Abstract
Objective
Hyperactivation of innate immunity by Toll-Like Receptors (TLR) can contribute to the development of autoinflammatory or autoimmune diseases. This study evaluated TAM receptor activation, physiological negative regulators of TLRs, by their agonists Growth arrest specific 6 (Gas6) and Protein S (Pros1) to prevent collagen-induced arthritis.
Methods
Adenoviruses overexpressing Gas6 and Pros1 were injected intravenously (i.v.) or intra-articularly (i.a.) into mice during collagen-induced arthritis. Splenic T-helper subsets of intravenously injected mice were studied by flow cytometry and knee joints of mice injected i.v. and i.a. were assessed histologically. Synovium of i.a injected mice was evaluated for cytokine and suppressor of cytokine signaling (SOCS) expression.
Results
Pros1 significantly reduced ankle joint swelling when overexpressed systemically. Further analysis of knee joints revealed a moderate reduction of joint pathology and a significant reduction of splenic T-helper 1 cells when Pros1 was overexpressed systemically. Local Gas6 overexpression decreased joint inflammation and joint pathology. Pros1 treatment showed a similar trend of protection. Consistently, Gas6 and Pros1 reduced cytokine production in synovium. Moreover, IL-12 and IL-23 mRNA levels were reduced by Gas6 and Pros1 with a corresponding decrease in IFNγ and IL-17 production. TAM ligand overexpression was associated with an increase in SOCS3, which likely contributed to the amelioration of arthritis
Conclusions
We provide the first evidence that TAM receptor stimulation by Gas6 and Pros1 can be used to ameliorate arthritis when applied systemically or locally. TAM receptor stimulation limits proinflammatory signaling and the adaptive immunity. This pathway provides a novel strategy to combat rheumatoid arthritis.
Rheumatoid arthritis is an auto-immune disease manifesting in articulating joints causing destruction of cartilage and bone. The cause of this disease is still unknown and treatment has focused on down regulating inflammation by blocking downstream signaling or neutralizing harmful cytokines. Although successful in the clinic, these therapies have substantial side effects and a high rate of non-responders among patients. Natural negative feedback mechanisms can potentially be used therapeutically to halt progression of the inflammatory process and initiate recovery. This approach could possibly limit side effects as the body’s own self-regulating responses are enhanced rather than uncontrolled and systemic blocking of cytokines, important in host defense.
One such controlling system of inflammation is that of the TAM receptors. Tyro3, Axl, and MerTK comprise a family of tyrosine kinase receptors and have been implicated in the negative regulation of inflammation. The regulatory role of TAM receptors in inflammation was found in triple knockout mice for the TAM receptors as these animals showed excessive lymphocyte proliferation and autoimmunity (1). Moreover, proinflammatory cytokine expression by macrophages is inhibited upon Gas6 treatment (2). Two ligands are described for the TAM receptor family, Gas6 and Pros1 (3). Both these ligands bind to phosphatidylserine on cell membranes and subsequently stimulate TAM receptors (4).
Gas6 has been shown to regulate Toll-Like Receptor (TLR) signaling in dendritic cells via activation of the Axl receptor (5). Stimulation of cells via the Axl receptor in conjunction with IFNAR leads to upregulation of suppressor of cytokine signaling (SOCS) proteins 1 and 3 (6;7), inhibitors of inflammation. SOCS1 blocks intracellular signaling e.g. NF-κB activation since SOCS1 can directly inhibit Mal, an adapter molecule for TLR2 and TLR4 (8). TLRs have also been implicated in maintaining the chronic inflammatory loop in RA synovium (9;10). and TLR2 and TLR4 play an important role in arthritis (11;12). SOCS3 also prevents binding of TRAF6 to TAK1, a key signaling molecule in e.g. TLR, IL-1 receptor and TNF receptor signaling (13;14). The protective role of SOCS proteins in experimental inflammatory mouse models has been shown by ectopic overexpression of SOCS3 in collagen-induced arthritis (15). This resulted in altered splenic T helper cell responses towards antigens and ameliorated arthritis.
Taking into account that inflammation can be resolved by SOCS3 in CIA, we set out to determine if overexpression of Gas6 or Pros1 can ameliorate experimental arthritis. Here, we report for the first time to our knowledge that TAM stimulation can ameliorate arthritis. Systemic overexpression of Pros1 decreases arthritis severity and is capable of reducing splenic Th1 cell numbers. Gas6 and Pros1 are both also capable of decreasing arthritis when overexpressed intra-articularly as joint pathology and synovial proinflammatory cytokine production were significantly reduced in the inflamed joint.
Material and Methods
Mice
Male DBA/1 mice aged 10–12 weeks (Janvier, Elavage, France) were housed in filter-top cages and fed a standard diet with freely available food and water. All in vivo studies complied with national legislation and were approved by local authorities for the care and use of animals with related codes of practice.
Cloning strategy
The constructs pCDNA6AmGas6 and pCDNA6AmProS were cloned with KpnI and XbaI in the pShuttle vector behind the cytomegalovirus promoter (CMV). The pShuttleCMVmGas6 and pShuttleCMVmProS were cloned into the E1 deleted region of the adeno-5 virus backbone pAdEasyI.
Construction of adenoviral vectors
Viral vectors were E1A,B and E3 deleted and were produced according to the method described by (16). The purified recombinant adenoviral vector DNA was transfected into N52E6 viral packaging cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Virus was purified using two CsCl gradient centrifugations and stored in small aliquots at −80°C. The viral titer of the purified viral vectors was determined in human embryonic retinoblastoma 911 indicator cells by immunohistochemical detection of viral capsid protein, 20 hours after transfection.
