Abstract
Background
Recognition of substantial immune-neural interactions is revising dogmas about their insular actions and revealing that immune-neural interactions can substantially impact CNS functions. The inflammatory cytokine interleukin-6 promotes susceptibility to depression and drives production of inflammatory T helper 17 (Th17) T cells, raising the hypothesis that in mouse models Th17 cells promote susceptibility to depression-like behaviors.
Methods
Behavioral characteristics were measured in male mice administered Th17 cells, CD4+ cells, or vehicle, and in RORγT+/GFP mice or male mice treated with RORγT inhibitor or anti-IL-17A antibodies.
Results
Mouse brain Th17 cells were elevated by learned helplessness and chronic restraint stress, two common depression-like models. Th17 cell administration promoted learned helplessness in 89% of mice in a paradigm where no vehicle-treated mice developed learned helplessness, and impaired novelty suppressed feeding and social interaction behaviors. Mice deficient in the RORγT transcription factor necessary for Th17 cell production exhibited resistance to learned helplessness, identifying modulation of RORγT as a potential intervention. Treatment with the RORγT inhibitor SR1001, or anti-IL-17A antibodies to abrogate Th17 cell function, reduced Th17-dependent learned helplessness.
Conclusions
These findings indicate that Th17 cells are increased in the brain during depression-like states, promote depression-like behaviors in mice, and specifically inhibiting the production or function of Th17 cells reduces vulnerability to depression-like behavior, suggesting antidepressant effects may be attained by targeting Th17 cells.
Keywords: antidepressant, depression, learned helplessness, neural-immune interactions, Th17 cells, RORgamma T
Introduction
Major depression is a progressive, debilitating disease that afflicts nearly 20% of people in the United States (1), yet available antidepressants are often inadequate (2). Progress in developing new antidepressants is hindered by a lack of understanding of the causes of depression, disease heterogeneity due to the influence of numerous genetic and environmental factors differentially affecting symptoms, limited animal models of depression, and often a narrow focus in drug development on traditional neurotransmitter systems.
In spite of these difficulties, potential new therapeutic targets are being identified, including a growing recognition that immune system actions profoundly impact mood regulation (3–6). This has been particularly well-established for inflammatory signals from the innate immune system being linked to depression in human and rodent studies (7). For example, the inflammatory cytokine interleukin-6 (IL-6) is elevated in the serum of a subset of depressed patients, is activated in conditions associated with depression in humans and in rodent models, and is sufficient to induce depressive-like behavior in rodent models, whereas deletion of the IL-6 gene is protective (3–5, 8). However, although anti-inflammatory interventions have been reported to provide some antidepressant actions in a portion of patients, they have not been found to be widely efficacious antidepressants on their own, possibly because inflammation only contributes to depression in a portion of patients and because inflammation also alters the adaptive immune system. This is exemplified by IL-6, which drives the production of T helper 17 (Th17) cells, a subset of CD4+ T cells that is a major contributor to the pathogenicity of several inflammatory and autoimmune conditions (9–11).
Since inflammatory cytokines that direct T cell subtype production are altered in depressed patients (12–14), it is likely that depression is associated with changes in T cell subtypes. However, little is known about the impact of T cells on mood regulation and depression, although alterations in T cell characteristics have been reported in patients with major depression (15–18), there is some evidence that mood-related behaviors in rodents are influenced by T cells (19–22), and T cells have been shown to directly impact the brain. This has been particularly well-established for cognitive functions, such as the finding that immune deficiency in mice causes cognitive impairments that are reversed by replenishment of CD4+ T cells (23–27). Furthermore, CD4+ T cells are necessary for hippocampal neurogenesis in adult rodents, a process linked to some forms of learning (24, 28–30). These and other findings have established that maintenance of certain brain functions is dependent on T cell actions in the CNS (31, 32). In opposition to this, inflammatory subtypes of T cells, particularly Th17 cells, are clearly deleterious in diseases such as multiple sclerosis (9–11, 24). Thus, it appears that T cells subtype-selectively bolster particular neural functions in a healthy CNS, and that disease- or environment-induced shifts in the balance of T cell subtypes can switch their actions from beneficial to detrimental.
Altogether, it is evident that T cells can modify CNS functions, and that depression is associated with an environment capable of altering the population of subsets of CD4+ T cells, raising the possibility that altered subtypes of T cells may impact mood regulation and mood disorders. Because the conditions to promote production of Th17 cells are prevalent in depressed patients, and Th17 cells are deleterious to the CNS, we tested the hypotheses that Th17 cells may promote the induction of depression-like behavior in mice.
Methods and materials
Mice and behavioral assessments
C57BL/6 and C57BL/6–129 Rorc(γT)+/GFP (6–12 weeks) male mice were from the Jackson Laboratories and GSK3S21A/S21A/S9A/S9A knockin mice were from Dr. Dario Alessi. 6 week old mice were used to prepared T cells while 8–12 week old mice were used for behavioral assessments. The Rorc(γT)+/GFP mice were obtained by crossing Rorc(γT)+/GFP × Rorc(γT)+/GFP, to produce 50% Rorc(γT)+/GFP, 25% wild-type, and 25% Rorc(γT)GFP/GFP mice, and behavioral experiments were carried out with littermates. Mice were housed in light and temperature controlled rooms and treated in accordance with NIH and university Institutional Animal Care and Use Committee regulations.
