Abstract
FGFs act in vertebrate mesoderm induction and also play key roles in early mesoderm formation in ascidians and amphioxus. However, in sea urchins initial characterizations of FGF function do not support a role in early mesoderm induction, making the ancestral roles of FGF signaling and mechanisms of mesoderm specification in deuterostomes unclear. In order to better characterize the evolution of mesoderm formation, we have examined the role of FGF signaling during mesoderm development in Saccoglossus kowalevskii, an experimentally tractable representative of hemichordates. We report the expression of an FGF ligand, fgf8/17/18, in ectoderm overlying sites of mesoderm specification within the archenteron endomesoderm. Embryological experiments demonstrate that mesoderm induction in the archenteron requires contact with ectoderm, and loss-of-function experiments indicate that both FGF ligand and receptor are necessary for mesoderm specification. fgf8/17/18 gain-of-function experiments establish that FGF8/17/18 is sufficient to induce mesoderm in adjacent endomesoderm. These experiments suggest that FGF signaling is necessary from the earliest stages of mesoderm specification and is required for all mesoderm development. Furthermore, they suggest that the archenteron is competent to form mesoderm or endoderm, and that FGF signaling from the ectoderm defines the location and amount of mesoderm. When considered in a comparative context, these data support a phylogenetically broad requirement for FGF8/17/18 signaling in mesoderm specification and suggest that FGF signaling played an ancestral role in deuterostome mesoderm formation.
Keywords: FGF, Deuterostome, Hemichordate, Mesoderm, Mesoderm induction
INTRODUCTION
Fibroblast growth factors (FGFs) and Nodals play crucial roles in mesoderm formation in many vertebrates (Ciruna and Rossant, 2001; Kimelman, 2006). In Xenopus laevis, FGF signaling is necessary for the initiation of Xbrachyury (Xbra) expression (Fletcher and Harland, 2008), which marks early dorsal and posterior mesoderm (Smith et al., 1991). FGF signaling also maintains mesodermal fate (Schulte-Merker and Smith, 1995; Casey et al., 1998), and loss of FGF signaling leads to reductions in posterior mesoderm (including somites and notochord) (Amaya et al., 1991; Conlon et al., 1996; Kumano and Smith, 2000; Kumano and Smith, 2002). The ligand FGF8 is particularly important for this process (Fletcher et al., 2006). FGF8 signaling is also necessary for mesoderm formation in zebrafish, and loss of both Fgf8 and its paralog Fgf24 reduces posterior mesoderm (Draper et al., 2003). FGF signaling is involved in mesoderm formation in invertebrate chordates. In amphioxus, FGF signaling is necessary for development of anterior somites (Bertrand et al., 2011), and in ascidians it is necessary for mesenchyme, notochord and secondary muscle development (Kim and Nishida, 1999; Kim et al., 2000; Darras and Nishida, 2001; Kim and Nishida, 2001; Imai et al., 2002; Miya and Nishida, 2003; Yasuo and Hudson, 2007). The widespread roles of FGF signaling in chordate mesoderm specification suggest that FGF signaling probably acted to specify mesoderm in stem chordates.
Although mesoderm induction is increasingly well-characterized in chordates, comprehensive studies in other deuterostomes are lacking and experimental analyses are limited to echinoderms. The limited functional studies of FGFs in sea urchins do not support an early role in mesoderm induction (McCoon et al., 1996; McCoon et al., 1998; Röttinger et al., 2008), and instead implicate Delta/Notch signaling (Sherwood and McClay, 1999; Range et al., 2008). Outside of deuterostomes, studies are limited to major ecdysozoan model systems. In Drosophila melanogaster, mesoderm is specified by high activity of the transcription factor Dorsal, which is activated by the Spatzle ligand and Toll receptor (Jiang et al., 1991; Ip et al., 1992), and FGFs act in mesoderm migration, but not in mesoderm induction (Stathopoulos et al., 2004; Kadam et al., 2009; McMahon et al., 2010; Tulin and Stathopoulos, 2010). In Caenorhabditis elegans, mesoderm specification requires Notch signaling (Good et al., 2004), and FGF signaling is involved only in the specification of larval sex myoblasts, a small subset of mesoderm (DeVore et al., 1995; Burdine et al., 1998; Goodman et al., 2003; Lo et al., 2008). Currently, there are no functional data on FGF signaling from any lophotrochozoan phyla. A rigorous test of the evolution of developmental mechanisms regulating mesoderm formation requires broader sampling at key phylogenetic positions.
As sister group to echinoderms and closely related to chordates (Turbeville et al., 1994; Bourlat et al., 2006; Dunn et al., 2008), hemichordates are in a key phylogenetic position to test hypotheses of the early evolution of deuterostome developmental mechanisms (Cameron et al., 2000; Lowe et al., 2006; Brown et al., 2008; Cannon et al., 2009). Despite differences in body plan organization between hemichordates and chordates, they share very similar anteroposterior (Lowe et al., 2003) and dorsoventral patterning (Lowe et al., 2006) and endomesoderm specification (Darras et al., 2011), suggesting that molecular comparisons can provide insights into early deuterostome evolution. In enteropneust hemichordates, a group of solitary, burrowing marine worms, mesoderm derives from five pouches that evaginate from the archenteron in a process called enterocoely (Bateson, 1884); this trait is shared with echinoderms and amphioxus (Conklin, 1932) and is suggested to be primitive for deuterostomes (Remane, 1963; Valentine, 2004). These morphogenetic similarities with echinoderms and basal chordates, and close similarities with chordates in early body plan patterning, suggest that analysis of mesoderm specification in enteropneusts could help reconstruct ancestral deuterostome developmental mechanisms for mesoderm induction.
To investigate a potentially conserved role of FGF signaling in deuterostome mesoderm induction, we examined the role of FGF signaling during early development of the direct-developing hemichordate Saccoglossus kowalevskii (Bateson, 1884; Bateson, 1886; Colwin and Colwin, 1953; Lowe et al., 2004; Gerhart et al., 2005; Röttinger and Lowe, 2012). We tested the function of the FGF ligand FGF8/17/18 and the FGF receptor FGFR-B (Rebscher et al., 2009) in hemichordate mesoderm formation, and our work demonstrates that FGF8/17/18 signals from ectoderm to the underlying archenteron to induce mesoderm. These findings suggest that an ortholog of the fgf8/17/18 subfamily was essential for mesoderm induction in the deuterostome common ancestor and have important implications for the evolution of mesoderm induction.
MATERIALS AND METHODS
Embryonic culture
Adult hemichordates were collected from Waquoit Bay, MA, USA. Fertilization and embryonic maintenance were performed as described by Lowe et al. (Lowe et al., 2004). For chemical treatments, embryos were immersed in filtered seawater containing either SU5402 (Mohammadi et al., 1997) or U0126 (Favata et al., 1998) (both Calbiochem) dissolved in DMSO. Control embryos were treated with DMSO. Inhibitor treatments were changed approximately every 12 hours.
Surgeries
Fertilization envelopes were removed with forceps and embryos were cultured in filtered seawater supplemented with 50 μg/ml gentamycin sulfate (FSW + GS). Embryos at the flat-plate gastrula stage were dissected on clay dishes in FSW + GS. The vegetal explants include archenteron endomesoderm and surrounding (posterior) ectoderm. Animal explants are composed only of ectoderm.
Alignment and phylogenetic trees
Protein sequences were obtained from Pubmed, and aligned using MegAlign ver. 8.1.4 (DNAStar), using a BLOSUM series and default CLUSTALW alignment parameters. Neighbor-joining cladograms for mPrx, mesp, six1, snail, mLim, mlca2, zic and foxA trees were made using MEGA 4.0 with a JTT model of molecular evolution, bootstrapped with 2000 iterations, and are shown in supplementary material Fig. S1 (Tamura et al., 2007). The fgf8/17/18 gene tree was made with MrBayes (Ronquist et al., 2012), using a mixed model of protein evolution, and with a Maximum Likelihood model, using PhyML 3.0 (Guindon and Gascuel, 2003). Accession numbers for all sequences are shown in supplementary material Table S1.
