Abstract
A plant’s surface is covered with epicuticular wax, which protects plants from inappropriate environmental conditions such as drought and pathogen attack. Very-long-chain fatty acids (VLCFAs) are the main component of epicuticular wax on the surface of above-ground organs. Here we show that a fatty acid elongase catalyzing an elongation reaction of VLCFAs is required for shoot development in rice. onion2 (oni2) mutants produced very small shoots in which leaves were fused to each other, and ceased growing after germination. The midrib of oni2 leaf blades was not developed correctly. Molecular cloning showed that ONI2 encodes a fatty acid elongase, which catalyzes the first step of elongation reactions of a carbon chain of VLCFAs, and oni2 had a reduced amount of VLCFAs. Expression analysis showed that ONI2 is specifically expressed in the outermost cell layer of young lateral organs. These results suggest that ONI2 is a layer 1-specific gene required for development of the entire shoot and that VLCFAs play an essential role in normal shoot development in rice.
Keywords: Fatty acid elongase, L1, Rice, Shoot, Very-long-chain fatty acid
Introduction
Epicuticular wax, which is present on the surface of the epidermis, protects plants from inappropriate environmental conditions producing biological and non-biological stresses. Very-long-chain fatty acids (VLCFAs) are the main component of the wax and are synthesized from C18 carbon chains by four-step sequential elongation reactions. The first step is a condensation reaction of a C2 unit to an acyl-CoA that is catalyzed by fatty acid elongase (β-ketoacyl-CoA synthase), the second step is a reduction reaction catalyzed by β-ketoacyl-CoA reductase, the third step is a dehydration reaction catalyzed by β-hydroxyl-CoA dehydratase and the last step is a reduction reaction catalyzed by enoyl-CoA reductase (Kunst and Samuels 2009). Several genes that encode fatty acid elongase have been identified, and their analyses showed that fatty acid elongase is necessary for the proper composition of VLCFAs and for avoiding ectopic organ fusions. For example, fiddlehead (fdh) mutants in Arabidopsis showed defective epidermal function, which brings about altered floral architecture due to abnormal floral organ formation, resulting in fusion of organs to one another (Yephremov et al. 1999, Pruitt et al. 2000). The fdh mutants also showed organ fusions in leaves, although vegetative shoot development appeared rather normal (Yephremov et al. 1999, Pruitt et al. 2000). fdh showed an altered composition, but not a reduced amount of VLCFAs (Voisin et al. 2009). In contrast to fdh mutants, mutations of the ONION1 (ONI1) gene in rice, which is an ortholog of FDH, showed severe morphological defects leading to seedling lethality (Ito et al. 2011). Although oni1 also showed organ fusions as is the case in Arabidopsis fdh, the oni1 mutants lacked a normal outermost cell layer (layer 1 or L1) and failed to maintain the shoot apical meristem (SAM). Along with the expression of ONI1 in embryogenesis, defects in the SAM were also observed even at this stage. In addition, oni1 had a reduced amount of VLCFAs. Consistent with these phenotypic differences, FDH failed to complement the oni1 mutation. These results suggest that rice fatty acid elongase ONI1 has a more critical function than Arabidopsis FDH in plant development, or ONI1 has an additional function to that of FDH.
To better understand the function of fatty acid elongase in plant development, we analyzed oni2 mutants, which were identified as mutants resembling the morphology of a KNOX gene-overexpressing plant and were morphologically similar to oni1 (Tsuda et al. 2009). The KNOX gene, which is specifically expressed in the SAM, is known to play an essential role in SAM formation and maintenance, and its ectopic expression brought about abnormal shoot morphologies (Ito et al. 2001, Tsuda et al. 2011). We previously reported that oni2 showed ectopic expression of KNOX genes in leaves, and ONI2 was mapped on the long arm of chromosome 10 (Tsuda et al. 2009). In this study, we carried out a detailed analysis of oni2 mutants and molecular characterization of ONI2. oni2 shoots showed various defects such as organ fusions between leaves and incorrect development of the midrib in the leaf blade. Molecular cloning showed that ONI2 encodes a fatty acid elongase similar to ONI1 and FDH, and is expressed specifically in L1 of young lateral organs throughout its life cycle. oni2 had a reduced amount of VLCFAs. Our results suggest that the fatty acid elongase and VLCFAs play an essential role in shoot development in rice.
Results
Morphological analysis of oni2
We previously reported identification of onion mutants (Tsuda et al. 2009). In this study we examined the morphology of oni2 seedlings. Since oni2 mutants were seedling lethal (Tsuda et al. 2009), we could not examine phenotypes at later developmental stages including reproductive stages, although ONI2 was expressed throughout its life cycle (see ‘Expression of ONI2’).
