adipokines
Introduction
Obesity is characterized by chronic low-grade systemic inflammation, which in addition to insulin resistance 1 is believed to contribute to several musculoskeletal diseases, such as osteoarthritis (OA) 2 and impaired tissue healing 3. Obesity due to a high-fat diet is associated with increased lipid deposits found not only in adipose tissue but also bone marrow 4, liver 5, and heart 6. Increased tissue adiposity is associated with elevation of several adipose-derived cytokines (adipokines), while apoptosis and lipolysis of adipocytes promote levels of circulating FFAs in the body. Importantly, altered cell functions have also been reported in obese individuals. For example, reduced numbers of endothelial cell have been observed in the bone marrow of obese patients 7. Wang et al. also found that contribution of bone marrow cells for tissue homeostasis was affected by diabetes and obesity 8. Results of these studies suggest altered tissue repair potential in obese patients. Furthermore, adipose tissue-resident macrophages in obese individuals appear to switch from an anti-inflammatory M2 phenotype to an inflammatory M1 phenotype, increasing inflammatory levels in obesity 9.
The mechanisms by which high fat diet-induced obesity alters cell function are not fully understood but may involve the chronic exposure to FFAs. FFAs can activate macrophages through JNK-dependent inflammatory pathways 10. Rat skeletal muscle cells cultured with FFAs have been reported to show impaired mitochondrial function 11. For osteoblasts and osteoclasts, FFAs have been suggested to modulate bone formation and resorption 12. Although it is still unclear whether FFAs have an impact on chondrocyte function, accumulation of lipids in the chondrocytes has been shown to correlate positively with the degree of OA in patients, implying possible involvement of FFAs in cartilage degeneration 13.
Mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into specific lineages including adipocytes, osteoblasts and chondrocytes14. This potential allows MSCs to play a significant role in tissue repair and remodeling, particularly within the marrow itself 15. In addition to their presence in bone marrow, similar but distinct populations of cells with multilineage potential have been recently identified in various tissues such as subcutaneous fat (sqASCs)16 and infrapatellar fat pad (IFP cells) 17. With high-fat diet induced obesity, these tissues are likely to be exposed to high concentrations of FFAs, and such a change of microenvironment may alter the characteristics of stem cells resident in these tissues. Indeed, stem cells harvested from the omental fat (visceral adipose tissue) of obese patients exhibit impaired multipotency 18. In a simulated obese environment containing the conditioned medium from FFA-treated adipocytes, MSCs isolated from lean mice demonstrated decreased adipogenesis but enhanced osteogenesis 19. However, the effects of obesity or FFAs on the intrinsic cellular properties of adult stem cells, such as in vivo frequency, self-renewal ability, or multilineage differentiation capacity, is still largely unknown.
In the present study, we investigated the effects of diet-induced obesity on the properties and function of several adult stem cell populations. We first isolated MSCs, sqASCs, and IFP cells from lean and high-fat diet induced obese mice and then compared their in vivo frequency, proliferation capacity, multipotency, and immunophenotype. To examine one potential mechanism by which a lard-enriched high-fat diet affects stem cell multipotency, we further differentiated lean stem cells in an in vitro environment rich in FFAs. We used a combination of palmitic acid, stearic acid (both saturated FA), and oleic acid (monounsaturated FA), as recent studies have shown that lard-enriched high-fat diet promotes levels of these FFAs in blood and adipose tissues 20, 21.
Materials and Methods
Animals
Male C57BL/6J mice fed either a high-fat diet (D12492, 60% energy from fat, Research Diets, Inc.) or a low-fat diet (D12450B, 10% energy from fat, Research Diets, Inc.) for 14 weeks were obtained from The Jackson Laboratory. Mice were sacrificed at 20 weeks of age in accordance with an Institutional Animal Care and Use Committee (IACUC) approved protocol at Duke University.
Cell isolation and expansion
Bones (femurs and tibias), subcutaneous adipose tissue (inguinal fat pad), and the IFP were collected from lean and obese mice and digested at 37 °C with 0.2% collagenase type I (Worthington) for 1–1.5 hours 22. MSCs were purified for Sca-1+ PDGFRα+ CD45− Ter119− from the bone fragments as previously described 23, 24 and sqASCs were purified for Sca-1+ CD34+ CD31− CD45− Ter119− from the digested inguinal fat by the method described by Rodeheffer et al. with a slight modification 25. In preliminary studies, the same marker combination as sqASCs was used to isolate a similar cell population from the epididymal fat pad (visASCs). However, due to their inability to differentiation into the chondrogenic or adipogenic lineages, these cells were not included in the overall analysis and are reported in the supplemental data. A Cytomation MoFlo® sorter (Beckman Coulter) with 100 μm nozzle was used to sort cells with designated markers (all antibodies from Biolegend). Due to the small size of the joint fat pad, stem cells were directly derived as the adherent cell fraction of the IFP after collagenase digestion 17.
Freshly sorted MSCs and sqASCs were plated at 100 cells/cm2 and 3,000 cells/cm2, respectively. IFP cells were seeded at 1,500 cells/cm2 for the primary passage. All the cells were cultured in expansion medium consisting of α-Modified Eagle’s Medium (αMEM, Invitrogen), 20% lot-selected fetal bovine serum (FBS, Sigma), and 1% penicillin/streptomycin/fungizone (P/S/F, Invitrogen) in hypoxic conditions (37 °C, 2% O2, 5% CO2, remaining gas N2). In previous studies, we have shown that these culture conditions allow for rapid expansion of mouse stem cells while maintaining their multipotency24. After 8 days with media changes every 3 days, cells were trypsinized using 0.25% trypsin-EDTA (Sigma) and plated at 3,000 cells/cm2. Cells were passaged every 5–6 days upon 90% confluence.
