Abstract
Hexabromocyclododecane (HBCDD), an additive brominated flame retardant routinely added to various consumer products, was reported to have toxic effects upon biota, including endocrine disruption. In this study, the potential toxicity of HBCDD was tested in peripubertal rat Leydig cells in vitro during 6 h exposure. HBCDD inhibited human chorionic gonadotropin- and forskolin-supported cAMP accumulation and steroidogenesis. It also inhibited basal cAMP production, but elevated basal steroidogenesis. The expression of several cAMP-dependent genes, including steroidogenic acute regulatory protein, cholesterol side chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase, was also inhibited by HBCDD treatment. Nevertheless, this was not accompanied by a decrease in steroidogenic acute regulatory protein expression, as documented by western blot analysis, and activity of steroidogenic enzymes, as documented by unaffected steroidogenesis in the presence of permeable 22(R)-hydroxycholesterol. However, HBCDD caused significant decrease in mitochondrial membrane potential in untreated and human chorionic gonadotropin-treated cells. This indicates that HBCDD acute toxicity in Leydig cells reflects changes in mitochondrial membrane potential-dependent cAMP production and basal and cAMP-regulated cholesterol transport. This in turn facilitates basal but inhibits cAMP-dependent steroidogenesis. Acute effects of HBCDD treatment on transcription are also indicative of its sustained effects on Leydig cell function.
Keywords: endocrine disrupters, hexabromocyclododecane, Leydig cell steroidogenesis, cAMP-signalling, mitochondrial membrane potential
1. Introduction
Hexabromocyclododecane (HBCDD) is an additive brominated flame retardant, routinely added to various consumer products because of its fire protecting properties. Its main application is in polystyrene foams used as thermal insulation in buildings, as a fire retardant in upholstery textiles, and to lesser extent in electronic equipment (Covaci, 2006). The substance was introduced as a commercial product in 1960s (Marvin et al., 2011). Its worldwide consumption in 2001 was estimated to be 16700 metric tons and about 57% demand was centered in Europe (Hale et al., 2006, Szabo et al., 2010). With the ban of all diphenyl ether commercial mixtures, HBCDD production and usage further increased, and the compound has been detected in all environmental matrices, including biota (Szabo et al., 2010). HBCDD is categorized as persistent organic pollutants and it is currently on the European Chemicals Agency candidate list of high concern substances, while the U.S. Environmental Protection Agency is considering adding it to the list of chemicals of concern (for references see Schecter et al., 2012).
The main route for human exposure is via dust inhalation and diet, especially fish consumption. It is shown that concentration of HBCDD in fish range from 9 to 1110 ng/g lipid. In general population, measurable HBCDD concentrations varied between 0.35 and 1.1 ng/g lipid of median blood values (Covaci et al. 2006), whereas the occupationally exposed workers showed higher concentrations in serum, ranging from 6 to 856 ng/g lipid (Thomsen et al., 2007). There is also experimental data showing toxicity of HBCDD. Most striking observations are enlargements of liver, with the induction of phases I and II drug metabolizing enzymes (Canton et al., 2008, Germer et al., 2006), and disrupted thyroid hormone homeostasis in rat (Canton et al., 2008, Ema et al., 2008, Hamers et al., 2006, van der Ven et al, 2006). Neurotoxicity of HBCDD is also shown in both in vitro (Mariussen and Fonnum, 2003, Reistad et al., 2006, Dingemans et al., 2009), and in vivo studies (Saegusa et al. 2009), including impaired spontaneous behavior, learning, and memory in mice (Eriksson et al., 2006).
It has also been suggested that HBCDD isomers could act as reproductive endocrine disruptors. Specifically, they could act as androgen, progesterone, and estrogen receptor antagonists as well as moderate to strong thyroid receptor agonists (Hamers et al., 2006). Reproductive toxicity was investigated in one-generation study in rats of both sexes (van der Ven et al., 2009), and HBCDD exhibited no significant disruption, except a decrease in the testis weight in F1 males. In the two-generation study done by Ema et al. (2008), decreased number of primordial follicles in ovary of F1 rats and higher mortality of F2 pups were observed when F0 female and male rats were treated by 1500 and 15000 ppm HBCDD. Furthermore, adult male rats exposed to technical HBCDD at 15000 ppm dose had decreased epididymides, reduced sperm counts, and the lateral sperm head displacement. In F1-generation male rats no alterations in gross testicular histology were noted in comparison with controls (Ema et al., 2008).
At the present time, there are no data regarding the mechanism of HBCDD action in gonads. This includes the lack of information about the status of testicular steroidogenesis in vivo and in vitro in the presence of HBCDD. Here we used the primary culture of rat Leydig cells from peripubertal rats to estimate the potential HBCDD toxicity on this testicular cell type. Animals in this developmental stage are frequently used to investigate in vivo effects of endocrine disruptive compounds (Stoker et al., 2000, Friedmann, 2002, Akingbemi et al., 2004, Pogrmic et al., 2009), because this is the most sensitive age when the interactions between hypothalamus, pituitary and gonads start to occur and changes in gonadal function have profound effects on establishment of this axis (Ge et al., 2007). The focus in our study was on basal and agonist-stimulated androgen production in Leydig cells with the emphasis on the cellular effectors for HBCDD, intracellular signaling pathways affected and the steroidogenic gene expression profile during the acute (6 h) exposure to this compound.
2. Material and Methods
2.1 Chemicals
1,2,5,6,9,10-Hexabromocyclododecane (95%), medium 199 with Earle’s salts and L-glutamine (M199), Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 Ham, 15 mM HEPES (DMEM/F12), percoll, bovine serum albumin (BSA) fraction V, collagenase type IA, cholesterol, 22(R)-hydroxycholesterol, 3-isobutyl-1-methylxanthine (IBMX), celecoxib, sulphorodamine B, BAPTA-AM, KT5823, penicillin, streptomycin, EDTA, phosphoric acid b-glycerophosphate, tergitol (Niaproof 4, type 4), dithiothreitol, leupeptin and aprotinin were obtained from Sigma-Aldrich Company (Munich, Germany). Tetramethylrhodamine ethyl ester perchlorate (TMRE) was obtained from Fluka (St. Gallen, Switzerland), and human chorionic gonadotropin (hCG; Pregnyl, 3000 IU/mg) was obtained from Organon (West Orange, NJ).
