Abstract
The mammalian skull vault consists of several intricately patterned bones that grow in close coordination. The growth of these bones depends on the precise regulation of the migration and differentiation of osteogenic cells from undifferentiated precursor cells located above the eye. Here, we demonstrate a role for Foxc1 in modulating the influence of Bmp signaling on the expression of Msx2 and the specification of these cells. Inactivation of Foxc1 results in a dramatic reduction in skull vault growth and causes an expansion of Msx2 expression and Bmp signaling into the area occupied by undifferentiated precursor cells. Foxc1 interacts directly with a Bmp responsive element in an enhancer upstream of Msx2, and acts to reduce the occupancy of P-Smad1/5/8. We propose that Foxc1 sets a threshold for the Bmp-dependent activation of Msx2, thus controlling the differentiation of osteogenic precursor cells and the rate and pattern of calvarial bone development.
Keywords: Skull vault, Foxc1, Msx2, Bmp responsive element, Mouse
INTRODUCTION
Knowing how signaling molecules elicit transcriptional responses of target genes at specific thresholds is crucial for understanding cell specification during embryonic patterning. A key element of such mechanisms is the complement of transcription factors that respond to the signal and influence the expression of downstream genes (Gurdon et al., 1995; Gurdon and Bourillot, 2001). Although a number of these factors have been identified, how their transcription regulatory activity is translated into events at the level of cell specification and patterned growth is still incompletely understood. Here, we investigate the role of Foxc1 in modulating the influence of Bmp signaling on the expression of the Bmp effector Msx2, and the specification of osteogenic precursor cells in the developing skull vault.
The mammalian skull vault consists of a group of intricately patterned bones that develop in close coordination (Morriss-Kay and Wilkie, 2005; Chai and Maxson, 2006). These include the paired frontal and parietal bones. The frontal bones are derived from cranial neural crest and the parietal bones from head mesoderm. In the mouse embryo, skull vault precursor cell populations migrate into positions above the eye by E12.5. There they condense and begin to differentiate into the calvarial rudiments. From E13.5 into postnatal development, the paired frontal and parietal bone rudiments grow apically, coming into apposition at the midline at the frontal and sagittal sutures, and laterally at the coronal suture.
The morphogenetic mechanisms underlying the patterned growth of the calvarial bones are under active investigation. Proliferation of osteogenic cells within the rudiments contributes to growth (Ishii et al., 2003), as does the apical migration of osteogenic precursor cells. DiI labeling experiments show that a population of such migratory osteogenic precursor (MOP) cells is located outside (ectocranial to) the rudiments; cells from this population migrate apically, insert into the leading edge of the growing rudiment and differentiate into osteoblasts (Yoshida et al., 2008; Ting et al., 2009; Roybal et al., 2010).
Msx genes, effectors of Bmp signaling, are crucial for calvarial development (Satokata et al., 2000; Wilkie et al., 2000; Chai and Maxson, 2006). Msx genes are an ancient and highly conserved homeobox gene family with a variety of functions in vertebrate organogenesis (Satokata and Maas, 1994; Phippard et al., 1996; Bach et al., 2003; Chen et al., 2007). Msx1 and Msx2 are well known as downstream targets of the Bmp signaling pathway, and at the same time can regulate the expression of Bmp ligands (Bei and Maas, 1998). In Msx2–/– embryos, the growth of the calvarial rudiments is deficient, resulting in a large ossification defect in the frontal bone (Ishii et al., 2003). Humans with heterozygous loss of MSX2 function are similarly affected (Wilkie et al., 2000). Combination Msx1/2 mutants exhibit agenesis of the frontal and parietal bones (Han et al., 2007).
Msx2 is regulated by the Bmp pathway through an upstream Bmp responsive enhancer element (Brugger et al., 2004). This element, ∼560 bp in length, contains a 52 bp fragment necessary for the Bmp responsiveness of an Msx2 transgene in embryos and in cultured cells. This fragment contains an AT-rich central sequence flanked by Smad-binding sites. Both the AT-rich site and the Smad sites are required for Bmp responsiveness. The AT-rich region contains a partial Fox consensus site (Gao et al., 2003; Brugger et al., 2004; Benayoun et al., 2011), leading us to propose that Fox proteins function together with Smads to regulate the ability of Msx2 to respond to Bmp signaling (Brugger et al., 2004). Here, we present genetic and molecular evidence that the Fox protein Foxc1 controls the Bmp responsiveness of Msx2.
Fox genes are involved in a variety of developmental processes during organogenesis, as well as in aspects of energy homeostasis and cancer (Hannenhalli and Kaestner, 2009; Benayoun et al., 2011). Both targeted (Foxc1lacZ) and spontaneous (Foxc1ch) Foxc1-null mice die perinatally with identical skeletal, ocular, genitourinary, cardiovascular and somitic defects, as well as hydrocephalus and cerebellar defects (Kume et al., 1998; Kume et al., 2000; Kume et al., 2001; Seo and Kume, 2006). The most striking skeletal phenotype in Foxc1 mutant mice is the lack of calvarial bones. This defect is not secondary to hydrocephalus (Rice et al., 2003). Recently, a new mouse model with a hypomorphic allele of Foxc1 (Foxc1hith) was found to have meningeal defects in association with frontal foramina and cortical dysplasia (Zarbalis et al., 2007). In humans, mutations in FOXC1 are associated with Axenfeld-Rieger and Dandy Walker syndromes, both inherited as autosomal dominant traits. Individuals affected with Axenfeld-Rieger syndrome exhibit a spectrum of malformations, including dysgenesis of the anterior segment of the eye, tooth abnormalities, maxillary hypoplasia, hypertelorism and cardiac outflow tract defects (Tümer and Bach-Holm, 2009). Dandy-Walker syndrome, the most common human cerebellar malformation, is associated with deletions or duplications spanning the FOXC1 gene (Aldinger et al., 2009).
We report here that Foxc1 controls the differentiation of osteogenic precursor cells that contribute to the frontal bone, and we present evidence that it does so by inhibiting the Bmp responsiveness of Msx2 and the activity of the Bmp pathway in such cells. We show further that Foxc1 interacts directly with a Bmp-responsive element in the Msx2 560 bp upstream enhancer, and we confirm, by means of gain- and loss-of-function experiments in a newly developed cranial neural crest cell line (Ishii et al., 2012), that Foxc1 acts as a negative regulator of the Bmp responsiveness of Msx2. We show finally that Foxc1 acts to restrict the occupancy of P-Smad1/5/8 on the Msx2 Bmp responsive element. We propose that the normal function of Foxc1 is to set a transcriptional threshold for the Bmp-dependent activation of Msx2, thus controlling the differentiation of osteogenic precursor cells and the initial phases of calvarial bone development.
MATERIALS AND METHODS
DNA constructs
Msx2 enhancer constructs pBSK-560bpMsx2-hsplacZ, pBSK-52bpMsx2-hsplacZ, pBSK-52bpMsx2-mut-hsplacZ and pGL2-560bpMsx2-tk-luciferase have been described previously (Brugger et al., 2004). The Foxc1 expression vector pcDNA3-mFoxc1 was a kind gift from Dr Tsutomu Kume (Northwestern University, Chicago, IL, USA).
Cell culture, co-transfections and luciferase assay
10T1/2 cells were propagated in DMEM with 10% FBS. O9-1 cells were maintained and differentiated into osteoblast as recently reported (Ishii et al., 2012). Lipofectamine 2000 (Life Technologies) was used to introduce pcDNA3-mFoxc1 or siFoxc1 with pGL2-560bpMsx2-tk-luc into 10T1/2 and O9-1 cells. BMP2 (100 ng/ml) treatment and luciferase assay was performed as described previously (Brugger et al., 2004).
Mouse strains and genotyping
Heterozygous Foxc1ch/+ (ch: congenital hydrocephalus) mice were purchased from Jackson Laboratory on the CHMU/Le background. Msx2 mutant mice, originally obtained from Dr Richard Maas (Harvard University, Boston, MA, USA), were crossed into a C57BL/6 background (Ishii et al., 2003). 560bpMsx2-hsplacZ transgenic mice have been described previously (Kwang et al., 2002). The ch allele of Foxc1 was identified by PCR amplification followed by Cac8I digestion (Kume et al., 2000). The Msx2 mutant allele and the lacZ transgene were identified by PCR (Satokata et al., 2000; Kwang et al., 2002).
Bead implantation
Preparation of the agarose beads and calvarial explant culture were performed as previously described (Rice et al., 2003; Brugger et al., 2004).
