Abstract
The neurohypophysis is a crucial component of the hypothalamo-pituitary axis, serving as the site of release of hypothalamic neurohormones into a plexus of hypophyseal capillaries. The growth of hypothalamic axons and capillaries to the forming neurohypophysis in embryogenesis is therefore crucial to future adult homeostasis. Using ex vivo analyses in chick and in vivo analyses in mutant and transgenic zebrafish, we show that Fgf10 and Fgf3 secreted from the forming neurohypophysis exert direct guidance effects on hypothalamic neurosecretory axons. Simultaneously, they promote hypophyseal vascularisation, exerting early direct effects on endothelial cells that are subsequently complemented by indirect effects. Together, our studies suggest a model for the integrated neurohemal wiring of the hypothalamo-neurohypophyseal axis.
Keywords: FGF, Guidance, Hypothalamus, Zebrafish, Chick
INTRODUCTION
Neurosecretory hypothalamo-neurohypophyseal (H-NH) neurons project axons to the neurohypophysis (NH), a richly vascularised protrusion of the ventral forebrain floor (Hokfelt and Fuxe, 1972; Gorbman et al., 1983; Markakis, 2002). Neurohormones and neurotransmitters are released from axon terminals into capillary networks and are transported, either locally to the anterior pituitary/adenohypophysis (AH) or to distant body targets. The close proximity of H-NH axons and capillary vessels in the NH thus underlies the future functioning of the H-NH axis and homeostatic balance.
NH architecture, and the projection patterns of hypothalamic axons have been well-described in many species (Cajal, 1894; Bargmann, 1949; Harris, 1955; Gorbman et al., 1983; Markakis, 2002). In amniotes, the NH is composed of the anterior median eminence (ME), the neural stalk (pituitary stalk/stem) and the posterior neural lobe (pars nervosa/posterior pituitary). Two major classes of H-NH neurons, parvocellular and magnocellular, project axons through the medial forebrain bundle, then exit and extend ventrally into the NH. H-NH axons do not cross the ventral midline. Instead, parvocellular axons project to the ME where they secrete neurohormones/neurotransmitters into portal vessels that transport them to a pituitary plexus (Green and Harris, 1947). By contrast, magnocellular axons traverse the ME and neural stalk to terminate on capillaries of the neural lobe, where they release oxytocin and vasopressin. The architecture of the NH in anamniotes is broadly similar. A neural lobe-like region contains nerve endings of magnocellular neurons, releasing Arginine-Vasopressin-like (Avpl) or Oxytocin-like (Oxtl) homologues. Anamniotes do not possess a well-defined ME and portal capillary system, but some species have an anterior neurohemal area (Nishioka and Bern, 1966). Thus, in anamniotes, parvocellular axons either synapse directly with cells in the AH or contact capillaries within the anterior neurohemal area.
Although structure-function analyses of the NH have been the focus of intensive research for the last century (Bargmann, 1949; Harris, 1955; Gorbman et al., 1983), little is understood of the mechanisms that establish H-NH axonal tracts in embryogenesis. Chemoattractive cues that direct the growth of axons to the ventral midline throughout most of the forming CNS, notably Netrin and Shh (Charron et al., 2003; Sánchez-Camacho and Bovolenta, 2009) are not expressed at the hypothalamic ventral midline, and so are absent in the forming NH (infundibulum) (Pearson et al., 2011). Instead, previous studies have suggested a role for other cues, reporting that Fgf and Notch signalling can influence H-NH axonal projections (Gill and Tsai, 2006; Aujla et al., 2011). Once at the NH, however, H-NH axons play a crucial role in shaping NH vasculature. Studies in zebrafish show that the neurotransmitter Oxytocin-like (referred to as Oxytocin), released by neurohypophyseal nerve endings, can promote vessel growth (Gutnick et al., 2011). Nevertheless, it remains unclear whether other factors operate alongside Oxytocin, potentially contributing to the H-NH vascularisation profile.
The evolutionarily conserved fibroblast growth factors (FGFs) (Ornitz and Itoh, 2001; Beenken and Mohammadi, 2009) play multiple roles in embryogenesis, including diverse roles in hypothalamic development (Thisse and Thisse, 2005; Tsai et al., 2011). Fgf3 and Fgf10, the subject of this work, belong to the same subfamily, the FGF7 subfamily. Previous studies have demonstrated a role for members of other FGF subfamilies in fashioning axonal and vasculature networks: Fgf8 selectively attracts spinal motor axons to the dermomyotome (Shirasaki et al., 2006), and Fgf2 exerts pro-angiogenic effects (Cross and Claesson-Welsh, 2001; Beenken and Mohammadi, 2009). However, it is unknown whether Fgf3 and Fgf10 play a direct role in axonal or vessel navigation, or can coordinate the concomitant innervation and vascularisation of neurohemal organs.
Here, we dissect the role of FGFs secreted from the forming NH in neurohypophyseal innervation and vascularisation in chick and zebrafish embryos. Ex vivo studies in chick and in vivo analyses of zebrafish fgf3–/– mutant and transgenic fish allowing temporally controlled, or cell type-specific, blockade of FGF signal reception reveal that Fgf3 in the zebrafish, and Fgf3 and Fgf10 in the chick, are crucial in directing NH innervation and vascularisation. Our studies describe an essential role for FGF signalling in the directed growth of early H-NH axons to the NH. We demonstrate a dose-dependent effect of Fgf10 on H-NH axons, its attractive function switching to repellant activity at high concentrations, suggesting a mechanism for the stalling of axons as they reach the NH. In contrast to its direct effects on NH innervation, FGF signalling operates in a largely indirect manner to govern H-NH vascularisation. Thus, although FGF signalling is transiently required for NH-evoked endothelial outgrowth in vitro, loss of FGF signal reception in endothelial cells in vivo leads to only minor perturbations. Together, these studies reveal multi-step roles for FGF7 subfamily members in the integrated wiring of the NH.
MATERIALS AND METHODS
Chick strains and fish lines
Bovan-Brown chicks and the zebrafish potential fgf3 null allele t24152 (Herzog et al., 2004) were used. Transgenic zebrafish lines used were: Tg(otpb:1EGFP)zc49 (Gutnick et al., 2011), Tg(pomca:GFP)zf44 (Liu et al., 2003), Tg(prl:RFP)zf144 (Liu et al., 2006), Tg(kdrl:Hsa.HRAS-mCherry)s896 (Chi et al., 2008), Tg(hsp70l:dnfgfr1-EGFP)pd1 (Lee et al., 2005), Tg(fliep:gal4ff)ubs4 (Gutnick et al., 2011). See below for generation of the tg(avpl:KaITA4)fr31 line. Heatshock induction was performed by transferring embryos from 28°C to 39°C for 30 minutes.
Constructs for transient zebrafish transgenesis and generation of stable tg(avlp:KalTA4) transgenic line
UAS:dnfgfr1-mCherry, UAS:dnfgfr1-egfp, UAS:egfp and UAS:mCherry constructs used for transient transgenesis were taken from (UAS:egfp) or generated (UAS:dnfgfr1-mCherry, UAS:dnfgfr1-egfp, UAS:mCherry) using the gateway-based Tol2 kit (Kwan et al., 2007). Following primer pairs were used to generate (1) UAS:dnfgfr1-mCherry, UAS:dnfgfr1-egfp: 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCCACCATGATAATGAAGACCACGCTG-3′, 5′-GGGGACCAAGTTTGTACAAGAAAGCTGGGTACAGAGCTGTGCATTTTGGCCAG-3′; (2) UAS:mCherry: 5′-GGGGACAAGTTTTACAAAAAAGCAGGCTCCATGGTGAGCAAGGGCGAGGAGGTC-3′, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTATCAGGAGAGCACACACTTGCAGCTCATGCAGCCGGGGCCACTCTCATCAGGAGGGTTCAGCTTCTTGTACACTCGTCCATGCC-3′.
avpl:eGFP and avpl:KaITA4 constructs were generated by homologous BAC recombination using the avpl-containing BAC CH73-129K1 and the Red/ET kit from Gene Bridges following the manufacturers’ instructions. The eGFP-containing reporter cassette was amplified from an eGFP-kanamycin construct (Shin et al., 2003) using the primers 5′-GCGCTGCTATATAAGAGTGTGTGTGTGTATGTGTCCGTCAGTGTG TCGAGACCatggtgagcaagggcgaggag-3′ and 5′-TCTTGCCTCCTCGTGGGCAGTTCTGGATGTAGCAGGCAGACGAGAGCGTGggactagt ctattccagaagtagtgaggag-3′ (capital roman letters: BAC homology arms; lower case italic letters: egfp-kanamycin sequences).
