Abstract
Melanocyte stem cells differ greatly from melanoma stem cells; the former provide pigmented cells during normal tissue homeostasis and repair, while the latter play an active role in a lethal form of cancer. These two cell types share several features and can be studied by similar methods. Aspects held in common by both melanocyte stem cells and melanoma stem cells include their expression of shared biochemical markers, a system of similar molecular signals necessary for their maintenance, and a requirement for an ideal niche microenvironment for providing these factors. This review provides a perspective of both these cell types and discusses potential models of stem cell growth and propagation. Recent findings provide a strong foundation for the development of new therapeutics directed at isolating and manipulating melanocyte stem cells for tissue engineering or at targeting and eradicating melanoma specifically, while sparing non-tumor cells.
Keywords: melanocyte, stem cell, cancer stem cell, melanoma, cancer therapy
An introduction to melanocytes, melanocyte stem cells, and melanoma
Our bodies are constantly under threat from external hazards. Skin, the main protection against many such environmental insults, is composed of three layers: the epidermis, dermis, and subcutaneous tissue. The main specialized cell types of the outermost layer, the epidermis, are the keratinocyte, Langerhans cell, and melanocyte. To guard against hazardous ultraviolet radiation (UVR) from sunlight, the melanocyte contains a unique organelle, the melanosome, that produces the pigment, melanin, to provide photoprotection.
The melanocyte is an appealing model for studying cellular function and differentiation, because they work as a single-cell unit and have a distinctive differentiation product in the form of melanosomal organelles and melanin. Interest in melanocytes and skin pigmentation can be traced back many centuries to Asia, where fancy mice were bred for their different coat colors (1). For many of these mouse strains, it was eventually discovered that the variation in coat color was the result of differences in genes involved in the production of melanin and the function of the melanocytes. These studies provide a foundation for our yet incomplete understanding of melanocyte function.
Melanocyte stem cells have specific qualities that make them an excellent model for the study of stem cells generally. First, they can be relatively non-invasively harvested from the skin. Within the skin, melanocyte stem cells are most likely located within a specific anatomic niche, making them easy to find and isolate; specifically, in murine skin, melanocyte stem cells reside within the bulge region of the hair follicle (2). A second valuable trait of the melanocyte stem cells is that expansion and differentiation of daughter cells are closely coupled to the hair growth cycle (at least in murine systems). Quiescence or growth of melanocytes may be easily controlled through hair depilation. Lastly, dysfunction of this population is easily identified by the resulting defects in pigmentation. Improper maintenance of melanocyte stem cells, such as through increased apoptosis as a consequence of Bcl2 deficiency, causes stem cell loss and hair graying (3).
Several intrinsic qualities of melanocytes in general are useful for any studies or techniques that require manipulation of these cells. These traits include the relatively long life of melanocyte stem cells in comparison to other skin populations, such as keratinocytes. In addition, melanocytes work as a single-cell unit, and growth and differentiation are tightly controlled through signals from surrounding cells. In addition, melanocytes are derived from the neural crest, a very plastic embryonic tissue.
Due to their developmental origins, melanocyte stem cells’ multipotency and ability to migrate readily to new locations may be innate properties. The multipotent and highly prolific melanocyte stem cells provide an important stem cell source for regenerative medicine applications. Such cells could be utilized for gene therapy through ex vivo gene delivery and re-transplantation. These excellent qualities of the melanocyte stem cell, including its long lifespan, ability to be manipulated in vivo and ex vivo, plasticity regarding its differentiation choices, and capability to migrate in vivo, open the doors to great opportunities for stem cell therapy.
The same traits that make the melanocyte stem cell so promising for stem cell therapeutics may also help explain why melanomas (tumors derived from melanocytes) are often so aggressive and deadly. Melanoma tends to metastasize early in the disease process and is often fatal. The incidence of melanoma, unlike that of many other cancer types, has been rising steadily for over half a century (4).
Therapeutic choices for melanoma are limited, and most treatments fail to improve quality of life or survival in a meaningful way (4, 5). In the past year, the development of a small compound (PLX4032/RG7204) targeting a specific V600E point mutation found in B-Raf kinase has produced exciting results, with an amazing 77–81% response rate for patients whose melanomas harbor this particular mutation (6, 7). These studies demonstrate that malignant melanoma can respond very well to targeted therapy. Unfortunately, PLX4032 is unhelpful or even harmful to the approximately 50% of melanoma patients whose tumors lack the V600E B-Raf mutations, and nearly all responding patients develop drug resistance followed by rapid disease progression (8–12). Additional molecular targets need to be identified and tested to develop new therapeutics to fight this highly lethal tumor.
Because some melanoma cells may be more able to promote and drive tumor formation than others, it would be more advantageous to target those tumor cells therapeutically to block cancer progression. Several years ago, it was discovered (or, more accurately, rediscovered (13)) that a small subset of tumors contains cancer cells that have qualities like their tissue-specific stem cells (14). These experiments determined that a small sub-population of acute myeloid leukemia cells could re-establish tumors in severe combined immunodeficiency (SCID) mice, while the majority of the tumor cells could not. This study laid the foundation for the cancer stem cell hypothesis, which states that a sub-population of the tumor cells can self-renew, differentiate, and initiate tumor formation. While these putative cancer stem cells may have properties similar to the stem cell population of their tissue of origin, the tumor need not arise from the normal stem cell pool. If melanoma cancer stem cells indeed exist, new therapeutics may be designed to specifically target these cells.
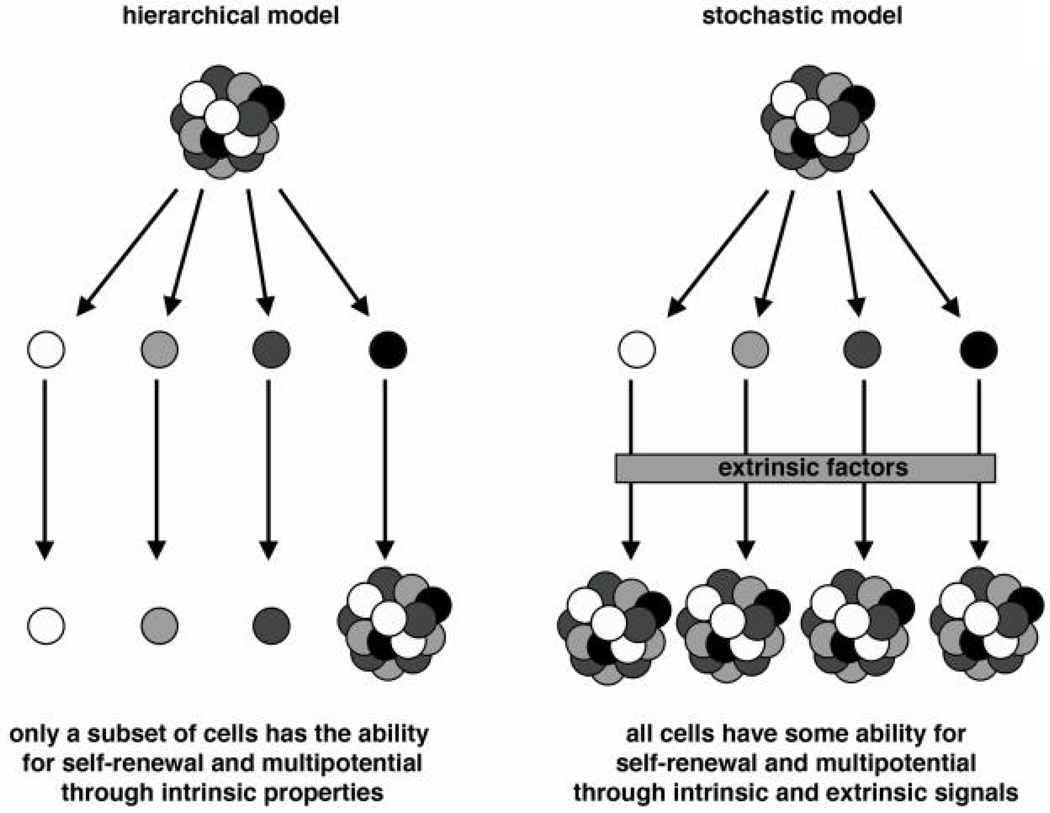
Many current chemotherapeutics target rapidly dividing cells, but cancer stem cells may be quiescent most of the time, thereby evading these treatments. While there is evidence that melanoma follows a cancer stem cell model for tumor development and a hierarchical model for tumor growth and progression, there is also support for a model wherein melanoma cells progress in a stochastic manner (Figure 1). Although there is still a debate on which model (or combination of models) melanoma follows, this point is crucial for designing new drug targets and therapeutics.
