Abstract
The alveolate superphylum includes many free-living and parasitic organisms, which are united by the presence of alveolar sacs lying proximal to the plasma membrane, providing cell structure. All species comprising the apicomplexan group of alveolates are parasites and have adapted to the unique requirements of the parasitic lifestyle. Here the evolution of apicomplexan secretory organelles that are involved in the critical process of egress from one cell and invasion of another is explored. The variations within the Apicomplexa and how these relate to species-specific biology will be discussed. In addition, recent studies have identified specific calcium-sensitive molecules that coordinate the various events and regulate the release of these secretory organelles within apicomplexan parasites. Some aspects of this machinery are conserved outside the Apicomplexa, and are beginning to elucidate the conserved nature of the machinery. Briefly, the relationship of this secretion machinery within the Apicomplexa will be discussed, compared with free-living and predatory alveolates, and how these might have evolved from a common ancestor.
Keywords: Toxoplasma, Plasmodium, Invasion, Myzocytosis, Microneme, Rhoptry
1. Introduction
All apicomplexans have a parasitic lifestyle and infect a wide variety of vertebrate and invertebrate hosts, often with complex multi-host life cycles. This group includes major human pathogens such as Plasmodium spp. causing malaria and opportunistic pathogens such as Toxoplasma gondii causing encephalitis and birth defects, and Cryptosporidium causing diarrhea. Other species like Theileria and Babesia result in large economic losses in livestock, mainly ruminants, whereas Eimeria spp. are a major scourge in poultry. A large subgroup of apicomplexans, the gregarines, are restricted to invertebrates, mostly in marine environments, and have received less attention by the medical field.
One feature that all apicomplexans share is their acquisition of nutrients from the host through invasion by different strategies (Fig. 1). Much progress has been made in our understanding of the mechanism of invasion into and egress from host cells by api-complexan parasites. Mostly from studies on T. gondii and Plasmodium falciparum, a broad mechanism for invasion has been elucidated. A process of gliding motility, using an actinomyosin motor, facilitates movement between host cells and appears to power invasion as well. Initial attachment of the parasite to the surface of the host cell is followed by the entry of the parasite into a newly formed parasitophorous vacuole within the host cell. Signal transduction ensures that the various mechanisms required at the different stages of egress and invasion progress in a coordinated fashion. Central to all of these processes is the release of specific effector molecules from secretory organelles – micronemes, rhoptries and dense granules – of which the first two are positioned at the apical end of the parasites.
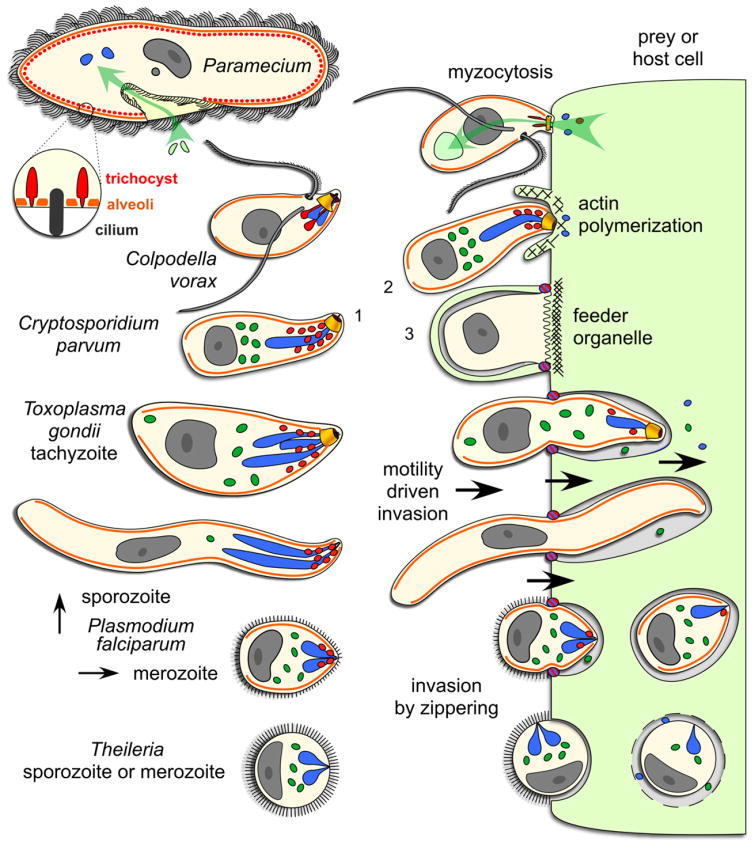
Fig 1.
Schematic comparison of organelles with a role in apicomplexan host cell invasion across the Alveolates. The surface of the free-living ciliate, Paramecium, is covered by cilia which it uses for swimming. Prey bacteria (light green) are taken up through the oral cavity by phagocytosis (green arrow) and merge with lysomes in the cytoplasm for digestion. The enlargement shows the alternating trichocysts (or dense core secretory vesicles (DCSVs)) and cilia underlying the plasma membrane and protruding from the alveolar vesicles. Colpodella vorax is a representative of a dinoflagellate lineage with two flagella that feeds by myzocytosis, also known as cellular vampirism. Note the open conoid structure that is in close apposition upon attachment to a prey cell (flagellate protists). Rhoptries and micronemes are secreted in the process. Prey cell cytoplasm is taken up by pinocytosis and accumulates in basally located vacuoles. Cryptosporidium parvum is an apicomplexan parasite closely related to the archigregarine lineage. A gliding motile sporozoite (1) is shown attaching with its apical end to an endothelial cell of a vertebrate host. Host actin polymerization is induced by the parasite and triggers pseudopod formation, which will engulf the parasite (3), as well as inducing an actin patch, keeping the parasite at the edge of the host cell. This results in extracytoplasmic, yet intracellular, residence of the vacuole. Note the single membrane separating the parasite and host cell cytoplasm, known as the feeder organelle, which is reminiscent of myzocytosis. Toxoplasma gondii tachyzoites and P. falciparum sporozoites display gliding motility, which drives invasion of vertebrate cells. A constriction known as the moving junction forms at the interface of the parasite and the host, and excludes plasma membrane proteins from the host entering into the parasitophorous vacuole membrane. Plasmodium falciparum merozoites enter red blood cells by a combination of gliding motility and zippering. Theileria sporozoites or merozoites are non-motile and can enter host cells in any orientation, a process whereby the dense coat of the zoite is shed. Evidence for micronemes has not been clearly established while no moving junction is formed and the rhoptries are only released upon completion of invasion. Secreted rhoptry and dense granule proteins dissolve the vacuolar membrane and results in cytoplasmic residence. Orange: alveoli or inner membrane complex (IMC); red: micronemes (trichocysts or DSCVs in Paramecium); blue: rhoptries (lysosomes in Paramecium); dark green: dense granules; yellow: conoid. Parasite cells are not drawn to scale.
Evidence is accumulating that apicomplexan parasites evolved from free-living, photosynthetic organisms into the diverse obligate intracellular parasites that is observed in extant parasites. Tracing the origins of the host cell invasion and egress processes can identify shared and unique aspects of these processes, particularly in relation to non-parasitic relatives. As part of the Alveolate phylum, the Apicomplexa share a defining structure with the ciliates and dinoflagellates, which all share an inner membrane complex (IMC) or alveolar membrane system (Keeling et al., 2005). Despite this shared feature, their life-styles are strikingly diverse, with the ciliates being mostly free-living predators whereas the dino-flagellates are split between photosynthetic organisms and predators, although most photosynthetic dinoflagellates can also forage by predation (mixotroph) (Cavalier-Smith, 1991; Stoecker, 1999). In recent years a handful of studies have identified shared features between these organisms, lifting the veil on their specific adaptations and refitting of organelles to a particular life style. For instance the alveolar system serves different functions depending on the life style. These functions include structural supports, cellulose-reinforced armor (Lau et al., 2007), and calcium storage (Stelly et al., 1991; Plattner and Klauke, 2001) in ciliates and dinoflagellates, and as support for glideosome mediated motility and as cytoskeletal scaffold in the cell division process in the Apicomplexa (Mann and Beckers, 2001; Gaskins et al., 2004). Although the alveolar element is the unifying feature among the Alveolates, this review will focus on several structures involved in apicomplexan host cell invasion, primarily the apical secretory organelles. The conserved features of their function and regulated release will be explored. In addition, recent findings will be placed in the context of the evolution of the secretory organelles within parasitic and related non-parasitic alveolates.
2. Cell biology of apicomplexan secretory organelles
Apicomplexans are so named because they possess a specialized apical end that is required for the many steps of movement between host cells. In particular, several functionally distinct secretory organelles have now been identified. Electron microscopic approaches have clearly identified electron-dense organelles at the apical end of all apicomplexan organisms. These have been well described in Plasmodium and Toxoplasma as micronemes, rhoptries and dense granules.