Induction of CIA
Induction of collagen-induced arthritis has been described before (17). Briefly, bovine type II collagen was dissolved in 0.05M acetic acid to a concentration of 2 mg/ml and was emulsified in equal volumes of Freund’s complete adjuvant (2mg/ml of Mycobacterium tuberculosis strain H37Ra) (Difco, Detroit, MI) Mice were immunized intradermally at the base of the tail with 100µl of emulsion (50µg of bovine type II collagen). Subsequently, mice were given an intra-peritoneal booster injection of 100µg of type II collagen dissolved in phosphate buffered saline (PBS) on day 21. One day after the booster injection, immunized mice were injected intravenously with 3x10E8 focus-forming units (FFU); for intra-articular injection into both knees with 1x10E7 FFU Ad5.Gas6 or Ad5.ProS or Ad5.Luciferase. Two independent observers monitored clinical signs of arthritis in paws and ankle joints, macroscopically. Cumulative scoring based on redness, swelling, and, in later stages, ankylosis was as follows: 0=no changes; 0.25=1–2 toes red or swollen; 0.5=3–5 toes red or swollen; 0.5= swollen ankle; 0.5=swollen footpad; 0.5=severe swelling and ankylosis (redness, excessive edema and deformation), with a maximal score of 2 per paw.
Histological analysis
Whole knee joints were dissected and fixed in phosphate buffered 4% paraformaldehyde followed by decalcification with 5% formic acid, and embedded in paraffin wax. Serial tissue sections (7µm) were stained with safranin O (BDH chemicals, Poole, UK) and counterstained with fast green (BHD Chemicals) or with hematoxylin / eosin (Merck, Germany) and eosin (Merck, Germany) (H&E). Serial sections were scored for histopathologic changes on a 0–3 scale, by 2 independent observers in a blinded manner. Joint inflammation was determined by the presence of synovial cell infiltrates and inflammatory cell exudates. Connective tissue destruction was determined by the depletion of cartilage proteoglycan (loss of safranin O staining of the non-calcified upper cartilage layer) and by cartilage and bone erosion.
RNA isolation and quantitative PCR analysis
Synovium and liver samples were disrupted using the MagNaLyser (Roche). Total RNA was extracted from the tissue homogenates and from cells using TRI reagent (Sigma) according to manufacturer’s protocol. Isolated RNA was treated with DNAse followed by reverse transcription of 1ug RNA into cDNA using Moloney murine leukemia virus reverse transcriptase 0.5µg/µl oligo(dT) primers, and 12.5mM dNTPSs (Invitrogen). Quantitative real-time PCR was performed using the StepOnePlus sequence detection system (Applied biosystems, Foster City, CA) PCR was performed in a total reaction volume of 12.5 µl consisting of appropriate cDNA. Five µM of forward and reverse primer and the sYBR green PCR master mix (Applied biosystems). PCR protocol consisted of 2 min at 50°C and 10 min of 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. Quantification of PCR signals was achieved by calculating the difference between the cycle threshold value (Ct) of the gene of interest with the Ct value of their reference gene glycerldehyde-3-phophate dehydrogenase (GAPDH) for each sample (delta Ct).
Immunohistochemistry
Local expression of SOCS3 was evaluated on paraffin sections of the knee joints. Sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked by 1% hydrogen peroxide for 15 minutes. Tissue sections were overnight incubated with anti-SOCS3 (Abbiotec, Emelca), followed by incubation with biotinylated goat α-rabbit. Peroxidase labeled streptavidine (Vectastain, Brunschwig) and diaminobenzidine were used for staining. Haematoxylin was used as counterstaining. Sections were arbitrarily scored on a scale from 0 to 3 on at least 2 sections per joint.
Asssessment of anti-collagen antibody titers
Concentrations of anti-bovine CII IgG1 and IgG2a antibodies were determined using ELISA. Briefly, 96-wells plates were coated with 0.1 µg of bovine type II collagen. Non-specific binding sites were blocked by a 5% solution of milk powder. Serial dilutions of mice sera were added followed by incubation with isotype-specific goat anti-mouse antibodies (peroxidase labeled) and 5-aminosalicylic acid as substrate. Absorbance was measured at 450 nm.
Flow cytometric analysis of splenic T-cells
Spleens from mice were mashed, filtered and erythrocytes were removed by osmotic shock. After washing CD3+ cells were isolated using the Pan T cell Isolation Kit (Miltenyi Biotec, Germany) according to manufacturer’s instructions. Purified CD3+ cells were stimulated in RPMI 1640 (Invitrogen) supplemented with 10% FCS penicillin/streptomycin and pyruvate with 50ng/ml PMA, 1µl/ml/10^6 cells Brefeldin A (BD Pharmingen) and 1µg/ml Ionomycin. After 4 hours of stimulation cells were stained with anti-mouse CD4-APC (1:200) (BD Pharmingen) or IgG2a-APC control (BD Pharmingen) for 30 minutes at 4°C. Subsequently, cells were stained intracellular with anti-IL17-FITC and anti-IFNγ-PE or with their controls respectively IgG1-PE and IgG1-FITC according to instructions by manufacturer (BD Pharmingen). Stained cells were analyzed using FACScalibur (Becton Dickinson) and analyzed with FlowJow software.
Fluorescent imaging
CIA mice received an i.v. injection with activatable ProSense 680 NIR-fluorescent probe (150 µl, 2nmol) (PerkinElmer, Bedford USA) at day 28 of CIA. Probe becomes activated upon enzymatic cleavage by cathepsins (B,K,L,S) present in arthritic joints. Cathepsin activity is a measure of inflammation and especially macrophage infiltration. At the time of imaging (24 hours post i.v. injection), mice were anesthetized with 2.5% isoflurane/oxygen, knee joints were shaved and placed on their back inside the light-tight chamber and imaged with the In Vivo Imaging System (IVIS) Lumina (Caliper Life Sciences), using the Cy5.5 filter. The collected data were analyzed using Living Image 3.0 (Caliper Life Sciences). Two-dimensional regions of interest (ROI) were drawn around the knee and ankle joints and fluorescent signal intensity was measured corrected for background and auto fluorescence signal.