Figure 1. Models for stem cell growth and progression.
For normal tissues as well as tumors, cells may grow in an asymmetrical (hierarchical) or a symmetrical pattern as a method for providing progeny cells for tissue remodeling or tumor growth (17, 81). In the hierarchical model, there is a defined subset of cells that are able to self-renew and to give rise to daughter cells. This quality is unidirectional and if this subset of cells, or the stem cell pool, is depleted, the tissue will be unable to replenish itself. In the stochastic model, all the cells in theory have some ability to behave as a stem cell in terms of self-renewal and multipotential. Extrinsic factors such as extracellular proteins, growth factors, and other cell types regulate the cell in terms of decisions for maintenance, differentiation, or cellular death.
This review provides an overview of the rapidly progressing fields of melanocyte stem cells and melanoma stem cells. While these two cell types are not interchangeable, some of the lessons learned for one may provide insights into the other.
Melanocyte stem cells
Adult stem cell definitions and criteria for determining stem cells
There is strong evidence to support that adult tissues harbor adult stem cells and that they are similar to their embryonic stem cell precursors. Broadly defined, stem cells have two essential characteristics (15, 16). First, stem cells self-renew; as they divide, they maintain the stem cell population. Second, stem cells provide the specialized and differentiated daughter cells of their specific tissue type. While the defining traits of self-renewal and multipotency are common to all stem cells, the degree of plasticity and the markers expressed differ between adult and embryonic stem cells, as well as between adult stem cells of different tissue types.
The mechanisms by which adult stem cells renew themselves and give rise to daughter cells are not well characterized and may be distinctive for each tissue type. In addition, circumstances in which the stem cells undergo division, for example during development, in response to environmental stress or injury, or in the course of normal tissue turn-over and remodeling, may also affect how the stem cell yields daughter cells. For example, the division of the stem cell may be asymmetrical or symmetrical (17) (Figure 1). In asymmetrical (or hierarchical) stem cell division, the stem cell yields two daughter cells, one of which maintains the stem cell qualities of the parent cell, while the other differentiates in order to perform the tasks of the tissue. The cells’ fates may be influenced by internal cues, with or without signals from the environment. In symmetrical (or stochastic) stem cell division, both daughter cells have the chance to be either a stem cell or a differentiating cell; again, this process maybe regulated through the stem cell environment. It is believed that many stem cells reside within a specific microenvironment called a “stem cell niche.” This specialized location may contain various cell types, including multiple kinds of stem cells as well as supportive cells that provide scaffolding or signaling to the stem cells (18). Most stem cells probably can undergo both asymmetric and symmetric division, and the method selected may be dependent on cell type, environmental signals, and circumstances (such as during development or injury) (17).
In order to realize the promise of stem cell therapeutics, it will be important to be able to identify and characterize tissue specific stem -cells. Multiple methods exist for these purposes, each with its own strengths and limitations; these include measurement of quiescence, behavior of the cells in culture, the ability to transplant the cells and observe them behaving as stem cells, in vivo lineage tracing, and the use of specific markers and anatomical location of cells (19). Cellular quiescence is a trait often attributed to stem cells, although it is unrelated to the major defining stem cell characteristics of self-renewal and multipotency. In terms of quiescence, it is often linked with stem cells along with self-renewal and multipotency; indeed, many adult stem cells exist in an overall quiescent state (20–23). Methods for marking cells that are non-proliferative and long-lived include label uptake and retention, most often through the incorporation of DNA analogues such as bromo-deoxyuridine (BrdU) (21); however, some stem cell populations are reportedly long-lived yet highly proliferative (24, 25). Growth-arrested and terminally differentiated cells with relatively long life spans may also retain BrdU (26). For these reasons, although a relatively long lifespan and quiescence are often associated with stem cells, quiescence and label retention should not be used as sole criteria for identifying stem cells.
A second method for identifying and characterizing stem cells is the study of isolated stem cells ex vivo. The advantage of this method is that the cells may be observed and tested in a controlled environment. The multipotency of the cells, as well as their responses to external stimuli, may be directly determined in cell culture. Many stem cells depend on the microenvironment within their natural niche; loss of the niche microenvironment may alter their natural behavior. In addition, stem cells grown on a totally artificial matrix, such as plastic, may undergo permanent alterations in phenotype and behavior (27).
A third standard method is to transplant donor stem cells into recipient mice. The advantage of this method is that it assesses both self-renewal and multipotency; however, once again, it removes the stem cells from their natural environment and the cells may behave differently. For example, in the skin, epithelial stem cells that endogenously provide only one or two of the cell types of the main skin structures (epidermis, hair follicle, and sebaceous gland) can yield all three cell types after transplantation (28–31). Perhaps, stress or injury induced by the transplantation leads to a different response that is seen in normal homeostasis. Indeed, some findings from the transplant studies are similar to responses seen after full-thickness skin wounds (28–34).
A fourth method for identifying and characterizing stem cells is lineage tracing; this method often relies on transgenic mouse models to genetically label a cell population (35). An example of this is an inducible Cre recombinase enzyme expressed from a gene or a genetic promoter that is active in stem cells. The inducible Cre will be expressed in the stem cell, but will become active only after exposure to an inducing agent. An example of an inducible Cre system is the CreERT2 expression gene, where the Cre enzyme is fused with a modified estrogen receptor moiety (36). Another example is CrePR1e, where Cre is fused to a modified progesterone receptor (37, 38). The resulting CreERT2 or CrePR1e enzymes will be sequestered in the cytoplasm until exposed to exogenous tamoxifen or anti-progestin homologues, resulting in a change in conformation of the protein and permitting entry into the nucleus. In addition to the Cre allele, the mice also possess some type of marker gene that will become active and express reporter protein after modification with active Cre enzyme. This method will label cells when the Cre enzyme is active as well as their resulting progeny. A main advantage of this method is that it tests stem cells within their physiological niche. The putative stem cells may be labeled, persistence of marker may be recorded as a test of self-renewal or quiescence, and the daughter cells can also be traced to measure multipotency. Because the cells may be labeled with a marker, cells may be isolated for ex vivo cultures or for gene expression experiments. While this method is a powerful tool for stem cell biology, it is limited by the promoter or gene locus used for the transgenics. It is difficult to find genes expressed specifically in the stem cell: often these genes are also expressed in daughter cells or other cell types.
While the four assays mentioned above test the main criteria of self-renewal and/or multipotential, other methods utilize protein expression or microanatomical location; these include the expression of potential stem cell markers and the location of cells within a stem-cell niche. Cell expression markers are valuable tools for stem cell biology, although expressing a marker cannot be used as a sole criterion, because it does not demonstrate either self-renewal or multipotency. Identification of markers from cells shown to have stem cell characteristics provides a short cut for the identification, isolation, and labeling of stem cells; however, markers may be expressed transiently in the stem cell population; only during certain conditions such as tissue injury or remodeling,; or in daughter cells and differentiated cells as well.
