Abstract
Mycobacterium avium complex (MAC) disease emerged early in the epidemic of AIDS as one of the common opportunistic infections afflicting human immunodeficiency virus-infected patients. However, only over the past few years has a consensus developed about its significance to the morbidity and mortality of AIDS. M. avium was well known to mycobacteriologists decades before AIDS, and the MAC was known to cause disease, albeit uncommon, in humans and animals. The early interest in the MAC provided a basis for an explosion of studies over the past 10 years largely in response to the role of the MAC in AIDS opportunistic infection. Molecular techniques have been applied to the epidemiology of MAC disease as well as to a better understanding of the genetics of antimicrobial resistance. The interaction of the MAC with the immune system is complex, and putative MAC virulence factors appear to have a direct effect on the components of cellular immunity, including the regulation of cytokine expression and function. There now is compelling evidence that disseminated MAC disease in humans contributes to both a decrease in the quality of life and survival. Disseminated disease most commonly develops late in the course of AIDS as the CD4 cells are depleted below a critical threshold, but new therapies for prophylaxis and treatment offer considerable promise. These new therapeutic modalities are likely to be useful in the treatment of other forms of MAC disease in patients without AIDS. The laboratory diagnosis of MAC disease has focused on the detection of mycobacteria in the blood and tissues, and although the existing methods are largely adequate, there is need for improvement. Indeed, the successful treatment of MAC disease clearly will require an early and rapid detection of the MAC in clinical specimens long before the establishment of the characteristic overwhelming infection of bone marrow, liver, spleen, and other tissue. Also, a standard method of susceptibility testing is of increasing interest and importance as new effective antimicrobial agents are identified and evaluated. Antimicrobial resistance has already emerged as an important problem, and methods for circumventing resistance that use combination therapies are now being studied.
Full text
PDF














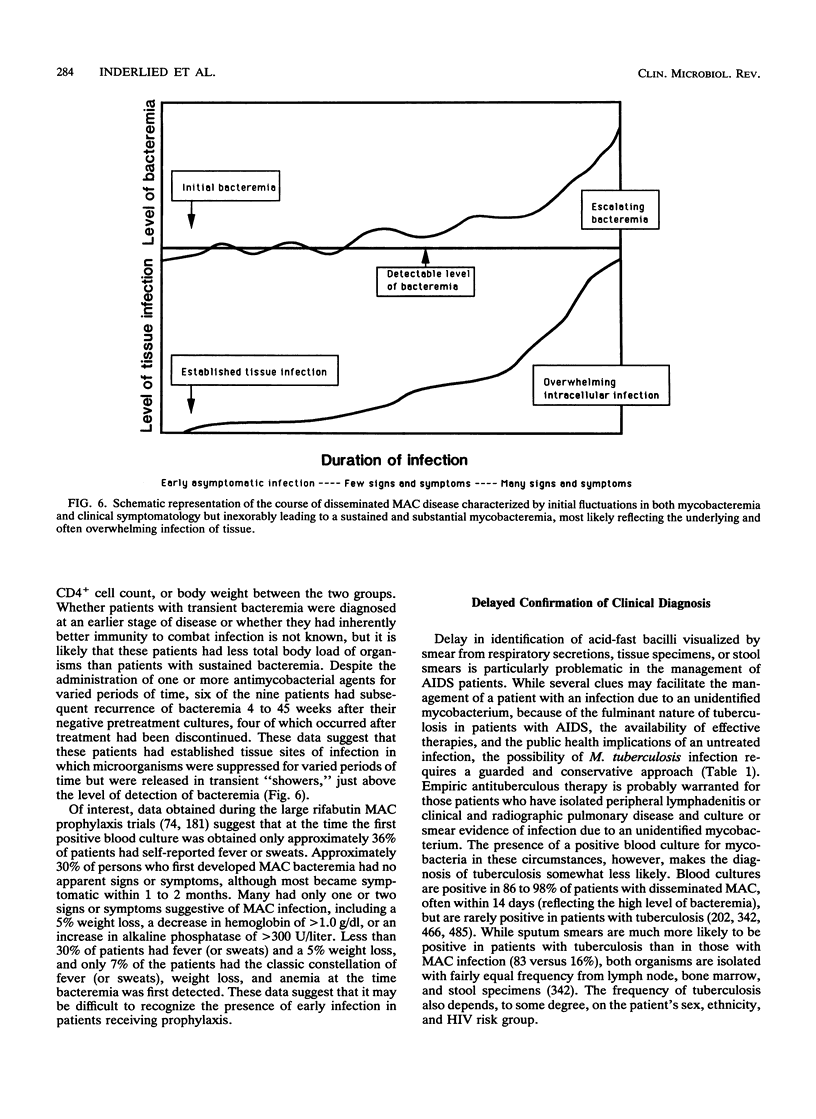

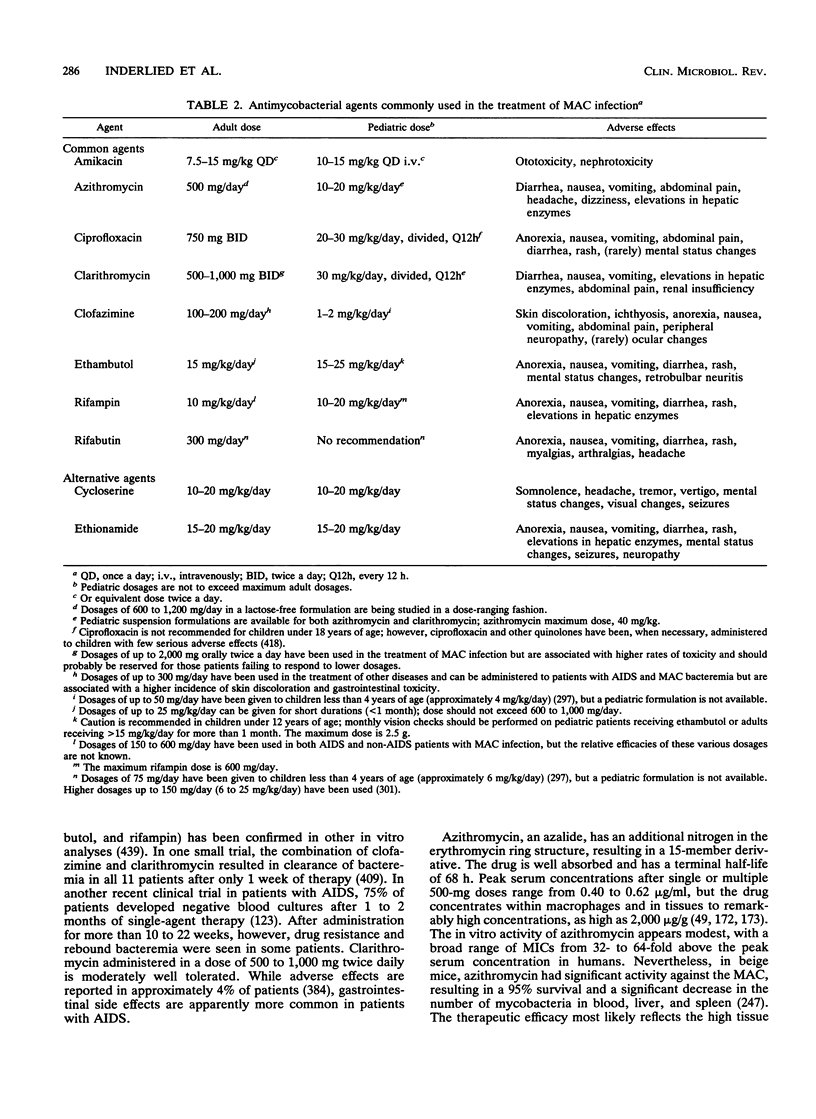

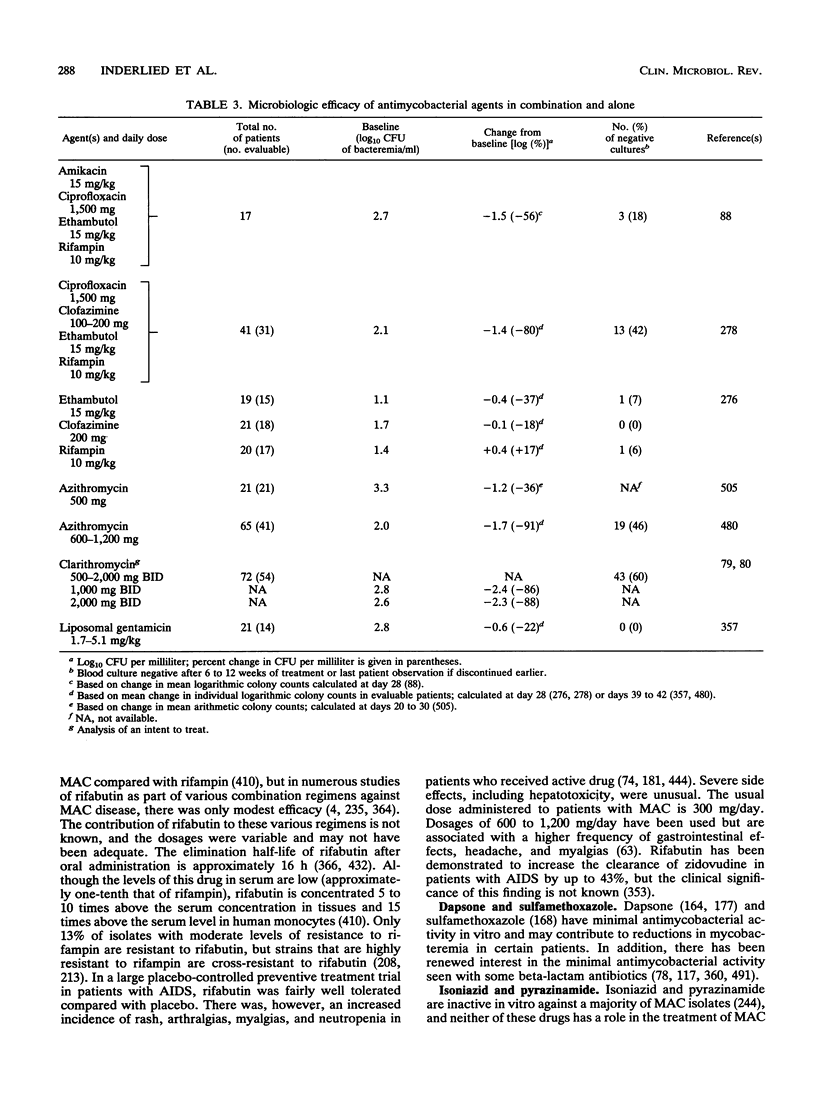






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams L. B., Franzblau S. G., Vavrin Z., Hibbs J. B., Jr, Krahenbuhl J. L. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991 Sep 1;147(5):1642–1646. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Agins B. D., Berman D. S., Spicehandler D., el-Sadr W., Simberkoff M. S., Rahal J. J. Effect of combined therapy with ansamycin, clofazimine, ethambutol, and isoniazid for Mycobacterium avium infection in patients with AIDS. J Infect Dis. 1989 Apr;159(4):784–787. doi: 10.1093/infdis/159.4.784. [DOI] [PubMed] [Google Scholar]
- Agy M. B., Wallis C. K., Plorde J. J., Carlson L. C., Coyle M. B. Evaluation of four mycobacterial blood culture media: BACTEC 13A, Isolator/BACTEC 12B, Isolator/Middlebrook agar, and a biphasic medium. Diagn Microbiol Infect Dis. 1989 Jul-Aug;12(4):303–308. doi: 10.1016/0732-8893(89)90094-1. [DOI] [PubMed] [Google Scholar]
- Ahn C. H., Ahn S. S., Anderson R. A., Murphy D. T., Mammo A. A four-drug regimen for initial treatment of cavitary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1986 Sep;134(3):438–441. doi: 10.1164/arrd.1986.134.3.438. [DOI] [PubMed] [Google Scholar]
- Ahn C. H., Lowell J. R., Onstad G. D., Shuford E. H., Hurst G. A. A demographic study of disease due to Mycobacterium kansasii or M intracellulare-avium in Texas. Chest. 1979 Feb;75(2):120–125. doi: 10.1378/chest.75.2.120. [DOI] [PubMed] [Google Scholar]
- Alessi D. P., Dudley J. P. Atypical mycobacteria-induced cervical adenitis. Treatment by needle aspiration. Arch Otolaryngol Head Neck Surg. 1988 Jun;114(6):664–666. doi: 10.1001/archotol.1988.01860180078035. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Slutsky A., Barber T. W., Maslow J. N., Niemczyk S., Falkinham J. O., 3rd, O'Connor G. T., von Reyn C. F. Genetic diversity among strains of Mycobacterium avium causing monoclonal and polyclonal bacteremia in patients with AIDS. J Infect Dis. 1993 Jun;167(6):1384–1390. doi: 10.1093/infdis/167.6.1384. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Gold J. W., Dryjanski J., Whimbey E., Polsky B., Hawkins C., Brown A. E., Bernard E., Kiehn T. E. Treatment of infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):738–743. doi: 10.7326/0003-4819-103-5-738. [DOI] [PubMed] [Google Scholar]
- Bach M. C., Bagwell S. P., Masur H. Utility of gallium imaging in the diagnosis of Mycobacterium avium-intracellulare infection in patients with the acquired immunodeficiency syndrome. Clin Nucl Med. 1986 Mar;11(3):175–177. doi: 10.1097/00003072-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Bach M. C. Treating disseminated Mycobacterium avium-intracellulare infection. Ann Intern Med. 1989 Jan 15;110(2):169–170. doi: 10.7326/0003-4819-110-2-169. [DOI] [PubMed] [Google Scholar]
- Baess I. Deoxyribonucleic acid relationships between different serovars of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):201–203. doi: 10.1111/j.1699-0463.1983.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Barbaro D. J., Orcutt V. L., Coldiron B. M. Mycobacterium avium-Mycobacterium intracellulare infection limited to the skin and lymph nodes in patients with AIDS. Rev Infect Dis. 1989 Jul-Aug;11(4):625–628. doi: 10.1093/clinids/11.4.625. [DOI] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., Pascopella L., Inamine J. M., Brennan P. J., Jacobs W. R., Jr Isolation and expression of a gene cluster responsible for biosynthesis of the glycopeptidolipid antigens of Mycobacterium avium. J Bacteriol. 1991 Nov;173(21):6991–6997. doi: 10.1128/jb.173.21.6991-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender B. L., Yunis E. J. Disseminated nongranulomatous Mycobacterium avium osteomyelitis. Hum Pathol. 1980 Sep;11(5):476–478. doi: 10.1016/s0046-8177(80)80057-8. [DOI] [PubMed] [Google Scholar]
- Benjamin D. R. Granulomatous lymphadenitis in children. Arch Pathol Lab Med. 1987 Aug;111(8):750–753. [PubMed] [Google Scholar]
- Bennett C., Vardiman J., Golomb H. Disseminated atypical mycobacterial infection in patients with hairy cell leukemia. Am J Med. 1986 May;80(5):891–896. doi: 10.1016/0002-9343(86)90634-0. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C. A method for testing for synergy with any number of agents. J Infect Dis. 1978 Feb;137(2):122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C. Correlations between methods for measurement of synergy. J Infect Dis. 1980 Sep;142(3):476–480. doi: 10.1093/infdis/142.3.476. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993 Feb;91(2):277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E. Infection of "nonprofessional phagocytes" with Mycobacterium avium complex. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):225–235. doi: 10.1016/s0090-1229(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Kolonoski P., Young L. S. Natural killer cell activity and macrophage-dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J Leukoc Biol. 1990 Feb;47(2):135–141. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Petrofsky M., Kolonoski P., Young L. S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992 Jan;165(1):75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993 Mar 1;150(5):1838–1845. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Enkel H., Young L. S. Naturally occurring antibodies against Mycobacterium avium complex. Ann Clin Lab Sci. 1989 Nov-Dec;19(6):435–443. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Martinelli J., Young L. S. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991 Oct;10(5):413–419. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Petrofsky M., Young L. S. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992 Oct;60(10):4245–4252. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988 Aug;32(8):1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991 May;59(5):1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Ethanol and survival of Mycobacterium avium complex within macrophages. Prog Clin Biol Res. 1990;325:383–391. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Ethanol augments intracellular survival of Mycobacterium avium complex and impairs macrophage responses to cytokines. J Infect Dis. 1991 Jun;163(6):1286–1292. doi: 10.1093/infdis/163.6.1286. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Killing of Mycobacterium avium: insights provided by the use of recombinant cytokines. Res Microbiol. 1990 Feb;141(2):241–243. doi: 10.1016/0923-2508(90)90037-q. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991 Jan 1;146(1):265–270. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Oxidative and non-oxidative intracellular killing of Mycobacterium avium complex. Microb Pathog. 1989 Oct;7(4):289–298. doi: 10.1016/0882-4010(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Phagocytosis and intracellular killing of Mycobacterium avium complex by human and murine macrophages. Braz J Med Biol Res. 1987;20(2):191–201. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bertram M. A., Inderlied C. B., Yadegar S., Kolanoski P., Yamada J. K., Young L. S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986 Jul;154(1):194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- Black C. M., Bermudez L. E., Young L. S., Remington J. S. Co-infection of macrophages modulates interferon gamma and tumor necrosis factor-induced activation against intracellular pathogens. J Exp Med. 1990 Sep 1;172(3):977–980. doi: 10.1084/jem.172.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., McMillen S., Hoffman S. L., Djeu J. Y. Mycobacterial induction of activated killer cells: possible role of tyrosine kinase activity in interleukin-2 receptor alpha expression. Infect Immun. 1992 Jul;60(7):2843–2849. doi: 10.1128/iai.60.7.2843-2849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Friedman H., Djeu J. Y. Lysis of mycobacteria-infected monocytes by IL-2-activated killer cells: role of LFA-1. Cell Immunol. 1989 Apr 1;119(2):402–411. doi: 10.1016/0008-8749(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., Freitag C. S., Djeu J. Y. Mycobacterium avium-intracellulare induces interleukin-6 from human monocytes and large granular lymphocytes. Blood. 1991 May 15;77(10):2218–2224. [PubMed] [Google Scholar]
- Blaser M. J., Cohn D. L. Opportunistic infections in patients with AIDS: clues to the epidemiology of AIDS and the relative virulence of pathogens. Rev Infect Dis. 1986 Jan-Feb;8(1):21–30. doi: 10.1093/clinids/8.1.21. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. R., Zucker J. R., Hawkins C. C. Mycobacterium avium complex-induced septic arthritis and osteomyelitis in a patient with the acquired immunodeficiency syndrome. Arthritis Rheum. 1990 May;33(5):757–758. doi: 10.1002/art.1780330522. [DOI] [PubMed] [Google Scholar]
- Bonavida B., Katz J., Gottlieb M. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. I. Defective trigger on NK cells for NKCF production by target cells, and partial restoration by IL 2. J Immunol. 1986 Aug 15;137(4):1157–1163. [PubMed] [Google Scholar]
- Bouza E., Burgaleta C., Golde D. W. Infections in hairy-cell leukemia. Blood. 1978 May;51(5):851–859. [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Heifets M., Ullom B. P. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982 Mar;15(3):447–455. doi: 10.1128/jcm.15.3.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S420–S430. doi: 10.1093/clinids/11.supplement_2.s420. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structures of the typing antigens of atypical mycobacteria: a brief review of present knowledge. Rev Infect Dis. 1981 Sep-Oct;3(5):905–913. doi: 10.1093/clinids/3.5.905. [DOI] [PubMed] [Google Scholar]