2.1. Micronemes
The micronemes are the smallest of the secretory organelles and are localized at the apical end of the parasite. They contain proteins that, following attachment to the host, release their contents to the surface of the parasites where they are available for binding to host cell receptors for invasion. Anterior to posterior movement of the ligand-receptor linkages through the action of the actinomyosin motor results in motility that powers invasion and also supports movement through tissues and on solid substrate. Proteases embedded in the plasma membrane on the basal end result in the shedding of the parasite ligands from the surface (Dowse et al., 2005; O’Donnell et al., 2006). Proteins that mark these organelles include several adhesins that bind to cognate receptors on the host cells (Carruthers and Tomley, 2008). These proteins have diversified between different species, reflecting the co-evolution with different host cell receptors. However, a few proteins are conserved between all apicomplexans, such as the AMA-1 protein that is thought to trigger rhoptry release (Tyler et al., 2011). Furthermore, a pore-forming protein secreted from the micronemes is required for Toxoplasma egress (Kafsack et al., 2009), illustrating an additional role for micronemes in egress, besides invasion.
2.2. Rhoptries
The rhoptries are the second key secretory organelle. They are larger than micronemes, pear- or club-shaped with one end attached to the very apical end of the parasite, and are thought to most resemble secretory lysosomal organelles (Ngo et al., 2004). The organelles are instrumental in the formation of the parasitophorous vacuolar membrane (PVM). The contents of the rhoptries are released following those of the micronemes, concomitant with a close interaction between the parasite and the host cell membranes. Rhoptries of some species also contain lamellar membranes that contribute to the formation of the PVM. Recent studies suggest that the rhoptry neck and rhoptry bulb are distinct compartments, which contain different complements of proteins, and are released differentially. For instance the RON proteins stored in the rhoptry neck are critical for the formation of the tight junction between the parasite and the host cell and are secreted before the rhoptry bulb proteins, which modify the vacuolar membrane and the host cell (Alexander et al., 2005; Proellocks et al., 2010; Tyler et al., 2011). During invasion the moving junction is also involved in the exclusion of host proteins from the PVM, which is largely made out of host cell plasma membrane. The characterized rhoptry proteome in T. gondii indicates that it contains many molecules that, once introduced into the cell, interact with host proteins and enhance parasitism (Bradley et al., 2005; Bradley and Sibley, 2007; Blader and Saeij, 2009). Rhoptry kinases in T. gondii have been characterized in particular and have been shown to be key modulators of virulence (Saeij et al., 2006; Taylor et al., 2006; Reese et al., 2011).
2.3. Dense granules
The other secretory organelles are known as dense granules. These are not located at the apical end but are instead found throughout the cell and are released immediately and constitutively after invasion and throughout intracellular replication. Recent evidence suggests that some of the effectors of this organelle are required for the modification of the host (Rosowski et al., 2011; Tobin and Knoll, 2012) but in the case of Toxoplasma, most of the proteins are involved in modifying the vacuolar compartment (Mercier et al., 2002, 2005). In Plasmodium dense granule proteins may modify the erythrocyte host cell (Culvenor et al., 1991).
3. Regulated release of secretory organelles
The secretory organelles are thought to be released in a sequential fashion in the host cell invasion process (Carruthers and Sibley, 1997). In Toxoplasma there is evidence for an initial distant attachment, after which a closer attachment is formed, where the micronemes are exocytosed to provide ligand–receptor interactions (Kafsack et al., 2007). The rhoptry neck proteins are released only after the micronemes are released, for which neither specific triggers nor the nature of the signal transduction pathway are currently known. Following completion of entry into the host cell, the dense granules are discharged. However, the Toxoplasma dense granules are also constitutively secreted at a low level. This sequence of secretory events is conserved for Plasmodium invasion (Singh et al., 2010).
Secretion in apicomplexan organisms is tightly controlled. The main signaling molecule is intracellular calcium. When calcium levels suddenly increase this can result in the release of the micronemes, followed by subsequent secretion of the rhoptries upon additional triggers, such as host cell recognition. This calcium-regulated exocytosis of the micronemes is akin to calcium-dependent secretion in other systems. There are numerous calcium binding proteins in apicomplexans (see reviews by Nagamune and Sibley, 2006; Nagamune et al., 2008b; Plattner et al., 2012). These include EF-hand and C2-domain containing proteins. Recent evidence suggests a central role for calcium-dependent protein kinases (CDPK) in orchestrating many of the processes required for invasion of host cells, including microneme release and the activation of the actinomyosin motor. Gene sequences encoding CDPKs have been identified in plants and Alveolate protists (Kim et al., 1998; Zhang and Choi, 2001). From an analysis of the intronic boundaries it appears that the protist and one subset of plant CDPKs are closely related, and were formed by the fusion of a protein kinase domain with calmodulin. Several studies have now indicated a role for CDPKs in the invasion and egress in both Toxoplasma and P. falciparum. The family has expanded to between seven and 12 members. It is likely that the different CDPK paralogs regulate different subsets of proteins. In T. gondii, TgCDPK1 is involved in the exocytosis of secretory organelles (Kieschnick et al., 2001; Lourido et al., 2010). CDPK1 in P. falciparum, which is not a direct ortholog of TgCDPK1, is involved in invasion and egress in blood-stage infections (Green et al., 2008; Kato et al., 2008). Plasmodium CDPK4 is involved in the formation of gametes (Billker et al., 2004), while CDPK3 is required for ookinete penetration of the midgut epithelium (Ishino et al., 2006; Siden-Kiamos et al., 2006). Plasmodium falciparum CDPK5 was shown to be essential for egress from red blood cells (Dvorin et al., 2010).
Recently, in a screen for molecules required for invasion of host cells by T. gondii, we found that a protein containing multiple calcium-binding C2 domains (DOC2.1) is required for regulated exocytosis of micronemes (Farrell et al., 2012). The P. falciparum ortholog of this protein is also required for the invasion of red blood cells and the release of micronemes. DOC2.1 likely functions in recruiting the membrane fusion machinery (SNAREs etc.) in a calcium-dependent fashion to the site of microneme secretion so that the vesicular membrane and the outer membrane of the parasite merge and the vesicular contents are released in the extracellular milieu. Several other C2 proteins are encoded in the genomes of Apicomplexa (e.g. ~10 with a single C2 domain and a handful with multiple C2 domains in Toxoplasma) but their roles remain to be elucidated.
Other signals that may be of importance are upstream to the release of calcium. Pharmacological evidence suggests that inositol trisphosphate (IP3), released by phosphatidylinositol-specific phospholipase C (PI-PLC), could result in the release of calcium through its action on IP3 receptors, and cyclic ADP Ribose (cADPR), produced by ADP-ribosyl cyclase, is also thought to act on ryanodine receptors (Carruthers et al., 1999; Moudy et al., 2001; Lovett et al., 2002; Chini et al., 2005). Surprisingly, these receptors do not appear to be encoded in the genomes of these parasites. Further, abscisic acid, a plant hormone, appears to play a role in the upstream initiation of egress from host cells for T. gondii parasites (Nagamune et al., 2008a). Independent of calcium release, chemical genetic evidence suggests that cyclic guanosine monophosphate (cGMP) and the cGMP-dependent kinase, PKG, are required for an essential role in microneme release, motility and invasion (Donald and Liberator, 2002; Wiersma et al., 2004; Moon et al., 2009).
In contrast to the calcium-based regulation of micronemes, the signals for rhoptry and dense granule release remain obscure. Evidence suggests that the AMA-1 protein is required for rhoptry release (Mital et al., 2005), but the mechanism for this regulation is unknown. In Toxoplasma Mic8 has been shown to be required for rhoptry secretion, but Mic8 itself does not play a role in microneme secretion (Kessler et al., 2008). Recently a specific inhibitor of rhoptry secretion was identified which may aid in identifying some of the components underlying the infrastructure of their release (Ravindran et al., 2009). The dense granules are secreted constitutively at a low level from both intracellular and extracellular Toxoplasma tachyzoites, although there is a spike shortly after completion of host cell invasion (Carruthers and Sibley, 1997).
4. Secretion in the evolution of parasitism from free-living organisms
Clearly, secretory organelles play a dominant role in the host cell invasion process. However, deviations from the outlined model become apparent when considering organisms outside the well-studied Toxoplasma and Plasmodium parasites described in the previous sections. When the field of view is expanded beyond the Apicomplexa to all Alveolates, several common themes emerge. By identifying the common evolutionary themes and variations in feeding behavior across the alveolates, the likely origin of defining processes can be reconstructed to provide key insights into basic mechanisms underlying the parasitic biology of the human pathogens.
4.1. Alveolate feeding strategies
It can be imagined that the development of the obligate intracellular lifestyle seen in the Toxoplasma and Plasmodium spp. today could have evolved from a predatory lifestyle. The ciliates are the alveolar lineage most distantly related to the Apicomplexa, yet many ciliates feed by capturing and ingesting bacteria. Many dinoflagellates are self-sufficient by relying on photosynthesis by their plastids, however just as many other dinoflagellates can also feed by, or are uniquely dependent on feeding by, predation of other cells. Significantly, the predatory feeding style most closely resembling apicomplexan invasion and intracellular parasitism is observed in the Colpodellida, which is a lineage at the base of the Apicomplexa (Leander and Keeling, 2003; Leander et al., 2003; Cavalier-Smith and Chao, 2004). This feeding strategy is also observed among the Archigregarine lineage of the Apicomplexa, parasites of marine invertebrates. It is of note that Cryptosporidium, an intracellular parasite of vertebrates, is closely related to the archigregarines yet resides in an extra-cytoplasmic, yet intra-cellular, vacuole with a feeding organelle resembling the strategy of the predatory relatives. As will be discussed in Sections 4.2–4.4, all of these strategies share several aspects with the intracellular strategies of Toxoplasma and Plasmodium. In the following sections a brief overview of the salient features of these feeding modes will be provided.