Unique stem cell markers are rare and are often not linked to stem cell function. For this reason, markers should be used stringently and only after being thoroughly tested biologically for being co-expressed specifically in cells that follow the strict definitions of being a stem cell. In addition, since stem-cell niches have been identified for a number of stem cells, cells within these niches may be stem cell candidates. Frequently, a cell is identified as a stem cell since it is in a niche and expressing putative stem cell markers; however, several other supportive cells also are found in the niche, and therefore anatomic location cannot be a sole standard for determining a cell’s status as a stem cell.
Melanocyte stem cells
Melanocyte stem cells are a source of transient amplifying cells and differentiated melanocytes. Melanocyte stem cell biology is a newly developing field. Not much is known of how melanocyte stem cells function within their niche, how external signals regulate their ability to remain quiescent or to divide, or how plastic this population is. In addition, the microanatomic location of melanocyte stem cells in various organisms, such as the m ouse, fly, zebrafish, and humans, is only now being explored in detail (39, 40).
To date, melanocyte stem cells have been studied most extensively in the mouse; while significant findings also exist in other organisms, this review will mostly focus on murine systems. An advantage to studying the murine melanocyte is that growth and apoptosis are paired to the growth cycle of the hair follicle. The hair follicle is a skin appendage composed of epidermal keratinocytes and follicular cells, mesenchymal cells, as well as the pigment-producing melanocytes. The hair follicle undergoes cyclic expansion and regression in response to normal growth or as a stress response due to hair removal. The phases of this follicular cycle are anagen (follicular growth and expansion), catagen (regression of the hair follicle) and telogen (resting follicle) (41). The anatomy and timing of the phases, as well as the location and differentiation of the melanocytes within the back-hairs of the C57B6 mouse strain are well characterized (42–47). The fate of the melanocytes within the follicle is tightly linked to the phases of the hair follicle, with expansion of the melanocyte pool and differentiation during anagen, and reduction of melanocytes through apoptosis in catagen.
In a major advance for melanocyte stem cell biology, a transgenic mouse model has been utilized, (2)in which the expression of a beta-galactosidase reporter gene was driven by a segment of the dopachrome tautomerase (Dct) gene promoter (Dct-LacZ) (48). Dct is an enzyme with a role in melanin synthesis, and is an early marker of melanoblasts. Endogenous Dct and the Dct-LacZ transgene are expressed within the matrix of the hair bulb and in the bulge region of the hair follicle. The bulge region, first characterized histologically many years ago as a thickened area in the upper portion of the follicle, was found to harbor slow-growing and BrdU-retaining cells (21, 49, 50). In this study (2), the anatomic location of the melanocyte stem cell niche was confirmed to be in the bulge region, also defined as the lower permanent portion (LPP), of the hair follicle (51). The Dct-LacZ expressing cells within the LPP region have stem cell traits of self-renewal and multipotency. To deplete dividing and pigment-producing melanocytes, an antibody targeting CD117 (Kit receptor) was applied to neonatal mouse skin (52, 53). Loss of these cells in the neonates led to completely unpigmented coat hairs in the C57B6 mouse strain; however, quiescent unpigmented melanocyte precursors remained, and after the first hair cycle there was a partial recovery of pigmentation; the hair follicles showing repigmentation were those that retained expression of the Dct-LacZ transgene (2). In addition, the LPP of vibrissal hair follicles, containing Dct-LacZ expressing cells, were transplanted and shown to be able to provide pigmented progeny cells. These melanocyte stem cells were slow-cycling cells that retained BrdU. This label retention was co-expressed with Dct-LacZ expressing cells. Dct was expressed in these melanocyte precursor cells, but this gene was also expressed in transient amplifying and differentiated cells, so it is not an exclusive melanocyte stem cell marker. While these two other Dct expressing cell populations were located in the hair matrix, the Dct positive cells in the LPP were unpigmented and the data strongly support that they were melanocyte stem cells. This study demonstrated that the Dct-LacZ expressing cells of the LPP have stem cell characteristics of self-renewal and multipotency, either in situ or after transplantation, and that these cells are relatively quiescent unless there is a need for melanocyte stem cell expansion.
The melanocyte stem cell niche
A niche for melanocyte stem cells has been discovered in the LPP of the hair follicle (2). This microenvironment regulates the stem cells through direct contact with adjacent cells, via scaffold proteins of the extracellular matrix, and through secreted signaling proteins. The niche may maintain the stem cell population avoiding loss of the stem cell pools, as well as promoting quiescence in order to prevent overgrowth of cells. Supportive cells within the niche, as well as the stem cells themselves, may express adhesion and extracellular matrix molecules including integrins or cadherins to support cells within the tissue-specific stem cell niche (54). For some stem cell niches (mostly known in specific Drosophila niches (55)), the only scaffold for the stem cell niche is the basement membrane, and supporting cells are absent. For the limited number of mammalian niches discovered, however, there usually are some additional support cells and extracellular matrix proteins.
In addition to the melanocyte stem cells, the hair follicle LPP is also home to at least one other stem cell population, the epithelial stem cells (30, 32). This epithelial stem cell is a multipotent stem cell that can give rise to a number of cell types, including keratinocytes and follicular cells. Because these two stem cell populations reside anatomically in the same neighborhood, it is plausible that there would be at least some interaction between these cell types. While keratinocytes tightly regulate cutaneous melanocytes in their pigment production, growth, and apoptosis, it is logical that the keratinocyte precursors would have a similar role. Indeed, this is supported by recent findings involving cells of the follicular LPP niche. In an elegant study utilizing transgenic mice, cross talk between these two stem cell populations within the niche has been demonstrated (56). The investigators activated or blocked canonical Wnt signaling in either epithelial or melanocyte stem cells through expression of a constitutively active beta-catenin, or via Cre-silencing of the beta-catenin locus. Intrinsically, when beta-catenin activity was either increased or decreased within melanocyte stem cells, hair pigmentation was lost. Forced activation induced differentiation at the expense of melanocyte stem cell renewal, phenotypically parallel to prior studies in which stem cells were depleted (3). Loss of beta-catenin also led to hair graying, albeit by a different mechanism. The LPP melanocytes were not depleted; these cells were similar to controls, except that they did not yield daughter cells. This report, as well as earlier studies (57), support that canonical Wnt signaling and beta-catenin become activated in the melanocyte stem cells progeny as they begin to proliferate and differentiate. Stabilization of beta-catenin in the epithelial stem cells caused an extrinsic effect on the melanoycte stem cells. This led to an enlargement of the bulge region in general, and an increase in proliferation of the melanocyte pool; melanocyte expansion was mediated by the epithelial stem cells through an endothelin-dependent signaling mechanism. This supports that the initiation of melanocyte stem cell proliferation is dictated at least in part by signals directed by epithelial cells.
These results add to the growing body of evidence that Wnt signaling is important in regulating the melanocyte stem cell within its niche (58). Some of this work may be indirectly applied to the melanocyte stem cell, and is primarily focused on melanoblast development or the effects on melanocytes in general. For example, two Wnt factors, Wnt1 and 3a, are necessary for melanoblast development in the embryo (59, 60). Although these experiments are only indirectly related to the melanocyte stem cell, they suggest that there is a differential response to Wnt signaling by the melanocyte stem cell and the differentiating melanocyte, and that this can be modulated through overall levels of signals that activate or inhibit the Wnt pathway. The importance of repressing Wnt signaling for the melanocyte stem cell is supported by the presence of several Wnt inhibitors in the niche, including DKK3, Sfrp1, and Dab2 (30, 61). The melanocyte stem cells themselves also express several Wnt inhibitors themselves, such as DKK4, Sfrp1, Dab2 and Wif1 (51, 62). These experiments support a model in which inhibition of Wnt signaling promotes the melanocyte stem cell phenotype, whereas activation leads to melanocyte differentiation.