Small interfering RNAs (siRNAs)
siRNA targets were identified using the siDESIGN Center (Dharmacon) and purchased from Ambion (Applied Biosystems). Coding domains of fgf8/17/18 were subcloned into pCS2+, and capped mRNAs were made with the mMessage Machine Kit (Ambion). siRNAs or mRNAs were injected as described by Lowe et al. (Lowe et al., 2006). fgf8/17/18 siRNA-a had the sense sequence 5′-AAAAAGCGGUACAAUUUAUGA-3′, and siRNA-b had the sense sequence 5′-AATGGAGATATTTACGCTAGA-3′. FGFR-B siRNA-a had the sense sequence 5′-CUAUACCAAUGAAACCAUATT-3′ and FGFRB-siRNA-b had the sense sequence 5′-GGAUUACCGAAAAACGUGATT-3′. siRNA was injected to a final dose of 100 pM, and mRNA was injected at ∼50 pg per embryo.
ESTs and in situ hybridization
snail and mLim cDNAs were generous gifts of John Gerhart (University of California, Berkeley, CA, USA). tdTomato cDNA was a gift of the Roger Tsien laboratory (University of California, San Diego, CA, USA). In situ hybridizations were performed as described (Lowe et al., 2004; Pani et al., 2012). GenBank IDs are supplied in supplementary material Table S1.
RT-PCR
Embryos were injected with either an siRNA targeting fgf8/17/18, or with a scrambled fgf8/17/18 control. Twenty embryos of each treatment were flash frozen at the postgastrula stage. Total RNA was isolated with the Ambion RNaqueous Kit (Applied Biosystems), and cDNA was made using Invitrogen Superscript III (Invitrogen) following the manufacturer’s instructions. Real time PCR was performed on a MyiQ Thermocycler (BIO-RAD), with the fgf8/17/18 forward primer TGCCCCATCGGTGCTACA, with the fgf8/17/18 reverse primer GCCGTCTCTGCCAAAACTGA, and fgfr-b forward primer AACGCCATATCCATCAGTTCCCGT and fgfr-b reverse primer AAAGGTCGGCCTGAGTTTCGGTAA. Data were analyzed in Microsoft Excel.
RESULTS
Mesoderm is specified during gastrulation
Mesoderm arises as enterocoelic evaginations from the archenteron in each of the three body regions: the proboscis, collar and trunk (Bateson, 1884) (Fig. 1, top row). To determine when mesoderm is specified in S. kowalevskii, we examined the expression patterns of hemichordate orthologs of genes with conserved roles in bilaterian mesoderm and endomesoderm formation (see supplementary material Fig. S1 for gene trees).
Fig. 1.
Enterocoely of mesoderm in S. kowalevskii, and expression of mesodermal and endodermal genes. (A-VV) Expression of mPrx (A-F), mesp (G-L), six1 (M-R), snail (S-X), mLim (muscle Lim protein) (Y-DD), mlca2 (EE-JJ), zic (KK-PP) and foxA (QQ-VV). Each column shows progressively later stages (from left to right): late blastula, midgastrula, early postgastrula, enterocoely, neurula and juvenile, as indicated by the diagrams of embryological development at the top. All embryos are cleared and shown as optical sections with anterior/animal to the upper left. In diagrams, mesoderm is red, ectoderm blue, endoderm yellow, and prospective endomesoderm is mixed yellow and red. A, anterior; An, animal pole; D, dorsal; P, posterior; V, ventral; Veg, vegetal pole. Scale bar: 100 μm.
The expression patterns of four transcription factors associated with early mesoderm development in other bilaterian phyla suggest that mesoderm is specified prior to the morphological segregation of mesoderm and endoderm. We examined mesoPrx (mPrx), a homolog of prx1 and prx2 (Cserjesi et al., 1992; de Jong and Meijlink, 1993; Leussink et al., 1995; Norris et al., 2000; Jones et al., 2001; Doufexi and Mina, 2008); mesp, a paralog to the vertebrate mesp and mesogenin (Saga et al., 1996; Saga et al., 1997; Sawada et al., 2000; Satou et al., 2004; Imai et al., 2006; Saga and Takahashi, 2008); six1 (Kozmik et al., 2007; Beaster-Jones et al., 2008; Gillis et al., 2012); and snail (Hammerschmidt and Nüsslein-Volhard, 1993; Erives et al., 1998; Fujiwara et al., 1998; Langeland et al., 1998; Cano et al., 2000; Wu and McClay, 2007; Rahimi et al., 2009). We did not detect expression of any of these genes in blastula-stage embryos (Fig. 1A,G,M,S). We first detected expression during gastrulation in anterior endomesoderm, in a site that later separates to become the proboscis mesoderm (Fig. 1B,H,N,T). Additional expression domains later appear in the presumptive trunk mesoderm and collar mesoderm (Fig. 1C,D,I,J,O,P,U,V). These early sites of expression coincide with sites of mesoderm structural gene expression (see below), suggesting that the expression of mPrx, mesp, six1 and snail are good markers of early mesoderm specification.
Expression patterns of mesoderm structural genes corroborate this view of mesoderm specification (Fig. 1Y-JJ). We examined expression of myosin light chain alkali 2 (mlca2) (Holland et al., 1995; Thézé et al., 1995; Thiébaud et al., 2001), an ortholog of other myosin alkali light chains, and muscle Lim (mLim) (Arber et al., 1994; Stronach et al., 1996; Stronach et al., 1999; Martindale et al., 2004). These genes are expressed in a pattern that closely matches the spatial pattern of transcription factor expression. However, the timing of structural gene expression is slightly delayed relative to the expression of transcription factors, consistent with structural genes acting downstream. mLim and mlca2 expression persists in mesoderm after enterocoely (Fig. 1L), indicating that these genes are excellent markers of late mesoderm.
Two other genes, though not expressed exclusively in mesoderm at early stages, are useful markers. Zic, a transcription factor with diverse developmental roles (Layden et al., 2010) is expressed in early mesoderm, in the vegetal plate and in anterior ectoderm (Fig. 1KK-PP). foxA is expressed in the vegetal plate (prospective endoderm and mesoderm) prior to gastrulation (Fig. 1QQ), similar to its expression in sea urchins (Harada et al., 1996; Oliveri et al., 2006) and frogs (Suri et al., 2004). During gastrulation, as endomesoderm invaginates to become the archenteron, foxA is maintained in endoderm, but downregulated in mesoderm (Fig. 1RR-VV; supplementary material Fig. S3B). It is not clear whether the vegetal plate cells marked by early foxA expression are endomesodermal precursors to both endoderm and mesoderm, or whether they are endodermal by default, but for the purposes of discussion we refer here to foxA-expressing cells from late blastula through early post-gastrula stages as endomesoderm. Once mesoderm markers are expressed, we describe internal foxA-positive cells as endoderm. The expression patterns of early transcription factors, structural genes and foxA suggest that mesoderm forms after vegetal archenteron endomesoderm contacts overlying ectoderm.
Ectoderm induces mesoderm
To test the hypothesis that hemichordate mesoderm requires ectodermal contact, we carried out surgical experiments on gastrulating embryos. When embryos begin gastrulation, their vegetal plate flattens, making the animal-vegetal axis obvious (Fig. 2A). Embryos at that stage were bisected into animal and vegetal explants (Fig. 2C). Blastomere isolation experiments (Colwin and Colwin, 1950), studies of vegetal β-catenin signaling (Darras et al., 2011) and gene expression (Fig. 1) indicate that animal explants are composed entirely of ectoderm, whereas the vegetal explants contain both vegetal plate endomesoderm and some posterior ectoderm. The vegetal plate normally invaginates to form the entire archenteron, and after surgery vegetal explants go through apparently normal cell movements to make an archenteron (Fig. 2D). In uncut embryos, the mesoderm marker snail is strongly expressed in anterior endomesoderm by mid-gastrula stage, following contact with anterior ectoderm (Fig. 2B). However, vegetal explants incubated to mid-gastrula stage lack snail expression (Fig. 2D). These data suggest that mesoderm does not form autonomously, but instead requires interaction with ectoderm.