The gal4 (KaITA4)-containing cassette was amplified from a KaITA4-kanamycin construct (Distel et al., 2009) using the primers 5′-GCGCTGCTATATAAGAGTGTGTGTGTGTATGTGTCCGTCAGTGTG TCGAGGCCGCCACCatgaaactgctctcatccatc-3′ and 5′-TCTTGCCTCCTCGTGGGCAGTTCTGGATGTAGCAGGCAGACGAGAGCGTGgg actagtctattccagaagtagtgaggag-3′ (capital roman letters: BAC homology arms; lower case italic letters: KaITA4-kanamycin sequences).
For generation of pPCR-KaITA4-Kan, the KaITA4 cassette was isolated from pCSKalTA4GI (Distel et al., 2009) by an EcoRI/HpaI digest. This fragment was in turn inserted into a pPCR-GFP-Kan construct (Shin et al., 2003) from which the EGFP cassette was removed by an EcoRI/SnaBI digest.
In addition, an iTol2-Amp cassette (Suster et al., 2009) was placed in the pTARBAC2.1 backbone of CH73-129K1. The iTol2-Amp cassette was amplified from pPCR8GW-itol2-Amp (Suster et al., 2009) plasmid, using the following primers: 5′-GTCGACGGCCAGGCGGCCGCCAGGCCTACCCACTAGTCAATTCGGGAGGACcctgctcgagccgggcccaagtg-3′, 5′-GTTCATGTCTCCTTCTGTATGTACTGTTTTTTGCGATCTGCCGTTTCGAattatgatcctctagatcagatct-3′ (capital roman letters: BAC homology arms; lower case italic letters: iTol2-Amp sequences). Injection of modified BACs and the isolation of stable transgenic lines was performed according to reported protocols (Bussmann and Schulte-Merker, 2011).
Explant and grafting cultures
Embryonic day (E) 4 or E7 hypothalamic neuroectoderm and E5 CAM vessels were dispase-isolated (1 mg/ml, Roche) and cultured in matrigel or collagen. In co-cultures, explants, or explants and beads, were positioned 100-200 μm apart and cultured for up to 48 hours. In grafts, hypothalamic neurons from Roslin GFP embryos were transplanted to lateral regions of wild-type hypothalami. FGFs (R&D Systems) were pre-soaked on beads (Affigel, Pharmacia Biotech). SU5402 (Calbiochem; 20 μM) was added at the start of culture. Fgf10 and Fgf3 blocking antibodies (Santa Cruz Biotechnology; 50 ng/μl) were added to medium or pre-incubated with tissue for 6-8 hours.
Immunofluorescence analyses
Immunohistochemical analysis of embryos (n=15-20) and explants was performed according to standard whole-mount or cryostat sectioning techniques (Pearson et al., 2011; Nowak and Hammerschmidt, 2006). In chick work, antibodies used were: anti-TUJ1 (anti neuron-specific class III β-tubulin; Calbiochem); anti-Neurofilament (3A10; DSHB); anti-Sox2 (Abcam); anti-TH rabbit polyclonal (Chemicon); anti-Tie1 (Santa Cruz Biotechnology); anti-Vasopressin (Bachem, Torrance, CA, USA); anti-VE cadherin (Abcam). In zebrafish work, antibodies used were: rabbit anti-Prl (Kawauchi et al., 1983); rabbit anti-(Arg8)-Vasopressin (Peninsula Laboratories) (Larson et al., 2006); anti-acetylated Tubulin (AcTub) (Sigma); rabbit anti-RFP (Abcam) and chicken anti-GFP (Molecular Probes). Secondary antibodies were conjugated to fluorescein isothyocyanate (FITC; Jackson ImmunoResearch), Alexa Fluor 555 or Alexa Fluor 488 (Molecular Probes). Nuclear counterstaining was performed with DAPI (Sigma). Fixation prior to anti-AcTub immunofluorescence was carried out in 2% trichloroacetic acid in PBS for 3 hours at room temperature.
In situ and double fluorescent hybridisations were carried out as described (Nica et al., 2004; Clay and Ramakrishnan, 2005; Pearson et al., 2011). For double analyses with AcTub, specimens were treated with collagenase (Sigma) instead of proteinase K. crabp1a probe was generated from EST clone IMAGp998C1511983 (RZPD) linearised with EcoRI and transcribed using SP6 polymerase. FITC-labelled riboprobes for prl, pomc and fgf3 were generated as described (Herzog et al., 2003; Herzog et al., 2004).
DiI tracing
The lipophilic carbocyanine dye DiI (Molecular Probes) was injected into the ventral-most region of E10 NH after dispase-isolation of whole hypothalamus; the explanted hypothalamus was cultured in matrigel until E12 and fixed in 4% paraformaldehyde.
Imaging
Chick tissue was imaged on a Zeiss Apotome or Olympus BX60 with Spot RT software v3.2 (Diagnostic Instruments). Zebrafish sections were imaged on a single-photon Zeiss LSM510 confocal. Live transgenic zebrafish larvae were imaged on a Zeiss LSM710 confocal using Non-Descanned-Detectors after excitation with a two-photon Chameleon laser.
Statistical analyses
Axon and endothelial process numbers were recorded after 48 hours, and data analysed using GraphPad Prism. Statistical significance of differences in means between groups was determined using a two-tailed Student’s t-test. Standard error bars are shown.
RESULTS
Innervation of chick neurohypohysis
In chick, the NH is first apparent at E4 as a button-like protrusion in the diencephalic ventral midline (Fig. 1A). By E7, it has grown, and projects ventrally above the adenohypophysis (Fig. 1E,F; supplementary material Fig. S1). Fate-mapping studies show that midline cells in the button-like protrusion give rise to the ventral NH (nh), whereas an overlapping/adjacent ‘collar region’ gives rise to the dorsal NH (Fig. 1A legend) (Pearson et al., 2011). A number of FGF genes are expressed in the developing NH. Fgf10 and Fgf19 are detected in the button-like cells at E4 (Fig. 1B,C) (Gimeno and Martinez, 2007). By E7, Fgf10 is detected throughout the NH (Fig. 1F,G) (Gimeno and Martinez, 2007; Pearson et al., 2011), with highest levels in the ventral NH (supplementary material Fig. S2A,B). Fgf3 and Fgf8 are detected in the collar region from E4 to E7 (Fig. 1D,H) (Pearson et al., 2011; Proszkowiec-Weglarz et al., 2011). Over this period, these FGF genes are not detected elsewhere in the chick diencephalon (Gimeno and Martinez, 2007; Pearson et al., 2011; Proszkowiec-Weglarz et al., 2011).
Fig. 1.