- Brooks R. W., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis. 1984 Oct;130(4):630–633. doi: 10.1164/arrd.1984.130.4.630. [DOI] [PubMed] [Google Scholar]
- Brown S. T., Edwards F. F., Bernard E. M., Niki Y., Armstrong D. Progressive disseminated infection with Mycobacterium avium complex after intravenous and oral challenge in cyclosporine-treated rats. J Infect Dis. 1991 Nov;164(5):922–927. doi: 10.1093/infdis/164.5.922. [DOI] [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger E. C., Teske A., Kirschner P., Bost S., Chang H. R., Beer V., Hirschel B. Disseminated "Mycobacterium genavense" infection in patients with AIDS. Lancet. 1992 Jul 11;340(8811):76–80. doi: 10.1016/0140-6736(92)90397-l. [DOI] [PubMed] [Google Scholar]
- CROW H. E., KING C. T., SMITH C. E., CORPE R. F., STERGUS I. A limited clinical, pathologic, and epidemiologic study of patients with pulmonary lesions associated with atypical acid-fast bacilli in the sputum. Am Rev Tuberc. 1957 Feb;75(2):199–222. doi: 10.1164/artpd.1957.75.2.199. [DOI] [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. Antigenic relationship between Mycobacterium paratuberculosis and Mycobacterium avium. Am J Vet Res. 1988 Aug;49(8):1307–1310. [PubMed] [Google Scholar]
- Cappell M. S., Hassan T., Rosenthal S., Mascarenhas M. Gastrointestinal obstruction due to Mycobacterium avium intracellulare associated with the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992 Dec;87(12):1823–1827. [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J., Favero M. S., Aguero S. M. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978 Dec;36(6):839–846. doi: 10.1128/aem.36.6.839-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M. J., Rodriguez F. C., Luna M. D., Benavente M. C. In vitro susceptibility of Mycobacterium tuberculosis, Mycobacterium africanum, Mycobacterium bovis, Mycobacterium avium, Mycobacterium fortuitum, and Mycobacterium chelonae to ticarcillin in combination with clavulanic acid. Antimicrob Agents Chemother. 1987 Jan;31(1):132–133. doi: 10.1128/aac.31.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson R. E., Hopewell P. C. Mycobacteria and AIDS mortality. Am Rev Respir Dis. 1989 Jan;139(1):1–3. doi: 10.1164/ajrccm/139.1.1. [DOI] [PubMed] [Google Scholar]
- Chaisson R. E., Moore R. D., Richman D. D., Keruly J., Creagh T. Incidence and natural history of Mycobacterium avium-complex infections in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. Am Rev Respir Dis. 1992 Aug;146(2):285–289. doi: 10.1164/ajrccm/146.2.285. [DOI] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S. The ecology of the atypicalmycobacteria. Arch Environ Health. 1971 Jan;22(1):41–46. doi: 10.1080/00039896.1971.10665813. [DOI] [PubMed] [Google Scholar]
- Chester A. C., Winn W. C., Jr Unusual and newly recognized patterns of nontuberculous mycobacterial infection with emphasis on the immunocompromised host. Pathol Annu. 1986;21(Pt 1):251–270. [PubMed] [Google Scholar]
- Chiu J., Nussbaum J., Bozzette S., Tilles J. G., Young L. S., Leedom J., Heseltine P. N., McCutchan J. A. Treatment of disseminated Mycobacterium avium complex infection in AIDS with amikacin, ethambutol, rifampin, and ciprofloxacin. California Collaborative Treatment Group. Ann Intern Med. 1990 Sep 1;113(5):358–361. doi: 10.7326/0003-4819-113-5-358. [DOI] [PubMed] [Google Scholar]
- Christensen E. E., Dietz G. W., Ahn C. H., Chapman J. S., Murry R. C., Anderson J., Hurst G. A. Pulmonary manifestations of Mycobacterium intracellularis. AJR Am J Roentgenol. 1979 Jul;133(1):59–66. doi: 10.2214/ajr.133.1.59. [DOI] [PubMed] [Google Scholar]
- Clark R., Cardona L., Valainis G., Hanna B. Genitourinary infections caused by mycobacteria other than Mycobacterium tuberculosis. Tubercle. 1989 Dec;70(4):297–300. doi: 10.1016/0041-3879(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Codias E. K., Reinhardt D. J. Distribution of serotypes of the Mycobacterium avium-intracellulare-scrofulaceum complex in Georgia. Am Rev Respir Dis. 1979 Jun;119(6):965–970. doi: 10.1164/arrd.1979.119.6.965. [DOI] [PubMed] [Google Scholar]
- Coffin J. W., Condon C., Compston C. A., Potter K. N., Lamontagne L. R., Shafiq J., Kunimoto D. Y. Use of restriction fragment length polymorphisms resolved by pulsed-field gel electrophoresis for subspecies identification of mycobacteria in the Mycobacterium avium complex and for isolation of DNA probes. J Clin Microbiol. 1992 Jul;30(7):1829–1836. doi: 10.1128/jcm.30.7.1829-1836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Saragas S. J. Endophthalmitis due to Mycobacterium avium in a patient with AIDS. Ann Ophthalmol. 1990 Feb;22(2):47–51. [PubMed] [Google Scholar]
- Coker R. J., Hellyer T. J., Brown I. N., Weber J. N. Clinical aspects of mycobacterial infections in HIV infection. Res Microbiol. 1992 May;143(4):377–381. doi: 10.1016/0923-2508(92)90049-t. [DOI] [PubMed] [Google Scholar]
- Cole G. W., Gebhard J. Mycobacterium avium infection of the skin resembling lepromatous leprosy. Br J Dermatol. 1979 Jul;101(1):71–74. doi: 10.1111/j.1365-2133.1979.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Collert S., Petrini B., Wickman K. Osteomyelitis caused by Mycobacterium avium. Acta Orthop Scand. 1983 Jun;54(3):449–451. doi: 10.3109/17453678308996600. [DOI] [PubMed] [Google Scholar]
- Colley E. W., Cox R. A. Axillary lymphadenitis due to Mycobacterium avium-intracellulare. Postgrad Med J. 1983 Sep;59(695):588–589. doi: 10.1136/pgmj.59.695.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989 Oct;2(4):360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle. 1985 Dec;66(4):267–276. doi: 10.1016/0041-3879(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Stokes R. W. Mycobacterium avium-complex infections in normal and immunodeficient mice. Tubercle. 1987 Jun;68(2):127–136. doi: 10.1016/0041-3879(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Immune responses to atypical mycobacterial lung infections. Rev Infect Dis. 1981 Sep-Oct;3(5):981–989. doi: 10.1093/clinids/3.5.981. [DOI] [PubMed] [Google Scholar]
- Contreras M. A., Cheung O. T., Sanders D. E., Goldstein R. S. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis. 1988 Jan;137(1):149–152. doi: 10.1164/ajrccm/137.1.149. [DOI] [PubMed] [Google Scholar]
- Corpe R. F. Surgical management of pulmonary disease due to Mycobacterium avium-intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):1064–1067. doi: 10.1093/clinids/3.5.1064. [DOI] [PubMed] [Google Scholar]
- Coyle M. B., Carlson L. C., Wallis C. K., Leonard R. B., Raisys V. A., Kilburn J. O., Samadpour M., Böttger E. C. Laboratory aspects of "Mycobacterium genavense," a proposed species isolated from AIDS patients. J Clin Microbiol. 1992 Dec;30(12):3206–3212. doi: 10.1128/jcm.30.12.3206-3212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Analysis of plasmids in Mycobacterium avium-intracellulare isolates from persons with acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986 Oct;134(4):659–661. doi: 10.1164/arrd.1986.134.4.659. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Bates J. H. Phage typing of the Mycobacterium avium-intracellulare-scrofulaceum complex. A study of strains of diverse geographic and host origin. Am Rev Respir Dis. 1985 Aug;132(2):386–389. doi: 10.1164/arrd.1985.132.2.386. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Cave M. D., Bates J. H. Characterization of plasmids from strains of Mycobacterium avium-intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):949–952. doi: 10.1093/clinids/3.5.949. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Cave M. D., Bates J. H. Evidence for plasmid-mediated restriction-modification in Mycobacterium avium intracellulare. J Gen Microbiol. 1981 Dec;127(2):333–338. doi: 10.1099/00221287-127-2-333. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Fitzhugh J. K., Bates J. H. Phage typing of the Mycobacterium avium-intracellulare-scrofulaceum complex. Am Rev Respir Dis. 1981 Nov;124(5):559–562. doi: 10.1164/arrd.1981.124.5.559. [DOI] [PubMed] [Google Scholar]
- Craythorn J. M., Swartz M., Creel D. J. Clofazimine-induced bull's-eye retinopathy. Retina. 1986 Winter-Spring;6(1):50–52. [PubMed] [Google Scholar]
- Crowle A. J., Cohn D. L., Poche P. Defects in sera from acquired immunodeficiency syndrome (AIDS) patients and from non-AIDS patients with Mycobacterium avium infection which decrease macrophage resistance to M. avium. Infect Immun. 1989 May;57(5):1445–1451. doi: 10.1128/iai.57.5.1445-1451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Dahl R., Ross E., May M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991 May;59(5):1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H., Palmer G. S., Sorg T. B. Comparative in vitro activities of ampicillin, BMY 28142, and imipenem against Mycobacterium avium complex. Diagn Microbiol Infect Dis. 1987 Feb;6(2):151–155. doi: 10.1016/0732-8893(87)90100-3. [DOI] [PubMed] [Google Scholar]
- Cynamon M. H., Swenson C. E., Palmer G. S., Ginsberg R. S. Liposome-encapsulated-amikacin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1989 Aug;33(8):1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato R. F., Isenberg H. D., Hochstein L., Mastellone A. J., Alperstein P. Evaluation of the Roche Septi-Chek AFB system for recovery of mycobacteria. J Clin Microbiol. 1991 Dec;29(12):2906–2908. doi: 10.1128/jcm.29.12.2906-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsker B., Bottone E. J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985 Jan;151(1):179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B., Saint Marc T., Meyohas M. C., Eliaszewitch M., Haniez F., Rogues A. M., De Wit S., Cotte L., Chauvin J. P., Grosset J. Clarithromycin and other antimicrobial agents in the treatment of disseminated Mycobacterium avium infections in patients with acquired immunodeficiency syndrome. Arch Intern Med. 1993 Feb 8;153(3):368–372. [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- David H. L. Basis for lack of drug susceptibility of atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):878–884. doi: 10.1093/clinids/3.5.878. [DOI] [PubMed] [Google Scholar]
- David H. L. Response of Mycobacteria to ultraviolet light radiation. Am Rev Respir Dis. 1973 Nov;108(5):1175–1185. doi: 10.1164/arrd.1973.108.5.1175. [DOI] [PubMed] [Google Scholar]
- Dawson D. J. Infection with Mycobacterium avium complex in Australian patients with AIDS. Med J Aust. 1990 Oct 15;153(8):466–468. doi: 10.5694/j.1326-5377.1990.tb126152.x. [DOI] [PubMed] [Google Scholar]
- De Paepe M. E., Guerrieri C., Waxman M. Opportunistic infections of the testis in the acquired immunodeficiency syndrome. Mt Sinai J Med. 1990 Jan;57(1):25–29. [PubMed] [Google Scholar]
- Debrunner M., Salfinger M., Brändli O., von Graevenitz A. Epidemiology and clinical significance of nontuberculous mycobacteria in patients negative for human immunodeficiency virus in Switzerland. Clin Infect Dis. 1992 Aug;15(2):330–345. doi: 10.1093/clinids/15.2.330. [DOI] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990 Sep;71(1):139–141. [PMC free article] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990 Oct;142(4):940–953. doi: 10.1164/ajrccm/142.4.940. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Herron R. M., Jr, Hindler J. A., Berlin O. G., Bruckner D. A. DNA probe reactivity of Mycobacterium avium complex isolates from patients without AIDS. Diagn Microbiol Infect Dis. 1988 Nov;11(3):125–128. doi: 10.1016/0732-8893(88)90013-2. [DOI] [PubMed] [Google Scholar]
- Dugdale D. C., Stevens D. L., Knight L. L. Mycotic aneurysm and disseminated Mycobacterium avium-intracellulare infection in a patient with hairy cell leukemia. West J Med. 1989 Feb;150(2):207–208. [PMC free article] [PubMed] [Google Scholar]
- Düzgüneş N., Perumal V. K., Kesavalu L., Goldstein J. A., Debs R. J., Gangadharam P. R. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988 Sep;32(9):1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L. B., Acquaviva F. A., Livesay V. T., Cross F. W., Palmer C. E. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969 Apr;99(4 Suppl):1–132. [PubMed] [Google Scholar]