4.2. Predation by ciliates
The ciliates make up a large group of free-living organisms, which forage on bacteria as well as other cells. The best-studied representatives of this group are Tetrahymena thermophila and Paramecium tetraurelia (Lynn, 2010). These organisms are relatively large (>100 μm) and their surface is covered with cilia, permitting them to swim directionally. Bacteria or other protists are taken up by phagocytosis into an invaginated pocket on the surface known as the oral cavity or cytostome (Fig. 1) (Ishida et al., 2001). The phagocytotic vesicles are transported along microtubules and fuse with lysosomes residing inside the cytoplasm to digest the bacteria (Allen and Fok, 2000). Although this is also what happens with phagocytosed materials in mammalian phagocytic cells, it is important to recognize that this is conserved in these eukaryotic lineages, particularly because the role of the lysosomes has been modified in Apicomplexa toward a specific role in host cell invasion.
Several features in the biology and feeding of these ciliates are shared in principle with the Apicomplexa. As in all alveolates, an alveolar vesicle network underlies the plasma membrane. The cilia are organized in regular arrays penetrating the alveoli. The cilia are alternating with secretory structures known as trichocysts or dense core secretory vesicles (DCSVs) (Plattner and Kissmehl, 2003). Interestingly, secretion or extrusion of these vesicles occurs upon contact with prey or to defend an attack by another organism. This is reminiscent of the triggers leading to microneme secretion in the invasion process of Apicomplexa, which depends on making contact with, and recognition of, its host cell. Moreover, like microneme secretion, trichocyst secretion is calcium-dependent. Not surprisingly, the contents of the trichocysts differ from those of the micronemes: trichocysts contain filaments anchored in the cell and extrusion resembles the launch of harpoons. Next to the trichocysts, a variety of other secretory organelles have been described across various representatives of this phylum. Their function, contents and nature vastly differ per species and illustrate the versatility of secretory organelles within this lineage (Rosati and Modeo, 2003).
4.3. Predatory dinoflagellates: myzocytosis or “cellular vampirism”
Feeding strategiesamongdinoflagellates are very diverse and can be both predatory and parasitic (Schnepf and Elbrachter, 1992; Coats, 1999; Schnepf, 2004). Feeding by phagocytosis is common, next to various more divergent feeding strategies. The focus here will be on two forms of feeding mediated by a cellular extrusion called a peduncle, since this feeding form shares several features with the Apicomplexa. The peduncle is a narrow membranous tube extending from the main cell body and attaches to a prey cell. In the first variation of peduncle feeding, the prey is captured with the peduncle upon which a pseudopod develops and engulfs the prey (dinoflagellates or other aquatic protozoa) as a veil. This feeding veil is called a pallium and this form of feeding is also known as pallium feeding (Gaines and Taylor, 1984; Gaines and Elbrächter, 1987; Jacobson and Anderson, 1992; Schnepf, 2004). Subsequently, the prey is digested within the feeding veil and nutrients are transported into the cytoplasm (extracellular digestion).
In the second variation of peduncle mediated feeding the prey is digested intracellularly. Again the peduncle is used to attach to the prey, however the cellular contents of the prey is sucked out and digested within the confinement of the predator’s main cell body. This process is called myzocytosis, also known as cellular vampirism (Schnepf and Deichgraber, 1984; Schnepf and Elbrachter, 1992; Stoecker, 1999). Upon contact of the peduncle with the prey, the plasma membrane of the prey cell is dissolved. This is quickly followed by the uptake of the prey’s cytoplasm through the peduncle, resembling drinking through a straw (Hausmann and Hülsmann, 2010). The prey’s cytoplasm is deposited in a food vacuole wherein it is digested. Furthermore, a related process has been described in a special group of the ciliates, the suctorial ciliates (Hausmann and Hülsmann, 2010). Instead of using a cytostome, this form of feeding employs tentacles. Each tentacle is equipped with extrusomes called haptocysts that are used to snare the prey. As in penduncle feeding, the cytoplasm of the prey it taken up through the tentacle-straw and deposited into a digestive vacuole. There is yet another variant of the straw-based feeding, which is found in nassophorean ciliates. These organisms feed by phagocytosis of a whole organism through a tube known as a cytopharyngeal basket. For instance Pseudomicrothorax dubius feeds on cyanobacteria. Although the structure of the straw is very different and it requires “sucking up” whole cells rather than cytoplasm, it has been argued this feeding method is mechanistically related to peduncle and tentacle feeding (Hausmann, 2002; Hausmann and Hülsmann, 2010).
4.4. Perkinsids: intracellular dinoflagellates
An intracellular feeding and replication strategy is found in Rastrimonas subtilis (originally named Cryptophagus subtilis). This feeding mechanism also uses apical secretory organelles (micronemes and rhoptry shaped organelles) as well an open pseudoconoid to fully invade the free-living Cryptomonad Chilomonas paramecium in which they replicate intracellularly (Brugerolle, 2002a, 2003; Cavalier-Smith and Chao, 2004). Rastrisomonas is part of the Perkinsozoa, which furthermore contains Perkinsus and Parvilucifera. Perkinsus spp. are facultative parasites of shellfish (e.g. Perkinsus marinus wreaks havoc on oysters) that can be taken up passively through phagocytosis by hemocytes (the macrophage ortholog cell type in shellfish) wherein they replicate. The Perkinsus marinus zoospore contains an open conoid, subpellicular microtubules, rhoptries, rectilinear micronemes and conoid-associated micronemes (Perkins, 1976, 1996; Simpson and Patterson, 1996). However, how these structures function in the life style of these organisms is not well understood, but they might be deployed for feeding intracellularly after phagocytosis by the hemocyte. Parvilucifera spp. parasitize dinoflagellates and replicate intracellularly. Again these organisms contain all of the apical organelles as seen in Perkinsus, although trichocysts are also present in some species, but the host cell invasion process has not been described (Noren et al., 1999; Leander and Hoppenrath, 2008). Phylogenetic analysis of the Perkinsids places them next to free-living dinoflagellates although statistical support is never very strong (Cavalier-Smith and Chao, 2004; Hoppenrath and Leander, 2009).
4.5. Colpodellid myzocytosis
Myzocytosis by the Colpodellida, which are considered very basal apicomplexans (Leander et al., 2003; Cavalier-Smith and Chao, 2004), unfolds without a peduncle. The Colpodellida attach directly with their apical end to the prey cell, which resembles the early steps in apicomplexan host cell invasion. The phylogenetic position of the Colpodellida warrants some further discussion in the light of recently identified free-living Apicomplexa, Chromera velia (Moore et al., 2008) and Vitrella brassicaformis (Obornik et al., 2012), both photosynthetic organisms living in coral reefs. The most recent interpretation of the phylogeny is that these free-living photosynthetic Apicomplexa branch off the evolutionary tree together with the Colpodella spp. but share a common ancestor with the Apicomplexa (Obornik et al., 2012). The Colpodellida and higher Apicomplexa appear to have lost their photosynthetic capacity independently and resorted to two different strategies, predation and parasitism, respectively.
The best-studied Colpodella spp. representatives are Colpodella edax and Colpodella vorax, which feed off Bodo and Spumella flagellated protists (Brugerolle, 2002b; Leander et al., 2003). Colpodellida myzocytosis unfolds by attachment of its apical end to the prey cell, piercing of the prey cell membrane, and secretion of vesicular contents from apical organelles into the prey cell (Fig. 1). Subsequently the prey cell contents are taken up by pinocytosis, transported into a basal food vacuole and digested, similar to myzocytosis in dinoflagellates (Brugerolle, 2002b; Leander et al., 2003). Several apically located secretory organelles have been described in this species, micronemes and distinct bulbous and lentil-shaped rhoptries (Brugerolle, 2002b). The bulbous yet elongated rhoptry shape is basically conserved in the Apicomplexa as is the localization at the very apical end of the cell. The molecular make up of the Colpodella rhoptries is unknown. Since the rhoptries cannot be discerned after attachment it is likely that they are secreted directly into the prey cell, which again is very reminiscent of the apicomplexan rhoptries (Brugerolle, 2002b). At the moment it is unclear what the contents of the micronemes are and whether they are secreted upon host cell recognition, so whether these are calcium-dependent secretory organelles related to the trichocysts in ciliates and/or the micronemes in Apicomplexa is unknown. Furthermore, secretion of rhoptries and prey cytoplasm uptake occur through a microtubular structure resembling the apicomplexan conoid, with the difference that the conoid is open at one end (Fig. 1) (Brugerolle, 2002b; Leander and Keeling, 2003). Other notable details include that at the attachment site the prey cell’s plasma membrane is dissolved so that the predator’s plasma membrane directly contacts the prey’s cytoplasm. Taken together, there is a striking conservation for several features of invasion and intracellular parasitism, suggesting the rudiments of these structures were already present in the free-living common ancestor (Leander and Keeling, 2003).
