Abstract
Ceramide-1-phosphate (C1P) is a bioactive lipid that, in contrast to ceramide, is an anti-apoptotic molecule released from cells that are damaged and “leaky”. As reported recently, C1P promotes migration of hematopoietic cells. In the current paper, we tested the hypothesis that C1P released upon tissue damage may play an underappreciated role in chemoattraction of various types of stem cells and endothelial cells involved in tissue/organ regeneration. We show for a first time that C1P is upregulated in damaged tissues and chemoattracts BM-derived multipotent stroma cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like stem cells (VSELs). Furthermore, compared to other bioactive lipids, C1P more potently chemoattracted human umbilical vein endothelial cells (HUVECs) and stimulated tube formation by these cells. C1P also promoted in vivo vascularization of Matrigel implants and stimulated secretion of stromal derived factor-1 (SDF-1) from BM-derived fibroblasts. Thus, our data demonstrate, for the first time, that C1P is a potent bioactive lipid released from damaged cells that potentially plays an important and novel role in recruitment of stem/progenitor cells to damaged organs and may promote their vascularization.
Keywords: C1P, MSC, HUVEC, chemoataxis, angiogenesis
Introduction
Evidence has accumulated that non-hematopoietic stem cells, including, multipotent stroma cells (MSCs), endothelial progenitor cells (EPCs), or very small embryonic-like stem cells (VSELs), are mobilized into peripheral blood in response to organ injuries (e.g., heart infarct or stroke) and can be subsequently recruited to the damaged tissues [1–14]. These circulating stem cells could potentially contribute to tissue repair directly by supporting formation of new blood vessels (e.g., EPCs) [8–10] or indirectly as a source of several growth factors and membrane-derived microvesicles (e.g., MSCs) that provide trophic signals that inhibit cell apoptosis and stimulate vascularization of damaged tissues [15–18].
It is well established that stem cells circulating in peripheral blood (PB) are recruited to damaged organs in response to chemotactic factors, including chemokines (e.g., stromal cell derived factor-1, SDF-1) and some growth factors (e.g., vascular endothelial growth factor, VEGF; basic fibroblast growth factor, bFGF; or hepatocyte growth factor, HGF) [19]. Evidence has accumulated that, in addition to chemotactic peptides, bioactive lipids also play an important role in the process of recruitment of circulating stem cells [20–23]. The best known of these is sphingosine-1-phosphate (S1P), which chemoattracts various types of stem cells, including MSCs and EPCs [24–27].
Although other mechanisms for C1P synthesis must exist, ceramide-1-phosphate (C1P) is a product of the phosphorylation of ceramide by ceramide kinase (CerK) and, like S1P, belongs to a family of bioactive sphingolipids [28]. Both S1P and C1P are important intracellular signaling molecules. However, in contrast to S1P, C1P is not secreted by intact cells, but released from “leaky” damaged cells [29]. It is also known that C1P, in contrast to its parent molecule ceramide, shows anti-apoptotic effects in several types of cells, including macrophages [30] and keratinocytes [31], and also plays a role in chemoattracting macrophages [32] and stimulating proliferation of myoblasts [33]. Recently, we have demonstrated that C1P is a potent chemoattractant for normal hematopoietic stem/progenitor cells (HSPCs) [29]. In particular, we demonstrated that C1P is elevated in bone marrow (BM) tissue damaged by myeloablative treatment and may stimulate homing of transplanted HSPCs [29].
In contrast to S1P, which binds to five different G protein-coupled receptors [34], the C1P receptor(s) have not yet been identified. However, based on its signaling sensitivity to pertussis toxin (PTx), the C1P receptor is most likely similar to the Gαi protein-coupled seven-transmembrane spanning S1P receptors [32]. Interestingly, it has been reported that dermal microendothelial cells from mice lacking CerK show profound defects in in vitro angiogenesis assays [35], which suggests that C1P could also be endowed with pro-angiopoietic properties.
In the current work, we became interested in whether C1P, like S1P [24–27], plays a role in trafficking of MSCs and EPCs. We observed that C1P i) becomes upregulated in damaged organs, ii) regulates migration and adhesion of stromal (MSCs) and endothelial cells (HUVECs), and iii) directly affects in vitro angiogenesis in the HUVEC model of tube formation and more important it promoted in vivo vascularization of Matrigel implants. Building on these earlier observations, we here demonstrate for the first time that C1P, like S1P [22], becomes overexpressed at sites of tissue/organ injury and also provides chemotactic homing signals for non-hematopoietic stem cells. Because of this finding, we postulate that modulation of C1P expression and signaling may play an important future role in regenerative medicine. Thus, more work is needed to identify C1P receptor/s and develop compounds that will modify in vivo biological availability of C1P.
Materials and Methods
Isolation of bone marrow cells
Murine bone marrow mononuclear cells (BMMNCs) were isolated by flushing the femurs and tibias of pathogen-free C57BL/6. Whole bone marrow cells were separated by Ficoll gradient, washed, and resuspended in RPMI medium (Thermo Fisher Scientific, South Logan, Utah, USA) containing 0.5% BSA (Sigma). Human BM samples were obtained from donors after informed consent, and all studies with human cells were approved by UK and UofL IRBs.
MSC and HUVEC culture
Bone marrow-derived stromal cells (MSCs) were isolated from whole murine or human bone marrow (BM) cells. Whole BM cells were cultured in DMEM with 20% FBS and 50 U/ml penicillin/streptomycin for more than 7 days at 37°C in a 5% CO2 incubator. Non-adherent hematopoietic cells were discarded and adherent cells were maintained. Human MSCs were expanded from BM samples isolated from donors after informed consent. Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical vein using collagenase I (Sigma) digestion and cultured in EBM (Lonza, Walkersville, MD, USA) supplemented with 2% fetal bovine serum (FBS), bovine brain extract (BBE), recombinant human epidermal growth factor (rhEGF), hydrocortisone (HC), and GA-1000 (all supplements to EBM reagents from Lonza) as described [36].
Cell migration assay
The 8-μm polycarbonate membranes were coated with 50 μL of 0.5% gelatin for 1 hour. MSCs and HUVECs were detached with Trypsin-EDTA, washed in DMEM (or EBM), resuspended in DMEM (or EBM) with 0.5% BSA, and seeded at a density of 3 × 104 cells/well into the upper chambers of Transwell inserts (Costar Transwell; Corning Costar). The lower chambers were filled with SDF-1 (5, 50, or 300 ng/mL, R&D systems, Minneapolis, MN, USA), sphingosine-1-phosphate (0.1 μM, Cayman Chemical, Michigan, USA), C16-ceramide-1-phosphate (1 μM, Avanti Polar Lipids, Alabaster, Alabama, USA), C18-ceramide-1-phosphate (0.1–10 μM, from bovine brain, Sigma, sonicated in distilled water), lysophosphatidic acid (LPA, 1 μM, Avanti Polar Lipids), or lysophosphatidylcholine (LPC, 1 μM, Avanti Polar Lipids) in 0.5% BSA DMEM or EBM (control). After 24 hours for HUVECs or 48 hours for MSCs, the inserts were removed from the Transwell plates. Cells remaining in the upper chambers were scraped off with cotton wool, and cells that had transmigrated were stained with HEMA 3 according to the manufacturer’s instructions (Fisher Scientific, Pittsburgh, PA, USA) and counted either on the lower side of the membranes or on the bottom of the lower Transwell chambers.