Multilineage differentiation
Passage three cells were pooled from 2 sets of isolations (n = 6 mice per isolation) and differentiated into adipo-, osteo-, and chondrogenic lineages to evaluate their multipotency. For adipogenesis, 10,000 cells were cultured in wells of 48 well plates for 2 days in expansion medium. Media was then switched to control medium consisting of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Lonza) with 10% FBS and 1% P/S/F or adipogenic differentiation medium consisting of control medium supplemented with (all from Sigma) 33 μM biotin, 17 μM pantothenate, 1 μM bovine insulin, 1 μM dexamethasone, and for the first three days 250 μM isobutylmethylxanthine and 2 μM rosiglitazone (Avandia™, GlaxoSmithKline). Cells were cultured either with control or adipogenic medium for 14 days with media changes every 3 days. Lipid droplets were stained by 0.5% Oil Red O (Sigma), which was released and quantified by absorbance at 535 nm and normalized to DNA content measured by Quant-iT™ PicoGreen® (Invitrogen). For osteogenesis, 10,000 cells were plated in wells of 48 well plates for 2 days in expansion medium. Media was then switched to control medium consisting of DMEM (4.5 g/L glucose, Invitrogen) with 10% FBS and 1% P/S/F or osteogenic differentiation medium consisting of control medium plus 10 mM β-glycerophosphate (Sigma), 250 μM ascorbate (Sigma), 2.5 μM retinoic acid (Sigma), and 50 ng/ml human bone morphogenetic protein-2 (BMP-2, R&D systems) for 21 days with media changes every 3 days. Mineral deposits were stained by 2% Alizarin Red S (Electron Microscopy Sciences). The stain was then released by heated acid extraction 26 and normalized to DNA. For chondrogenesis, 250,000 cells were centrifuged in 15 ml polypropylene tubes at 300 g for 5 minutes to form pellets. After 2 days, media were switched to control medium consisting of DMEM (4.5 g/L glucose, Invitrogen), 1% insulin-transferrin-selenous acid (ITS+, BD), 50 μg/ml ascorbate (Sigma), 40 μg/ml proline (Sigma), and 1% P/S (Sigma) or chondrogenic differentiation medium consisting of control medium supplemented with 10 ng/ml human transforming growth factor-β3 (TGF-β3, R&D systems) and 500 ng/ml mouse bone morphogenetic protein-6 (BMP-6, R&D systems). For MSCs and IFP cells, serum free control and chondrogenic medium were used but for sqASCs both media were supplemented with 10% FBS. After 28 days, pellets were analyzed for their glycosaminoglycan (GAG) and DNA content by 1,9-dimethylmethylene blue (DMB) and PicoGreen assay, respectively. Some pellets were also processed for histochemical staining for sulfated GAGs by 1% Alcian Blue (pH = 1, Acros) and immunohistochemical labeling for collagen type II (Hybridoma Bank). For FFA treated groups, cells were performed as the same differentiation methods as described above but with the supplement of FFA mixture or vehicle control as appropriate in their differentiation medium.
FFA preparation and treatment
To simulate an obese environment rich in saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA), a FFA mixture (SA/MUFA) containing palmitic, stearic and oleic acids (NuChek Prep) was used. 3.3 mM stock FFA mixture was prepared by a method described by Nguyen et al. 10. Briefly, FFA was dissolved in ethanol and mixed with DMEM (4.5 g/L glucose, Invitrogen) supplemented with fatty acid-free bovine serum albumin (BSA, Sigma). A ratio of 5:1 FFA:BSA was used to mimic elevated FFA levels. The FFA-BSA solution was then conjugated at 37°C for 1.5 hr until homogeneous. A vehicle control (Control) that contained BSA with the same volume of ethanol but no FFA was also prepared. The FFA mixture was aliquoted and stored at −20 °C until use. In preliminary studies, we examined effects of several different concentrations (150 μM, 250 μM and 500 μM) of FFA to test the toxicity of FFA on stem cells. We did not observe any cell death under these conditions. These cells also maintained their spindle-shaped cell morphology and were able to reach confluence at a similar rate, independent of FFA concentration (Supplemental Figure 1). The final concentration of individual FFA used in the differentiation culture medium was 500 μM.
Immunophenotype analysis
Passage three cells were divided into aliquots of 100,000 cells, treated with Fc block (CD16/32) for 10 minutes at 4 °C to reduce unspecific binding, then incubated for 30 minutes at 4 °C with antibodies against following cell surface markers or appropriate isotype controls (all from Biolegend): CD45, CD31, Ter119, CD44, CD11b, platelet-derived growth factor receptor α (PDGFRα), CD34, CD105, stem cell antigen-1 (Sca-1). A C6 benchtop flow cytometer (Accuri Cytometers) was used for analysis and percentages obtained by subtracting the value of isotype controls.
Statistical analysis
Statistical analysis was carried out using a 2-tailed Student’s t-test for comparison of two groups (α = 0.05). Values are expressed as mean ± SEM.
Results
Obesity alters stem cell percentage in bone marrow and adipose tissues
Obese mice weighed significantly more than lean mice (40.25 ± 1.17g obese vs. 31.14 ± 0.35g lean, p < 0.001). Inguinal fat pads from obese mice were a larger percentage of total body weight as compared to lean mice (2.43% ± 0.17% obese vs. 0.66% ± 0.03% lean, p < 0.05). Results were averaged from ≥ 15 mice per group with mean ± SEM displayed.
A highly purified population of MSCs was isolated based on a specific combination of cell surface markers 23. MSCs were identified as cells that are double-negative for CD45 and Ter119 (hematopoietic cell markers) and double-positive for Sca-1 and PDGFRα (stem cell markers) (Figure 1A). Obesity showed a trend toward increased in vivo frequency of MSCs in obese mice (p = 0.07; Figure 1B). There was no significant difference in the percentage of CD45− Ter119− population in bone marrow cells between lean and obese mice (Figure 1C). Interestingly, among this double-negative cell population, obese mice had a significantly higher percentage of Sca-1+ PDGFRα+ cells. Results were averaged from 5 independent isolations with mean ± SEM displayed (n ≥ 4 mice per isolation).
Figure 1.