Fig. 2.
Anterior ectoderm induces mesodermal snail expression in archenteron endomesoderm. (A) Diagram of lateral view of an uncut embryo at flat-plate gastrula stage of S. kowalevskii. (B) snail expression at mid-gastrula stage. (C) Method of making animal ectodermal and vegetal endomesodermal explants by dissection of a flat-plate gastrula embryo. (D) snail expression in a vegetal explant at mid-gastrula stage. (E) Method of making an animal-vegetal conjugate. The concave vegetal piece has begun archenteron formation, and ectoderm is placed on the blastocoel-facing side of the vegetal piece. (F) snail expression in an animal-vegetal conjugate. (G) Method of making a vegetal-vegetal conjugate. (H) snail expression in a vegetal-vegetal conjugate. Right-hand column shows schematics of the fate of the embryo shown to its left. All images show optical sections of cleared embryos. Anterior is to the top left. Mesoderm is red, ectoderm blue, endoderm yellow, and endomesoderm is mixed yellow and red. Red lines indicate dissection. An, animal pole; Veg, vegetal pole.
To test whether ectoderm is capable of inducing mesoderm, we grafted small pieces of ectoderm cut from animal explants onto vegetal explants of equivalent stage, making animal/vegetal conjugates that were incubated until mid-gastrula stage (Fig. 2E). Ectoderm from the animal pole induced snail expression in the vegetal tissue directly underlying it (Fig. 2F). Precise placement of animal tissue varied, but snail expression was always adjacent to the animal tissue graft (supplementary material Fig. S3C), suggesting that ectoderm induces endomesoderm to become mesoderm. To test whether mesoderm can be induced by contact with any embryonic tissue, we made vegetal-vegetal conjugates (Fig. 2G) and examined them for mesodermal expression. However, vegetal endomesoderm could not induce snail expression (Fig. 2H). These data are consistent with the model that ectoderm provides a signal(s) to the underlying endomesoderm to induce mesoderm.
Expression of fgf8/17/18 and fgfr-B are consistent with roles in mesoderm formation
To examine the possible roles of FGF signaling in hemichordate mesoderm formation, we investigated the expression of FGF ligands and FGF receptors. We isolated fgf8/17/18 (Pani et al., 2012), an ortholog of the FGFD family, which is implicated in mesoderm development in several groups (see Discussion). We identified two splice forms (fgf8/17/18.1 and fgf8/17/18.2) that differ only in the predicted N-terminal signal peptide, but are not orthologs of vertebrate fgf8 splice variants (Fletcher et al., 2006) (supplementary material Fig. S2B). Expression of fgf8/17/18 was examined by in situ hybridization using a probe for fgf8/17/18.1, which hybridizes to both splice forms. Expression is detected at blastula stage (Fig. 3A; supplementary material Fig. S3A), and during gastrulation becomes increasingly restricted to the anterior ectoderm, the region that is contacted by the anterior tip of the archenteron (Fig. 3B). Following gastrulation, additional expression domains are detected at intermediate positions along the anterior-posterior (A/P) axis, in the collar region (Fig. 3C,D). Here, fgf8/17/18 is expressed in two slim, lateral, ectodermal bands immediately overlying sites of mesoderm specification in the collar. Further posterior, fgf8/17/18 is expressed in a weak circumferential ring overlying the site of the trunk mesoderm (Fig. 3D,E). Thus, the expression of fgf8/17/18 in the ectoderm is associated with the induction of all three regions of mesoderm in the archenteron.
Fig. 3.
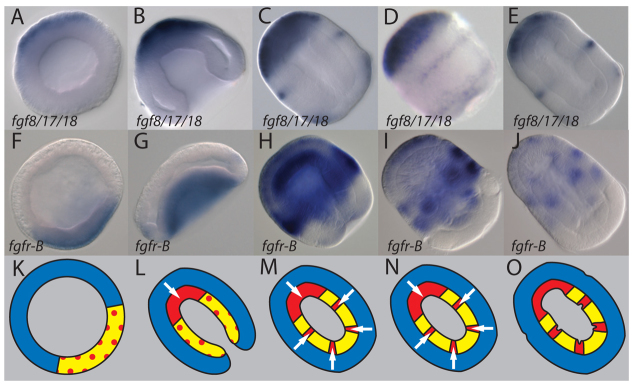
Expression of FGF signaling components in relation to endomesodermal and mesodermal gene expression. (A-J) Expression of fgf8/17/18 (A-E) and fgfr-B (F-J) during early stages of S. kowalevskii development. The animal/anterior pole is to the upper left. All images are dorsal views of median optical sections of cleared embryos, except for D, which is a lateral surface view of an uncleared embryo. (K-O) Model of hypothetical interactions during mesoderm formation. White arrows indicate direction of FGF signaling from ectoderm to archenteron endomesoderm. Mesoderm is red, ectoderm blue, endoderm yellow, and endomesoderm is mixed yellow and red.
S. kowalevskii has two FGF receptors, FGFR-A and FGFR-B, which arose from a hemichordate-specific duplication (Rebscher et al., 2009) (Fig. 3F-J). fgfr-B is expressed in endomesoderm of the archenteron at early gastrula stage and is later upregulated in nascent mesoderm. It is also expressed in ectoderm beginning at late gastrula (not shown) and persisting into later stages (Fig. 3H-J). Expression of fgfr-B is both temporally and spatially coincident with upregulation of mesoderm specification markers. Thus, the expression of both fgf8/17/18 and fgfr-B are consistent with the model that FGF8/17/18 is secreted from ectoderm to induce mesoderm in underlying endomesoderm (Fig. 3K-O).
Endomesodermal fgfr-B is necessary for mesoderm specification
To test whether fgfr-B is necessary for mesoderm specification, we injected zygotes with custom-designed siRNAs. Expression of fgfr-b is reduced following siRNA injection (supplementary material Fig. S4A; ∼40% reduction relative to controls). Expression of the mesodermal specification markers snail and mLim is strongly reduced at early gastrula stages (compare Fig. 4A,F with 4C,H), suggesting that fgfr-B is necessary for mesoderm specification. Mesodermal zic expression is also reduced, but expression in anterior ectoderm is unaffected (Fig. 4B,G), indicating that ectodermal expression is not FGF dependent. At later juvenile stages, the differentiated mesoderm marker mLim is strongly reduced (Fig. 4E,J), suggesting a failure of muscle formation. Furthermore, the endodermal epithelium extends abnormally far into the proboscis and contacts the anterior-most ectoderm (Fig. 4E,J, arrow), suggesting that anterior endomesoderm differentiates into endoderm in the absence of FGF signaling. A second siRNA for fgfr-B gave a consistent but milder phenotype, supporting the specificity of the knockdown (supplementary material Fig. S3D). Similarly, treating embryos with 20 μM of the FGFR inhibitor SU5402, (Mohammadi et al., 1997) or 8 μM of the MEK inhibitor U0126 (Favata et al., 1998) beginning at late blastula stage either completely inhibits or severely reduces mesoderm (supplementary material Fig. S5), indicating that FGFR and MAPK signaling are necessary for mesoderm specification during gastrulation. Treating embryos at progressively later stages suggests that FGFR and MAPK signaling are necessary early during proboscis mesoderm specification but are dispensable at later stages (supplementary material Fig. S6), but we have not assayed the more posterior mesoderm of the collar and trunk. These phenotypes are consistent with the model that reception of FGF signaling is necessary for mesoderm specification.
Fig. 4.