Morphologically, Colpodella resemble the Perkinsids: their motile stages have a pair of anterior flagella, an open conoid, micronemes and rhoptries. However, at the molecular phylogenetic level Colpodella sides with the Apicomplexa, whereas the Perkinsids are clearly separated and are associated with the dinoflagellates. Furthermore, although the Colpodellids are highlighted due to their kinship to the Apicomplexa, it should be noted that several dinoflagellate ectoparasites feed in an attached state very similarly to Colpodellids (e.g. Apodinium floodi feeding on sea squirt larvae (McLean and Galt, 1990; Coats, 1999)). Collectively, these observations strongly argue for ancient ancestry of the apical organelles. In addition, these observations argue that the cell biological basis for the intracellular parasitic life style as observed among the Perkinsids far predates the radiation of the Apicomplexa (Hoppenrath and Leander, 2009).
4.6. Myzocytosis by archigregarines
Gregarines are a diverse group of apicomplexans mostly parasitizing the gut of marine invertebrates (see Leander, 2008 for an extensive review). The archigregarines comprise a sub-group wherein the feeding behavior appears to link the Colpodellida predation strategy with cell invasion and intracellular parasitism of Plasmodium and Toxoplasma. The best studied representative is Selenidium orientale isolated from the Pacific sipunculid, Themiste pyroides, also known as the peanut worm (Simdyanov and Kuvardina, 2007).
Oocyst forms of Selenidium are taken up by the peanut worm and excyst in the gut to liberate the sporozoites. The sporozoites contain a typical apical complex and invade cells of the host (Leander, 2008). Subsequently developing trophozoites are free in the gut of the host and feed by myzocytosis on ciliated epithelial cells lining the gut. The same structures are involved: there are small vesicles orthologous to micronemes and rhoptries secreted into the cytoplasm of the prey cell through a conoid, through which prey cell cytoplasm is taken up by pinocytosis. A notable difference with Colpodella is that Selenidium further invades into the cytoplasm (Barta and Thompson, 2006; Simdyanov and Kuvardina, 2007).
Myzocytosis is therefore found in both Colpodellida and the Archigregarines (Brugerolle, 2002b; Simdyanov and Kuvardina, 2007; Leander, 2008), but the phylogenetic position of the free-living, photosynthetic Apicomplexa appears to separate these lineages (Obornik et al., 2012). This could hint at convergent evolution of myzocytosis, in support of the hypothesis that the basic structures were already present in the last common ancestor, and that Plasmodium and Toxoplasma extended on this theme.
4.7. Crytosporidium: intracellular myzocytosis
In recent years it hasbecomeapparent that Cryptosporidium occupies a special position among the Apicomplexa. Phylogenetically it is more akin to the gregarines, yet it is a parasite of vertebrates and appears to be an intracellular parasite, although its intracellular parasitism status is being re-evaluated (recently reviewed by Barta and Thompson, 2006; Borowski et al., 2008). Cryptosporidium invades endothelial cells in the gut, but does this in a manner quite different from Toxoplasma and Plasmodium (Fig. 1). Excysted sporozoites contain all of the typical organelles (micronemes, rhoptries, conoid (Tetley et al., 1998)), display robust gliding motility (Deng et al., 2002; Wetzel et al., 2005; Putignani et al., 2008) and attach apical end first to an endothelial cell to make a tight connection like ToxoplasmaandPlasmodium, likely basedonortholgous rhoptry neck proteins (Valentini et al., 2012). However, rather than invaginating the host cell to form the vacuole, the parasite induces actin polymerization in the host cell at the site of attachment to generate pseudopods and is encapsulated by induced phagocytosis (Elliott and Clark, 2000; Chen et al., 2004a,b). This strategy is therefore a combination of active and induced invasion. Furthermore, the formation of an actin patch or pedestal below the parasite is triggered, which keeps the parasite pushed toward the very edge of the endothelial cell (Elliott and Clark, 2000; O’Hara et al., 2008). In essence, this actin patch sequesters the parasite from the host cell cytoplasm resulting in its residence in an extracytoplasmic niche. Notably, the interface with the host cell consists of only a single membrane known as the feeder organelle (Fig. 1). This architecture resembles the single membrane separating predator and prey, as observed in myzocytosis. However, Cryptosporidium does not ‘gulp’ its host’s cytoplasm but imports nutrients through membrane embedded transporters (Sauvage et al., 2009). In conclusion, the behavior of Crytosporidium is generally more related to myzocytosis than to invasion and intracellular residence of Toxoplasma and Plasmodium, which is consistent with their phylogenetic position (Barta and Thompson, 2006; Borowski et al., 2008). These findings illustrate the versatile deployment of a conserved arsenal of predation/invasion organelles with until recently underappreciated shared features.
5. Adaptations in organelles and function
It is evident from the conserved organelles involved in the feeding behavior of alveolates with shared apicomplexan ancestry that these observations could be relevant in dissecting and advancing our understanding of Plasmodium and Toxoplasma host cell invasion. In this section we will attempt to deduce the functions of various structures and organelles by comparing these within, as well as outside, the Apicomplexa.
5.1. Functional specialization of the micronemes
The different apicomplexans of medical importance use the same general scheme for invasion, however clear distinctions exist both in the quantity of the secretory organelles present in each apicomplexan, as well as in the molecular contents of these organelles. Several species-specific adaptations within apicomplexans reflect the different host cells that are parasitized by each of the different parasites as well as differences in their invasion mode. The microneme contents, which mainly consist of host cell recognition ligands, differ per parasite and even per life cycle stage within the same parasite, reflecting the host tropisms of each parasite (reviewed in Tomley and Soldati, 2001; Carruthers and Tomley, 2008). While many of these proteins share conserved domain structures, variations on this theme allow them to recognize species and cell-type specific host cell molecules.
Another variable is the number of micronemes, which generally correlates with the dependence of a particular life stage on gliding motility (Fig. 1). At the low end of the spectrum lie Theileria sporozoites and merozoites, whose invasion does not depend on gliding motility and which do not appear to contain micronemes (Shaw and Tilney, 1995; Shaw, 2003). The tick transmitted Theileria sporozoites enter the bovine host during a blood meal and invade leukocytes. Neither the sporozoites nor the merozoites, which invade red blood cells, contain micronemes (Shaw and Tilney, 1995; Shaw, 2003). Neither do they contain a conoid, sub-pellicular microtubules, nor an inner membrane complex. The absence of a cortical cytoskeleton results in a globular rather than elongated shape. Consistent with this shape, Theileria sporozoites and merozoites can enter the host cell in any orientation, rather than the “apical-end-first” mode observed for gliding based invasion (Fig. 1). Theileria enters the host cell by a zippering process, which sheds the shaggy surface coat in absence of a moving junction, which is dependent on protease activity (Shaw et al., 1991). Critically, motile Theileria kinetes that cross the gut wall of the tick do contain micronemes. How the micronemes play a role in Theileria invasion is not well understood.
In the closely related Babesia parasites (both are piroplasms), micronemes cannot be discerned at the ultrastructural level. However, their genomes encode proteins with microneme protein adhesion domain signatures, suggesting the presence of a functionally comparable compartment (Gaffar et al., 2004a,b). Moreover, gliding motility of Babesia merozoites was recently demonstrated (Asada et al., 2012). Whether Theileria and Babesia share this feature remains to be determined.
Residing in the middle of the spectrum are Plasmodium blood-stage merozoites, which do not exhibit gliding motility but rely on microneme exocytosis for invasion. These merozoites contain fewer micronemes than Plasmodium sporozoites, coccidian parasites such as T. gondii and Eimeria tenella, or Cryptosporidium sporozoites, which all contain numerous micronemes and all display extensive gliding motility (Tetley et al., 1998; Sibley, 2004; Soldati et al., 2004).
Furthermore, in Plasmodium parasites the micronemes have been found to be surprisingly diverse. In addition to traditional micronemes, exonemes have been identified that are thought to be released during egress and contain proteases required for maturation of the parasite and dissolution of the PVM (Yeoh et al., 2007). In addition, a single thread-like organelle named the mononeme has been identified that contains the ROM1 protease, which is secreted upon host cell invasion (Singh et al., 2007). Although distinct micronemes have not been definitively reported in T. gondii, there is some circumstantial evidence for different classes. For instance, a perforin that dissolves the vacuolar membrane is required for egress but has no documented role in invasion (Kafsack et al., 2009). It is therefore likely that perforin is secreted before AMA1, which is strictly required for host cell invasion. Some evidence for different microneme compartments has surfaced from dissection of the Toxoplasma secretory pathway (Gaji et al., 2011) whereas microneme proteins localizing to different vesicles have been reported in the related coccidian parasite, Eimeria (Lai et al., 2011).
