Abstract
Mutations of the TARDBP gene encoding TDP-43 protein have been shown to cause amyotrophic lateral sclerosis and have been reported to present with clinical heterogeneity including parkinsonism. In addition, TDP-43 pathology has been observed across a spectrum of neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease. Herein we report the presence of a TDP-43 mutation in a patient with a clinical diagnosis of Parkinson’s disease. The TDP-43 p.N267S substitution has been previously implicated in both amyotrophic lateral sclerosis and behavioral variant frontotemporal dementia. Our findings widen the phenotypic presentation for the TDP-43 p.N267S substitution and support a possible role for rare TDP-43 mutations presenting with Parkinson’s disease.
Keywords: TDP-43, amyotrophic lateral sclerosis, Parkinson’s disease
Introduction
Tar-DNA binding protein of 43kDA (TDP-43) was identified as the major protein component of the ubiquitin immune-positive inclusions that are the pathologic substrate of common forms of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) [1]. Subsequent studies identified genetic mutations inherited in an autosomal dominant fashion in the TARDBP gene as a cause of both familial and sporadic ALS [2–4]. Although the vast majority of TDP-43 research has focused on ALS and FTD, it has become clear that TDP-43 pathology is observed in other neurodegenerative disorders, including Alzheimer’s (AD) and Parkinson’s disease (PD) [5–7]. These observations suggest that, perhaps as with tau and α-synuclein, the TDP-43 protein may be generally involved in neurodegenerative processes.
Recently Quadri and colleagues reported that the phenotype associated with a TARDBP mutation (c.G1144A; p.A382T) that is common in the Sardinian population can present clinically as PD [8]. The TDP-43 p.A382T substitution, which appears to originate from a common founder, accounts for upwards of 30% of familial and sporadic ALS patients in Sardinia and parkinsonism has been reported as a clinical manifestation [9, 10]. The study of a series of patients with PD from Sardinia identified 8 (2.5%) p.A382T carriers from a total of 327 patients [8]. All 8 mutation carriers were diagnosed with typical late-onset “sporadic” PD (8/231; 3.5%) but notably no mutation carriers were identified in their subgroup of early onset familial PD (n=88). The TDP-43 p.A382T substitution was identified in one healthy control (1/578); however, this individual reported a family history of motor neuron disease and dementia.
Pathogenic TARDBP mutations cluster in the C-terminal glycine-rich domain, and the underlying pathomechanism, whether a toxic gain or loss of function, remains to be resolved [11]. The mutations occur in conserved codons, and the amino acid substitutions are hypothesized to disrupt protein-protein interactions, localization, ubiquitination or phosphorylation. Given the recent insights and links with PD, we screened a PD patient-control series for mutations in exon 6 of the TARDBP gene, which encodes the Glycine-rich domain.
Subjects and methods
We employed the Mayo Clinic Florida PD patient-control series. The series consists of 692 patients (63% male) with PD and an average age of 75 years (±12 years; 33–101 years) and age-at-onset of 67 years (±11 years; 28–98 years). Healthy control subjects (n=689; 41% male) with an average age of 70 years (±12 years; 21–94 years) were used for comparison. All patients were examined and observed longitudinally by a movement disorders neurologist and diagnosed with PD according to published criteria [12]. PD was considered familial when ≥1 first- or second-degree relatives were reportedly affected. Unrelated control individuals were free of personal or familial history suggesting parkinsonism. The ethical review board at the Mayo Clinic approved the study and all participants provided informed consent.
We performed bidirectional DNA sequencing on exon 6 of the TARDBP gene using an ABI 3730 DNA sequencer with BigDye terminator chemistry and analyzed using SeqScape v2.5 software (Applied Biosystems) [13]. Primers and conditions are available upon request.
Results
We identified two variants in our series of patients with PD, p.N267S (c.800 A>G; rs80356718) and p.A315A (c.945G>A). The silent p.A315A is located in a known disease-associated mutation site p.A315T and p.A315E who have presented with parkinsonism (Table 1); however, the clinical/functional relevance of this synonymous variant remains unclear. The silent p.A315A was observed in a sporadic late-onset patient diagnosed at age 69 years with typical PD. Neuropsychological testing performed early in the disease course showed mild cognitive impairment. He later developed REM sleep behavior disorder documented by polysomnography, restless legs syndrome, visual hallucinations, and fluctuations in cognition. Shortly before his death seven years after symptom onset, he also developed some aggressive behavior requiring therapy with antipsychotic medications. The final clinical diagnosis was consistent with Parkinson disease dementia and no autopsy was performed.