Histochemistry, immunostaining and in situ hybridization
lacZ, ALP histochemistry and immunofluorescent detection for P-Smad1/5/8 (Cell Signaling) have been described previously (Ishii et al., 2003; Ting et al., 2009). Msx2 full-length cDNA was cloned into pBSKII(+) (Kwang et al., 2002). A cloned Foxc1 cDNA was a kind gift from Dr David Rice (University of Helsinki, Finland). Bmp2 and Bmp4 full-length cDNAs were obtained from Dr Malcolm Snead (USC, Los Angeles, CA, USA). Noggin cDNA was a gift from Dr Richard Harland (UCLA, Berkeley, CA, USA). Runx2 cDNA was obtained from Dr Gérard Karsenty (Columbia University Medical Center, NY, USA). Ribonucleotide probes were generated as described previously (Kwang et al., 2002). Fluorescent in situ hybridization followed by tyramide signal amplification (TSA PLUS, Perkin Elmer) was carried out as reported previously (Paratore et al., 1999; Ting et al., 2009).
To quantify ALP, Msx2, Bmp4 and Bmp2 expression, continuous sections from at least two wild-type and two mutant embryos were photographed and analyzed with Adobe Photoshop. We used the magic wand tool to select the region of interest and calculate the pixel numbers of selected area. Data show the average pixel count of ∼10-15 pictures from one representative pair. For Foxc1 and Msx2 colocalization (Fig. 2G), yellow pixels were selected by color and counted from a total 16 sections of three pairs of control and mutant embryos. At least 10 pictures from three individuals of each genotype were analyzed to quantify the influence of Msx2 dose on ectopic ALP (Fig. 4I) and P-Smad1/5/8 (Fig. 4N) expression.
Fig. 2.
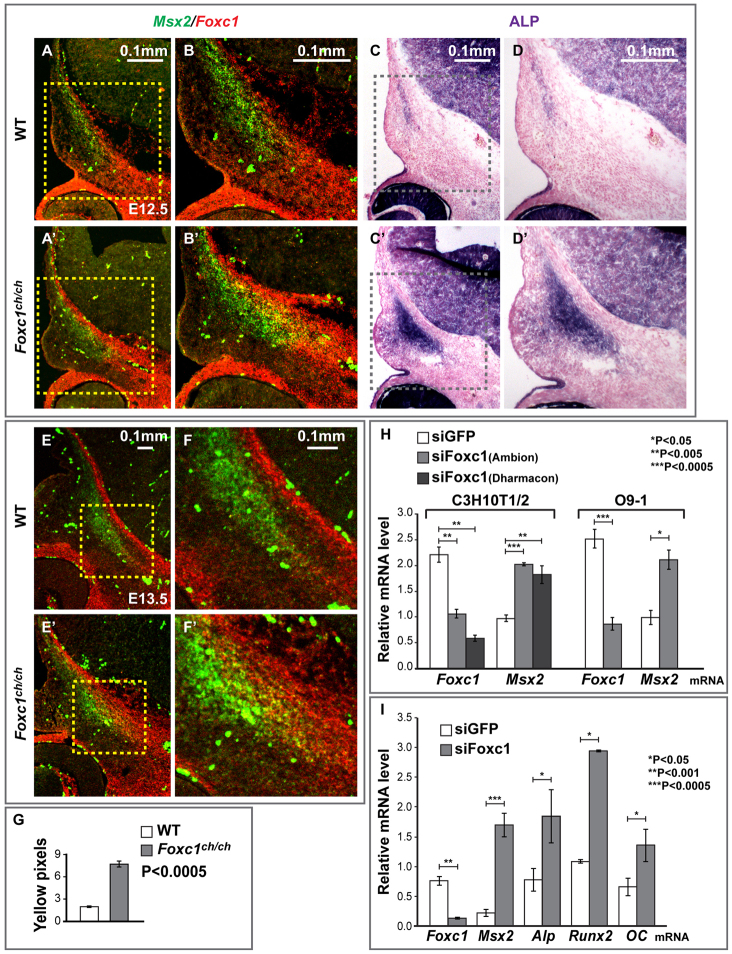
Foxc1 inhibits Msx2 expression in the supraorbital ridge and in cultured 10T1/2 and O9-1 cranial neural crest cells. (A,A′,B,B′,E,E′,F,F′) Control and Foxc1ch/ch embryos at E12.5 or E13.5 were sectioned as in Fig. 1A and subjected to in situ hybridization for Msx2 and Foxc1 mRNAs simultaneously. Msx2 is in green; Foxc1 in red. (C-D′) Sections from the same embryo were stained for ALP. Boxed areas are shown at higher magnification on the right. (G) The area of Msx2 and Foxc1 co-expression, indicated by the yellow color in B and B′, is significantly larger (P<5.00×10–4) in Foxc1 mutants. (H) Effect of siRNA-mediated knockdown of Foxc1 on Msx2 mRNA levels in C3H10T1/2 cells and O9-1 cranial neural crest cells. mRNA levels of Foxc1 and Msx2 were measured by qPCR and normalized to Gapdh. siRNA against EGFP provided a negative control. (I) We assessed the influence of Foxc1 knockdown on the rate of osteogenic differentiation of cultured O9-1 cells. Cells were treated with siRNA against Foxc1, and qPCR was used to measure levels of Msx2, ALP, Runx2 and osteocalcin mRNAs. Significant (P<0.05) upregulation of each of these osteogenic differentiation markers was observed after 48 hours of Foxc1 siRNA treatment. Significance was assessed by Student’s t-test. Error bars indicate s.d.
Fig. 4.
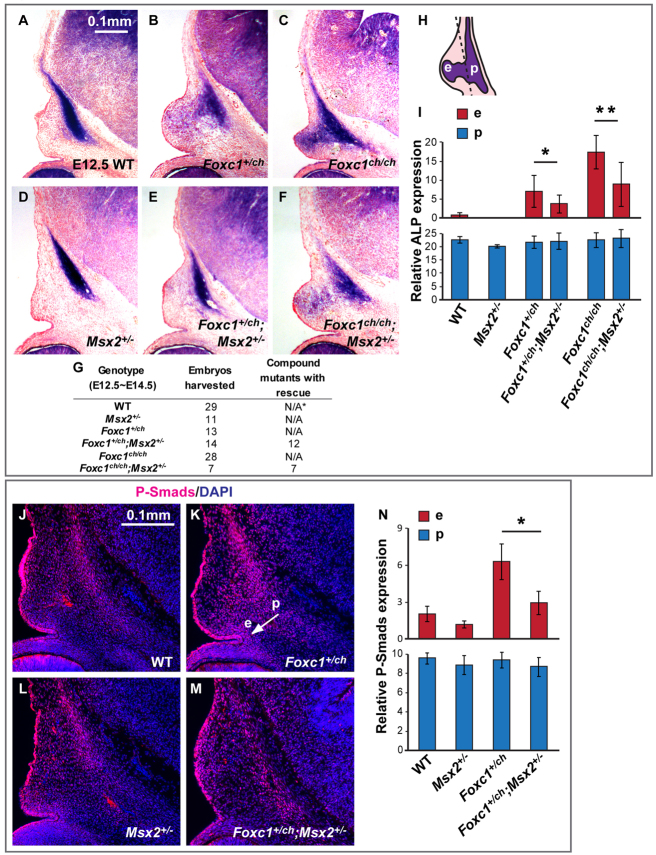
Reduced dose of Msx2 rescues ectopic osteogenesis and Bmp signaling in the supraorbital ridge of Foxc1 mutant embryos. (A-F) ALP expression in the supraorbital ridge in E12.5 wild-type, Foxc1, Msx2 and compound mutant embryos. There is near-normal expression of ALP in the Foxc1ch/+; Msx2+/– compound heterozygous mutant (E) versus the Foxc1 heterozygous mutant (B). Also note the partial rescue in the Foxc1ch/ch; Msx2+/– mutant (F) compared with the Foxc1 homozygous mutant (C). (G) Numbers of embryos harvested for this experiment. *N/A, not applicable. (H) We measured the level of ALP expression in the supraorbital ridge by counting the purple pixels in two areas: p (primordium) and e (ectopic). (I) The influence of Msx2 dose on ALP expression in Foxc1 mutants was statistically significant in area e (*P<0.05, **P<0.005). (J-M) Bmp signaling activity in E13 embryos was detected by P-Smad1/5/8 immunohistochemistry. P-Smad1/5/8 expansion is partially rescued: compare the area spanned by the white arrow from p to e in K with the corresponding area in M. (N) Significant reduction of P-Smad1/5/8 signal in area e in Foxc1ch/+; Msx2+/– mutant embryo in comparison with Foxc1ch/+ mutant (*P<5.00×10–6). Significance was assessed by Student’s t-test. Error bars indicate s.d.
RNAi and qRT-PCR
siRNAs targeting Foxc1 (Ambion, SMARTpool Dharmacon) were introduced into O9-1 or C3H10T1/2 cells by Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol. siGFP was used as control (supplementary material Table S1). Cells were collected ∼24-48 hours after transfection and subjected to total RNA extraction with the RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized with the SuperScript III system (Life Technologies) for RT-PCR analysis. Relative expression levels were normalized to Gapdh mRNA. Primer sequences are given in supplementary material Table S1.