In chemotaxis assays performed on sorted by FACS VSELs, EPCs and MSCs we employed Costar Transwell 24 well plates with 5-μm filters (Costar Corning). Cells after sorting were resuspended in RPMI with 0.5% BSA and loaded to upper chamber, whereas the lower chamber contained medium alone (RPMI + 0.5% BSA) or medium supplemented with C1P (50 μM). After 3 h incubation in 37 °C, 95% humidity, and 5% CO2, cells that migrated to lower chamber were counted using inverted microscope.
FACS analysis
Cells migrating in response to C18-C1P were stained in medium containing 2% fetal bovine serum (FBS). All monoclonal antibodies (mAbs) were added at saturating concentrations and the cells incubated for 30 min on ice, washed twice, resuspended in staining buffer at a concentration of 5×106 cells/ml, and analyzed using an LSR II (BD Biosciences, Mountain View, CA). The following anti-mouse monoclonal antibodies were purchased from BD Pharmingen (San Diego, CA, USA): Ly-GA/E (Sca-1, FITC, clone D7), lineage marker (CD45R [also known as B220], PE, clone RA3-6B2), Ly-6G/Ly-6C (PE, clone RB6-8C5), T-cell receptor β (PE, clone H57-597), T-cell receptor-γδ (PE, clone GL3), CD11b (PE, clone M1/70), Ter119 (PE, clone TER-119), CD51 (biotin, clone RMV-7, with streptavidin-conjugated to PE-Cy5 as secondary Ab), CD31 (APC, clone 390), and CD45 (APC-Cy7, clone 30-F11). The Sca-1+Lin−CD45− cells (VSELs), Lin−CD45+CD31+ (EPCs), and Lin−CD45−CD31−CD51+Sca-1+ (MSCs) were isolated as described [37].
Western blotting
Bone marrow-derived MSCs and HUVECs were starved overnight in DMEM or EBM containing 0.5% BSA at 37°C and stimulated with S1P (0.1 μM), C16-C1P (10 μM), C18-C1P (10, 20, or 50 μM), LPA (1 μM), or LPC (1 μM ) for 5 min at 37°C and then lysed for 30 min on ice in M-PER lysing buffer (Pierce, Rockford, IL, USA) containing protease and phosphatase inhibitors (Sigma-Aldrich, St Louis, MO, USA). The extracted proteins (30 μg) were separated and analyzed for phosphorylation of MAPKp44/42 and AKT (Ser473). Phospho-specific antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Equal loading in the lanes was evaluated by stripping the blots and reprobing with the monoclonal or polyclonal antibodies against total MAPKp44/42 and total Akt. For blocking signaling, cells were treated with pertussis toxin (0.05 μg/ml, Sigma-Aldrich) for 1 hour. The membranes were developed with an enhanced chemiluminescent reagent (Amersham Life Sciences, Arlington Heights, IL, USA) and exposed to film (HyperFilm, Amersham). SDF-1 and COX-2 were analyzed in cell extract prepared from murine stroma after overnight starvation followed 24 stimulation with C18-C1P.
SDF-1 and COX-2 were analyzed in cell extract prepared from murine stroma after overnight starvation followed by 36h stimulation with C18-C1P. Extracts were prepared as described above and blots were developed with anti-SDF-1 (R&D, Minneapolis, MN) and anti-COX-2 (Abcam Cambridge, MA, USA). Equal loading in the lanes was evaluated with anit-β-actin antibody (Sigma).
Measurement of C1P in irradiated tissues and myocardium after experimental ex vivo heart ischemia
Pathogen-free C57BL/6 mice were utilized for measurements of C1P in irradiated organs and in the myocardial hypoxia experiments. In irradiation experiments mice were irradiated by 500 cGy form γ-irradiator source and 24 hours later major organs were extracted.
In ex vivo heart ischemia experiments whole hearts were excised and cannulated for the Langendorf perfusion technique using perfusion buffer containing 4.7 mM K+ and 1.8 mM Ca++. The control hearts were allowed to beat for 50 minutes in warmed and oxygenated perfusion buffer, then removed and flash frozen in liquid nitrogen. The ischemic hearts were hung and allowed to beat for 5 or 6 minutes with flow in the warmed and oxygenated perfusion buffer, then the flow was stopped for 30 minutes and the hearts bathed in the warmed buffer. Following ischemia, the flow was restarted, and the hearts started beating in about 0.5 to 1 minute after onset of flow and continued beating for 20 minutes to simulate reperfusion injury. Ischemic hearts were then flash frozen as detailed above. Frozen myocardial samples were utilized for total C1P measurements using samples of equal weight. Following homogenization, the lipids were extracted as detailed above. In parallel, pathogen-free C57BL/6 mice were irradiated with 500 cGy (γ-irradiation), the organs removed, and, following homogenization, lipid extraction and mass spectrometry measurements performed by employing liquid chromatography electrospray ionization tandem mass spectrometry (HPLC ESI MS/MS), as described [29].
Zymography
To evaluate secretion of matrix metalloproteinase-9 (MMP-9) by stromal cells, conditioned media harvested from these cells were analyzed by zymography, as described previously by us [29].
Messenger RNA expression quantification
Total RNA was isolated using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) from MSCs after stimulation with C18-C1P for 3 hours to evaluate SDF-1 and Cox-2 expression levels. Messenger RNA was reverse transcribed with 500 U of Moloney murine leukemia virus reverse transcriptase primed with oligo-dT. The resulting cDNA fragments were amplified using the SYBR green system (Applied Biosystems, Carlsbad, CA, USA). The primer sequences for mouse β-2 microglobulin (β2m) were 5′-CAT ACG CCT GCA GAG TTA AGC-3′ (forward) and 5′-GAT CAC ATG TCT CGA TCC CAG TAG-3′ (reverse). For SDF-1, the primer sequences were 5′-CGT GAG GCC AGG GAA GAG T-3′ (forward) and 5′-TGA TGA GCA TGG TGG GTT GA-3′ (reverse). For Cox-2, 5′-TGA GCA ACT ATT CCA AAC CAG C-3′ (forward) and 5′-GCA CGT AGT CTT CGA TCA CTA TC-3′ (reverse). For mouse MMP2, 5′-GGA CCC CGG TTT CCC TAA G-3′ (forward) and 5′-GTC CAC GAC GGC ATC CA-3′ (reverse). For mouse MMP9, 5′-CCT GGA ACT CAC ACG ACA TCT TC-3′ (forward) and 5′-TGG AAA CTC ACA CGC CAG AA-3′ (reverse). For human β–actin, 5′-GGC CCA CAG AAT GCA GAA GGA-3′ (forward), 5′-CGA TCC ACA CGG AGT ACT TG-3′ (reverse). For human MMP2, 5′-TCA AGG GCA TTC AGG AGC TC-3′ (forward), 5′-TGC CAA GGT CAA TGT CAG GAG-3′ (reverse). For human MMP9, 5′-TGG TTA CAC TTCG GGT GGC A-3′ (forward), 5′-GGC CCC AGA GAT TTC GAC TC-3′ (reverse). The relative quantity of the target, normalized to an endogenous control gene (β2m or β-actin) and relative to a calibrator, was expressed as 2−ddCt (fold difference), in which dCt equals the Ct of the target genes minus the Ct of the endogenous control gene, and ddCt equals the dCt of the samples for the target gene minus the dCt of the calibrator for the target gene.