Table 1.
Summery of clinical phenotypes and genetic information of the previously reported cases and our case with TDP-43 mutations manifesting Parkinsonism
| Reference | Familial /sporadic |
Amino acid change |
AAO (year) |
DD (year) |
Sex | Initial symptoms |
P | LR | Other clinical features |
|---|---|---|---|---|---|---|---|---|---|
| Quadri, et al. | Familial | p.A382T | 76 | A | F | P | B, RT | + ^ | Hallucination, FTD |
| Familial | p.A382T | 68 | 2 | M | P | B, R | + ^ | FTD | |
| Familial | p.A382T | 74 | 3 # | F | P | B, R | + | - | |
| Sporadic | p.A382T | 79 | A | M | P | B, R, RT | + | Hyposmia, MCI | |
| Sporadic | p.A382T | 51 | A | M | P | RT | + | RBD | |
| Sporadic | p.A382T | 65 | A | M | P | B, R, RT | + | RBD, hyposmia | |
| Sporadic | p.A382T | 78 | A | M | P | B, R, RT | + | RBD, hyposmia | |
| Sporadic | p.A382T | 75 | A | M | P, MCI | B, R, RT | + | Dementia (MMSE: 18/30) | |
| Sporadic | p.A382T | 71 | A | M | P | B, R | + | MCI | |
| Sporadic | p.A382T | 65 | A | F | P | B, R, RT | + | RBD | |
| Sporadic | p.A382T | 76 | A | M | P, MCI | B, R, RT | + | Dementia (MMSE: 20/30) | |
| Borghero, et al. | Familial | p.A382T | 49 | A | M | MND, FTD | B, R | + | Dystonic movement, tic |
| Mosca, et al. | Familial | p.A382T | 61 | 8 | M | P | B, R | + | MND, falls, FTD |
| Fujita, et al. | Familial | p.A315E | 57 | 4 | F | P | B, R, RT | NA | MND |
| Familial | p.A315E | 40 | 2* | M | MND | B, R, RT | − | Depression | |
| Our case | Familial | p.N267S | 42 | A | M | P | B, R, RT | + | Falls, hyposmia, OH, RBD, anxiety, depression, behavioral and personality changes |
A: alive at the point of publication or the last examination, AAO: age at onset, B: bradykinesia, DD: disease duration, F: female, FTD: frontotemporal dementia, LR: levodopa responsiveness, M: male, MMSE: mini-mental state examination, NA: not available, OH: orthostatic hypotension, P: parkinsonism, R: rigidity, RBD: REM-sleep behavior disorder, RT: resting tremor, disease duration was calculated by subtracting the date of age of symptomatic disease onset from date of death,
died of acute myocardial infarction,
died of suicide,
with only a mild improvement
The proband harboring the TDP-43 p.N267S substitution was diagnosed with PD at age 42; screening for early-onset PD genes PARKIN, PINK1 and DJ-1 was negative and the patient did not carry any known pathogenic LRRK2 mutations. His first symptom was difficulty performing precision movements with his left hand. This was followed by hypomimia, left hand rest tremor, and shuffling gait. With progression of his illness all cardinal signs of PD including rigidity, bradykinesia, rest tremor, and late on postural instability developed. Although his symptoms eventually become bilateral, persistent asymmetry with the left side more affected persisted. He initially responded well to therapy with dopamine agonists, but eventually he developed severe compulsive behavior (gambling). He was than placed on levodopa-carbidopa therapy, again with beneficial response. His condition was slowly progressive. At age 57 years he had a surgery for lumber stenosis. This was complicated by the occurrence of a large wound hematoma that required surgical evacuation and left him with persistent weakness of his right leg. Over the last three years falling became an increasing problem that led to severe left traumatic ulnar palsy. The last examination performed at age 59 years revealed advanced parkinsonism treated with 1200 mg of levodopa per day complicated by wearing off but no dyskinesias, dystonia, or hallucinations. The score on his UPDRS PART III was 19 points in “on” state and 57 points in “off” state (Video Supplement). Non-motor symptoms reported at the time of the last visit included the hyposmia, mild orthostatic hypotension, anxiety, depression, excessive daytime sleepiness, and REM-sleep behavior disorder. There were no clinical or electromyographic signs of motor neuron disease. He admitted to some irritability and compulsive ice cream eating in the “off” state. On neuropsychological evaluation, there was no evidence of cognitive dysfunction, with a normal mini mental status examination (MMSE) of 30/30, normal memory Weschler Memory Scale –Revised (WMS-R), Logical Memory I: 26, WMS-R, Logical Memory II: 24), no behavioral abnormality (Behavioral Inhibition Scale: 25/28), normal language (Boston Naming Test: 57/60, Sentence Repetition: 10/10, Auditory Comprehension: 37/37, Multilingual Aphasia Examination VII-Token Test: 43/44, Semantic Fluency: 37), and normal praxis (Motor Praxis: 60/60). In addition, the questionnaires aimed to assess cognitive functions completed by the patient demonstrated no behavioral abnormalities or personality changes. The questionnaires complete by his partner, however, indicated that the patient had apathy, loss of spontaneity, personal neglect, disorganization, impulsivity, and hypersexuality.