2.2 Primary Leydig cell culture and treatments
Experiments were conducted on Leydig cells obtained from 51 days old Wistar rats raised in animal facility at the Department of Biology and Ecology (University of Novi Sad) under controlled environmental conditions (temperature 22 ± 2 °C and 14 h light/10 h dark) with food and water ad libitum. Experiments were approved by the Ethics Committee for Protection and Welfare of Experimental Animals at University of Novi Sad and conducted in accordance with the statements of National Law for Animal Welfare (copyright March 2009). Isolation and purification of Leydig cells was performed as previously described (Andric et al., 2008, Pogrmic-Majkic et al., 2010). Leydig cells were seeded in 24-well plates (250000 cells/500 μl DMEM/F12 /well and allowed 3 h to attach before applying HBCDD for 6 h at doses: 0, 1, 2, 5 and 10 μM in the absence (basal) and presence of hCG (0.125, 0.25, 1 and 10 ng/ml). Incubations were done at 35 °C, in 5% CO2 atmosphere. Treatments in the presence of cholesterol (20 μM) or 22(R)-hydroxycholesterol (20 μM) were performed to localize the step in steroidogenic process that is affected by the treatment. For testing involvement of different signaling pathways in toxic response, experiments were conducted in the presence of 1 mM IBMX, a non-specific inhibitor of phosphodiesterases (PDEs), forskolin (0.1, 1, 10 μM), an adenylyl cyclase (AC) activator, BAPTA-AM, an intracellular Ca2+ chelator, KT5823, a protein kinase G inhibitor, or celecoxib (5 and 15 μM), an inhibitor of cyclooxygenase-2 (COX-2) activity. HBCDD and different inhibitors/activator were dissolved in dimethyl sulfoxide in a way that enables its final percentage in incubation medium (0.1% BSA DMEM/F12) to be 0.1% in all groups, including control. After the treatment, cell-free media were collected and stored at −70 °C prior to measurement of cAMP, cGMP, progesterone and androgens. For the measurement of the cellular content of cAMP in both basal and hCG (0.125 ng/ml)-treated cells in the absence and presence of 10 μM HBCDD, Leydig cells (250000 cells/well) were scraped and lyzed in absolute ethanol, dried and dissolved in 0.25 ml of 0.1% BSA phosphate buffer containing 1 mM IBMX. For gene expression, Leydig cells were seeded in petri dishes (1×106/2 ml DMEM/F12/dish) and treated according to conditions described above.
2.3 Hormone and cAMP/cGMP measurements
Androgen and progesterone levels in the collected incubation medium were estimated by radioimmunoassay as described in Andric et al. (2000). Anti-testosterone-11-BSA serum no. 250 and anti-progesteron-11BSA serum no. 337 were kindly supplied by Dr. Niswender (Colorado State University, Fort Collins, CO), while [1,2,6,73H(N)] labeled testosterone and [1,2,6,73H(N)] labeled progesterone were obtained from the New England Nuclear (Brussels, Belgium). Each experiment was run in a single assay (sensitivity 6 pg/tube; intra-assay variation coefficient was 5–8%). Androgen levels are referred to as testosterone + dihydrotestosterone (T + DHT) levels, because the anti-testosterone serum used in the assay has a high cross-reactivity with DHT. Progesterone measurements were also done in one assay (sensitivity 6 pg/tube; intra-assay variation coefficient 6–8%). Levels of cAMP and cGMP accumulated in incubation medium were estimated by the cAMP and cGMP EIA Kit (Cayman Chemicals, Ann Arbor, MI), which typically display an IC50 (50% B/B0) value of approximately 0.5 pmol/ml for acetylated cAMP/cGMP samples and permits a detection limit of 0.1 pmol/ml (at 80% B/B0).
2.4 Quantitative RT-PCR analysis
The total RNA was isolated from Leydig cells incubated for 6 h in the absence or presence of different concentrations of HBCDD using RNAqueous-4PCR Kit (Ambion, Austin, TX), and subsequently treated with DNase I in order to eliminate any residual genomic DNA. 1 μg of total RNA was reverse transcribed to cDNA using High Capacity Reverse Transcription Kit with RNase inhibitor (Applied Biosystems, Foster City, CA). All procedures were conducted according to manufacturer’s instructions. Quantitative RT-PCR was performed on 7900HT Fast Real-Time PCR System (Applied Biosystems), using standard PCR settings in SDS Software (Applied Biosystems). The primers were designed by using software Primer Express 3.0 (Applied Biosystems) and full gene sequences from National Center for Biotechnology Information (NCBI) Entrez Nucleotide database (www.ncbi.nlm.nih.gov/sites/entrez). The Power SYBR Green PCR Master Mix was used according to supplier’s instruction for gene expression analysis. The reaction volume was set to 12.5 μl, with 2.5 μl of template cDNA. Primers were used in final concentration of 500 nM. After PCR reaction was finished, a melting curve analysis was performed to ensure that single product was generated. Relative gene expression calculation was performed by using RQ Manager Software (Applied Biosystems), which utilizes 2−ΔΔCt quantification method. Expression of all genes was normalized to the expression of Ectonucleotide pyrophosphatase/phosphodiesterase 1 (Enpp1), which was used as endogenous control. Each sample was run in duplicate for each of the four independent experiments.
2.5 Mitochondrial membrane potential assay
Change in the mitochondrial membrane potential (ΔΨm) was determined by measuring uptake and accumulation of potentiometric dye, tetramethylrhodamine ethyl ester perchlorate (TMRE). Assay was done according to Allen et al. (2006) with slight modifications. Briefly, cells were seeded in 96-well fluorescence assay plate (100.000 cells/200 μl culture medium/well), allowed to attach and treated for 6 h with different doses of HBCDD in the absence and presence of 0.125 ng/ml hCG. After the treatment, culture media were removed and cells were incubated in 200 μl of 10 μM TMRE at 35 °C for 20 min. TMRE media were aspirated, cells were washed twice with warm 0.1% BSA-phosphate buffer and then 200 μl of this buffer was added for fluorescence measurement using a Fluoroscan Ascent FL plate reader (ThermoFisher, Waltham, MA). TMRE fluorescence was measured using an excitation of 550 nm and an emission at 590 nm. Obtained fluorescence values are proportional to the magnitude of ΔΨm. Results are presented as percentage of fluorescence in treated cells versus fluorescence in control group, which is expressed as 100%.
2.6 Cell viability test - sulforhodamine B assay
To determine whether HBCDD treatment was cytotoxic, sulforhodamine B assay was carried out. Briefly, cells were seeded in 96-well plate (105 cells/200 μl culture medium/well), treated for 6 h with different doses of HBCDD and at the end of the treatment fixed for 1 h at 4 °C by 50 μl of 50% w/v trichloroacetic acid per well. After fixation, the cells were washed five times with distilled water, and stained with 50 μl of 0.4% sulphorhodamine B in 1% acetic acid for 30 min. The cells were washed five times with 1% acetic acid and air-dried. The stain was solubilized in 10 mM tris(hydroxymethyl)aminomethane (pH 10.5) and light absorption was measured using the plate reader set on 492 nm, with reference wavelength 690 nm. Neither of the tested HBCDD doses caused cytotoxicity.
2.7. Western blot analysis
This analysis was carried out according to the previously published procedure (Kostic et al, 2011). Briefly, after lysis of Leydig cells, protein concentration was determined using BCA protein assay kit (BioRad, Hercules, CA). Proteins were separated by SDS page and transferred to PVDF membrane. Membrane was blocked in 5% non-fat dry milk following overnight incubation at 4 °C with StAR antibody kindly provided by Dr. D. M. Stocco (Texas Tech University, Health Science Center, Lubbock, TX, USA). Immunoreactive bands were visualized using a chemiluminescence system ECL (GE Healthcare, Pittsburgh, PA). The intensity of protein bands was quantified by using ImageJ software (http://rsbweb.nih.gov/ij) and normalized against GAPDH (Cell Signaling, Denvers, MA).