Ectodermal fgf8/17/18 and endomesodermal fgfr-B are necessary for mesoderm specification. (A-J) fgfr-B is necessary for mesoderm specification. Control S. kowalevskii embryos (A-E) show mesodermal (snail, zic, mLim) and endodermal (foxA) expression, altered in corresponding fgfr-B siRNA-injected matching stage embryos (F-J). (K-T) fgf8/17/18 is necessary for mesoderm specification. Control embryos (K-O) show mesodermal and endodermal expression. Corresponding matching stage embryos (P-T) injected with fgf8/17/18 siRNA show reduced mesoderm and expanded endoderm. (U-Y) Conjugates of animal ectoderm and vegetal archenteron endomesoderm pieces taken from siRNA-injected and control embryos, stained for snail expression. All are shown at late gastrula stage. (U) Uncut control. (V) Animal-vegetal (A/V) conjugate, with animal portion from an fgfr-B siRNA-injected embryo. (W) A/V conjugate, with vegetal portion from an fgfr-B siRNA-injected embryo. (X) A/V conjugate with animal tissue from an fgf8/17/18 siRNA-injected embryo. (Y) A/V conjugate, with vegetal tissue from an fgf8/17/18 siRNA-injected embryo. All are optical sections of cleared embryos, with anterior to upper left.
fgfr-B is expressed in endomesoderm during early gastrulation, but is also detectable at lower levels in ectoderm (not shown). Therefore, FGF signaling might act directly on presumptive mesoderm, indirectly by secondary signals from the ectoderm, or both. To test for direct effects on mesoderm induction, we bisected gastrulating embryos injected with fgfr-B siRNA into animal and vegetal pieces, and recombined each one with tissue from uninjected sibling embryos, resulting in animal-vegetal conjugates that contained fgfr-B siRNA in either animal or vegetal tissue. Loss of fgfr-B in ectoderm does not affect mesoderm (Fig. 4V), but loss of fgfr-B in the underlying endomesoderm leads to complete loss (4/8) or reduction (4/8) of snail expression (Fig. 4W). This suggests that mesoderm induction requires reception of FGF signals by endomesodermal FGFR-B.
Ectodermal fgf8/17/18 is necessary for mesoderm specification
In order to test the hypothesis that FGF8/17/18 induces mesoderm specification, we injected embryos with either of two siRNAs (fgf8a, fgf8b), each targeting both fgf8/17/18 splice variants. RT-PCR analyses of fgf8/17/18 levels demonstrate that expression is strongly reduced following siRNA injection (supplementary material Fig. S5; ∼77% reduction relative to controls). Embryos fixed during enterocoely show reduced expression of the mesodermal markers zic, mLim and myosin (Fig. 4K-M,P-R), and the endomesodermal/endodermal marker foxA continues to be expressed at the sites where mesoderm would normally form (Fig. 4N,S). At the juvenile stage, mLim expression is reduced, proboscis muscle is largely absent and the anterior endodermal epithelium expands into the proboscis (Fig. 4O,T; supplementary material Fig. S3E,F). This is similar to the fgfr-B siRNA phenotype, and consistent with FGF8/17/18 inducing mesoderm.
To test whether fgf8/17/18 is required in ectoderm or endomesoderm for mesoderm specification, we made conjugates by combining animal and vegetal explants from siRNA-injected and uninjected embryos as already described for FGFR-B. Following knockdown of fgf8/17/18 in vegetal tissue, ectoderm explants induce snail expression normally (Fig. 4Y), but if fgf8/17/18 is knocked down in the animal explants and grafted onto normal endomesoderm, mesoderm specification fails (Fig. 4X). This is consistent with the model that FGF8/17/18 secreted from ectoderm is required to specify mesoderm in underlying endomesoderm.
fgf8/17/18 is sufficient for mesoderm specification
To test whether fgf8/17/18 is sufficient to induce mesoderm, we injected capped fgf8/17/18 mRNA or a control tdtomato mRNA into zygotes. At early developmental stages, fgf8/17/18 mRNA expanded mesodermal markers (snail and zic) (Fig. 5A,C,I,K) and reduced expression of the endoderm/endomesoderm marker foxA (Fig. 5B,J). In juveniles, mesoderm is expanded (mLim; Fig. 5D,L) and endoderm is significantly reduced (foxA; Fig. 5E,M), suggesting that fgf8/17/18 can induce archenteron endomesoderm to become mesoderm. If FGF8/17/18 signals through FGFR-B, then mesoderm expansion should require fgfr-B expression. In embryos co-injected with fgf8/17/18 mRNA and fgfr-B siRNA, archenteron expression of the mesoderm marker zic is reduced (Fig. 5F,N) relative to its expression in embryos injected with fgf8/17/18 mRNA alone (Fig. 5K), suggesting that fgfr-B is necessary to transduce the inductive effect of the exogenous fgf8/17/18 mRNA. Furthermore, if FGF8/17/18 induces mesoderm directly, then it should have the capacity to do so in the absence of ectoderm. In order to test the sufficiency of fgf8/17/18, we cut vegetal explants from embryos injected with fgf8/17/18 mRNA. The resulting explants expressed zic throughout most of the endomesoderm (Fig. 5O). By contrast, vegetal explants made from embryos injected with control tdtomato mRNA did not show any zic expression (Fig. 5G). Next, we tested whether overexpression of fgf8/17/18 in vegetal explants conveys mesodermal inductive properties. To this aim, we made vegetal-vegetal conjugates between explants from uninjected embryos and fgf8/17/18 mRNA-injected embryos. Resulting conjugates show mesoderm induction in the half composed of the injected explant (3/3), and either partial (1/3) or complete (2/3) mesoderm induction in the half composed of the uninjected explant (Fig. 5P). By contrast, injection of tdtomato mRNA failed to induce zic expression in either explant (Fig. 5H). These data suggest that FGF8/17/18 signaling is sufficient to induce mesoderm from endomesoderm.
Fig. 5.

fgf8/17/18 overexpression is sufficient to induce mesoderm in archenteron endomesoderm, but requires fgfr-B activity. (A-E,I-M) Control embryos (A-E) stained for mesodermal (snail, zic, mLim) or endodermal (foxA) gene expression, and matching stage experimental embryos injected with fgf8/17/18 mRNA at fertilization and stained for the same markers (I-M). (F) Control embryo stained for zic expression at enterocoely stage. (G,H) zic expression in vegetal explants and vegetal-vegetal conjugates. (G) Vegetal explant injected with the control mRNA tdtomato. (H) Vegetal-vegetal conjugate made from vegetal archenteron pieces from uninjected and tdtomato-injected embryos. (N) Embryo co-injected with fgf8/17/18 mRNA and fgfr-B siRNA. (O) Vegetal explant injected with fgf8/17/18.1 mRNA. (P) Vegetal-vegetal conjugate made from uninjected and fgf8/17/18.1 mRNA injected embryos. All embryos and conjugates are cleared and shown as median optical sections. Anterior is to the upper left and posterior to the lower right, except for H and P. In H, vegetal explants contact at their anterior tips. In P, the injected explant is in typical orientation, and the uninjected explant is oriented with anterior towards the viewer. Gray dotted line indicates plane of contact between explants.
DISCUSSION
Hemichordate mesoderm is specified from endomesoderm
Expression analyses indicate that the mesodermal markers snail, mLim, mlcal2, mesoprx, six1 and mesp are co-expressed in tissues clearly identifiable as mesoderm. Expression analyses also show that foxA is expressed first throughout the vegetal plate at early gastrula, and that its expression is lost in cells that initiate expression of mesodermal markers. It remains to be experimentally determined whether this early foxA-positive domain represents endomesoderm or an endodermal default. The mesodermal expression arises in a characteristic pattern towards the end of gastrulation, first anteriorly in the proboscis mesoderm, then posteriorly in the trunk and collar mesoderm (Fig. 1). We do not detect expression of any gene at blastula stage. We cannot rule out the possibility that there is earlier expression of other genes, but based on these data it appears that hemichordate mesoderm is specified during gastrulation, later in development compared with chordates and sea urchins.