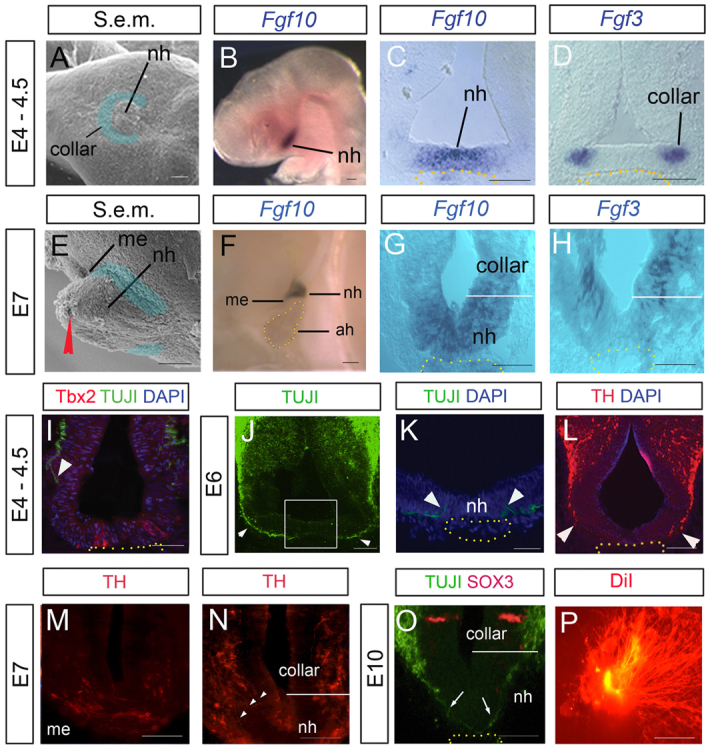
Chick hypothalamic axons project to the NH. (A,E) Scanning electron micrographs (S.e.m.), ventral (A) and side (E) views, showing prospective (A) and definitive (E) ventral NH (nh) and the median eminence (me) region of the dorsal NH. Dorsal NH derives from collar cells (blue shaded region). Anterior is to the left. (B-D,F-H) Fgf10 and Fgf3 expression in the forming NH, shown in lateral whole-mount views (B,F; anterior to left) or transverse sections. nh points to prospective/definitive ventral NH. Expression of Fgf3 (H) distinguishes collar/dorsal NH from ventral NH (nh). White bars indicate approximate boundary between collar/dorsal NH and ventral NH. Yellow dots outline adenohypophysis. (I-N) Transverse sections show that hypothalamic pioneers project to the dorsal NH, but do not initially project into the ventral NH. At E4.5, TUJ1+ pioneers (arrowhead in I) project towards the Tbx2+ midline (Manning et al., 2006) above the adenohypophysis (outlined by yellow dots). By E6, TUJ1+ pioneers project through the collar region (arrowheads in J) but turn (arrowheads in K), rather than project into/beneath the prospective ventral NH. (L) TH+ axons project to the collar region (arrowheads) at E6. Boxed area in J indicates area shown in K. (M,N) Serial adjacent transverse sections at E7 at the level of the me (M) or 50 μm more posterior (N). Arrowheads point to axons that have projected through the collar, but have not penetrated the ventral NH. (O) Transverse section of E10 NH. TUJ1+ axons (arrows) project beyond the Sox3+ collar region (Pearson et al., 2011) into/beneath the ventral NH. (P) Retrograde DiI labelling (position of DiI injection indicated by red arrowhead in E) reveals that many axons project into the NH by E12. ah, adenohypophysis/anterior pituitary; me, median eminence; nh, ventral NH. Scale bars: 100 μm in A-E,I-L; 50 μm in G,H,M-P; 150 μm in F.
TUJ1+ pioneering axons project towards the prospective NH (prosp-NH) at ∼E4.5 (Fig. 1I), project through the collar region by E6 (Fig. 1J), but turn before they reach the ventral NH, rather than projecting into/beneath it (Fig. 1K, arrowheads). Analysis of Tyrosine hydroxylase (TH), a marker of parvocellular dopaminergic neurons, and of Vasopressin (Vp), a marker of magnocellular neurons, confirms that H-NH neurosecretory cells differentiate some time after pioneers, then follow a similar trajectory (Fig. 1L; supplementary material Fig. S2C; data not shown). Thus, TH+ axons project to dorsal NH regions (the median eminence and collar) but do not initially project into/beneath the ventral-most NH (Fig. 1M,N). The first TUJ1+ pioneering axons project into/beneath the ventral NH only at E10 (Fig. 1O; supplementary material Fig. S2D,E). Retrograde DiI labelling reveals that in the subsequent 48 hours, many axons project deep into the NH (Fig. 1P)
Together, our results suggest that, in chick, the first H-NH axons respond to potential guidance cues from the prosp-NH at E4-E5, project to dorsal regions of the NH by E6, but extend into the ventral NH only at E10.
Chemoattractive effects of the prosp-NH
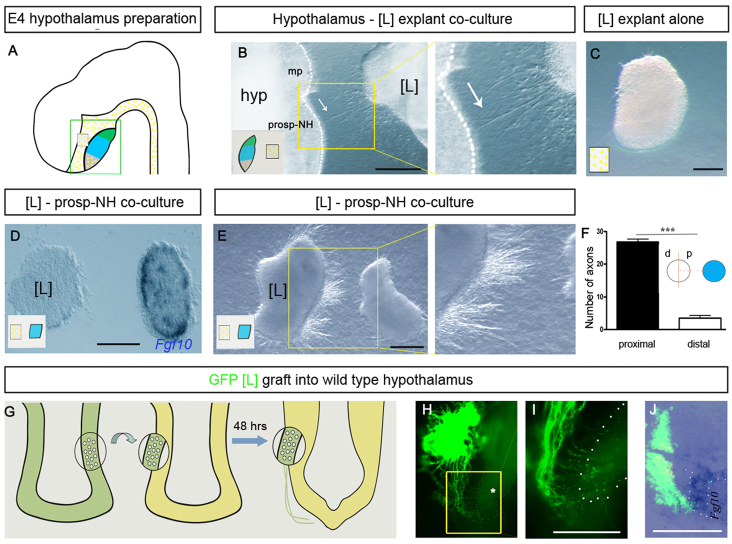
To investigate whether the ventral hypothalamus exerts a long-range guidance effect on H-NH axons, chick lateral [L] hypothalamic explants containing nascent H-NH neurons were cultured at a distance from E4 ventral hypothalamic explants in vitro (Fig. 2A,B). Long axons/fascicles emerge, almost exclusively from the proximal face of [L] explants (Fig. 2B, arrow; data not shown) and project to the ventral hypothalamus. Notably, axons project to the prosp-NH and not to retrochiasmatic or mammillary pouch regions (Fig. 2B). By contrast, few axons emerge from [L] explants cultured alone (Fig. 2C). These results indicate that the prosp-NH secretes a diffusible molecule(s) that exerts a long-range effect on H-NH axons.
Fig. 2.
Forming NH directs H-NH axons in chick. (A) Schematic depicting hypothalamus (green, mammillary pouch; blue, prosp-NH; grey, retrochiasmatic area; yellow dots, Shh+ region) and indicating regions of hypothalamic and [L] dissections (green and black boxes, respectively). Schematic insets in B-E show cultures. (B) Co-culture of ventral hypothalamus (hyp) and [L] explants. Axons project to prosp-NH (white arrow); right-hand panel shows high-power view of boxed region. Dotted line indicates ventral edge of hypothalamic explant. (C) No outgrowth is detected from [L] explant cultured alone. (D-F) Axons extend from [L] explant co-cultured with Fgf10+ prosp-NH explant; right-hand panel in (E) shows high-powered view of boxed region. Significantly more (***P<0.0001) axons extend from proximal (p) versus distal (d) face of [L] explants. Error bars represent s.e.m. (G-J) [L] explant from E4 Roslin GFP-chick, grafted homotypically to wild-type hypothalamus (shown schematically in G). After 2 days, axons extend from the graft towards the nascent NH (H,I; shown schematically in right-hand panel of G), re-orienting their growth as they approach NH (outlined by white dots). (I) High-powered view of boxed region in H. Axons extend towards the NH (dotted outline), but do not project deeply into it (asterisk in H). (J) Explant shown in H after fixation and in situ hybridisation with Fgf10. mp, mammillary pouch. Scale bars: 100 μm.
To test this idea, Fgf3+Fgf10+ E4 prosp-NH explants were cultured a short distance from [L] explants (Fig. 2D; data not shown). Robust axon outgrowth is elicited by the prosp-NH, with significantly more axons projecting from the proximal than the distal face of [L] explants (Fig. 2E,F; supplementary material Table S1).