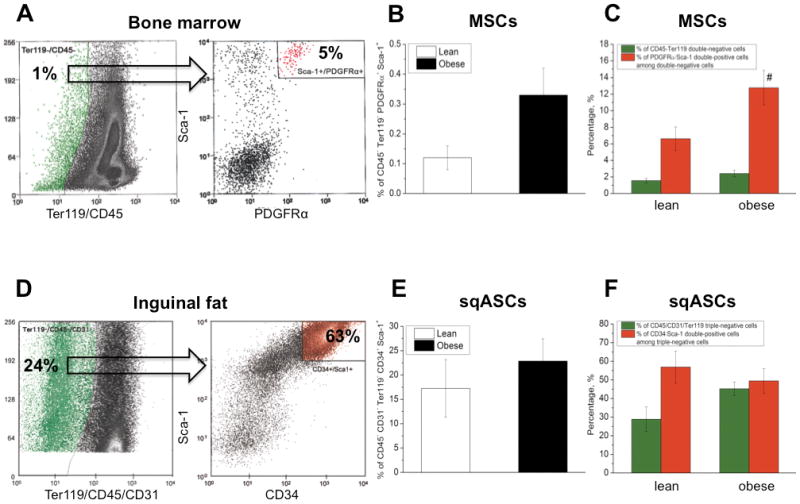
Representative stem cell sorting result of (A–C) bone marrow and (D–F) inguinal fat from lean mice. In the bone marrow of lean mice, approximately 1% of stromal cells were Ter119/CD45 double-negative (green dots). Only 5% of these cells were Sca-1/PDGFRα double-positive (red dots) and the cells that were Sca-1+ PDGFRα+ Ter119− CD45− were identified as MSCs. In inguinal fat of lean mice, around 24% of adipose-tissue stromal cells were CD31/Ter119/CD45 triple-negative (green dots). Among this population, 63% were Sca-1/CD34 double-positive (red dots) and sqASCs were designated as the cells that were Sca-1+ CD34+ CD31− Ter119− CD45−. Obese mice showed a trend toward increased in vivo frequency of (B) MSCs (n = 5 isolations) and (E) sqASCs (n = 3 isolations). n ≥ 4 mice per isolation. In the stem cell population, obesity significantly increased (C) the percentage of Sca-1+ PDGFRα+ cells (red bar) among Ter119− CD45− cell population (green bar) in the bone marrow (# p < 0.05 vs. corresponding lean cell population). (F) Obese mice showed a trend toward increased CD45− CD31− Ter119− cells (green bar) in the inguinal fat.
To harvest a pure stem cell population from the inguinal fat, a similar sorting strategy was used but a slightly different combination of cell markers. CD31 has been used to distinguish endothelial progenitor cells from stem cells 27, while CD34 has been reported to be expressed on freshly isolated adipose-derived stem cells 28. Therefore, we defined sqASCs as cells triple-negative for CD31, CD45 and Ter119 but double-positive for Sca-1 and CD34 (Figure 1D). In inguinal fat, obese mice showed a moderated increase in sqASCs although not significant (Figure 1E). Obesity also had a trend toward an increased percentage of CD45− CD31− Ter119− cells in the inguinal fat compared to lean mice (p = 0.09; Figure 1F) but among this triple-negative cell population, no significant difference was observed in CD34 and Sca-1 double-positive cells between lean and obese mice. Results were averaged from 3 independent isolations with mean ± SEM displayed (n ≥ 4 mice per isolation). The cell sorting yield of MSCs and sqASCs per mouse is summarized in Supplemental Table 1.
MSCs, sqASCs and IFP cells exhibit similar rates of proliferation in vitro
The overall morphology of stem cells was similar between lean and obese mice. All stem cells exhibited fibroblastic-like morphologies, although sqASCs displayed larger cell protrusions than MSCs and IFP cells (Figure 2A–F). Cells were cultured through five passages to investigate their in vitro proliferation (n = 3 independent experiments). All the cell types proliferated robustly under hypoxic conditions. Lean MSCs exhibited greater expansion, but it was not significantly different from obese MSCs (Figure 2G). Both obese sqASCs and IFP cells showed a trend toward increased proliferation as compared to their corresponding lean cell types (Figure 2H–I).
Figure 2.
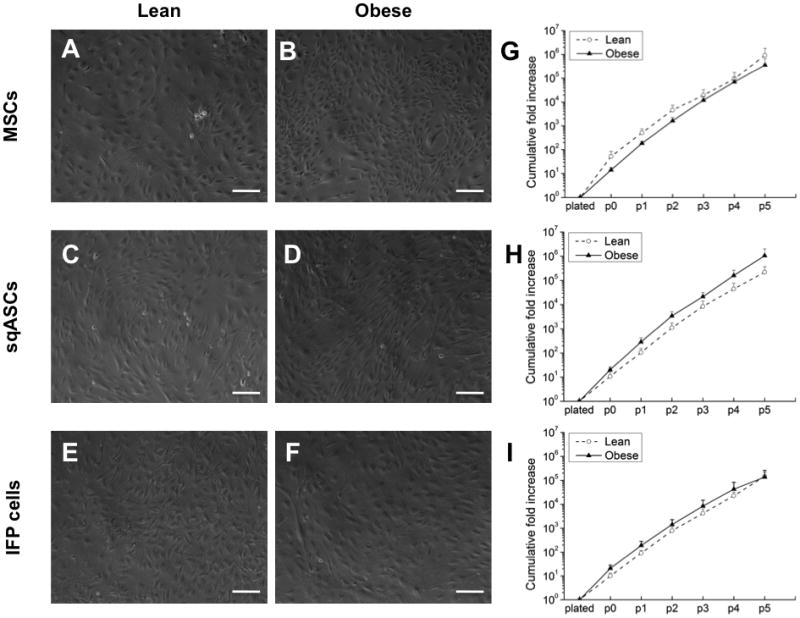
Morphology of (A, C, E) lean and (B, D, F) obese stem cells at passage 3. The cumulative fold increase during expansion under hypoxic conditions through passage 5 of (G) MSCs (H) sqASCs and (I) IFP cells harvested from lean and obese mice. Obese sqASCs and IFP cells showed a trend toward increased proliferation, while obese MSCs had a trend toward decreased cell growth. Results averaged from 3 independent isolations with mean ± SEM displayed (n ≥ 3 mice per isolation). Scale bar is 100 μm.
MSCs, sqASCs and IFP cells exhibit distinct levels of surface antigens
Immunophenotype analysis was performed at passage three for each cell type (Table 1). All the stem cells were negative for hematopoietic cell markers (CD11b, CD45 and Ter119; all < 1%) and endothelial progenitor cell marker (CD31; all < 1%) but positive for Sca-1 (all ≥ 99%). Most cell types were negative for CD34, although IFP cells showed some CD34 expression. Both MSCs and IFP cells showed high percentage of cells expressing CD44 and PDGFRα, while sqASCs had fewer cells expressing these two markers. Obese MSCs showed a trend towards a lower percentage of cells expressing CD105 but a significantly higher percentage expressing PDGFRα as compared to lean MSCs (for CD105, p = 0.06; for PDGFRα, p < 0.05).