In S. kowalevskii, mesoderm arises as subsets of the vegetal, endomesodermal tissue that forms the archenteron. These tissues are visible at early gastrula stage as a thick vegetal plate that is marked by expression of foxA and zic. As mesoderm specification begins, foxA is restricted to the remaining endomesoderm/endoderm, whereas zic is first restricted to the proboscis mesoderm, but later is expressed in the trunk and collar mesoderm. The endomesoderm might be either endoderm by default, or a distinct precursor cell type that requires signaling to adopt either fate. In either model, these data imply that the vegetal endomesoderm has competence to form mesoderm or endoderm.
zic and mesoderm specification
In S. kowalevskii, zic is the only gene in our study expressed throughout the vegetal plate and then restricted to mesoderm at later stages. It is possible that vegetal plate expression of zic might be necessary for appropriate mesoderm specification. This possibility deserves special consideration, as zic is a homolog of the ascidian gene macho-1 (Layden et al., 2010), which acts in ascidian cell-autonomous mesoderm specification (Nishida and Sawada, 2001; Sawada et al., 2005). However, S. kowalevskii zic is expressed throughout the vegetal plate, unlike ascidian macho-1. This means that hemichordate zic is also expressed in precursors of all endoderm, indicating that zic expression in the vegetal plate cannot be sufficient for mesoderm specification, but the subsequent loss of zic expression in endoderm might be necessary for endoderm differentiation. Loss of zic expression following knockdown of fgf8/17/18 (Fig. 4K-M,P-R) and expanded zic expression in response to fgf8/17/18 misexpression (Fig. 5C,K,G,H,O,P) suggests that late zic expression is actively induced or maintained by ectodermal FGF signaling. We believe that the expression of zic in the vegetal plate probably represents a distinct role, perhaps in specification of endomesoderm, or patterning of the animal-vegetal axis. Analyses of zic loss of function and misexpression will be necessary to test this hypothesis.
Hemichordate FGF8/17/18 induces endomesoderm to become mesoderm
Three kinds of evidence presented here indicate that FGF signaling induces mesoderm in the hemichordate S. kowalevskii. First, fgf8/17/18 expression in the ectoderm overlies regions of the archenteron with upregulated expression of the FGF receptor gene fgfr-B (Fig. 3) and a battery of mesodermal markers (Fig. 1). Second, in loss-of-function experiments, knockdown of either fgf8/17/18 or fgfr-B greatly reduces or eliminates mesoderm formation, leaving endoderm formation intact or expanded (Fig. 3). Surgical experiments indicate that mesoderm can be induced in archenteron presumptive endomesoderm by signals released from attached pieces of ectoderm (Fig. 2) that express fgf8/17/18 (Fig. 3). Similar experiments that combine surgical grafting with knockdown of either ligand or receptor further refine the basic loss-of-function approach and indicate that fgfr-B is required in the presumptive mesoderm, and that fgf8/17/18 is required in the adjacent ectoderm (Fig. 4). Third, in gain-of-function sufficiency tests, fgf8/17/18 overexpression induces archenteron endomesoderm to become mesoderm, even in the absence of ectoderm (Fig. 5), and this mRNA gives endomesoderm the capacity to induce mesoderm in other explants (Fig. 5). These data provide strong evidence that FGF8/17/18 signals from the ectoderm to adjacent endomesoderm to induce mesoderm, and also imply that spatially regulated fgf8/17/18 expression in the ectoderm determines the location and amount of mesoderm induced from the archenteron.
FGF8/17/18 and deuterostome mesoderm specification
Our findings contribute to a growing body of evidence indicating that FGF8/17/18 signaling is necessary for mesoderm specification in many deuterostomes. FGF signaling is required for posterior mesoderm (somites, notochord) in Xenopus laevis and the zebrafish Danio rerio (Amaya et al., 1991; Draper et al., 2003; Fletcher et al., 2006; Fletcher and Harland, 2008), for induction of mesenchyme, notochord and some tail muscles in ascidians (Kim and Nishida, 1999; Kim et al., 2000; Darras and Nishida, 2001; Kim and Nishida, 2001; Imai et al., 2002; Miya and Nishida, 2003), and for anterior somites in amphioxus (Bertrand et al., 2011). fgf8/17/18 orthologs are crucial for these signaling events in frog (Fletcher et al., 2006), zebrafish (Draper et al., 2003) and ascidians (Yasuo and Hudson, 2007), but the FGF ligand acting in amphioxus is unknown. Echinoderms are the only deuterostome phylum without experimental data supporting a role of FGF in mesoderm specification. However, our study suggests that a more comprehensive experimental analysis of the FGF complement in echinoderms will be required before it can be conclusively ruled out as a regulator of early mesoderm fate in this group. We can now state that FGF8/17/18 signaling is also essential in mesoderm specification in a representative of another deuterostome phylum, the hemichordates. It will now be interesting to test further the requirement of FGF signaling more broadly in hemichordates by investigating its role during the development of enteropneust species characterized by indirect development and a distinct larval body plan, more similar to echinoderm early developmental strategies. Nevertheless, comparison of experimental data between deuterostome species suggests that there is a widespread requirement for FGF8/17/18 signaling in mesoderm specification.
Hemichordate FGF8/17/18 ligand signals from ectoderm to prospective endomesoderm
Our data suggest that FGF8/17/18 is produced in ectoderm and transfers patterning information from ectoderm to the underlying prospective endomesoderm. This is different from vertebrates, in which FGF signals required for mesoderm fate are instead produced within the mesoderm itself in response to signaling from endoderm. However, in both cases FGF signaling is received within the endomesoderm, suggesting that this is a conserved feature of deuterostomes. The site of signal production might be more evolutionarily flexible than the site where signaling is received, and so it is possible that hemichordates lost mesoderm-specific FGF gene expression. Alternatively, chordates might have gained FGF expression in mesoderm. One informative outgroup, the dipteran D. melanogaster, requires FGF signaling for mesoderm migration, and, as in hemichordates, FGF ligands are produced in ectoderm (Stathopoulos et al., 2004; Kadam et al., 2009; McMahon et al., 2010; Tulin and Stathopoulos, 2010). It is possible that FGF signals to mesoderm originally signaled from ectoderm to endomesoderm, but shifted to a production site in the mesoderm during the evolution of early chordates. Testing this hypothesis will require examination of other deuterostome invertebrates and other protostome phyla.
Inductive signaling in deuterostome mesoderm specification
In vertebrates, several other signaling pathways have particularly important roles in mesoderm induction. Nodal signaling is generally required for vertebrate mesoderm (Kimelman, 2006). However, there is limited comparative support for a conserved role of this pathway outside of vertebrates. Nodal does not induce mesoderm in amphioxus (Onai et al., 2010) or sea urchins (Duboc et al., 2005).
Another major signaling pathway, Delta/Notch signaling, appears to have at least two distinct and relevant roles in deuterostome early endomesoderm development. There is a widespread requirement for Delta/Notch signaling in endomesoderm segregation in frog, zebrafish and sea urchins (Kikuchi et al., 2004; Contakos et al., 2005; Revinski et al., 2010; Sethi et al., 2012), but it generally seems to promote endoderm at the expense of mesoderm. In sea urchins, Delta/Notch signaling also plays a key role in mesoderm induction (Sherwood and McClay, 1999; Range et al., 2008; Materna and Davidson, 2012). This would appear to be more comparable to the role of hemichordate FGF signaling that we observe here, but the inductive role of Delta/Notch signaling has not been observed in other animals. It does not have this role in the closely related asteroid echinoderms (Hinman and Davidson, 2007) or in mouse and zebrafish (Sherwood and McClay, 1999; Shi et al., 2005; Range et al., 2008).
The general distribution of Nodal and Notch requirements is consistent with those pathways acquiring roles in mesoderm induction during the evolution of stem vertebrates and echinoderms, respectively. Examining these signaling pathways within hemichordates will help to test these hypotheses. In contrast to Nodal and Delta/Notch signaling, FGF signaling has phylogenetically broad support for a role in mesoderm specification. We propose that FGF8/17/18-mediated mesoderm specification was a primitive trait of deuterostomes and that FGF signaling might have acted non-autonomously to induce mesoderm, as it does in S. kowalevskii.