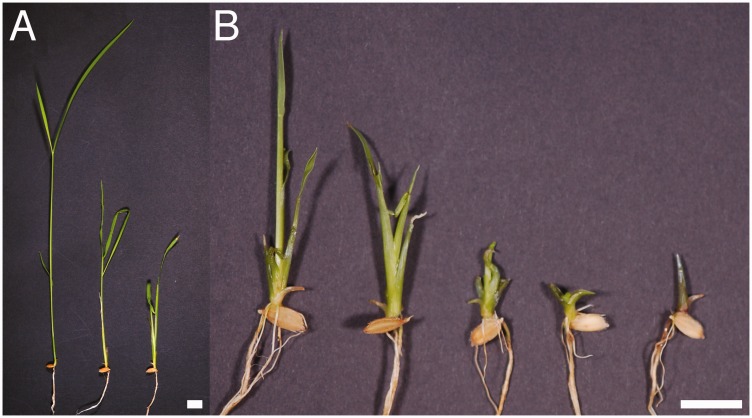
The severity of oni2 phenotypes was very different among individuals even in the same allele (Fig. 1). In the most severe case, oni2-1 mutant seeds produced dark green and very small shoots, and ceased growing soon after germination (Fig. 1B). In milder cases, oni2-1 mutant shoots could elongate, and their leaf blade and leaf sheath could be clearly distinguished from each other, albeit that oni2 mutants were still shorter than wild-type shoots and did not reach maturity (Fig. 1A, B).
Fig. 1.
Various gross morphologies of oni2 shoots. (A) The wild-type (left) and mild oni2-1 (middle and right) mutant shoots. (B) Moderate (left) to severe (right) mutant oni2-1 shoots. Bars = 1 cm.
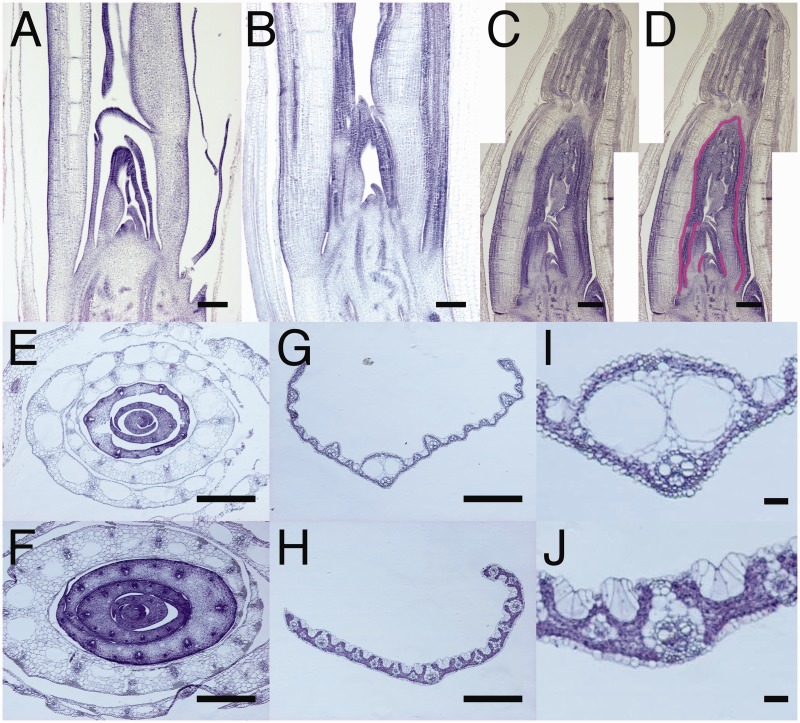
Longitudinal and transverse sections of oni2-1 showed organ fusions between neighboring leaves, which were observed in both severe and mild mutant shoots (Fig. 2A–F). Organ fusions were observed in continuous regions along the proximo-distal and lateral axes of the leaf, but not in an entire region of the leaf (Fig. 2B–D, F). In contrast, no organ fusion was observed in the wild type, and instead space was clearly observed between leaves (Fig. 2A, E). Transverse sections of leaf blades of mild mutant shoots showed abnormal midribs (Fig. 2G–J). In the wild type, two lacunae were observed in the midrib, whereas in the oni2-1 midrib no lacuna was observed. The overall leaf architecture was quite discordant in oni2-1, and the oni2-1 leaf was thick, except for the midribs (Fig. 2I, J).
Fig. 2.
Histological observation of oni2 shoots. (A–D) Longitudinal sections of shoots of the wild type (A), and mild oni2-1 (B) and severe oni2-1 mutants (C and D). Organ fusions between neighboring leaves were observed in oni2-1. Fused regions are labeled in magenta (D). (E and F) Transverse sections of wild-type (E) and oni2-1 (F) shoots. Organ fusions between neighboring leaves were observed. (G and H) Transverse sections of the third leaf of the wild type (G) and oni2-1 (H). The midrib was not well developed in oni2-1. (I and J) Enlargement of the midribs of the wild type (I) and oni2-1 (J). Lacunae were not observed in oni2-1. Bars = 200 µm in (A–D), 1 mm in (E–H) and 100 µm in (I) and (J).