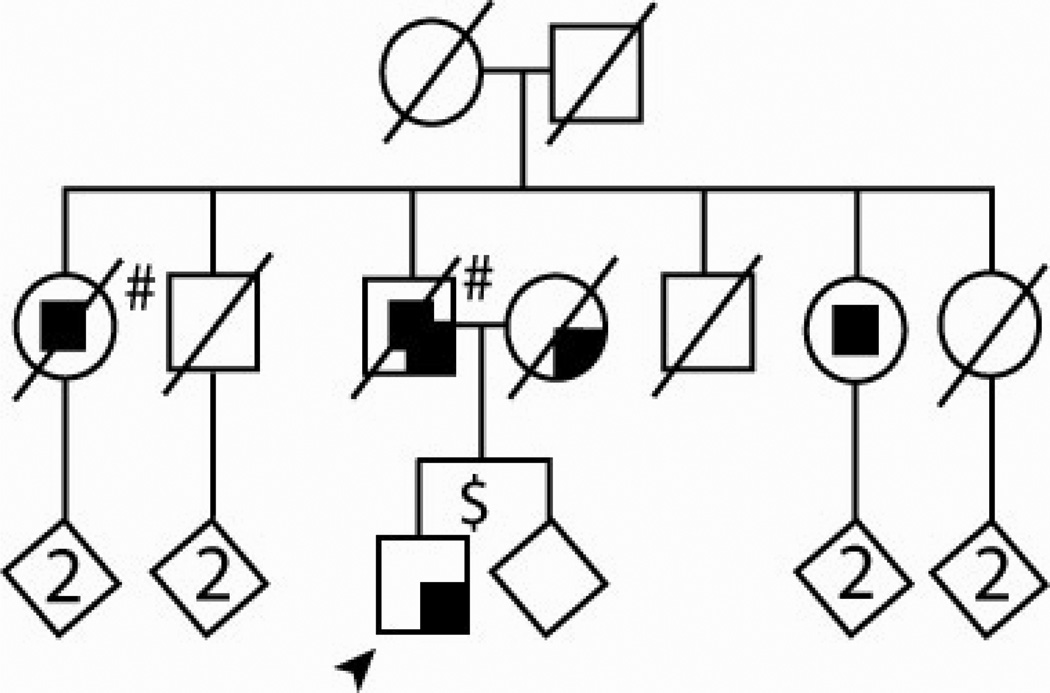
Genetic analyses demonstrated the TDP-43 p.N267S mutation was inherited from the proband’s father. Based on history obtained from the family, throughout his life the father was irritable, short-tempered and had frequent crying spells. He suffered a four-year course of progressive memory decline, disorientation, and mood swings until death at the age of 81. Approximately 2 years prior to his death, he also developed some parkinsonian features including bradykinesia and asymmetrical tremor in his hands. He did not take any medications for his parkinsonism. At the end of his life, he exhibited aggressive behavior and showed features of hypersexuality. An MRI scan showed white matter changes and moderate cortical atrophy, including the temporal areas. The proband’s mother had a five year history of asymmetrical parkinsonism. She presented with bradykinesia, asymmetrical tremor, rigidity, falling, and dysphagia, and her symptoms were responsive to levodopa-carbidopa therapy. She died of stroke at age 81 years. Two of the proband’s paternal aunts developed dementia, the older one of frontotemporal type, and the younger of Alzheimer type based on family history, but no details of their clinical course and symptoms are available. No post-mortem studies were conducted on deceased affected family members. The pedigree is presented in Figure 1.
Figure 1. Pedigree of the TDP-43 p.N267S proband.