We propose two broad evolutionary models of the roles of FGF8/17/18 signaling in deuterostome mesoderm specification. First, FGF signaling might have been required for formation of all or most mesoderm. This scenario implies that other signaling pathways, including Nodal (Kimelman, 2006) in vertebrates and Notch signaling in echinoderms (Sherwood and McClay, 1999; Range et al., 2008), gained additional prominence during later evolution, but it is also consistent with multiple signals being required for mesoderm specification. It would also imply lineage-specific secondary loss of FGF-dependent induction in subpopulations of mesoderm in different chordate groups, and potentially complete loss in echinoderms. A second possibility is that FGF signaling was ancestrally associated with induction of a specific subset of mesoderm (either a specific tissue or axial level), but gained additional importance during hemichordate evolution.
We consider the first model, that FGF8/17/18 signaling was required for all mesoderm in early deuterostomes, to be the best fit for the available comparative developmental data, for several reasons. First, FGF signaling acts broadly in mesoderm induction in both hemichordates and ascidians, where it is required in multiple mesodermal tissues at all axial levels (Lemaire et al., 2008). Second, in chordates, in which FGF signaling is required in only a subset of mesoderm, there is little commonality in the precise location of the requirement: FGF signaling is required for posterior mesoderm in frogs (Kumano and Smith, 2000; Kumano and Smith, 2002; Kimelman, 2006; Bertrand et al., 2011) and zebrafish (Draper et al., 2003), but is required for anterior somites in amphioxus (Bertrand et al., 2011). This condition seems more likely to result from a selective loss of FGF function than from multiple independent gains of function, suggesting that FGF signaling might have had ancestral roles in mesoderm specification.
FGF signaling and the origins of mesoderm
Mesoderm evolved prior to the divergence of deuterostomes and protostomes, but the changes in gene regulation that led to the innovation of a distinct mesodermal germ layer remain unknown. Our data suggest that FGF signaling acted to induce mesoderm in early deuterostomes, but determining whether this was an ancestral bilaterian trait requires additional data from protostomes. Currently, studies from ecdysozoan model systems do not support this model; FGFs act in protostome mesoderm patterning and migration, but we are not aware of any evidence of a role in mesoderm induction. Data from the other main protostome lineage, the lophotrochozoans, will be important for testing this hypothesis. Currently there are no experimental analyses of lophotrochozoan FGF signaling, but experiments from gastropod molluscs have implicated MAPK signaling in formation of larval mesoderm derived from the 3D lineage (Lambert and Nagy, 2001; Lambert and Nagy, 2003; Koop et al., 2007), and FGFs are good candidates for this signaling event.
Supplementary Material
Acknowledgments
We would like to thank the staff of the Marine Biological Laboratory (Woods Hole, MA, USA), particularly Martha Peterson and Herb Luther; Zeiss and Nikon for their generous microscopy support; and the Waquoit Bay Estuarine Research Reserve. We thank Bob Freeman for assistance with bioinformatics; John Gerhart for helpful comments on the manuscript; Ariel Pani for generating the fluorescent fgf8 in situ hybridizations; Laura Kerosuo and Saku Jayasena for assistance with RT-PCR; and Sebastien Darras, Laurinda Jaffe, Marc Kirschner, Andrew Gillis, the Lowe lab and the Casey lab for their support during the field season.
Footnotes
Funding
This work was supported by a National Science Foundation (NSF) award [0818679 to C.J.L.]. S.A.G. was supported by a National Institutes of Health (NIH) training grant [T32GM07197] and an NSF predoctoral fellowship [DGE0638477]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.083790/-/DC1
References
- Amaya E., Musci T. J., Kirschner M. W. (1991). Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66, 257–270 [DOI] [PubMed] [Google Scholar]
- Arber S., Halder G., Caroni P. (1994). Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 79, 221–231 [DOI] [PubMed] [Google Scholar]
- Bateson W. (1884). The early stages in the development of Balanoglossus (sp. Incert.). Q. J. Microsc. Sci. 25, 208–236 [Google Scholar]
- Bateson W. (1886). Continued account of the later stages in the development of Balanoglossus kowalevskii, and of the morphology of the enteropneusta. Q. J. Microsc. Sci. 26, 511–534 [Google Scholar]
- Beaster-Jones L., Kaltenbach S. L., Koop D., Yuan S., Chastain R., Holland L. Z. (2008). Expression of somite segmentation genes in amphioxus: a clock without a wavefront? Dev. Genes Evol. 218, 599–611 [DOI] [PubMed] [Google Scholar]
- Bertrand S., Camasses A., Somorjai I., Belgacem M. R., Chabrol O., Escande M. L., Pontarotti P., Escriva H. (2011). Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc. Natl. Acad. Sci. USA 108, 9160–9165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlat S. J., Juliusdottir T., Lowe C. J., Freeman R., Aronowicz J., Kirschner M., Lander E. S., Thorndyke M., Nakano H., Kohn A. B., et al. (2006). Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444, 85–88 [DOI] [PubMed] [Google Scholar]
- Brown F. D., Prendergast A., Swalla B. J. (2008). Man is but a worm: chordate origins. Genesis 46, 605–613 [DOI] [PubMed] [Google Scholar]
- Burdine R. D., Branda C. S., Stern M. J. (1998). EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125, 1083–1093 [DOI] [PubMed] [Google Scholar]
- Cameron C. B., Garey J. R., Swalla B. J. (2000). Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proc. Natl. Acad. Sci. USA 97, 4469–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. T., Rychel A. L., Eccleston H., Halanych K. M., Swalla B. J. (2009). Molecular phylogeny of hemichordata, with updated status of deep-sea enteropneusts. Mol. Phylogenet. Evol. 52, 17–24 [DOI] [PubMed] [Google Scholar]
- Cano A., Pérez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 [DOI] [PubMed] [Google Scholar]
- Casey E. S., O’Reilly M. A., Conlon F. L., Smith J. C. (1998). The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development 125, 3887–3894 [DOI] [PubMed] [Google Scholar]
- Ciruna B., Rossant J. (2001). FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37–49 [DOI] [PubMed] [Google Scholar]
- Colwin A. L., Colwin L. H. (1950). The developmental capacities of separated early blastomeres of an enteropneust, Saccoglossus kowalevskii. J. Exp. Zool. 115, 263–295 [Google Scholar]
- Colwin A. L., Colwin L. H. (1953). The normal embryology of Saccoglossus kowalevskii. J. Morphol. 92, 401–453 [Google Scholar]
- Conklin E. G. (1932). The embryology of Amphioxus. J. Morphol. 54, 69–151 [Google Scholar]
- Conlon F. L., Sedgwick S. G., Weston K. M., Smith J. C. (1996). Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122, 2427–2435 [DOI] [PubMed] [Google Scholar]
- Contakos S. P., Gaydos C. M., Pfeil E. C., McLaughlin K. A. (2005). Subdividing the embryo: a role for Notch signaling during germ layer patterning in Xenopus laevis. Dev. Biol. 288, 294–307 [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. (1992). MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development 115, 1087–1101 [DOI] [PubMed] [Google Scholar]
- Darras S., Nishida H. (2001). The BMP signaling pathway is required together with the FGF pathway for notochord induction in the ascidian embryo. Development 128, 2629–2638 [DOI] [PubMed] [Google Scholar]
- Darras S., Gerhart J., Terasaki M., Kirschner M., Lowe C. J. (2011). β-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development 138, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R., Meijlink F. (1993). The homeobox gene S8: mesoderm-specific expression in presomite embryos and in cells cultured in vitro and modulation in differentiating pluripotent cells. Dev. Biol. 157, 133–146 [DOI] [PubMed] [Google Scholar]