Human endothelial tube formation
HUVECs were assessed on a synthetic basement membrane according to the manufacturer’s protocol (Matrigel, BD Biosciences). Briefly, the matrix was thawed at 4°C and polymerized at 37°C for 30 min before use. HUVECs (less than passage 4) were resuspended in reduced-serum medium with 0.5% BSA (negative control), FGF2 (50 ng/ml, Pepro Tech, RockyHill, NJ, USA), SDF-1 (50 ng/ml), S1P (0.1 μM), C16-C1P (1 μM), C18-C1P (1 μM ), LPA (1 μM), or LPC (1 μM). The cells were seeded (2 × 104 cells/well, in each of 24 wells) on the Matrigel and placed in a 37°C, 5% CO2 incubator. The tubes in each well were counted and phototraphed. All conditions were tested in duplicate wells in three separate experiments.
Enzyme-linked immunosorbent assay for SDF-1
Level of SDF-1 in cell extracts as well as in conditioned medium harvested from MSCs after 36h stimulation with C1P (1 μM) were analyzed by sensitive ELISA assay as described previously by us [29].
CFU-F assay
Whole bone-marrow cells (1 × 106 cells/well, in each of 6 wells) were stimulated with C1P (1 or 10 μM). On the next day, non-adherent cells were removed, and each culture was incubated in DMEM plus 20% FBS for 14 days as described [38]. The adherent cells were washed twice with PBS, fixed, and stained with the HEMA 3 staining kit.
Cell proliferation assay
Cells were seeded in culture plates at an initial density of 3 × 104/well in the presence or absence of C18-C1P (0.1 or 1 μM). The cell number was determined at 1, 2, 3, and 4 days after culture initiation. At the indicated time points, cells were harvested from the wells by trypsinization and the number of cells was determined using a Bürker hemocytometer or LSR II flow cytometer (BD Biosciences).
In vivo angiogenesis assay
SCID mice were injected subcutaneously in the abdominal region with 0.4 ml Matrigel/mouse (BD Bioscience) containing C1P (1 μM or 10 μM), FGF-2 (50 ng/ml, positive control) or Matrigel without any additives (negative control). After 2 weeks implants were isolated, homogenized at 4°C, centrifuged and hemoglobin concentration in supernatant was determined using Drabkin method according to manufacture protocol (Sigma-Aldrich).
Statistical analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA). Statistical significance was defined as P<0.05. Data were analyzed using Student’s t-test for unpaired samples and the t-test for paired samples.
Results
C1P chemoattracts BM-residing MSCs, EPCs, and VSELs
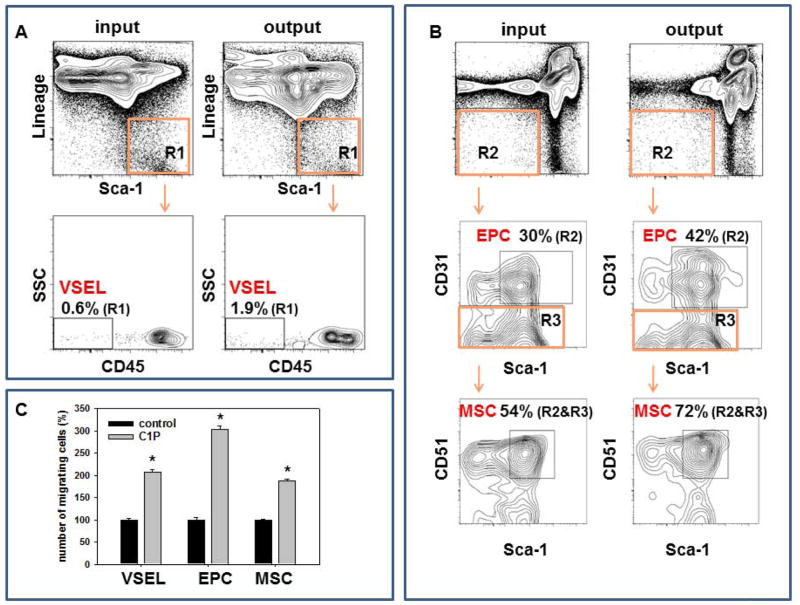
C1P has been demonstrated to be a chemoattractant for macrophages [32] and bone marrow (BM)-residing hematopoietic stem/progenitor cells (HSPCs) [29]. Thus, we became interested in whether C1P chemoattracts other types of non-hematopoietic stem cells residing in BM, including multipotent stroma cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like stem cells (VSELs). By employing the Transwell migration assay combined with FACS analysis of cells collected from the lower chambers of Transwell plates after migration we observed that C1P (chemoattracts BM-residing VSELs (Sca-1+Lin−CD45−, Figure 1A), MSCs (Sca-1+CD51+CD31−Lin−CD45−, Figure 1B middle panel) and EPCs (CD31+Lin−CD45−, Figure 1B lower panel), which suggests that C1P may promote egress of several types of stem cells into peripheral blood (PB). Overall we noticed that the number of murine BM-derived VSELs, EPCs and MSCs in lower chambers of Transwell system containing C1P was higher × 3.1+/−0.3, 1.4+/−0.1% and 1.3+/−0.1 respectively as compared to control chambers with no C1P.
Figure 1. Mesenchymal stem cells (MSCs), endothelial cells (ECs), and very small embryonic-like stem cells (VSELs) migrate in response to ceramide-1-phosphate (C1P).
Whole BM cells (input) were loaded into the upper Transwell chamber, C18-C1P (50 μM) was loaded into the lower chamber, and chemotaxis allowed to proceed for 3 hours. Migrated cells (output) found in the lower chamber were collected and stained for detection of various populations. Panel A: Lin−Sca-1+CD45− (VSELs). Panel B: Lin−CD45−CD31+ (EPCs) and Lin−CD45−CD31− Sca-1+ CD51+ (MSCs). The data are expressed as % of cells in indicted gates detected in population of BMMNC (R1, R2 or R3) before chemotaxis in upper chambers (input) and after chemotaxis in lower chambers (output). Representative analysis out of three independent experiments is shown. Panel C: Transwell chemotaxis assay to C18-C1P (50 μM) or medium alone (control) performed on purified by FACS VSELs, EPCs and MSCs * p<0.05. There are shown combined data from three independent experiments.
Since chemotaxis of rare population of cells could be somehow affected in Transwell assay by admixture of high number of other nucleated cells present in suspension of BM cells, we purified by FACS population of BM-derived VSELs, EPCs and MSCs and performed chemotaxis of these cells to C1P gradient (Figure 1C). We noticed that all these cell populations were chemoattracted by C1P.
C1P is upregulated in damaged tissues
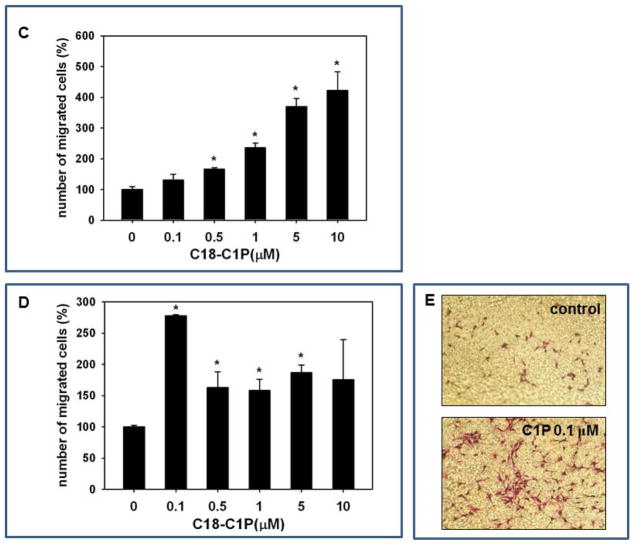
We next became interested in whether C1P, which promotes egress of BM-derived stem cells into PB, could also be potentially involved in chemoattracting circulating stem cells to damaged tissues. Since several clinical trials using stem cells have been performed in patients after acute myocardial infarction (AMI) [39,40], we employed a murine model of acute heart hypoxia to test this hypothesis. As shown in Figure 2A, by employing HPLC ESI MS/MS analysis, we observed a significant increase in C1P level in murine hypoxic hearts.