2.8. Statistical analysis
Each experiment was run at least three times. Data are expressed as mean ± SEM. Comparison between multiple groups was done by one-way ANOVA, followed by Dunnett’s, or Newman–Keuls multiple-range post-hoc test, while Mann-Whitney test was used when only two groups were compared, with p < 0.05 considered as a significant difference.
3. Results
3.1 HBCDD stimulates basal and inhibits hCG-supported steroidogenesis
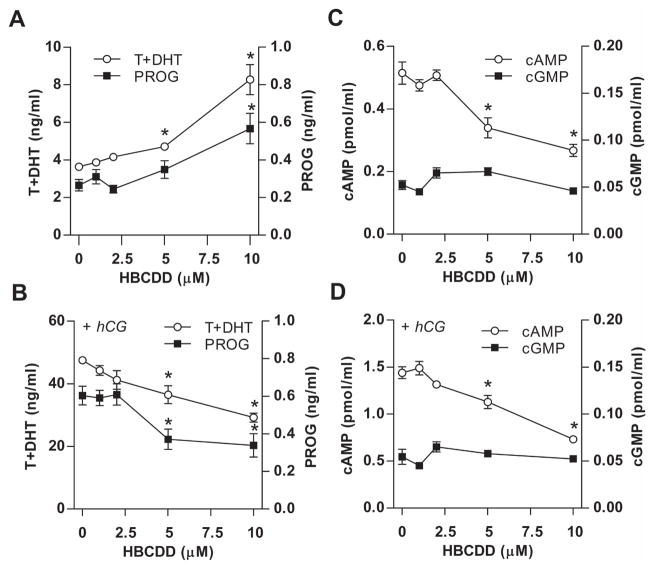
The potential toxicity of HBCDD on steroidogenesis in peripubertal rat Leydig cells in vitro was tested in the absence (basal) and presence of hCG, an agonist for luteinizing hormone (LH) receptor expressed in Leydig cells. HBCDD was applied in 1 to 10 μM concentration range for 6 h. When Leydig cells were incubated in HBCDD-containing medium without hCG, a concentration-dependent increase in androgen and progesterone levels in culture medium was observed (Table 1, Fig. 1A). However, HBCDD was without effect when applied in the presence of medium (1 ng/ml) and high/saturated (10 ng/ml) concentrations of hCG. Furthermore, in the presence of low hCG (0.125 and 0.25 ng/ml), HBCDD significantly decreased androgen production in a concentration-dependent manner (Table 1). hCG-supported progesterone production was also significantly inhibited (Fig. 1B). These results indicate the opposite effects of HBCDD on steroidogenesis in the presence and absence of hCG.
Table 1.
Concentration-dependent effect of HBCDD on basal and hCG-supported androgen (T+DHT) production by Leydig cells in vitro.
| hCG (ng/ml) | Control | 1 μM HBCDD | 5 μM HBCDD | 10 μM HBCDD |
|---|---|---|---|---|
| 0 (basal) | 3.6±0.2 | 3.9±0.1 | 4.7±0.2* | 8.3±0.8* |
| 0.125 | 43.5±1.1 | 41.2±2.6 | 32.1±1.3* | 29.1±1.0* |
| 0.25 | 47.2±1.3 | 46.1±1.0 | 39.9±2.5* | 36.8±1.1* |
| 1 | 58.1±0.7 | 56.5±1.6 | 61.4±1.7 | 59.4±2.6 |
| 10 | 81.2±6.0 | 80.9±7.3 | 78.5±5.1 | 79.9±5.8 |
Leydig cells (250000/500 μl/well) were seeded in 24-well plates and treated with HBCDD and hCG in different concentrations for 6 h. Value shown are mean ± SEM values of T+DHT (ng/ml) obtained in one out of three similar experiments.
p < 0.05 vs. corresponding control.
Fig. 1.
Concentration-dependent effects of HBCDD on basal and hCG-supported androgen, progesterone, cAMP and cGMP production in Leydig cells. (A and B) Effects on basal (A) and hCG-stimulated (B) androgen (T+DHT) and progesterone (PROG) accumulation in the culture medium. (C and D) Effects on basal (C) and hCG-supported (D) cAMP accumulation in culture medium. Notice the lack of effects on cGMP levels. In this and following figures, cells (250000 in 500 μl/well) were seeded in 24-well plates, and treated during 6 h with increasing HBCDD concentrations in the absence or presence of 0.125 ng/ml hCG. Data shown represents mean ± SEM from 3–4 independent experiments. * p < 0.05 vs. corresponding control (0).
3.2. HBCDD inhibits basal and hCG-supported cAMP accumulation
Because the cAMP pathway in Leydig cells dominantly regulates steroidogenesis in Leydig cells (Stocco et al., 2005) and cGMP contributes to this process (Andric et al., 2007), we examined cAMP and cGMP accumulation in culture medium in basal and low hCG-stimulated conditions in the presence and absence of HBCDD. In contrast to steroidogenesis, HBCDD caused significant reduction of cAMP accumulation in culture medium in the absence and presence of hCG. The inhibitory effect of HBCDD on cAMP accumulation was also observed in the presence of saturated concentration of hCG (two fold decrease compared to control), although the androgen production was not affected (Table 1). Furthermore, the level of extracellular cGMP was affected by HBCDD neither in the absence or presence of hCG (Fig. 1C and D). Finally, HBCDD stimulatory effect on steroidogenesis was also visible in the presence of KT5823, a specific protein kinase G inhibitor (data not shown). Thus, HBCDD specifically inhibited cAMP accumulation in medium and there was dissociation between the status of cAMP and progesterone/androgen accumulation in medium in HBCDD-treated cells in the absence of hCG.
3.3 HBCDD does not affect phosphodiesterase and cAMP efflux pump activities
In general, the released cAMP levels reflect a balance between three cellular processes: cAMP release from the cells by cyclic nucleotide transporters, intracellular degradation of cAMP by PDEs, and de novo production of cAMP by ACs. A concentration-dependent decrease in cAMP release in HBCDD treated cells could reflect inhibitory effect of this compound on cyclic nucleotide pump activity, leading to accumulation of cAMP and cGMP in cells. Contrary to this prediction, cellular content of cAMP in both basal and hCG-supported conditions showed similar fold reduction: basal = 1.5 ± 0.1 (controls) vs. 1 ± 0.1 pmol/ml (10 μM HBCDD-treated group); hCG-supported = 2.4 ± 0.1 (controls) vs. 1.6 ± 0.1 pmol/ml (10 μM HBCDD-treated group).
If HBCDD acts as a facilitator of cAMP-specific PDEs, this should decrease cAMP intracellular levels and subsequently the released levels of this messenger. To test the relevance of PDEs in HBCDD inhibitory action on cAMP accumulation intra and extracellular, we used 1 mM IBMX, a concentration that inhibits the majority of these enzymes. Due to the inhibition of PDE activity, an increase in cAMP and therefore increase in androgen production should be expected in Leydig cells incubated in IBMX-containing medium. Obtained results confirmed this hypothesis, i.e., cAMP and androgen levels increased in both basal and hCG-supported conditions in the presence of IBMX. However, the inhibitory effect of HBCDD on cAMP production and the opposite effects of this compound on basal and hCG-supported steroidogenesis were still visible (Fig. 2), similarly to those observed in IBMX-free medium (Fig. 1). Thus, it is unlikely that HBCDD facilitates the cAMP-specific PDE activity.