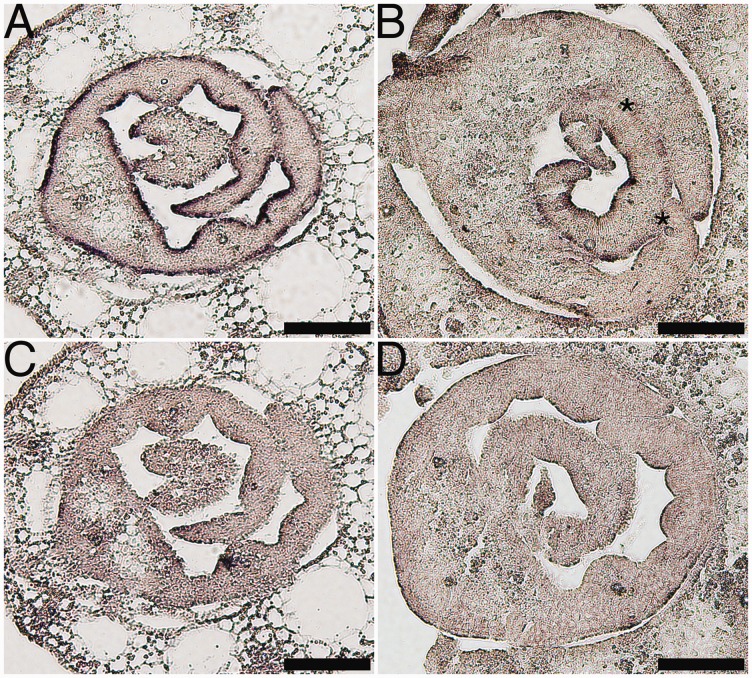
Since organ fusions suggested a defect in L1, we examined expression of ROC1 in oni2-1. ROC1 is an ortholog of an Arabidopsis L1-specific gene ATML1 required for L1 identification and is specifically expressed in L1 in rice (Ito et al. 2002, Abe et al. 2003). In situ hybridization detected ROC1 expression in L1 of both the abaxial and adaxial sides in the wild-type leaf, but in the oni2-1 mutant leaf ROC1 expression in the abaxial side was hardly detected, and in the adaxial side it was reduced (Fig. 3). Perturbed expression of ROC1 suggested that the differentiation and/or functionality of L1 was compromised in oni2. Thus, oni2 seemed to lack normal L1 in the leaf.
Fig. 3.
Expression of ROC1 in oni2. Transverse sections of the wild type (A and C) or oni2 (B and D) were hybridized with the antisense probe (A and B) or sense probe (C and D) of ROC1. Asterisks in (B) indicate the sites of organ fusions. Bars = 100 µm.
Molecular cloning of ONI2
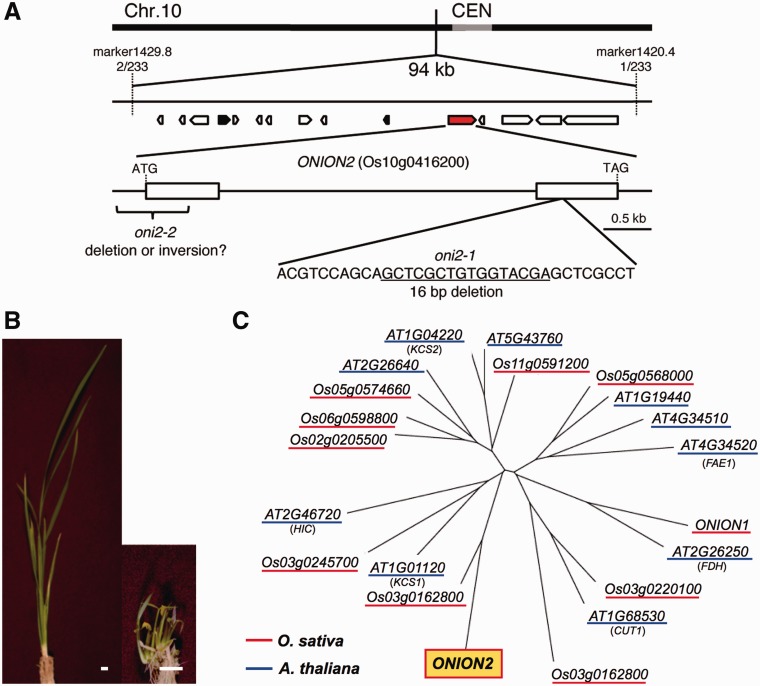
We previously mapped ONI2 on the long arm of chromosome 10 (Tsuda et al. 2009). Fine mapping using 233 F2 plants derived from a crossing of an oni2 heterozygous plant and the indica cultivar Kasalath narrowed down the candidate region within 94 kb, and in this region 15 genes were predicted (Fig. 4A). Among them, Os10 g0416200 was predicted to encode fatty acid elongase. Since a fatty acid elongase mutant oni1 showed a very similar morphology to that of severe oni2 mutants (Tsuda et al. 2009, Ito et al. 2011), we analyzed the sequence of Os10 g0416200 in oni2. PCR amplification and sequence analysis showed that Os10 g0416200 has a 16 bp deletion in the second exon in oni2-1 (Fig. 4A). In addition, we failed to amplify the first exon of Os10 g0416200 by PCR in oni2-2, probably due to deletion of the exon, a large insertion in the exon or an inversion (Fig. 4A). These results indicated that Os10 g0416200 is a strong candidate for ONI2. To examine this possibility, a complementation test was carried out. When oni2-1 mutant calli were transformed with a vector containing a wild-type ONI2 genome, normal shoots were regenerated. They reached maturity and set seeds. On the other hand, when oni2-1 mutant calli were transformed with an empty vector, mutant shoots were regenerated and stopped growing before maturity (Fig. 4B). From these results, we concluded that Os10g0416200 is ONI2.
Fig. 4.