Table 1.
Immunophenotype analysis for passage 3 stem cells from lean and obese mice. In response to obesity, sqASCs and IFP cells did not significantly alter surface marker expression. Interestingly, however, obesity significantly increased percentage of the MSCs expressing PDGFRα but a trend toward to decrease CD105 level. Results from 3 independent experiments for PDGFRα and CD105 in lean and obese MSCs with mean ± SEM displayed (n ≥ 3 mice per experiment. Values with different superscript letters are significantly different; p < 0.05). For other cell types, results averaged from of 2 independent experiments (n ≥ 3 mice per experiment).
| Surface Marker | MSCs | sqASCs | IFP cells | |||
|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Lean | Obese | |
| CD11b | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.5% | ≤ 0.3% |
|
| ||||||
| CD45 | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.3% | ≤ 0.3% |
|
| ||||||
| TER119 | ≤ 0.1% | ≤ 0.1% | ≤ 0.3% | ≤ 0.3% | ≤ 0.3% | ≤ 0.1% |
|
| ||||||
| CD31 | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.1% | ≤ 0.3% | ≤ 0.3% |
|
| ||||||
| CD34 | ≤ 0.5% | ≤ 0.5% | 1.95% | ≤ 0.5% | 1.65% | 1.27% |
|
| ||||||
| Sca-1 | ≥ 99% | ≥ 99% | ≥ 99% | ≥ 99% | ≥ 99% | ≥ 99% |
|
| ||||||
| CD44 | > 95% | > 95% | 90% | 61% | > 95% | > 95% |
|
| ||||||
| PDGFRα | 68.9±6%a | 82.7±7%b | 27.3% | 27.5% | 95% | 93.5% |
|
| ||||||
| CD105 | 68.9±11%a | 31.3±10%a | 64.2% | 60.8% | ≥ 85% | ≥ 85% |
Obesity alters the multipotency of adult stem cells in a manner that depends on the tissue source of the cells
Passage three cells were differentiated into adipo-, osteo- and chondrogenic lineages (each cell type was pooled from two independent isolations, n = 6 mice per isolation). For MSCs, obese cells showed significantly reduced adipogenic (Figure 3A–B) and osteogenic potential (Figure 3C–D) compared to lean MSCs (for adipogenesis, Figure 3E,; for osteogenesis, Figure 3F). When MSCs were differentiated into chondrocytes, obese MSCs showed a trend toward reduced GAG/DNA ratio, less Alcian Blue and collagen type II staining intensity compared to lean MSCs (p = 0.07; Figure 4A–E). When the GAG/DNA ratio was normalized to the GAG/DNA ratio of pellets under control conditions, this trend was significant (data not shown).
Figure 3.
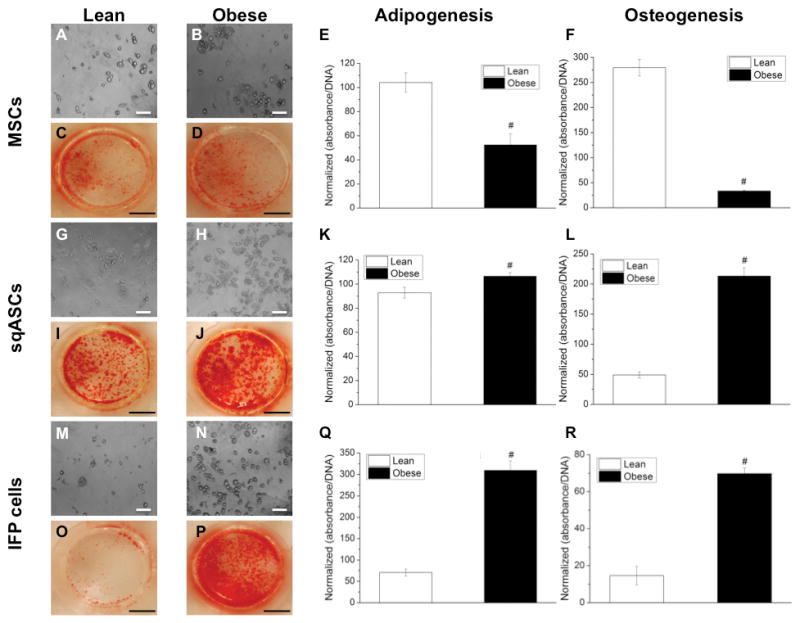
Adipogenesis and osteogenesis of stem cells harvested from lean and obese mice. Lipid droplets accumulation in (A, G, M) lean and (B, H, N) obese stem cells after 14 days culture in adipogenic medium. Cells were stained with 0.5% Oil Red O. Stain was then released and normalized to DNA content to quantify adipogenic potential of (E) MSCs, (K) sqASCs and (Q) IFP cells. For osteogenesis, calcium mineral deposits stained with 2% Alizarin Red S in (C, I, O) lean and (D, J, P) obese stem cells after 21 days culture in osteogenic medium. Stain was then extracted and normalized to DNA content to determine osteogenic capacity of (F) MSCs, (L) sqASCs and (R) IFP cells. Results from≥ 5 samples per group of the cells pooled from two independent isolations (n = 6 mice per isolation) with mean ± SEM displayed. # p < 0.05 vs. corresponding lean cell type by t-test. Scale bar is 100 μm for adipo- and 5 mm for osteogenesis, respectively.
Figure 4.