Fig. 2.
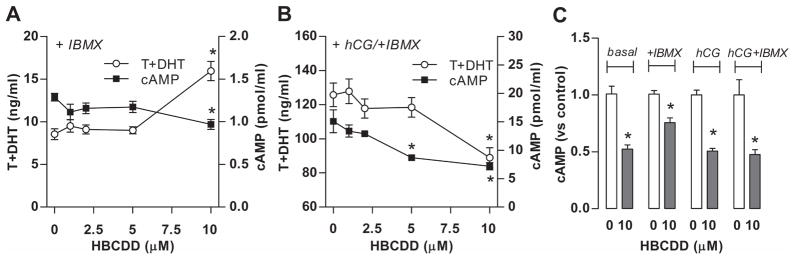
Effects of HBCDD on androgen and cAMP accumulation in culture medium of Leydig cells with inhibited PDEs by 1 mM IBMX. (A) Effects on basal androgen and cAMP accumulation in the incubation medium. (B) Effects on hCG (0.125 ng/ml)-supported androgen and cAMP accumulation in the incubation medium. (C) HBCDD effects on cAMP levels when normalized to corresponding control. Results shown are means ± SEM from three independent experiments. * p < 0.05 vs. corresponding control (0).
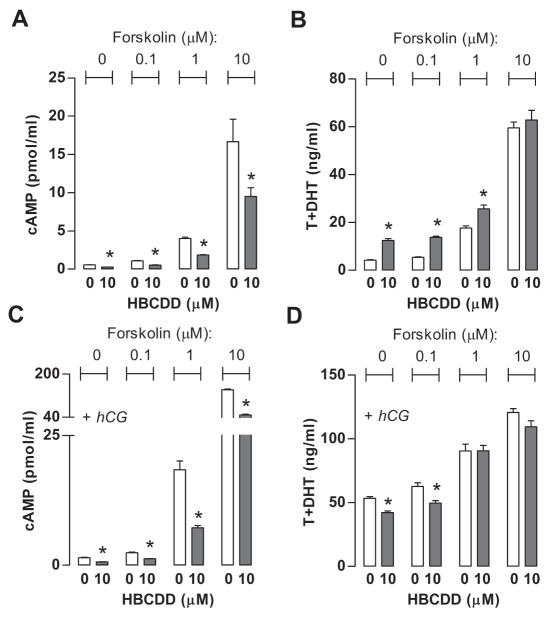
3.4 HBCDD inhibits forskolin-supported AC activity
Inhibitory effect of HBCDD on hCG-supported cAMP accumulation does not clarify how it inhibits cAMP accumulation, at LH/hCG receptor level, signaling from receptors to G proteins to AC, or inhibiting AC activity independently of G protein activation of this enzyme. To study whether HBCDD affects AC activity, we checked the effect of HBCDD application on cAMP accumulation in the presence of 0.1, 1.0 and 10 μM forskolin, a well-known AC activator, in basal and hCG-supported conditions. Figure 3 shows that forskolin stimulated cAMP production in a concentration-dependent manner (A) and enhanced hCG-stimulated cAMP production (B). The inhibitory effect of HBCDD on cAMP accumulation in medium was preserved in cells treated with forskolin in all concentrations under basal and hCG-supported conditions, clearly indicating its direct inhibitory effect on AC activity. HBCDD-induced facilitation of basal and inhibition of hCG-stimulated steroidogenesis was also observed but only at low forskolin concentrations (Fig. 3B and D). The lack of modulation of steroidogenesis by HBCDD at higher forskolin concentration is consistent with finding that maximal streoidogenesis is reached before maximal production of cAMP (Mendelen et al., 1975). In basal conditions, 10 μM forskolin-stimulated cAMP production was 12-fold higher than that induced by applied hCG concentration and enhancement for 0.1, 1 and 10 μM forskolin was 2, 11- and 77-fold higher than hCG stimulated cAMP production, respectively.
Fig 3.
Effects of HBCDD on androgen and cAMP accumulation in culture medium of Leydig cells treated with forskolin, an activator of ACs. (A and C) Effects of forskolin (0.1, 1 and 10 μM) on basal (A) and hCG (0.125 ng/ml)-supported (C) cAMP accumulation in the incubation medium. (B and D), Effects on basal (B) and hCG-supported (D) androgen production. Results shown are means ± SEM from three independent experiments. * p < 0.05 vs. corresponding control (0).
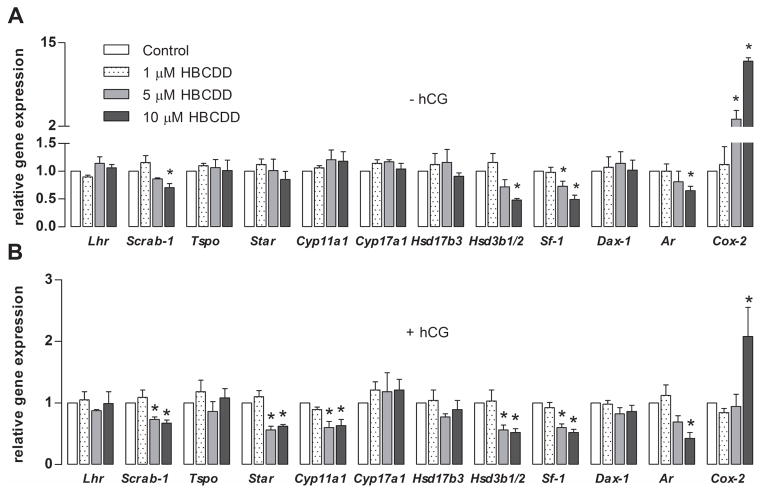
3.5 HBCDD alters the steroidogenic gene expression profile
qRT-PCR analysis of basal gene expression of steroidogenic machinery elements revealed significant inhibition of scavanger receptor B1 (Scarb-1), steroidogenic factor 1 (Sf-1), androgen receptor (Ar) and 3β-hydroxysteroid dehydrogenase 1/2 (Hsd3b1/2). The only gene whose expression was stimulated was cyclooxygenase 2 (Cox-2) by 12 fold. Unaffected were: LH receptor (Lhr), translocator protein (Tspo), steroidogenic acute regulatory protein (Star), cholesterol side chain cleavage enzyme (Cyp11a1), 17α-hydroxylase/C17-20-lyase (Cyp17a1), 17β-hydroxysteroid dehydrogenase 3 (Hsd17b3) and dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (Dax-1) (Fig. 4A). In hCG-supported conditions, there were number of genes, which expression was significantly downregulated by HBCDD treatment, including ones that were shown to be affected in basal conditions. Downregulated genes were: Scarb-1, Star, Sf-1, Ar, Cyp11a1 and Hsd3b1/2 (Fig. 4B).
Fig. 4.
Effects of HBCDD on expression of genes controlling steroidogenesis. (A and B). Gene expression in basal (A) and hCG (0.125 ng/ml)-supported (B) conditions. The mRNA transcripts were analyzed with qRT-PCR. Relative gene expression was calculated by equation RQ=2−ΔΔCt, whereas Enpp1 was used as an endogenous control. Results shown are means ± SEM from three independent experiments. * p < 0.05 vs. corresponding control (white bars).