Cloning of ONI2. (A) The ONI2 locus on chromosome 10. Mapping with 233 F2 plants narrowed down the ONI2 locus to within a 94 kb region between marker 1429.8 and marker1420.4 near the centromere. In this region, 15 genes were predicted, one of which, Os10g0416200 marked in red, encodes a protein similar to fatty acid elongase. Os10g0416200 had mutations in two oni2 mutant alleles examined. (B) Complementation of oni2 by the fatty acid elongase gene Os10g0416200. The oni2-1 mutant calli transformed with the wild-type genomic fragment (left) showed regeneration of wild-type shoots, whereas the mutant calli transformed with an empty vector showed regeneration of mutant shoots (right). Bars = 1 cm. (C) A phylogenetic tree of fatty acid elongases of rice and Arabidopsis drawn on the basis of the entire amino acid sequences. Rice and Arabidopsis proteins are underlined in red and blue, respectively. ONI2 is shaded in yellow. AT1G01120, KCS1; AT1G04220, KCS2; AT1G68530, CUT1; AT2G26250, FDH; AT4G34520, FAE1.
Structural characteristics of ONI2
ONI2 consists of two exons separated by an intron and encodes a protein with 523 amino acid residues. A similarity search and phylogenetic analysis showed that ONI2 is homologous to fatty acid elongase (β-ketoacyl-CoA synthase) that catalyzes the first step of elongation reactions of the carbon chain of VLCFAs (Fig. 4C). We previously identified ONI1, which also encodes fatty acid elongase and is required for proper shoot development (Ito et al. 2011). ONI2 was 44% identical to ONI1 in their entire amino acid sequences. No close homolog of ONI2 was identified in Arabidopsis (Fig. 4C).
Expression of ONI2
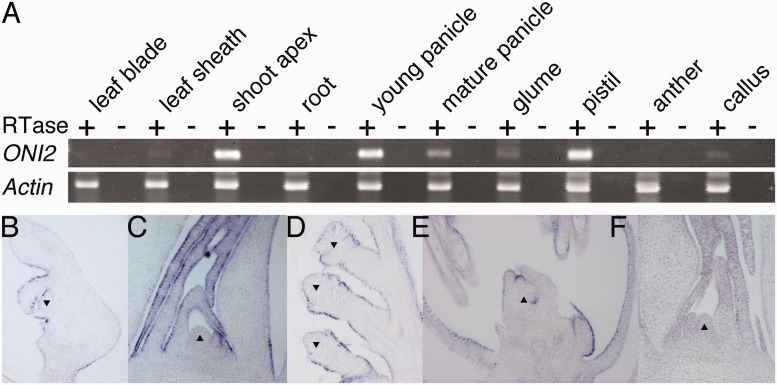
Reverse transcription–PCR (RT–PCR) analysis showed that ONI2 is expressed in the shoot apex, panicle and pistil, and weakly in the leaf sheath, glume and callus, but not in the leaf blade, anther or root (Fig. 5A). RNA in situ hybridization showed that ONI2 was expressed in L1 of the embryo, young leaf and flower. In the embryo, ONI2 expression was detected in L1 of developing organs such as leaves and coleoptile (Fig. 5B). The expression was hardly detected in the SAM, and no signal was detected in the radicle. In the vegetative growth stage, ONI2 expression was specifically detected in L1 of young developing leaves, but no signal was detected in the SAM (Fig. 5C). In the reproductive growth phase, ONI2 expression was detected in L1 of the developing panicle and floral organs (Fig. 5D, E). No signal was detected in the inner cells in any organs examined. A sense probe also produced no signal above the background (Fig. 5F). These expression analyses revealed that ONI2 is an L1-specific gene of the young above-ground lateral organs.
Fig. 5.
Expression pattern of ONI2. (A) RT–PCR. RNAs isolated from the indicated organs were reverse transcribed with the oligo(dT) primer and amplified by ONI2 or actin-specific primers (Supplementary Table S1). + and – indicate whether reverse transcriptase was added to or omitted from the reaction mixture, respectively. (B–F) RNA in situ hybridization. Longitudinal sections were hybridized with the antisense probe of ONI2 (B–E). (B) Embryo. (C) Vegetative shoot apex. (D) Panicle branches. (E) Developing flower. (F) Vegetative shoot apex hybridized with the sense probe of ONI2 as a negative control. Triangles indicate the SAM (B, C and F), rachis branch meristem (D) and developing pistil (E).
Fatty acid composition of oni2
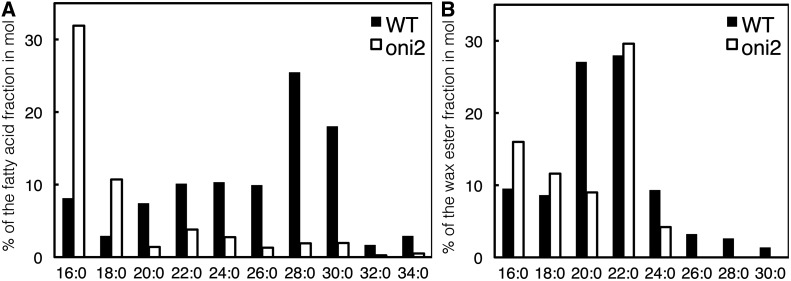
To examine whether ONI2 encodes a functional fatty acid elongase, we examined the composition of VLCFAs in a 2-week-old oni2 shoot. In the oni2-1 shoot, the amount of free saturated VLCFAs with a carbon number of 20 (C = 20) or more was reduced compared with that of the wild-type shoot. In particular, VLCFAs of C = 32 and C = 34 were barely detected (Fig. 6A).