Arrow indicates proband. Squares represent males, circles represent females. Darkened symbols indicate affected individuals (phenotypes in the right upper corner). A diagonal line through a symbol means deceased. Number indicates number of offspring. To protect patient confidentiality, the birth order was modified, gender was masked in some individuals and mutation status is not highlighted. Subjects with clinical phenotype accompanied by mild behavioral problems$, or accompanied by severe behavioral problems# are noted.
Discussion
The observation of TDP-43 as a major protein component of the proteinaceous inclusions in ALS and FTD and subsequent identification of pathogenic mutations in the TARDBP gene resulting in ALS has placed it at the center of neurodegenerative disease research [14]. Conditions displaying predominant TDP-43 pathology are now termed TDP-43 proteinopathies, due to its critical role in the pathogenesis of these diseases. Most of the patients with TDP-43 mutations present with typical ALS, occasionally accompanied by dementia [15]. Other clinical presentations such as FTD or parkinsonism were described only in single cases or very small families. Most TDP-43 mutations have incomplete age-related penetrance that makes precise genotype-phenotype correlations difficult [8, 9, 16–21].
To date, 15 cases presenting with parkinsonism as part of their disease phenotype caused by TDP-43 mutations have been reported (Table 1) [8, 20, 21, 22]. Quadri and colleagues reported two cases manifesting parkinsonism and FTD, and a single case with PD from the same family due to TDP-43 p.A382T mutation [8]. Borghero and colleagues reported a family with three affected members due to the same mutation [20]. Recently, we reported a family with parkinsonism and TDP-43 pathology, all known PD genes have been excluded as the genetic cause and exome sequencing has not yet identified any mutations in genes related to TDP-43 pathology [23]. In addition, TDP-43 pathology is observed in Perry syndrome, a rare disorder typified by parkinsonism, weight loss, depression and hypoventilation [24, 25].
The present study has identified an ALS-associated TDP-43 mutation (p.N267S) in a PD patient with a family history of dementia and/or parkinsonism; the father who carried the TDP-43 p.N267S suffered from dementia, followed by parkinsonism and behavioral changes. Interestingly, the TDP-43 p.N267S substitution was previously observed not only in an ALS patient, but also in a patient with behavioral variant FTD [19, 26]. Unfortunately, no autopsy was performed on the father of our proband to confirm the pathologic basis of his dementia, leaving the possibility that he might have had FTLD. The proband’s mother and paternal aunt presented with neurodegenerative disease that appears unrelated to the TDP-43 p.N267S substitution. This means the possibility remains that the parkinsonism in the proband is not due to his carrier status for TDP-43 p.N267S.
In silico and functional studies suggests the TDP-43 p.N267S substitution creates a novel phosphorylation site that can result in reduced protein levels [19]. TDP-43 is a nuclear protein that binds DNA and RNA and has multiple functions as a transcriptional repressor and a modulator of the alternative mRNA splicing [11]. Disruption of these normal TDP-43 functions leads to disease; however, the cause remains elusive in the vast majority of patients with TDP-43 proteinopathy, as they do not carry the known rare TDP-43 mutations. Taken together, these findings suggest intrafamilial variability of clinical phenotype due to TDP-43 p.N267S mutation and provide further evidence to support the possible role of TDP-43 in parkinsonism.
Supplementary Material
This PROBAND’S video WAS obtained during the last clinical examination performed at age 59 years. At the time, he suffered from PD for 17 years. He developed posttraumatic left unlar palsy two yeas and a mild weakness of right leg due to sequelea of surgery performed for lumbar stenos complicated by surgical wound hematoma two years ago.
Video Section 1: Off state (−54 seconds). The patient presents with persistent resting tremor in his left hand and intermittent resting tremor in his right hand. Bradykinesia is severe and more pronounced on the left side. His walking is slow and shuffling with reduced arm swing bilaterally. On pull test, he can not recover on his own.
Video Section 2: On state (−1 minute and 38 seconds). The patient does not have any tremors in his hands. He has a mild bradykinesia. When walking, his gait is much improved. On pull test, he still shows some postural instability but less severe.