5.2. Relationship between trichocysts and micronemes
The origin of the micronemes likely lies very early in the Alveolate lineage. This insight is based on the conservation of a comparable calcium-dependent secretory compartment in some ciliates: the apicomplexan micronemes and the ciliate trichocysts or DCSVs (Plattner and Kissmehl, 2003; Plattner et al., 2012).
Many dinoflagellates contain trichocysts as well, next to a variety of other secretory organelles (Livolant, 1982a,b; Westfall et al., 1983). These trichocysts also span the alveolar sacs such that they are in contact with the plasma membrane, but in many cases their presence is restricted to the area around the cytostome. Trichocysts are absent from the Colpodellida (Cavalier-Smith and Chao, 2004). However, there are predatory dinoflagellates that contain both trichocysts and organelles named micronemes, such as Voromonas pontica (Cavalier-Smith and Chao, 2004). In this respect the position of C. edax has been disputed since it contains both trichocysts and micronemes (Leander et al., 2003). Based on 18S rRNA phylogeny it is also more distant from the other Colpodella spp., resulting in its proposed renaming as Alphamonas edax and together with Voromanas was defined as the Myzomonadaea, closely related to the Apicomplexa (Cavalier-Smith and Chao, 2004). Whether the micronemes described in these organisms, based solely on morphology, are truly orthologous to the apicomplexan micronemes has not been experimentally validated.
Regardless of the exact phylogenetic position and terminology, a string of discoveries has reported a shared biology in the calcium-dependent secretion of ciliate trichocysts and apicomplexan micronemes. Three sets of genes support a shared machinery between ciliate trichocysts and apicomplexan micronemes. Firstly, CDPKs are conserved in the ciliates (Gundersen and Nelson, 1987; Son et al., 1993; Kim et al., 1998) and some function in trichocyst release (Plattner et al., 2012). Secondly, the DOC2 protein required for microneme secretion of Toxoplasma and Plasmodium is also conserved in Paramecium (Farrell et al., 2012). The third notable gene supporting this evolutionary relationship is a conserved glycolytic enzyme with a moonlighting function. Paralogs of the enzyme phosphoglucomutase (PGM) exist in various eukaryotes and function in calcium-mediated signaling events (Kim et al., 1992). This PGM paralog is called PFUS in Paramecium (Satir et al., 1989) and PRP1 in Toxoplasma (Matthiesen et al., 2001, 2003), with a putative ortholog also being present in the Plasmodium genome (Kats et al., 2008). RNA interference (RNAi) knock-down of PFUS in P. tetraurelia results in failed assembly of the DCSVs, which is consistent with a function in the secretory vesicle scaffold of DCSVs (Liu et al., 2011). PFUS and PRP1 function via post-translational modifications in membrane scaffolds of the secretory vesicle. Interestingly, calcium-stimulated secretion results in release of PFUS/PRP1 from the vesicle scaffold into the cytoplasm, coinciding with a calcium-dependent dephosphoglucosylation. PFUS/PRP1 re-associates with newly forming vesicles in the cytosol. Isolated DCSVs contain glycosylated PFUS in their scaffold (Liu et al., 2009). Although the details of the mechanism wherein PFUS/PRP1 functions has not yet been extensively studied, the available data in combination with the CDPKs and DOC2 support the evolutionary relationship between the two organelles. It has to be kept in mind, however, that calcium-dependent secretion is wide-spread in the eukaryotic lineage and that DOC2 proteins, for instance, are also required for calcium-dependent neurotransmitter release (Friedrich et al., 2010; Groffen et al., 2010). It is therefore likely that the relationship between the machinery controlling trichocyst release and microneme secretion far predates the occurrence of alveolates.
5.3. Rhoptries
Rhoptry organelles are conserved in shape and apical localization from early branching dinoflagellates, the Colpodellids, and across the Apicomplexa (Okamoto et al., 2012). Mechanistically, their contents are injected in the host or prey cell upon attachment of the apical end. In Colpodellids this likely involves piercing of the host cell membrane or dissolving the host’s plasma membrane from the outside, whereas the injection mechanism in Apicomplexa has not been well resolved. Patch-clamp capacitance studies have provided evidence that there is a breach in host cell plasma membrane during the invasion process, which likely involves the release of rhoptries (Suss-Toby et al., 1996). The injection of Toxoplasma rhoptry contents occurs in the form of evacuoles which can be observed in the host cell cytosol (Hakansson et al., 2001). Furthermore, it has been demonstrated that the rhoptries are acidic and based on that have been suggested to be related to the lysosomes in other eukaryotes (Ngo et al., 2004). Therefore a relationship, albeit tenuous, can be drawn between the rhoptries as secreted lysosomes and the fusion of phagocytosed bacteria with the lysosomes within the ciliates. Taken together, in both cases the gaining of access to nutrients, either from the outside or within, relies on likely ancestral, acidic organelles.
There is one known variation to the function and timing of rhoptry release. Theileria invades by a zippering mechanism, in which the shaggy surface coat of the parasite is stripped in the invasion process and the host and parasite plasma membranes are in very close apposition (Fig. 1). Upon completion of invasion Theileria escapes from the nascent vacuoles and lives free in the cytoplasm (Shaw and Tilney, 1995; Shaw, 2003). These parasites do not form a moving junction, composed of rhoptry neck proteins, to exclude host cell proteins from the nascent vacuole. This is consistent with the quick escape from the vacuole: there is no need to exclude plasma membrane markers from the nascent vacuole if the parasites escape before the host cell can target lysosomes or other control processes to the compartment for destruction. In contrast, Theileria rhoptries are secreted only after completion of invasion, which coincides with dissolution of the vacuolar membrane (Shaw and Tilney, 1995; Shaw, 2003). This dissolution is most likely mediated by a large family of perforins encoded in the Theileria genome (Roiko and Carruthers, 2009). The timing of rhoptry release and their likely contents are consistent with a function in escape of the Theileria parasites from the vacuole.
5.4. Conoid
The presence of rhoptries in an alveolate organism is with few exceptions correlated with the presence of a conoid (Leander and Keeling, 2003). The apically located conoid plays a key role in attachment of Colpodellids to the prey cell. Moreover, secretion and cytoplasm uptake take place through this structure. However, the open appearance of the conoid in Colpodellids and dinoflagellate-related organisms is different from the closed architecture in Apicomplexa: a closed conoid is an innovation in the Apicomplexa (Leander and Keeling, 2003; Hoppenrath and Leander, 2009). Although this difference is striking, its functional significance is unclear. Most of the Apicomplexa contain a conoid, with the exception of hemosporidians (Plasmodium spp.) and the related piroplasms (Theileria and Babesia spp.), although their kinetes have a rudimentary conoid. Since these are related organisms, loss likely only occurred once and is therefore an exception rather than the rule. The conoid is also conserved in photosynthetic C. velia (Obornik et al., 2011) suggesting that either its ancestral function might not be related to feeding or that it is vestigial in these organisms.
In coccidians such as Toxoplasma, the conoid extends and retracts repeatedly while the parasite appears to be probing for the surface of a host cell. This extension and retraction is calcium-dependent (Mondragon and Frixione, 1996; Gonzalez Del Carmen et al., 2009). Furthermore, conoid extension is essential for the invasion process and independent of microneme secretion, since specific inhibitors of conoid extrusion, which block invasion, have been identified (Carey et al., 2004). Moreover, conoid extension was not compromised in a microneme secretion mutant (Farrell et al., 2012). Taken together, these findings identify the conoid as an ancient ancestral feature, yet its function and origin are still largely unknown.
5.5. Motility
Since several microneme proteins mediate contact between receptors outside the parasite and actin required for gliding motility on the cytoplasmic side, gliding motility is often considered critical to the invasion process. However, this requirement depends on the mode of invasion. For instance, Theileria sporozoites and merozoites are not motile, Plasmodium merozoite invasion depends only moderately on motility, whereas motility is a strict requirement for the invasion process of Plasmodium sporozoites. Two observations coincide with the complex relation between motility and invasion. First, Plasmodium merozoites and Theileria sporozoites/merozoites invade by a poorly understood zippering mechanism. Second, these parasite stages are small and relatively round compared with the motile and elongated sporozoite stages relying on motility (e.g. Cryptosporidium, Plasmodium and Toxoplasma) (Fig. 1). The nature of these putative relationships is presently not well understood.
When looking at which apicomplexans display gliding motility, an evolutionary perspective can be provided. The unique apicomplexan gliding motility is based on the ancestral morphological alveoli, known as the IMC, in the Apicomplexa. It has been argued that the loss of trichocysts coincided with the flattening of the alveoli, which facilitated the development of gliding motility (Cavalier-Smith and Chao, 2004). Among the gregarines, motility is not a common feature, and the vast diversity observed across gregarines allowed Leander (2008) to propose a scenario underlying its origin and function. Several representatives of the archigregarines developed stiffer cytoskeletons and an expansion of surface area by membrane folds supported by subpellicular microtubules. This attribute appears to coincide with a shift from myzocytosis to nutrient uptake through the expanded plasma membrane surface area. This shift in feeding behavior is also associated with the loss of a conoid. An important additional innovation facilitated by the stiffer cytoskeleton is the repurposing of a myosin motor to power gliding motility (Leander, 2008). Since intracellular replication of the archigregarines is ancestral to the development of gliding motility, gliding appears not to be a strict requirement for intracellular parasitism. Rather than powering host cell invasion, the innovation of gliding motility may have initially permitted migration outside the gut by crossing endothelia, which leads to the conquest of new cell and tissue types.