For the novelty-suppressed feeding (NSF) test (33), mice were weighed before and after food deprivation for 24 hr, and placed for 10 min in a brightly lit open field apparatus with a food pellet at the center on a slightly (1 cm) elevated platform. Behavior was monitored, and the latency to begin feeding was recorded. Upon returning to their home cage, the total amount of food consumed during a 5 min period was measured to test whether feeding differences in the novel environment were due to differences in hunger or motivation.
Learned helplessness was measured using a standard learned helplessness paradigm or a modified inescapable shock protocol, referred to as the reduced intensity inescapable shock protocol, and 24 hr later mice were tested with 30 escape trials as described previously (34). Mice were placed in one side of a Gemini Avoidance system shuttle box (San Diego Instruments, San Diego, CA, USA) with the gate between chambers closed. For standard learned helplessness, 180 inescapable foot shocks were delivered at an amplitude of 0.3 mA, a duration of 3–5 s per shock, and a randomized inter-shock interval of 5–45 s (35). In a modified inescapable shock protocol, referred to as the reduced intensity inescapable shock protocol, mice were given 180 foot shocks with amplitude of 0.3 mA and fixed 3 s shock duration, and a randomized inter-shock interval of 5–25 s (34). Twenty-four hours after inescapable foot shocks, mice were returned to the shuttle box and the escape task was tested by giving 30 escape trials with each trial preceded by a 0.3 mA foot shock for a maximum duration of 24 s. The door of the chamber opens at the beginning of the foot shock administration to allow mice to escape. Latency to escape the shock was recorded using Gemini software, and trials in which the mouse did not escape within the 24 s time limit were counted as escape failures. Mice with greater than 15 escape failures were defined as learned helpless. When indicated mice were injected i.p. once with 100 mg/kg lithium chloride as described (36) 24 h after the inescapable foot shocks. Mice used for learned helplessness were not used for other behavior measurements, whereas the same mice were used for the social interaction measurements and the NSF test. For chronic restraint stress, mice were placed individually in a 50 ml ventilated conical tube for 2 hrs daily for 2 weeks.
Th17 cell preparation and transfer
CD4+ T cells were isolated as described previously (37) and in the Supplementary Materials. CD4+ T cells cultured with splenic feeder cells were activated with 2.5 µg/mL of anti-CD3 (clone 145–11), and differentiated to Th17 cells by addition of IL-6 (20 ng/mL; Peprotech), TGFβ (5 ng/mL, Peprotech), anti-IL-4 (10 µg/mL; clone 11B11, UAB core facility) and anti-IFNγ (10 µg/mL; clone XMG1.2, UAB core facility). After 5 days of differentiation toward Th17 cells, cells were recovered after histopaque gradient purification, and resuspended in PBS. An aliquot of cells was used to evaluate the percent of Th17 cells (~40%) and ~10–20×106 undifferentiated CD4+ T or Th17 cells were injected in 500 µL PBS by tail vein 48 h prior to behavioral testing. Where indicated, mice were injected intraperitoneally with 100 µg anti-IL-17A, or 125 µg SR1001 daily beginning 1 day before Th17 cell transfer, and this was continued throughout the experiment. As controls, mice were injected i.v. with ~10–20×106 CD4+ T cells to evaluate the effect of non-differentiated cells, or with 500 µL PBS to mimic the stress of tail vein i.v. injection 48 h prior to behavioral testing. Intracellular cytokine staining was carried out as described previously (37) and in the Supplementary Information.
Statistical analysis
Histograms represent Means±SEM. Statistical significance was analyzed with a one-way multiple range analysis of variance (ANOVA) for multiple comparisons or with unpaired/paired t-test using Prism software. A p value <0.05 was considered significant.
Results
Stress is a critical factor in promoting susceptibility to depression (2, 38), and is modeled in rodents with the learned helplessness paradigm of depression-like behavior (39) to assess modulators of mood, while limitations of animal models are also well-recognized (38, 40, 41). The traditional learned helplessness paradigm involves stressing mice with mild, unpredictable and inescapable foot shocks (IES), and typically 24 hr later exposing mice to mild foot shocks from which they are free to escape. Due to the initial stressful period, many mice fail to escape even when it is readily available, and learned helplessness is defined as failure to escape in >15 out of 30 escapable trials. Learned helplessness is also advantageous to many models of depression-like behavior because the parameters can be adjusted to measure either resistance to learned helplessness using the traditional protocol, or increased susceptibility to learned helplessness using diminished aversive stimuli (34).