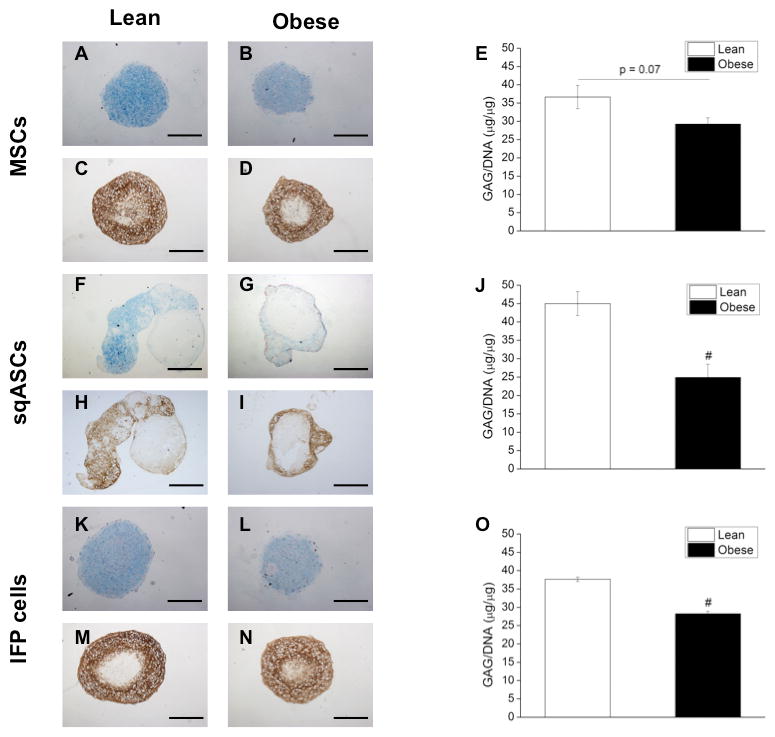
Sulfated GAGs and proteoglycans of chondrogenic pellets from (A, F, K) lean stem cells and (B, G, L) obese stem cells after 28 days pellet culture in chondrogenic medium was detected by 1% Alcian Blue staining (pH = 1). Collagen II immunohistochemical staining was also performed for the pellets from (C, H, M) lean stem cells and (D, I, N) obese stem cells. Quantification of GAG content was performed by DMB assay and the value was then further normalized to DNA to determine chondrogenic potential of (E) MSCs, (J) sqASCs and (O) IFP cells. Obese MSCs exhibited a trend toward decreased chondrogenesis (p = 0.07 vs. lean MSCs), while obese sqASCs and IFP cells showed significantly decreased chondrogenic capacity. Results from ≥ 4 pellets per group of the cells pooled from two independent isolations (n = 6 mice per isolation) with mean ± SEM displayed. # p < 0.05 vs. corresponding lean cell type by t-test. Scale bar is 500 μm.
For sqASCs, obese cells showed significantly enhanced adipogenic (Figure 3G–H) and osteogenic differentiation (Figure 3I–J) compared to lean sqASCs (for adipogenesis, Figure 3K; for osteogenesis, Figure 3L). However, lean sqASCs exhibited greater chondrogenic potential than obese sqASCs (Figure 4F–J).
Similar to sqASCs, obese IFP cells demonstrated significantly increased adipogenesis (Figure 3M–N) and osteogenesis (Figure 3O–P) compared to lean IFP cells (for adipogenesis, Figure 3Q; for osteogenesis, Figure 3R). When differentiated into the chondrogenic lineage, lean IFP cells showed significantly higher GAG/DNA ratio than obese IFP cells (Figure 4K–O).
While lean IFP cells had significantly higher GAG content per pellet compared to obese IFP cells, other lean cell types exhibited a trend toward higher GAG content per pellet compared to corresponding obese cell types (Supplemental Figure 2A–C).
visASCs from the epididymal fat pad showed poor in vitro differentiation capacity for adipogenic and chondrogenic lineages, and thus the effects of obesity were not examined in this cell type (Supplemental Figure 3).
A table summarizing the multilineage differentiation capacity of lean and obese stem cells isolated from different tissues is provided in Supplemental Table 2(A).
FFA treatment alters the multipotency of lean adult stem cells
To examine the potential influence of increased FFAs, high concentrations of palmitic and stearic acid as well as oleic acid (SA/MUFA mixture) were supplemented into the differentiation media of lean stem cells. When treated with SA/MUFA, lean MSCs demonstrated significantly enhanced adipogenesis and osteogenesis compared to vehicle control (for adipogenesis, Figure 5A; for osteogenesis, Figure 5B). However, there was no significant difference in chondrogenesis between SA/MUFA treated and vehicle control (Figure 5C).
Figure 5.
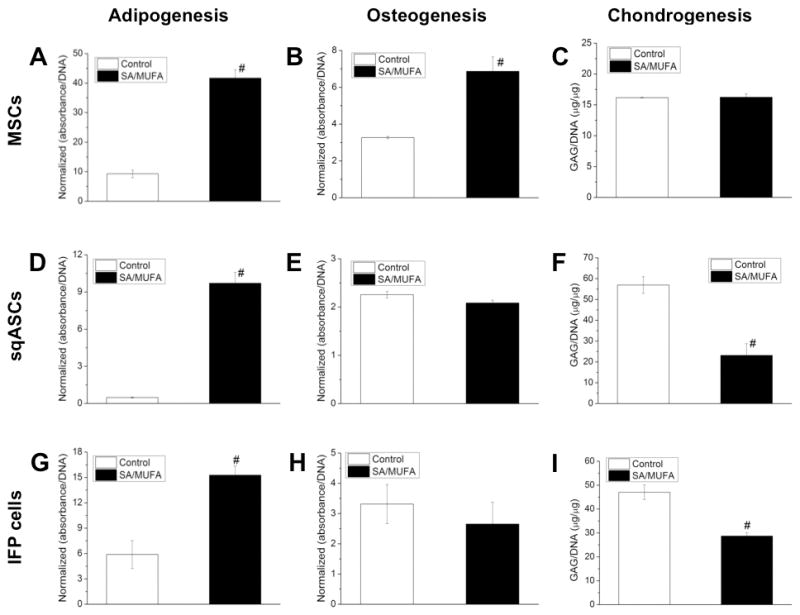
Multi-lineage differentiation of lean stem cells with supplement of SA/MUFA. (A, D, G) all the stem cells treated with SA/MUFA demonstrated increased adipogenesis. SA/MUFA also significantly enhanced (B) osteogenesis of MSCs but did not significantly affect (E, H) osteogenic potential of sqASCs and IFP cells. However, MSCs did not alter (C) chondrogenic potential in response to SA/MUFA but the treatment of SA/MUFA significantly decreased (F, I) chondrogenic capacity of sqASCs and IFP cells. Results from 5 samples (for adipogenesis and osteogenesis) or ≥ 4 pellets (for chondrogenesis) per group of the cells pooled from two independent isolations (n = 6 mice per isolation) with mean ± SEM displayed. # p < 0.05 vs. vehicle control by t-test.