While an active canonical Wnt signaling pathway plays a role in proliferation and differentiation of the melanocyte stem cells within the niche, other factors, such as transforming growth factor-beta (TGF-beta), regulate melanocyte stem cell quiescence and maintenance of an undifferentiated state (63). TGF-beta is a signaling molecule that can regulate a number of cellular responses, such as cell growth and survival (64). In the hair follicle, TGF-beta proteins are expressed as the hair follicle regresses during catagen and promote apoptosis of the epithelial-derived components of the follicle (65, 66). In addition, the bulge cells of the hair follicle express nuclear phospho-Smad2 (indicating active TGF-beta signaling) (61). This exposure to TGF-beta results in cell-cycle arrest and immaturity in melanocytes, both in vitro and in vivo (63). Changes in TGF-beta expression are seen in normal physiological hair growth and follicular cycling as well as in response to UVR, and key transcription factors such as MITF and PAX3, as well as melanin-associated enzymes such as tyrosinase are down-regulated in response to TGF-beta (63, 67). TGF-beta signaling is a major mechanism for the maintenance of the melanocyte stem cell, as well as the production of pigment-producing melanocyte progeny.
Disruption of the Notch canonical signaling pathway also disrupts the ability of melanocyte stem cells to self-renew. There are four Notch receptors, which interact with specific ligands (Jagged 1 and 2, Delta-like 1,3,4) through cell-cell interactions (68). Once activated, the Notch intracellular domain (NID) is cleaved by gamma secretase. The now-freed NID can disassociate from the membrane and translocate into the nucleus. The NID interacts with the transcription factor called RBP-Jκ in mice (an ortholog to CBF1 in humans) and activates Notch down-stream genes. Inhibition of the Notch signaling cascade, through a gamma secretase inhibitor (GSI) mediated block of Notch cleavage, leads to hair graying. Unlike the use of Kit inhibitor antibodies, this graying is not reversible, suggesting a permanent effect on the melanocyte stem cell population (69). GSI treatment leads to apoptosis of the melanocytes and depletion of the melanocyte stem cell pool (69, 70). This effect is mediated through Notch receptors 1 and 2, but not 3 or 4. Notch1 and Notch2 compensate for each other; while deletion of one receptor in the melanocyte population leads to a partial pigmentation defect, the double Notch1/2 mutant has a fully gray coat (71). The phenotype of the Notch1/2 mutant is similar to that of mice with a melanocyte-targeted deletion of the RBP-Jκ gene.
Examination of the skin in both of these murine models demonstrated that Dct expressing cells in the LPP had been depleted. The phenotypes for the GSI treatment, Notch1/2 deletion, and RBP-Jκ were most likely due to the same pathway, since the addition of Notch intracellular protein in the Notch1/2 melanocyte-deleted mice rescued the graying phenotype and protected the melanocyte stem cell pool (72). This raises many interesting questions about how the Notch pathway is regulated in the stem cell niche, and what are the ligand-expressing cells that mediate the signal. Perhaps, again, the epithelial stem cells will be shown to have a role in Notch pathway regulated self-renewal melanocyte stem cells.
The epithelial stem cells within the follicular niche also regulate themselves and the melanocyte stem cells through the production of extracellular matrix protein. The epithelial stem cells highly express the hemidesmosomal transmembrane collagen called collagen XVII (also called COL17A1, BP180 or BPAG2) (73). This protein attaches to the underlying basement membrane, and deficiency of this protein leads to a reduction of cellular anchorage, hair graying and loss, and follicular atrophy (74–79). In Col17a1-deficient transgenic mice, the hair loses pigmentation prior to the occurrence of extensive hair loss (77). Collagen XVII provides the stem cell niche architecture within the LPP for normal maintenance of both the epithelial and melanocyte stem cells (73). Dysregulation of collagen XVII leads to premature differentiation of melanocytes within the niche, possibly due to direct loss of proper microenvironmental structure, and/or loss of signaling from the epithelial stem cells. This work provides insight into the architecture of the follicular stem cell niche, and may provide the foundation for creating artificial niches for growing stem cells ex vivo.
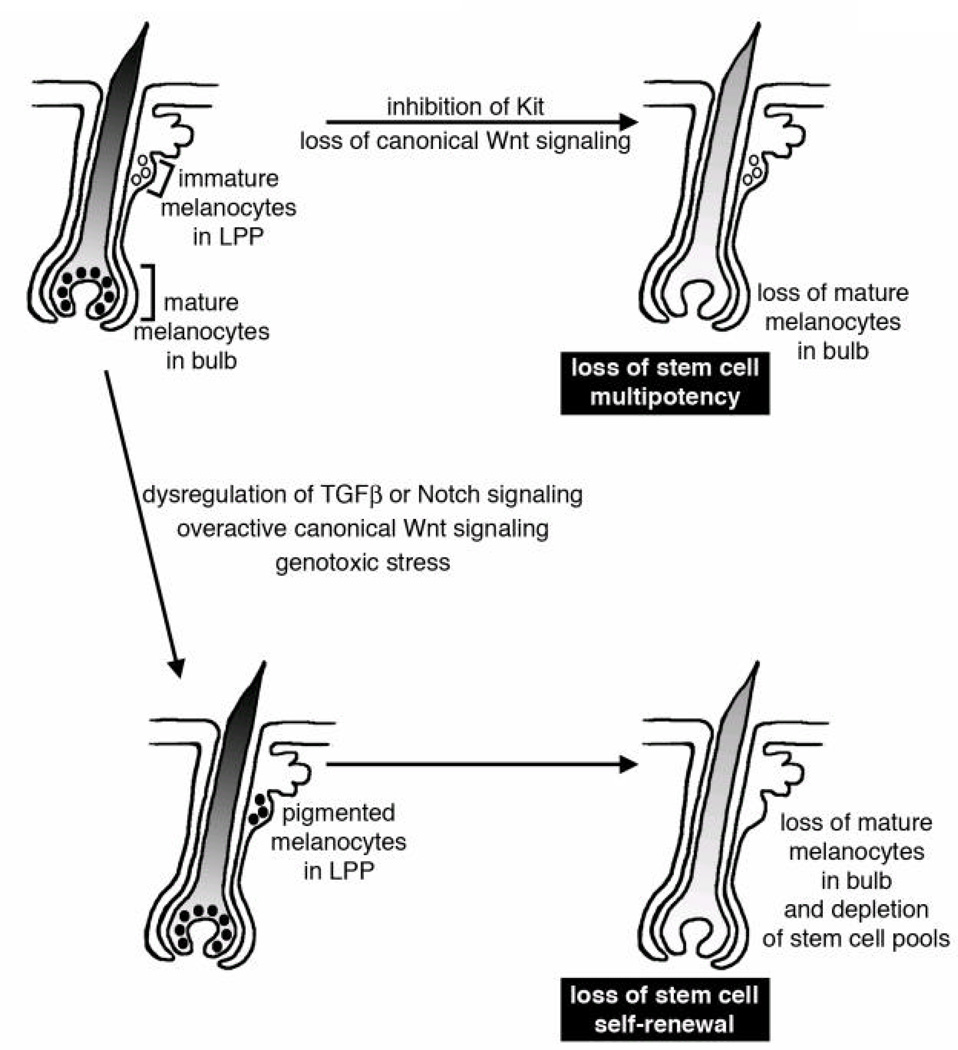
A picture of how melanocyte stem cells function and are maintained is starting to come into focus. They are sustained and regulated in a distinct location of the LPP of the hair follicle, and are influenced by other cell types such as the epithelial stem cells, extracellular matrix proteins, and a number of secreted factors that promote or restrict multipotency or self-renewal (Figure 2). Disruption of these regulatory signals lead to errors in the ability of the stem cell to maintain their stem cell pools or provide pigmented progeny.