Fig. 6.
Amount of saturated VLCFAs in oni2. (A) Amount of free saturated VLCFAs. The percentages of saturated VLCFAs per total fatty acids in a molar ratio are shown. The values are the average of two independent experiments. (B) Amount of saturated VLCFAs in the alkyl ester fraction of epicuticular wax. The percentages of methyl esters of saturated VLCFAs per total fatty acid methyl esters in a molar ratio are shown.
We further examined the composition of VLCFAs in the alkyl ester fraction of the epicuticular wax of a 2-week-old oni2-1 shoot. We found that the amount of saturated VLCFAs of C = 20, 24, 26, 28 and 30 was reduced in the oni2-1 shoot compared with the wild type shoot. In particular, VLCFAs of C = 26 and longer were not detected (Fig. 6B). These results indicate that VLCFAs in the alkyl ester fraction of epicuticular wax were reduced in the oni2-1 shoot.
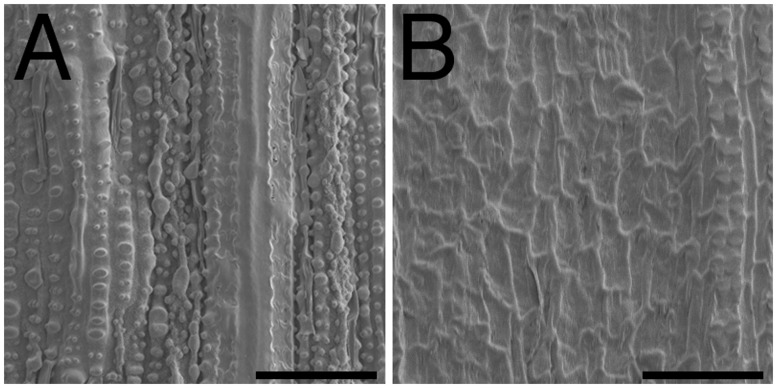
Since VLCFAs are the main components of cuticle wax, we examined protrusions of cuticle on the abaxial surface of the leaf sheath. Scanning electron microscopy (SEM) observation showed that the cuticular architecture with protrusions was well developed on the surface of the leaf epidermis in the wild type, but such protrusions were not observed on the surface of oni2-1 epidermis (Fig. 7). These results indicate that oni2 had a reduced amount of VLCFAs, which is consistent with the idea that ONI2 encodes functional fatty acid elongase.
Fig. 7.
SEM observation of the leaf surface. (A) Wild type. (B) oni2. The surface was rough in the wild type due to cuticle protrusions, but it was rather smooth in oni2. Bars = 50 µm.
Expression of auxin-related genes
Since expression of KNOX genes, which are ectopically expressed in oni2, is known to be negatively regulated by auxin (Hay et al. 2006, Tsuda et al. 2009, Perez-Perez 2010, Tabata et al. 2010), and oni1 showed altered expression of auxin-inducible IAA genes, suggesting altered distribution of auxin in its shoot apex (Takasugi and Ito 2011), we examined their expression in oni2. Quantitiative real-time PCR analysis showed that OsIAA7 was up-regulated in oni2-1, whereas OsIAA14 was down-regulated (Fig. 8). These results suggest that the expression of auxin-inducible genes is perturbed in oni2 shoots.
Fig. 8.
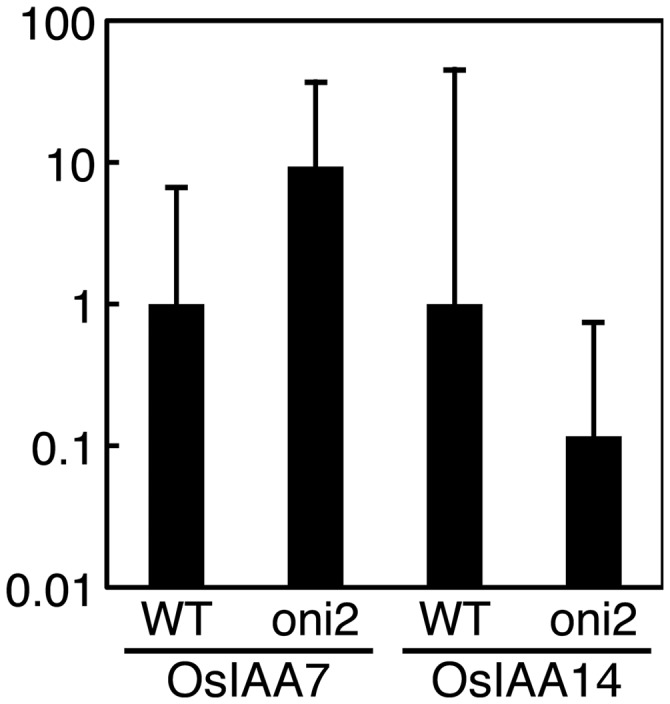
Expression of auxin-related genes in oni2 shoots. Expression of OsIAA7 and OsIAA14. RNAs were isolated from shoots of 1-week-old seedlings grown in a growth chamber at 30°C with continuous light, and subjected to quantitative real-time PCR. Actin was used as a reference, and the relative expression level is shown.