- Eggelmeijer F., Kroon F. P., Zeeman R. J., Dijkmans B. A., van 't Wout J. W. Tenosynovitis due to Mycobacterium avium-intracellulare: case report and a review of the literature. Clin Exp Rheumatol. 1992 Mar-Apr;10(2):169–171. [PubMed] [Google Scholar]
- Ellner P. D., Kiehn T. E., Cammarata R., Hosmer M. Rapid detection and identification of pathogenic mycobacteria by combining radiometric and nucleic acid probe methods. J Clin Microbiol. 1988 Jul;26(7):1349–1352. doi: 10.1128/jcm.26.7.1349-1352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Mangia A. Diagnosis of Mycobacterium bacteremia in patients with acquired immunodeficiency syndrome by direct examination of blood films. J Clin Microbiol. 1989 Apr;27(4):768–769. doi: 10.1128/jcm.27.4.768-769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbaek H. C., Vergmann B., Bentzon M. W. Lung disease caused by Mycobacterium avium/Mycobacterium intracellulare. An analysis of Danish patients during the period 1962-1976. Eur J Respir Dis. 1981;62(2):72–83. [PubMed] [Google Scholar]
- Etzkorn E. T., Aldarondo S., McAllister C. K., Matthews J., Ognibene A. J. Medical therapy of Mycobacterium avium-intracellulare pulmonary disease. Am Rev Respir Dis. 1986 Sep;134(3):442–445. doi: 10.1164/arrd.1986.134.3.442. [DOI] [PubMed] [Google Scholar]
- Evans K. D., Nakasone A. S., Sutherland P. A., de la Maza L. M., Peterson E. M. Identification of Mycobacterium tuberculosis and Mycobacterium avium-M. intracellulare directly from primary BACTEC cultures by using acridinium-ester-labeled DNA probes. J Clin Microbiol. 1992 Sep;30(9):2427–2431. doi: 10.1128/jcm.30.9.2427-2431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G. A., Hadley S. J., Sharkey F. E., Liss M., Muschenheim C. Mycobacterium avium infections in man. Am J Med. 1973 Jun;54(6):801–810. doi: 10.1016/0002-9343(73)90069-7. [DOI] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Parker B. C., Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980 Jun;121(6):931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- Farhi D. C., Mason U. G., 3rd, Horsburgh C. R., Jr Pathologic findings in disseminated Mycobacterium avium-intracellulare infection. A report of 11 cases. Am J Clin Pathol. 1986 Jan;85(1):67–72. doi: 10.1093/ajcp/85.1.67. [DOI] [PubMed] [Google Scholar]
- Farhi D. C., Mason U. G., 3rd, Horsburgh C. R., Jr The bone marrow in disseminated Mycobacterium avium-intracellulare infection. Am J Clin Pathol. 1985 Apr;83(4):463–468. doi: 10.1093/ajcp/83.4.463. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Franzblau S. G., Takeda T., Nakamura M. Mycobacterial plasmids: screening and possible relationship to antibiotic resistance in Mycobacterium avium/Mycobacterium intracellulare. Microbiol Immunol. 1986;30(9):903–907. doi: 10.1111/j.1348-0421.1986.tb03017.x. [DOI] [PubMed] [Google Scholar]
- Frehel C., Ryter A., Rastogi N., David H. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann Inst Pasteur Microbiol. 1986 Nov-Dec;137B(3):239–257. doi: 10.1016/s0769-2609(86)80115-6. [DOI] [PubMed] [Google Scholar]
- Friedman B. F., Edwards D., Kirkpatrick C. H. Mycobacterium avium-intracellulare: cutaneous presentations of disseminated disease. Am J Med. 1988 Aug;85(2):257–263. doi: 10.1016/s0002-9343(88)80357-7. [DOI] [PubMed] [Google Scholar]
- Fry K. L., Meissner P. S., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. VI. Identification and use of epidemiologic markers for studies of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Am Rev Respir Dis. 1986 Jul;134(1):39–43. doi: 10.1164/arrd.1986.134.1.39. [DOI] [PubMed] [Google Scholar]
- Furney S. K., Roberts A. D., Orme I. M. Effect of rifabutin on disseminated Mycobacterium avium infections in thymectomized, CD4 T-cell-deficient mice. Antimicrob Agents Chemother. 1990 Sep;34(9):1629–1632. doi: 10.1128/aac.34.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Candler E. R. Activity of some antileprosy compounds against Mycobacterium intracellulare in vitro. Am Rev Respir Dis. 1977 Apr;115(4):705–708. doi: 10.1164/arrd.1977.115.4.705. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd, Murthy P. S., Pratt P. F. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983 May;127(5):648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Kesavalu L., Rao P. N., Perumal V. K., Iseman M. D. Activity of amikacin against Mycobacterium avium complex under simulated in vivo conditions. Antimicrob Agents Chemother. 1988 Jun;32(6):886–889. doi: 10.1128/aac.32.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Crawford J. T., Bates J. H. Association of plasmids and virulence of Mycobacterium avium complex. Am Rev Respir Dis. 1988 Jan;137(1):212–214. doi: 10.1164/ajrccm/137.1.212. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Podapati N. R., Kesavalu L., Iseman M. D. In vivo activity of amikacin alone or in combination with clofazimine or rifabutin or both against acute experimental Mycobacterium avium complex infections in beige mice. Antimicrob Agents Chemother. 1988 Sep;32(9):1400–1403. doi: 10.1128/aac.32.9.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrelts J. C. Clofazimine: a review of its use in leprosy and Mycobacterium avium complex infection. DICP. 1991 May;25(5):525–531. doi: 10.1177/106002809102500513. [DOI] [PubMed] [Google Scholar]
- Georghiou P. R., Mollee T. F., Tilse M. H. Pasteurella multocida infection after a Tasmanian devil bite. Clin Infect Dis. 1992 Jun;14(6):1266–1267. doi: 10.1093/clinids/14.6.1266. [DOI] [PubMed] [Google Scholar]
- Ghossein R. A., Ross D. G., Salomon R. N., Rabson A. R. Rapid detection and species identification of mycobacteria in paraffin-embedded tissues by polymerase chain reaction. Diagn Mol Pathol. 1992 Sep;1(3):185–191. [PubMed] [Google Scholar]
- Gill M. J., Fanning E. A., Chomyc S. Childhood lymphadenitis in a harsh northern climate due to atypical mycobacteria. Scand J Infect Dis. 1987;19(1):77–83. doi: 10.3109/00365548709032381. [DOI] [PubMed] [Google Scholar]
- Gill V. J., Park C. H., Stock F., Gosey L. L., Witebsky F. G., Masur H. Use of lysis-centrifugation (isolator) and radiometric (BACTEC) blood culture systems for the detection of mycobacteremia. J Clin Microbiol. 1985 Oct;22(4):543–546. doi: 10.1128/jcm.22.4.543-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow B. J., Layfield L. J., Anders K. H. Mycobacterium avium-intracellulare and adrenal insufficiency in AIDS. Hum Pathol. 1988 Feb;19(2):245–246. doi: 10.1016/s0046-8177(88)80358-7. [DOI] [PubMed] [Google Scholar]
- Godwin J. H., Stopeck A., Chang V. T., Godwin T. A. Mycobacteremia in acquired immune deficiency syndrome. Rapid diagnosis based on inclusions in the peripheral blood smear. Am J Clin Pathol. 1991 Mar;95(3):369–375. doi: 10.1093/ajcp/95.3.369. [DOI] [PubMed] [Google Scholar]
- Gomez J., Pohajdak B., O'Neill S., Wilkins J., Greenberg A. H. Activation of rat and human alveolar macrophage intracellular microbicidal activity by a preformed LGL cytokine. J Immunol. 1985 Aug;135(2):1194–1200. [PubMed] [Google Scholar]
- Gonzalez A. H., Berlin O. G., Bruckner D. A. In-vitro activity of dapsone and two potentiators against Mycobacterium avium complex. J Antimicrob Chemother. 1989 Jul;24(1):19–22. doi: 10.1093/jac/24.1.19. [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Hanna B. A. Evaluation of Gen-Probe DNA hybridization systems for the identification of Mycobacterium tuberculosis and Mycobacterium avium-intracellulare. Diagn Microbiol Infect Dis. 1987 Oct;8(2):69–77. doi: 10.1016/0732-8893(87)90152-0. [DOI] [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Good R. C., Snider D. E., Jr Isolation of nontuberculous mycobacteria in the United States, 1980. J Infect Dis. 1982 Dec;146(6):829–833. doi: 10.1093/infdis/146.6.829. [DOI] [PubMed] [Google Scholar]
- Goslee S., Wolinsky E. Water as a source of potentially pathogenic mycobacteria. Am Rev Respir Dis. 1976 Mar;113(3):287–292. doi: 10.1164/arrd.1976.113.3.287. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Markesich D. C., Yoshimura H. H. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987 Feb;92(2):436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Graham L., Jr, Warren N. G., Tsang A. Y., Dalton H. P. Mycobacterium avium complex pseudobacteriuria from a hospital water supply. J Clin Microbiol. 1988 May;26(5):1034–1036. doi: 10.1128/jcm.26.5.1034-1036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. R., Rabeneck L. Atypical mycobacterial infection of the gastrointestinal tract in AIDS patients. Am J Gastroenterol. 1989 Dec;84(12):1521–1524. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Gribetz A. R., Damsker B., Bottone E. J., Kirschner P. A., Teirstein A. S. Solitary pulmonary nodules due to nontuberculous mycobacterial infection. Am J Med. 1981 Jan;70(1):39–43. doi: 10.1016/0002-9343(81)90409-5. [DOI] [PubMed] [Google Scholar]
- Grice K. Sarcoidosis and Mycobacterium avium-intracellulare cutaneous abscesses. Clin Exp Dermatol. 1983 May;8(3):323–327. doi: 10.1111/j.1365-2230.1983.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Gruft H., Katz J., Blanchard D. C. Postulated source of Mycobacterium intracellulare (Battey) infection. Am J Epidemiol. 1975 Oct;102(4):311–318. doi: 10.1093/oxfordjournals.aje.a112166. [DOI] [PubMed] [Google Scholar]
- Guthertz L. S., Damsker B., Bottone E. J., Ford E. G., Midura T. F., Janda J. M. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J Infect Dis. 1989 Dec;160(6):1037–1041. doi: 10.1093/infdis/160.6.1037. [DOI] [PubMed] [Google Scholar]
- Hallander H. O., Dornbusch K., Gezelius L., Jacobson K., Karlsson I. Synergism between aminoglycosides and cephalosporins with antipseudomonal activity: interaction index and killing curve method. Antimicrob Agents Chemother. 1982 Nov;22(5):743–752. doi: 10.1128/aac.22.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson S. J., Portaels F., Thompson J., Green E. P., Moss M. T., Hermon-Taylor J., McFadden J. J. DNA probes demonstrate a single highly conserved strain of Mycobacterium avium infecting AIDS patients. Lancet. 1989 Jan 14;1(8629):65–68. doi: 10.1016/s0140-6736(89)91427-x. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlir D., Kemper C. A., Deresinski S. C. Reproducibility of lysis-centrifugation cultures for quantification of Mycobacterium avium complex bacteremia. J Clin Microbiol. 1993 Jul;31(7):1794–1798. doi: 10.1128/jcm.31.7.1794-1798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D. Choice of antimicrobial agents for M. avium disease based on quantitative tests of drug susceptibility. N Engl J Med. 1990 Aug 9;323(6):419–420. doi: 10.1056/NEJM199008093230615. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D. Determination of in vitro susceptibility of mycobacteria to ansamycin. Am Rev Respir Dis. 1985 Sep;132(3):710–711. doi: 10.1164/arrd.1985.132.3.710. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Combinations of rifampin or rifabutine plus ethambutol against Mycobacterium avium complex. Bactericidal synergistic, and bacteriostatic additive or synergistic effects. Am Rev Respir Dis. 1988 Mar;137(3):711–715. doi: 10.1164/ajrccm/137.3.711. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Ethambutol MICs and MBCs for Mycobacterium avium complex and Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1986 Dec;30(6):927–932. doi: 10.1128/aac.30.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J., Comstock R. D. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J., Iseman M. D. Rifabutine: minimal inhibitory and bactericidal concentrations for Mycobacterium tuberculosis. Am Rev Respir Dis. 1988 Mar;137(3):719–721. doi: 10.1164/ajrccm/137.3.719. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J. MICs and MBCs of Win 57273 against Mycobacterium avium and M. tuberculosis. Antimicrob Agents Chemother. 1990 May;34(5):770–774. doi: 10.1128/aac.34.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. B. Synergistic effect of rifampin, streptomycin, ethionamide, and ethambutol on Mycobacterium intracellulare. Am Rev Respir Dis. 1982 Jan;125(1):43–48. doi: 10.1164/arrd.1982.125.1.43. [DOI] [PubMed] [Google Scholar]