- DeVore D. L., Horvitz H. R., Stern M. J. (1995). An FGF receptor signaling pathway is required for the normal cell migrations of the sex myoblasts in C. elegans hermaphrodites. Cell 83, 611–620 [DOI] [PubMed] [Google Scholar]
- Doufexi A. E., Mina M. (2008). Signaling pathways regulating the expression of Prx1 and Prx2 in the chick mandibular mesenchyme. Dev. Dyn. 237, 3115–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper B. W., Stock D. W., Kimmel C. B. (2003). Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development 130, 4639–4654 [DOI] [PubMed] [Google Scholar]
- Duboc V., Röttinger E., Lapraz F., Besnardeau L., Lepage T. (2005). Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev. Cell 9, 147–158 [DOI] [PubMed] [Google Scholar]
- Dunn C. W., Hejnol A., Matus D. Q., Pang K., Browne W. E., Smith S. A., Seaver E., Rouse G. W., Obst M., Edgecombe G. D., et al. (2008). Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 [DOI] [PubMed] [Google Scholar]
- Erives A., Corbo J. C., Levine M. (1998). Lineage-specific regulation of the Ciona snail gene in the embryonic mesoderm and neuroectoderm. Dev. Biol. 194, 213–225 [DOI] [PubMed] [Google Scholar]
- Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 [DOI] [PubMed] [Google Scholar]
- Fletcher R. B., Harland R. M. (2008). The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Dev. Dyn. 237, 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R. B., Baker J. C., Harland R. M. (2006). FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development 133, 1703–1714 [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Corbo J. C., Levine M. (1998). The snail repressor establishes a muscle/notochord boundary in the Ciona embryo. Development 125, 2511–2520 [DOI] [PubMed] [Google Scholar]
- Gerhart J., Lowe C., Kirschner M. (2005). Hemichordates and the origin of chordates. Curr. Opin. Genet. Dev. 15, 461–467 [DOI] [PubMed] [Google Scholar]
- Gillis J. A., Fritzenwanker J. H., Lowe C. J. (2012). A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc. R. Soc. Biol. 279, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K., Ciosk R., Nance J., Neves A., Hill R. J., Priess J. R. (2004). The T-box transcription factors TBX-37 and TBX-38 link GLP-1/Notch signaling to mesoderm induction in C. elegans embryos. Development 131, 1967–1978 [DOI] [PubMed] [Google Scholar]
- Goodman S. J., Branda C. S., Robinson M. K., Burdine R. D., Stern M. J. (2003). Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development 130, 3757–3766 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M., Nüsslein-Volhard C. (1993). The expression of a zebrafish gene homologous to Drosophila snail suggests a conserved function in invertebrate and vertebrate gastrulation. Development 119, 1107–1118 [DOI] [PubMed] [Google Scholar]
- Harada Y., Akasaka K., Shimada H., Peterson K. J., Davidson E. H., Satoh N. (1996). Spatial expression of a forkhead homologue in the sea urchin embryo. Mech. Dev. 60, 163–173 [DOI] [PubMed] [Google Scholar]
- Hinman V. F., Davidson E. H. (2007). Evolutionary plasticity of developmental gene regulatory network architecture. Proc. Natl. Acad. Sci. USA 104, 19404–19409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. Z., Pace D. A., Blink M. L., Kene M., Holland N. D. (1995). Sequence and expression of amphioxus alkali myosin light chain (AmphiMLC-alk) throughout development: implications for vertebrate myogenesis. Dev. Biol. 171, 665–676 [DOI] [PubMed] [Google Scholar]
- Imai K. S., Satoh N., Satou Y. (2002). Early embryonic expression of FGF4/6/9 gene and its role in the induction of mesenchyme and notochord in Ciona savignyi embryos. Development 129, 1729–1738 [DOI] [PubMed] [Google Scholar]
- Imai K. S., Levine M., Satoh N., Satou Y. (2006). Regulatory blueprint for a chordate embryo. Science 312, 1183–1187 [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., Levine M. (1992). dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 6, 1518–1530 [DOI] [PubMed] [Google Scholar]
- Jiang J., Kosman D., Ip Y. T., Levine M. (1991). The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 5, 1881–1891 [DOI] [PubMed] [Google Scholar]
- Jones F. S., Meech R., Edelman D. B., Oakey R. J., Jones P. L. (2001). Prx1 controls vascular smooth muscle cell proliferation and tenascin-C expression and is upregulated with Prx2 in pulmonary vascular disease. Circ. Res. 89, 131–138 [DOI] [PubMed] [Google Scholar]
- Kadam S., McMahon A., Tzou P., Stathopoulos A. (2009). FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 136, 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Verkade H., Reiter J. F., Kim C. H., Chitnis A. B., Kuroiwa A., Stainier D. Y. (2004). Notch signaling can regulate endoderm formation in zebrafish. Dev. Dyn. 229, 756–762 [DOI] [PubMed] [Google Scholar]
- Kim G. J., Nishida H. (1999). Suppression of muscle fate by cellular interaction is required for mesenchyme formation during ascidian embryogenesis. Dev. Biol. 214, 9–22 [DOI] [PubMed] [Google Scholar]
- Kim G. J., Nishida H. (2001). Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev. Growth Differ. 43, 521–533 [DOI] [PubMed] [Google Scholar]
- Kim G. J., Yamada A., Nishida H. (2000). An FGF signal from endoderm and localized factors in the posterior-vegetal egg cytoplasm pattern the mesodermal tissues in the ascidian embryo. Development 127, 2853–2862 [DOI] [PubMed] [Google Scholar]
- Kimelman D. (2006). Mesoderm induction: from caps to chips. Nat. Rev. Genet. 7, 360–372 [DOI] [PubMed] [Google Scholar]
- Koop D., Richards G. S., Wanninger A., Gunter H. M., Degnan B. M. (2007). The role of MAPK signaling in patterning and establishing axial symmetry in the gastropod Haliotis asinina. Dev. Biol. 311, 200–212 [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Holland N. D., Kreslova J., Oliveri D., Schubert M., Jonasova K., Holland L. Z., Pestarino M., Benes V., Candiani S. (2007). Pax-Six-Eya-Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev. Biol. 306, 143–159 [DOI] [PubMed] [Google Scholar]
- Kumano G., Smith W. C. (2000). FGF signaling restricts the primary blood islands to ventral mesoderm. Dev. Biol. 228, 304–314 [DOI] [PubMed] [Google Scholar]
- Kumano G., Smith W. C. (2002). The nodal target gene Xmenf is a component of an FGF-independent pathway of ventral mesoderm induction in Xenopus. Mech. Dev. 118, 45–56 [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Nagy L. M. (2001). MAPK signaling by the D quadrant embryonic organizer of the mollusc Ilyanassa obsoleta. Development 128, 45–56 [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Nagy L. M. (2003). The MAPK cascade in equally cleaving spiralian embryos. Dev. Biol. 263, 231–241 [DOI] [PubMed] [Google Scholar]
- Langeland J. A., Tomsa J. M., Jackman W. R., Jr, Kimmel C. B. (1998). An amphioxus snail gene: expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Dev. Genes Evol. 208, 569–577 [DOI] [PubMed] [Google Scholar]
- Layden M. J., Meyer N. P., Pang K., Seaver E. C., Martindale M. Q. (2010). Expression and phylogenetic analysis of the zic gene family in the evolution and development of metazoans. EvoDevo 1, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Smith W. C., Nishida H. (2008). Ascidians and the plasticity of the chordate developmental program. Curr. Biol. 18, R620–R631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussink B., Brouwer A., el Khattabi M., Poelmann R. E., Gittenberger-de Groot A. C., Meijlink F. (1995). Expression patterns of the paired-related homeobox genes MHox/Prx1 and S8/Prx2 suggest roles in development of the heart and the forebrain. Mech. Dev. 52, 51–64 [DOI] [PubMed] [Google Scholar]
- Lo T. W., Branda C. S., Huang P., Sasson I. E., Goodman S. J., Stern M. J. (2008). Different isoforms of the C. elegans FGF receptor are required for attraction and repulsion of the migrating sex myoblasts. Dev. Biol. 318, 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. J., Wu M., Salic A., Evans L., Lander E., Stange-Thomann N., Gruber C. E., Gerhart J., Kirschner M. (2003). Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853–865 [DOI] [PubMed] [Google Scholar]
- Lowe C. J., Tagawa K., Humphreys T., Kirschner M., Gerhart J. (2004). Hemichordate embryos: procurement, culture, and basic methods. Methods Cell Biol. 74, 171–194 [DOI] [PubMed] [Google Scholar]