When treated with SA/MUFA, lean sqASCs demonstrated significantly enhanced adipogenesis compared to vehicle control (Figure 5D). However, SA/MUFA did not alter the osteogenesis of lean sqASCs (Figure 5E). Supplementation with SA/MUFA significantly decreased the chondrogenic capacity (Figure 5F).
Similar to lean sqASCs, lean IFP cells exhibited enhanced adipogenesis but maintained osteogenic capacity when treated with SA/MUFA (for adipogenesis, Figure 5G; for osteogenesis, Figure 5H). SA/MUFA treatment also decreased chondrogenesis of lean IFP cells (Figure 5I).
A table summarizing the multilineage differentiation capacity of FFA-treated lean stem cells and vehicle control is provided in Supplemental Table 2(B).
Discussion
The findings of this study show that high-fat diet induced obesity significantly alters a number of cellular properties of adult stem cells derived from bone marrow, subcutaneous fat, and the infrapatellar fat pad. Obesity appeared to increase the in vivo frequency of stem cells and alter their multilineage potential in a manner that was dependent on the cell source. Some of these effects were reproduced by culture with FFAs, suggesting that the increase systemic levels of FFAs associated with a high-fat diet may be responsible in part for the observed effects.
Our finding of an increased sqASC population is consistent with a previous study showing that the percentage of proliferating CD34+/CD31− adipose-tissue progenitors was increased in class I obese patients (BMI 30–34.9 kg/m2) 29. Increased proliferation of CD34+/Sca-1+ adipose tissue progenitors was also observed in subcutaneous fat using an in vivo bromodeoxyuridine labeling method after mice were exposed to a high-fat diet 30. Our obese sqASCs also showed a trend toward increased in vitro proliferation capacity compared to lean sqASCs. This result is supported by a previous study showing adipose progenitor cells obtained from subcutaneous fat pad of high BMI individuals exhibited higher proliferation than those from low BMI individuals 31. The expanded stem cell populations may reflect increased adipogenic differentiation in the marrow and possibly a commitment into an endothelial lineage for adapting hypoxic conditions in vivo 32. There is also evidence that stem cells are associated with vasculature 33, and increased tissue adiposity may increase vascularity and thus the stem cell pool.
Obesity had a consistent inhibitory effect on multilineage potential of MSCs. Our results are in agreement with a recent study showing that stem cells isolated from lean individuals demonstrated better multipotency in mesodermal lineages than those from obese individuals 18. MSCs are the common precursor cells for both adipocytes and osteoblasts in bone marrow 34. The relationships between marrow fat and bone density in response to obesity is not fully understood and is an area of intensive investigations. Increased body mass seems to encourage bone formation, but inflammation due to excessive fat tissues may be detrimental to osteogenesis 35, 36. Our findings provide evidence that obesity results in reduced in vitro adipogenic and osteogenic capacity of MSCs.
Obese sqASCs and IFP cells both exhibited significantly enhanced adipogenesis and a trend toward higher proliferation as compared to their corresponding lean cell types. These findings imply that subcutaneous fat and infrapatellar fat pad may have increased fat-storing capacities during weight gain. Several studies have shown that subcutaneous adipose tissues expand fat mass by hyperplasia (increased adipocyte numbers) instead of hypertrophy (increased adipocyte size) which particularly occurs in visceral fat depot such as epididymal fat pad 30. Increased adipocytes can arise from the proliferation and adipogenic differentiation of sqASCs, as adipocytes are terminally differentiated cells and incapable of proliferation 37. In our preliminary studies, we also observed that adult stem cells isolated from epididymal fat pad had lower adipogenesis compared to sqASCs (Supplemental Figure 3). Compared to large adipocytes, new smaller adipocytes have better capacity in up-taking excess FFAs and therefore protect adipocytes from apoptosis 38, which may inhibit infiltration of inflammatory macrophages into adipose tissues 39. To investigate whether macrophages infiltrate into obese joint fat pads, inguinal fat, and epididymal fat, these tissues harvested from lean and obese mice were stained with an antibody against epidermal growth factor seven transmembrane protein (F4/80) expressed on macrophages. Interestingly, in contrast to obese visceral fat, we did not observe massively infiltrated macrophages in the obese IFP and inguinal fat pad (Supplemental Figure 4).
In this study, we also observed that both sqASCs and IFP cells from obese mice exhibited significantly higher in vitro osteogenesis. Recent studies have suggested that osteogenesis is closely linked to Wnt/β-catenin signaling pathways 40 and microRNA expressions such as miR-26a, -133 and -135 41, 42. Whether obese sqASCs and IFP cells have dysregulated Wnt signaling or altered microRNA levels requires further investigation.
Several approaches for cartilage repair and regeneration rely on chondrogenesis of stem cells. For example, microfracture is a procedure to stimulate MSC migration directly from bone marrow into focal cartilage defects 43. Scaffolds seeded with culture-expanded autologous adult stem cells for cartilage repair are also currently undergoing intensive investigation44. However, the potential impact of obesity on the intrinsic chondrogenic ability of these cells is not well understood. Our data show that obese MSCs, sqASCs and IFP cells exhibit decreased production of GAGs and collagen type II, implying that obesity might interfere with cartilage repair during autologous stem cell therapy.
While our results indicate that obesity significantly affected the multipotency of stem cells, we did not observe changes in antigen expression levels in sqASCs and IFP cells after exposed to a high-fat diet. Nevertheless, we cannot exclude possible alterations in other antigens such as Toll-like receptors (TLRs), which have been shown to modulate stem cell functions 45. Interestingly, obese MSCs did show a trend toward decreased percentage of the cells expressing CD105. CD105, also known as endoglin, is an accessory protein in mediating signaling of TGF-β superfamily 46, and it is well known that TGF-β up-regulates the key transcription factor Sox9, critical for the commitment of MSCs to the chondrogenic lineage 47. It has been shown that downstream SMAD signaling of CD105 is required for onset of chondrogenesis of MSCs 48. Furthermore, we also found that obese MSCs had a significantly increased percentage of cells expressing PDGFRα. Previous studies have suggested that PDGF-AA promotes early stages of cartilage development of chicken embryo but may inhibit chondrogenesis at later stages 49. Recent studies have shown that hypoxia-conditioned human embryonic stem cells chondrogenesis was associated with a high CD105 but low PDGRFα expression profile 50. Our results in accordance with above studies indicate that the decreased chondrogenic capacity of obese MSCs could be potentially due to down-regulated expression of CD105 but up-regulated expression of PDGFRα.