- Heifets L., Lindholm-Levy P. Comparison of bactericidal activities of streptomycin, amikacin, kanamycin, and capreomycin against Mycobacterium avium and M. tuberculosis. Antimicrob Agents Chemother. 1989 Aug;33(8):1298–1301. doi: 10.1128/aac.33.8.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988 Aug;32(8):1131–1136. doi: 10.1128/aac.32.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988 May;137(5):1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- Helbert M., Robinson D., Buchanan D., Hellyer T., McCarthy M., Brown I., Pinching A. J., Mitchell D. M. Mycobacterial infection in patients infected with the human immunodeficiency virus. Thorax. 1990 Jan;45(1):45–48. doi: 10.1136/thx.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer T. J., Brown I. N., Dale J. W., Easmon C. S. Plasmid analysis of Mycobacterium avium-intracellulare (MAI) isolated in the United Kingdom from patients with and without AIDS. J Med Microbiol. 1991 Apr;34(4):225–231. doi: 10.1099/00222615-34-4-225. [DOI] [PubMed] [Google Scholar]
- Herrod H. G., Rourk M. H., Jr, Spock A. Pulmonary disease in children caused by nontuberculous mycobacteria. J Pediatr. 1979 Jun;94(6):915–917. doi: 10.1016/s0022-3476(79)80213-9. [DOI] [PubMed] [Google Scholar]
- Hoffman G. S., Myers R. L., Stark F. R., Thoen C. O. Septic arthritis associated with mycobacterium avium: a case report and literature review. J Rheumatol. 1978 Summer;5(2):199–209. [PubMed] [Google Scholar]
- Hoffner S. E. Improved detection of Mycobacterium avium complex with the Bactec radiometric system. Diagn Microbiol Infect Dis. 1988 May;10(1):1–6. doi: 10.1016/0732-8893(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Hoffner S. E., Kratz M., Olsson-Liljequist B., Svenson S. B., Källenius G. In-vitro synergistic activity between ethambutol and fluorinated quinolones against Mycobacterium avium complex. J Antimicrob Chemother. 1989 Sep;24(3):317–324. doi: 10.1093/jac/24.3.317. [DOI] [PubMed] [Google Scholar]
- Hoffner S. E., Källenius G., Petrini B., Brennan P. J., Tsang A. Y. Serovars of Mycobacterium avium complex isolated from patients in Sweden. J Clin Microbiol. 1990 Jun;28(6):1105–1107. doi: 10.1128/jcm.28.6.1105-1107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffner S. E., Svenson S. B., Beezer A. E. Microcalorimetric studies of the initial interaction between antimycobacterial drugs and Mycobacterium avium. J Antimicrob Chemother. 1990 Mar;25(3):353–359. doi: 10.1093/jac/25.3.353. [DOI] [PubMed] [Google Scholar]
- Hoffner S. E., Svenson S. B., Källenius G. Synergistic effects of antimycobacterial drug combinations on Mycobacterium avium complex determined radiometrically in liquid medium. Eur J Clin Microbiol. 1987 Oct;6(5):530–535. doi: 10.1007/BF02014241. [DOI] [PubMed] [Google Scholar]
- Hooper L. C., Barrow W. W. Decreased mitogenic response of murine spleen cells following intraperitoneal injection of serovar-specific glycopeptidolipid antigens from the Mycobacterium avium complex. Adv Exp Med Biol. 1988;239:309–325. doi: 10.1007/978-1-4757-5421-6_31. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Cohn D. L., Roberts R. B., Masur H., Miller R. A., Tsang A. Y., Iseman M. D. Mycobacterium avium-M. intracellulare isolates from patients with or without acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1986 Dec;30(6):955–957. doi: 10.1128/aac.30.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Havlik J. A., Ellis D. A., Kennedy E., Fann S. A., Dubois R. E., Thompson S. E. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):557–559. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Mason U. G., 3rd, Farhi D. C., Iseman M. D. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine (Baltimore) 1985 Jan;64(1):36–48. doi: 10.1097/00005792-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Metchock B. G., McGowan J. E., Jr, Thompson S. E. Clinical implications of recovery of Mycobacterium avium complex from the stool or respiratory tract of HIV-infected individuals. AIDS. 1992 May;6(5):512–514. [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- Hoy J., Mijch A., Sandland M., Grayson L., Lucas R., Dwyer B. Quadruple-drug therapy for Mycobacterium avium-intracellulare bacteremia in AIDS patients. J Infect Dis. 1990 Apr;161(4):801–805. doi: 10.1093/infdis/161.4.801. [DOI] [PubMed] [Google Scholar]
- Hubbard R. D., Flory C. M., Collins F. M. T-cell immune responses in Mycobacterium avium-infected mice. Infect Immun. 1992 Jan;60(1):150–153. doi: 10.1128/iai.60.1.150-153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudolin V. Tuberculosis and alcoholism. Ann N Y Acad Sci. 1975 Apr 25;252:353–364. doi: 10.1111/j.1749-6632.1975.tb19179.x. [DOI] [PubMed] [Google Scholar]
- Hudson V., Cox F., Taylor L., Guill M., Wray B., Steele J. Pulmonary clofazimine crystals in a child with acquired immunodeficiency syndrome and disseminated Mycobacterium avium-intracellulare infection. Pediatr Infect Dis J. 1988 Dec;7(12):880–882. [PubMed] [Google Scholar]
- Hunter A. M., Campbell I. A., Jenkins P. A., Smith A. P. Treatment of pulmonary infections caused by mycobacteria of the Mycobacterium avium-intracellulare complex. Thorax. 1981 May;36(5):326–329. doi: 10.1136/thx.36.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama S., Shimokata K., Tsukamura M. The isolation of Mycobacterium avium complex from soil, water, and dusts. Microbiol Immunol. 1988;32(7):733–739. doi: 10.1111/j.1348-0421.1988.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Kemper C. A. Disseminated Mycobacterium avium complex infection. AIDS Clin Rev. 1992:131–172. [PubMed] [Google Scholar]
- Inderlied C. B., Kolonoski P. T., Wu M., Young L. S. Amikacin, ciprofloxacin, and imipenem treatment for disseminated Mycobacterium avium complex infection of beige mice. Antimicrob Agents Chemother. 1989 Feb;33(2):176–180. doi: 10.1128/aac.33.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderlied C. B., Kolonoski P. T., Wu M., Young L. S. In vitro and in vivo activity of azithromycin (CP 62,993) against the Mycobacterium avium complex. J Infect Dis. 1989 May;159(5):994–997. doi: 10.1093/infdis/159.5.994. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Young L. S. Disseminated Mycobacterium avium complex infection. AIDS Clin Rev. 1990:165–191. [PubMed] [Google Scholar]
- Inderlied C. B., Young L. S., Yamada J. K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987 Nov;31(11):1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac-Renton J. L., Allen E. A., Chao C. W., Grzybowski S., Whittaker E. I., Black W. A. Isolation and geographic distribution of Mycobacterium other than M. tuberculosis in British Columbia, 1972-81. CMAJ. 1985 Sep 15;133(6):573–576. [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990 Feb;7(1):73–82. [PubMed] [Google Scholar]
- Iseman M. D., Corpe R. F., O'Brien R. J., Rosenzwieg D. Y., Wolinsky E. Disease due to Mycobacterium avium-intracellulare. Chest. 1985 Feb;87(2 Suppl):139S–149S. doi: 10.1378/chest.87.2.139s. [DOI] [PubMed] [Google Scholar]
- Iseman M. D. Mycobacterium avium complex and the normal host: the other side of the coin. N Engl J Med. 1989 Sep 28;321(13):896–898. doi: 10.1056/NEJM198909283211310. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., D'Amato R. F., Heifets L., Murray P. R., Scardamaglia M., Jacobs M. C., Alperstein P., Niles A. Collaborative feasibility study of a biphasic system (Roche Septi-Chek AFB) for rapid detection and isolation of mycobacteria. J Clin Microbiol. 1991 Aug;29(8):1719–1722. doi: 10.1128/jcm.29.8.1719-1722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Kalpana G. V., Cirillo J. D., Pascopella L., Snapper S. B., Udani R. A., Jones W., Barletta R. G., Bloom B. R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Hopewell P. C., Yajko D. M., Hadley W. K., Lazarus E., Mohanty P. K., Modin G. W., Feigal D. W., Cusick P. S., Sande M. A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991 Nov;164(5):994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Jenkin D. J., Dall G. Lesions of bone in disseminated infection due to the mycobacterium avium-intracellulare group. Report of a case. J Bone Joint Surg Br. 1975 Aug;57(3):373–375. [PubMed] [Google Scholar]
- Jensen A. G., Bennedsen J., Rosdahl V. T. Plasmid profiles of mycobacterium avium/intracellulare isolated from patients with AIDS or cervical lymphadenitis and from environmental samples. Scand J Infect Dis. 1989;21(6):645–649. doi: 10.3109/00365548909021692. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Shiratsuchi H., Toba H., Ellner J. J. Preservation of monocyte effector functions against Mycobacterium avium-M. intracellulare in patients with AIDS. Infect Immun. 1991 Oct;59(10):3639–3645. doi: 10.1128/iai.59.10.3639-3645.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi W., Davidson P. M., Jones P. G., Campbell P. E., Roberton D. M. Non-tuberculous mycobacterial lymphadenitis in children. Eur J Pediatr. 1989 Aug;148(8):751–754. doi: 10.1007/BF00443102. [DOI] [PubMed] [Google Scholar]
- Jucker M. T., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria IX. Evidence for two DNA homology groups among small plasmids in Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. Am Rev Respir Dis. 1990 Oct;142(4):858–862. doi: 10.1164/ajrccm/142.4.858. [DOI] [PubMed] [Google Scholar]
- Kahn S. A., Saltzman B. R., Klein R. S., Mahadevia P. S., Friedland G. H., Brandt L. J. Hepatic disorders in the acquired immune deficiency syndrome: a clinical and pathological study. Am J Gastroenterol. 1986 Dec;81(12):1145–1148. [PubMed] [Google Scholar]
- Katila M. L., Mattila J. Enhanced isolation of MOTT on egg media of low pH. APMIS. 1991 Sep;99(9):803–807. doi: 10.1111/j.1699-0463.1991.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Katz P., Yeager H., Jr, Whalen G., Evans M., Swartz R. P., Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. J Clin Immunol. 1990 Jan;10(1):71–77. doi: 10.1007/BF00917500. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Role of T-cell subsets in bacterial infections. Curr Opin Immunol. 1991 Aug;3(4):465–470. doi: 10.1016/0952-7915(91)90004-k. [DOI] [PubMed] [Google Scholar]
- Kaur I., Ram J., Kumar B., Kaur S., Sharma V. K. Effect of clofazimine on eye in multibacillary leprosy. Indian J Lepr. 1990 Jan-Mar;62(1):87–90. [PubMed] [Google Scholar]
- Kelsey D. S., Chambers R. T., Hudspeth A. S. Nontuberculous mycobacterial infection presenting as a mediastinal mass. J Pediatr. 1981 Mar;98(3):431–432. doi: 10.1016/s0022-3476(81)80713-5. [DOI] [PubMed] [Google Scholar]
- Kemper C. A., Meng T. C., Nussbaum J., Chiu J., Feigal D. F., Bartok A. E., Leedom J. M., Tilles J. G., Deresinski S. C., McCutchan J. A. Treatment of Mycobacterium avium complex bacteremia in AIDS with a four-drug oral regimen. Rifampin, ethambutol, clofazimine, and ciprofloxacin. The California Collaborative Treatment Group. Ann Intern Med. 1992 Mar 15;116(6):466–472. doi: 10.7326/0003-4819-116-6-466. [DOI] [PubMed] [Google Scholar]
- Kerlikowske K. M., Katz M. H. Mycobacterium avium complex and Mycobacterium tuberculosis in patients infected with the human immunodeficiency virus. West J Med. 1992 Aug;157(2):144–148. [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Cammarata R. Comparative recoveries of Mycobacterium avium-M. intracellulare from isolator lysis-centrifugation and BACTEC 13A blood culture systems. J Clin Microbiol. 1988 Apr;26(4):760–761. doi: 10.1128/jcm.26.4.760-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Cammarata R. Laboratory diagnosis of mycobacterial infections in patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1986 Nov;24(5):708–711. doi: 10.1128/jcm.24.5.708-711.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Brannon P., Tsang A. Y., Maio M., Gold J. W., Whimbey E., Wong B., McClatchy J. K., Armstrong D. Infections caused by Mycobacterium avium complex in immunocompromised patients: diagnosis by blood culture and fecal examination, antimicrobial susceptibility tests, and morphological and seroagglutination characteristics. J Clin Microbiol. 1985 Feb;21(2):168–173. doi: 10.1128/jcm.21.2.168-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby J. M., Gilligan P. H., Yankaskas J. R., Highsmith W. E., Jr, Edwards L. J., Knowles M. R. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest. 1992 Jul;102(1):70–75. doi: 10.1378/chest.102.1.70. [DOI] [PubMed] [Google Scholar]