Figure 2. Mechanisms for pigmentation loss and hair graying through extrinsic signals.
In a hair follicle (shown as a schematic for an anagen follicle) immature melanocytes are located in a stem cell niche within the bulge region of the hair follicle, or in a region defined as the lower permanent portion (LPP) (51). Loss of stem cell multipotency occurs when the stem cells fail to differentiate, either permanently or transiently. While immature melanocytes are still found in the LPP, there is a loss of pigmented melanocytes in the bulbar region of the hair follicle. Loss of stem cell multipotency may be caused by inhibiting Kit receptor signaling (52, 53) or through loss of canonical Wnt signaling (56). Loss of stem cell self-renewal occurs when the stem cells prematurely differentiate in the LPP and the stem cell pool is depleted. An error in self-renewal first leads to pigmented melanocytes in the LPP, followed by exhaustion of the stem cells and subsequently by a loss of differentiated melanocytes. A loss of melanocyte stem cell self-renewal may be induced by overactive canonical Wnt signaling (56), dysregulation of TGFβ or Notch signaling (63, 69–72), or through genotoxic stress (124).
Melanoma stem cells
Cancer stem cell definitions and models for tumor development and progression
To standardize the definition of the cancer stem cell, in 2006 the American Association of Cancer Research stated, "The cancer stem cell is a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor (80).” This statement defines cancer stem cells as having the essential properties of stem cells: self-renewal and the ability to give rise to progeny that can grow and differentiate. This definition implies that the cancer stem cells possess a hierarchical nature; however, it is not clear whether all tumors pursue a hierarchically organized pathway, in which the stem cell trait is stable, or rather follow a stochastic model, where all tumor cells are equivalent but their fates are governed by environmental factors (81)(Figure 1).
Tumors are heterogeneous, undergo uncontrolled proliferation and exhibit some self-renewal properties. Two major models, both of which incorporate versions of a cell with stem cell-like qualities, could explain how tumors grow and progress (17, 81). In the stochastic model, all the tumor cells are equivalent but their fates are governed by intrinsic signals as well as environmental factors. Not only do all the cancer cell progeny have the potential to behave like a cancer stem cell but they also retain plasticity to go from a non-stem cell to a stem cell-like precursor. In the hierarchical model, the cancer stem cells are biologically distinct, can renew themselves, and give rise to various progeny cells that lack the ability to self-renew. In this model, the cancer stem cell phenotype is a stable characteristic. The hierarchical model is often considered the “cancer stem cell model” for sustained tumor propagation, whereas the stochastic model does not posit a separate and specific stem cell population but rather assumes the existence of many cells that may act as cancer stem cells. The stochastic model is not considered to include cancer stem cells as narrowly defined, because it lacks some characteristics that are also linked to cancer stem cells, namely rarity and quiescence or dormancy. The latter feature allows cancer stem cells to resist standard cancer therapies, leading to clinical recurrence of tumors after an initial response to treatment regimens; however, under the broad definitions provided by the AACR workshop, none of these attributes define the cancer stem cell. While the stochastic model of tumor propagation may not be considered to include cancer stem cells in a narrow sense, alternatively, it may be argued that all the cells of the tumor according to this model have the ability to act as a cancer stem cell.
At present, methods for testing and determining cancer stem cells are limited. The assay utilized most widely is to xenotransplant the cells into an immunodeficient mouse model. As with the methods described for stem cell biology, this method will reveal whether the transplanted cells possess the ability both to self-renew and to generate progeny in vivo. Again, the major drawback to this method is that it removes the cells from their natural environment. This method is often further complicated by the use of various murine strains and multiple techniques for isolating the cell population to be tested and transplanted. At present, there is little direct evidence of a separate stem cell pool within unmanipulated solid tumors.
Melanoma cancer stem cell markers and evidence for the hierarchical model of tumor growth
A major focus of melanoma stem cell research is the identification of markers for the cancer stem cell population. Note that markers should not be used as a sole criterion for identifying cancer stem cells. Many of the markers utilized in cancer stem cell research have the advantage of being cell-surface proteins that allow cells to be separated by fluorescence-activated cell sorting, and these factors may not have any role in cancer stem cell biology. An additional complication is that expression of these markers may not be stable, and cells that are either positive or negative for a particular marker may yield both positive and negative daughter cells (82–84). The markers may not be unique to the cancer stem cells, being expressed in other cell types as well. Despite these caveats, some important insights have been obtained through the use of these markers and the identification of new potential markers may provide future therapeutic targets.
The first characterization of potential cancer stem cells in a solid tumor was accomplished in breast cancer, and these cells expressed CD44 but were CD24-negative (85). This finding was followed by similar reports in other solid cancers, such as brain and colon cancer (86–89). All these studies utilized a xenograft transplantation mouse model for testing their hypotheses, and they demonstrated that the tumors contained a rare subpopulation that was hierarchically organized. The resulting tumors after transplantation resembled the original tumors both morphologically and in heterogeneity.
CD20, a cell surface marker normally found on B-cells, has been associated with melanoma cells having stem cell characteristics (90). The investigators isolated cell populations from established cell lines that grew in non-adherent spheroids in stem cell culture medium. The cells derived from these spheroids were multipotent and able to differentiate into multiple neural crest-like progenitor cells, and also were self-renewing both in culture and in xenograft transplants in SCID mice. The spheroid cells were enriched with cells that expressed CD20 and demonstrated a much higher degree of multipotency in comparison to the CD20-negative cell population. In patient samples, CD20 expression was found in a very limited population (less than 2% of the tumor) in 4/5 primary tumor tissues (91). T-cells, genetically engineered to recognize and have specificity for CD20, were transplanted into immunodeficient NIHIII mice harboring these CD20+ melanoma tumors (91). The T-cells were able to eradicate the tumors in the mouse tumor model, although the CD20 antigen was present on the surface of only a small minority of tumor cells. In addition, the use of the anti-CD20 antibody rituximab produced a lasting remission in a patient whose melanoma had failed to respond to immunotherapeutics and dacarbazine treatments (92). Rituximab produced complete regression of all but one of the melanoma metastases, and the remaining tumor showed a partial and stable regression. Rituximab has also been utilized in a small study in nine patients without evident residual disease after standard therapies but are high risk for melanoma recurrence; 5/9 of the patients were tumor-free for over 42 months (93). CD20 needs more investigation as a putative cancer stem cell marker in melanoma and as a potential therapeutic target. However, these recent studies are promising, and may provide a paradigm shift in cancer therapeutics, in which targeting only a small minority population within the tumor could be effective in reducing the entire tumor mass.
CD133 (Prominin-1), a cell surface protein of unknown function first found on the surface of immature hematopoietic and neural stem cells (94, 95), has also been linked to cancer stem cells in a wide variety of different tumor types including melanoma (88, 89, 96, 97). CD133-expressing cells from primary melanoma tumor tissue had enhanced ability to form tumors in NOD/SCID mice (98). In addition, an established melanoma cell line (WM115), with the ability to form multiple cellular phenotypes, highly expressed CD133 and was able to form spheroids in stem-cell medium. More rigorous tests linking CD133 expression to self-renewal and multipotency need to be performed in melanoma. In addition, other studies have found that both CD133-expressing and non-expressing tumor cells can initiate tumors (83); however, CD133 expression has been linked to an increased disease progression, while inhibition of CD133 leads to a decrease in tumor cell growth and metastatic progress, suggesting some role in melanoma advancement (99, 100).