In summary, gliding motility is clearly involved in host cell invasion by many critical parasites and life stages, whereas others rely minimally or not at all on gliding. The bigger common denominator appears to be that the innovation of gliding was a development that permitted the crossing of biological barriers, greatly expanding host range and tissue tropism.
6. Conclusions and perspectives
In conclusion, the alveolates demonstrate a remarkable diversity in the strategies that they use for acquiring nutrients and interacting with other cells. Indeed, even within apicomplexan parasites there are numerous adaptations by which parasites invade host cells, presumably in response to the colonization of different cell types and tissues. This has been achieved by specialization and species-specific elaborations of the core calcium-based machinery and secretory organelles. The result is a highly regulated expansive exocytic/endocytic system as it is known today. In addition, a relationship of these protozoan secretory mechanisms was recently suggested to the cnidocyte cells of the cninidarians (jellyfish, sea anemomes and fresh water polyps). This cell type is among the most complex animal cell types known and unifies both sensory and secretory functions by secreting toxins in a light-activated, ion channel-mediated fashion (Holstein, 2012; Plachetzki et al., 2012). Hence, much remains to be discovered, especially with regard to the molecules involved, both to define a core-conserved machinery and to understand its diversification. For instance, what are the specific roles for the expansions of the calcium-responsive regulators of exocytosis such as the CDPK and C2 domain containing proteins? What are the molecular triggers to secrete rhoptries? What is the cell biology of microneme fusion; does this occur at the rhoptry neck? Where/how do dense granules secrete? Furthermore, considering that there is a significant amount of secretion, how is the surface area kept constant since there is no evidence for endocytosis? How are rhoptry contents injected into the host cell? This appears not to happen through the use of a Type III secretion needle. The unique cell biology of the different parasites at each stage of their life cycles reflects the very different environmental obstacles they need to overcome. We anticipate that an evolutionary assessment of the ontogeny of the secretory organelles and other structures will provide an exciting framework for studying apicomplexan parasites in the future.
Acknowledgments
We thank Drs. Aditya Paul and Bradley Coleman for critical reading of the manuscript. Due to considerations of brevity, we apologize for not citing all manuscripts critical to the described insights. This work was supported by National Institutes of Health, USA Grants AI081220 to M.J.G., and AI057919 and AI088314 to M.T.D., a Burroughs Wellcome Fund, USA, New Investigator in the Pathogenesis of Infectious Disease Fellowship to M.T.D., a Research Scholar Grant (RSG-12-175-01-MPC) from the American Cancer Society to M.J.G., and a Humboldt Stiftung, Germany, Research Fellowship to M.J.G.
References
- Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the Moving Junction Complex of Toxoplasma gondii: A Collaboration between Distinct Secretory Organelles. PLoS Pathog. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RD, Fok AK. Membrane trafficking and processing in Paramecium. Int Rev Cytol. 2000:198. doi: 10.1016/s0074-7696(00)98007-0. [DOI] [PubMed] [Google Scholar]
- Asada M, Goto Y, Yahata K, Yokoyama N, Kawai S, Inoue N, Kaneko O, Kawazu S. Gliding motility of Babesia bovis merozoites visualized by time-lapse video microscopy. PLoS ONE. 2012;7:e35227. doi: 10.1371/journal.pone.0035227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta JR, Thompson RC. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006;22:463–468. doi: 10.1016/j.pt.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS: acta pathologica, microbiologica, et immunologica. Scandinavica. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski H, Clode PL, Thompson RC. Active invasion and/or encapsulation? A reappraisal of host–cell parasitism by Cryptosporidium. Trends Parasitol. 2008;24:509–516. doi: 10.1016/j.pt.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, Boothroyd JC. Proteomic analysis of rhoptry organelles reveals many novel constituents for host–parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugerolle G. Cryptophagus subtilis: a new parasite of cryptophytes affiliated with the Perkinsozoa lineage. Eur J Protistol. 2002a;37:379–390. [Google Scholar]
- Brugerolle G. Colpodella vorax: ultrastructure, predation, life-cycle, mitosis, and phylogenetic relationships. Eur J Protistol. 2002b;38:113–126. [Google Scholar]
- Brugerolle G. Apicomplexan parasite Cryptophagus renamed Rastrimonas gen. nov. Eur J Protistol. 2003;39:101. [Google Scholar]
- Carey KL, Westwood NJ, Mitchison TJ, Ward GE. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc Natl Acad Sci USA. 2004;101:7433–7438. doi: 10.1073/pnas.0307769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342 (Pt 2):379–386. [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Tomley FM. Microneme proteins in apicomplexans. Subcell Biochem. 2008;47:33–45. doi: 10.1007/978-0-387-78267-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Cell diversification in heterotrophic flagellates. In: Patterson DJ, Larsen J, editors. The Biology of Free-living Heterotrophic Flagellates. Oxford University Press; Oxford: 1991. pp. 113–131. [Google Scholar]
- Cavalier-Smith T, Chao EE. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom nov.) Eur J Protistol. 2004;40:185–212. [Google Scholar]
- Chen XM, Huang BQ, Splinter PL, Orth JD, Billadeau DD, McNiven MA, LaRusso NF. Cdc42 and the actin-related protein/neural Wiskott–Aldrich syndrome protein network mediate cellular invasion by Cryptosporidium parvum. Infect Immun. 2004a;72:3011–3021. doi: 10.1128/IAI.72.5.3011-3021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Splinter PL, Tietz PS, Huang BQ, Billadeau DD, LaRusso NF. Phosphatidylinositol 3-kinase and frabin mediate Cryptosporidium parvum cellular invasion via activation of Cdc42. J Biol Chem. 2004b;279:31671–31678. doi: 10.1074/jbc.M401592200. [DOI] [PubMed] [Google Scholar]
- Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem J. 2005;389:269–277. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats DW. Parasitic life styles of marine dinoflagellates. J Eukaryot Microbiol. 1999;46:402–409. [Google Scholar]
- Culvenor JG, Day KP, Anders RF. Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect Immun. 1991;59:1183–1187. doi: 10.1128/iai.59.3.1183-1187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Templeton TJ, London NR, Bauer C, Schroeder AA, Abrahamsen MS. Cryptosporidium parvum genes containing thrombospondin type 1 domains. Infect Immun. 2002;70:6987–6995. doi: 10.1128/IAI.70.12.6987-6995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Liberator PA. Molecular characterization of a coccidian parasite cGMP dependent protein kinase. Mol Biochem Parasitol. 2002;120:165–175. doi: 10.1016/s0166-6851(01)00451-0. [DOI] [PubMed] [Google Scholar]
- Dowse TJ, Pascall JC, Brown KD, Soldati D. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Int J Parasitol. 2005;35:747–756. doi: 10.1016/j.ijpara.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DA, Clark DP. Cryptosporidium parvum induces host cell actin accumulation at the host–parasite interface. Infect Immun. 2000;68:2315–2322. doi: 10.1128/iai.68.4.2315-2322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A, Thirugnanam S, Lorestani A, Dvorin JD, Eidell KP, Ferguson DJ, Anderson-White BR, Duraisingh MT, Marth GT, Gubbels MJ. A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science. 2012;335:218–221. doi: 10.1126/science.1210829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R, Yeheskel A, Ashery U. DOC2B, C2 domains, and calcium: a tale of intricate interactions. Mol Neurobiol. 2010;41:42–51. doi: 10.1007/s12035-009-8094-8. [DOI] [PubMed] [Google Scholar]
- Gaffar FR, Yatsuda AP, Franssen FF, de Vries E. Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1. Infect Immun. 2004a;72:2947–2955. doi: 10.1128/IAI.72.5.2947-2955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffar FR, Yatsuda AP, Franssen FF, de Vries E. A Babesia bovis merozoite protein with a domain architecture highly similar to the thrombospondin-related anonymous protein (TRAP) present in Plasmodium sporozoites. Mol Biochem Parasitol. 2004b;136:25–34. doi: 10.1016/j.molbiopara.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Gaines G, Taylor FJR. Extracellular digestion in marine dinoflagellates. J Plankton Res. 1984;6:1057–1061. [Google Scholar]
- Gaines G, Elbrächter M. Heterotrophic nutrition. In: Taylor FJR, editor. The biology of dinoflagellates, Botanical Monographs. Vol. 65. Blackwell Scientific Publications; Oxford: 1987. [Google Scholar]