The prosp-NH could, in principle, promote axon extension without influencing direction. To seek evidence that it can govern axonal trajectory, [L] explants from GFP-transgenic chicks (McGrew et al., 2004) were grafted to E4 wild-type hypothalami (n=7; Fig. 2G, schematic) and cultured ex vivo. The NH develops in the first 48 hours (Fig. 2G, schematic) and promotes extensive directed outgrowth from grafted [L] explants. GFP+ axons emerging from the graft show oriented growth, extending down the side of the host hypothalamus, towards the developing NH. As they reach the NH, axons further re-orient their trajectories, growing towards it (Fig. 2H,I). As in vivo, axons project to the Fgf10+ NH but do not invade deep into it (Fig. 2I,J; supplementary material Fig. S2C,D). This suggests that the developing NH exerts a long-range chemoattractive effect on hypothalamic axons, but exerts a local stalling/halting effect once axons reach it.
NH-derived FGFs direct H-NH axons
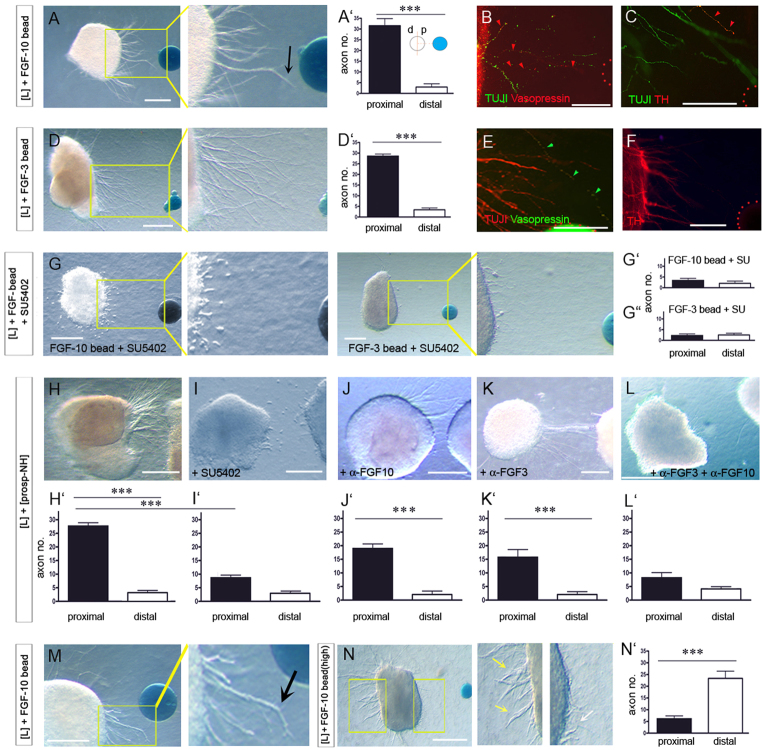
FGFs can direct motor axons (Shirasaki et al., 2006) and show restricted expression within the prosp-NH. Given this, we examined whether FGF-soaked beads mimic the chemoattractive effect of the NH. Fgf3- and Fgf10-, but not PBS- or Fgf8-soaked beads elicit axon outgrowth from chick E4-E5 [L] explants, with the vast majority of axons projecting from the face of the explant proximal to the FGF source (Fig. 3A,A′,D,D′; supplementary material Fig. S3A,B and Table S1). Immunolabelling shows that many axons grow within [L] explants, but only project out in the presence of FGFs (supplementary material Fig. S3C,D). Both parvocellular (TH+) and magnocellular (Vp+) axons respond to FGFs (Fig. 3B,C,E,F). An inherent bias in axon growth in [L] explants (supplementary material Fig. S3C; F.L. and M.P., unpublished) enables FGF-soaked beads to be positioned orthogonal to the normal axis of axon growth. In this configuration, axons re-orient to grow towards the bead (supplementary material Fig. S3B,D), suggesting an axon-guiding effect of FGFs. Fgf3 and Fgf10 do not alter the numbers of Nkx2.1+ progenitors or TH+ neurons (supplementary material Fig. S3E-L; data not shown), consistent with findings that the ventral hypothalamus does not exert neurogenic or trophic effects on hypothalamic neurons at these stages (Rogers et al., 1997; Gibson et al., 2000).
Fig. 3.
FGFs orient H-NH axons. (A,A′,D,D′) Chick [L] explants cultured with protein-soaked beads shown in whole-mount view (A,D). Significantly more (***P<0.0001) axons extend from the proximal (p) versus distal (d) face of [L] explants (A′,D′). (B,C,E,F) High-powered views of boxed regions in A,D, after immunolabelling. Axons, including those expressing Vasopressin and TH (arrowheads) project from [L] explants towards Fgf10- (B,C) or Fgf3- (E,F) soaked beads. (Beads outlined by red dots.) (G-G″) Treatment with SU5402 abolishes FGF-stimulated outgrowth. (H-L′) [L] explants cultured with prosp-NH explants alone (H,H′) or with inhibitors (I-L′). (M) Local chemorepulsion (arrow) in proximity to an Fgf10-soaked bead. (N,N′) [L] explants cultured with high concentrations of FGF. In high powered views (N) white arrow points to short axons that emerge from proximal face, yellow arrows point to axons emerging from distal face. Error bars represent s.e.m. ***P<0.005. d, distal; p, proximal. Scale bars: 100 μm in A,D,G,H-L,M,N; 50 μm in B,C,E,F.
To address whether the chemoattractive effect of prosp-NH explants involves FGF signalling, explants were exposed to SU5402, a small molecule inhibitor that suppresses tyrosine kinase activity of FGF and vascular endothelial growth factor (VEGF) receptors (Mohammadi et al., 1997). SU5402 does not provoke outgrowth of axons from [L] explants cultured alone (supplementary material Fig. S3M), and does not alter inherent axonal growth within [L] explants, or Nkx2.1+ and TH+ cell numbers (supplementary material Fig. S3H,L-P). However, SU5402 eliminates Fgf10- and Fgf3-evoked axon extension (Fig. 3G-G″; supplementary material Table S1). Furthermore, SU5402 significantly reduces extension evoked by the prosp-NH; the few axon fascicles that do emerge are significantly shorter than normal (Fig. 3H,I).
To address the specific requirements for Fgf3 and Fgf10, we analysed the effects of Fgf10- or Fgf3-blocking antibodies on [L]-prosp-NH co-cultures, and on [L] hypothalamic grafts. In vitro, blockade of either Fgf10 or Fgf3 results in a marked decrease in axon outgrowth evoked by the prosp-NH, and this effect is potentiated by blockade of both (Fig. 3J-L; supplementary material Table S1). Similarly, blockade of Fgf3 and Fgf10 eliminates the chemoattractive effect of the prosp-NH ex vivo (supplementary material Fig. S2H). Therefore, both Fgf3 and Fgf10 secreted by the forming NH might act as long-range chemoattractants for H-NH axons.
In the collagen culture system, we noted that as axons approach Fgf10-soaked beads, they frequently re-orient their growth, turning away from the beads (Fig. 3A,M, arrows). To test whether this is a direct effect of high concentrations of Fgf10, [L] explants were challenged with beads soaked in high concentrations of Fgf10 (supplementary material Table S1). Under these conditions, the chemoattractive effect of Fgf10 is eliminated or reversed (Fig. 3N,N′). Only a few short axons emerge from the proximal side of the [L] explant (Fig. 3N, white arrow; data not shown); instead, many axons now emerge from the distal side (Fig. 3N, yellow arrows; supplementary material Table S1). This effect was not observed with Fgf3 (supplementary material Table S1). Thus, Fgf10 might exert a graded action, stimulating the outgrowth of H-NH axons at lower concentrations, but stalling/repelling them at higher concentrations.