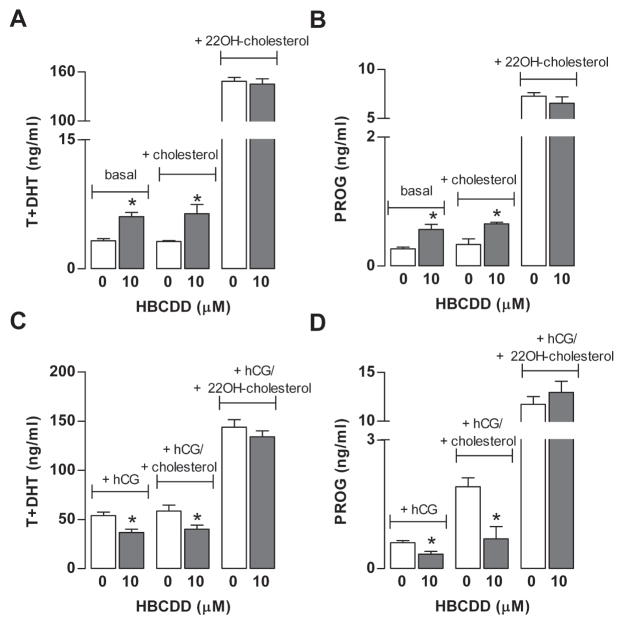
3.6. HBCDD affects cholesterol transport to mitochondria
To test whether HBCDD affects cholesterol transport into mitochondria, a rate-limiting step in steroidogenesis, two experiments were performed. Because StAR regulates cholesterol transports and our gene expression analysis indicated a decrease in StAR mRNA transcription, suggesting that downregulation of this protein may account for inhibited hCG-supported steroidogenesis. However, our Western blot analysis for StAR protein expression from three independent experiments indicated no obvious differences in the expression of 30 kDa form of StAR in HBCDD-treated cells compared to controls hCG-stimulated conditions (data not shown), arguing against this hypothesis.
In the second experiment, cells were bathed in the presence and absence of hCG in combination with cholesterol (20 μM) or 22(R)-hydroxycholesterol (20 μM). It is known that cholesterol needs to be actively transported into mitochondria, whereas 22(R)-hydroxycholesterol easily diffuses to inner membrane of mitochondria (Liu and Stocco, 1997). In the presence of 22(R)-hydroxycholesterol, steroidogenesis was normal, indicating that CYP11A1 and 3βHSD activities were not affected during 6 h exposure to this compound. In contrast, in the presence of cholesterol significant inhibition of hCG-supported and stimulation of basal steroidogenesis remained (Fig. 5). This data indicate that HBCDD toxicity could be associated with cholesterol transport to inner mitochondrial membrane independently of the status of StAR protein expression.
Fig. 5.
Effect of 10 μM HBCDD on Leydig cell steroidogenesis in the absence and presence of 20 μM cholesterol and 20 μM 22R-hydroxycholesterol. (A and B) Effects on basal androgen (A) and progesterone (B) production. (C and D) Effects on hCG (0.125 ng/ml) supported androgen (C) and progesterone (D) production. Results shown are means ± SEM from three independent experiments. * p < 0.05 vs. corresponding control (0).
3.7 HBCDD alters steroidogenesis in a calcium and COX-2-independent manner
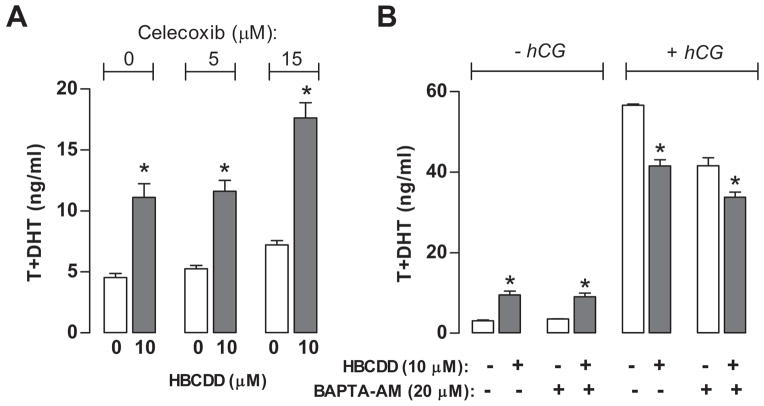
COX-2 is an arachidonic acid metabolizing enzyme and its products, prostaglandins, exhibit constitutive inhibitory action on steroidogenesis (Wang et al., 2003). Because our results indicated to a 12-fold up-regulation of Cox-2 expression in basal conditions, we examined the potential physiological relevance. To do this, we tested the effect of HBCDD on steroidogenesis in the presence of celecoxib, an inhibitor of COX-2. Observed results indicated that celecoxib did not interfere with HBCDD-induced stimulation of basal androgen production. However, at high concentration (15 μM) celecoxib facilitated basal steroidogenesis in the presence and absence of HBCDD (Fig. 6A), which is consistent with a finding that inhibition of COX-2 activity increases the sensitivity of steroidogenesis to cAMP (Wang et al., 2003, Stocco et al., 2005).
Fig. 6.
Independence of HBCDD action on basal Leydig cell steroidogenesis of COX-2 expression and intracellular calcium. (A) Effects of celecoxib (5 μM and 15 μM), a COX-2 inhibitor, on in vitro steroidogenesis. Cells were treated during 6 h with 10 μM HBCDD in the absence and presence of celecoxib (B) HBCDD effects on Leydig cell basal and hCG (0.125 ng/ml)-supported androgen production when challenged with BAPTA-AM (20 μM), an intracellular Ca2+ chelator. Notice that BAPTA-AM did not diminish HBCDD toxic effects. Data points represent means ± SEM in one out of three similar experiments. * p < 0.05 vs. corresponding control (0).
We also tested possibility whether it is disrupted level of intracellular calcium that is more directly involved in stimulated basal steroidogenesis, by performing treatments in the presence of intracellular calcium chelator BAPTA-AM (20 μM). Figure 6B shows that in the presence of BAPTA-AM, HBCDD induced stimulation was retained, indicating that calcium signaling is not critical for the action of this compound.
3.8 HBCDD inhibits ΔΨm in Leydig cells
According to literature data (Midzak et al., 2006), stimulation of basal steroidogenesis accompanied with inhibition of hCG stimulated steroidogenesis may occur due to disruption of ΔΨm. Led by these findings, we examined ΔΨm in Leydig cells after 6 h of HBCDD treatment in basal and hCG (0.125 ng/ml)-stimulated conditions, using the potentiometric dye TMRE. Data indeed revealed that HBCDD is causing a significant decrease in ΔΨm by 14% and 29% in basal and by 33% and 42% in hCG-stimulated conditions when Leydig cells are treated with 5 μM and 10 μM, respectively (Table 2). The values of ΔΨm in control cells incubated either in the absence or presence of 0.125 ng hCG were similar (fluorescence, arbitrary units: 3605±240 vs. 3047±210 (data from 3 independent experiments).
Table 2.