- Kinsella J. P., Culver K., Jeffrey R. B., Kaplan M. J., Grossman M. Extensive cervical lymphadenitis due to Mycobacterium avium-intracellulare. Pediatr Infect Dis J. 1987 Mar;6(3):289–291. doi: 10.1097/00006454-198703000-00017. [DOI] [PubMed] [Google Scholar]
- Kinsella J. P., Grossman M., Black S. Otomastoiditis caused by Mycobacterium avium-intracellulare. Pediatr Infect Dis. 1986 Nov-Dec;5(6):704–706. doi: 10.1097/00006454-198611000-00023. [DOI] [PubMed] [Google Scholar]
- Kirihara J. M., Hillier S. L., Coyle M. B. Improved detection times for Mycobacterium avium complex and Mycobacterium tuberculosis with the BACTEC radiometric system. J Clin Microbiol. 1985 Nov;22(5):841–845. doi: 10.1128/jcm.22.5.841-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt E. C., Jensen D. F., Meyer P. R. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Hum Pathol. 1987 Jul;18(7):709–714. doi: 10.1016/s0046-8177(87)80242-3. [DOI] [PubMed] [Google Scholar]
- Knapp A., Stern G. A., Hood C. I. Mycobacterium avium-intracellulare corneal ulcer. Cornea. 1987;6(3):175–180. doi: 10.1097/00003226-198706030-00004. [DOI] [PubMed] [Google Scholar]
- Kuitert L. M., Thomas M. G., Ellis-Pegler R. B. Outcome of untreated Mycobacterium avium-intracellulare complex infection in AIDS. AIDS. 1991 Aug;5(8):1036–1038. [PubMed] [Google Scholar]
- Kunze Z. M., Portaels F., McFadden J. J. Biologically distinct subtypes of Mycobacterium avium differ in possession of insertion sequence IS901. J Clin Microbiol. 1992 Sep;30(9):2366–2372. doi: 10.1128/jcm.30.9.2366-2372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze Z. M., Wall S., Appelberg R., Silva M. T., Portaels F., McFadden J. J. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol Microbiol. 1991 Sep;5(9):2265–2272. doi: 10.1111/j.1365-2958.1991.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Zander A., Vellekoop L., Kanojia M., Luna M., Dicke K. Mycobacterial pulmonary infections after allogeneic bone marrow transplantation. Am J Med. 1984 Jul;77(1):35–40. doi: 10.1016/0002-9343(84)90432-7. [DOI] [PubMed] [Google Scholar]
- Källenius G., Koivula T., Rydgård K. J., Hoffner S. E., Valentin A., Asjö B., Ljungh C., Sharma U., Svenson S. B. Human immunodeficiency virus type 1 enhances intracellular growth of Mycobacterium avium in human macrophages. Infect Immun. 1992 Jun;60(6):2453–2458. doi: 10.1128/iai.60.6.2453-2458.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Hoffner S. E. Ethambutol: a key for Mycobacterium avium complex chemotherapy? Am Rev Respir Dis. 1989 Jul;140(1):264–264. doi: 10.1164/ajrccm/140.1.264. [DOI] [PubMed] [Google Scholar]
- LEWIS A. G., Jr, LASCHE E. M., ARMSTRONG A. L., DUNBAR F. P. A clinical study of the chronic lung disease due to nonphotochromogenic acid-fast bacilli. Ann Intern Med. 1960 Aug;53:273–285. doi: 10.7326/0003-4819-53-2-273. [DOI] [PubMed] [Google Scholar]
- Lai K. K., Stottmeier K. D., Sherman I. H., McCabe W. R. Mycobacterial cervical lymphadenopathy. Relation of etiologic agents to age. JAMA. 1984 Mar 9;251(10):1286–1288. doi: 10.1001/jama.251.10.1286. [DOI] [PubMed] [Google Scholar]
- Lebrun L., Espinasse F., Poveda J. D., Vincent-Levy-Frebault V. Evaluation of nonradioactive DNA probes for identification of mycobacteria. J Clin Microbiol. 1992 Sep;30(9):2476–2478. doi: 10.1128/jcm.30.9.2476-2478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. Y., Chatterjee D., Bozic C. M., Brennan P. J., Cohn D. L., Bales J. D., Harrison S. M., Andron L. A., Orme I. M. Prevalence of serum antibody to the type-specific glycopeptidolipid antigens of Mycobacterium avium in human immunodeficiency virus-positive and -negative individuals. J Clin Microbiol. 1991 May;29(5):1026–1029. doi: 10.1128/jcm.29.5.1026-1029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. H., Bolinger A. M. Treatment of nontuberculous mycobacterial infections in pediatric patients. Clin Pharm. 1988 Jul;7(7):545–551. [PubMed] [Google Scholar]
- Levy H., Wayne L. G., Anderson B. E., Barnes P. F., Light R. W. Antimycobacterial antibody levels in pleural fluid as reflection of passive diffusion from serum. Chest. 1990 May;97(5):1144–1147. doi: 10.1378/chest.97.5.1144. [DOI] [PubMed] [Google Scholar]
- Lewis L. L., Butler K. M., Husson R. N., Mueller B. U., Fowler C. L., Steinberg S. M., Pizzo P. A. Defining the population of human immunodeficiency virus-infected children at risk for Mycobacterium avium-intracellulare infection. J Pediatr. 1992 Nov;121(5 Pt 1):677–683. doi: 10.1016/s0022-3476(05)81892-x. [DOI] [PubMed] [Google Scholar]
- Leysen D. C., Haemers A., Pattyn S. R. Mycobacteria and the new quinolones. Antimicrob Agents Chemother. 1989 Jan;33(1):1–5. doi: 10.1128/aac.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. D., Todd J., Lopez J., Ford E., Janda J. M. Genotypic identification of pathogenic Mycobacterium species by using a nonradioactive oligonucleotide probe. J Clin Microbiol. 1991 Jun;29(6):1276–1278. doi: 10.1128/jcm.29.6.1276-1278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln E. M., Gilbert L. A. Disease in children due to mycobacteria other than Mycobacterium tuberculosis. Am Rev Respir Dis. 1972 May;105(5):683–714. doi: 10.1164/arrd.1972.105.5.683. [DOI] [PubMed] [Google Scholar]
- Lindholm-Levy P. J., Heifets L. B. Clofazimine and other rimino-compounds: minimal inhibitory and minimal bactericidal concentrations at different pHs for Mycobacterium avium complex. Tubercle. 1988 Sep;69(3):179–186. doi: 10.1016/0041-3879(88)90019-0. [DOI] [PubMed] [Google Scholar]
- Love G. L. Nontuberculous mycobacterial skin infection resembling lepromatous leprosy. South Med J. 1987 Aug;80(8):1060–1061. doi: 10.1097/00007611-198708000-00033. [DOI] [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Thorel M. F., Varnerot A., Gicquel B. DNA polymorphism in Mycobacterium paratuberculosis, "wood pigeon mycobacteria," and related mycobacteria analyzed by field inversion gel electrophoresis. J Clin Microbiol. 1989 Dec;27(12):2823–2826. doi: 10.1128/jcm.27.12.2823-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra C. M., Erickson A. D., Feinsilver S. H., Spraragen S. C., McCully K. S., Claunch B. C. Ga-67 studies in a patient with acquired immunodeficiency syndrome and disseminated mycobacterial infection. Clin Nucl Med. 1985 Feb;10(2):96–98. doi: 10.1097/00003072-198502000-00007. [DOI] [PubMed] [Google Scholar]
- Mapother M. E., Songer J. G. In vitro interaction of Mycobacterium avium with intestinal epithelial cells. Infect Immun. 1984 Jul;45(1):67–73. doi: 10.1128/iai.45.1.67-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchevsky A., Damsker B., Gribetz A., Tepper S., Geller S. A. The spectrum of pathology of nontuberculous mycobacterial infections in open-lung biopsy specimens. Am J Clin Pathol. 1982 Nov;78(5):695–700. doi: 10.1093/ajcp/78.5.695. [DOI] [PubMed] [Google Scholar]
- Margileth A. M., Chandra R., Altman R. P. Chronic lymphadenopathy due to mycobacterial infection. Clinical features, diagnosis, histopathology, and management. Am J Dis Child. 1984 Oct;138(10):917–922. doi: 10.1001/archpedi.1984.02140480019007. [DOI] [PubMed] [Google Scholar]
- Margileth A. M. Management of nontuberculous (atypical) mycobacterial infections in children and adolescents. Pediatr Infect Dis. 1985 Mar-Apr;4(2):119–121. doi: 10.1097/00006454-198503000-00002. [DOI] [PubMed] [Google Scholar]
- Maricic M. J., Alepa F. P. Reactive arthritis after Mycobacterium avium-intracellulare infection: Poncet's disease revisited. Am J Med. 1990 May;88(5):549–550. doi: 10.1016/0002-9343(90)90444-i. [DOI] [PubMed] [Google Scholar]
- Marinelli D. L., Albelda S. M., Williams T. M., Kern J. A., Iozzo R. V., Miller W. T. Nontuberculous mycobacterial infection in AIDS: clinical, pathologic, and radiographic features. Radiology. 1986 Jul;160(1):77–82. doi: 10.1148/radiology.160.1.3715048. [DOI] [PubMed] [Google Scholar]
- Martin J. L. Drinking patterns and drinking problems in a community sample of gay men. Prog Clin Biol Res. 1990;325:27–34. [PubMed] [Google Scholar]
- Masaki S., Sugimori G., Okamoto A., Imose J., Hayashi Y. Effect of Tween 80 on the growth of Mycobacterium avium complex. Microbiol Immunol. 1990;34(8):653–663. doi: 10.1111/j.1348-0421.1990.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Masur H., Tuazon C., Gill V., Grimes G., Baird B., Fauci A. S., Lane H. C. Effect of combined clofazimine and ansamycin therapy on Mycobacterium avium-Mycobacterium intracellulare bacteremia in patients with AIDS. J Infect Dis. 1987 Jan;155(1):127–129. doi: 10.1093/infdis/155.1.127. [DOI] [PubMed] [Google Scholar]
- Maurice P. D., Bunker C., Giles F., Goldstone A., Holton J. Mycobacterium avium-intracellulare infection associated with hairy-cell leukemia. Arch Dermatol. 1988 Oct;124(10):1545–1549. [PubMed] [Google Scholar]
- Mayer B. K., Falkinham J. O., 3rd Superoxide dismutase activity of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Infect Immun. 1986 Sep;53(3):631–635. doi: 10.1128/iai.53.3.631-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek G. H., Hartman S., Zhang Y., Brown B. A., Hector J. S., Murphy D., Wallace R. J., Jr Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993 Feb;31(2):390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. M. Utilization of nitrate or nitrite as single nitrogen source by Mycobacterium avium. J Clin Microbiol. 1987 Feb;25(2):263–267. doi: 10.1128/jcm.25.2.263-267.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Ammonium ion requirement for the cell cycle of Mycobacterium avium. Infect Immun. 1978 Jan;19(1):304–311. doi: 10.1128/iai.19.1.304-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Ashbaugh P. Factors that affect the cell cycle of Mycobacterium avium. Rev Infect Dis. 1981 Sep-Oct;3(5):914–925. doi: 10.1093/clinids/3.5.914. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Effect of palmitic acid utilization on cell division in Mycobacterium avium. Infect Immun. 1974 Feb;9(2):363–372. doi: 10.1128/iai.9.2.363-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth andcell division. Infect Immun. 1976 Nov;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Kunze Z. M., Portaels F., Labrousse V., Rastogi N. Epidemiological and genetic markers, virulence factors and intracellular growth of Mycobacterium avium in AIDS. Res Microbiol. 1992 May;143(4):423-30, discussion 430-6. doi: 10.1016/0923-2508(92)90057-u. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Brennan P. J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol. 1991 May;142(4):451–463. doi: 10.1016/0923-2508(91)90120-y. [DOI] [PubMed] [Google Scholar]
- Mehle M. E., Adamo J. P., Mehta A. C., Wiedemann H. P., Keys T., Longworth D. L. Endobronchial Mycobacterium avium-intracellulare infection in a patient with AIDS. Chest. 1989 Jul;96(1):199–201. doi: 10.1378/chest.96.1.199. [DOI] [PubMed] [Google Scholar]
- Meissner G., Anz W. Sources of Mycobacterium avium complex infection resulting in human diseases. Am Rev Respir Dis. 1977 Dec;116(6):1057–1064. doi: 10.1164/arrd.1977.116.6.1057. [DOI] [PubMed] [Google Scholar]
- Mercier B., Gaucher C., Feugeas O., Mazurier C. Direct PCR from whole blood, without DNA extraction. Nucleic Acids Res. 1990 Oct 11;18(19):5908–5908. doi: 10.1093/nar/18.19.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan P. R., Munis J. R., Richman D. D., Kornbluth R. S. Concurrent human immunodeficiency virus and mycobacterial infection of macrophages in vitro does not reveal any reciprocal effect. J Infect Dis. 1992 Jan;165(1):80–86. doi: 10.1093/infdis/165.1.80. [DOI] [PubMed] [Google Scholar]