Our findings also demonstrated that SA/MUFA strongly affected the multilineage potential of lean stem cells. MSCs treated with SA/MUFA up-regulated both adipogenic and osteogenic potentials but showed no marked alteration in chondrogenic ability. Our result of enhanced osteogenesis by SA/MUFA is supported by previous findings that oleic acid significantly promoted osteogenesis of mouse mesenchymal cells in the presence of BMP-2 51. Saturated FFAs, acting as lipopolysaccharide (LPS), can activate TLR4 via triggering MyD88-dependent pathways 52, shifting cytokine secretion profile in MSCs 53. Furthermore, human MSCs with prolonged LPS treatment have been shown to exhibit enhanced osteogenic capacity 54. It is therefore plausible that palmitic and stearic acids in the FFA mixture we used both contributed to promote osteogenic differentiation of lean MSCs. However, murine MSCs showed enhanced osteogenesis but decreased adipogenesis when cultured in a simulated obese environment containing the conditioned medium of palmitic and oleic acid-treated adipocytes 19. This discrepancy may be a result of the different culture methods. The conditioned medium secreted by FFA-treated adipocytes might contain other cytokines that modulate stem cell functions.
We also observed that lean sqASCs and IFP cells had a similar response to FFA treatment in differentiation into three mesodermal lineages, although these cells exhibit distinct phenotypes. To date, few studies have investigated how SFA and MUFA modulate functions of adipose tissue stem cells, despite the fact that these two types of FFAs constitute an important part of our diets 55. ManicKam et al. reported that no significant alteration in lipid accumulation was observed when 3T3-L1 cells were treated with either stearic or oleic acid56. However, pre-adipocyte cell lines might have different responses to obesity compared to multipotent stem cells that are higher in the stem cell hierarchy. Future investigations are necessary to elucidate the molecular mechanism(s) by which SFA and MUFA modulate multipotency of stem cells.
Another significant finding of this study is that FFA-altered multipotency of lean stem cells did not completely recapitulate the multipotency of stem cells directly harvested from obese mice. This result suggests that the exposure to FFAs alone cannot explain the alterations of stem cell functions in the obese environment, and it also implies that other obesity-associated cytokines might act synergically with FFAs on stem cells. Indeed, leptin, an adipokine often up-regulated in obesity, has been shown to modulate the balance between adipogenesis and osteogenesis of mouse and human mesenchymal progenitor cells 57. In addition to adipokines, a number of studies have also reported that inflammatory cytokines such as TNF-α can inhibit osteogenesis of stem cells58. Moreover, that IL-17A produced by CD4+ Th17 cells, a possible infiltrating immune cells during weight gain, significantly suppresses adipogenic differentiation of human MSCs via the COX-2/prostaglandin E2 pathway59.
One potential limitation of the current study is that multipotency of the stem cells was evaluated only by using histological and biochemical assays. Although previous studies have shown that these assays are reflected by similar changes in gene expression 60, detailed gene expression analysis may provide insight into whether lean and obese stem cells have a temporal difference in response to differentiation-inducing signals. To examine the specific mechanism(s) underlying the alteration of stem cell functions caused by obesity or FFA treatment, future studies may wish to investigate the genetic and/or epigenetic profile of these cells following differentiation into various lineages.
Conclusions
Our results indicate that obesity significantly alters the characteristics of stem cells resident in various tissues. This study is significant for the development of autologous stem cell therapy for obese patients as obesity is highly prevalent in the US and continuously increasing in other countries. An improved understanding of the effects of obesity on the adult stem cell pool may help in optimizing the response of obese stem cells, which may be necessary to enhance their therapeutic capacity. Our finding of an increased stem cell pool with altered properties in various obese tissues extends our understanding of the mechanisms underlying the remodeling of musculoskeletal tissues in obesity.
Supplementary Material
Acknowledgments
Funding sources:
Supported by Taiwan GSSA graduate fellowship, NIH grants AR50245, AG15768, AR48852, AR48182, the Arthritis Foundation, the Howard Hughes Medical Institute, and a NSF graduate fellowship.
The authors thank Nancy Martin of the Flow Cytometry Shared Resource of the Duke Cancer Institute, as well as Steve Johnson of Orthopaedic Research Laboratories for assistance with animal handling.