Measurement serum levels IL-6 and KC
IL-6 and KC levels in serum were measured on a Luminex-100 System (Luminex corp.) using a magnetic bead-based multiplex immunoassay (Milliplex, Merck Millipore). Data analysis was performed with Bio-Plex Manager software (Bio-Rad Laboratories).
Statistics
All data is represented as mean ± SEM and analyzed with GraphPad 5.0 software. Statistical significance was determined by either 1-Way ANOVA or Two-Way ANOVA with Bonferroni post test, comparing Ad-Gas6 and Ad-Pros1 groups with Ad-Luciferase.
Results
Systemic overexpression of Gas6 and Pros1 moderately reduces arthritis
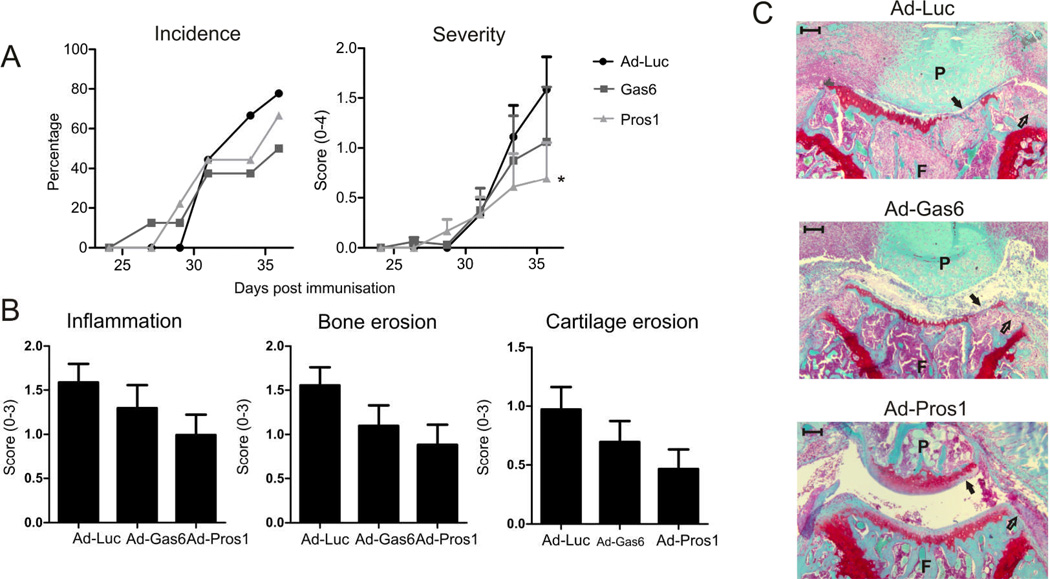
Adenoviruses expressing Luciferase, Gas6, or Pros1 were administered intravenously to mice immunized with bovine collagen type II. As shown in Figure 1 overexpression of either Gas6 or Pros1 did not affect arthritis incidence. However, arthritis severity was slightly reduced 36 days post immunization when Gas6 was overexpressed. Moreover, Pros1 treatment resulted in a significant decrease in arthritis severity. In addition to scoring the macroscopic swelling and redness of the joints, knee joints were isolated to enable detailed examination on the effects of TAM activation on cell influx, bone and cartilage. This revealed a trend in decreased inflammation, cartilage erosion, and bone erosion when Gas6 or Pros1 were overexpressed systemically (Figure 1B–C). These data point towards a protective role of TAM activation in experimental arthritis.
Figure 1.
Effect of systemically overexpressed Gas6 or Pros1 on arthritis. Mice with CIA intravenously injected with adenovirus encoding for either luciferase, Gas6, or Pros1 after immunization and arthritis development was monitored over time. (A) Arthritis incidence and severity of hind paws. Severity was determined macroscopically; mean of the cumulative score of ankle and wrist joints per animal. Statistics: two-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (* p<0.05). (B) Histological analysis of knee joints for inflammation (HE stained sections), bone and cartilage erosion (safranin O stained sections). Data represented as mean ± SEM, n=9 per group. (C) Representative safranin-O stained sections indicating cartilage erosion (closed arrows) and bone erosions (open arrows). Scale bar = 100 µm. P = patella, F = femur.