Members of the ATP-binding cassette (ABC) transporter family of proteins have also been reported to be expressed in melanoma stem cells. These transmembrane proteins hydrolyze ATP to perform functions such as the transportation of various substrates (101). Four of the ABC transporter proteins have known roles in multidrug resistance to cancer therapeutics, and two of these, ABCG2 and ABCB5, have been identified in potential melanoma stem cells. ABCG2 (also known as breast cancer resistance protein, BCRP) provides a mechanism of resistance against a wide variety of drugs, including the tyrosine kinase inhibitors imatinib and gefitinib, antibiotics, and HMG-CoA inhibitors (102). While ABCG2 is expressed in a number of epithelial tissues, it has not been detected in cutaneous melanocytes or melanoma cells in primary tissues (103). In WM115 cells, ABCG2 is co-expressed with CD133, although both are down-regulated in cell spheroids (98). At present, the data are too preliminary to strongly link ABCG2 to melanoma stem cells.
A 2008 report provides strong support for ABCB5 expression in a melanoma stem cell population (104). ABCB5 shares similarity with other B family ABC transporter proteins ABCB1 (Multi-Drug Resistant protein 1, MDR1) and ABCB4 (105). ABCB5 is co-expressed in an overlapping but not identical pattern with CD133 in G3361 melanoma cells and primary melanoma tissue samples, and in a subpopulation of CD271/VEGFR-expressing cells (106, 107). ABCB5 also mediates doxorubicin chemoresistance in melanoma cell lines (106). In addition, ABCB5 expression was found in melanoma cells that had qualities of cancer stem cells (104).
Here, both melanoma stem cell qualities of self-renewal and multipotency were demonstrated through serial dilutions in an NOD/SCID immunodeficient mouse xenotransplant model and in vivo lineage tracking. Primary melanoma tissues from patients were sorted for expression of ABCB5; only those cells that expressed this factor were able to yield both ABCB5-positive and negative cells, and regenerated tumors with a similarly heterogeneous phenotype after multiple rounds of serial dilutions. For the lineage tracing studies, the melanoma cell line G3361 was stably transfected with constructs expressing either DsRed or yellow-green EYFP proteins. Each cell line was sorted, and ABCB5-positive cells were isolated from G3361-DsRed cells, and ABCB5-negative cells were isolated from G3361-EYFP cells. The yellow-green cells demonstrated weak growth in Balb/c nude mice and gave rise only to ABCB5-non-expressing daughter cells, while the DsRed cells were able to generate new tumors and cells that were either ABCB5-positive or negative. In addition, the stem cell qualities of the ABCB5-expressing population were suppressed if the expression of ABCB5 was directly inhibited with a specific monoclonal antibody.
Despite these strong findings, other groups havefound tumor initiation to be ABCB5-independent, and could generate melanomas with the use of ABCB5 non-expressing cells (82). Clinical data support a role for ABCB5, in that expression of ABCB5 is linked to disease progression. Expression of ABCB5 is generally low in pigmented melanocytic nevus tissue, but is found in a subpopulation of thick primary melanomas and metastases (104, 108, 109). Furthermore, induction of terminal differentiation is correlated with decreasing levels of ABCB5 (110). An intriguing potential mechanism for melanoma promotion by ABCB5 has been proposed through an immune-invasive capacity of the ABCB5-expressing population through suppression of T-cell activation (111). The ABCB5-positive population displayed lower levels of MHC class I receptors and melanoma-associated antigens as well as co-stimulatory molecules B7.2 and PD-1 in comparison to the ABCB5-non-expressing melanoma cells. This finding may prove to be beneficial in melanoma therapeutics, including ongoing clinical trials targeting the PD-1 receptor (112).
CD271 expression has also been described in melanoma stem cells. CD271, also known as low-affinity nerve growth factor receptor (NGFR) or p75NTR, has been identified as a marker of neural crest cells (113). CD271 expression has been linked to perineural invasion and migration in melanomas (114). A subpopulation of primary melanomas may be expressed as CD271, comprising approximately 2.5% to 41% of the total tumor cell population (115). The CD271-expressing population was able to establish tumors from 70% of the transplanted cells in a Rag−/−γc−/− mouse model, which is deficient in T-, B-, and Natural Killer (NK) cells. In comparison, CD271-non-expressing tumor cells were able to establish tumors from only approximately 7% of cells transplanted into this highly tolerant mouse model. In addition, the ability of the CD271-positive population to act as a cancer stem cell was tested in a model more physiologically relevant to human disease, where melanoma cells were seeded in normal human skin or bone tissue that was then grafted on both the Rag−/−γc−/ and NOD/LTSscid/IL2Rγnull immunodeficient mouse models. The resultant tumors regenerated the heterogeneity of the parental tumors.
Other investigators have found a similar phenomenon when CD271-positive cells were transplanted into NOD/SCID mice (116). Alternatively, other studies discovered that both CD271+ and CD271- melanoma cells were equally competent to produce tumors after transplantation into NOD/LTSscid/IL2Rγnull mice (82, 116). In addition, the expression of CD271 is negatively correlated with the ability of mouse melanoma cells to form tumors in Nu/Nu mice (117).
Evidence that melanoma cells follow a stochastic model for tumor propagation
While there is ample evidence for the hierarchical model for tumor propagation in melanoma, there is also strong support for a stochastic pattern in melanoma or, perhaps stated more eloquently, a model whereby melanoma is dynamically regulated (84). Two seminal publications demonstrate that the ability to self-renew and provide new cells to the population is a common trait in melanoma cells (82, 83). The investigators demonstrated that more than over 25% of the cells from the tumors were able to generate new tumors in xenotransplantation assays (83). The cells could generate tumors whether or not they expressed various putative melanoma stem cell markers, including CD133/Prominin-1, ABCB5, and CD271/NGFR (82, 83).
Additional studies provide further evidence that melanomas are dynamically regulated. The histone demethylase JARID1B is expressed in a melanoma cell subpopulation that promotes continuous tumor growth, self-renewal, and multipotency, but unlike other markers its expression does not follow a hierarchical model of cancer stem cells (84). JARID1B is a member of the jumonji/ARID1 histone 3 K4 (H3K4) demethylases, and through its enzymatic function it can reawaken previously silenced genes. JARID1B is expressed at low levels or not expressed in many normal tissues, but is abundant in regenerative tissues and in melanocytic nevi (118). In melanoma, however, JARID1B-positive cells constitute only a small subset (5–10%) of the tumor cells (118). Cells expressing JARID1B exhibited self-renewal and multipotency in vitro (84).
When the cells were sorted for JARID1B expression and xenotransplanted in NOD/LTSscid/IL2Rγnull mice, there was no difference between the JARID1B+ and JARID1B− groups in the ability to regenerate tumors. The expression of JARID1B was able to arise from populations either that expressed this factor or did not. The connection between JARID1B and cancer stem cells is illustrated in experiments where the expression was inhibited; while there was an initial increase in cellular growth after blocking JARID1B expression, there was an eventual exhaustion of the population both in vitro and in vivo. While expression of JARID1B does not follow a hierarchical model for cancer stem cells, its expression is needed for self-renewal and the subsequent ability to generate daughter cells. These studies support that JARID1B is a cancer stem cell associated gene.
Melanoma – hierarchical or stochastic tumor propagation?
The studies presented here offer, at least on the surface, contradictory evidence to support either the hierarchical or stochastic model for melanoma propagation. Because these reports demonstrate high standards of scientific methodology, the findings are hard to dismiss as misinterpreted or incorrect. One likely explanation for these differences is the variety of methods used to determine the ability of cells to self-renew and propagate. One such variable is the choice of mouse strain for in vivo transplantation studies (Table 1). The use of xenotransplantation is presently the gold standard for determining cancer stem cells, but the natural environment for the tumors is not maintained and there are variances in the levels of immune cells present. While all of the studies incorporate immunocompromised mouse models, different strains were utilized. In addition, many of the studies utilized severe combined immunodeficient (SCID) mice, or mice with both a non-obese diabetic (NOD) and SCID background, the studies demonstrating a stochastic tumor propagation utilized even more profoundly immunodeficient NOD/LTSscid/IL2Rγnull mice. These mice lack natural killer cell activity through the deletion of IL2Rγ in addition to their NOD/SCID background (119). In addition, some of the studies utilized Matrigel to aid in the transplantation, a substance that may also promote the establishment and growth of tumors (120). There were subtle but significant differences in methods for purifying cells, in terms of enzymes used (for example, trypsin and collagenase), concentrations of enzymes, and the duration of exposure of the cells to these enzymes.