Effects of HBCDD on mitochondrial membrane potential (ΔΨm).
| HBCDD (μM) |
ΔΨm (% vs control)
|
|
|---|---|---|
| Basal | hCG-stimulated | |
| 0 | 100.4±3.1 | 100.0±11.0 |
| 1 | 102.8±2.5 | 103.6±5.7 |
| 5 | 86.5±2.5* | 70.7±5.8* |
| 10 | 66.5±2.3** | 58.1±5.2** |
For the purpose of measuring ΔΨm, cells (100000/200 μl/well) were seeded in 96-well fluorescence assay plates and treated for 6 h with different doses of HBCDD in the absence or presence of 0.125 ng/ml hCG. After the treatment, ΔΨm was evaluated with TMRE assay. TMRE fluorescence is directly proportional to ΔΨm and is presented as percentage vs. fluorescence of corresponding control (0). Data represent means ± SEM from eight independent experiments in absence and three independent experiments in the presence of 0.125 ng hCG.
p < 0.05 and
p < 0.01 and vs. corresponding control (0).
4. Discussion
Here we show for the first time that exposure to HBCDD has several adverse effects on Leydig cell function in the absence and presence of hCG. In both experimental conditions, it inhibits cAMP production. However, it has bidirectional effect on steroidogenesis, stimulating basal and inhibiting hCG-supported progesterone and androgen accumulation. Because activation of cAMP/PKA pathway by LH/hCG receptor agonists is the main pathway regulating steroidogenesis (Stocco et al., 2005), it is logical to conclude that reduced cAMP levels in HBCDD-treated cells challenged with hCG account for or contributes to downregulated steroidogenesis, whereas stimulation of basal androgen production at reduced cAMP levels indicates an additional, cAMP-independent action of this compound. Consistent with down-regulation of cAMP production, HBCDD treatment also inhibited expression of several genes, but this effect appears not to play a role in acute modulation of steroidogenesis. Although very complex, these direct acute effects on Leydig cell function could be explained by a single action of HBCDD on ΔΨm.
To clarify a mechanism by which HBCDD inhibits cAMP accumulation, we analyzed several steps in this signaling pathway. In general, levels of cellular cyclic nucleotides are determined by the rate of their synthesis by ACs and guanylyl cyclases, the rate of their degradation by PDEs, and the activity of specific efflux pumps (Stojilkovic et al., 2012). It is unlikely that HBCDD is affecting the functionality of cAMP efflux pumps, because similar fold reduction in cAMP accumulation was detected in cellular content and in culture medium, both in basal and in hCG-supported condition. In IBMX-treated cells the effect of HBCDD was also not abolished, further indicating that it does not stimulates PDEs.
The inhibitory effect of HBCDD on cAMP accumulation in the presence of low and high forskolin concentrations clearly indicates that the catalytic activity of these enzymes is affected by HBCDD in a concentration-dependent manner. All classes of AC catalyze the conversion of ATP to cAMP and pyrophosphate and HBCDD significantly reduces ATP production in other cell types (Hinkson and Whalen, 2009), suggesting that the reduced intracellular ATP could accoun for reduction in cAMP production in Leydig cells. In parallel to our experiments, others have shown that myxothiazol, a mitochondrial transport chain inhibitor, attenuated LH-stimulated cAMP production and steroidogenesis in Leydig cells. They also detected a strong inhibition of ATP production in the presence of myxothiazol, suggesting that downregulation of cAMP production could be the consequence of diminished ATP production (Midzak et al. 2006).
More recently, the Midzak’s group also reported that decrease in ΔΨm by blocking the electron transport chain by carbonyl cyanide m-chlorophenyl hydrazone leads to decreased synthesis of ATP, and testosterone in LH-stimulated primary rat Leydig cells. The authors also stressed that primary Leydig cells dependent almost completely on mitochondrial respiration for their ATP supply, and therefore electron transport chain, ΔΨm, and ATP synthesis cooperatively affected steroidogenesis (Midzak et al., 2011). Consistent with these data, our results show that HBCDD caused drop in ΔΨm by 33% in basal conditions and by 42% in hCG-stimulated conditions. Thus, it is reasonable to conclude that HBCCD-induced drop in ΔΨm causes downregulation of ATP production, which in turn reduced agonist- and forskolin-stimulated cAMP production and cAMP-dependent steroidogenesis.
cAMP dependence of steroidogenesis is related to StAR function, a protein that is involved in cholesterol transfer to inner mitochondrial membrane, which is the rate-limiting step in the production of steroid hormones. The transfer occurs through a complex termed the “transduceosome”, in which cytosolic StAR interacts with outer mitochondrial membrane translocator protein and voltage-dependent anion channel to assist with the transfer of cholesterol (Rone et al., 2012). Because expression of Star is a cAMP-dependent process, we examined the status of Star expression. Downregulation of StAR mRNA expression was observed after 6 h HBCDD treatment in the presence of hCG, but was not accompanied by a significant decrease in StAR protein expression, suggesting that preexisting mRNA is sufficient to keep normal expression of StAR protein. However, phosphorylation of StAR by PKA is required for maximal steroidogenic activity (Rone et al. 2009) and thus it is reasonable to conclude that decreased level of cAMP in HBCCD-treated cells account for downregulation of StAR function, causing inhibition of facilitated steroidogenesis. Certainly, it is also reasonable to suggest that sustained HBCDD treatment will affect StAR protein expression and have more severe effects on Leydig cell steroidogenesis, which should be tested in further experiments.
At inner mitochondrial membrane, conversion of cholesterol to pregnenolone is controlled by CYP11A1, pregnonolone is further metabolized by the action of 3βHSD to progesterone, conversion of progesterone to androstenedione is catalyzed by CYP17A1, and 17βHSD converts androstenedione to testosterone (Payne and Youngblood, 1995). PKA also up-regulates transcription of genes for these enzymes through the orphan receptor SF-1, which requires at least four hours for increase in mRNA transcripts to be observed and 12 hour to reach its maximum (John et al., 1986; Sewer and Waterman, 2001). The HBCDD induced downregulation of several genes, including Sf-1, shown here is in accordance with decreased levels of cAMP. However, steroidogenesis was not altered by HBCDD in cells bathed in medium containing the mitochondria permeable 22(R)-hydroxycholesterol, indicating that steroidogenic enzyme expression at protein levels and their activity was not significantly affected during 6 h exposure. However, this does not exclude the sustained effects of HBCDD on steroidogenesis due to down regulation of steroidogenic enzyme expression at protein level, an issue that should be addressed in future experiments.
The opposite effect of HBCDD on cAMP and androgen productions in the absence of hCG clearly indicates the existence of an additional mechanism of HBCDD action in Leydig cells. Consistent with our data, myxothiazol has bidirectional effects on steroidogenesis; it inhibits facilitated but stimulates basal steroidogenes in Leydig cells, the latter in a calcium-dependent mechanism (Midzak et al. 2006). This is consistent with a role of mitochondria in cellular calcium homeostasis (Saotome et al., 2004). In our experimental conditions, treatment of Leydig cells by BAPTA-AM, a calcium chelator, did not change effects of HBCDD on basal and hCG-supported steroidogenesis. Furthermore, cGMP is known to stimulate steroidogenesis via cGMP-protein kinase G signaling pathway (Andric et al., 2007), but two lines of evidence also argue against involvement of this pathway in HBCCD action. First, 6 h HBCDD treatment did not cause any change in cGMP level in basal and hCG-supported cells. Second, HBCDD stimulatory effect on steroidogenesis was also visible in the presence of a PKG inhibitor. Cholesterol transport through transduceosome in basal conditions (Rone, 2010) should also be dependent on ΔΨm (Midzak et al., 2011). It is possible that HBCDD induced drop in this potential facilitates basal cholesterol transport, leading to elevation in steroidogenesis. Because basal steroidogenesis is less than 10% of hCG-stimulated steroidogenesis, we speculate that such facilitation could not compensate for the cAMP-PKA-dependent transport of cholesterol in agonist-stimulated cells.