Because the conditions to promote differentiation of Th17 cells are present in depressed patients, and Th17 cells are deleterious to the CNS, we tested if learned helplessness resulted in increased brain Th17 cells in wild-type mice and if this was blocked by administration of the mood stabilizer and glycogen synthase kinase-3 (GSK3) inhibitor lithium, which has an antidepressant effect in the learned helplessness paradigm (36). After exposure to IES, the subsequent escapable test was used to separate mice into two cohorts, those that exhibited learned helplessness and those that escaped (Figure 1A). Immediately after the escapable shock session, cells isolated from the brain were analyzed by FACS. The number of Th17 cells, which are identified by positive co-staining for CD4+ and for IL-17A (i.e., IL-17A+ CD4+ T cells) was increased by 78% in the learned helplessness mouse brains compared to the non-depressed mice, even though all mice were exposed to the same foot shock protocol. A 27% increase in brain Th1 cells, which are identified by positive co-staining for CD4+ and IFNγ (IFNγ+ CD4+ T cells), was found in learned helpless mice, whereas there was no difference between groups in T regulatory cells (Tregs; FoxP3+ CD4+ T cells) (Figure 1B). Lithium pretreatment reduced the percent of mice meeting learned helplessness criteria from 60% to 10%, and blocked the increases in brain Th17 and Th1 cells, consistent with a previous report that lithium reduces the production of Th17 cells in mice in vivo (42). There were no significant differences among these groups in the total numbers in brain of CD4+ T cells, CD8+ T cells, or activated microglia (identified by staining for F4/80+CD45int cells) (Figure 1C). Furthermore, there were no differences in the number of brain CD11c+CD45high dendritic cells (DCs) or F4/80+CD45high cells (activated macrophages) (Figure 1C), indicating there was no difference in blood brain barrier permeability between mice that were or were not learned helpless, which would be evident by an increase in the number of infiltrated DCs and macrophages. The numbers of Th17, but not Th1 cells or Tregs, also were greater in the spleens of learned helpless mice than in nonlearned helpless mice or in mice treated with lithium (Figure 1D).
Figure 1.
Th17 cells accumulate in the brains of mice exhibiting learned helplessness. Wild-type mice were subjected to mild inescapable foot shocks, 24 h later lithium chloride (LiCl; 100 mg/kg; i.p.) was administered, and after 24 h mice were tested with escapable foot shocks. (A) Escape failures after standard IES. Each symbol represents total escape failures in 30 trials for an individual mouse, bars represent group means ± sem (n=10–21 mice/group, t-test *p<0.05, t=3.030, df=31). Mice were separated into 3 groups: mice that failed to escape <15 of 30 trials (nondepressed, ND), mice that failed to escape >15 of 30 trials (depressed, D), and non-depressed lithium-treated mice. After behavior measurements, the indicated (B) Th cells or (C) immune cells from the brain, or (D) Th cells from the spleen, were stained and analyzed by flow cytometry. Bars represent mean ± sem. (ANOVA, *p<0.05 compared with values from non-depressed mice, df=10, F=8.466 (Th17 cells), *p<0.05 compared to ND+Li, df=7, F=9.614 (Th1 cells)). (E) Exposure to chronic restraint stress (CS) for two weeks increased the number of Th17 cells and (F) Th1 cells in the brains of mice compared to non-stressed mice (n=5/group, Mann-Whitney test *p<0.05, † p=0.06). (G) GSK3 knockin (KI) mice received escapable shocks only, and 46% of the mice failed to escape >15 of 30 trials (depressed, D). Each symbol represents total escape failures in 30 trials for an individual mouse (n=13). Bars in panels A–G are representative of 3 independent experiments. (H) After behavior measurements, Th cells recovered from 5 brains per group (ND and D) of GSK3 knockin mice were pooled together, stained as indicated, and analyzed by flow cytometry.
To test if brain Th17 and Th1 cells increased in another model of depression, mice were subjected to restraint stress for two weeks. This is widely used to induce a depressive phenotype (33), substantiated by a 15% lowered body weight (Supplemental Figure S1), a marker of chronic stress. As with learned helplessness, chronic restraint stress resulted in increased brain levels of Th17 cells (Figure 1E) and Th1 cells (Figure 1F). Taken together, these results suggest that increased Th17 or Th1 cells in the brain may contribute to, or be an outcome of, depression-like behavior.
Genetically predisposed individuals exposed to environmental factors, such as stress, may be especially vulnerable to major depression. Glycogen synthase kinase 3 (GSK3) knockin mice possess a genetic predisposition that increases deleterious responses to stress, which is evident upon exposure to mild aversive stimuli (34). GSK3 knockin mice express normal levels of both GSK3 isoforms, GSK3α and GSK3β, but with serine-to-alanine mutations, S9A-GSK3β and S21A-GSK3α, to maintain GSK3 maximally active, since it cannot be inhibited by serinephosphorylation (43, 44). Exposure to only escapable foot shocks, without a prior session of inescapable foot shocks, causes learned helplessness behavior in a portion of GSK3 knockin mice, but not wild-type mice (34). A single session of only escapable foot shocks induced learned helplessness in ~40% of GSK3 knockin mice (Figure 1G). Comparing the two cohorts of GSK3 knockin mice that received the same foot shock protocol revealed that the numbers of Th17 and Th1 cells were significantly higher in the brains of GSK3 knockin mice that failed to escape than in those that escaped, while the numbers of macrophages and DCs were the same in both groups (Figure 1H). Thus, conditions causing depression-like behavior consistently increased mouse brain Th17 and Th1 cells.