Chromatin immunoprecipitation (ChIP) assays
ChIP was performed as described previously (Ma et al., 2003; Brugger et al., 2004) with minor modifications. Cells were treated with 60 ng/ml BMP2 for 30 minutes prior to fixation. Anti-Foxc1 pull-down was achieved by two sessions of incubation: first with a goat anti-Foxc1 antibody (Abcam), then with a rabbit anti-goat IgG (Sigma-Aldrich). Rabbit IgG was used for mock immunoprecipitation (Sigma-Aldrich). qPCR was performed to amplify a 350 bp region of the endogenous Msx2 enhancer. For transfected cells, DNA constructs or siRNA were introduced into cells 24 hours prior to BMP2 treatment. A 200 bp region of the hsp68 mini-promoter immediately downstream of the 52 bp Msx2 Bmpre in pBSK was amplified by qPCR. Each result is controlled by IgG, and is from a single experiment representative of three independent experiments. Primer sequences are given in supplementary material Table S1.
RESULTS
Foxc1 regulates the size of the frontal bone rudiment and the osteogenic differentiation of cultured cranial neural crest cells
We first examined the expression of an early osteoblast marker, alkaline phosphatase (ALP), in Foxc1 mutant and control embryos at E12.5 (Fig. 1B-C′), E13.5 (Fig. 3D,D′,F,F′) and E14 (supplementary material Fig. S1A-B″). Consistent with previous findings (Ishii et al., 2003; Rice et al., 2003), ALP was expressed in control embryos in a group of cells that make up the frontal and parietal bone rudiments (Fig. 1B,C; Fig. 3D,F; supplementary material Fig. S1A,B). In Foxc1ch/ch mutants, apical growth did not progress beyond the primordium stage, and the rudiments mineralized by E15 with little or no further growth (supplementary material Fig. S1A″,B″) (Rice et al., 2003). These results are consistent with those of Rice and colleagues (Rice et al., 2003). In addition to this previously reported lack of apical growth, we found that the ALP-positive area expanded laterally toward the epidermis and ventrally towards the midline of the cranial base (arrows, Fig. 1C′). Quantitation of the ALP signal in serial sections through the supraorbital ridge confirmed this expansion (P<5.00×10–5, Fig. 1F). Runx2, also a pre-osteoblast marker, exhibited a similar expansion in Foxc1 mutant embryos (supplementary material compare Fig. S1C with S1C″). Foxc1 mutants have hydrocephalus, precluding analysis of skull vault growth and patterning in embryos after E15.
Fig. 1.
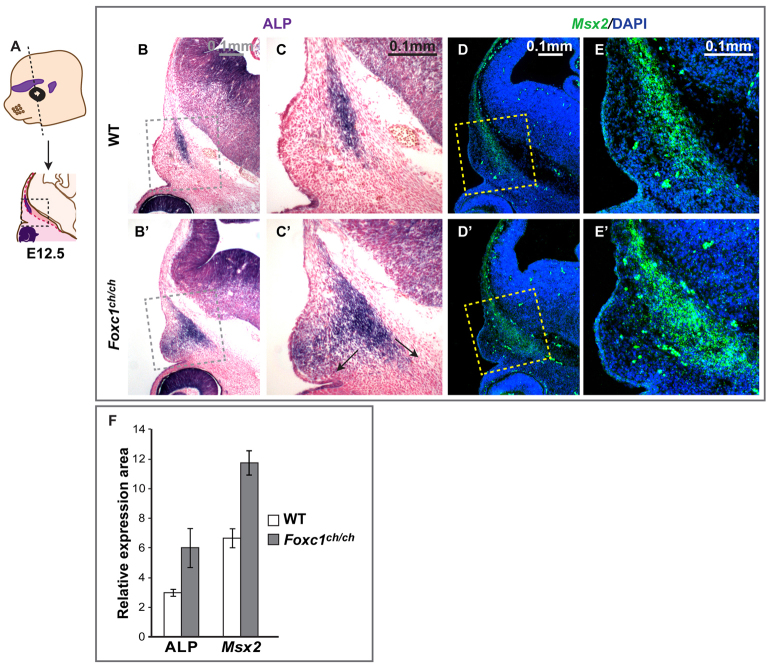
Expansion of frontal bone osteogenic domain in the supraorbital ridge of Foxc1 mutant embryos. (A-E′) Control (B-E) and Foxc1ch/ch mutant (B′-E′) embryos at E12.5 were sectioned in the indicated (coronal) plane (A) and stained for ALP to mark osteogenic cells (B-C′) or were subjected to in situ hybridization for Msx2 (D-E′). Boxed areas are shown at higher magnification on the right. (F) Increased ALP-positive osteogenic progenitor domain and Msx2 mRNA signal inside the boxed area in Foxc1 mutant embryos (Student’s t-test, P<5.00×10–5). Error bars indicate s.d.
Fig. 3.
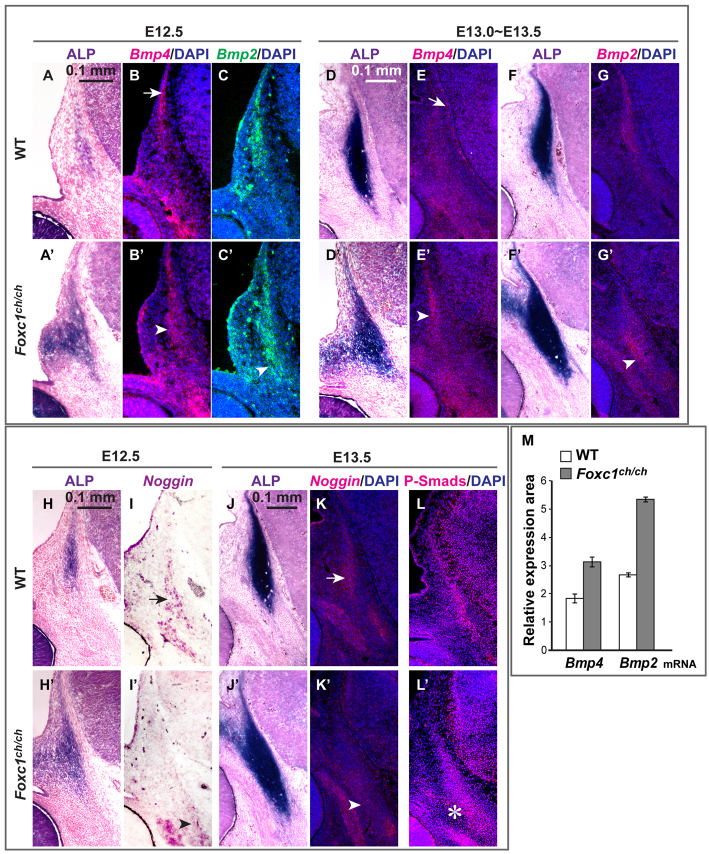
Elevated Bmp signaling activity in the supraorbital ridge of Foxc1 mutant embryos. (A-G′,M) We detected Bmp4 and Bmp2 expression by in situ hybridization in coronal sections of embryos at E12.5 and E13.5 wild-type and Foxc1 mutant embryos. There is Bmp4 expression at the apex of the osteogenic area and in the meninges of wild-type embryos (arrows, B and E), and in the rudiment of Foxc1 mutants (arrowheads, B′ and E′). Bmp2 expression is increased in the rudiment in Foxc1 mutants (arrowheads, C′ and G′). The increases in both Bmp4 and Bmp2 expression in these areas were statistically significant at E12.5 (Student’s t-test, P<1.00×10–8, M). Error bars indicate s.d. (H-K′) Expression of a Bmp antagonist, Noggin, assessed by in situ hybridization at E12.5 and E13.5. ALP was used to visualize the signal at E12.5 because it provided a more robust signal than fluorescent detection with extended developing time. Noggin transcripts are present in the rudiment and in an adjacent cartilage in wild-type embryos (arrows, I and K), but reduced in Foxc1 mutants (arrowheads, I′ and K′). (L,L′) Immunodetection of P-Smad 1/5/8 served to indicate the overall activity of the canonical Bmp pathway. The number of P-Smad1/5/8-positive nuclei is increased in Foxc1 mutant (asterisk, L′).
Lineage tracing with Wnt1-Cre/R26R (neural crest) and Mesp1-Cre/R26R (mesoderm) showed no major differences between Foxc1 mutant and control embryos in the distribution of neural crest and mesoderm in the head region of E9.5 or E12.5 embryos (supplementary material Fig. S2). Thus, the lack of apical extension of the frontal and parietal bones is not likely to be caused by a failure of osteogenic precursor cells to migrate into position in the supraorbital ridge.