Previous experiments have shown that perfluorinated carboxylic acids, used as commercial wetting agents and flame retardants, reduces both in vivo and in vitro testosterone production by acting at the Leydig cells (Bookstaff et al., 1990; Boujrad et al., 2000). Moreover, the data of Boujrad et al., (2000) showed that perfluorinated carboxylic acids inhibits hCG-stimulated steroidogenesis in immortalized mouse Leydig cells by affecting translocator protein mRNA stability, thus inhibiting its expression, cholesterol transport into mitochondria, and subsequent progesterone production. However, we have not observed a decrease in translocator protein mRNA transcript levels in HBCDD-treated rats. Similarly, the expression of Dax1, which protein is a negative regulator of Star expression (Jo and Stocco, 2004), was not affected by the treatment. We also excluded a hypothesis that COX-2, an arachidonic acid metabolizing enzyme exhibiting constitutive inhibitory action on steroidogenesis (Wang et al., 2003), is involved in HBCDD-dependent stimulation of androgen production, because in the presence of celecoxib, an inhibitor of COX-2, stimulatory effect was not abolished. Finally, further studies are needed to clarify the physiological role of inhibition of Scarb-1, which is responsible for selective cellular import of cholesterol from circulating high density lipoproteins (Rone et al., 2009), and Ar, which also serves as an ultra short feed back loop in repressing steroidogenic gene expression (Eacker and Braun, 2007).
In conclusion, in this study we have shown that HBCDD inhibits basal and hCG-supported synthesis of cAMP and therefore inhibiting PKA activation, which is a dominant pathway regulating steroidogenesis. Consequently, it caused downregulation of multiple genes involved in this process. HBCDD has a dual effect on in vitro steroidogenesis, stimulatory in the absence of hCG and inhibitory in the presence of hCG. We also showed that HBCDD affects ΔΨm, which provides a rationale for downregulation of cAMP production and cAMP-dependent steroidogenesis and gene expression, as well as for the ΔΨm-controlled cholesterol transport. Our experiments also argue against the contribution of HBCDD-induced inhibition of hCG-stimulated gene expression in acute effects on steroidogenesis, but pointed to further investigation on evaluation of expression of steroidogenic enzymes and related proteins and their activities during chronic exposure to HBCDD.
Highlights.
Hexabromocyclododecane (HBCDD) is an additive brominated flame retardant.
HBCDD increased basal and decreased stimulated androgen production in Leydig cell.
In contrast, HBCDD inhibited cAMP accumulation in basal and stimulated conditions.
In the presence of 22(R)-hydroxycholesterol HBCDD effects were abolished.
HBCDD caused significant decrease in mitochondrial membrane potential.
Acknowledgments
We are grateful to Drs. D. M. Stocco for providing StAR antiserum and G. D. Niswender for the supply of testosterone and progesterone antiserums. This work is supported by the Ministry of Science, Republic of Serbia (grant no. 173037), by the Autonomic Province of Vojvodina (grant no. 2570) and a Eunce Kennedy Shiver National Institute of Health and Human Development intramural grant.
Abbreviations
- ACs
adenylyl cyclases
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxigenase-2
- HBCDD
hexabromocyclododecane
- hCG
human chorionic gonadotropin
- IBMX
3-isobutyl-1-methylxanthine
- LH
luteinizing hormone
- PDEs
phosphodiesterases
- PKA
cAMP-dependent protein kinase
- StAR
steroidogenic acute regulatory protein
- T + DHT
testosterone + dihydrotestosterone
- TMRE
tetramethylrhodamine ethyl ester perchlorate
- ΔΨm
mitochondrial membrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006;147:3924–3935. doi: 10.1210/en.2005-1204. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CMAI, Klinefelter GR, Hardy MP. Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Andric N, Kostic T, Kaisarevic S, Fa S, Pogrmic K, Kovacevic R. In vivo and in vitro effects of PCB126 and PCB153 on rat testicular androgenesis. Environ Toxicol Pharmacol. 2008;25:222–226. doi: 10.1016/j.etap.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Andric SA, Kostic TS, Stojilkovic SS, Kovacevic RZ. Inhibition of rat testicular androgenesis by polychlorinated biphenyl mixture Aroclor 1248. Biol Reprod. 2000;62:1882–1888. doi: 10.1095/biolreprod62.6.1882. [DOI] [PubMed] [Google Scholar]
- Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Protein kinase G-mediated stimulation of basal Leydig cell steroidogenesis. Am J Physiol Endocrinol Metab. 2007;293:1399–1408. doi: 10.1152/ajpendo.00482.2007. [DOI] [PubMed] [Google Scholar]
- Boiujard N, Vidic B, Gazouli M, Cluty M, Papadopoulos V. The peroxisome proliferator perfluorodecanoic Acid Inhibits the peripheral-Type benzodiazepine receptor (PBR) expression and Hormone-Stimulated Mitochondrial Cholesterol transport and Steroid Formation in Leydig Cells. doi: 10.1210/endo.141.9.7678. [DOI] [PubMed] [Google Scholar]
- Bookstaff RC, Moore RW, Ingall GB, Peterson RE. Androgeenic deficiency in Male rats treated with Perfluorodecanoic Acid. Toxicol App Pharmacol. 1990;104:322–333. doi: 10.1016/0041-008x(90)90306-f. [DOI] [PubMed] [Google Scholar]
- Cantón RF, Peijnenburg ACM, Hoogenboomb RLAP, Piersma AH, van der Ven LTM, van den Berg M, Heneweer M. Subacute effects of hexabromocyclododecane (HBCD) on hepatic gene expression profiles in rats. Toxicol Appl Pharmacol. 2008;231:267–272. doi: 10.1016/j.taap.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Heather L, Allchin CR, de Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environ Sci Technol. 2006;40:3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Heusinkveld HJ, de Groot A, Bergman A, van den Berg M, Westerink RHS. Hexabromocyclododecane inhibits depolarization-induced increase in intracellular calcium levels and neurotransmitter release in PC12 Cells. Toxicol Sci. 2009;107:490–497. doi: 10.1093/toxsci/kfn249. [DOI] [PubMed] [Google Scholar]
- Eacker SM, Braun RE. Androgen receptor in Leydig cell function and development. In: Payne AH, Hardy MP, editors. The Leydig cell in health and disease. Humana Press Inc; Totowa, New Jersey: 2007. pp. 345–362. [Google Scholar]
- Ema M, Fujii S, Hirata-Koizumi M, Matsumoto M. Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats. Reprod Toxicol. 2008;25:335–351. doi: 10.1016/j.reprotox.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Fischer C, Wallin M, Jakobsson E, Fredriksson A. Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD) Environ Toxicol Pharmacol. 2006;21:317–322. doi: 10.1016/j.etap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Ge R, Hardy MP. Regulation of Leydig cells during pubertal development. In: Payne AH, Hardy MP, editors. Contemporary Endocrinology: The Leydig cells in health and desease. Human Press Inc; 2007. pp. 55–71. [Google Scholar]