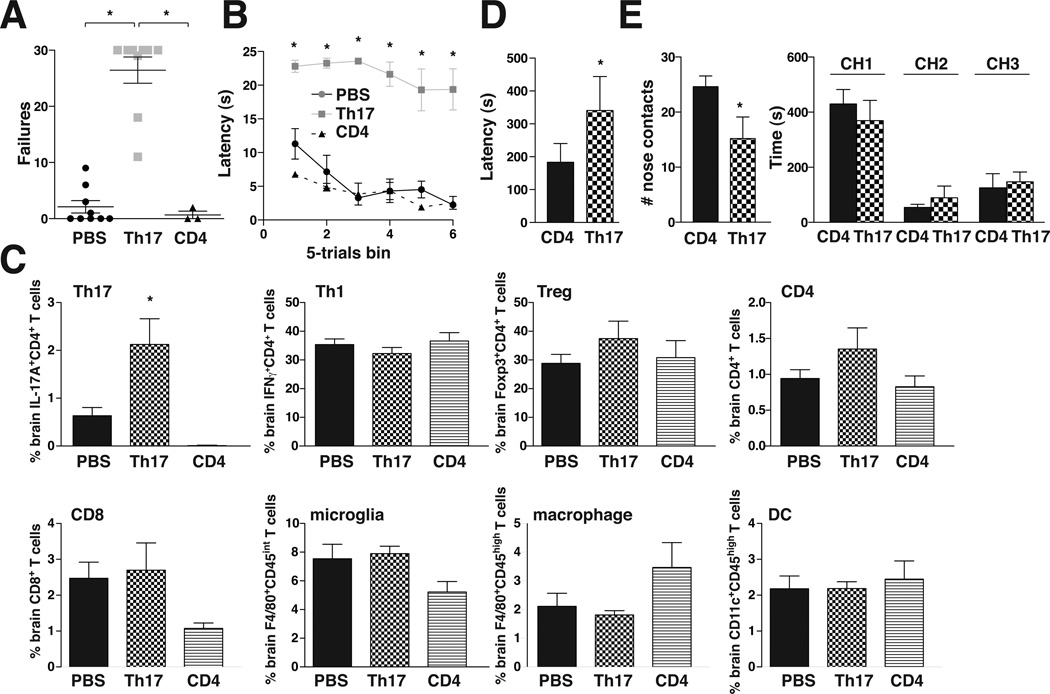
The well-established pathological actions of Th17 cells in other diseases (45–47) and their increases in the brains and spleens of learned helpless and stressed mice raised the possibility that Th17 cells may promote susceptibility to depression-like behavior. Therefore, we tested if administration of Th17 cells to wild-type mice was sufficient to promote depression-like behaviors, including learned helplessness, impaired novelty-suppressed feeding (NSF), and impaired social interactions. Th17-recipient mice did not differ from vehicle-treated controls in locomotor activity in a novel open field (Supplemental Figure S2A) or pain sensitivity (Supplemental Figure S2B). Mice were subjected to inescapable foot shocks of reduced intensity that did not induce learned helplessness in vehicle- or undifferentiated CD4+ T cell-treated control mice, whereas 89% of Th17-recipient mice met the learned helplessness criteria of >15 failures out of 30 trials (Figure 2A). The latency time to escape demonstrated rapid escape times by control mice, whereas Th17 recipient mice consistently failed to escape (Figure 2B). The Th17-recipient mice contained elevated brain levels of IL-17A+ CD4+ T cells (Th17 cells), but no differences from control mice in IFNγ+ CD4+ T cells (Th1 cells), FoxP3+ CD4+ T cells (Tregs), total CD4+ T cells, CD8+ T cells, F4/80+CD45int cells (activated microglia), F4/80+CD45high cells (activated macrophages), and CD11c+CD45high cells (DCs) (Figure 2C), confirming that Th17 cells increase in the brain of learned helpless mice. The spleens of Th17 recipient mice also contained elevated levels of IL-17A+ CD4+ T cells, and a slight but statistically significant increase in total CD4+ T cells, but no changes of other cell types (Supplemental Figure 3), confirming that sufficient injected cells were viable after administration to increase the overall populations of CD4+ and Th17 cells in the spleen. These results demonstrate that elevation of Th17 cells is sufficient to increase susceptibility to the learned helplessness model of depression-like behavior.
Figure 2.