- Gaji RY, Flammer HP, Carruthers VB. Forward targeting of Toxoplasma gondii proproteins to the micronemes involves conserved aliphatic amino acids. Traffic. 2011;12:840–853. doi: 10.1111/j.1600-0854.2011.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins E, Gilk S, DeVore N, Mann T, Ward G, Beckers C. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J Cell Biol. 2004;165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Del Carmen M, Mondragon M, Gonzalez S, Mondragon R. Induction and regulation of conoid extrusion in Toxoplasma gondii. Cell Microbiol. 2009;11:967–982. doi: 10.1111/j.1462-5822.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- Green JL, Rees-Channer RR, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J Biol Chem. 2008;283:30980–30989. doi: 10.1074/jbc.M803129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen RE, Nelson DL. A novel Ca2+-dependent protein kinase from Paramecium tetraurelia. J Biol Chem. 1987;262:4602–4609. [PubMed] [Google Scholar]
- Hakansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 2001;20:3132–3144. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann K. Food acquisition, food ingestion and food digestion by protists. Jpn J Protozool. 2002;35:85–94. [Google Scholar]
- Hausmann K, Hülsmann N. Ultrastructural and functional aspects of static and motile systems in two taxa of the Alveolata: Dinoflagellata and Ciliata. Protistology. 2010;6:139–146. [Google Scholar]
- Holstein TW. A view to kill. BMC Biol. 2012;10:18. doi: 10.1186/1741-7007-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenrath M, Leander BS. Molecular phylogeny of Parvilucifera prorocentri (Alveolata, Myzozoa): Insights into perkinsid character evolution. J Eukaryot Microbiol. 2009;56:251–256. doi: 10.1111/j.1550-7408.2009.00395.x. [DOI] [PubMed] [Google Scholar]
- Ishida M, Allen RD, Fok AK. Phagosome formation in Paramecium: roles of somatic and oral cilia and of solid particles as revealed by video microscopy. J Eukaryot Microbiol. 2001;48:640–646. doi: 10.1111/j.1550-7408.2001.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Jacobson DM, Anderson DM. Ultrastructure of the feeding apparatus and myonemal system of the heterotrophic dinoflagellate Protoperidinium spinulosum. J Phycol. 1992;28:69–82. [Google Scholar]
- Kafsack BF, Carruthers VB, Pineda FJ. Kinetic modeling of Toxoplasma gondii invasion. J Theor Biol. 2007;249:817–825. doi: 10.1016/j.jtbi.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, Bursulaya B, Henson K, Johnson J, Kumar KA, Marr F, Mason D, McNamara C, Plouffe D, Ramachandran V, Spooner M, Tuntland T, Zhou Y, Peters EC, Chatterjee A, Schultz PG, Ward GE, Gray N, Harper J, Winzeler EA. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4:347–356. doi: 10.1038/nchembio.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats LM, Cooke BM, Coppel RL, Black CG. Protein trafficking to apical organelles of malaria parasites – building an invasion machine. Traffic. 2008;9:176–186. doi: 10.1111/j.1600-0854.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, Burger G, Durnford DG, Lang BF, Lee RW, Pearlman RE, Roger AJ, Gray MW. The tree of eukaryotes. Trends Ecol Evol. 2005;20:670–676. doi: 10.1016/j.tree.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, Frischknecht F, Meissner M. Microneme protein 8 – a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121:947–956. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lee YS, Landry AB., 3rd Regulation of Ca2+ release from sarcoplasmic reticulum in skeletal muscles. Mol Cell Biochem. 1992;114:105–108. doi: 10.1007/BF00240304. [DOI] [PubMed] [Google Scholar]
- Kim K, Messinger LA, Nelson DL. Ca2+-dependent protein kinases of Paramecium – cloning provides evidence of a multigene family. Eur J Biochem. 1998;251:605–612. doi: 10.1046/j.1432-1327.1998.2510605.x. [DOI] [PubMed] [Google Scholar]
- Lai L, Bumstead J, Liu Y, Garnett J, Campanero-Rhodes MA, Blake DP, Palma AS, Chai W, Ferguson DJ, Simpson P, Feizi T, Tomley FM, Matthews S. The role of sialyl glycan recognition in host tissue tropism of the avian parasite Eimeria tenella. PLoS Pathog. 2011;7:e1002296. doi: 10.1371/journal.ppat.1002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau RK, Kwok AC, Chan WK, Zhang TY, Wong JT. Mechanical characterization of cellulosic thecal plates in dinoflagellates by nanoindentation. J Nanosci Nanotechnol. 2007;7:452–457. [PubMed] [Google Scholar]
- Leander BS, Keeling PJ. Morphostasis in alveolate evolution. Trends Ecol Evol. 2003;18:395–402. [Google Scholar]
- Leander BS, Kuvardina ON, Aleshin VV, Mylnikov AP, Keeling PJ. Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): insights into the phagotrophic ancestry of apicomplexans. J Eukaryot Microbiol. 2003;50:334–340. doi: 10.1111/j.1550-7408.2003.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Leander BS. Marine gregarines: evolutionary prelude to the apicomplexan radiation? Trends Parasitol. 2008;24:60–67. doi: 10.1016/j.pt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Leander BS, Hoppenrath M. Ultrastructure of a novel tube-forming, intracellular parasite of dinoflagellates: Parvilucifera prorocentri sp nov (Alveolata, Myzozoa) Eur J Protistol. 2008;44:55–70. doi: 10.1016/j.ejop.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Liu L, Tucker SC, Satir BH. Toxoplasma PRP1 is an ortholog of parafusin (PFUS) in vesicle scaffold assembly in Ca(2+)-regulated exocytosis. Eur J Cell Biol. 2009;88:301–313. doi: 10.1016/j.ejcb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Liu L, Wyroba E, Satir BH. RNAi knockdown of parafusin inhibits the secretory pathway. Eur J Cell Biol. 2011;90:844–853. doi: 10.1016/j.ejcb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Livolant F. Dinoflagellate trichocyst ultrastructure I. The shaft. Biol Cell. 1982a;43:201–210. [Google Scholar]
- Livolant F. Dinoflagellate trichocyst ultrastructure II. Existence of a sheath. Biol Cell. 1982b;43:211–216. [Google Scholar]
- Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- Lynn DH. The ciliated protozoa. Springer Science; Dordrecht, Heidelberg, London, New York: 2010. [Google Scholar]
- Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–268. doi: 10.1016/s0166-6851(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Matthiesen SH, Shenoy SM, Kim K, Singer RH, Satir BH. A parafusin-related Toxoplasma protein in Ca2+-regulated secretory organelles. Eur J Cell Biol. 2001;80:775–783. doi: 10.1078/0171-9335-00214. [DOI] [PubMed] [Google Scholar]
- Matthiesen SH, Shenoy SM, Kim K, Singer RH, Satir BH. Role of the parafusin orthologue, PRP1, in microneme exocytosis and cell invasion in Toxoplasma gondii. Cell Microbiol. 2003;5:613–624. doi: 10.1046/j.1462-5822.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- McLean N, Galt CP. Apodinium floodi n. sp., a dinoflagellate (Dinoflagellata: Apodinidae) ectoparasitic on Oikopleura labradoriensis (Urochordata: Larvacea) Dis Aquat Org. 1990;9:213–219. [Google Scholar]
- Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol Biol Cell. 2002;13:2397–2409. doi: 10.1091/mbc.E02-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Adjogble KD, Daubener W, Delauw MF. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int J Parasitol. 2005;35:829–849. doi: 10.1016/j.ijpara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mital J, Meissner M, Soldati D, Ward GE. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell. 2005;16:4341–4349. doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon R, Frixione E. Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol. 1996;43:120–127. doi: 10.1111/j.1550-7408.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- Moon RW, Taylor CJ, Bex C, Schepers R, Goulding D, Janse CJ, Waters AP, Baker DA, Billker O. A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog. 2009;5:e1000599. doi: 10.1371/journal.ppat.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RB, Obornik M, Janouskovec J, Chrudimsky T, Vancova M, Green DH, Wright SW, Davies NW, Bolch CJ, Heimann K, Slapeta J, Hoegh-Guldberg O, Logsdon JM, Carter DA. A photosynthetic alveolate closely related to apicomplexan parasites. Nature. 2008;451:959–963. doi: 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol. 2006;23:1613–1627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008a;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008b;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- Ngo HM, Yang M, Joiner KA. Are rhoptries in Apicomplexan parasites secretory granules or secretory lysosomal granules? Mol Microbiol. 2004;52:1531–1541. doi: 10.1111/j.1365-2958.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- Noren F, Moestrup Ø, Rehnstam-Holm AS. Parvilucifera infectans Noren et Moestrup gen et sp nov (Perkinsozoa phylum nov): a parasitic flagellate capable of killing toxic microalgae. Eur J Protistol. 1999;35:233–254. [Google Scholar]
- O’Donnell RA, Hackett F, Howell SA, Treeck M, Struck N, Krnajski Z, Withers-Martinez C, Gilberger TW, Blackman MJ. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol. 2006;174:1023–1033. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara SP, Small AJ, Chen XM, LaRusso NF. Host cell actin remodeling in response to Cryptosporidium. Subcell Biochem. 2008;47:92–100. doi: 10.1007/978-0-387-78267-6_7. [DOI] [PubMed] [Google Scholar]