Figure 2. C1P increases in damaged tissues.
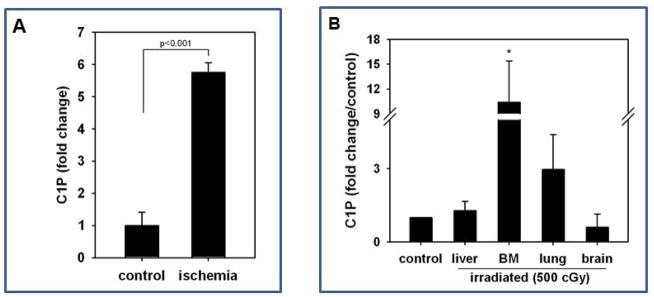
Panel A – An increase in C1P concentration in ischemic murine myocardium. Panel B – Mice were irradiated and organs isolated 24 hr after irradiation (500cGy), and C18-C1P measured by mass spectrophotometry. The data shown represent the combined results from three independent experiments carried out in duplicate per group. * p<0.001 Panel C and D - Dose-dependent effect of ceramide-1-phosphate on MSC and HUVEC migration. MSCs (Panel C) and HUVECs (Panel D) were loaded into the upper Transwell chamber (8-μm), C18-C1P (0.1, 0.5, 1, 5, and 10 μM) was loaded into the lower chamber, and chemotaxis allowed to proceed for 24 hours. Cells remaining in the upper chambers were scraped off with cotton wool, and cells that had transmigrated were stained by HEMA 3 according to the manufacturer’s instructions (Fisher Scientific, Pittsburgh, PA, USA) and counted either on the lower side of the membranes or on the bottom of the Transwell chambers. The data shown represent the combined results from two independent experiments carried out in quadruplicate per group. * p<0.05 Panel E – representative image showing Transwell insets from migration assay of HUVECs to medium alone (control) and 0.1 μM C1P.
Next, based on our observation that C1P and S1P are upregulated in irradiated murine bone marrow [29], we next analyzed C1P levels in other murine organs by HPLC ESI MS/MS and observed that sublethal irradiation by 500 cGy upregulates the level of this bioactive lipid in irradiated BM and lungs (Figure 2B and C).
This data suggests that C1P may play a novel (and until now, underappreciated) role in chemoattracting stem cells circulating in PB to damaged tissues.
C1P chemoattracts ex vivo-expanded MSCs and HUVECs
Since MSCs and other types of stem cells are employed in regenerative medicine [1–14], we performed dose-response chemotactic studies on ex vivo-expanded MSCs and purified HUVECs with C1P and observed that it strongly chemoattracts these cells (Figure 2C and D). As shown in Figure 2C, while the optimal chemotactic dose for MSCs was ~10 μM, the responsiveness of HUVECs was highest at a 100-fold lower concentration of this bioactive lipid (0.1 μM, Figure 2D and E). Since the receptor(s) for C1P have not yet been identified [29,32], we could not determine whether these differences were related to differences in receptor density on the target cells. Furthermore, by employing varying concentrations of C1P in the upper and lower Transwell chambers, we excluded the possibility that the C1P effect is not mediated by directional gradient migration, but is instead due to chemokinesis [29] of MSCs and HUVECs (data not shown).
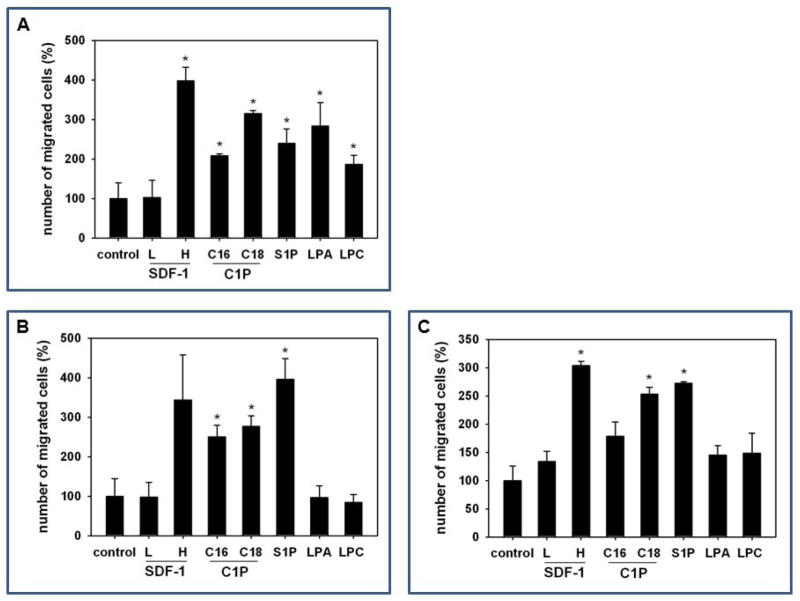
In parallel studies, we next compared the chemotactic activity of C1P to that of other bioactive lipids (sphingosine-1-phosphate, S1P; lysophosphatidylic acid, LPA; and lysophosphatidylocholine, LPC) against murine MSCs (Figure 3A), HUVECs (Figure 3B), as well as human MSCs (Figure 3C). All these bioactive lipids were employed at doses corresponding to their PB plasma levels [29]. In parallel, we also evaluated the responsiveness of these cells to both low (10 ng/ml) and high doses of the α-chemokine SDF-1 (300 ng/ml), which has been demonstrated to be a potent chemoattractant for both MSCs and HUVECs [41,42].
Figure 3. MSCs and HUVECs migrate in response to bioactive lipids, and C1P increases adhesion of MSCs.
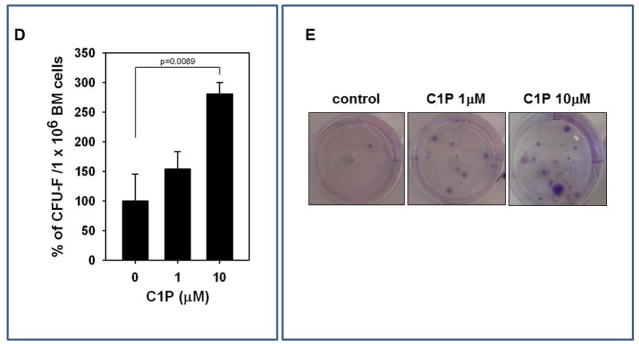
Murine bone marrow-derived multipotent stroma cells (msMSCs, Panel A), HUVECs (Panel B), and human BM-derived multipotent stroma cells (huMSCs, Panel C) were loaded into an upper Transwell chamber (8-μm), with the lower chamber containing different bioactive lipids, such as S1P (0.1 μM), C16-C1P (1 μM), C18-C1P (1 μM), LPA (1 μM), LPC (1 μM) or SDF-1 (low dose (L), 10 ng/ml and high dose (H), 300 ng/ml). Twenty-four hours later, cells remaining in the upper chambers were scraped off with cotton wool, and cells that had transmigrated were stained by HEMA 3 according to the manufacturer’s instructions (Fisher Scientific, Pittsburgh, PA, USA) and counted either on the lower side of the membranes or on the bottom of the Transwell chambers. The data shown represent the combined results from two independent experiments carried out in quadruplicate per group. *p<0.05 Panel D: For the CFU-F assay, whole BM cells were stimulated with C1P and seeded into culture plates. Mouse whole bone marrow cells were stimulated with C18-C1P (1 or 10 μM) for 1 hour and seeded (1 × 106 cells/well, into each of 6 wells). On the next day, non-adherent cells were removed and cultured for 14 days in complete medium. Adherent cells were washed twice with PBS, stained with the HEMA 3 staining kit, and the colonies counted. The data shown represent the combined results from three independent experiments carried out in quadruplicate per group. *p<0.0089 Panel E: Representative stained colonies of adherent cells are shown.