Transfer of Th17 cells increased susceptibility to depression-like behavior. Wild-type mice received either PBS, Th17 cells, or CD4+ T cells, 48 h later mice were subjected to reduced-intensity inescapable foot shocks that did not induce learned helplessness in vehicle- or CD4+ T cell-treated mice, and 24 h later mice were tested with escapable foot shocks. (A) Escape failures after reduced intensity IES. Each symbol represents total escape failures in 30 trials for an individual mouse, bars represent group means±sem (n=3–9 mice/group, ANOVA *p<0.05, df=20, F=58.67). (B) Average escape latency during 5-trial blocks after reduced intensity IES. WT mice injected with PBS or CD4+ cells had an average of 5.5±1.4 or 4±0.7 sec escape latency, respectively, whereas mice injected with Th17 cells had an average of 22.7±0.8 sec escape latency. Bars represent group means±sem (n=3–9 mice/group, ANOVA *p<0.05, df=17, F=98.30). After behavior measurements, the indicated (C) Th cells or immune cells were stained and analyzed by flow cytometry. Bars represent mean±sem (n=3–9 mice/group ANOVA *p<0.05 compared with PBS-treated mice, df=21, F=10.84). (D) In the NSF test, Th17-recipient mice displayed significantly longer latencies to feed when placed in a novel arena with food following 24 hr food deprivation compared with control CD4+ T cell-treated mice (n=4 mice/group, *p<0.05 compared with CD4-treated mice, paired t-test, df=3, t=3.200). (E) Th17-recipient mice demonstrated significantly fewer nose contacts with a novel conspecific mouse than did CD4+ T cell-treated mice but did not differ in the time spent in each chamber of the apparatus (CH1, chamber 1 containing the conspecific mouse; CH2, empty middle chamber 2; CH3, chamber 3, equivalent to CH1 without a mouse) (n=5 mice/group, *p<0.05 compared with CD4-treated mice, paired t-test, df=4, t=3.670).
Administration of Th17 cells also affected behavior in the NSF test and in social interaction. In the NSF test, Th17-recipient mice displayed significantly longer latencies to feed when placed in a novel arena with food following 24 hr food deprivation compared with control CD4+ T cell-treated mice (Figure 2D). This was not due to suppressed appetite and there was no difference in weight loss between groups (Supplemental Figure S3B). Although Th17-recipient mice did not exhibit overt changes in sociability as estimated by time spent in the chamber with a novel conspecific mouse, reduced nose contacts indicated diminished social interaction (Figure 2E). Thus, administration of Th17 cells was sufficient to impair several behaviors that have been associated with depression.
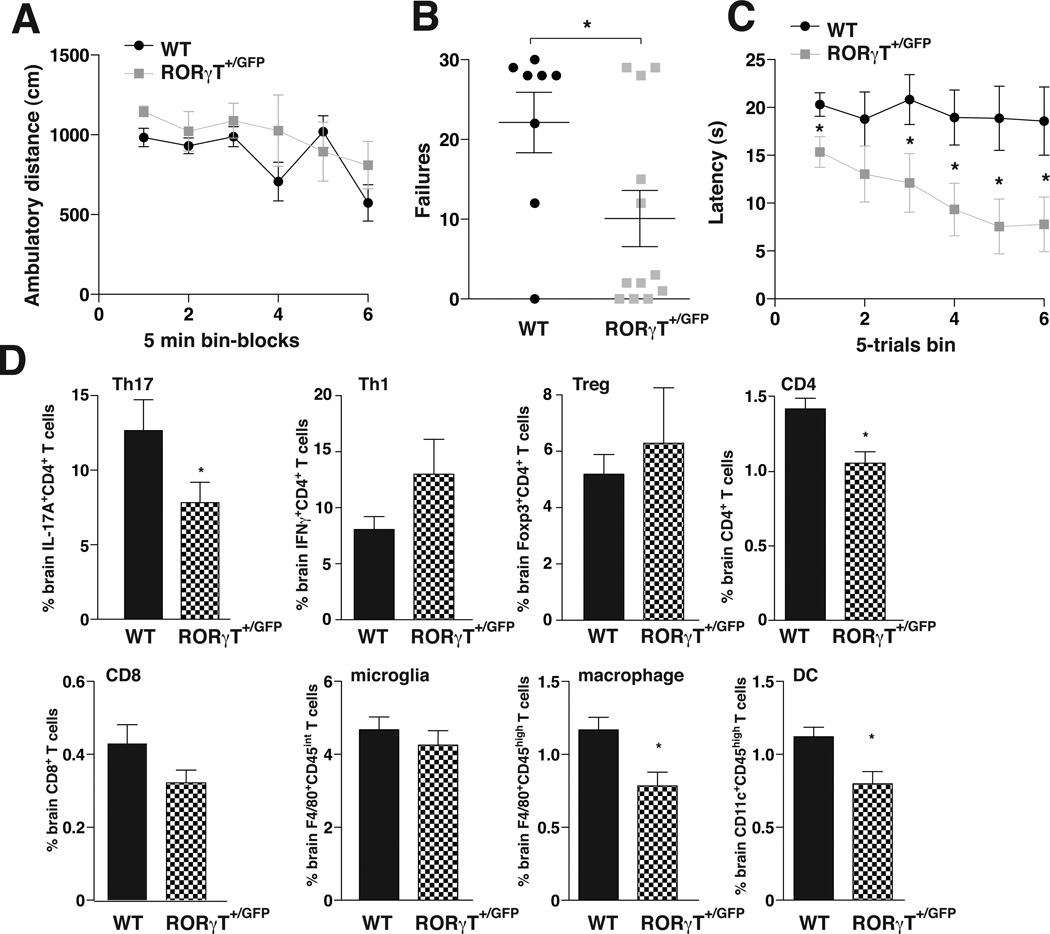