An early role for the prosp-H-NH in chick endothelial growth
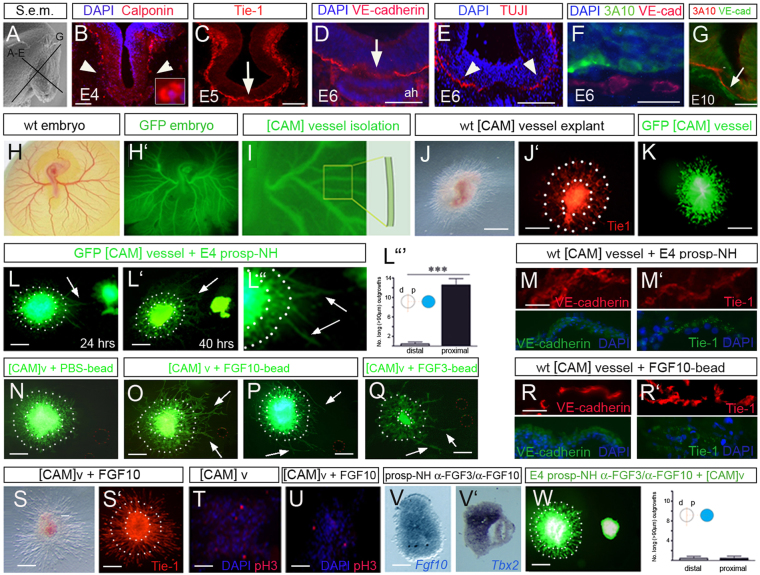
The functioning of the NH depends on the intimate association of axonal terminals with vascular capillaries. In chick, calponin+ endothelial cells are first detected close to the forming NH at E4 (Fig. 4A,B) and by E5, Tie1+ VE-cadherin+ cells are aggregating to vessel-like structures that lie between the NH and underlying adenohypophysis (Fig. 4C). Analysis of serial adjacent sections at E6 reveals that endothelial cells project into/under the NH before pioneering axons (Fig. 4D,E), but are closely associated with, and just ventral to, H-NH neurons/axons (Fig. 4F) and maintain this close association at E10, when H-NH pioneers project into the ventral NH (Fig. 4G).
Fig. 4.
Prosp-NH-derived FGFs stimulate outgrowth of endothelial processes. (A-G) Transverse (B-F) and sagittal (G) sections through chick NH at planes indicated in A. D,E show serial adjacent sections. Arrowheads and arrows point to endothelial processes/axons outside (arrowheads) or within/beneath (arrow) ventral NH. (H-I) [CAMv] isolation from wild-type or Roslin-GFP embryos. (J-K) Short radial projections emerge from [CAMv] explants cultured alone for 48 hours. Whole-mount immunolabelling shows that these are Tie1+ (J′). Dotted circles in J′ outline explant and ring of short processes. (L-L″) [CAMv]-E4 prosp-NH co-culture after 24 (L) and 40 hours (L′). Arrows indicate long endothelial processes. (L′″) Quantitative analysis of long processes from proximal versus distal faces of [CAMv] explants. Error bars represent s.e.m. ***P<0.001. d, distal; p, proximal. (M,M′) High-power views, after sectioning; extending processes are DAPI+VE-cadherin+Tie1+. (N-R′) GFP [CAMv] explants cultured with PBS- (N), Fgf10- (O,P) or Fgf3- (Q) soaked beads for 42 hours. FGF-beads promote significant numbers of long projections from proximal faces of [CAMv] explants. Analyses of wild-type co-cultures reveals that endothelial projections are DAPI+VE-cadherin+Tie1+ (R,R′). (S,S′) Wild-type [CAMv] explant cultured with Fgf10 for 40 hours. Whole-mount immunohistochemical analyses show many long (>90 μm) Tie1+ projections. Dotted circles show explant outline and reference for control process lengths (see J′). (T,U) Transverse sections through [CAMv] explants cultured with/without Fgf10. No significance difference is detected in DAPI+ or pH3+ cells. (V,V′) Transverse sections through prosp-NH explants pre-soaked in anti-Fgf3 and anti-Fgf10 antibodies. Fgf10 and Tbx2 are expressed normally (compare with Fig. 2D). (W) Prosp-NH explant pre-soaked in anti-Fgf3 and anti-Fgf10 fails to provoke long process outgrowth from GFP [CAMv] explants, which appear similar to controls (compare with K). Righthand panel shows quantitative analysis. Scale bars: 70 μm in A-E,G-L″,N-Q,S,S′,V,W; 45 μm in T,U; 25 μm in M,M′,R,R′; 20 μm in F.
Given the role of FGFs in angiogenesis and vasculogenesis (Cross and Claesson-Welsh, 2001; Beenken and Mohammadi, 2009), we speculated that NH-derived Fgf3 and Fgf10 might simultaneously stimulate NH innervation and vascularisation. To test this, we adapted the in vitro culture system, challenging enzymatically isolated chorioallantoic membrane endothelial capillary vessel [CAMv] explants with prosp-NH explants. When [CAMv] explants from E4-E5 wild-type or Roslin GFP-transgenic chicks are enzymatically isolated (Fig. 4H,I) and cultured alone (Fig. 4J,K), a ring of short Tie1+ (68.8±4.9 μm; supplementary material Table S2) processes extend radially from them (Fig. 4J-K). However, when confronted with E4 prosp-NH explants, long (>90 μm) processes emerge from the [CAMv] explant, specifically on the side proximal to the prosp-NH (Fig. 4L; supplementary material Table S2). Processes are composed of VE-cadherin+ and Tie1+ cellular assemblies, confirming their endothelial character (Fig. 4M,M′).
To examine whether the endothelial outgrowth might reflect an FGF-stimulated effect, we cultured [CAMv] explants close to beads soaked in PBS (Fig. 4N), Fgf10 (Fig. 4O,P) or Fgf3 (Fig. 4Q). Exposure of [CAMv] explants to a point-source of FGFs results in the appearance of long (>90 μm) projections, preferentially on the side of the [CAMv] explant facing the bead (Fig. 4O-Q; supplementary material Table S2). Section analyses reveal these are VE-cadherin+Tie1+ endothelial cellular assemblies (Fig. 4R,R′). When presented uniformly, FGFs similarly lead to a significant lengthening of Tie1+ process outgrowth (Fig. 4S) without altering the number of DAPI+ cells, or M-phase cells within [CAMv] explants (Fig. 4T,U; supplementary material Table S2). To investigate whether prosp-NH-evoked outgrowth requires FGFs, E4 prosp-NH explants were pre-soaked in blocking antibodies to Fgf3 and Fgf10, and then co-cultured with [CAMv] explants. Pre-treatment with anti-Fgf3 or anti-Fgf10 does not alter the character of the prosp-NH explants (they continue to express characteristic markers, including Fgf10 and Tbx2; Fig. 4V; data not shown) but eliminates their ability to promote endothelial outgrowth. Thus, although short radial outgrowths occur from [CAMv] explants, no long cellular projections are detected (Fig. 4W, compare with 4L; supplementary material Table S2).
To gain insights into the temporal requirements of FGFs for NH vascularisation, we analysed the endothelial outgrowth-promoting activity of E6 NH explants (in which innervation has begun). E6 explants show a diminished capacity to promote endothelial outgrowth, compared with E4 explants (supplementary material Fig. S4A, compare with Fig. 4L; supplementary material Table S2). Furthermore, in contrast to E4 explants, E6 NH-stimulated outgrowth cannot be completely abolished upon pre-treatment with Fgf3 or Fgf10 antibodies (supplementary material Fig. S5B, compare with Fig. 4W; supplementary material Table S2).
Together, these analyses indicate that FGFs deriving from the prosp-NH play a direct role in promoting endothelial growth to the chick NH, but suggest that this role might be transient and partly complemented by other factors during later stages of hypothalamo-hypophyseal development.