Figure 3 shows that if employed at physiological doses, C1P, (including both C16 and C18 analogues) is a very potent chemottractant for murine MSCs, HUVECs, and human MSCs. Of note, the chemotactic activity of a low dose of SDF-1 (10 ng/ml) was not effective in our assays to chemoattract these cells. This is an important observation, because the SDF-1 physiological concentration in PB plasma has been reported to be <3 ng/ml [29]. Therefore, our data indicate that C1P, at the physiological level found in PB [43], is a more potent chemoattractant for these cells.
C1P increases adhesion of MSCs
We next became interested in whether C1P increases adhesion of MSCs. We employed the colony forming unit of fibroblasts (CFU-F) assay to evaluate the effect of C1P on adhesion of MSCs present among the populations of murine BMMNCs. Accordingly, BMMNCs were stimulated or not (control) by C1P and plated at a low concentration in petri dishes. The number of CFU-F colonies established was evaluated after 14 days as described [38]. Figure 3D shows that C1P increases adhesion of clonogenic CFU-F, which are true clonogenic progenitor cells for MSCs, in a dose-dependent manner. This effect was more pronounced for CFU-F with high proliferation potential (Figure 3E). At the same time, we observed that stimulation of MSCs and HUVECs by C1P does not affect expression of adhesion molecules, including VLA-4 or VCAM-1 (data not shown).
C1P induces signaling in ex vivo-expanded MSCs and HUVECs
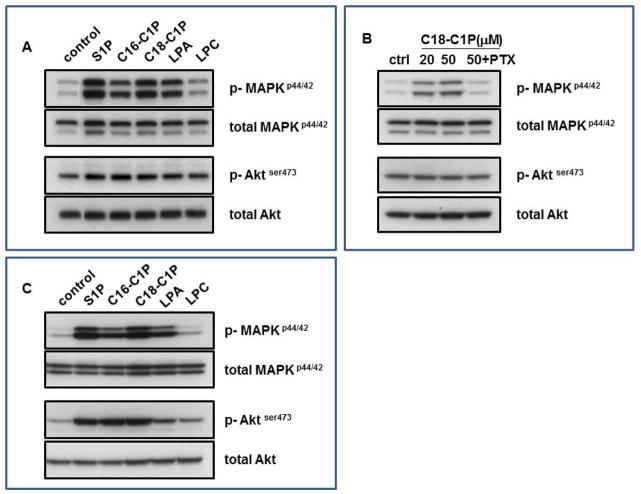
While the receptor(s) for C1P have been not identified, we evaluated whether this bioactive lipid activates signaling in MSCs (Figure 4A and B) and HUVECs (Figure 4C). We observed that C1P strongly activates phosphorylation of MAPKp42/44 in MSCs (Figure 5A) and that this signaling is pertussis toxin (PTx)-sensitive (Figure 4B), which suggests that it is mediated by receptor/s coupled to a Gαi signaling protein. At the same time, we observed stronger phosphorylation of MAPKp42/44 and AKT stimulated by C1P in HUVECs (Figure 4C) than in MSCs.
Figure 4. Bioactive lipids stimulate signaling pathways in MSCs and HUVECs.
Bioactive lipids activate two signaling pathways in mouse MSCs (Panel A and B) and HUVECs (Panel C) that are crucial for cell migration and adhesion: MAPKp44/42 and Aktser473. Before stimulation, cells were starved overnight in DMEM (or EBM for HUVECs) containing 0.5% BSA in an incubator and subsequently stimulated with S1P (0.1 μM), C16-C1P (1 μM), C18-C1P (1 μM), LPA (1 μM), or LPC (1 μM) for 5 min. Panel B: MSCs were pretreated with pertussis toxin (0.05 μg/ml) for 1 hour before C1P stimulation. Experiments were repeated independently four times with similar results. A representative western blot is shown.
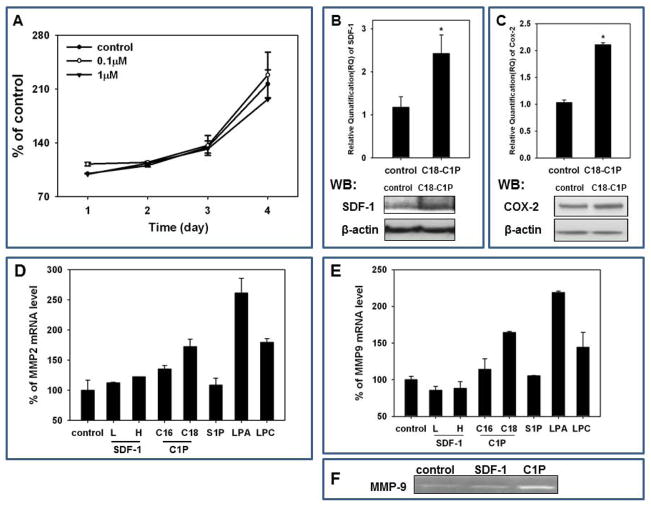
Figure 5. C1P does not affect proliferation of MSCs, but increases SDF-1, Cox-2, MMP2, and MMP9 expression.
Panel A: MSCs were cultured with C18-C1P (0.1 or 1 μM) for 1, 2, 3, and 4 days and cells counted at the indicated time points. The data shown represent the combined results from two independent experiments carried out in quadruplicate per group. MSCs were stimulated with C18-C1P for 3 hours, and the mRNA levels for SDF-1 (Panel B, upper panel), Cox-2 (Panel C, upper panel), MMP2 (Panel D), and MMP9 (Panel E) were measured. Experiments were repeated independently three times with similar results. * p<0.05. For protein level analysis, Western blots were performed on extracts prepared 36 h after stimulation with C18-C1P (Panel B and C, lower panels). Analysis was performed three times with similar results and representative results are shown. Panel F Expression of MMP-9 measured by zymography in supernatants from MSCs starved for 24 h before stimulation by SDF-1 (300 ng/ml; positive control) or C18-C1P (1 μm). Representative result out of three is shown.
C1P does not affect proliferation of MSCs, but increases SDF-1, Cox-2, and MMPs expression
We next became interested in the biological effects of C1P that could be related to organ tissue repair. Our direct proliferation studies, however, revealed that C1P does not significantly affect MSC proliferation (Figure 5A). At the same time, however, we observed that stimulation of MSCs by C1P increases expression of SDF-1 in these cells at mRNA and protein level (Figure 5B). Furthermore, increase in level of SDF1 in cell extracts as well as in conditioned media from MSCs stimulated by C1P was subsequently confirmed by ELISA assay (Supplementary Figure 1 A and B). Similarly, C1P also enhanced in MSCs expression of cyclooxygenase-2 (Cox-2) both at mRNA and protein level (Figure 5C). Of note, both SDF-1 and the Cox-2 product prostaglandin E2 (PGE2) [44] play an important role in chemottracting circulating stem cells to the damaged organs, as previously reported [45–48].