- Obornik M, Vancova M, Lai DH, Janouskovec J, Keeling PJ, Lukes J. Morphology and ultrastructure of multiple life cycle stages of the photosynthetic relative of apicomplexa, Chromera velia. Protist. 2011;162:115–130. doi: 10.1016/j.protis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Obornik M, Modry D, Lukes M, Cernotikova-Stribrna E, Cihlar J, Tesarova M, Kotabova E, Vancova M, Prasil O, Lukes J. Morphology, ultrastructure and life cycle of Vitrella brassicaformis n. sp., n gen., a novel chromerid from the Great Barrier. Reef Protist. 2012;163:306–323. doi: 10.1016/j.protis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Horak A, Keeling PJ. Description of Two Species of Early Branching Dinoflagellates, Psammosa pacifica n. g., n sp and P atlantica n sp. PLoS ONE. 2012;7:e34900. doi: 10.1371/journal.pone.0034900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins FO. Zoospores of the oyster pathogen, Dermocystidium marinum I Fine structure of the conoid and other sporozoan-like organelles. J Parasitol. 1976;62:959–974. [Google Scholar]
- Perkins FO. The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine, 1978 with comments on taxonomy and phylogeny of Perkinsus spp. J Shellfish Res. 1996;15:67–87. [Google Scholar]
- Plachetzki DC, Fong CR, Oakley TH. Cnidocyte discharge is regulated by light and opsin-mediated phototransduction. BMC Biol. 2012;10:17. doi: 10.1186/1741-7007-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H, Klauke N. Calcium in ciliated protozoa: sources, regulation, and calcium-regulated cell functions. Int Rev Cytol. 2001;201:115–208. doi: 10.1016/s0074-7696(01)01003-8. [DOI] [PubMed] [Google Scholar]
- Plattner H, Kissmehl R. Dense-core secretory vesicle docking and exocytotic membrane fusion in Paramecium cells. Biochim Biophys Acta. 2003;1641:183–193. doi: 10.1016/s0167-4889(03)00092-2. [DOI] [PubMed] [Google Scholar]
- Plattner H, Sehring IM, Mohamed IK, Miranda K, De Souza W, Billington R, Genazzani A, Ladenburger EM. Calcium signaling in closely related protozoan groups (Alveolata): non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma) Cell Calcium. 2012;51:351–382. doi: 10.1016/j.ceca.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Proellocks NI, Coppel RL, Waller KL. Dissecting the apicomplexan rhoptry neck proteins. Trends Parasitol. 2010;26:297–304. doi: 10.1016/j.pt.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Putignani L, Possenti A, Cherchi S, Pozio E, Crisanti A, Spano F. The thrombospondin-related protein CpMIC1 (CpTSP8) belongs to the repertoire of micronemal proteins of Cryptosporidium parvum. Mol Biochem Parasitol. 2008;157:98–101. doi: 10.1016/j.molbiopara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ravindran S, Lodoen MB, Verhelst SH, Bogyo M, Boothroyd JC. 4-Bromophenacyl bromide specifically inhibits rhoptry secretion during Toxoplasma invasion. PLoS ONE. 2009;4:e8143. doi: 10.1371/journal.pone.0008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci USA. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko MS, Carruthers VB. New roles for perforins and proteases in apicomplexan egress. Cell Microbiol. 2009;11:1444–1452. doi: 10.1111/j.1462-5822.2009.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati G, Modeo L. Extrusomes in ciliates: diversification, distribution, and phylogenetic implications. J Eukaryot Microbiol. 2003;50:383–402. doi: 10.1111/j.1550-7408.2003.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir BH, Hamasaki T, Reichman M, Murtaugh TJ. Species distribution of a phosphoprotein (parafusin) involved in exocytosis. Proc Natl Acad Sci USA. 1989;86:930–932. doi: 10.1073/pnas.86.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage V, Aubert D, Escotte-Binet S, Villena I. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol Biochem Parasitol. 2009;167:81–94. doi: 10.1016/j.molbiopara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Schnepf E, Deichgraber G. “Myzocytosis”, a kind of endocytosis with implications to compartmentalization in endosymbiosis. Naturwissenschaften. 1984;71:218–219. [Google Scholar]
- Schnepf E, Elbrachter M. Nutritional strategies in dinoflagellates: a review with emphasis on cell biological aspects. Eur J Protistol. 1992;28:3–24. doi: 10.1016/S0932-4739(11)80315-9. [DOI] [PubMed] [Google Scholar]
- Schnepf E. Protoctists and microalgae: antagonistic and mutualistic associations and the symbiogenesis of plastids. In: Esser K, Luttge U, Beyschlag W, Murata J, editors. Progress in Botany. Vol. 65. Springer-Verlag; Berlin, Heidelberg, New York: 2004. pp. 1–11. [Google Scholar]
- Shaw MK, Tilney LG, Musoke AJ. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J Cell Biol. 1991;113:87–101. doi: 10.1083/jcb.113.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MK, Tilney LG. The entry of Theileria parva merozoites into bovine erythrocytes occurs by a process similar to sporozoite invasion of lymphocytes. Parasitology. 1995;111 (Pt 4):455–461. doi: 10.1017/s0031182000065951. [DOI] [PubMed] [Google Scholar]
- Shaw MK. Cell invasion by Theileria sporozoites. Trends Parasitol. 2003;19:2–6. doi: 10.1016/s1471-4922(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol. 2006;60:1355–1363. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simdyanov TG, Kuvardina ON. Fine structure and putative feeding mechanism of the archigregarine Selenidium orientale (Apicomplexa: Gregarinomorpha) Eur J Protistol. 2007;43:17–25. doi: 10.1016/j.ejop.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Simpson AGB, Patterson DJ. Ultrastructure and identification of the predatory flagellate Colpodella pugnax Cienkowski (Apicomplexa) with a description of Colpodella turpis n sp and a review of the genus. Syst Parasitol. 1996;33:187–198. [Google Scholar]
- Singh S, Plassmeyer M, Gaur D, Miller LH. Mononeme: a new secretory organelle in Plasmodium falciparum merozoites identified by localization of rhomboid-1 protease. Proc Natl Acad Sci USA. 2007;104:20043–20048. doi: 10.1073/pnas.0709999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati D, Foth BJ, Cowman AF. Molecular and functional aspects of parasite invasion. Trends Parasitol. 2004;20:567–574. doi: 10.1016/j.pt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Son M, Gundersen RE, Nelson DL. A second member of the novel Ca(2+)-dependent protein kinase family from Paramecium tetraurelia Purification and characterization. J Biol Chem. 1993;268:5940–5948. [PubMed] [Google Scholar]
- Stelly N, Mauger JP, Claret M, Adoutte A. Cortical alveoli of Paramecium: a vast submembranous calcium storage compartment. J Cell Biol. 1991;113:103–112. doi: 10.1083/jcb.113.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecker DK. Mixotrophy among dinoflagellates. J Eukaryot Microbiol. 1999;46:397–401. [Google Scholar]
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Tetley L, Brown SM, McDonald V, Coombs GH. Ultrastructural analysis of the sporozoite of Cryptosporidium parvum. Microbiology. 1998;144:3249–3255. doi: 10.1099/00221287-144-12-3249. [DOI] [PubMed] [Google Scholar]
- Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in a nitric oxide-dependent manner. Infect Immun. 2012;80:55–61. doi: 10.1128/IAI.05543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley FM, Soldati DS. Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 2001;17:81–88. doi: 10.1016/s1471-4922(00)01761-x. [DOI] [PubMed] [Google Scholar]
- Tyler JS, Treeck M, Boothroyd JC. Focus on the ringleader: the role of AMA1 in apicomplexan invasion and replication. Trends Parasitol. 2011;27:410–420. doi: 10.1016/j.pt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini E, Cherchi S, Possenti A, Dubremetz JF, Pozio E, Spano F. Molecular characterisation of a Cryptosporidium parvum rhoptry protein candidate related to the rhoptry neck proteins TgRON1 of Toxoplasma gondii and PfASP of Plasmodium falciparum. Mol Biochem Parasitol. 2012;183:94–99. doi: 10.1016/j.molbiopara.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Westfall JA, Bradbury PC, Townsend JW. Ultrastructure of the dinoflagellate Polykrikos. I Development of the nematocyst-taeniocyst complex and morphology of the site for extrusion. J Cell Sci. 1983;63:245–261. doi: 10.1242/jcs.63.1.245. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Schmidt J, Kuhlenschmidt MS, Dubey JP, Sibley LD. Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infect Immun. 2005;73:5379–5387. doi: 10.1128/IAI.73.9.5379-5387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma HI, Galuska SE, Tomley FM, Sibley LD, Liberator PA, Donald RG. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int J Parasitol. 2004;34:369–380. doi: 10.1016/j.ijpara.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Yeoh S, O’Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, Hackett F, Withers-Martinez C, Mitchell GH, Bannister LH, Bryans JS, Kettleborough CA, Blackman MJ. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Zhang XS, Choi JH. Molecular evolution of calmodulin-like domain protein kinases (CDPKs) in plants and protists. J Mol Evol. 2001;53:214–224. doi: 10.1007/s002390010211. [DOI] [PubMed] [Google Scholar]