These results raised the possibility that reducing Th17 cells may provide a feasible intervention to bolster resistance to depressive-like conditions. Th17 cells are absent in mice with depleted retinoid-related orphan receptor-γT (RORγT), a transcription factor that drives the differentiation of Th17 cells that is thought to be expressed almost exclusively in Th17 cells (48), and is a widely used marker of Th17 cells (49). RORγT knockout mice exhibited strong resistance to learned helplessness (Supplemental Figure S4A), consistent with the postulate that Th17 cells promote this behavior. However, examination of the immune system of these mice revealed multiple alterations (48), Supplemental Figure S4B). Therefore, we examined heterozygote Rorc(γT)+/GFP mice, which produce a greatly diminished Th17 cell population because of the reduction of RORγT expression (48), as we recently confirmed (37). Rorc(γT)+/GFP mice did not differ from littermate wild-type mice in locomotor activity in a novel open field (Figure 3A). To test for an anti-depressant effect of reduced Th17 cells, mice were exposed to IES, and 24 hr later, the escapable shock test was used to identify mice exhibiting learned helplessness. Rorc(γT)+/GFP mice exhibited a strong resistance to the learned helplessness paradigm, with only 25% of mice meeting the learned helplessness criteria, compared with 75% of littermate wild-type mice (Figure 3B). The latency time to escape also demonstrated that the wild-type mice maintained learned helpless behavior throughout the test in contrast to the Rorc(γT)+/GFP mice (Figure 3C).
Figure 3.
Depletion of Th17 cells in Rorc(γT)+/GFP mice reduces acquisition of learned helplessness. (A) Basal activity of wild-type or Rorc(γT)+/GFP mice was measured in an open field for 30 min. (B–D) Mice were subjected to standard IES, and 24 h later tested with escapable foot shocks. (B) Escape failures after standard IES. Each symbol represents total escape failures in 30 trials for an individual mouse, bars represent group means (n=8–12 mice/group, t-test *p<0.05, df=18, t=2.267). (C) Average escape latency during 5-trial blocks after standard IES. WT and Rorc(γT)+/GFP mice had an average of 19.4±0.4 and 10.9±1.3 sec escape latency, respectively. (n=8–12 mice/group, Mann Whitney *p<0.05). After behavior measurements, the indicated (D) Th cells or immune cells were stained and analyzed by flow cytometry. Bars represent mean ± sem. (n=8–12 mice/group Mann Whitney *p<0.05).
Evaluation of cells in the brains of mice after the learned helplessness paradigm confirmed that, compared with wild-type mice, Rorc(γT)+/GFP mice had lower levels of IL-17A+ CD4+ T cells (Th17 cells), a slight but not statistically significant increase in IFNγ+ CD4+ T cells (Th1 cells), no difference in FoxP3+ CD4+ T cells (Tregs), and lower total CD4+ T cells (Figure 3D). Rorc(γT)+/GFP mouse brain CD8+ T cells and F4/80+CD45int cells (activated microglia) did not differ from controls, but F4/80+CD45high cells (activated macrophages) and CD11c+CD45high cells (DCs) were lower in Rorc(γT)+/GFP mice than wild-type mice (Figure 3E). Thus, Rorc(γT)+/GFP mice displayed a remarkable resistance to learned helplessness behavior in this paradigm that is robustly effective in wild-type mice, further indicating that reduction of Th17 cells increases resistance to learned helplessness behavior.