We also tested whether C1P enhances expression of MMP-2 and MMP-9, two proteolytic enzymes that are involved in cell trafficking [29]. As shown in Figure 5D and E, C1P (the C18 analogue) stimulates within 3 h expression of mRNA for MMP2 and MMP9 in MSCs that were starved before stimulation for 24 h. Furthermore, we employed zymography assay to demonstrate increase in MMP-9 protein in cell supernatants stimulated by C1P (Figure 5E) Expression of MMP-2 Figure 5D and MMP-9 Figure 5E was upregulated after stimulation by LPA and LPC, but, to our surprise, neither by S1P nor by SDF-1. Both these factors required longer stimulation time (6 h) to upregulate MMPs in starved MSCs (data not shown).
C1P does not affect proliferation of HUVECs, but triggers capillary-like structure formation in 3D-Matrigel assay and induces angiogenesis in vivo in Matrigel implants
In parallel experiments, we observed that, as in MSCs (Figure 5A), C1P does not affect proliferation of HUVECs (Figure 6A). However, Figure 6B and C demonstrate that C1P, in our hands, is a potent inducer of tube formation by HUVECs in vitro in Matrigel assay. In particular, we observed that the C18 isoform, known to be released by cells in response to tissue damage and stress [29], is the most potent inducer of tube formation. Of note, this tube-formation effect was even stronger than an optimal dose of potent pro-angiopoietic factor that is FGF-2.
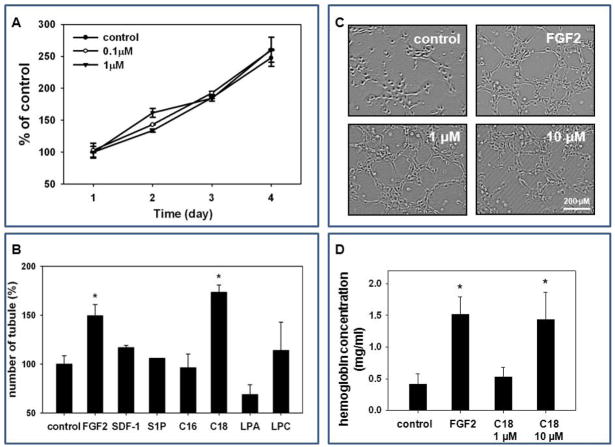
Figure 6. C1P does not affect proliferation of HUVECs, but triggers capillary-like structure formation in 3D-Matrigel cultures, angiogenesis in vivo in Matrigel implants and enhances adhesion of HUVECs.
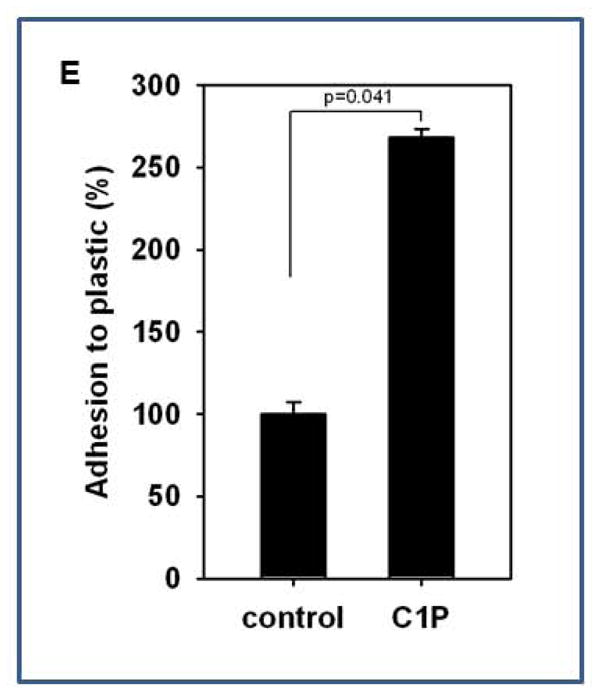
Panel A: HUVECs were cultured with C18-C1P (0.1 or 1 μM) for 1, 2, 3, and 4 days and cells were counted at the indicated time points. The data shown represent the combined results from two independent experiments carried out in quadruplicate per group. Panel B: HUVECs were seeded into Matrigel cultures in the presence of bioactive lipids S1P (0.1 μM), C16-C1P (1 μM), C18-C1P (1 μM), LPA (1 μM), LPC ( 1 μM), SDF-1 (50 ng/ml), or FGF2 (50 ng/ml, positive control) in serum-free media, incubated for 4 hours at 37°C, and the number of tubules counted. *p<0.05 Panel C: Tube formation in the presence of C18-C1P. Panel D: Hemoglobin level in Matrigel implants supplemented with C1P (1 or 10 μM), FGF2 (50 ng/ml, positive control) or in presence of medium alone. Matrigel implants were removed and analyzed 2 weeks later. *p<0.05 Magnification × 100. Panel E: C1P increases HUVEC adhesion. HUVECs were stimulated with C18-C1P (1 μM) for 1 hour and seeded (5 × 104 cells/well, in each of 6 wells); after 3 minutes, non-adherent cells were removed by washing twice with PBS.
To support this better we performed in vivo Matrigel implants vascularization assay and noticed again a potent vascularization effect of C1P that was comparable to FGF-2 (Figure 6D). Finally, we also observed that C1P increases similarly as for MSCs adhesion of HUVECs to plastic dishes (Figure 6E). Thus, C1P seems to be an underappreciated regulator of angiogenesis.
Discussion
Mounting evidence indicates that the bioactive sphingolipids, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P), play an important role in several biological processes, including cell trafficking [27,29]. As reported, S1P acts as an intracellular second messenger molecule and is secreted from cells as a potent anti-apoptotic factor that stimulates angiogenesis and chemoattracts several types of cells [49,50]. Murine embryos that lack sphingosine kinase-1 and -2 and thus lack S1P die at early stages of embryonic development [51–53].
Unlike S1P, the biological effects of C1P are still largely unstudied. C1P is a product of the phosporylation of ceramide, which is a known mediator that promotes cell cycle arrest [54] and induces apoptosis [55]. By contrast, C1P can act as an intracellular second messenger to promote cell survival [30,56], or, if released by the cells, can act as an extracellular chemottractant for monocytes [32] and hematopoietic stem progenitor cells (HSPCs), as recently demonstrated [29]. Dermal microendothelial cells isolated from ceramide kinase-deficient mice showed profound in vitro defects in angiogenesis [35], which suggested that C1P is endowed with pro-angiopoietic properties.
In this report, we demonstrate for the first time that C1P is a strong chemoattractant for several types of BM-residing non-hematopoietic stem cells, including MSCs, EPCs, and VSELs. It may thus play a role in mobilization/egress of these cells from BM into PB. Furthermore, since C1P, as we show here, is released from damaged “leaky” cells (e.g., heart tissue during acute hypoxia), it may create a chemotactic gradient in damaged organs for stem cells circulating in PB, and such cells could be involved in tissue/organ repair. The same mechanism, however, may also be involved in an unwanted recruitment of circulating EPCs and MSCs to expanding tumor tissue [29].