In situ hybridization revealed that Msx2, which is normally expressed in the osteogenic cells of the frontal and parietal rudiments (Fig. 1E), expanded laterally in Foxc1ch/ch mutants, coincident with the expansion in the ALP domain (arrows, Fig. 1C′; P<1.00×10–16, Fig. 1F). We used Foxc1 and Msx2 probes simultaneously to determine the relationship between the Foxc1 expression domain and the expansion of Msx2 expression (Fig. 2). Because the Foxc1 ch mutation is a single base change, the Foxc1 transcript is detectible in sections of mutant embryos. At E11.5, Msx2 was expressed broadly in cranial mesenchyme. Foxc1 transcripts were detected mainly in the periocular mesenchyme and not in the mesenchyme where the frontal bone rudiment forms (data not shown). By E12.5, in control embryos, Foxc1 was expressed in the meninges and in the cranial mesenchyme, overlapping partially with Msx2 in the lateral region of the Foxc1 domain (Fig. 2A,B). The number of cells expressing both Foxc1 and Msx2 transcripts (yellow pixels, Fig. 2G) increased in Foxc1 mutants (P<5.00×10–4), consistent with an increase in Msx2 transcripts in cells expressing the mutant Foxc1 mRNA. Msx2 expression also increased laterally outside the Foxc1 domain (Fig. 2B′), suggesting that loss of Foxc1 results in non-cell autonomous effects on Msx2 expression. Similar changes in Msx2 expression in Foxc1 mutants were evident at E13.5 (Fig. 2E′,F′). These results suggest that Foxc1 acts to restrict the osteogenic domain and the Msx2 expression domain within the supraorbital ridge. We note that Msx2 expression does not expand into the innermost (endocranial) region of the Foxc1 domain, suggesting that additional factors may be required for Msx2 expression in this region.
To test directly whether Foxc1 negatively regulates Msx2, we used siRNA to knock down Foxc1 in cultured cells (Fig. 2H). In initial experiments, we used C3H10T1/2 cells, which are multipotent mesenchymal cells capable of differentiating into muscle cells, chondrocytes, adipocytes and osteoblasts (Pinney and Emerson, 1989; Katagiri et al., 1990). Because the mesenchyme of the supraorbital ridge at the level of the frontal bone rudiment is derived from cranial neural crest (Jiang et al., 2002; Ishii et al., 2003), we also used a cranial neural crest (CNC) cell line, O9-1, that we developed recently (Ishii et al., 2012). This cell line was derived from Wnt1-Cre; R26R-EGFP-expressing cells from the head region of E8.5 mouse embryos. We established culture conditions that allow O9-1 cells to be grown as multipotent stem-like cells, maintaining an ability to differentiate into osteoblasts, chondrocytes, smooth muscle cells and glial cells. O9-1 cells can be propagated and passaged indefinitely, and can contribute to bone and smooth muscle after injection into mouse embryos (Ishii et al., 2012). O9-1 cells undergo differentiation into osteoblasts with high efficiency after being placed into an osteogenic medium.
We transfected either a control siRNA or a Foxc1 siRNA into 10T1/2 and O9-1 cells, and assessed endogenous Foxc1 and Msx2 mRNA levels by real-time PCR (Fig. 2H). In 10T1/2 cells, Foxc1 mRNA declined to 50% of its level in control cells, and Msx2 transcript levels increased approximately twofold, consistent with Foxc1 exerting an inhibitory effect on Msx2 expression. In O9-1 cells, Foxc1 siRNA caused a reduction of Foxc1 mRNA to ∼30% of its level in control siRNA-treated cells by 24 hours after transfection, and caused Msx2 transcript levels to increase by approximately twofold. To rule out off-target effects, we used siRNAs against distinct sequences in the Foxc1 transcript (Fig. 2H). These siRNAs (a mixture of four, none of which had sequences that overlapped with the siRNA used in the initial experiments) caused a similar reduction of Foxc1 mRNA and increase in Msx2 mRNA in both 10T1/2 (Fig. 2H) and O9-1 cells (data not shown). These results support the hypothesis that Foxc1 negatively regulates Msx2 in the supraorbital ridge.
We next asked whether knockdown of Foxc1 augmented the osteogenic differentiation of O9-1 cells (Fig. 2I). We assessed the expression of the osteogenic markers ALP, Runx2 and osteocalcin in Foxc1-knockdown and control O9-1 cells cultured in osteogenic medium. We found that the expression of each marker was increased relative to controls, suggesting that knockdown of Foxc1 not only upregulates Msx2, but also accelerates the osteogenic differentiation of O9-1 cells. These data support the hypothesis that reduced Foxc1 function can increase the rate of osteogenic differentiation of cranial neural crest cells.
Ectopic osteogenesis and Msx2 expression correlate with ectopic Bmp signaling in calvarial rudiments of Foxc1 mutants
We assessed the expression of Bmp2, Bmp4, P-Smad1/5/8 and the extracellular Bmp inhibitor Noggin in the calvarial rudiments and supraorbital ridge of Foxc1ch/ch mutant and control embryos (Fig. 3). We found that Bmp2 and Bmp4 expression domains were expanded at E12.5 and E13.5, similar to ALP (arrowheads, Fig. 3B′,C′,E′,G′; quantitation in 3M). In control embryos, Noggin transcripts were located ventral to the Bmp/ALP/Msx2 domain (arrows, Fig. 3I,K). In mutants, Noggin expression declined substantially (arrowheads, Fig. 3I′,K′). P-Smad1/5/8, the downstream effector of canonical Bmp signaling (Massagué, 1998), was distributed in a pattern that largely coincided with the Bmp/ALP/Msx2 domain (Fig. 3L,L′). Expression expanded ventrally in the mutant (star, Fig. 3L′), consistent with a net increase in Bmp signaling in the area of ectopic ALP expression.
Reduced Msx2 gene dose rescues ectopic osteogenesis and Bmp signaling in Foxc1 mutants
To determine whether Msx2 has a functional role downstream of Foxc1 in the regulation of osteogenic precursor differentiation, we asked whether reduced Msx2 dosage mitigated ectopic osteogenesis and Bmp signaling in Foxc1 mutants (Fig. 4). We crossed Msx2 and Foxc1 mutants, producing embryos with the genotype Foxc1ch/+; Msx2+/– and Foxc1ch/ch; Msx2+/–. We did not recover double homozygous null embryos from double heterozygous matings (n=59), consistent with this genotype causing lethality prior to E13. We assessed the size and growth of the calvarial rudiments by ALP staining of serial sections through the supraorbital ridge at E12.5 (Fig. 4A-F). Foxc1ch/+ embryos exhibited a subtle but reproducible expansion of ALP, suggesting that this phenotype is Foxc1 dose dependent at early developmental stages (supplementary material Fig. S1). However, this deficiency was transient, as frontal bone development was indistinguishable from wild type at E17 and P0 (Rice et al., 2003; J.S., M.I., M.-C.T. and R.M., unpublished).
The ectopic ALP staining evident in E12.5 Foxc1ch/+ and Foxc1ch/ch mutant embryos was reduced or absent in Foxc1/Msx2 double mutants (Fig. 4B-F). Quantitation of the ectopic ALP signal (e.g. Fig. 4H) by enumerating ALP pixels revealed reductions of 50% in both Foxc1ch/+; Msx2+/– and Foxc1ch/ch; Msx2+/– combinations. Similarly, ectopic P-Smad1/5/8 activity was reduced (Fig. 4J-N). These results suggest that the ectopic expression of Msx2 resulting from loss of Foxc1 function is causally related to ectopic osteogenesis and to Bmp signaling in the supraorbital ridge. Because Foxc1ch/+ animals do not have a frontal bone phenotype at late stages of embryogenesis or in early postnatal life, we were unable to ask whether reduced Msx2 dose rescued such animals. In addition, reduced Msx2 dose did not rescue the hydrocephalus defect in Foxc1ch/ch embryos, precluding examination of such embryos at late stages.
Foxc1 acts through an upstream Msx2 enhancer to regulate the Msx2 expression domain and Bmp responsiveness in the supraorbital ridge
We next asked whether Foxc1 influences Msx2 expression via an effect on an upstream Msx2 enhancer (Fig. 5). ChIP experiments in ES cells and adult heart tissue showed that this enhancer is enriched for the transcription co-activator p300, as well as histone marks associated with active enhancers (Santos-Rosa et al., 2002; Bernstein et al., 2005; Chen et al., 2008; Visel et al., 2009) (Encode Project, UCSC Genome Browser).
Fig. 5.