- Meylan P. R., Richman D. D., Kornbluth R. S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990 Aug;58(8):2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolich D. J., Mates S. M. Granulomatous prostatitis due to Mycobacterium avium complex. Clin Infect Dis. 1992 Feb;14(2):589–591. doi: 10.1093/clinids/14.2.589. [DOI] [PubMed] [Google Scholar]
- Miranda D., Vuletin J. C., Kauffman S. L. Disseminated histiocytosis and intestinal malakoplakia. Occurrence due to Mycobacterium intracellulare infection. Arch Pathol Lab Med. 1979 Jun;103(6):302–305. [PubMed] [Google Scholar]
- Miyachi T., Shimokata K., Dawson D. J., Tsukamura M. Changes of the biotype of Mycobacterium avium-Mycobacterium intracellulare complex causing lung disease in Japan. Tubercle. 1988 Jun;69(2):133–137. doi: 10.1016/0041-3879(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Fukunaga M., Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981 May;146(2):656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Ogawa M., Udou T. Morphological changes induced by beta-lactam antibiotics in Mycobacterium avium-intracellulare complex. Antimicrob Agents Chemother. 1985 Apr;27(4):541–547. doi: 10.1128/aac.27.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Udou T., Yamada T. Mechanism of antibiotic resistance in Mycobacterium intracellulare. Microbiol Immunol. 1983;27(5):425–431. doi: 10.1111/j.1348-0421.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Modilevsky T., Sattler F. R., Barnes P. F. Mycobacterial disease in patients with human immunodeficiency virus infection. Arch Intern Med. 1989 Oct;149(10):2201–2205. [PubMed] [Google Scholar]
- Morris S. L., Bermudez L., Chaparas S. D. Mycobacterium avium complex disease in patients with AIDS: seroreactivity to native and recombinant mycobacterial antigens. J Clin Microbiol. 1991 Dec;29(12):2715–2719. doi: 10.1128/jcm.29.12.2715-2719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. L., Rouse D. A., Malik A., Chaparas S. D., Witebsky F. G. Characterisation of plasmids extracted from AIDS--associated Mycobacterium avium isolates. Tubercle. 1990 Sep;71(3):181–185. doi: 10.1016/0041-3879(90)90073-h. [DOI] [PubMed] [Google Scholar]
- Morrissey A. B., Aisu T. O., Falkinham J. O., 3rd, Eriki P. P., Ellner J. J., Daniel T. M. Absence of Mycobacterium avium complex disease in patients with AIDS in Uganda. J Acquir Immune Defic Syndr. 1992;5(5):477–478. [PubMed] [Google Scholar]
- Motyl M. R., Saltzman B., Levi M. H., McKitrick J. C., Friedland G. H., Klein R. S. The recovery of Mycobacterium avium complex and Mycobacterium tuberculosis from blood specimens of AIDS patients using the nonradiometric BACTEC NR 660 medium. Am J Clin Pathol. 1990 Jul;94(1):84–86. doi: 10.1093/ajcp/94.1.84. [DOI] [PubMed] [Google Scholar]
- Mufarrij A. A., Greco M. A., Antopol S. C., Borkowsky W. The histopathology of cervical lymphadenitis caused by Mycobacterium avium-Mycobacterium intracellulare complex in an immunocompromised host. Hum Pathol. 1982 Jan;13(1):78–81. doi: 10.1016/s0046-8177(82)80142-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Scavuzzo D. A., Chaparas S. D., Roberts R. B. T lymphocyte responses to mycobacterial antigen in AIDS patients with disseminated Mycobacterium avium-Mycobacterium intracellulare infection. Chest. 1988 May;93(5):922–925. doi: 10.1378/chest.93.5.922. [DOI] [PubMed] [Google Scholar]
- Musial C. E., Tice L. S., Stockman L., Roberts G. D. Identification of mycobacteria from culture by using the Gen-Probe Rapid Diagnostic System for Mycobacterium avium complex and Mycobacterium tuberculosis complex. J Clin Microbiol. 1988 Oct;26(10):2120–2123. doi: 10.1128/jcm.26.10.2120-2123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S. P., Samsonoff W. A., Ruck R. E. Effects of surface-active agents on drug susceptibility levels and ultrastructure of Mycobacterium avium complex organisms isolated from AIDS patients. Diagn Microbiol Infect Dis. 1988 Sep;11(1):11–19. doi: 10.1016/0732-8893(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Goto Y., Kitamura K., Tokunaga T. Two types of suppressor T cells that inhibit delayed-type hypersensitivity to Mycobacterium intracellulare in mice. Infect Immun. 1989 Mar;57(3):779–784. doi: 10.1128/iai.57.3.779-784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassos P. S., Yajko D. M., Sanders C. A., Hadley W. K. Prevalence of Mycobacterium avium complex in respiratory specimens from AIDS and non-AIDS patients in a San Francisco hospital. Am Rev Respir Dis. 1991 Jan;143(1):66–68. doi: 10.1164/ajrccm/143.1.66. [DOI] [PubMed] [Google Scholar]
- Nel E. E. Mycobacterium avium-intracellulare complex serovars isolated in South Africa from humans, swine, and the environment. Rev Infect Dis. 1981 Sep-Oct;3(5):1013–1020. doi: 10.1093/clinids/3.5.1013. [DOI] [PubMed] [Google Scholar]
- Nightingale S. D., Byrd L. T., Southern P. M., Jockusch J. D., Cal S. X., Wynne B. A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992 Jun;165(6):1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- Noel S. B., Ray M. C., Greer D. L. Cutaneous infection with Mycobacterium avium-intracellulare scrofulaceum intermediate: a new pathogenic entity. J Am Acad Dermatol. 1988 Sep;19(3):492–495. doi: 10.1016/s0190-9622(88)70203-0. [DOI] [PubMed] [Google Scholar]
- Nozawa R. T., Kato H., Yokota T., Sugi H. Susceptibility of intra- and extracellular Mycobacterium avium-intracellulare to cephem antibiotics. Antimicrob Agents Chemother. 1985 Jan;27(1):132–134. doi: 10.1128/aac.27.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum J. M., Dealist C., Lewis W., Heseltine P. N. Rapid diagnosis by buffy coat smear of disseminated Mycobacterium avium complex infection in patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990 Mar;28(3):631–632. doi: 10.1128/jcm.28.3.631-632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez J. M., Monteagudo I., López-Longo F. J., Cobeta J. C., Rivera J. Disseminated Mycobacterium avium-intracellulare infection in a patient with polymyositis. Arthritis Rheum. 1989 Jul;32(7):934–936. [PubMed] [Google Scholar]
- Nyberg D. A., Federle M. P., Jeffrey R. B., Bottles K., Wofsy C. B. Abdominal CT findings of disseminated Mycobacterium avium-intracellulare in AIDS. AJR Am J Roentgenol. 1985 Aug;145(2):297–299. doi: 10.2214/ajr.145.2.297. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Geiter L. J., Lyle M. A. Rifabutin (ansamycin LM427) for the treatment of pulmonary Mycobacterium avium complex. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):821–826. doi: 10.1164/ajrccm/141.4_Pt_1.821. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Geiter L. J., Snider D. E., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987 May;135(5):1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Lyle M. A., Snider D. E., Jr Rifabutin (ansamycin LM 427): a new rifamycin-S derivative for the treatment of mycobacterial diseases. Rev Infect Dis. 1987 May-Jun;9(3):519–530. doi: 10.1093/clinids/9.3.519. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Cranfill R., Hall L., Lang J., Fu Y. X., Kubo R., Born W. Recognition of a single hsp-60 epitope by an entire subset of gamma delta T lymphocytes. Immunol Rev. 1991 Jun;121:155–170. doi: 10.1111/j.1600-065x.1991.tb00827.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Prophylactic effect in mice of BCG vaccination against nontuberculous mycobacterial infections. Tubercle. 1985 Jun;66(2):117–120. doi: 10.1016/0041-3879(85)90076-5. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortbals D. W., Marr J. J. A comparative study of tuberculous and other mycobacterial infections and their associations with malignancy. Am Rev Respir Dis. 1978 Jan;117(1):39–45. doi: 10.1164/arrd.1978.117.1.39. [DOI] [PubMed] [Google Scholar]
- Packer S. J., Cesario T., Williams J. H., Jr Mycobacterium avium complex infection presenting as endobronchial lesions in immunosuppressed patients. Ann Intern Med. 1988 Sep 1;109(5):389–393. doi: 10.7326/0003-4819-109-5-389. [DOI] [PubMed] [Google Scholar]
- Panaccio M., Lew A. PCR based diagnosis in the presence of 8% (v/v) blood. Nucleic Acids Res. 1991 Mar 11;19(5):1151–1151. doi: 10.1093/nar/19.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. C., Ford M. A., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential aerosolization of Mycobacterium intracellulare from natural waters. Am Rev Respir Dis. 1983 Oct;128(4):652–656. doi: 10.1164/arrd.1983.128.4.652. [DOI] [PubMed] [Google Scholar]
- Pergament M., Gonzalez R., Fraley E. E. Atypical mycobacteriosis of the urinary tract. A case report of extensive disease caused by the Battey bacillus. JAMA. 1974 Aug 12;229(7):816–817. [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Pocidalo J. J., Vilde J. L. Activities of clarithromycin, sulfisoxazole, and rifabutin against Mycobacterium avium complex multiplication within human macrophages. Antimicrob Agents Chemother. 1990 Aug;34(8):1508–1511. doi: 10.1128/aac.34.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. R., Jaques D. P., Lesar M. S., d'Avis J. C., Peterson H. D. Mycobacterium avium infection in a silicone-injected breast. Plast Reconstr Surg. 1985 Jan;75(1):104–106. doi: 10.1097/00006534-198501000-00024. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Lu R., Floyd C., Nakasone A., Friedly G., de la Maza L. M. Direct identification of Mycobacterium tuberculosis, Mycobacterium avium, and Mycobacterium intracellulare from amplified primary cultures in BACTEC media using DNA probes. J Clin Microbiol. 1989 Jul;27(7):1543–1547. doi: 10.1128/jcm.27.7.1543-1547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Gekker G., Chao C. C., Schut R., Verhoef J., Edelman C. K., Erice A., Balfour H. H., Jr Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J Immunol. 1992 Jul 15;149(2):676–680. [PubMed] [Google Scholar]
- Pethel M. L., Falkinham J. O., 3rd Plasmid-influenced changes in Mycobacterium avium catalase activity. Infect Immun. 1989 Jun;57(6):1714–1718. doi: 10.1128/iai.57.6.1714-1718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken R. N., Plotch S. J., Wang Z., Lin B. C., Donegan J. J., Yang H. L. DNA probes for mycobacteria. I. Isolation of DNA probes for the identification of Mycobacterium tuberculosis complex and for mycobacteria other than tuberculosis (MOTT). Mol Cell Probes. 1988 Jun;2(2):111–124. doi: 10.1016/0890-8508(88)90033-3. [DOI] [PubMed] [Google Scholar]
- Piscitelli S. C., Danziger L. H., Rodvold K. A. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm. 1992 Feb;11(2):137–152. [PubMed] [Google Scholar]
- Pitchenik A. E., Cole C., Russell B. W., Fischl M. A., Spira T. J., Snider D. E., Jr Tuberculosis, atypical mycobacteriosis, and the acquired immunodeficiency syndrome among Haitian and non-Haitian patients in south Florida. Ann Intern Med. 1984 Nov;101(5):641–645. doi: 10.7326/0003-4819-101-5-641. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. B., Plikaytis B. D., Yakrus M. A., Butler W. R., Woodley C. L., Silcox V. A., Shinnick T. M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992 Jul;30(7):1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohajdak B., Gomez J. L., Wilkins J. A., Greenberg A. H. Tumor-activated NK cells trigger monocyte oxidative metabolism. J Immunol. 1984 Nov;133(5):2430–2436. [PubMed] [Google Scholar]