Furthermore, we have also shown that C1P upregulates expression of SDF-1 and Cox-2 in stromal cells. Thus, the chemotactic effect of C1P against circulating stem cells may be additionally potentiated by SDF-1, a known chemoattractant for stem cells [57] and PGE2 (a Cox2 product), which enhances responsiveness of stem cells to an SDF-1 gradient [44]. Thus, we present for the first time evidence that C1P, like other chemoattractants released in damaged tissue, such as SDF-1, VEGF, HGF, or S1P, could be directly or indirectly (by upregulating SDF-1 and Cox-2) involved in recruitment of BM-derived stem cells circulating in PB.
It has been demonstrated in several animal models [58–61] as well as in clinical settings [62–66] that stem cells become mobilized into PB in response to tissue/organ injury. However, the potential involvement of these circulating stem cells in organ tissue regeneration is still not fully understood [67]. While circulating EPCs could supply new vessels in damaged tissues, and VSELs could contribute to replacement of damaged cells, other stem cell types, such as MSCs or HSPCs, could play an important role as a source of several soluble paracrine factors (e.g., growth factors, cytokines, or chemokines) that may play a role in inhibiting apoptosis of damaged cells [68,69]. On the other hand, evidence has accumulated that an important role in stem cell therapies for damaged organs may be played by membrane-derived microvesicles or exosomes, which promote survival of damaged cells and stimulate angiogenesis by delivering to the damaged tissues several proteins, mRNAs, and miRNAs that stimulate angiogenesis [70,71] and inhibit apoptosis [72,73]. Microvesicles are highly enriched for S1P [74], and further studies are needed to measure the content of C1P in these bioactive, spherical membrane fragments.
Our experiments performed in serum-free media indicate that C1P, in contrast to macrophages [32] and myoblastic cells [33], does not affect proliferation of MSCs and HUVECs. Thus, the responsiveness of cells to C1P seems to be cell-specific. In further support of this observation, neither human nor murine HSPCs respond to C1P by increasing proliferation, as we have already reported [29]. Therefore, in our adhesion assay experiments, the observed increase in the appearance of large CFU-F colonies after 14 days in in vitro culture (Figure 3E) is best explained by preferential adhesion of CFU-F endowed with high proliferative potential.
We also observed that murine and human MSCs and HUVECs, like monocytes [32], myoblasts [33], and HSPCs, respond robustly to C1P in chemotactic assays, which supports an important role for C1P in cell trafficking.
In this report, we also demonstrate that C1P enhances adhesiveness of MSCs and HUVECs. Thus, in a similar way as reported for HSPCs [29], C1P stimulates adhesion of stromal cells. Moreover, like HSPCs, the enhanced adhesion of MSCs and HUVECs did not depend on an increase in expression of VLA4 or VCAM-1 on MSCs and HUVECs [75].
As mentioned above, receptors for C1P have not yet been identified [29,32]. However, our signaling studies revealed that stimulation of MSCs and HUVECs by C1P induces phosphorylation of MAPKp42/44 and AKT. The fact that this signaling is PTx-sensitive indicates involvement of Gαi protein-coupled receptors in this phenomenon. The sensitivity of C1P to PTx signaling has been previously demonstrated in monocytes [32] and in murine and human HSPCs [29].
An important step in regeneration of damaged organ tissues is promotion of neovascularization or angiogenesis [76,77]. In this paper, for the first time we demonstrate that C1P is a very strong pro-angiopoietic factor by being a chemoattractant for endothelial cells. Furthermore, in in vitro functional assays we confirmed that C1P directly promotes tube formation and our data in vivo in murine model demonstrate that C1P promotes also vasculariztion of Matrigel implants. This observation supports a previous report where it was shown that skin microendothelial cells isolated from CerK-deficient mice show defects in angiogenesis in vitro [35]. Thus, C1P, like S1P [69], plays an important novel role as a pro-angiopoietic factor.
Our data demonstrate for the first time that C1P is a potent bioactive lipid released from damaged cells and could play an important and novel role in regulating repair of other damaged organs and tissues. Thus, it may be involved both in recruitment of circulating stem cells to regenerating tissues as well as in unwanted recruitment of EPCs and MSCs to an expanding tumor. This possibility, however, requires further study.
In conclusion, as demonstrated in Figure 7, cells in damaged organs release C1P, which together with other factors not shown in this scheme (e.g., SDF-1, S1P, VEGF, and HGF) creates a chemotactic gradient for MSCs, EPCs, and VSELs circulating in PB. Based on this mechanism, we propose that modification of C1P signaling in damaged tissues will help in developing more optimal therapeutic strategies in regenerative medicine. On the other hand, inhibition of a C1P gradient may prevent unwanted recruitment of stem cells circulating in PB into expanding tumor tissue. Therefore, it is important to clone the C1P receptor(s) and then to develop small-molecule C1P receptor agonists and antagonists that could be employed in the clinical setting.
Figure 7. The role of C1P in chemoattracting circulating stem cells to damaged tissues.
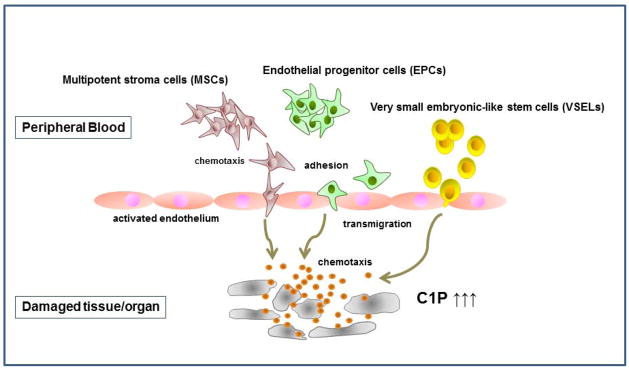
Cells in damaged organs release C1P, which creates a chemotactic gradient for stem cells circulating in PB, such as multipotent stroma cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like (VSEL) stem cells. For reasons of simplicity, other potential chemoattractants, such as S1P and SDF-1, are not shown in this scheme. The organ tissue damage may, for example, be the result of ischemia (e.g., stroke or heart infarct) or the result of tumor expansion. Therefore, C1P could, on the one hand, chemoattract circulating MSCs and EPCs for regeneration and, on the other hand, play a role in unwanted recruitment of these cells to the expanding tumor.
Supplementary Material
MSCs were stimulated with C1P for 36 hours, and the level of SDF-1 was measured in cell extracts (A) and in conditioned medium (B) by sensitive ELISA assay. The data shown represent the combined results from two independent experiments carried out in duplicate. *p<0.05
Acknowledgments
This work was supported by NIH grant 2R01 DK074720, the Stella and Henry Endowment, the European Union structural funds (Innovative Economy Operational Program POIG.01.01.02-00-109/09-00) and grant Maestro 2011/02/A/NZ4/00035.