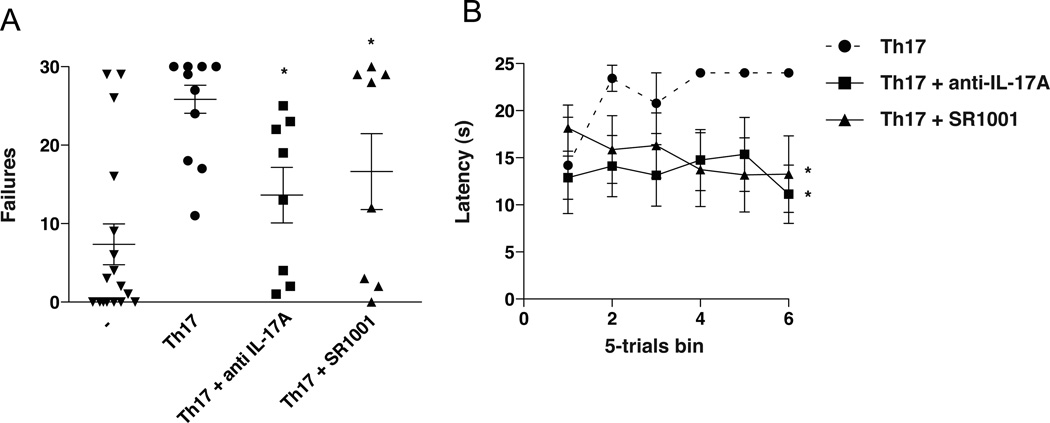
Two approaches were used to test the feasibility of pharmacologically reducing the pathogenecity of transferred Th17 cells as a therapeutic intervention for depression. Using the model of Th17 cell administration, mice were treated with anti-IL-17A antibodies to neutralize actions of Th17 cells, and to reduce the differentiation of Th17 cells mice were treated with SR1001, a recently identified RORγT inhibitor (50). Anti-IL17A therapy and inhibition of RORγT did not alter locomotor activity (Figure 4A), but each treatment significantly reduced learned helplessness and reduced the latency time to escape (Figure 4B,C). Measurements of Th17 cells in the spleen and brain demonstrated a significant reduction of Th17 cells after SR1001 treatment (Supplemental Figure S5). These findings confirmed that blocking Th17 actions provide antidepressant effects in Th17-dependent learned helplessness. Thus, brain Th17 cells increase with depressive-like behavior, and intervening at the level of IL-17A or RORγT is sufficient to neutralize the Th17-dependent depression-like behavior effect.
Figure 4.
Inhibiting RORγT or IL-17A has antidepressant effects in Th17-dependent learned helplessness. Wild-type mice pretreated where indicated with anti-IL-17A antibodies or SR1001, a RORγT inhibitor, received Th17 cells. (A–B) Mice were subjected to reduced intensity IES, and 24 h later tested with escapable foot shocks. (A) Escape failures after reduced IES. Each symbol represents total escape failures in 30 trials for an individual mouse, bars represent group means (n=8–10 mice/group, ANOVA *p<0.05 compared with untreated Th17 recipient mice, df=45, F=8.220). (B) Average escape latency during 5-trial blocks after reduced intensity IES. Mice receiving Th17, Th17+ anti-IL17A, and Th17 + SR1001 mice had an average of 21.8±1.6, 13.6±0.6 and 15.1±0.8 sec escape latency, respectively. (n=8–10 mice/group, ANOVA *p<0.05 compared with untreated Th17 recipient mice, df=17, F=15.77).
Discussion
The growing evidence of causative links between inflammation and depression (3, 4) indicates that targets of inflammatory molecules contribute to establishing a pro-depressant environment. This study focused on T cells because they are major sensors of the cytokine environment, each subtype of T helper CD4+ cell responds to a different cytokine signature, and, importantly, T cells are more enduring than transiently expressed cytokines, potentially enabling T cells to contribute to the prolonged and recurrent episodes characteristic of major depression. IL-6 is an inflammatory cytokine with well-established links to depression, stress induces IL-6 expression, and IL-6 is a crucial driver of Th17 cells that are detrimental to the CNS, raising the possibility that Th17 cells contribute to mediating the depressive outcome of excessive inflammation. We found that Th17 cells accumulate in the brains of mice subjected to two models of depression-like behavior, administration of Th17 cells promoted three behavioral models of depression, and reducing Th17 cells provided resistance to learned helplessness. This extends to mood regulation previous findings that T cells are present and functional in the brain (32) where they modulate higher functions, such as cognition.
The initial direct evidence that Th17 cells may promote depression-like behavior was the increased Th17 cell numbers found in mouse brain after depression-inducing stimuli, including a 78% increase of Th17 cells in the brains of mice that displayed learned helplessness. Importantly, in this paradigm all mice received the same aversive stimuli, which is well-established to produce learned helplessness in only a portion of treated mice. Thus, despite matching treatments, only those mice with increased Th17 cells displayed learned helplessness, raising the possibility that Th17 cells may contribute to the development of depression-like behavior. Th1 cells also may play a regulatory role since they increased in mouse brains after depression-inducing stimuli, and these associated increases in Th17 and Th1 cells may be linked to recent evidence that Th1 cells can be derived from Th17 cells (51). In contrast, anti-inflammatory Treg levels were not altered in these conditions, suggesting that proinflammatory cytokines are driving the pathogenic T cells.
A causative role for Th17 cells in depression-like behaviors is supported by the findings that altering Th17 cells in mice modified the behavioral outcomes. Administration of Th17 cells was used to test if pre-existing elevated Th17 cells promoted learned helplessness by taking advantage of the fact that untreated wild-type mice seldom develop learned helplessness after subjection to a mild foot shock paradigm. Remarkably, 89% of mice developed learned helplessness after receiving Th17 cells two days previously, a time that allows for recovery from the acute treatment and proliferation of administered T cells. Th17-recipient mice also displayed deficits in NSF and social interactions, two other behaviors that have been linked to a depressive phenotype. Thus, the adaptive immune system, in particular the Th17 cell burden, profoundly affects stress-induced behavioral responses that may model mood dysregulation. However, this study was for the most part limited to evaluations of the learned helplessness model, so it remains to be determined if Th17 cells also promote other models of depression-like behavior in rodents and major depression in humans.