Simultaneous innervation and vascularisation of the zebrafish neurohypophysis
To test whether the described roles of FGFs are conserved among vertebrates and to investigate their in vivo relevance, we analysed FGF gene expression profiles and neurohypophyseal innervation and vascularisation in zebrafish. fgf10 is not expressed in the developing hypothalamo-hypophyseal system of the zebrafish, but functions elsewhere (Nechiporuk and Raible, 2008). However, from 18 hours post-fertilisation (hpf), fgf3 is expressed in the ventral hypothalamic floor, extending anteriorly to the prosp-NH (Toro et al., 2009), directly dorsal to AH precursors (Herzog et al., 2004). At 32 and 33 hpf, fgf3 expression persists in posterior-most cells of the prosp-NH, characterised by co-expression of crabp1a (Liu et al., 2005), and in a stripe of ventral hypothalamic cells just posterior to the prosp-NH (Fig. 5A,D). fgf3 expression becomes restricted to regions posterior to the prosp-NH at 36 hpf and later (Fig. 5B,C,E), indicating a progressive posterior shift of the fgf3 expression domain.
Fig. 5.
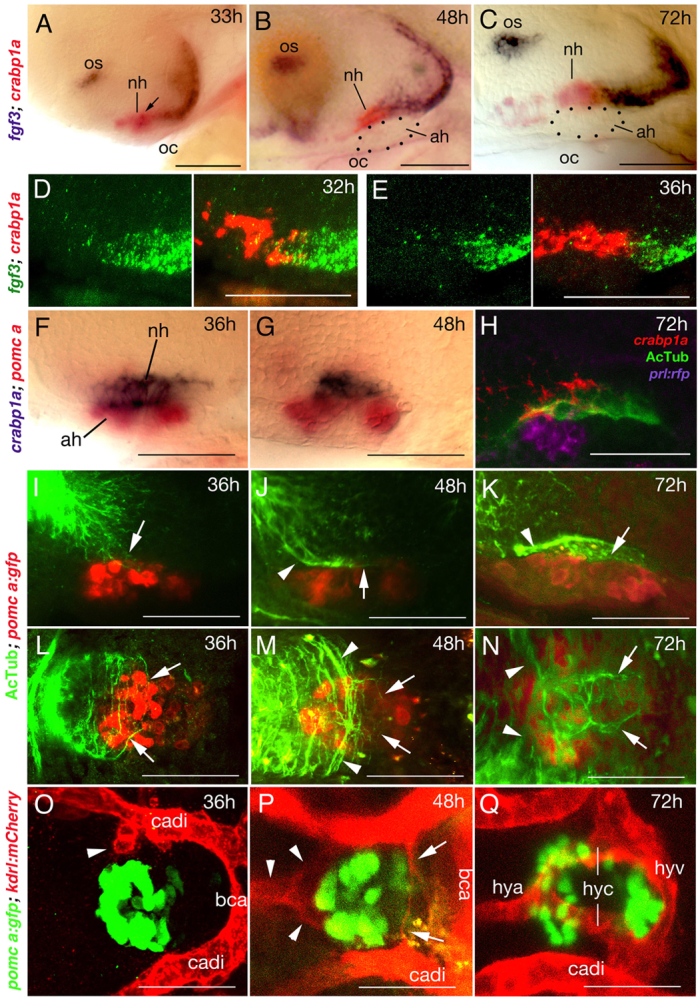
fgf3 expression and NH innervation and vascularisation in zebrafish. Lateral (A-K) or ventral (L-Q) views of wild-type zebrafish; anterior to the left. (A-C) fgf3 expression in posterior cells of the crabp1a+ NH (overlap in A indicated by arrow) and ventral-most cells of the posterior hypothalamus from 33 to 72 hpf. Dotted outline in B,C shows AH. (D,E) Double fluorescent in situ hybridisation reveals a partial overlap of fgf3 (green) and crapb1a (red) expression in posterior regions of the crabp1a domain at 32 hpf (D), whereas at 36 hpf, fgf3+ cells lie exclusively posterior of the crabp1a domain (E). (F,G) The crabp1a+ NH lies directly dorsal to the pomca+ AH. (H) Triple staining reveals innervation (AcTub) of NH (crabp1a) directly dorsal to AH (prl:RFP transgene product in lactotropes). Note that expression of AcTub and crabp1a signals are suboptimal owing to different fixation requirements. (I-N) Pioneering axons enter the anterior/dorsal NH anlage at 36 hpf (I,L). Axons are detected in the anterior half of the NH at 48 hpf (J,M), and throughout the NH at 72 hpf (K,N). Axons within the NH are indicated by arrows, axons outside the NH with arrowheads. For spatial reference, specimens are counterstained for the AH marker pomca. (O-Q) Endothelial cells (arrowhead) are detected at 36 hpf close to anterior regions of the pituitary (marked by pomca expression in AH) (O). At 48 hpf, endothelial cells assembling to vessels are detected anteriorly (arrowheads) and posteriorly (arrows) (P). At 72 hpf, the hypophyseal blood vessel system is completed (Q). ah, adenohypophysis; bca, basal communicating artery; cadi, caudal division of internal carotid artery; h, hours post fertilisation (hpf); hya, hypophyseal artery; hyc; hypophyseal capillary; hyv, hypophyseal vein; nh, neurohypophysis; oc, oral cavity; os, optic stalk. Scale bars: 50 μm.
Innervation of the NH starts at 36 hpf, when anti-acetylated Tubulin (AcTub) labels axons that have entered the anterior half of the NH anlage (Fig. 5I,L, compare with 5F for position of NH). By 48 hpf, axons have started to grow posteriorly within the NH (Fig. 5G,J,M), and are present along its entire length by 72 hpf (Fig. 5H,K,N).
NH vascularisation is initiated concomitant with its innervation. At 72 hpf, Tg(kdrl:Has.HRAS-mCherry); Tg(pomca:GFP) double transgenic fish display a circular blood vessel system at the interface of the AH and the NH. The hypophyseal artery (hya) runs along the midline from anterior to posterior regions of the hypothalamus and splits into two capillary branches (hyc) at the anterior aspect of the pituitary to bilaterally encompass the gland. Directly posterior to the pituitary, the two capillaries fuse with the hypophyseal vein (hyv), which in turn is connected to the primary head sinus (Fig. 5Q; supplementary material Fig. S5) (Isogai et al., 2001). Endothelial cells are already apparent close to anterior regions of the pituitary at 36 hpf (Fig. 5O, arrowhead) and have started to form the hypophyseal artery and bilateral capillaries by 48 hpf (Fig. 5P, arrowheads). In addition, thin endothelial structures, probably contributing to the hypophyseal vein, are apparent close to posterior regions of the pituitary at 48 hpf (Fig. 5P, arrows).
Zebrafish fgf3 mutants lack NH innervation and vascularisation
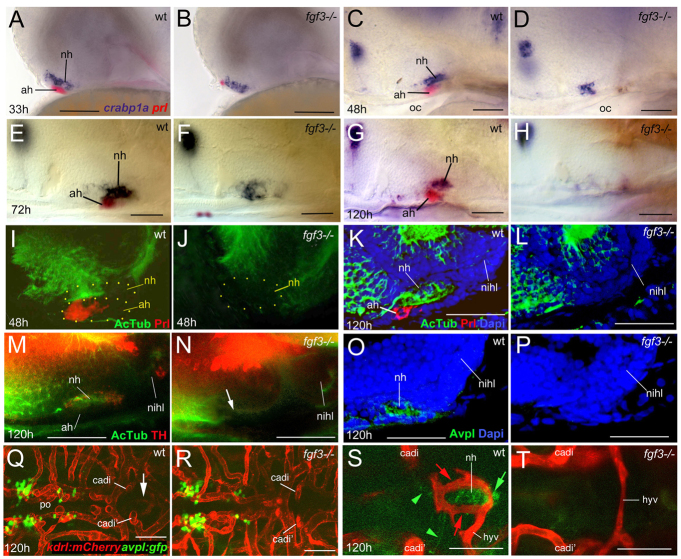
As a first step to investigate whether Fgf3 signalling is required for zebrafish NH innervation and vascularisation in vivo, we studied fgf3 loss-of-function mutants (Herzog et al., 2004). In these mutants, most adenohypophyseal cells undergo apoptosis between 28 and 32 hpf (Herzog et al., 2004), shortly before hypothalamic axons normally reach and enter the NH. In comparison, neurohypophyseal cells are affected much later, as judged by expression of crabp1a. At 33 hpf, fgf3 mutants display normal crabp1a expression in the NH anlage, and crabp1a expression is only moderately reduced at 48 hpf (Fig. 6C,D). Severe reduction/spatial disorganisation of crabp1a expression is observed later, at 72 hpf and 120 hpf (Fig. 6E-H). This suggests that, as in chick (Pearson et al., 2011), the zebrafish NH initially develops independently of Fgf3, but requires it for later steps of its development and/or maintenance (see Discussion).