Systemically overexpressed Gas6 and Pros1 suppress the proinflammatory immune response
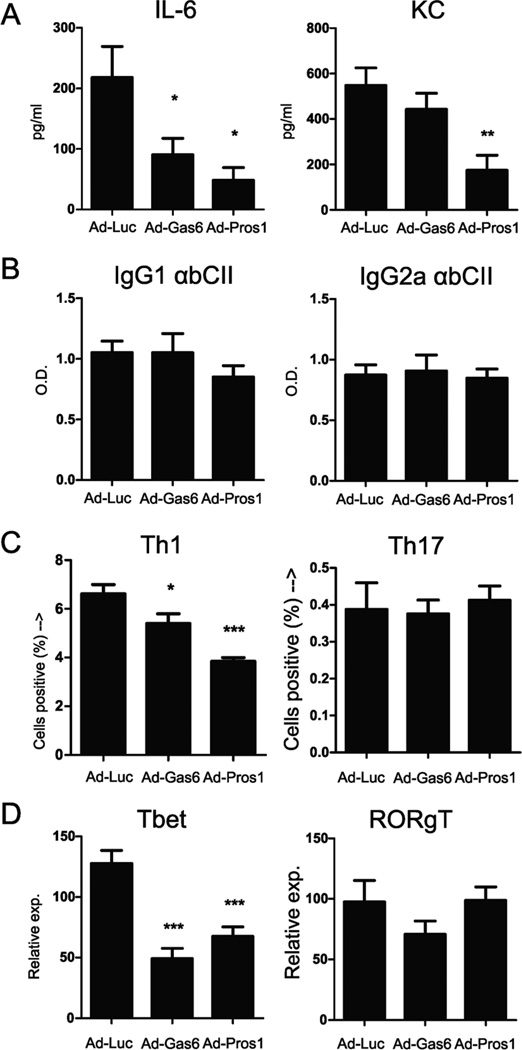
To study the effects on macrophage activity, serum was taken and evaluated for circulating cytokine levels. The TLR-inducible IL-6 and KC were detected in serum and Gas6 and Pros1 overexpression reduced circulating IL-6 levels in serum significantly by 59% and 78%, respectively. In addition, Pros1 caused a 68% decline in circulating KC levels (Figures 2A), potentially explaining a decrease in inflammatory cell influx into the inflamed joints. Moreover, serum IL-6 and KC levels significantly correlated with macroscopic arthritis scores (IL-6 correlation: R2 = 0.41, p value 0.001. KC correlation: R2 = 0.33, p value 0.004). This indicates that Gas6 and Pros1 decreased systemically produced cytokines during inflammatory conditions and possibly control antigen presenting cell (APC) activation and function. To study the effect of TAM ligand overexpression systemically on B-cells the antibody titers against bovine collagen type II were determined (Figure 2B). Both Gas6 and Pros1 did not have an effect on collagen type II specific IgG1 or IgG2a antibody titers. This suggests that TAM ligands did not influence B-cell function. To further analyze the effects of Gas6 and Pros1 overexpression on the adaptive immunity, splenic CD3+ cells were isolated and T cell differentiation was determined. TAM receptor stimulation significantly reduced Th1 levels, whereas Th17 levels were unaffected by either treatment (Figure 2C). In accordance, mRNA expression level of T-bet was decreased significantly, whereas RORγT expression was unchanged (Figure 2D). This indicates that TAM activation has a clear effect on T-cell immunity by diminishing the development of Th1 cells, resulting in a reduction of arthritis.
Figure 2.
Serum cytokines, antibody titers, and splenic Th1 and Th17 analysis. (A) Serum was harvested at time of sacrifice (day 36 of CIA) and serum levels of IL-6 and KC were measured by multiplex bead array. (B) Anti-bovine collagen type II antibodies in serum measured by ELISA. O.D. = Optical density. (C) Spleens from CIA mice were isolated at time of sacrifice (day 36 of CIA) and CD3+ cells were isolated by negative MACS selection. T-cells were subsequently stimulated with PMA and ionomycin for 4 hours and stained for CD4, IFNγ, and IL-17 and percentage of positive cells were determined by flowcytometry. (C) Percentages of splenic Th1 (CD4+/IFNγ+) and Th17 (CD4+/IL-17+) cells, gated on CD4+ cells. (D) mRNA expression analysis of transcription factors Tbet (Th1) and RORγT (Th17). Relative expression=2-dCt. Statistics: one-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (* p<0.05, ** p<0.01, *** p<0.001). Data represented as mean ± SEM, n=9 per group.
Local overexpression of TAM ligands decreases inflammation and joint pathology
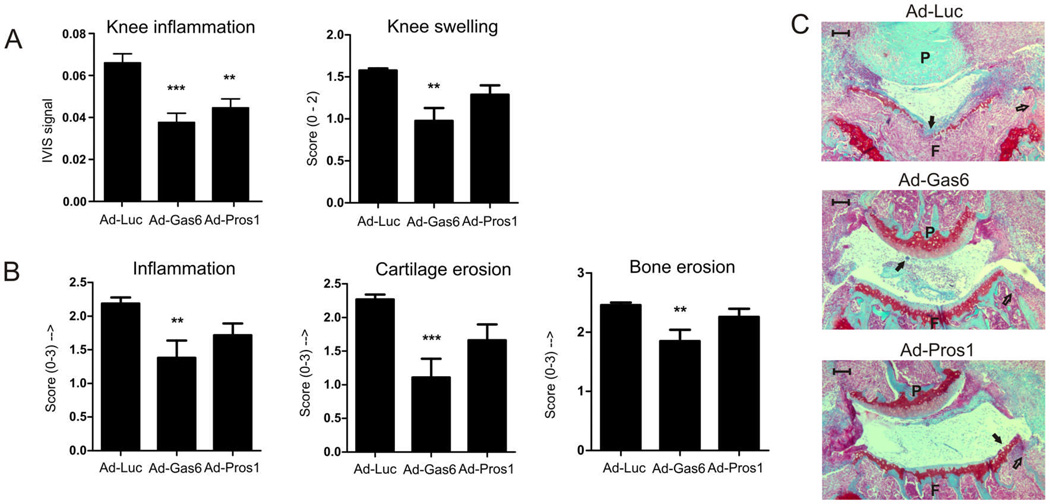
Gas6 and Pros1 show clear effects on Th1 development, but failed to ameliorate inflammation and joint pathology significantly. To study the effect of Gas6 and Pros1 directly at the inflammatory site, adenoviruses were injected intra-articularly in both knee joints before onset of CIA. During arthritis development the inflammation was measured with the ProSense probe at day 29 (Figure 3A), and TAM activation significantly reduced inflammation in the treated knee joints. Further analysis of inflammation, cartilage, and bone destruction revealed that TAM activation is beneficial for halting joint destruction (Figure 3B–C). Inflammation of the non-treated (ankle) joints was unaltered by either treatment (data not shown) indicating that TAM activation occurred only locally in the knee joint. This indicates that TAM activation directly at the site of inflammation can be applied to treat inflammatory diseases.
Figure 3.