Discussion
In this study, we showed that ONI2 encodes a fatty acid elongase catalyzing an elongation reaction of VLCFAs and is specifically expressed in L1 in the shoot. In spite of the L1-specific expression of ONI2, the effects of oni2 mutations were not restricted to L1, but were expanded over the entire shoot. From these results, we suggest that ONI2 and VLCFAs or VLCFA-containing compound(s) are required for L1 development, and L1 supports the development of the entire shoot possibly by producing or transmitting a signal required for inner cell development, in agreement with the previous suggestion that L1 both promotes and restricts shoot growth by providing a non-autonomous signal to inner cells (Savaldi-Goldstein et al. 2007).
VLCFA biosynthesis genes and shoot development
In addition to oni2, several mutants involved in the biosynthesis of VLCFAs have been analyzed thus far. For example, oni1, another fatty acid elongase mutant of rice, showed very similar phenotypes to oni2, including organ fusions, lack of normal L1, a reduced amount of VLCFAs and perturbed expression of auxin-related genes and KNOX genes (Tsuda et al. 2009, Ito et al. 2011, Takasugi and Ito 2011). In Arabidopsis, fdh showed organ fusions mainly in the reproductive phase and some defects of epidermal cell differentiation (Yephremov et al. 1999, Pruitt et al. 2000). In addition to genes encoding fatty acid elongase, which catalyzes the first step of the elongation reactions of VLCFAs, some genes involved in other steps of elongation reactions have also been shown to play an essential role in plant development. A double mutant of maize GL8a and GL8b, both of which encode 3-ketoacyl reductase catalyzing the second step of the elongation reactions, has been shown to result in embryonic lethality (Dietrich et al. 2005). A null mutation in Arabidopsis PASTICCINO2 (PAS2), which encodes a hydroxy fatty acyl-CoA dehydratase catalyzing the third step of the elongation reactions, has also been shown to result in embryonic lethality (Bach et al. 2008). pas2 mutants also showed defects in plastid division (Nobusawa and Umeda 2012). Since several genes necessary for different steps of VLCFA biosynthesis are required for normal shoot development in different plant species, it is suggested that VLCFAs or VLCFA-containing compounds and their biosynthesis genes play indispensable roles in plant growth and development.
How do VLCFAs affect shoot development? One of the simple explanations is that VLCFAs or VLCFA-containing compounds have a physiological role required for proper L1 development. In this case, altered L1 development is directly affected by a change in the amount or composition of VLCFAs, and a lack of normal L1 consequently affects development of the entire shoot. However, one could argue that perturbing the amount or composition of VLCFAs removes natural barriers that normally block inappropriate information exchange between cells or between a cell and the environment. If signaling is abnormal, downstream developmental events may be abnormal, and this cascade of signaling events results in the evident phenotype. In this case, the primary defect can bring about downstream consequences, and those consequences manifest as a defect in shoot development.
Importance of L1 for shoot development
oni2 showed organ fusions, lack of cuticle protrusions on the surface of the epidermis and perturbed expression of an L1-specific gene ROC1. These phenotypes suggest that the differentiation and/or functionality of L1 is impaired in oni2. However, in spite of L1-specific expression of ONI2, the effects of the oni2 mutations were not restricted to L1 as oni2 did not show simply an epidermis-less or abnormal epidermis phenotype, but expanded to an entire shoot to produce a small shoot and abnormal development of the midrib. Thus, ONI2 function could be non-cell autonomous. Another fatty acid elongase gene ONI1 also showed L1-specific expression, and oni1 mutants had abnormal L1, which brought about abnormal development of the entire shoot. These mutant analyses suggest that L1 is necessary for normal development of inner cells and supports development of the entire shoot. A non-autonomous signal provided from L1 to the inner cells has been suggested in this process in Arabidopsis (Savaldi-Goldstein et al. 2007).
Difference in phenotypic severity between oni1 and oni2
oni2 showed various degrees of phenotypic severity even in the same allele, from severe phenotypes with very small shoots to mild phenotypes with more or less elongated shoots. A simple explanation for the variation in morphological severity is developmental timing and the environment that induce organ fusions. The fusion events may occur only if sustained surface contact is achieved. Otherwise, epidermal cells do not adhere, and growth continues normally.
In contrast to oni2, which showed various degrees of phenotypic severity, oni1 showed only severe phenotypes with very small shoots (Tsuda et al. 2009, Ito et al. 2011). Two possible reasons may be able to account for this difference. One is the difference in substrate specificities between ONI1 and ONI2, and the other is the difference in expression patterns of these two genes.
Analysis of the fatty acid composition showed that oni2 had a reduced amount of VLCFAs with a carbon number of ≥20. This suggests that ONI2 catalyzes an elongation reaction of VLCFAs with a carbon number of ≥20. ONI1 was also suggested to catalyze an elongation reaction of VLCFAs with a carbon number of ≥20 (Ito et al. 2011). Thus, ONI and ONI2 seemed to have similar substrate specificities, at least when focusing on saturated VLCFAs. Therefore, substrate specificities for saturated VLCFAs may not be the cause of the difference in the phenotypic severity between oni1 and oni2, although the difference in the composition of the non-saturated VLCFAs between these two mutants cannot be ruled out.