Fig. 6.
NH innervation and vascularisation are compromised in zebrafish fgf3 mutants. Whole-mount in situ hybridisation (A-H), or immunohistochemical analyses (whole-mounts: I,J,M,N; sections: K,L,O,P), and confocal images (Q-T) of wild-type (wt) and fgf3–/– zebrafish; lateral (A-P) or ventral (Q-T) views; anterior to the left. (A-H) fgf3–/– display robust crabp1a expression in the NH anlage at 33 hpf (B) and 48 hpf (D); prl+ AH cells are largely (B) or completely (D) lost. At 72 hpf, crapb1a expression is moderately reduced (E,F); only a remnant of diffuse expression detected at 120 hpf (G,H). (I-L) Axons enter the NH and then extend throughout its antero-posterior length in wt larvae, but remain outside the fgf3 mutant NH. AH and NH outlined by yellow dots. (M-P) At 120 hpf, AcTub+ axons in the wt NH include both TH+ (M) and Avpl+ (O) axons. Neither is detected in fgf3 mutant NH (N,P). (Q-T) fgf3 mutants display normal numbers of Avpl+ neurons in the hypothalamus (Q,R), but lose Avpl+ axonal NH innervation (T), the hypophyseal artery and the two bilateral hypophyseal capillaries (T). The hypophyseal vein remains present (T). Q and R display maximal confocal projections through midbrain area. Arrow (Q) points to pituitary region, magnified and intensified in S. Panels S and T show single confocal sections at higher magnification in same specimens as in Q and R. Red arrows (S) point to hypophyseal capillaries; green arrowheads to GFP+ axons of avpl:egfp+ neurons projecting to NH; green arrow to GFP+ axonal termini within NH. ah, adenohypophysis; cadi, caudal division of internal carotid artery; hyv, hypophyseal vein; nihl, nucleus of the inferior hypothalamic lobe; nh, neurohypophysis; oc, oral cavity; po, preoptic area. Scale bars: 50 μm.
Despite the presence of crabp1a+ cells, hypothalamic axons fail to enter the NH of fgf3 mutants, but remain in the anterior/dorsal hypothalamus both at 48 hpf (Fig. 6I,J) and later (Fig. 6K,L). Both TH+ parvocellular and Arginine-vasopressin-like (Avpl) peptide+ magnocellular axons are affected. At 120 hpf, neither TH nor Avpl peptide is visible in the region containing the NH-remnant (Fig. 6N,P), whereas in wild-type siblings they can be readily detected (Fig. 6M,O). Consistent results were obtained with transient transgenic zebrafish that express eGPF under the control of cis-regulatory elements of the avpl gene (avpl:eGFP). Whereas GFP+ axons project into the NH in wild-type zebrafish (Fig. 6S), such axons are absent in fgf3 mutants (Fig. 6T), although mutants and wild types contain indistinguishable numbers of GFP-positive avpl neurons in the hypothalamus (Fig. 6Q,R). Together, these data indicate that both parvocellular and magnocellular neurosecretory axons require Fgf3 to project to the NH. In addition, fgf3 mutants lack proper vascularisation of the NH. The hypophyseal artery and bilateral capillaries do not form, although the hypophyseal vein remains present (Fig. 6S,T).
Together, analyses of these mutants suggest that Fgf3 is needed for both NH innervation and vascularisation. However, it remains unclear whether these effects are direct, and when and in which cell types Fgf3 signalling is required.
The role of zebrafish FGF signalling in NH innervation and vascularisation is independent of its role in AH development
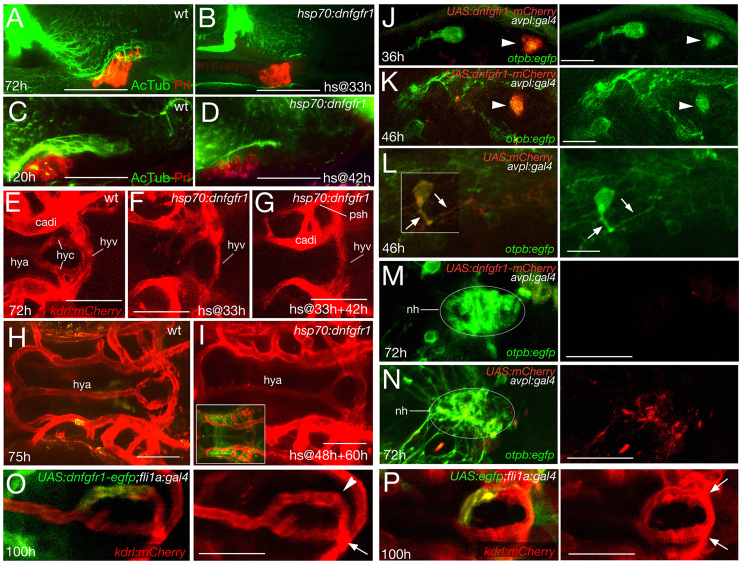
To dissect temporally the effect of Fgf3 on NH innervation and vascularisation from its earlier effect on AH development, we used the transgenic line Tg(hsp70l:dnfgfr1-EGFP), in which a dominant-negative, C-terminally truncated FGF receptor fused to GFP is expressed under the control of the heat-inducible hsp70 promoter, allowing temporal control of transgene activation (Lee et al., 2005). Heatshock application at 33 hpf, shortly after the requirement of Fgf3 during AH development, but before the onset of NH innervation (see Fig. 5I), yields embryos with normal Prolactin expression in the AH at 72 hpf, but with much-reduced NH innervation (Fig. 7A,B), comparable to untreated fish at 36 hpf (Fig. 5I). Similarly, application of the heatshock at 42 hpf, after pioneering hypothalamic axons have reached the NH, causes stalling of hypothalamic axons in intermediate positions of the NH at 120 hpf (Fig. 7C,D), resembling the situation in untreated fish at 48 hpf (Fig. 5J). This indicates that FGF signalling is required both for the early projection of hypothalamic axons towards the NH and for later extension of axons within the NH.
Fig. 7.
FGF signalling affects NH innervation and vascularisation independently of its effect on AH development and via direct effects on hypothalamic neurons and endothelial cells. Whole-mount immunolabelling (A-D) and confocal in vivo images (E-P) of wild-type (wt) or transgenic fish, as indicated. A-D: lateral views; E-I,M-P: ventral views, anterior to left; J-L: ventral views, anterior to top. (A-D) Double-labelled specimens for AcTub and Prolactin. (A,B) Maximal confocal projections at 72 hpf (A,B) or 120 hpf (C,D) in wt or after heatshock-activated dnFgfr1 expression at 33 hpf (B) or 42 hpf (D). Prolactin expression in the AH is normal, whereas NH axons appear to stall (compare B with Fig. 5I, and D with Fig. 5J). (E-I) Maximal projections of kdrl-driven mCherry fluorescence in endothelial cells at 72 hpf (E-G) or 75 hpf (H,I). Heatshock-activated dnFgfr1 expression at 33 hpf and 48 hpf leads to loss of hypophyseal artery and capillaries, but the hypophyseal vein remains present (compare panel G with E and with Fig. 6T). Single heatshock application at 33 hpf leads to an intermediate vessel phenotype (F), whereas heatshock applications at 48 hpf and 60 hpf leave hypophyseal vascularisation unaffected (compare panel I with H; inset in I shows strong and widespread expression of dnFgfr1-GFP). (J-N) Injection of UAS:dnfgfr1-mcherry plasmid (J,K,M) or UAS:mCherry plasmid (L,N) into tg(otpb:egfp);tg(avlp:gal4) double transgenic animals leads to mosaic expression of dominant-negative Fgfr1-mCherry fusion protein (J,K) or mCherry protein (L) in a subset of pre-optic hypothalamic cells (marked by otpb-driven EGFP expression, green). Avpl+ neurons expressing dnFgfr1-mCherry (arrowheads in J,K) fail to project axons and to innervate the NH (M), whereas neurons lacking dnFgfr1-mCherry display normal axogenesis (J,K). Axogenesis (L) and NH innervation (N) are also normal in Avpl+ neurons of control transgenics expressing mCherry (arrows in L). (O,P) Injection of UAS:dnfgfr1-egfp plasmid into tg(fli1:gal4);tg(kdrl:mcherry) double transgenic animals leads to mosaic expression of dominant-negative Fgfr1-EGFP fusion protein (green) in a subset of endothelial cells (marked by kdrl-driven mCherry expression, red). Contribution of cells expressing dnFgfr1 to one of the hypophyseal capillaries abrogates its fusion with the hypophyseal vein (O; arrowhead), whereas the fusion is not affected by cells carrying a UAS.egfp control plasmid (instead of UAS:dnfgfr1-egfp) (P; arrows). Arrow in O indicates normally wired non-transgenic endothelial cells. cadi, caudal division of internal carotid artery; hya, hypophyseal artery; hyc; hypophyseal capillary; hyv, hypophyseal vein; nh, neurohypophysis; phs, primary head sinus. Scale bars: 50 μm in A-I,M-P; 15 μm in J-L.