Effects of local overexpressed Gas6 and Pros1 on joint inflammation and histology. CIA mice were injected intra-articularly into the left and right knee joint with adenovirus encoding for either Luciferase, Gas6, or Pros1 and arthritis development was monitored over time. (A) Knee inflammation on day 29 of CIA measured with a cathepsin inducible fluorescent probe (ProSense) and knee swelling at time of sacrifice (day 31 of CIA) determined macroscopically (scale 0–2). (B) Histological analysis of knee joints for inflammation (HE stained sections), bone and cartilage erosion (safranin O stained sections). Statistics: one-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (** p<0.01, *** p<0.001). Data represented as mean±SEM. ProSense measurement n=20 per group, knee swelling n=10 per group, histology n=14 per group. (C) Representative safranin-O stained sections indicating cartilage erosion (closed arrows) and bone erosions (open arrows). Scale bar = 100 µm. P = patella, F = femur.
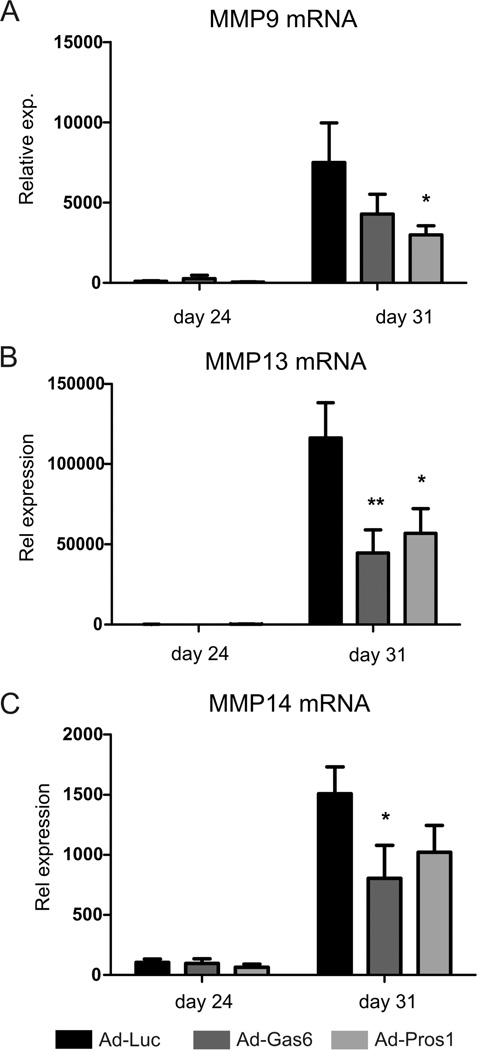
Messenger RNA expression analysis of synovium showed that both Gas6 and Pros1 mRNA were upregulated two days after virus injection (data not shown). Further analysis revealed that both Gas6 and Pros1 reduced matrix metalloproteinase (MMP) expression in synovium (Figure 4A–C). Gas6 and Pros1 significantly reduced MMP13 mRNA expression, whereas MMP14 and MMP9 expression were diminished significantly by overexpressing Gas6 or Pros1 respectively. Altogether, these data show that local TAM activation directly in inflamed joints decreases joint destruction by reduced MMP expression.
Figure 4.
MMP expression analysis in synovium of locally treated CIA mice. Matrix metallo-proteinase expression in synovium harvested at day 24 and day 31 of CIA. (A) MMP9, (B) MMP13, (C) MMP14. Statistics: two-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (* p<0.05, ** p<0.01). Data represented as mean ± SEM, n=4 on day 24 and n=6 on day 31.
Gas6 and Pros1 decrease cytokine production in synovium
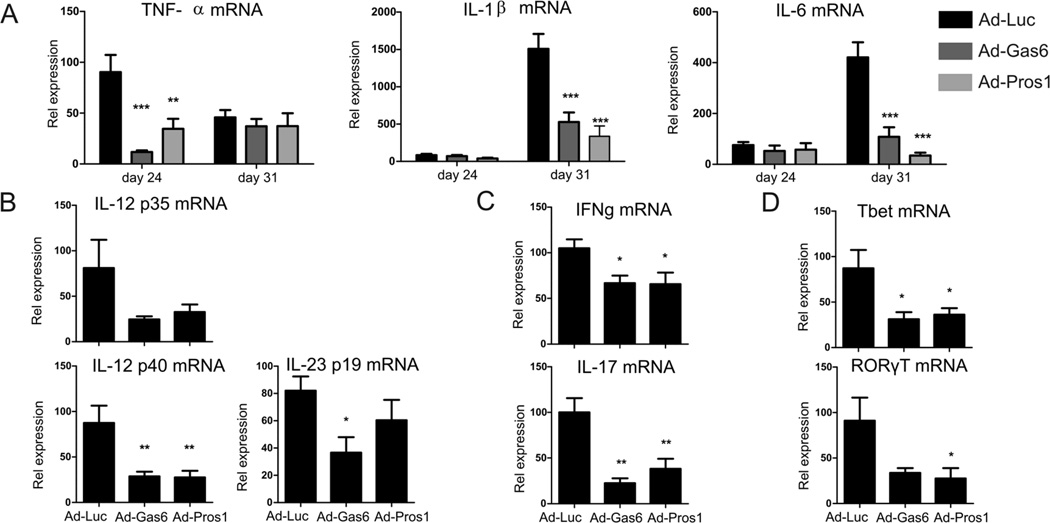
To study the effects of TAM activation on local cytokine production before clinical manifestation was observed, synovium was isolated at day 24 of CIA. Interestingly, TNFα production was detected before clinical manifestation and was significantly inhibited 87% and 62% by Pros1 and Gas6, respectively. IL-1β and IL-6 were only marginally produced on day 24, but were markedly induced when synovitis occurred (Figures 5A). Gas6 and Pros1 decreased IL-1β production at day 31 of CIA by the inflamed synovium by 65% and 78% respectively. In addition, IL-6 production returned to near basal expression levels by overexpression of Gas6 and Pros1 as IL-6 mRNA expression was significantly reduced by 74% and 92% respectively. The anti-inflammatory effects of Gas6 and Pros1 were also observed in production of T-cell activating cytokines IL-12 and IL-23. Figure 5B shows that overexpression of Gas6 and Pros1 caused a decline in IL-12 and IL-23 production in synovium resulting in reduced IFNγ and IL-17 levels in the synovium (Figure 5C). In addition, Figure 5D shows that T-cell transcription factors mRNA expression of T-bet and RORγT, responsible for Th1 and Th17 development respectively, was significantly reduced by Gas6 and Pros1. These cytokine expression profiles support the findings of reduced joint pathology, since IL-1 and IL-17 are key factors in cartilage and bone destruction. These data show that local TAM activation by Gas6 and Pros1 reduce proinflammatory cytokine production in inflamed synovium. This probably led to subsequently hampered T-cell activation and proliferation at the site of inflammation.