In contrast, the difference in expression patterns may be able to explain the difference of phenotypic severity. ONI1 is strongly expressed in the entire L1 of the SAM and young lateral organs in the shoot (Ito et al. 2011). In contrast, ONI2 was expressed in young lateral organs in the shoot, but not in the SAM. The differences in expression patterns may be a possible cause of the difference in the phenotypic severities between oni1 and oni2. Analysis of the oni1 oni2 double mutant would be a useful approach to understand the functional differences, if any, between ONI1 and ONI2.
Perturbed expression of auxin-inducible genes
Auxin is known to play indispensable roles in various aspects of plant growth and development (Shani et al. 2006). oni2 lacked normal L1, which is a preferential pathway of polar auxin transport in the rice shoot (Miyashita et al. 2010). This may result in altered auxin distribution in oni2. Consistent with this, oni2 showed altered expression of IAA genes whose expression is generally induced by auxin (Jain et al. 2006). KNOX genes, which are specifically expressed in the SAM and bring about abnormal shoot morphologies when overexpressed, are ectopically expressed in oni2 leaves (Tsuda et al. 2009). It is also known that auxin negatively regulates expression of KNOX genes, and enhanced auxin response is reported to change leaf morphologies by interacting with a KNOX repressing pathway (Hay et al. 2006, Perez-Perez 2010, Tabata et al. 2010). The altered expression of IAA and KNOX genes is consistent with the idea that auxin distribution might be altered in oni2 shoots. If this is the case, this might be one of the possible causes of the abnormal shoot morphology of oni2. Analysis of auxin distribution in the oni2 shoot will clarify this notion.
Materials and Methods
Plant materials
oni2 mutants were identified from Tos17-mutant populations derived from Oryza sativa L. cultivar Nipponbare (Tsuda et al. 2009). Nipponbare was used as the wild type.
Histological analysis
Samples were fixed in FAA [formaldehyde : glacial acetic acid : 50% ethanol (1 : 1 : 18)] overnight at 4°C and then dehydrated in a graded ethanol series. The absolute ethanol in dehydrated samples was replaced with xylene and the samples were embedded in Paraplast plus (McCormick Scientific). Microtome sections (10 µm thick) were stained with Delafield’s hematoxylin and observed with a light microscope.
SEM analysis
The leaf sheaths of 10-day-old seedlings grown in an incubator at 30°C under 24 h light conditions were used for SEM analysis. The samples were freeze-dried, coated with platinum–palladium and observed using a Hitachi SU8000.
Cloning of ONI2
ONI2 was mapped on the long arm of chromosome 10 (Tsuda et al. 2009). Fine mapping was carried out using an F2 population of 233 individuals derived from a crossing of an oni2 heterozygous plant and the indica cultivar Kasalath. Coding regions of a candidate gene were amplified by PCR with the primer combinations e1-2f and e1-2r, and e2-2f and e2-2r, and then sequenced directly. Primer sequences are shown in Supplementary Table S1.
Complementation of oni2
Selfed seeds of an oni2 heterozygous plant were used for callus induction on an N6CI medium, and a small part of each callus was used for DNA isolation and subsequently for genotyping. For genotyping, PCR was carried out with the primer combination of ONI2-3 and ONI2-4 (Supplementary Table S1). The PCR product amplified from the mutant allele was 16 bp shorter than that from the wild-type allele due to deletion. Homozygous mutant calli were used for Agrobacterium-mediated transformation (Hiei et al. 1994). A 10.1 kb NcoI fragment, which covers ONI2 with 5.0 kb upstream and 0.2 kb downstream regions, was cloned into the NcoI site of a derivative of pENTR4 (Invitrogen) in which ccdB was removed by cutting with EcoRI followed by self-ligation, and then cloned into a binary vector plasmid pGWB by LR reaction (Nakagawa et al. 2007). The resultant vector was used for the transformation of the mutant calli. An empty vector was used as a control.
Expression analysis
RT–PCR and quantitative real-time PCR analyses were carried out as described previously using RNAs isolated from the indicated organs and calli (Ito et al. 2001, Ito et al. 2011). The expression level of actin was used as the internal control. Specific primers used for each gene are listed in Supplementary Table S1.
RNA in situ hybridization was carried out using paraffin sections prepared as described above (Ito et al. 2001). An RT–PCR product amplified by the primer combination of ONI2-19 and ONI2-20, which covers a complete coding region, was cloned into pCR-BluntII-TOPO and used as a template for transcription of digoxigenin (DIG)-labeled ONI2 RNA probes. A full-length cDNA of ROC1 (AK120496) was used for preparation of DIG-labeled ROC1 RNA probes, which were hybridized with 10-day-old shoot sections.
VLCFA analysis
Analysis of fatty acids was carried out as described previously using shoots of 2-week-old seedlings grown in an incubator at 30°C under constant light (Ito et al. 2011). For analysis of free fatty acids, the sample was ground in methanol and then methanolysed at 80°C for 1.5 h, followed by extraction with n-hexane. The n-hexane-soluble fraction was subjected to gas chromatography.