Methods capable of reducing Th17 cell levels in vivo are limited, with RORγT knockout mice being the most widely used approach (48, 49). Although RORγT is a characteristic marker of Th17 cells, since it may have unknown additional functions in other cell types, results with RORγT knockout mice must be considered in conjunction with other means of modulating Th17 cells, such as antibodies that block the actions of IL-17A or recently described RORγT inhibitors that limit differentiation of Th17 cells (50). Indeed, we found significant immune alterations in RORγT knockout mice, which led us to focus on Rorc(γT)+/GFP mice with only partial depletion of RORγT. Although in these mice Th17 cells were only partially depleted, not abolished, the mice nevertheless displayed a robust 3-fold increase in resistance to acquisition of learned helplessness, with 75% of the Rorc(γT)+/GFP mice resistant to a paradigm in which only 25% of wild-type mice were resistant. Thus, even partial reduction of Th17 cell production provides significant protection from the vulnerability of mice to learned helplessness. It is also notable that the mood stabilizer lithium both blocked learned helplessness and Th17 cell accumulation in the brain, which may be due to the previously identified action of GSK3 inhibitors to block Th17 cell production (37). Moreover, inhibition of RORγT confirmed that pharmacological intervention aimed at Th17 cell production provides resistance to Th17 cell-dependent depressive-like behavior, providing a potential new avenue of intervention. The antidepressant effect of anti-IL-17A therapy demonstrated that IL-17A is a critical cytokine mediating Th17-dependent learned helplessness, in accordance with its detrimental effects in autoimmune diseases, although it remains to be determined if this results from peripheral actions or effects on cells in the CNS, such as its previously identified actions on astrocytes that may alter astrocyteneuronal interactions (52).
These findings indicate for the first time a significant contribution of Th17 cells to promote susceptibility to depressive-like behavior. Th17 cells are likely only one factor among many that modify susceptibility to depression-like behavior. Thus, interfering with the production or actions of Th17 cells may prove most effective in patients with depression that are pre-identified as having elevated levels of IL-6, which drives Th17 cell production, or of IL-17A, which is produced by Th17 cells. These findings demonstrate that the adaptive immune system significantly impacts susceptibility to learned helplessness and other behaviors that may be relevant to mood regulation, suggesting that interventions being developed for suppressing Th17 cells in autoimmune diseases may provide a starting point to develop new treatments for mood disorders.
Supplementary Material
Acknowledgements
This research was supported by grants from the NIMH (MH038752, MH090236, MH095380).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 7.Licinio J, Mastronardi C, Wong ML. Pharmacogenomics of neuroimmune interactions in human psychiatric disorders. Exp Physiol. 2007;92:807–811. doi: 10.1113/expphysiol.2007.038471. [DOI] [PubMed] [Google Scholar]
- 8.Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 12.Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee LF, Lih CJ, Huang CJ, Cao T, Cohen SN, McDevitt HO. Genomic expression profiling of TNF-alpha-treated BDC2.5 diabetogenic CD4+ T cells. Proc Natl Acad Sci U S A. 2008;105:10107–10112. doi: 10.1073/pnas.0803336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, et al. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Irwin MR, Miller AH. Depressive disorders and immunity 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 18.Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 20.Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Hickie I, Hickie C, Lloyd A, Silove D, Wakefield D. Impaired in vivo immune responses in patients with melancholia. Br J Psychiatry. 1993;162:651–657. doi: 10.1192/bjp.162.5.651. [DOI] [PubMed] [Google Scholar]
- 22.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 23.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 25.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and 'chemo-brain' have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Ron-Harel N, Segev Y, Lewitus GM, Cardon M, Ziv Y, Netanely D, et al. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008;11:903–913. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 29.Ziv Y, Schwartz M. Orchestrating brain-cell renewal: the role of immune cells in adult neurogenesis in health and disease. Trends Mol Med. 2008;14:471–478. doi: 10.1016/j.molmed.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Cardon M, Ron-Harel N, Cohen H, Lewitus GM, Schwartz M. Dysregulation of kisspeptin and neurogenesis at adolescence link inborn immune deficits to the late onset of abnormal sensorimotor gating in congenital psychological disorders. Mol Psychiatry. 2010;15:415–425. doi: 10.1038/mp.2009.66. [DOI] [PubMed] [Google Scholar]
- 31.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 33.Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 36.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beurel E, Yeh WI, Michalek SM, Harrington LE, Jope RS. Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol. 2011;186:1391–1398. doi: 10.4049/jimmunol.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. 2010;15:883–895. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 41.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beurel E. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci. 2011;4:18. doi: 10.3389/fnmol.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eom TY, Jope RS. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol Psychiatry. 2009;66:494–502. doi: 10.1016/j.biopsych.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 46.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Huang Z, Xie H, Wang R, Sun Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin Ther Targets. 2007;11:737–743. doi: 10.1517/14728222.11.6.737. [DOI] [PubMed] [Google Scholar]
- 50.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.