Figure 5.
Cytokine expression in synovium of locally treated CIA mice. Synovium was isolated two days after virus injection (day 24 of CIA) and at time of sacrifice (day 31 of CIA) and mRNA was isolated. (A) mRNA expression of TNFα, IL-1β, and IL-6 was determined by qPCR. Statistics: two-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (** p<0.01, *** p<0.001). Data represented as mean±SEM, n=4 on day 24 and n=6 on day 31. mRNA expression of cytokines (B-C) and transcription factors (D) at day 31 of CIA. Statistics: one-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (* p<0.05, ** p<0.01). Data represented as mean ± SEM, n=6 per group.
SOCS1 mediated anti-inflammatory effects of Gas6 and Pros1
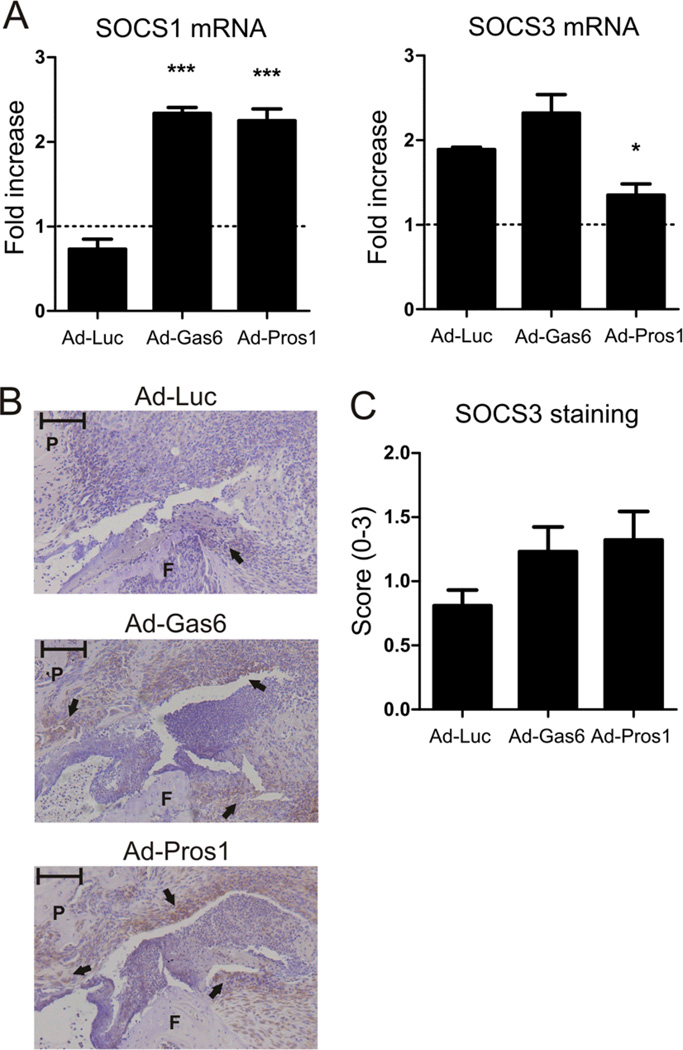
To unravel the inhibitory mechanism of TAM receptor stimulation, mRNA expression of SOCS1 and SOCS3 was evaluated (Figure 6A). SOCS1 mRNA expression was upregulated 2.3 fold in synovium of mice injected with Gas6 or Pros1 virus, whereas control animals showed a slight down regulation. In contrast, SOCS3 mRNA regulation was marginally affected by Gas6 overexpression and even slightly downregulated by Pros1 overexpression. Since this is in contrast with previous results (18), we determined SOCS3 protein levels by immunohistochemistry. Figure 6B shows representative images of the SOCS3 staining and a clear trend is observed in upregulation of SOCS3 protein by Gas6 and Pros1 (Figure 6C). This suggests that SOCS1 and SOCS3 mediate the anti-inflammatory effects of TAM activation by Gas6 and Pros1.
Figure 6.
SOCS proteins are up regulated by Gas6 and Pros1. mRNA was isolated from synovium isolated two days after virus injection (day 24 of CIA) and at time of sacrifice (day 31 of CIA). (A) Fold increase in mRNA expression of SOCS1 and SOCS3 on day 31 of CIA compared to day 24 of CIA. Statistics: one-way ANOVA with Bonferroni post test, compared to Ad-Luciferase (* p<0.05, *** p<0.001). Data represented as mean±SEM, n=4 on day 24 and n=6 on day 31. (B) Representative images of immunohistochemistry staining of SOCS3 in intraarticular injected knee joints. (closed arrows). Scale bar = 100 µm. P = patella, F = femur. (C) Arbitrary score of SOCS3 staining (scale 0-3). Data represented as mean±SEM, n=14 per group.