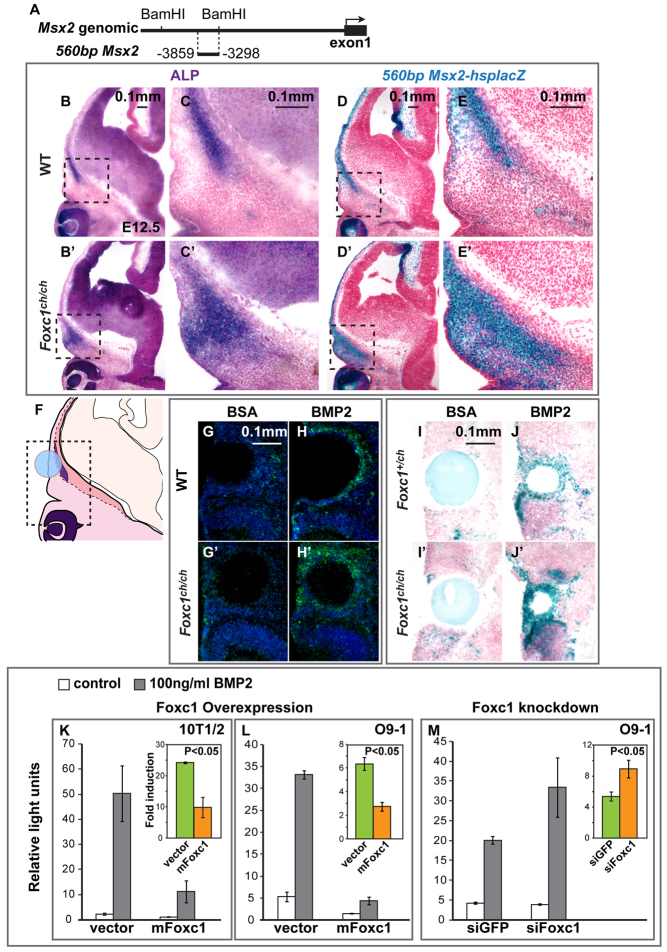
Foxc1 inhibits the Bmp responsiveness of Msx2 in embryos and cultured cells. (A) Msx2 locus showing 560 bp Msx2 upstream enhancer. (B-E′) ALP and lacZ staining was performed on adjacent sections of E12.5 560bpMsx2-hsplacZ transgenic embryos carrying either the wild-type (B-E) or Foxc1ch mutant allele (B′-E′). Boxed areas are shown at higher magnification on the right. The 560bpMsx2 transgene is sufficient to respond to loss of Foxc1 function. (F-J′) Influence of BMP2-soaked beads on endogenous Msx2 (G-H′) assessed by in situ hybridization and 560bpMsx2-hsplacZ expression (I-J′). The signal intensity in the supraorbital ridge is increased in both the endogenous Msx2 (H versus H′) and the transgene (J versus J′) in Foxc1 mutants compared with control embryos. (K-M) We tested the effect of overexpression (K,L) and siRNA-mediated knockdown (M) of Foxc1 on the Bmp inducibility of the 560bpMsx2-tk-luc construct in 10T1/2 and O9-1 cranial neural crest cells. The insets show the fold change in luciferase expression. The results represent means of three biological replicates. Error bars show one s.d. Significance was assessed using Student’s t-test.
We crossed mice carrying the 560bpMsx2-hsplacZ transgene with Foxc1 mutants (Foxc1ch/+) and assessed its expression in the area of the frontal and parietal bone rudiments. lacZ expression expanded, similar to endogenous Msx2 expression (Fig. 5D-E′). Therefore the 560 bp enhancer is sufficient to respond to a loss of Foxc1 function.
Foxc1 negatively regulates Msx2 expression and Msx2 is an immediate early target of the Bmp signaling pathway (Brugger et al., 2004) suggesting that Foxc1 might inhibit the Bmp responsiveness of Msx2. To test this hypothesis, we implanted BMP2-soaked beads in the supraorbital ridge of explants of embryonic heads of E12.5 embryos (Fig. 5F). We cultured the explants and, after 2 days, carried out in situ hybridization with a probe for Msx2 (Fig. 5G-H′). We found that the BMP2 beads elicited a greater increase in Msx2 expression in Foxc1 mutant embryos than in control embryos.
This result is in contrast to the findings of Rice et al. (Rice et al., 2003) who found that a BMP2-soaked bead implanted in the dorsum of the head of E15 embryos elicited a reduced Bmp response in Foxc1 mutants compared with wild-type embryos. We repeated their experiment and obtained closely similar results; i.e. a reduction in Bmp responsiveness of Msx2 (supplementary material Fig. S3). Therefore, the difference between our findings in the supraorbital ridge and those of Rice et al. (Rice et al., 2003) in the head mesenchyme is likely a consequence of the difference in the sites and developmental stages of bead implantation.
To further test the hypotheses that Foxc1 modulates the Bmp responsiveness of Msx2, and that the 560 bp enhancer is sufficient for this effect, we carried out a BMP2 bead implantation experiment in embryonic heads (Fig. 5I-J′) and limbs (data not shown) of mice carrying the 560bpMsx2-hsplacZ transgene. Staining for β-galactosidase activity revealed that the 560 bp enhancer was indeed sufficient for the increase in the Bmp responsiveness of Msx2 in Foxc1 mutants in the supraorbital ridge.
Finally, we used both gain- and loss-of-function approaches in cultured cells to assess the influence of Foxc1 on the Bmp-responsiveness of Msx2 (Fig. 5K-M). We transfected a construct bearing the 560 bp enhancer driving tk-luciferase (560bpMsx2-tk-luc) into 10T1/2 cells and O9-1 CNC cells, and tested the effect of overexpressing Foxc1 on the Bmp-inducibility of 560bpMsx2 (Fig. 5K,L). In both 10T1/2 and O9-1 cells, Bmp inducibility was more than halved (P<0.05). Reciprocally, transfection of siRNA against Foxc1 caused an increase in 560bpMsx2 Bmp inducibility in O9-1 cells of approximately twofold (P<0.05, Fig. 5M). These data support the hypothesis that Foxc1 regulates the Bmp responsiveness of Msx2 through an effect on the 560 bp upstream enhancer.
Foxc1 is associated with the Bmp responsive element within the Msx2 560bp upstream enhancer
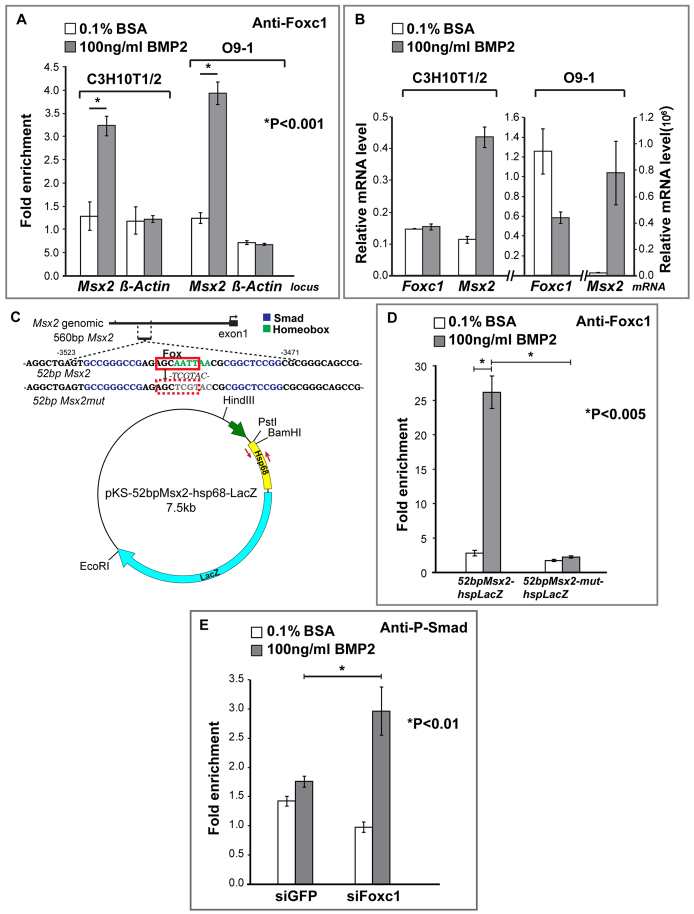
We next performed ChIP experiments to determine whether Foxc1 interacts with the endogenous Msx2 locus in the area of the 560 bp enhancer when induced by BMP2 (Fig. 6). Using primers that flank the genomic region containing the 560 bp enhancer, and control primers against the β-actin promoter, we performed ChIP with a Foxc1 antibody on chromatin extracts derived from control or BMP2-treated O9-1 CNC and 10T1/2 cells (Fig. 6A). Treatment of both O9-1 cranial neural crest and 10T1/2 cells with BMP2 resulted in a substantial (approximately two- to threefold) enrichment of Foxc1 at the endogenous Msx2 locus but not at the β-actin promoter (P<0.001). Quantitative RT-PCR showed that Foxc1 mRNA is not increased upon treatment of O9-1 or 10T1/2 cells with BMP2 (Fig. 6B). The increase in Foxc1 at the Msx2 enhancer is thus not controlled at the level of transcript accumulation.