Acknowledgements
We would like to thank all those who have contributed to our research, particularly the patients and families who donated DNA samples for this work. This work is supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence (NINDS P50NS072187), NINDS R01NS078086 and the Mayo Clinic Clinical Research Program (MCF #90052030). RR is supported by NIH grants R01AG026251 and the ALS Therapy Alliance. LP is supported by NINDS [R01NS063964, R01NS077402], Amyotrophic Lateral Sclerosis Association, and Department of Defense [W81XWH-10-1-0512-1 and W81XWH-09-1-0315AL093108].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 4.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 8.Quadri M, Cossu G, Saddi V, Simons EJ, Murgia D, Melis M, et al. Broadening the phenotype of TARDBP mutations: the TARDBP Ala382Thr mutation and Parkinson's disease in Sardinia. Neurogenetics. 2011;12:203–209. doi: 10.1007/s10048-011-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chio A, Borghero G, Pugliatti M, Ticca A, Calvo A, Moglia C, et al. Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch Neurol. 2011;68:594–598. doi: 10.1001/archneurol.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orru S, Manolakos E, Orru N, Kokotas H, Mascia V, Carcassi C, et al. High frequency of the TARDBP PAla382Thr mutation in Sardinian patients with amyotrophic lateral sclerosis. Clin Genet. 2012;81:172–178. doi: 10.1111/j.1399-0004.2011.01668.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 2012;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Ross OA, Soto AI, Vilarino-Guell C, Heckman MG, Diehl NN, Hulihan MM, et al. Genetic variation of Omi/HtrA2 and Parkinson's disease. Parkinsonism Relat Disord. 2008;14:539–543. doi: 10.1016/j.parkreldis.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–615. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 16.Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J Med Genet. 2010;47:554–560. doi: 10.1136/jmg.2010.077180. [DOI] [PubMed] [Google Scholar]
- 17.Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–473. doi: 10.1002/ana.21612. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord. 2009;24:1843–1847. doi: 10.1002/mds.22697. [DOI] [PubMed] [Google Scholar]
- 19.Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–E983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 20.Borghero G, Floris G, Cannas A, Marrosu MG, Murru MR, Costantino E, et al. A patient carrying a homozygous p.A382T TARDBP missense mutation shows a syndrome including ALS, extrapyramidal symptoms, and FTD. Neurobiol Aging. 2011;32:2327, e1–e5. doi: 10.1016/j.neurobiolaging.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosca L, Lunetta C, Tarlarini C, Avemaria F, Maestri E, Melazzini M, et al. Wide phenotypic spectrum of the TARDBP gene: homozygosity of A382T mutation in a patient presenting with amyotrophic lateral sclerosis, Parkinson's disease, and frontotemporal lobar degeneration, and in neurologically healthy subject. Neurobiol Aging. 2012;33:1846, e1–e4. doi: 10.1016/j.neurobiolaging.2012.01.108. [DOI] [PubMed] [Google Scholar]
- 22.Fujita Y, Ikeda M, Yanagisawa T, Senoo Y, Okamoto K. Different clinical and neuropathologic phenotypes of familial ALS with A315E TARDBP mutation. Neurology. 2011;77:1427–1431. doi: 10.1212/WNL.0b013e318232ab87. [DOI] [PubMed] [Google Scholar]
- 23.Puschmann A, Pfeiffer RF, Stoessl AJ, Kuriakose R, Lash JL, Searcy JA, et al. A family with Parkinsonism, essential tremor, restless legs syndrome, and depression. Neurology. 2011;76:1623–1630. doi: 10.1212/WNL.0b013e318219fb42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, et al. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41:163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wider C, Dachsel JC, Farrer MJ, Dickson DW, Tsuboi Y, Wszolek ZK. Elucidating the genetics and pathology of Perry syndrome. J Neurol Sci. 2010;289:149–154. doi: 10.1016/j.jns.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrado L, Ratti A, Gellera C, Buratti E, Castellotti B, Carlomagno Y, et al. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Hum Mutat. 2009;30:688–694. doi: 10.1002/humu.20950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This PROBAND’S video WAS obtained during the last clinical examination performed at age 59 years. At the time, he suffered from PD for 17 years. He developed posttraumatic left unlar palsy two yeas and a mild weakness of right leg due to sequelea of surgery performed for lumbar stenos complicated by surgical wound hematoma two years ago.
Video Section 1: Off state (−54 seconds). The patient presents with persistent resting tremor in his left hand and intermittent resting tremor in his right hand. Bradykinesia is severe and more pronounced on the left side. His walking is slow and shuffling with reduced arm swing bilaterally. On pull test, he can not recover on his own.
Video Section 2: On state (−1 minute and 38 seconds). The patient does not have any tremors in his hands. He has a mild bradykinesia. When walking, his gait is much improved. On pull test, he still shows some postural instability but less severe.