Table 1.
Studies supporting stable markers in melanoma stem cells.
| SCID or NOD/SCID (mice with severe impairment of T- and B- cells) |
NOD/LTSscid/IL2Rγnull (mice with severe impairment of T- and B-cells and an absence of NK-cells) |
Rag−/−γc−/ (mice with an absence of T-, B-, and NK- cells) |
“Humanized” mouse models |
|
|---|---|---|---|---|
| CD20 | Yes (90) | NT | NT | NT |
| CD133/Prominin-1 | Yes (98) | No (83) | NT | NT |
| ABCG2 | NT | NT | NT | NT |
| ABCB5 | Yes (104) | No (82) | NT | NT |
| CD271/NGFR/p75NTR | Yes (116) | No (82, 116) | Yes (115) | Yes (115) |
| JARID1B | NT | No (84) | NT | NT |
NT=not tested
It may be argued that the perceived ability of some of the cells to function as cancer stem cells may simply reflect the capability of the cells to survive the isolation methods (although some of the studies controlled against this possibility). In addition, the differences may reflect how tolerant and fertile the environment is for the transplanted cells. The main question remains, what should be the criteria for defining a cancer stem cell? If the test is purely the ability of the cells to act as a tumor-initiating cell, then the use of the most tolerant of environments is most efficient. If, on the other hand, the test is to measure the ability of the cells to self-renew and efficiently propagate progeny cells in a more native environment for tumors, then the model systems utilized are limited.
Melanoma often engages the immune system, and it develops in an environment containing stromal and immune cells (121). Accordingly, the present model systems for xenotransplantation have several shortcomings. New models need to be developed, employing techniques presently utilized for melanocyte stem cell research. Fortunately, several transgenic models for melanoma already exist, including many that resemble human disease in their progression and metastasis (122, 123).
Conclusions
Our understanding of how melanomas arise and progress has advanced greatly, and with this knowledge new therapeutic strategies are being developed. These findings have also raised many new questions, and a more complicated picture is emerging. As more insight is gained into how melanoma cells grow and thrive, it is hoped that new drugs will be created to target the cells that promote the tumor, and spare normal tissues, thereby efficiently targeting the tumor without the bystander side-effects that often complicate present-day drug options.
Acknowledgements
The authors are grateful to our colleagues whose works we cite here, and we apologize for the omission of other relevant works due to space restrictions. Supported by The University of Chicago Department of Medicine/Section of Dermatology, University of Chicago Cancer Center Pilot program (P30 CA014599), Friends of Dermatology-University of Chicago, the American Cancer Society (RSG CSM 121505), and the National Institutes of Health (R01CA130202).
Financial support: This work was supported by the University of Chicago, Pritzker School of Medicine, Department of Medicine/Section of Dermatology, University of Chicago Cancer Center Pilot program (P30 CA014599), Friends of Dermatology-University of Chicago, the American Cancer Society (RSG CSM 121505), and the National Institutes of Health (R01CA130202).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare that they have no conflict of interest.
Reerences
- 1.Silvers WK. The coat colors of mice. New York: Springer-Verlag; 1972. [Google Scholar]
- 2.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002 Apr 25;416(6883):854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005 Feb 4;307(5710):720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000 Jan;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 6.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010 Sep 30;467(7315):596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010 Apr;23(2):190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010 Dec 16;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 Mar 18;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010 Dec 14;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011 Mar;17(3):313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 14.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994 Feb 17;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 15.Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989 Aug;106(4):619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 16.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 17.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006 Jun 29;441(7097):1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010 Jan 29;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. 2011 Feb;12(2):113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004 Jul 23;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990 Jun 29;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 22.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997 Aug;78(4):219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008 Dec 12;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010 Jan 8;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007 Oct 25;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 26.Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007 Sep 13;449(7159):238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010 Aug 27;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008 Nov;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 29.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009 May 8;4(5):427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004 Apr;22(4):411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 31.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010 Mar 12;327(5971):1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 32.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004 Sep 3;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007 May 17;447(7142):316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 34.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. Faseb J. 2007 May;21(7):1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 35.Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012 Jan 20;148(1–2):33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997 Aug 28;237(3):752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 37.Chen MR, Liu SW, Wu TC, Kao VY, Yu HC, Chen FH, et al. RU486-inducible recombination in the salivary glands of lactoferrin promoter-driven green fluorescent Cre transgenic mice. Genesis. 2010 Oct 1;48(10):585–595. doi: 10.1002/dvg.20666. [DOI] [PubMed] [Google Scholar]
- 38.Tsujita M, Mori H, Watanabe M, Suzuki M, Miyazaki J, Mishina M. Cerebellar granule cell-specific and inducible expression of Cre recombinase in the mouse. J Neurosci. 1999 Dec 1;19(23):10318–10323. doi: 10.1523/JNEUROSCI.19-23-10318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011 Jun;24(3):401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 40.Tu S, Johnson SL. Clonal analyses reveal roles of organ founding stem cells, melanocyte stem cells and melanoblasts in establishment, growth and regeneration of the adult zebrafish fin. Development. 2010 Dec;137(23):3931–3939. doi: 10.1242/dev.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999 Aug 12;341(7):491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 42.Botchkareva NV, Botchkarev VA, Gilchrest BA. Fate of melanocytes during development of the hair follicle pigmentary unit. J Investig Dermatol Symp Proc. 2003 Jun;8(1):76–79. doi: 10.1046/j.1523-1747.2003.12176.x. [DOI] [PubMed] [Google Scholar]
- 43.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001 Jul;117(1):3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 44.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999 Oct;113(4):523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 45.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993 Jul;101(1 Suppl):90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 46.Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D, et al. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994 Jun;102(6):862–869. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005 Jan;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997 Dec 1;192(1):99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 49.Unna PG. Beiträge zur histologie und entwicklungsgeschichte der menschlichen oberhaut und ihrer anhangsgebilde. Arch Mikroskop Anat entwicklungsmech. 1876;12:665–741. [Google Scholar]
- 50.Pasolli HA. The hair follicle bulge: a niche for adult stem cells. Microsc Microanal. 2011 Aug;17(4):513–519. doi: 10.1017/S1431927611000419. [DOI] [PubMed] [Google Scholar]
- 51.Nishikawa S, Osawa M. Generating quiescent stem cells. Pigment Cell Res. 2007 Aug;20(4):263–270. doi: 10.1111/j.1600-0749.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, et al. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. Embo J. 1991 Aug;10(8):2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okura M, Maeda H, Nishikawa S, Mizoguchi M. Effects of monoclonal anti-c-kit antibody (ACK2) on melanocytes in newborn mice. J Invest Dermatol. 1995 Sep;105(3):322–328. doi: 10.1111/1523-1747.ep12319939. [DOI] [PubMed] [Google Scholar]
- 54.Raymond K, Deugnier MA, Faraldo MM, Glukhova MA. Adhesion within the stem cell niches. Curr Opin Cell Biol. 2009 Oct;21(5):623–629. doi: 10.1016/j.ceb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 55.O'Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008 Aug 25;182(4):801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011 Jun 10;145(6):941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005 Feb 24;433(7028):884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 58.Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008 Dec;21(6):627–645. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997 Oct 30;389(6654):966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 60.Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998 Nov 26;396(6709):370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 61.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004 Jan 16;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osawa M, Egawa G, Mak SS, Moriyama M, Freter R, Yonetani S, et al. Molecular characterization of melanocyte stem cells in their niche. Development. 2005 Dec;132(24):5589–5599. doi: 10.1242/dev.02161. [DOI] [PubMed] [Google Scholar]