Discussion
A novel inhibitory pathway on TLR and cytokine signaling by TAM receptor activation has been exploited in this study to inhibit experimental arthritis. Here, we show that enhancing the negative feedback on inflammation by TAM receptor activation can be used to treat arthritis in a prophylactic setting. Systemic overexpression of Pros1 affected the T-cell immune response by decreasing Th1 and ameliorated experimental arthritis moderately. Intra-articular overexpression of Gas6 and Pros1 reduced proinflammatory cytokine production in synovium, which was likely to be mediated by SOCS1 and SOCS3. Gas6 also significantly decreased joint destruction when overexpressed in the inflamed joint. We show for the first time that TAM receptor activation by Gas6 and Pros1 in vivo ameliorates arthritis. This puts the TAM pathway forward as a new therapeutic pathway to be exploited to treat arthritis.
In our study Pros1 decreased splenic Th1 cells by 40% while leaving Th17 levels unaffected. This is in accordance with previous studies in Axl and MerTK double knockout animals. Naïve splenic CD4+ T cells from double knockout mice show a remarkable increase in IFNγ production when stimulated with anti-CD3 and anti-CD28 and no change in IL-17 production. In addition, immunized double knockout mice show increased Th1 development and normal Th17 levels in spleen and DLN (19). In animals that lack the MerTK receptor in the diabetes prone NOD background, a strong Th1 response was observed when β-cells underwent apoptosis (20). Combined with our data, it appears that TAM activation on APCs primarily affects Th1 response in vivo while not influencing Th17 response. Since circulating IL-6 levels were significantly decreased by Gas6 or Pros1 overexpression in our study an effect on Th17 could be expected. However, previous studies have shown that Gas6 can regulate TGF-β expression. Clauser et al. (21) showed that increased Gas6 secretion from carotid plaques correlates with increased TGF-β secretion. In addition, Gas6 knockout animals produce less TGF-β upon induction of liver damage (22). If Gas6, and perhaps Pros1, increase TGF-β levels this could compensate for the reduced IL-6 levels and leaving Th17 levels unaffected.
Gas6 and Pros1 appear to have differential effects depending on local or systemic overexpression. When overexpressed systemically, Pros1 seems slightly more efficacious than Gas6 and locally the reverse effect has been observed. But in fact, no significant differences between Gas6 and Pros1 were found on arthritis. The trends observed between Gas6 and Pros1 could be attributable to different target cells. Systemic overexpressed TAM ligands will affect systemic adaptive immunity by APC activity modulation in the spleen, which was also observed in our study. At the site of inflammation on the other hand, TAM ligands are expressed and secreted into the joint cavity affecting all the cells present, such as infiltrated macrophages, T-cells, and the synovial lining. Fibroblasts in the synovial lining are active contributors to the inflammation (23) and the effects of TAM ligands and TAM receptor expression on synovial fibroblasts is unknown and warrants further investigation.
The anti-inflammatory effects of TAM receptors has been reported to be mediated by SOCS1 and SOCS3 (24;25). Rothlin et al. found that stimulation of the Axl receptor in conjunction with the IFNARI lead to an upregulation of SOCS1 and SOCS3 in dendritic cells, which interfere with intracellular signaling and NF-κB activation. The effects of local Gas6 or Pros1 overexpression appear to be mediated via SOCS1 and SOCS3. Overexpression resulted in upregulation of SOCS1 expression during arthritis, whereas control animals showed a slight downregulation of SOCS1. The pivotal role of SOCS1 in controlling inflammation has been shown in macrophages from SOCS1 conditional knockout animals, in which TNF-α and IL-6 expression was down regulated upon LPS challenge (26). In our study we also observed a decrease in proinflammatory cytokine production in synovium by overexpressing Gas6 or Pros1 in the joint cavity. In contrast to SOCS1 up regulation, little regulation of SOCS3 mRNA by TAM receptor activation was found. However, immunohistological staining revealed a trend towards increased SOCS3 protein after Gas6 or Pros1 overexpression. SOCS3 mRNA levels are partly controlled by TNF-α (27) and Il-6 (28), of which we found significant differences at day 24 and day 31 of CIA respectively. Therefore, mRNA expression at time of sacrifice could deviate from protein levels. In addition, cytokine signaling has been suggested to prevent SOCS3 turnover (29). The increase in SOCS1 and SOCS3 are also in line with previous studies (30), showing the involvement of SOCS1 and SOCS3 in TAM mediated downregulation of inflammation. Taken together, a significant increase in SOCS1 mRNA in synovium and a clear trend in increased SOCS3 protein could partly account for the anti-inflammatory effects observed by Gas6 and Pros1.
Another possible mechanism by which Gas6 and Pros1 exert their anti-inflammatory effects is by inducing phagocytosis. Gas6 and Pros1 can opsonize apoptotic cells by binding to phosphatidylserine displayed on apoptotic cells. It has been shown before that joint inflammation can be reduced by prophylactic injection of apoptotic cells directly into the joint (31). Clearance of apoptotic leukocytes by lining macrophages decreases their chemotactic activity and thereby limits inflammation. MerTK is predominantly involved in phagocytosis (32) and plays a role in inflammation as well. It has been shown that MerTK downregulates TNF-α production upon LPS stimulation (33) and MerTK is also involved in LPS induced lung injury (34). Both Gas6 and Pros1 are ligands for the MerTK receptor and could therefore increase TAM signaling, via apoptotic cells or direct stimulation of the MerTK receptor on macrophages. However, the exact role of exogenous Gas6 and Pros1 in mediating phagocytosis of apoptotic cells to facilitate resolution of joint inflammation needs further investigation.
Axl and Gas6 have been implicated in maintaining the abnormal vasculature in RA (35) and thereby contributing to inflammation. Here, we show that the net effect of increasing TAM signaling is beneficial for experimental arthritis. TAM ligands could potentially induce SOCS1 and SOCS3 expression in human RA synovium and thereby decreasing inflammation. With decreasing inflammation also the process of angiogenesis will halt and TAM stimulation by Gas6 or Pros1 could potentially treat RA by controlling inflammation irrespective of its putative effect on angiogenesis.