Fig. 6.
Foxc1 interacts with a Bmp-responsive element in the 560 bp Msx2 enhancer and inhibits the recruitment of P-Smad1/5/8. (A) Chromatin immunoprecipitation experiments in C3H10T1/2 and O9-1 cranial neural crest cells. We performed ChIP on control and BMP2-treated cells with an antibody against Foxc1 or rabbit IgG as a control. qPCR was used to amplify the 560 bp Msx2 enhancer as well as a control β-actin promoter region. We show results of a single experiment (three PCR amplifications) representative of three independent experiments. Student’s t-test was used to evaluate the strength of the difference between starred groups. Error bars represent one s.d. Foxc1 is enriched on the Msx2 enhancer. (B) Effect of a 24-hour BMP2 treatment on Foxc1 and Msx2 mRNA level was evaluated by qPCR in both 10T1/2 and O9-1 cells. (C) The 52 bp Msx2 Bmp-responsive element (Bmpre) (Brugger et al., 2004). Smad1 binding sites (blue) flank an AT-rich sequence (green). A partial Fox consensus binding site, AGCAATT [matching the consensus (G/A)(T/C)(C/A)AA(T/C)A in 5/7 positions (underlined)], overlapping the AT-rich sequence is boxed in red. (D) 10T1/2 cells were transfected with plasmids containing wild type (52bpMsx2-hsplacZ) or a mutant (52bpMsx2-mut-hsplacZ) in which the Fox site was mutated, and treated with BSA or BMP2 for 30 minutes. They were analyzed by ChIP with an anti-Foxc1 antibody or rabbit IgG as a control. qPCR was used to amplify a 200 bp hsp68 fragment immediately downstream of the 52 bp Msx2 (red arrows). We show results from at least three independent transfections. The interaction of Foxc1 with the Bmpre was almost completely abrogated by the mutation in the Fox/AT-rich element. (E) O9-1 cranial neural crest cells were treated with an siRNA against Foxc1 or EGFP. Cells were then treated with BSA or BMP2 and subjected to ChIP with an anti-P-Smad1 antibody or rabbit IgG. qPCR was used to amplify a 350 bp fragment in the 560 bp Msx2 enhancer. Student’s t-test was used to evaluate the strength of the difference between asterisked groups. Error bars represent one s.d.
To test whether Foxc1 interacts with the 52 bp Bmpre, we transfected into 10T1/2 cells a plasmid carrying only the 52 bp Bmpre driving lacZ (52bpMsx2-hsplacZ). ChIP assays with primers hybridizing with the plasmid sequence adjacent to the Bmpre (red arrows, Fig. 6C) showed that Foxc1 was enriched substantially at the Bmpre and that its occupancy at this site increased upon BMP2 treatment (P<0.005, Fig. 6D).
The AT-rich domain of the Msx2 Bmpre has both an Antennapedia superclass recognition sequence (AATTAA) and an overlapping partial Fox site (AGCAATT, underlines) that matched the consensus (G/A)(T/C)(C/A)AA(T/C)A in 5/7 positions (Gao et al., 2003; Benayoun et al., 2011). To determine whether the interaction of Foxc1 with the Bmpre requires the AT-rich domain, we tested the effect of a mutation (52bpMsx2-mut-hsplacZ) in this domain on the association of Foxc1 with the 52 bp element. We transfected mutant and control constructs into 10T1/2 cells and asked whether we could detect Foxc1 by ChIP. As is evident in Fig. 6D, Foxc1 occupancy was reduced substantially relative to that of the control construct (P<0.005). Together, our findings support the view that Foxc1 acts directly on a Bmpre in the 560 bp enhancer upstream of Msx2 to attenuate its Bmp responsiveness in the mesenchyme of the supraorbital ridge.
Foxc1 functions to exclude P-Smad1/5/8 from the Msx2 Bmp responsive element
A simple mechanism that could explain the increased Bmp responsiveness of Msx2 in Foxc1 mutants is that Foxc1 attenuates the interaction of P-Smad1/5/8 with the Msx2 Bmpre. Thus, loss of Foxc1 should result in an increase in P-Smad1/5/8 levels within the 560 bp enhancer. We used ChIP to assess P-Smad1/5/8 levels within the 560 bp enhancer in O9-1 cranial neural crest cells in which Foxc1 was knocked down by siRNA. Consistent with our hypothesis, we found that in Foxc1 knockdown cells, P-Smad1/5/8 levels were substantially higher than in control cells (P<0.01, Fig. 6E).
DISCUSSION
Here, we demonstrate a role for Foxc1 in regulating the influence of Bmp signaling on the expression of Msx2 and the specification of osteogenic precursor cells in the developing skull vault. Using both mouse embryos and a cranial neural crest cell line, we show that Foxc1 acts directly on a Bmp-responsive enhancer to reduce the occupancy of P-Smad1/5/8 and thus restrict Msx2 expression to an osteogenic zone fated to become the developing frontal bone. We propose that within the supraorbital ridge, Foxc1 functions through Msx2 to set a threshold level of Bmp signaling activity, thus controlling the differentiation of osteogenic precursor cells and the development of the calvarial bones.
Rice and colleagues first suggested a relationship between Foxc1 and Msx2 (Rice et al., 2003). These authors showed that BMP2 beads implanted in the head mesenchyme of E15 Foxc1 mutants stimulated expression of Msx2 to a lesser extent than in wild-type mice, leading to the conclusion that Bmp-induced expression of Msx2 requires Foxc1. We were thus surprised at first by our finding that Foxc1 negatively regulates Msx2. We note, however, that Rice et al. (Rice et al., 2003) implanted Bmp beads in the dorsum of the head of E15 embryos, whereas we implanted them in the supraorbital ridge of E12.5 embryos. We repeated the bead implantation performed by Rice et al. (Rice et al., 2003) and obtained similar results (supplementary material Fig. S3). Thus, our finding of increased Msx2 expression and Bmp responsiveness in the supraorbital ridge of Foxc1 mutants is not in conflict with the findings of Rice et al. (Rice et al., 2003), but instead is likely a result of the different site or developmental stage of bead implantation. While this work was under review, Mirzayans and colleagues, working in several mouse and human cell lines, reported that FOXC1 positively regulates Msx2 (Mirzayans et al., 2012). They identified a 480 bp proximal promoter fragment that contains a FOXC1-binding site and is sufficient to respond to FOXC1. This fragment is distinct from the 560 bp Msx2 enhancer. These findings underline our conclusion that the nature of the interaction between Foxc1 and Msx2 is highly dependent on cellular context. The molecular details of how Foxc1 represses Msx2 gene activity in one context and activates it in another remain obscure, but are likely to reflect the ability of Foxc1 to recruit either co-activators or co-repressors to target promoters. It is thus interesting that in zebrafish podocytes, Foxc1a can both activate and repress the Podocalyxin promoter, depending on the dose of Foxc1 relative to an interacting transcription factor, Wt1 (O’Brien et al., 2011).
The supraorbital ridge domain is crucial for the development of the skull vault. DiI labeling and recent Cre-based lineage-tracing experiments (Yoshida et al., 2008; Ting et al., 2009; Roybal et al., 2010; Deckelbaum et al., 2012) show that at E11.5 undifferentiated precursor cells of the frontal and parietal bones, as well as the coronal suture, are located in the supraorbital ridge. These cells subsequently migrate apically and contribute to the growing bones and suture. These cells are defined functionally by their ability to contribute to the ALP-positive frontal and parietal bone rudiments and the ALP-negative coronal suture between them. Our data show that the distribution of neural crest and mesoderm within the supraorbital ridge is not grossly changed in Foxc1 mutants compared with controls. Given this, our finding that the frontal bone rudiment begins to develop in Foxc1 mutants but does not elongate suggests that the deficiency in Foxc1 mutants is not in the migration of the precursors into the supraorbital ridge, but in their development during the apical growth phase of calvarial development. The expansion of ALP across the ridge strongly suggests a mechanism that could slow or prevent elongation – the depletion of precursors by differentiation. That knockdown of Foxc1 in cultured neural crest cells accelerates the differentiation of such cells also supports this model. We note that Rice and colleagues documented a reduction in proliferation of cells within the rudiments of Foxc1 mutants (Rice et al., 2003). Such a reduction could also contribute to the slowed calvarial growth. We point out, finally, that as Foxc1 homozygous mutants do not survive into late embryogenesis and heterozygotes do not have a skull phenotype, we do not yet know the consequences of the premature differentiation of osteogenic precursor cells in supraorbital ridge on the later development of skull vault. Addressing this issue will have to await the conditional inactivation of Foxc1 in prospective calvarial tissues, an approach made possible by the recent generation of a conditional Foxc1 mutant allele (Sasman et al., 2012).