- 63.Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010 Feb 5;6(2):130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009 Jan;19(1):103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 65.Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. Faseb J. 2000 Apr;14(5):752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 66.Soma T, Dohrmann CE, Hibino T, Raftery LA. Profile of transforming growth factorbeta responses during the murine hair cycle. J Invest Dermatol. 2003 Nov;121(5):969–975. doi: 10.1046/j.1523-1747.2003.12516.x. [DOI] [PubMed] [Google Scholar]
- 67.Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, et al. Inhibition of PAX3 by TGFbeta modulates melanocyte viability. Mol Cell. 2008 Nov 21;32(4):554–563. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011 Nov;43(11):1550–1562. doi: 10.1016/j.biocel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumano K, Masuda S, Sata M, Saito T, Lee SY, Sakata-Yanagimoto M, et al. Both Notch1 and Notch2 contribute to the regulation of melanocyte homeostasis. Pigment Cell Melanoma Res. 2008 Feb;21(1):70–78. doi: 10.1111/j.1755-148X.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 70.Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006 May 8;173(3):333–339. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, Radtke F, et al. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn. 2007 Jan;236(1):282–289. doi: 10.1002/dvdy.21000. [DOI] [PubMed] [Google Scholar]
- 72.Schouwey K, Larue L, Radtke F, Delmas V, Beermann F. Transgenic expression of Notch in melanocytes demonstrates RBP-Jkappa-dependent signaling. Pigment Cell Melanoma Res. 2010 Feb;23(1):134–136. doi: 10.1111/j.1755-148X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 73.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011 Feb 4;8(2):177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Darling TN, Bauer JW, Hintner H, Yancey KB. Generalized atrophic benign epidermolysis bullosa. Adv Dermatol. 1997;13:87–119. discussion 20. [PubMed] [Google Scholar]
- 75.Hintner H, Wolff K. Generalized atrophic benign epidermolysis bullosa. Arch Dermatol. 1982 Jun;118(6):375–384. [PubMed] [Google Scholar]
- 76.Masunaga T, Shimizu H, Yee C, Borradori L, Lazarova Z, Nishikawa T, et al. The extracellular domain of BPAG2 localizes to anchoring filaments and its carboxyl terminus extends to the lamina densa of normal human epidermal basement membrane. J Invest Dermatol. 1997 Aug;109(2):200–206. doi: 10.1111/1523-1747.ep12319337. [DOI] [PubMed] [Google Scholar]
- 77.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med. 2007 Mar;13(3):378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 78.Nishizawa Y, Uematsu J, Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem. 1993 Apr;113(4):493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- 79.Powell AM, Sakuma-Oyama Y, Oyama N, Black MM. Collagen XVII/BP180: a collagenous transmembrane protein and component of the dermoepidermal anchoring complex. Clin Exp Dermatol. 2005 Nov;30(6):682–687. doi: 10.1111/j.1365-2230.2005.01937.x. [DOI] [PubMed] [Google Scholar]
- 80.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006 Oct 1;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 81.Dick JE. Stem cell concepts renew cancer research. Blood. 2008 Dec 15;112(13):4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 82.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010 Nov 16;18(5):510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008 Dec 4;456(7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010 May 14;141(4):583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004 Nov 18;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 87.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007 Jan 4;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 89.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007 Jan 4;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 90.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005 Oct 15;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schlaak M, Schmidt P, Bangard C, Kurschat P, Mauch C, Abken H. Regression of metastatic melanoma in a patient by antibody targeting of cancer stem cells. Oncotarget. 2012 Jan;3(1):22–30. doi: 10.18632/oncotarget.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinc A, Somasundaram R, Wagner C, Hormann M, Karanikas G, Jalili A, et al. Targeting CD20 in Melanoma Patients at High Risk of Disease Recurrence. Mol Ther. 2012 Feb 21; doi: 10.1038/mt.2012.27. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res. 2004 Aug 15;77(4):475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- 95.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005 Apr;37(4):715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005 Dec 1;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 97.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003 Sep 15;63(18):5821–5828. [PubMed] [Google Scholar]
- 98.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007 Mar;43(5):935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 99.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007 Jan;20(1):102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 100.Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008 Dec;26(12):3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robey RW, Massey PR, Amiri-Kordestani L, Bates SE. ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem. 2010 Oct 1;10(8):625–633. doi: 10.2174/187152010794473957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007 Mar;26(1):39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 103.Deichmann M, Thome M, Egner U, Hartschuh W, Kurzen H. The chemoresistance gene ABCG2 (MXR/BCRP1/ABCP1) is not expressed in melanomas but in single neuroendocrine carcinomas of the skin. J Cutan Pathol. 2005 Aug;32(7):467–473. doi: 10.1111/j.0303-6987.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 104.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008 Jan 17;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003 Nov 21;278(47):47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 106.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005 May 15;65(10):4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 107.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011 Feb 15;71(4):1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010 Sep;163(1):e11–e15. doi: 10.1016/j.jss.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 109.Vasquez-Moctezuma I, Meraz-Rios MA, Villanueva-Lopez CG, Magana M, Martinez- Macias R, Sanchez-Gonzalez DJ, et al. ATP-binding cassette transporter ABCB5 gene is expressed with variability in malignant melanoma. Actas Dermosifiliogr. 2010 May;101(4):341–348. doi: 10.1016/j.ad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Botelho MG, Wang X, Arndt-Jovin DJ, Becker D, Jovin TM. Induction of terminal differentiation in melanoma cells on downregulation of beta-amyloid precursor protein. J Invest Dermatol. 2010 May;130(5):1400–1410. doi: 10.1038/jid.2009.296. [DOI] [PubMed] [Google Scholar]
- 111.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010 Jan 15;70(2):697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2012 Sep;47(14):2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 113.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992 Dec 11;71(6):973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 114.Iwamoto S, Odland PB, Piepkorn M, Bothwell M. Evidence that the p75 neurotrophin receptor mediates perineural spread of desmoplastic melanoma. J Am Acad Dermatol. 1996 Nov;35(5 Pt 1):725–731. doi: 10.1016/s0190-9622(96)90728-8. [DOI] [PubMed] [Google Scholar]
- 115.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010 Jul 1;466(7302):133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011 Apr 15;71(8):3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 117.Held MA, Curley DP, Dankort D, McMahon M, Muthusamy V, Bosenberg MW. Characterization of melanoma cells capable of propagating tumors from a single cell. Cancer Res. 2010 Jan 1;70(1):388–397. doi: 10.1158/0008-5472.CAN-09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roesch A, Becker B, Meyer S, Wild P, Hafner C, Landthaler M, et al. Retinoblastomabinding protein 2-homolog 1: a retinoblastoma-binding protein downregulated in malignant melanomas. Mod Pathol. 2005 Sep;18(9):1249–1257. doi: 10.1038/modpathol.3800413. [DOI] [PubMed] [Google Scholar]
- 119.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002 Nov 1;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 120.Mortarini R, Gismondi A, Maggioni A, Santoni A, Herlyn M, Anichini A. Mitogenic activity of laminin on human melanoma and melanocytes: different signal requirements and role of beta 1 integrins. Cancer Res. 1995 Oct 15;55(20):4702–4710. [PubMed] [Google Scholar]
- 121.Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008 Jun 1;68(11):4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 122.Becker JC, Houben R, Schrama D, Voigt H, Ugurel S, Reisfeld RA. Mouse models for melanoma: a personal perspective. Exp Dermatol. 2009 Feb;19(2):157–164. doi: 10.1111/j.1600-0625.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 123.Larue L, Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res. 2007 Dec;20(6):485–497. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 124.Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009 Jun 12;137(6):1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]