- Poropatich C. O., Labriola A. M., Tuazon C. U. Acid-fast smear and culture of respiratory secretions, bone marrow, and stools as predictors of disseminated Mycobacterium avium complex infection. J Clin Microbiol. 1987 May;25(5):929–930. doi: 10.1128/jcm.25.5.929-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince D. S., Peterson D. D., Steiner R. M., Gottlieb J. E., Scott R., Israel H. L., Figueroa W. G., Fish J. E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- Pulliam J. P., Vernon D. D., Alexander S. R., Hartstein A. I., Golper T. A. Nontuberculous mycobacterial peritonitis associated with continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1983 May;2(6):610–614. doi: 10.1016/s0272-6386(83)80040-7. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990 May;34(5):759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Hellio R., David H. L. A new insight into the mycobacterial cell envelope architecture by the localization of antigens in ultrathin sections. Zentralbl Bakteriol. 1991 Aug;275(3):287–302. doi: 10.1016/s0934-8840(11)80292-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V., Goh K. S., De Sousa J. P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2473–2480. doi: 10.1128/aac.35.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich J. M., Johnson R. E. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992 Jun;101(6):1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- Reznikov M., Leggo J. H., Dawson D. J. Investigation by seroagglutination of strains of the Mycobacterium intracellulare-M. scrofulaceum group from house dusts and sputum in Southeastern Queensland. Am Rev Respir Dis. 1971 Dec;104(6):951–953. doi: 10.1164/arrd.1971.104.6.951. [DOI] [PubMed] [Google Scholar]
- Rice L., Shenkenberg T., Lynch E. C., Wheeler T. M. Granulomatous infections complicating hairy cell leukemia. Cancer. 1982 May 1;49(9):1924–1928. doi: 10.1002/1097-0142(19820501)49:9<1924::aid-cncr2820490928>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Riesbeck K., Andersson J., Gullberg M., Forsgren A. Fluorinated 4-quinolones induce hyperproduction of interleukin 2. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2809–2813. doi: 10.1073/pnas.86.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoire B., Ranchoff B. J., Chatterjee D., Gaylord H., Tsang A. Y., Kolk A. H., Aspinall G. O., Brennan P. J. Generation of monoclonal antibodies to the specific sugar epitopes of Mycobacterium avium complex serovars. Infect Immun. 1989 Oct;57(10):3147–3158. doi: 10.1128/iai.57.10.3147-3158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., McMillan C., Coyle M. B. Whole chromosomal DNA probes for rapid identification of Mycobacterium tuberculosis and Mycobacterium avium complex. J Clin Microbiol. 1987 Jul;25(7):1239–1243. doi: 10.1128/jcm.25.7.1239-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein J. A., Swartz R. P., Yeager H., Jr Nonopsonic uptake of Mycobacterium avium complex by human monocytes and alveolar macrophages. J Lab Clin Med. 1992 Jun;119(6):772–781. [PubMed] [Google Scholar]
- Rogers P. L., Walker R. E., Lane H. C., Witebsky F. G., Kovacs J. A., Parrillo J. E., Masur H. Disseminated Mycobacterium haemophilum infection in two patients with the acquired immunodeficiency syndrome. Am J Med. 1988 Mar;84(3 Pt 2):640–642. doi: 10.1016/0002-9343(88)90150-7. [DOI] [PubMed] [Google Scholar]
- Romanus V. Childhood tuberculosis in Sweden. An epidemiological study made six years after the cessation of general BCG vaccination of the newborn. Tubercle. 1983 Jun;64(2):101–110. doi: 10.1016/0041-3879(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig D. Y., Schlueter D. P. Spectrum of clinical disease in pulmonary infection with Mycobacterium avium-intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):1046–1051. doi: 10.1093/clinids/3.5.1046. [DOI] [PubMed] [Google Scholar]
- Rotterdam H., Sommers S. C. Alimentary tract biopsy lesions in the acquired immune deficiency syndrome. Pathology. 1985 Apr;17(2):181–192. doi: 10.3109/00313028509063754. [DOI] [PubMed] [Google Scholar]
- Rulong S., Aguas A. P., da Silva P. P., Silva M. T. Intramacrophagic Mycobacterium avium bacilli are coated by a multiple lamellar structure: freeze fracture analysis of infected mouse liver. Infect Immun. 1991 Nov;59(11):3895–3902. doi: 10.1128/iai.59.11.3895-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon E. H. Ten mycobacterial pathogens. Tubercle. 1974 Sep;55(3):235–240. doi: 10.1016/0041-3879(74)90051-8. [DOI] [PubMed] [Google Scholar]
- Saito H., Sato K., Tomioka H. Comparative in vitro and in vivo activity of rifabutin and rifampicin against Mycobacterium avium complex. Tubercle. 1988 Sep;69(3):187–192. doi: 10.1016/0041-3879(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K., Tasaka H., Dawson D. J. Identification of various serovar strains of Mycobacterium avium complex by using DNA probes specific for Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1990 Aug;28(8):1694–1697. doi: 10.1128/jcm.28.8.1694-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Susceptibilities of transparent, opaque, and rough colonial variants of Mycobacterium avium complex to various fatty acids. Antimicrob Agents Chemother. 1988 Mar;32(3):400–402. doi: 10.1128/aac.32.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Therapeutic efficacy of liposome-entrapped rifampin against Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1989 Apr;33(4):429–433. doi: 10.1128/aac.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfinger M., Stool E. W., Piot D., Heifets L. Comparison of three methods for recovery of Mycobacterium avium complex from blood specimens. J Clin Microbiol. 1988 Jun;26(6):1225–1226. doi: 10.1128/jcm.26.6.1225-1226.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson T. L., Moskowitz L., Hensley G. T., Cleary T. J., Penneys N. Disseminated Mycobacterium avium-intracellulare infection appearing as a panniculitis. Arch Pathol Lab Med. 1982 Mar;106(3):112–114. [PubMed] [Google Scholar]
- Sandler E. D., Ng V. L., Hadley W. K. Clofazimine crystals in alveolar macrophages from a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1992 May;116(5):541–543. [PubMed] [Google Scholar]
- Schaad U. B., Votteler T. P., McCracken G. H., Jr, Nelson J. D. Management of atypical mycobacterial lymphadenitis in childhood: a review based on 380 cases. J Pediatr. 1979 Sep;95(3):356–360. doi: 10.1016/s0022-3476(79)80506-5. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood J. Lack of quinolone-induced arthropathy in children. J Antimicrob Chemother. 1992 Oct;30(4):414–416. doi: 10.1093/jac/30.4.414. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J Clin Invest. 1990 Apr;85(4):1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit K. E., Hendley J. O. Primary mycobacterial infection of the pharynx. Am J Dis Child. 1978 Feb;132(2):210–210. doi: 10.1001/archpedi.1978.02120270108026. [DOI] [PubMed] [Google Scholar]
- Schurr E., Malo D., Radzioch D., Buschman E., Morgan K., Gros P., Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991 Mar;12(3):A42–A45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- Sherman I., Harrington N., Rothrock A., George H. Use of a cutoff range in identifying mycobacteria by the Gen-Probe Rapid Diagnostic System. J Clin Microbiol. 1989 Feb;27(2):241–244. doi: 10.1128/jcm.27.2.241-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Silva C. L., Lukacs K., Lowrie D. B. Major histocompatibility complex non-restricted presentation to CD4+ T lymphocytes of Mycobacterium leprae heat-shock protein 65 antigen by macrophages transfected with the mycobacterial gene. Immunology. 1993 Jan;78(1):35–42. [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Palacios A., Colston M. J., Lowrie D. B. Mycobacterium leprae 65hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb Pathog. 1992 Jan;12(1):27–38. doi: 10.1016/0882-4010(92)90063-t. [DOI] [PubMed] [Google Scholar]
- Simony N., Basset A., Ehrhardt J. D., Porte A., Brini A. Pigmentation cornéo-conjonctivale au lamprène. Bull Mem Soc Fr Ophtalmol. 1986;97:215–217. [PubMed] [Google Scholar]
- Simpson G. L., Raffin T. A., Remington J. S. Association of prior nocardiosis and subsequent occurrence of nontuberculous mycobacteriosis in a defined, immunosuppressed population. J Infect Dis. 1982 Aug;146(2):211–219. doi: 10.1093/infdis/146.2.211. [DOI] [PubMed] [Google Scholar]
- Skinner M. H., Hsieh M., Torseth J., Pauloin D., Bhatia G., Harkonen S., Merigan T. C., Blaschke T. F. Pharmacokinetics of rifabutin. Antimicrob Agents Chemother. 1989 Aug;33(8):1237–1241. doi: 10.1128/aac.33.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small P. M., Schecter G. F., Goodman P. C., Sande M. A., Chaisson R. E., Hopewell P. C. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1991 Jan 31;324(5):289–294. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- Snider D. E., Jr, Jones W. D., Good R. C. The usefulness of phage typing Mycobacterium tuberculosis isolates. Am Rev Respir Dis. 1984 Dec;130(6):1095–1099. doi: 10.1164/arrd.1984.130.6.1095. [DOI] [PubMed] [Google Scholar]
- Soini H., Skurnik M., Liippo K., Tala E., Viljanen M. K. Detection and identification of mycobacteria by amplification of a segment of the gene coding for the 32-kilodalton protein. J Clin Microbiol. 1992 Aug;30(8):2025–2028. doi: 10.1128/jcm.30.8.2025-2028.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J. G. Environmental sources of Mycobacterium avium for infection of animals and man. Proc Annu Meet U S Anim Health Assoc. 1980;84:528–535. [PubMed] [Google Scholar]
- Stacey A. R. Isolation of Mycobacterium avium-intracellulare-scrofulaceum complex from faeces of patients with AIDS. Br Med J (Clin Res Ed) 1986 Nov 8;293(6556):1194–1194. doi: 10.1136/bmj.293.6556.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Urbance J. W. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990 Jan;172(1):116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. W., Horwitz C. A., Burton L. G., Weisser J. A. Negative images of bacilli and mycobacterial infection: a study of fine-needle aspiration smears from lymph nodes in patients with AIDS. Diagn Cytopathol. 1990;6(2):118–121. doi: 10.1002/dc.2840060209. [DOI] [PubMed] [Google Scholar]
- Stauffer F., Dörtbudak O., Lahonik E. In vitro testing of clarithromycin in combination with ethambutol and rifampicin against Mycobacterium avium complex. Infection. 1991 Sep-Oct;19(5):343–345. doi: 10.1007/BF01645362. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Bodey G. P. Infections in hairy cell leukemia (leukemic reticuloendotheliosis). Cancer. 1981 Feb 15;47(4):801–805. doi: 10.1002/1097-0142(19810215)47:4<801::aid-cncr2820470428>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Stone A. B., Schelonka R. L., Drehner D. M., McMahon D. P., Ascher D. P. Disseminated Mycobacterium avium complex in non-human immunodeficiency virus-infected pediatric patients. Pediatr Infect Dis J. 1992 Nov;11(11):960–964. doi: 10.1097/00006454-199211110-00011. [DOI] [PubMed] [Google Scholar]
- Stormer R. S., Falkinham J. O., 3rd Differences in antimicrobial susceptibility of pigmented and unpigmented colonial variants of Mycobacterium avium. J Clin Microbiol. 1989 Nov;27(11):2459–2465. doi: 10.1128/jcm.27.11.2459-2465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand C. L., Epstein C., Verzosa S., Effatt E., Hormozi P., Siddiqi S. H. Evaluation of a new blood culture medium for mycobacteria. Am J Clin Pathol. 1989 Mar;91(3):316–318. doi: 10.1093/ajcp/91.3.316. [DOI] [PubMed] [Google Scholar]
- Sutker W. L., Lankford L. L., Tompsett R. Granulomatous synovitis: the role of atypical mycobacteria. Rev Infect Dis. 1979 Sep-Oct;1(5):729–735. doi: 10.1093/clinids/1.5.729. [DOI] [PubMed] [Google Scholar]
- TIMPE A., RUNYON E. H. The relationship of atypical acid-fast bacteria to human disease; a preliminary report. J Lab Clin Med. 1954 Aug;44(2):202–209. [PubMed] [Google Scholar]
- Takashima T., Collins F. M. T-cell-mediated immunity in persistent Mycobacterium intracellulare infections in mice. Infect Immun. 1988 Nov;56(11):2782–2787. doi: 10.1128/iai.56.11.2782-2787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassell S. K., Pourshafie M., Wright E. L., Richmond M. G., Barrow W. W. Modified lymphocyte response to mitogens induced by the lipopeptide fragment derived from Mycobacterium avium serovar-specific glycopeptidolipids. Infect Immun. 1992 Feb;60(2):706–711. doi: 10.1128/iai.60.2.706-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Himes E. M. Mycobacterial infections in animals. Rev Infect Dis. 1981 Sep-Oct;3(5):960–972. doi: 10.1093/clinids/3.5.960. [DOI] [PubMed] [Google Scholar]