Footnotes
Authors contribution
ChiHwa Kim - Collection and/or assembly of data, Final approval of manuscript
Ahmed Abdel-Latif - Collection and/or assembly of data, Final approval of manuscript
Kasia Mierzejewska - Collection and/or assembly of data
Gabriela Schneider - Collection and/or assembly of data, Final approval of manuscript
Manjula Sunkara - Collection of data
Sylwia Borkowska - Collection of data
Janina Ratajczak - Collection and/or assembly of data
Andrew J. Morris - Collection and/or assembly of data, Final approval of manuscript
Magda Kucia - Collection and/or assembly of data
Mariusz Z. Ratajczak - Conception and design, Financial support, Manuscript writing
References
- 1.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz LA, DuTreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the anti-inflammatory and anti-fibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–1007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunter U, Rong S, Djuric Z, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 4.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringden O, Uzunel M, Rasmusson L, et al. Mesenchymal stem cells for the treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 6.Minguell JJ, Erices A. Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med (Maywood) 2006;231:39–9. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 7.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 8.Dome B, Timar J, Dobos J, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 9.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, Nolan D, McDonnell K, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Liu R, Ratajczak J, et al. The role of pluripotent embryonic-like stem cells residing in adult tissues in regeneration and longevity. Differentiation. 2011;81:153–161. doi: 10.1016/j.diff.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Zuba-Surma EK, Wojakowski W, Ratajczak MZ, et al. Very small embryonic-like stem cells: biology and therapeutic potential for heart repair. Antioxid Redox Signal. 2011;15:1821–1834. doi: 10.1089/ars.2010.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojakowski W, Kucia M, Liu R, et al. Circulating very small embryonic-like stem cells in cardiovascular disease. J Cardiovasc Transl Res. 2011;4:138–144. doi: 10.1007/s12265-010-9254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drukała J, Paczkowska E, Kucia M, et al. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2012;8:184–194. doi: 10.1007/s12015-011-9272-4. [DOI] [PubMed] [Google Scholar]
- 15.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera MB, Fonsato V, Gatti S, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and il-1 alpha. J Cellular Physiol. 1996;166:585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 19.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 20.Hanel P, Andréani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa R, Nakamura K, Okubo S, et al. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–63. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 22.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–97. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Kong Y, Wang H, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak J, Kucia M, Mierzejewska K, et al. A novel view of paroxysmal nocturnal hemoglobinuria pathogenesis: more motile PNH hematopoietic stem/progenitor cells displace normal HSPCs from their niches in bone marrow due to defective adhesion, enhanced migration and mobilization in response to erythrocyte-released sphingosine-1 phosphate gradient. Leukemia. 2012 doi: 10.1038/leu.2012.46.. [DOI] [PubMed] [Google Scholar]
- 26.Calise S, Blescia S, Cencetti F, et al. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439–450. doi: 10.1016/j.bbamcr.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Ratajczak MZ, Kim CH, Abdel-Latif A, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arana L, Gangoiti P, Ouro A, et al. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CH, Wu W, Wysoczynski M, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Muñoz A, Kong JY, Parhar K, et al. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005;579:3744–3750. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji K, Mitsutake S, Yokose U, et al. Role of ceramide kinase in peroxisome proliferator-activated receptor beta-induced cell survival of mouse keratinocytes. FEBS J. 2008;275:3815–3826. doi: 10.1111/j.1742-4658.2008.06527.x. [DOI] [PubMed] [Google Scholar]
- 32.Granado MH, Gangoiti P, Ouro A, et al. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Gangoiti P, Bernacchioni C, Donati C, et al. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie. 2012;94:597–607. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niwa S, Graf C, Bornancin F. Ceramide kinase deficiency impairs microendothelial cell angiogenesis in vitro. Microvasc Res. 2009;77:389–393. doi: 10.1016/j.mvr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Majka M, Kijowski J, Lesko E, et al. Evidence that platelet-derived microvesicles may transfer platelet-specific immunoreactive antigens to the surface of endothelial cells and CD34+ hematopoietic stem/progenitor cells--implication for the pathogenesis of immune thrombocytopenias. Folia Histochem Cytobiol. 2007;45:27–32. [PubMed] [Google Scholar]
- 37.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 38.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 39.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 40.Samper E, Diez-Juan A, Montero JA, et al. Cardiac Cell Therapy: Boosting Mesenchymal Stem Cells Effects. Stem Cell Rev. 2012 doi: 10.1007/s12015-012-9353-z. [DOI] [PubMed] [Google Scholar]
- 41.Zheng H, Fu G, Dai T, et al. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- 42.Ryu CH, Park SA, Kim SM, et al. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun. 2010;398:105–110. doi: 10.1016/j.bbrc.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Hammad SM, Pierce JS, Soodavar F, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 46.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia. 2011;25:1674–1686. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim RH, Takabe K, Milstien S, et al. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Jin ZQ, Zhang J, Huang Y, et al. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 52.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 53.Vessey DA, Li L, Jin ZQ, et al. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 55.Sanvicens N, Cotter TG. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem. 2006;98:1432–1444. doi: 10.1111/j.1471-4159.2006.03977.x. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Munoz A. Ceramide-1-phosphate: a novel regulator of cell activation. FEBS Lett. 2004;562:5–10. doi: 10.1016/s0014-5793(04)00211-x. [DOI] [PubMed] [Google Scholar]
- 57.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 58.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 59.Mark AL, Sun Z, Warren DS, et al. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg. 2010;252:591–596. doi: 10.1097/SLA.0b013e3181f4e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yannaki E, Athanasiou E, Xagorari A, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108–119. doi: 10.1016/j.exphem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Yannaki E, Psatha N, Athanasiou E, et al. Mobilization of hematopoietic stem cells in a thalassemic mouse model: implications for human gene therapy of thalassemia. Hum Gene Ther. 2010;21:299–310. doi: 10.1089/hum.2009.077. [DOI] [PubMed] [Google Scholar]
- 62.Gordon MY, Levicar N, Pai M, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 63.Pasquet S, Sovalat H, Hénon P, et al. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy. 2009;11:1002–1015. doi: 10.3109/14653240903164963. [DOI] [PubMed] [Google Scholar]
- 64.Zohlnhöfer D, Ott I, Mehilli J, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 65.Malafronte C, Achilli F. Stem cells mobilization in acute myocardial infarction (stem-AMI trial): preliminary data of a perspective, randomized, single blind trial. Minerva Cardioangiol. 2007;55:721–731. [PubMed] [Google Scholar]
- 66.George JC. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Transl Res. 2010;155:10–19. doi: 10.1016/j.trsl.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Ratajczak MZ. Spotlight series on stem cell mobilization: many hands on the ball, but who is the quarterback? Leukemia. 2010;24:1665–1666. doi: 10.1038/leu.2010.181. [DOI] [PubMed] [Google Scholar]
- 68.Ioannidou E. Therapeutic modulation of growth factors and cytokines in regenerative medicine. Curr Pharm Des. 2006;12:2397–2408. doi: 10.2174/138161206777699007. [DOI] [PubMed] [Google Scholar]
- 69.Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2011 doi: 10.1038/leu.2011.389.. [DOI] [PubMed] [Google Scholar]
- 70.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 71.Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 72.Camussi G, Deregibus MC, Bruno S, et al. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 73.Camussi G, Cantaluppi V, Deregibus MC, et al. Role of microvesicles in acute kidney injury. Contrib Nephrol. 2011;174:191–199. doi: 10.1159/000329397. [DOI] [PubMed] [Google Scholar]
- 74.Baj-Krzyworzeka M, Majka M, Pratico D, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 75.Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 76.Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 77.Casteilla L, Planat-Bénard V, Cousin B, et al. Vascular and endothelial regeneration. Curr Stem Cell Res Ther. 2010;5:141–144. doi: 10.2174/157488810791268546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MSCs were stimulated with C1P for 36 hours, and the level of SDF-1 was measured in cell extracts (A) and in conditioned medium (B) by sensitive ELISA assay. The data shown represent the combined results from two independent experiments carried out in duplicate. *p<0.05