- Germer S, Piersma AH, van der Ven L, Kamyschnikow A, Fery Y, Schmitz HJ, Schrenk D. Subacute effects of the brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on hepatic cytochrome P450 levels in rats. Toxicology. 2006;218:229–236. doi: 10.1016/j.tox.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 2006;64:181–186. doi: 10.1016/j.chemosphere.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hinkson NC, Whalen MM. Hexabromocyclododecane decreases the lytic function and ATP levels of human natural killer cells. J Appl Toxicol. 2009;29:656–661. doi: 10.1002/jat.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y, Stocco DM. Regulation of steroidogenesis and steroidogenic acute regulatory protein in R2C Cells by DAX-1 (Dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene-1) Endocrinology. 2004;145:5629–5637. doi: 10.1210/en.2004-0941. [DOI] [PubMed] [Google Scholar]
- John EM, John CM, Bogaram V, Simpson RE, Waterman NMR. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci U S A. 1997;83:4715–4719. doi: 10.1073/pnas.83.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic TS, Stojkov NJ, Bjelic MM, Mihajlovic AI, Janjic MM, Andric SA. Pharmacological Doses of Testosterone Upregulated Androgen Receptor and 3-Beta Hydroxysteroid Dehydrogenase/Delta-5-Delta-4 Isomerase and Impaired Leydig Cells Steroidogenesis in Adult Rats. Tox Sci. 2011;121:397–407. doi: 10.1093/toxsci/kfr063. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stocco DM. Heat shock-induced inhibition of acute steroidogenesis in MA-10 cells is associated with inhibition of the synthesis of the steroidogenic acute regulatory protein. Endocrinology. 1997;138:2722–2728. doi: 10.1210/endo.138.7.5278. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Marvin CH, Tomy GT, Armitage JM, Arnot JA, McCarty L, Covaci A, Palace V. Hexabromocyclododecane: current understanding of chemistry, environmental fate and toxicology and implications for global management. Environ Sci Technol. 2011;45:8613–8623. doi: 10.1021/es201548c. [DOI] [PubMed] [Google Scholar]
- Mendelson C, Dufau M, Catt K. Gonadotropin Binding and stimulation of cyclic adenosine 3′:5′-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975;250:8818–8823. [PubMed] [Google Scholar]
- Midzak AS, Chen H, Aon MA, Papadopoulos V, Zirkin BA. ATP Synthesis, Mitochondrial Function, and Steroid Biosynthesis in Rodent Primary and Tumor Leydig Cells. Biol Reprod. 2011;84:976–985. doi: 10.1095/biolreprod.110.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak AS, Liu J, Zirkin BR, Chen H. Effect of myxothiazol on Leydig cell steroidogenesis: inhibition of luteinizing hormone-mediated testosterone synthesis but stimulation of basal steroidogenesis. Endocrinology. 2006;148:2583–2590. doi: 10.1210/en.2006-1488. [DOI] [PubMed] [Google Scholar]
- Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–222. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- Pogrmic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Atrazine Oral Exposure of Peripubertal Male Rats Downregulates Steroidogenesis Gene Expression in Leydig Cells. Tox Sci. 2009;111:189–197. doi: 10.1093/toxsci/kfp135. [DOI] [PubMed] [Google Scholar]
- Pogrmic-Majkic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Upregulation of peripubertal rat Leydig cell steroidogenesis following 24 h in vitro and in vivo exposure to atrazine. Toxicol Sci. 2010;118:52–60. doi: 10.1093/toxsci/kfq227. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Rone BM. Role of protein-protein interactions in mitochondrial protein import, cholesterol transport and steroid biosynthesis. PhD thesis. 2010 https://repository.library.georgetown.edu/handle/10822/552850.
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Andrew S, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V. Identification of a Dynamic Mitochondrial Protein Complex Driving Cholesterol Import, Trafficking, and Metabolism to Steroid Hormones. Mol Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa Y, Fujimoto H, Woo GH, Inoue K, Takahashi M, Mitsumori K, Hirose M, Nishikawa A, Shibutani M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol. 2009;28:456–467. doi: 10.1016/j.reprotox.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Saotome M, Katoh H, Satoh H, Nagasaka S, Yoshihara S. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;288:1820–1828. doi: 10.1152/ajpheart.00589.2004. [DOI] [PubMed] [Google Scholar]
- Schecter A, Szabo DT, Miller J, Gent TL, Malik-Bass N, Petersen M, Paepke O, Colacino JA, Hynan LS, Harris TR, Malla S, Birnbaum LS. Hexabromocyclododecane (HBCD) stereoisomers in U.S. food from Dallas, Texas. Environ Health Perspect. 2012;120:1260–1264. doi: 10.1289/ehp.1204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. Insight into the transcriptional regulation of steroidogenic enzymes and StAR. Rev Endocr Metab Disord. 2001;2:269–274. doi: 10.1023/a:1011516532335. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang XJ, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Kretschmannova K, Tomi M, Stratakis CA. Dependence of the excitability of pituitary cells on cyclic nucleotides. J Neuroendocrinol. 2012;24:1–18. doi: 10.1111/j.1365-2826.2012.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Parks LG, Gray LE, Cooper RL. Endocrine disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Crit Rev Toxicol. 2000;30:197–252. doi: 10.1080/10408440091159194. [DOI] [PubMed] [Google Scholar]
- Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: effect of dose, timing, repeated exposure and metabolism. Toxicol Sci. 2010;117:282–293. doi: 10.1093/toxsci/kfq183. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Molander P, Daae HL, Janák K, Froshaug M, Liane VH, Thorud S, Becher G, Dybing E. Occupational exposure to hexabromocyclododecane at an industrial plant. Environ Sci Technol. 2007;41:5210–5216. doi: 10.1021/es0702622. [DOI] [PubMed] [Google Scholar]
- van der Ven LT, van de Kuil T, Leonards PE, Slob W, Lilienthal H, Litens S, Herlin M, Håkansson H, Cantón RF, van den Berg M, Visser TJ, van Loveren H, Vos JG, Piersma AH. Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats. Toxicol Lett. 2009;185:51–62. doi: 10.1016/j.toxlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- van der Ven LTM, Verhoef A, van de Kuil T, Slob W, Leonards PEG, Visser TJ, Hamers T, Herlin M, Hakansson H, Olausson H, Piersma AH, Vos JG. A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats. Toxicol Sci. 2006;94:281–292. doi: 10.1093/toxsci/kfl113. [DOI] [PubMed] [Google Scholar]
- Wang X, Dyson MT, Jo Y, Stocco DM. Inhibition of cyclooxygenase-2 activity enhances steroidogenesis and steroidogenic acute regulatory gene expression in MA-10 mouse Leydig cells. Endocrinology. 2003;144:3368–3375. doi: 10.1210/en.2002-0081. [DOI] [PubMed] [Google Scholar]