It is well established that Bmp signaling controls the differentiation of osteogenic precursor cells in the frontal and parietal bone rudiments (Kim et al., 1998). There is also evidence that reduced dose of Msx1/2 results in reduced numbers of osteogenic precursor cells in calvarial bone rudiments, and reduced calvarial bone growth (Ishii et al., 2003; Han et al., 2007). Given that Msx genes are known to be transcriptional effectors of Bmp signaling (Vainio et al., 1993; Graham et al., 1994; Bei and Maas, 1998), our finding that Foxc1 negatively regulates the Bmp inducibility of Msx2 in the mesenchyme of the supraorbital ridge and in cultured cranial neural crest cells suggests that Foxc1 controls a Bmp threshold response of Msx2. Reduced Foxc1 activity lowers the threshold (i.e. makes Msx2 more sensitive to Bmp induction); increased Foxc1 activity raises it. This threshold, we propose, regulates the balance between the maintenance of an undifferentiated precursor cell population on the one hand, and the differentiation of osteogenic precursors as they move into the high Bmp environment of the frontal bone rudiment, on the other.
Our data suggest that the expression domain of Foxc1 has only a narrow region of overlap with that of Msx2, yet the influence of Foxc1 on Msx2 expression spans a much wider area. The overlapping region includes parts of the dura and the mesenchyme immediately adjacent to the dura. We were surprised to find that in Foxc1 mutants, Msx2 is upregulated over the major part of the supraorbital ridge, not just in the Foxc1-Msx2 overlapping region. ALP, Bmp2 and Bmp4 expand similarly across this region. Thus, loss of Foxc1 results in a non-autonomous cascade of changes in gene expression across the supraorbital ridge. We propose that the expansion of Msx2, as well as that of ALP, Bmp2 and Bmp4, is the result of a positive-feedback loop between Bmp signaling and Msx2: in the absence of Foxc1, Msx2 is upregulated in the Foxc1 domain, resulting in upregulation of Bmp genes in this same domain. Bmp proteins are able to signal adjacent cells, resulting in upregulation of Msx2, and in turn a further expansion of Bmp gene expression. The net result is a wave of Msx2 and Bmp expression that spreads from medial to lateral across the supraorbital ridge. This, we suggest, results in the ectopic differentiation of supraorbital mesenchyme to ALP-positive osteogenic cells.
Direct functional evidence for a Foxc1-Msx2-Bmp loop comes from the genetic reduction of Msx2 activity in the Foxc1 mutant. This results not only in a rescue of Msx2 expression, but also of P-Smad1/5/8 activity. Thus, our results show that Msx2 is required downstream of Foxc1 to maintain cells of the supraorbital ridge in an undifferentiated state. It is intriguing that loss of Foxc1 also results in reduced Noggin expression and increased P-Smad1/5/8 activity in a localized area medial to the growing rudiment. We do not know that fate of these Noggin-expressing cells, but speculate that they may be osteogenic precursors. DiI labeling experiments show such precursor cells are present in the supraorbital ridge (Yoshida et al., 2008; Ting et al., 2009).
The Foxc1-Msx2-Bmp regulatory loop has some features of a bistable system (Ferrell, 2002). Such systems contain positive or double-negative feedback loops whose component genes typically respond to their upstream regulators in an ultrasensitive manner (Furtado et al., 2008). Bistable systems function to convert graded inputs into switch-like responses (Ferrell, 2002). In the case of the Foxc1/Msx2/Bmp axis, we propose that a positive loop between Bmp and Msx2 is modulated by negative regulation of Msx2 by Foxc1. The net result of this Foxc1-dependent regulation of the Msx2-Bmp loop is a sharp boundary between differentiating osteogenic cells of the rudiments and undifferentiated cells of the mesenchyme lateral to the rudiments (Fig. 7). An apparently graded Bmp input is thus converted into a sharp functional boundary. Loss of Foxc1 function results in a blurring of the boundary.
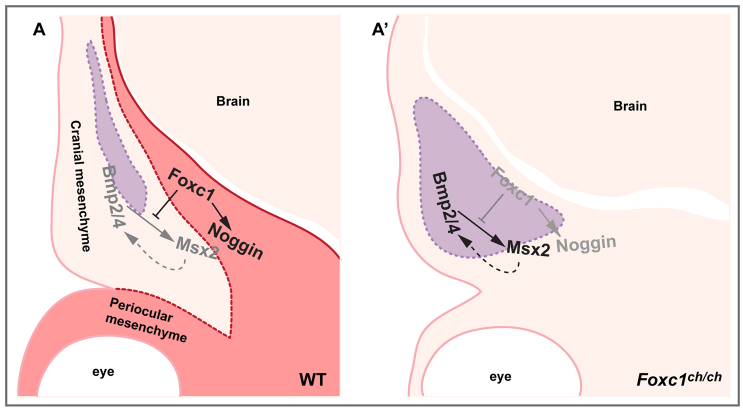
Fig. 7.
Schematic model showing role of Foxc1 regulating Bmp signaling and osteoprogenitor development in the supraorbital ridge. Foxc1 is expressed in the meninges and adjacent mesenchyme (red in A). Osteogenic cells are marked with purple shading. (A) In wild-type embryos, Foxc1 negatively regulates Msx2 and Bmp2/4, and positively regulates Noggin, thereby maintaining levels of Bmp signaling appropriate for the migration of progenitor cells into the frontal bone rudiment and their differentiation into osteoblasts. (A′) In Foxc1 mutants, loss of Foxc1 results in upregulation of Msx2, which initiates a positive-feedback loop between Msx2 and Bmp2/4. The result is the propagation of a wave of Msx2 and Bmp2/4 expression from medial to lateral across the supraorbital ridge, and the lateral displacement of the boundary between ALP-expressing cells of the frontal bone rudiment and adjacent non-ALP expressing mesenchyme.
Experiments in cultured CNC cells and 10T1/2 cells support the view that Foxc1 can act directly on Msx2. These experiments show: (1) that Foxc1 is associated with chromatin in the area of a 52 bp Bmp-responsive element within an upstream Msx2 enhancer; and (2) that the Foxc1 interaction is abrogated by a mutation that targets both a Foxc1 consensus site and an overlapping AATTAA site. Moreover, loss of Foxc1 results in increased Bmp-inducibility of endogenous Msx2. That we obtained similar results in 10T1/2 cells and in the O9-1 cranial neural crest cell line strengthens the argument for a direct, negative regulatory interaction between Foxc1 and Msx2.
Our data suggest a molecular mechanism by which reduced Foxc1 activity could influence Msx2 transcription. We have found that loss of Foxc1 function in cranial neural crest and 10T1/2 cells results in an increase in P-Smad1/5/8 in the region of the 560 bp enhancer. Given the crucial function of P-Smad1/5/8 in Bmp-dependent transcription (Massagué, 1998; Massagué, 2000), this increase could explain the enhanced Bmp inducibility of Msx2 when Foxc1 activity is reduced genetically or by siRNA. How this reciprocal relationship between Foxc1 and P-Smad1/5/8 enhancer occupancy might work on a molecular level is not yet clear. Foxc1 and Smad1 are not known to interact. However, FoxO proteins can interact with Smad4 to modulate Tgfβ signaling (Seoane et al., 2004; Gomis et al., 2006). Moreover, Foxc1 can associate with Smad4, which is also required for Bmp-dependent signaling and is found in a transcriptional complex with Smad1 (Lagna et al., 1996; Kretzschmar et al., 1997). Thus, loss of Foxc1 could act through Smad4 to affect indirectly the ability of Smad1 to participate in a complex on the enhancer.