For analysis of fatty acids in epicuticular wax, free VLCFAs and alkyl VLCFAs of chloroform-extracted wax were separated by thin-layer chromatography (hexane : ethyl ether : acetic acid, 9 : 1 : 0.1), and derivatized to the methyl ester by an HCl/methanol method (Lepage and Roy 1986). The fatty acid methyl ester composition was analyzed by gas chromatography with a flame ionization detector (GC-380, GL Sciences) and column (ZB-5 ms, 30 m × 0.25 mm internal diameter, 0.2 µm film thickness, Phenomenex). The column temperature program was as follows; 170°C for 1 min, to 200°C at 2°C min–1, to 320°C at 10°C min−1 and a hold at 320°C for 10 min.
Supplementary data
Supplementary data are available at PCP online.
Funding
This study was supported by the Japan Society for the Promotion of Science [a Grant-in-Aid for Scientific Research (B) grant No. 24380003 to Y.I. and F.K.];. the Japan Society for the Promotion of Science for Young Scientists [Research Fellowship (22-1871) to K.T.].
Supplementary Material
Acknowledgments
pGWB was a gift from Dr. Nakagawa (Shimane University, Japan). ROC1 full-length cDNA was obtained from the Rice Genome Resource Center, Japan.
Glossary
Abbreviations
- DIG,digoxigenin
FDH
- FIDDLEHEAD
L1
- layer 1
ONION2
- ONI2
RT–PCR
- reverse transcription–PCR
SAM
- shoot apical meristem
SEM
- scanning electron microscopy
VLCFA
- very-long-chain fatty acid.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;130:635–643. doi: 10.1242/dev.00292. [DOI] [PubMed] [Google Scholar]
- Bach L, Michaelson LV, Haslam R, Bellec Y, Gissot L, Marion J, et al. The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc. Natl Acad. Sci. USA. 2008;105:14727–14731. doi: 10.1073/pnas.0805089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich CR, Perera MADN, Yandeau-Nelson MD, Meeley RB, Nikolau BJ, Schnable PS. Characterization of two GL8 paralogs reveals that the 3-ketoacyl reductase component of fatty acid elongase is essential for maize (Zea mays L.) development. Plant J. 2005;42:844–861. doi: 10.1111/j.1365-313X.2005.02418.x. [DOI] [PubMed] [Google Scholar]
- Hay A, Barkoulas M, Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–3961. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Sentoku N, Nishimura A, Hong S-K, Sato Y, Matsuoka M. Position dependent expression of GL2-type homeobox gene, Roc1: significance for protoderm differentiation and radial pattern formation in early rice embryogenesis. Plant J. 2002;29:497–507. doi: 10.1046/j.1365-313x.2002.01234.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Eiguchi M, Kurata N. KNOX homeobox genes are sufficient in maintaining cultured cells in an undifferentiated state in rice. Genesis. 2001;30:231–238. doi: 10.1002/gene.1069. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kimura F, Hirakata K, Tsuda K, Takasugi T, Eiguchi M, et al. Fatty acid elongase is required for shoot development in rice. Plant J. 2011;66:680–688. doi: 10.1111/j.1365-313X.2011.04530.x. [DOI] [PubMed] [Google Scholar]
- Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa) Funct. Integr. Genomics. 2006;6:47–59. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels L. Plant cuticles shine: advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009;12:721–727. doi: 10.1016/j.pbi.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- Miyashita Y, Takasugi T, Ito Y. Identification and expression analysis of PIN genes in rice. Plant Sci. 2010;178:424–428. [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nobusawa T, Umeda M. Very-long-chain fatty acids have an essential role in plastid division by controlling Z-ring formation in Arabidopsis thaliana. Genes Cells. 2012;17:709–719. doi: 10.1111/j.1365-2443.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- Perez-Perez JM, Candela H, Robles P, Lopez-Torrejon G, del Pozo JC, Micol JL. A role for AUXIN RESISTANT3 in the coordination of leaf growth. Plant Cell Physiol. 2010;51:1661–1673. doi: 10.1093/pcp/pcq123. [DOI] [PubMed] [Google Scholar]
- Pruitt RE, Vielle-Calzada J-P, Ploense SE, Grossniklaus U, Lolle SJ. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc. Natl Acad. Sci. USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;466:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- Shani E, Yanai O, Ori N. The role of hormones in shoot apical meristem function. Curr. Opin. Plant Biol. 2006;9:484–489. doi: 10.1016/j.pbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y, et al. Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol. 2010;51:164–175. doi: 10.1093/pcp/pcp176. [DOI] [PubMed] [Google Scholar]
- Takasugi T, Ito Y. Altered expression of auxin-related genes in the fatty acid elongase mutant oni1 of rice. Plant Signal. Behav. 2011;6:887–888. doi: 10.4161/psb.6.6.15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Ito Y, Sato Y, Kurata N. Positive autoregulation of a KNOX gene is essential for shoot apical meristem maintenance in rice. Plant Cell. 2011;23:4368–4381. doi: 10.1105/tpc.111.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Ito Y, Yamaki S, Miyao A, Hirochika H, Kurata N. Isolation and mapping of three rice mutants that showed ectopic expression of KNOX genes in leaves. Plant Sci. 2009;177:131–135. [Google Scholar]
- Voisin D, Nawrath C, Kurdyukov S, Franke RB, Reina-Pinto JJ, Efremova N, et al. Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator. PLOS Genet. 2009;5:e1000703. doi: 10.1371/journal.pgen.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell. 1999;11:2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.