In summary, we provide the first evidence that enhancing natural negative feedback on inflammation by TAM stimulation is efficacious to treat inflammatory arthritis. TAM receptors and their ligands Gas6 and Pros1 offer many possibilities and options to fine tune the negative feedback on inflammation to resolve auto-inflammatory and autoimmune diseases.
Acknowledgements
We would like to thank Richard Huijbens for performing the Luminex assay.
Financial support: Top Institute Pharma, project D1-101-0. VIDI grant (917.46.363) of the Netherlands Organization for Scientific Research. BTCure (grant agreement 115142-2), National Institutes of Health R01 AI089824 to C.V.R
Reference List
- 1.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 2.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87(5):869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 3.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 4.Uehara H, Shacter E. Auto-oxidation and oligomerization of protein S on the apoptotic cell surface is required for Mer tyrosine kinase-mediated phagocytosis of apoptotic cells. J Immunol. 2008;180(4):2522–2530. doi: 10.4049/jimmunol.180.4.2522. [DOI] [PubMed] [Google Scholar]
- 5.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Lee YJ, Han JY, Byun J, Park HJ, Park EM, Chong YH, et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-kappaB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc Biol. 2012;91(6):921–932. doi: 10.1189/jlb.0611289. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 9.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50(12):3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 10.Abdollahi-Roodsaz S, van de Loo FA, van den Berg WB. Trapped in a vicious loop, Toll-like receptors sustain the spontaneous cytokine production by rheumatoid synovium. Arthritis Res Ther. 2011;13(2):105. doi: 10.1186/ar3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56(9):2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118(1):205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279(52):54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen ML, Ronn SG, Bruun C, Larsen CM, Eizirik DL, Mandrup-Poulsen T, et al. IL-1beta-induced chemokine and Fas expression are inhibited by suppressor of cytokine signalling-3 in insulin-producing cells. Diabetologia. 2009;52(2):281–288. doi: 10.1007/s00125-008-1199-1. [DOI] [PubMed] [Google Scholar]
- 15.Veenbergen S, Bennink MB, de Hooge AS, Arntz OJ, Smeets RL, van den Berg WB, et al. Splenic suppressor of cytokine signaling 3 transgene expression affects T cell responses and prevents development of collagen-induced arthritis. Arthritis Rheum. 2008;58(12):3742–3752. doi: 10.1002/art.24072. [DOI] [PubMed] [Google Scholar]
- 16.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70(7):4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenbergen S, Bennink MB, de Hooge AS, Arntz OJ, Smeets RL, van den Berg WB, et al. Splenic suppressor of cytokine signaling 3 transgene expression affects T cell responses and prevents development of collagen-induced arthritis. Arthritis Rheum. 2008;58(12):3742–3752. doi: 10.1002/art.24072. [DOI] [PubMed] [Google Scholar]
- 18.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Ye F, Han L, Lu Q, Dong W, Chen Z, Shao H, et al. Retinal self-antigen induces a predominantly Th1 effector response in Axl and Mertk double-knockout mice. J Immunol. 2011;187(8):4178–4186. doi: 10.4049/jimmunol.1101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205(1):219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clauser S, Meilhac O, Bieche I, Raynal P, Bruneval P, Michel JB, et al. Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques. Thromb Haemost. 2012;107(1):140–149. doi: 10.1160/TH11-05-0368. [DOI] [PubMed] [Google Scholar]
- 22.Fourcot A, Couchie D, Chobert MN, Zafrani ES, Mavier P, Laperche Y, et al. Gas6 deficiency prevents liver inflammation, steatohepatitis, and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1043–G1053. doi: 10.1152/ajpgi.00311.2010. [DOI] [PubMed] [Google Scholar]
- 23.Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis, toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58(12):3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 24.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Han JY, Byun J, Park HJ, Park EM, Chong YH, et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-kappaB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc Biol. 2012;91(6):921–932. doi: 10.1189/jlb.0611289. [DOI] [PubMed] [Google Scholar]
- 26.Sachithanandan N, Graham KL, Galic S, Honeyman JE, Fynch SL, Hewitt KA, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60(8):2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlting C, Lai WS, Schaper F, Brenndorfer ED, Matthes RJ, Heinrich PC, et al. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178(5):2813–2826. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- 28.Yang XP, Schaper F, Teubner A, Lammert F, Heinrich PC, Matern S, et al. Interleukin-6 plays a crucial role in the hepatic expression of SOCS3 during acute inflammatory processes in vivo. J Hepatol. 2005;43(4):704–710. doi: 10.1016/j.jhep.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Babon JJ, McManus EJ, Yao S, DeSouza DP, Mielke LA, Sprigg NS, et al. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol Cell. 2006;22(2):205–216. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 31.van Lent PL, Licht R, Dijkman H, Holthuysen AE, Berden JH, van den Berg WB. Uptake of apoptotic leukocytes by synovial lining macrophages inhibits immune complex-mediated arthritis. J Leukoc Biol. 2001;70(5):708–714. [PubMed] [Google Scholar]
- 32.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 33.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162(6):3498–503. [PubMed] [Google Scholar]
- 34.Lee YJ, Han JY, Byun J, Park HJ, Park EM, Chong YH, et al. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-kappaB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. J Leukoc Biol. 2012;91(6):921–932. doi: 10.1189/jlb.0611289. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell K, Harkes IC, Dougherty L, Wicks IP. Expression of receptor tyrosine kinase Axl and its ligand Gas6 in rheumatoid arthritis, evidence for a novel endothelial cell survival pathway. Am J Pathol. 1999;154(4):1171–1180. doi: 10.1016/S0002-9440(10)65369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]