- Lowe C. J., Terasaki M., Wu M., Freeman R. M., Jr, Runft L., Kwan K., Haigo S., Aronowicz J., Lander E., Gruber C., et al. (2006). Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale M. Q., Pang K., Finnerty J. R. (2004). Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131, 2463–2474 [DOI] [PubMed] [Google Scholar]
- Materna S. C., Davidson E. H. (2012). A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Dev. Biol. 364, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoon P. E., Angerer R. C., Angerer L. M. (1996). SpFGFR, a new member of the fibroblast growth factor receptor family, is developmentally regulated during early sea urchin development. J. Biol. Chem. 271, 20119–20125 [DOI] [PubMed] [Google Scholar]
- McCoon P. E., Blackstone E., Angerer R. C., Angerer L. M. (1998). Sea urchin FGFR muscle-specific expression: posttranscriptional regulation in embryos and adults. Dev. Biol. 200, 171–181 [DOI] [PubMed] [Google Scholar]
- McMahon A., Reeves G. T., Supatto W., Stathopoulos A. (2010). Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development 137, 2167–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya T., Nishida H. (2003). An Ets transcription factor, HrEts, is target of FGF signaling and involved in induction of notochord, mesenchyme, and brain in ascidian embryos. Dev. Biol. 261, 25–38 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 [DOI] [PubMed] [Google Scholar]
- Nishida H., Sawada K. (2001). macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature 409, 724–729 [DOI] [PubMed] [Google Scholar]
- Norris R. A., Scott K. K., Moore C. S., Stetten G., Brown C. R., Jabs E. W., Wulfsberg E. A., Yu J., Kern M. J. (2000). Human PRRX1 and PRRX2 genes: cloning, expression, genomic localization, and exclusion as disease genes for Nager syndrome. Mamm. Genome 11, 1000–1005 [DOI] [PubMed] [Google Scholar]
- Oliveri P., Walton K. D., Davidson E. H., McClay D. R. (2006). Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development 133, 4173–4181 [DOI] [PubMed] [Google Scholar]
- Onai T., Yu J. K., Blitz I. L., Cho K. W., Holland L. Z. (2010). Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev. Biol. 344, 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani A. M., Mullarkey E. E., Aronowicz J., Assimacopoulos S., Grove E. A., Lowe C. J. (2012). Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R. A., Allmond J. J., Wagner H., McCauley D. W., Langeland J. A. (2009). Lamprey snail highlights conserved and novel patterning roles in vertebrate embryos. Dev. Genes Evol. 219, 31–36 [DOI] [PubMed] [Google Scholar]
- Range R. C., Glenn T. D., Miranda E., McClay D. R. (2008). LvNumb works synergistically with Notch signaling to specify non-skeletal mesoderm cells in the sea urchin embryo. Development 135, 2445–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebscher N., Deichmann C., Sudhop S., Fritzenwanker J. H., Green S., Hassel M. (2009). Conserved intron positions in FGFR genes reflect the modular structure of FGFR and reveal stepwise addition of domains to an already complex ancestral FGFR. Dev. Genes Evol. 219, 455–468 [DOI] [PubMed] [Google Scholar]
- Remane A. (1963). The enterocelic origin of the celom. In The Lower Metazoa (ed. Dougherty E.). Berkeley, CA: University of California Press; [Google Scholar]
- Revinski D. R., Paganelli A. R., Carrasco A. E., López S. L. (2010). Delta-Notch signaling is involved in the segregation of the three germ layers in Xenopus laevis. Dev. Biol. 339, 477–492 [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttinger E., Lowe C. J. (2012). Evolutionary crossroads in developmental biology: hemichordates. Development 139, 2463–2475 [DOI] [PubMed] [Google Scholar]
- Röttinger E., Saudemont A., Duboc V., Besnardeau L., McClay D., Lepage T. (2008). FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development 135, 353–365 [DOI] [PubMed] [Google Scholar]
- Saga Y., Takahashi Y. (2008). Mesp-family genes are required for segmental patterning and segmental border formation. Adv. Exp. Med. Biol. 638, 113–123 [DOI] [PubMed] [Google Scholar]
- Saga Y., Hata N., Kobayashi S., Magnuson T., Seldin M. F., Taketo M. M. (1996). MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122, 2769–2778 [DOI] [PubMed] [Google Scholar]
- Saga Y., Hata N., Koseki H., Taketo M. M. (1997). Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 11, 1827–1839 [DOI] [PubMed] [Google Scholar]
- Satou Y., Imai K. S., Satoh N. (2004). The ascidian Mesp gene specifies heart precursor cells. Development 131, 2533–2541 [DOI] [PubMed] [Google Scholar]
- Sawada A., Fritz A., Jiang Y. J., Yamamoto A., Yamasu K., Kuroiwa A., Saga Y., Takeda H. (2000). Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development 127, 1691–1702 [DOI] [PubMed] [Google Scholar]
- Sawada K., Fukushima Y., Nishida H. (2005). Macho-1 functions as transcriptional activator for muscle formation in embryos of the ascidian Halocynthia roretzi. Gene Expr. Patterns 5, 429–437 [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Smith J. C. (1995). Mesoderm formation in response to Brachyury requires FGF signalling. Curr. Biol. 5, 62–67 [DOI] [PubMed] [Google Scholar]
- Sethi A. J., Wikramanayake R. M., Angerer R. C., Range R. C., Angerer L. M. (2012). Sequential signaling crosstalk regulates endomesoderm segregation in sea urchin embryos. Science 335, 590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood D. R., McClay D. R. (1999). LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703–1713 [DOI] [PubMed] [Google Scholar]
- Shi S., Stahl M., Lu L., Stanley P. (2005). Canonical Notch signaling is dispensable for early cell fate specifications in mammals. Mol. Cell. Biol. 25, 9503–9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. (1991). Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67, 79–87 [DOI] [PubMed] [Google Scholar]
- Stathopoulos A., Tam B., Ronshaugen M., Frasch M., Levine M. (2004). pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 18, 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B. E., Siegrist S. E., Beckerle M. C. (1996). Two muscle-specific LIM proteins in Drosophila. J. Cell Biol. 134, 1179–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B. E., Renfranz P. J., Lilly B., Beckerle M. C. (1999). Muscle LIM proteins are associated with muscle sarcomeres and require dMEF2 for their expression during Drosophila myogenesis. Mol. Biol. Cell 10, 2329–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C., Haremaki T., Weinstein D. C. (2004). Inhibition of mesodermal fate by Xenopus HNF3beta/FoxA2. Dev. Biol. 265, 90–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 [DOI] [PubMed] [Google Scholar]
- Thézé N., Hardy S., Wilson R., Allo M. R., Mohun T., Thiebaud P. (1995). The MLC1f/3f gene is an early marker of somitic muscle differentiation in Xenopus laevis embryo. Dev. Biol. 171, 352–362 [DOI] [PubMed] [Google Scholar]
- Thiébaud P., Rescan P. Y., Barillot W., Rallière C., Thézé N. (2001). Developmental program expression of myosin alkali light chain and skeletal actin genes in the rainbow trout Oncorhynchus mykiss. Biochim. Biophys. Acta 1519, 139–142 [DOI] [PubMed] [Google Scholar]
- Tulin S., Stathopoulos A. (2010). Extending the family table: Insights from beyond vertebrates into the regulation of embryonic development by FGFs. Birth Defects Res. C Embryo Today 90, 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbeville J. M., Schulz J. R., Raff R. A. (1994). Deuterostome phylogeny and the sister group of the chordates: evidence from molecules and morphology. Mol. Biol. Evol. 11, 648–655 [DOI] [PubMed] [Google Scholar]
- Valentine J. W. (2004). On the Origin of Phyla. Chicago, IL, USA: University of Chicago Press; [Google Scholar]
- Wu S. Y., McClay D. R. (2007). The Snail repressor is required for PMC ingression in the sea urchin embryo. Development 134, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo H., Hudson C. (2007). FGF8/17/18 functions together with FGF9/16/20 during formation of the notochord in Ciona embryos. Dev. Biol. 302, 92–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