Supplementary Material
Acknowledgments
J.S. thanks Dr Mamoru Ishii for the hypothesis that led to her fellowship from the California Institute of Regenerative Medicine. We also thank Dr Deborah Johnson for advice on siRNA and members of Dr Michael Stallcup’s laboratory for help with ChIP and qPCR analyses.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [R01DE016320 and R01DE019650 to R.M.]. J.S. was supported by a fellowship from the California Institute of Regenerative Medicine [T100004]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.085225/-/DC1
References
- Aldinger K. A., Lehmann O. J., Hudgins L., Chizhikov V. V., Bassuk A. G., Ades L. C., Krantz I. D., Dobyns W. B., Millen K. J. (2009). FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat. Genet. 41, 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach A., Lallemand Y., Nicola M. A., Ramos C., Mathis L., Maufras M., Robert B. (2003). Msx1 is required for dorsal diencephalon patterning. Development 130, 4025–4036 [DOI] [PubMed] [Google Scholar]
- Bei M., Maas R. (1998). FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125, 4325–4333 [DOI] [PubMed] [Google Scholar]
- Benayoun B. A., Caburet S., Veitia R. A. (2011). Forkhead transcription factors: key players in health and disease. Trends Genet. 27, 224–232 [DOI] [PubMed] [Google Scholar]
- Bernstein B. E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D. K., Huebert D. J., McMahon S., Karlsson E. K., Kulbokas E. J., 3rd, Gingeras T. R., et al. (2005). Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 [DOI] [PubMed] [Google Scholar]
- Brugger S. M., Merrill A. E., Torres-Vazquez J., Wu N., Ting M. C., Cho J. Y., Dobias S. L., Yi S. E., Lyons K., Bell J. R., et al. (2004). A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131, 5153–5165 [DOI] [PubMed] [Google Scholar]
- Chai Y., Maxson R. E., Jr (2006). Recent advances in craniofacial morphogenesis. Dev. Dyn. 235, 2353–2375 [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Ishii M., Sun J., Sucov H. M., Maxson R. E., Jr (2007). Msx1 and Msx2 regulate survival of secondary heart field precursors and post-migratory proliferation of cardiac neural crest in the outflow tract. Dev. Biol. 308, 421–437 [DOI] [PubMed] [Google Scholar]
- Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V. B., Wong E., Orlov Y. L., Zhang W., Jiang J., et al. (2008). Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. A., Holmes G., Zhao Z., Tong C., Basilico C., Loomis C. A. (2012). Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1. Development 139, 1346–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr (2002). Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 [DOI] [PubMed] [Google Scholar]
- Furtado M. B., Solloway M. J., Jones V. J., Costa M. W., Biben C., Wolstein O., Preis J. I., Sparrow D. B., Saga Y., Dunwoodie S. L., et al. (2008). BMP/SMAD1 signaling sets a threshold for the left/right pathway in lateral plate mesoderm and limits availability of SMAD4. Genes Dev. 22, 3037–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., Zhang J., Rao M. A., Case T. C., Mirosevich J., Wang Y., Jin R., Gupta A., Rennie P. S., Matusik R. J. (2003). The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol. Endocrinol. 17, 1484–1507 [DOI] [PubMed] [Google Scholar]
- Gomis R. R., Alarcón C., He W., Wang Q., Seoane J., Lash A., Massagué J. (2006). A FoxO-Smad synexpression group in human keratinocytes. Proc. Natl. Acad. Sci. USA 103, 12747–12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Francis-West P., Brickell P., Lumsden A. (1994). The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 372, 684–686 [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Bourillot P. Y. (2001). Morphogen gradient interpretation. Nature 413, 797–803 [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Mitchell A., Mahony D. (1995). Direct and continuous assessment by cells of their position in a morphogen gradient. Nature 376, 520–521 [DOI] [PubMed] [Google Scholar]
- Han J., Ishii M., Bringas P., Jr, Maas R. L., Maxson R. E., Jr, Chai Y. (2007). Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech. Dev. 124, 729–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannenhalli S., Kaestner K. H. (2009). The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet. 10, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M., Merrill A. E., Chan Y. S., Gitelman I., Rice D. P., Sucov H. M., Maxson R. E., Jr (2003). Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 130, 6131–6142 [DOI] [PubMed] [Google Scholar]
- Ishii M., Arias A. C., Liu L., Chen Y. B., Bronner M. E., Maxson R. E. (2012). A stable cranial neural crest cell line from mouse. Stem Cells Dev. 21, 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Iseki S., Maxson R. E., Sucov H. M., Morriss-Kay G. M. (2002). Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 241, 106–116 [DOI] [PubMed] [Google Scholar]
- Katagiri T., Yamaguchi A., Ikeda T., Yoshiki S., Wozney J. M., Rosen V., Wang E. A., Tanaka H., Omura S., Suda T. (1990). The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem. Biophys. Res. Commun. 172, 295–299 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Rice D. P., Kettunen P. J., Thesleff I. (1998). FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 125, 1241–1251 [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Liu F., Hata A., Doody J., Massagué J. (1997). The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 11, 984–995 [DOI] [PubMed] [Google Scholar]
- Kume T., Deng K. Y., Winfrey V., Gould D. B., Walter M. A., Hogan B. L. (1998). The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 93, 985–996 [DOI] [PubMed] [Google Scholar]
- Kume T., Deng K., Hogan B. L. (2000). Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127, 1387–1395 [DOI] [PubMed] [Google Scholar]
- Kume T., Jiang H., Topczewska J. M., Hogan B. L. (2001). The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 15, 2470–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwang S. J., Brugger S. M., Lazik A., Merrill A. E., Wu L. Y., Liu Y. H., Ishii M., Sangiorgi F. O., Rauchman M., Sucov H. M., et al. (2002). Msx2 is an immediate downstream effector of Pax3 in the development of the murine cardiac neural crest. Development 129, 527–538 [DOI] [PubMed] [Google Scholar]
- Lagna G., Hata A., Hemmati-Brivanlou A., Massagué J. (1996). Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature 383, 832–836 [DOI] [PubMed] [Google Scholar]
- Ma H., Shang Y., Lee D. Y., Stallcup M. R. (2003). Study of nuclear receptor-induced transcription complex assembly and histone modification by chromatin immunoprecipitation assays. Methods Enzymol. 364, 284–296 [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791 [DOI] [PubMed] [Google Scholar]
- Massagué J. (2000). How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 [DOI] [PubMed] [Google Scholar]
- Mirzayans F., Lavy R., Penner-Chea J., Berry F. B. (2012). Initiation of early osteoblast differentiation events through the direct transcriptional regulation of Msx2 by FOXC1. PLoS ONE 7, e49095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay G. M., Wilkie A. O. (2005). Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J. Anat. 207, 637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L. L., Grimaldi M., Kostun Z., Wingert R. A., Selleck R., Davidson A. J. (2011). Wt1a, Foxc1a, and the Notch mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev. Biol. 358, 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratore C., Suter U., Sommer L. (1999). Embryonic gene expression resolved at the cellular level by fluorescence in situ hybridization. Histochem. Cell Biol. 111, 435–443 [DOI] [PubMed] [Google Scholar]
- Phippard D. J., Weber-Hall S. J., Sharpe P. T., Naylor M. S., Jayatalake H., Maas R., Woo I., Roberts-Clark D., Francis-West P. H., Liu Y. H., et al. (1996). Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development 122, 2729–2737 [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Emerson C. P., Jr (1989). 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ. Health Perspect. 80, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R., Rice D. P., Olsen B. R., Thesleff I. (2003). Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Dev. Biol. 262, 75–87 [DOI] [PubMed] [Google Scholar]
- Roybal P. G., Wu N. L., Sun J., Ting M. C., Schafer C. A., Maxson R. E. (2010). Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev. Biol. 343, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002). Active genes are tri-methylated at K4 of histone H3. Nature 419, 407–411 [DOI] [PubMed] [Google Scholar]
- Sasman A., Nassano-Miller C., Shim K. S., Koo H. Y., Liu T., Schultz K. M., Millay M., Nanano A., Kang M., Suzuki T., et al. (2012). Generation of conditional alleles for Foxc1 and Foxc2 in mice. Genesis 50, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I., Maas R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, 348–356 [DOI] [PubMed] [Google Scholar]
- Satokata I., Ma L., Ohshima H., Bei M., Woo I., Nishizawa K., Maeda T., Takano Y., Uchiyama M., Heaney S., et al. (2000). Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 24, 391–395 [DOI] [PubMed] [Google Scholar]
- Seo S., Kume T. (2006). Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev. Biol. 296, 421–436 [DOI] [PubMed] [Google Scholar]
- Seoane J., Le H. V., Shen L., Anderson S. A., Massagué J. (2004). Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 [DOI] [PubMed] [Google Scholar]
- Ting M. C., Wu N. L., Roybal P. G., Sun J., Liu L., Yen Y., Maxson R. E., Jr (2009). EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development 136, 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer Z., Bach-Holm D. (2009). Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur. J. Hum. Genet. 17, 1527–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S., Karavanova I., Jowett A., Thesleff I. (1993). Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, 45–58 [PubMed] [Google Scholar]
- Visel A., Blow M. J., Li Z., Zhang T., Akiyama J. A., Holt A., Plajzer-Frick I., Shoukry M., Wright C., Chen F., et al. (2009). ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457, 854–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie A. O., Tang Z., Elanko N., Walsh S., Twigg S. R., Hurst J. A., Wall S. A., Chrzanowska K. H., Maxson R. E., Jr (2000). Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat. Genet. 24, 387–390 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Vivatbutsiri P., Morriss-Kay G., Saga Y., Iseki S. (2008). Cell lineage in mammalian craniofacial mesenchyme. Mech. Dev. 125, 797–808 [DOI] [PubMed] [Google Scholar]
- Zarbalis K., Siegenthaler J. A., Choe Y., May S. R., Peterson A. S., Pleasure S. J. (2007). Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc. Natl. Acad. Sci. USA 104, 14002–14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.