Footnotes
Author contributions:
CLW planned and performed the experiments and wrote the paper, BOD assisted with cell isolations, DJ assisted and performed the experiments, FG conceived of the experiments, directed the project, and wrote the paper.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose
References
- 1.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121(6):2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12 (4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo JH, Wong MS, Perez RV, Li CS, Lin TC, Troppmann C. Renal Transplant Wound Complications in the Modern Era of Obesity. J Surg Res. 2011;173(2):216–223. doi: 10.1016/j.jss.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56(4):952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O’Donnell CJ, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging. 2010;3(5):559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire TR, Brusnahan SK, Bilek LD, Jackson JD, Kessinger MA, Berger AM, et al. Inflammation associated with obesity: relationship with blood and bone marrow endothelial cells. Obesity (Silver Spring) 2011;19(11):2130–2136. doi: 10.1038/oby.2011.246. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Seifert RA, Bowen-Pope DF, Kregel KC, Dunnwald M, Schatteman GC. Diabetes and aging alter bone marrow contributions to tissue maintenance. Int J Physiol Pathophysiol Pharmacol. 2009;2(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic Switching of Adipose Tissue Macrophages With Obesity Is Generated by Spatiotemporal Differences in Macrophage Subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen MTA, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. Journal of Biological Chemistry. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 11.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010;222(1):187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- 12.Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res. 2010;49(4):438–449. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Lippiello L, Walsh T, Fienhold M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism. 1991;40 (6):571–576. doi: 10.1016/0026-0495(91)90046-y. [DOI] [PubMed] [Google Scholar]
- 14.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20(5):1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheum. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 16.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–82. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 18.Roldan M, Macias-Gonzalez M, Garcia R, Tinahones FJ, Martin M. Obesity short-circuits stemness gene network in human adipose multipotent stem cells. FASEB J. 2011;25(12):4111–4126. doi: 10.1096/fj.10-171439. [DOI] [PubMed] [Google Scholar]
- 19.Lv S, Wu L, Cheng P, Yu J, Zhang AS, Zha JM, et al. Correlation of obesity and osteoporosis: Effect of free fatty acids on bone marrow-derived mesenchymal stem cell differentiation. Exp Ther Med. 2010;1(4):603–610. doi: 10.3892/etm_00000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tallman DL, Taylor CG. Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. J Nutr Biochem. 2003;14(1):17–23. doi: 10.1016/s0955-2863(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 21.Kleemann R, van Erk M, Verschuren L, van den Hoek AM, Koek M, Wielinga PY, et al. Time-Resolved and Tissue-Specific Systems Analysis of the Pathogenesis of Insulin Resistance. PLoS ONE. 2010;5(1):e8817. doi: 10.1371/journal.pone.0008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diekman BO, Wu CL, Louer CR, Furman BD, Huebner JL, Kraus VB, et al. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents post-traumatic arthritis. Cell Transplant. doi: 10.3727/096368912X653264. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodeheffer MS, Birsoy K, Friedman JM. Identification of White Adipocyte Progenitor Cells In Vivo. Cell. 2008;135(2):240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Liang SX, Khachigian LM, Ahmadi Z, Yang M, Liu S, Chong BH. In vitro and in vivo proliferation, differentiation and migration of cardiac endothelial progenitor cells (SCA1+/CD31+ side-population cells) J Thromb Haemost. 2011;9(8):1628–1637. doi: 10.1111/j.1538-7836.2011.04375.x. [DOI] [PubMed] [Google Scholar]
- 28.Maumus M, Peyrafitte JA, D’Angelo R, Fournier-Wirth C, Bouloumie A, Casteilla L, et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond) 2011;35(9):1141–1153. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maumus M, Sengenes C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, et al. Evidence of in Situ Proliferation of Adult Adipose Tissue-Derived Progenitor Cells: Influence of Fat Mass Microenvironment and Growth. J Clin Endocr Metab. 2008;93(10):4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 30.Joe AWB, Yi L, Even Y, Vogl AW, Rossi FMV. Depot-Specific Differences in Adipogenic Progenitor Abundance and Proliferative Response to High-Fat Diet. Stem Cells. 2009;27(10):2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez M, Acuna MJ, Reyes M, Olivares D, Hirsch S, Bunout D, et al. Proliferation and differentiation of human adipocyte precursor cells: differences between the preperitoneal and subcutaneous compartments. J Cel Biochem. 2010;111(3):659–664. doi: 10.1002/jcb.22753. [DOI] [PubMed] [Google Scholar]
- 32.Baptista LS, Silva KR, Pedrosa CSG, Claudio-da-Silva C, Carneiro JRI, Aniceto M, et al. Adipose Tissue of Control and Ex-Obese Patients Exhibit Differences in Blood Vessel Content and Resident Mesenchymal Stem Cell Population. Obes Surg. 2009;19(9):1304–1312. doi: 10.1007/s11695-009-9899-2. [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 35.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, et al. Obesity reduces bone density associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats. PLoS ONE. 2010;5(10):e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majka SM, Barak Y, Klemm DJ. Concise review: adipocyte origins: weighing the possibilities. Stem Cells. 2011;29(7):1034–1040. doi: 10.1002/stem.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 39.Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, et al. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab. 2007;293(1):E210–218. doi: 10.1152/ajpendo.00645.2006. [DOI] [PubMed] [Google Scholar]
- 40.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23 (2):287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105(37):13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutzner J, Kasten P, Gunther KP, Kirschner S. Surgical options for patients with osteoarthritis of the knee. Nat Rev Rheumatol. 2009;5(6):309–316. doi: 10.1038/nrrheum.2009.88. [DOI] [PubMed] [Google Scholar]
- 44.Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468(9):2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwa Cho H, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24(12):2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 46.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, et al. Endoglin (CD105): A Marker of Tumor Vasculature and Potential Target for Therapy. Clin Cancer Res. 2008;14(7):1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 47.Quintana L, zur Nieden NI, Semino CE. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15(1):29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellingman CA, Davidson EN, Koevoet W, Vitters EL, van den Berg WB, van Osch GJ, et al. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17(7–8):1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 49.Ataliotis P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Develop. 2000;94(1–2):13–24. doi: 10.1016/s0925-4773(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 50.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthr Cartilage. 2008;16(12):1450–1456. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Deshimaru R, Ishitani K, Makita K, Horiguchi F, Nozawa S. Analysis of fatty acid composition in human bone marrow aspirates. Keio J Med. 2005;54(3):150–155. doi: 10.2302/kjm.54.150. [DOI] [PubMed] [Google Scholar]
- 52.Yu S, Cho HH, Joo HJ, Bae YC, Jung JS. Role of MyD88 in TLR agonist-induced functional alterations of human adipose tissue-derived mesenchymal stem cells. Mol Cell Biochem. 2008;317(1–2):143–150. doi: 10.1007/s11010-008-9842-1. [DOI] [PubMed] [Google Scholar]
- 53.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109(4):1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 54.Mo IF, Yip KH, Chan WK, Law HK, Lau YL, Chan GC. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008;9:52. doi: 10.1186/1471-2121-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.German JB, Dillard CJ. Saturated fats: what dietary intake? Am J Clin Nutr. 2004;80(3):550–559. doi: 10.1093/ajcn/80.3.550. [DOI] [PubMed] [Google Scholar]
- 56.Manickam E, Sinclair AJ, Cameron-Smith D. Suppressive actions of eicosapentaenoic acid on lipid droplet formation in 3T3-L1 adipocytes. Lipids Health Dis. 2010;9:57. doi: 10.1186/1476-511X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28(6):1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao L, Huang J, Zhang H, Wang Y, Matesic LE, Takahata M, et al. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells. 2011;29(10):1601–1610. doi: 10.1002/stem.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77(12):1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Noth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62(7–8):765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