- Thorel M. F., David H. L. Specific surface antigens of SmT variants of Mycobacterium avium. Infect Immun. 1984 Jan;43(1):438–439. doi: 10.1128/iai.43.1.438-439.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel M. F., Krichevsky M., Lévy-Frébault V. V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990 Jul;40(3):254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Sato K., Saito H., Yamada Y. Susceptibility of Mycobacterium avium and Mycobacterium intracellulare to various antibacterial drugs. Microbiol Immunol. 1989;33(6):509–514. doi: 10.1111/j.1348-0421.1989.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Torres R. A., Nord J., Feldman R., LaBombardi V., Barr M. Disseminated mixed Mycobacterium simiae-Mycobacterium avium complex infection in acquired immunodeficiency syndrome. J Infect Dis. 1991 Aug;164(2):432–433. doi: 10.1093/infdis/164.2.432. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Miyachi T. Correlations among naturally occurring resistances to antituberculosis drugs in Mycobacterium avium complex strains. Am Rev Respir Dis. 1989 Apr;139(4):1033–1035. doi: 10.1164/ajrccm/139.4.1033. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Mizuno S., Murata H. Occurrence of Mycobacterium tuberculosis and strains of the Mycobacterium avium-M. intracellulare complex together in the sputum of patients with pulmonary tuberculosis. Tubercle. 1981 Mar;62(1):43–46. doi: 10.1016/0041-3879(81)90034-9. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Okuda Y., Yamamoto Y. An analysis of in vitro T cell responsiveness in nontuberculous mycobacterial infection. Chest. 1988 Oct;94(4):822–829. doi: 10.1378/chest.94.4.822. [DOI] [PubMed] [Google Scholar]
- Tuffley R. E., Holbeche J. D. Isolation of the Mycobacterium avium-M. intracellulare-M. scrofulaceum complex from tank water in Queensland, Australia. Appl Environ Microbiol. 1980 Jan;39(1):48–53. doi: 10.1128/aem.39.1.48-53.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry P. A., Bogousslavsky J., Regli F., Chave J. P., Beer V. Chronic Mycobacterium avium complex infection of the central nervous system in a nonimmunosuppressed woman. Eur Neurol. 1992;32(5):285–288. doi: 10.1159/000116843. [DOI] [PubMed] [Google Scholar]
- Uribe-Botero G., Prichard J. G., Kaplowitz H. J. Bone marrow in HIV infection. A comparison of fluorescent staining and cultures in the detection of mycobacteria. Am J Clin Pathol. 1989 Mar;91(3):313–315. doi: 10.1093/ajcp/91.3.313. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Deans R. J., Band H., Ohmen J., Panchamoorthy G., Morita C. T., Rea T. H., Modlin R. L. Evidence for clonal selection of gamma/delta T cells in response to a human pathogen. J Exp Med. 1991 Sep 1;174(3):683–692. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. E., Robbins A. H. Mycobacterium avium-intracellulare complex enteritis: pseudo-Whipple disease in AIDS. AJR Am J Roentgenol. 1985 May;144(5):921–922. doi: 10.2214/ajr.144.5.921. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Wallace J. M., Hannah J. B. Mycobacterium avium complex infection in patients with the acquired immunodeficiency syndrome. A clinicopathologic study. Chest. 1988 May;93(5):926–932. doi: 10.1378/chest.93.5.926. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr Nontuberculous mycobacteria and water: a love affair with increasing clinical importance. Infect Dis Clin North Am. 1987 Sep;1(3):677–686. [PubMed] [Google Scholar]
- Wardrop P. A., Pillsbury H. C., 3rd Mycobacterium avium acute mastoiditis. Arch Otolaryngol. 1984 Oct;110(10):686–687. doi: 10.1001/archotol.1984.00800360058014. [DOI] [PubMed] [Google Scholar]
- Wasem C. F., McCarthy C. M., Murray L. W. Multilocus enzyme electrophoresis analysis of the Mycobacterium avium complex and other mycobacteria. J Clin Microbiol. 1991 Feb;29(2):264–271. doi: 10.1128/jcm.29.2.264-271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Anderson B., Chetty K., Light R. W. Antibodies to mycobacterial peptidoglycolipid and to crude protein antigens in sera from different categories of human subjects. J Clin Microbiol. 1988 Nov;26(11):2300–2306. doi: 10.1128/jcm.26.11.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Good R. C., Krichevsky M. I., Blacklock Z., David H. L., Dawson D., Gross W., Hawkins J., Levy-Frebault V. V., McManus C. Fourth report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1991 Oct;41(4):463–472. doi: 10.1099/00207713-41-4-463. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992 Jan;5(1):1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G. The "atypical" mycobacteria: recognition and disease association. Crit Rev Microbiol. 1985;12(3):185–222. doi: 10.3109/10408418509104429. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Young L. S., Bertram M. Absence of mycobacterial antibody in patients with acquired immune deficiency syndrome. Eur J Clin Microbiol. 1986 Jun;5(3):363–365. doi: 10.1007/BF02017802. [DOI] [PubMed] [Google Scholar]
- Weinstein R. A., Golomb H. M., Grumet G., Gelmann E., Schechter G. P. Hairy cell leukemia: association with disseminated atypical mycobacterial infection. Cancer. 1981 Jul 15;48(2):380–383. doi: 10.1002/1097-0142(19810715)48:2<380::aid-cncr2820480226>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Wendt S. L., George K. L., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous Mycobacteria. III. Isolation of potentially pathogenic mycobacteria from aerosols. Am Rev Respir Dis. 1980 Aug;122(2):259–263. doi: 10.1164/arrd.1980.122.2.259. [DOI] [PubMed] [Google Scholar]
- Wiley E. L., Mulhollan T. J., Beck B., Tyndall J. A., Freeman R. G. Polyclonal antibodies raised against Bacillus Calmette-Guerin, Mycobacterium duvalii, and Mycobacterium paratuberculosis used to detect mycobacteria in tissue with the use of immunohistochemical techniques. Am J Clin Pathol. 1990 Sep;94(3):307–312. doi: 10.1093/ajcp/94.3.307. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Wolke A., Meyers S., Adelsberg B. R., Bottone E. J., Damsker B., Schwartz I. S., Janowitz H. D. Mycobacterium avium-intracellulare-associated colitis in a patient with the acquired immunodeficiency syndrome. J Clin Gastroenterol. 1984 Jun;6(3):225–229. [PubMed] [Google Scholar]
- Wong B., Edwards F. F., Kiehn T. E., Whimbey E., Donnelly H., Bernard E. M., Gold J. W., Armstrong D. Continuous high-grade mycobacterium avium-intracellulare bacteremia in patients with the acquired immune deficiency syndrome. Am J Med. 1985 Jan;78(1):35–40. doi: 10.1016/0002-9343(85)90458-9. [DOI] [PubMed] [Google Scholar]
- Woodley C. L., David H. L. Effect of temperature on the rate of the transparent to opaque colony type transition in Mycobacterium avium. Antimicrob Agents Chemother. 1976 Jan;9(1):113–119. doi: 10.1128/aac.9.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Washington J. A., 2nd Mycobacteria other than Mycobacterium tuberculosis: review of microbiologic and clinical aspects. Rev Infect Dis. 1987 Mar-Apr;9(2):275–294. doi: 10.1093/clinids/9.2.275. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagupsky P., Menegus M. A. Cumulative positivity rates of multiple blood cultures for Mycobacterium avium-intracellulare and Cryptococcus neoformans in patients with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1990 Sep;114(9):923–925. [PubMed] [Google Scholar]
- Yajko D. M., Kirihara J., Sanders C., Nassos P., Hadley W. K. Antimicrobial synergism against Mycobacterium avium complex strains isolated from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1988 Sep;32(9):1392–1395. doi: 10.1128/aac.32.9.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Therapeutic implications of inhibition versus killing of Mycobacterium avium complex by antimicrobial agents. Antimicrob Agents Chemother. 1987 Jan;31(1):117–120. doi: 10.1128/aac.31.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Sanders C. A., Gonzalez P. C., Hadley W. K. Comparison of the intracellular activities of clarithromycin and erythromycin against Mycobacterium avium complex strains in J774 cells and in alveolar macrophages from human immunodeficiency virus type 1-infected individuals. Antimicrob Agents Chemother. 1992 May;36(5):1163–1165. doi: 10.1128/aac.36.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Sanders C. A., Hadley W. K. Killing by antimycobacterial agents of AIDS-derived strains of Mycobacterium avium complex inside cells of the mouse macrophage cell line J774. Am Rev Respir Dis. 1989 Nov;140(5):1198–1203. doi: 10.1164/ajrccm/140.5.1198. [DOI] [PubMed] [Google Scholar]
- Yakrus M. A., Good R. C. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990 May;28(5):926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori S., Tsukamura M. Comparison of prognosis of pulmonary diseases caused by Mycobacterium avium and by Mycobacterium intracellulare. Chest. 1992 Jul;102(1):89–90. doi: 10.1378/chest.102.1.89. [DOI] [PubMed] [Google Scholar]
- Yamori S., Tsukamura M. Paradoxical effect of Tween 80 between the susceptibility to rifampicin and streptomycin and the susceptibility to ethambutol and sulfadimethoxine in the Mycobacterium avium-Mycobacterium intracellulare complex. Microbiol Immunol. 1991;35(10):921–926. doi: 10.1111/j.1348-0421.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Yanagihara D. L., Barr V. L., Knisley C. V., Tsang A. Y., McClatchy J. K., Brennan P. J. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J Clin Microbiol. 1985 Apr;21(4):569–574. doi: 10.1128/jcm.21.4.569-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y. Nucleic acid hybridization studies of mycobactin-dependent mycobacteria. J Clin Microbiol. 1988 Jul;26(7):1309–1312. doi: 10.1128/jcm.26.7.1309-1312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3086–3093. doi: 10.1128/iai.59.9.3086-3093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Berlin O. G., Inderlied C. B. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 1987 Apr 27;82(4A):23–26. [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]
- Young L. S., Wiviott L., Wu M., Kolonoski P., Bolan R., Inderlied C. B. Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet. 1991 Nov 2;338(8775):1107–1109. doi: 10.1016/0140-6736(91)91965-w. [DOI] [PubMed] [Google Scholar]
- Zimmer B. L., DeYoung D. R., Roberts G. D. In vitro synergistic activity of ethambutol, isoniazid, kanamycin, rifampin, and streptomycin against Mycobacterium avium-intracellulare complex. Antimicrob Agents Chemother. 1982 Jul;22(1):148–150. doi: 10.1128/aac.22.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalla F., Maserati R., Scarpellini P., Marone P., Nicolin R., Caccamo F., Rigoli R. Clarithromycin-ciprofloxacin-amikacin for therapy of Mycobacterium avium-Mycobacterium intracellulare bacteremia in patients with AIDS. Antimicrob Agents Chemother. 1992 Jul;36(7):1567–1569. doi: 10.1128/aac.36.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamballerie X., Zandotti C., Vignoli C., Bollet C., de Micco P. A one-step microbial DNA extraction method using "Chelex 100" suitable for gene amplification. Res Microbiol. 1992 Oct;143(8):785–790. doi: 10.1016/0923-2508(92)90107-y. [DOI] [PubMed] [Google Scholar]
- du Moulin G. C., Sherman I. H., Hoaglin D. C., Stottmeier K. D. Mycobacterium avium complex, an emerging pathogen in Massachusetts. J Clin Microbiol. 1985 Jul;22(1):9–12. doi: 10.1128/jcm.22.1.9-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Moulin G. C., Stottmeier K. D., Pelletier P. A., Tsang A. Y., Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA. 1988 Sep 16;260(11):1599–1601. doi: 10.1001/jama.260.11.1599. [DOI] [PubMed] [Google Scholar]
- von Reyn C. F., Hennigan S., Niemczyk S., Jacobs N. J. Effect of delays in processing on the survival of Mycobacterium avium-M. intracellulare in the isolator blood culture system. J Clin Microbiol. 1991 Jun;29(6):1211–1214. doi: 10.1128/jcm.29.6.1211-1214.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]