Furthermore, heatshock application at 33 hpf leads to a strong perturbation, and repetitive heatshocks at 33 hpf and 42 hpf to a complete loss of the hypophyseal artery and the hypophyseal capillaries at 72 hpf, with the hypophyseal vein remaining unaffected (Fig. 7E-G), reminiscent of the vascular defects of fgf3 mutants (Fig. 6T). By contrast, heatshock application at 48 hpf and 60 hpf leaves the vascular system unaffected (Fig. 7H,I), indicating that FGF signalling promoting NH vascularisation is required between 33 and 48 hpf.
Together, these results point to a concomitant role of FGF signalling in NH innervation and vascularisation that is independent of its earlier role in AH development.
Cell type-specific blockade of FGF signal reception affects both NH innervation and vascularisation
To determine whether FGF signalling exerts a direct effect, both on hypothalamic neurons projecting to the NH and on endothelial cells contributing to the hypophyseal vascular system, we employed Gal4-UAS transgenesis for specific expression of the dominant-negative FGF receptor in the respective cell types. For expression in hypothalamic Avpl neurons, UAS:dnfgfr1-mcherry responder DNA was co-injected into tg(avpl:KaITA4) (attenuated Gal4); tg(otpb:gfp) double-transgenic embryos; for expression in endothelial cells, UAS:dnfgfr1-gfp DNA was injected into tg(fli1a:gal4); tg(krdl:mcherry) double transgenics. In agreement with previous experiences, this approach yields mosaic transgene expression (Gutnick et al., 2011).
Hypothalamic (otpb-positive) neurons carrying the UAS:dnfgfr1-mCherry transgene fail to extend axons at 36 and 46 hpf (Fig. 7J,K) and to innervate the NH (Fig. 7M) (n=9/9). By contrast, axon formation and NH innervation is normal in otpb neurons carrying a UAS:mCherry control transgene (Fig. 7L,N; n=6/6), suggesting that they need to receive FGF signals directly to project towards the NH.
Mosaic expression of the UAS:dnfgfr1-gfp transgene in endothelial cells results in a reduced contribution of transgenic cells to the hypophyseal blood vessel system (in 12/30 embryos: 40%), compared with cells carrying a UAS-egfp control transgene (in 25/32 embryos: 78%), despite similar contribution rates to other cephalic vessels (supplementary material Fig. S6A,B). Furthermore, when UAS:dnfgfr1-gfp cells contribute to one of the two hypophyseal capillaries, this branch fails to fuse with the posteriorly located hypophyseal vein, but instead fuses with the opposing capillary (Fig. 7O; n=8/8). Capillaries lacking transgenic cells, or capillaries containing UAS-egfp control cells (n=13/13) fuse in the normal pattern (Fig. 7P). The hypophyseal vein itself, which forms normally in fgf3 mutants (see above; Fig. 6T) is unaffected by incorporated UAS:dnfgfr1-gfp cells (supplementary material Fig. S6C,D). Together, this suggests that direct reception of FGF signals by endothelial cells has an impact on their recruitment to the NH vasculature and on the wiring of the system. However, the direct effect on endothelial cell recruitment is not absolutely essential, but is complemented by indirect mechanisms and other (Fgf3-dependent) factors (see Discussion).
DISCUSSION
The functioning of neurohemal organs depends on the intimate association of axonal terminals with vascular capillaries. Many studies have pointed to the common usage of guidance cues in the directed growth of axons and blood vessels (Carmeliet and Tessier-Lavigne, 2005; Adams and Eichmann, 2010). Here, we investigated whether Fgf3 and Fgf10 might display such a dual role during the formation of the neurohypophyseal neurohemal system to ensure proper convergence of hypothalamic nerves and capillaries. Our studies reveal a direct effect of FGFs in H-NH axonal guidance. Fgf3 and Fgf10 exert striking orienting effects on hypothalamic axons in chick explant cultures, and analyses of zebrafish fgf3 mutants and transgenic fish after temporally controlled or cell type-specific blockade of FGF signalling reveal a direct and indispensible effect of FGFs on hypothalamic axons in vivo. By contrast, our studies suggest that, although FGFs can exert a direct effect on endothelial cells, this role is not absolutely essential to the formation of a H-NH vasculature. Thus, FGFs secreted from the chick NH can promote endothelial cellular outgrowth in vitro, but this effect is weak. Consistent with this, in zebrafish, endothelial cells expressing a dominant-negative FGF receptor display a reduced, but not completely abrogated, contribution to the hypophyseal blood vessels that are missing in fgf3 mutants. These results, together with the demonstration that Oxtl released by neurohypophyseal nerve endings is required for proper hypophyseal vascularisation (Gutnick et al., 2011), suggest a multi-step model in which FGFs have direct effects both on NH innervation and on NH vascularisation, but in which vascularisation involves the concerted action of FGFs and hormones from (FGF-dependent) neurohypophyseal axon terminals, thereby ensuring proper integration of the neuro-vascular wiring of this neurohemal organ.
NH-derived FGFs mediate a direct chemoattractive effect on developing H-NH axons
Molecular gradients play a pivotal role in directing axonal growth (Charron and Tessier-Lavigne, 2007; Sánchez-Camacho and Bovolenta, 2009; Chédotal and Richards, 2010). Although not as well-studied as other gradient-forming ligands, FGFs may operate as a gradient cue (Irving et al., 2002; Shirasaki et al., 2006) and can govern axonal extension (McFarlane et al., 1995; Szebenyi et al., 2001; Gill et al., 2004; Webber et al., 2005; Shirasaki et al., 2006). Early reports, moreover, suggest a role for FGF signalling in H-NH pathfinding, with compromised FGF signal transduction leading to defects in GnRH axonal extension (Gill and Tsai, 2004).
Our studies support and extend these observations, suggesting that an FGF ligand gradient forms in the hypothalamus that directs nascent H-NH axons towards their target area, the forming NH. In the chick, the FGF7 subfamily members Fgf3 and Fgf10 are expressed appropriately, confined to the forming NH when pioneering H-NH axons extend. In vitro, point-sources of Fgf3 or Fgf10 exert a chemotropic effect on chick H-NH axons, and can stimulate and re-orient their growth. In vitro, the NH exerts a chemoattractive effect on H-NH axons. Temporal blockade of NH-derived Fgf3 or Fgf10 results in a significant decrease in the directed growth of H-NH axons; this effect is potentiated by simultaneous blockade of Fgf3 and Fgf10. Abrogation of FGF signalling does not lead to a simultaneous decline in progenitor cells or differentiated neurons. Thus, although we cannot exclude some involvement of other factors (as small numbers of short fascicles continue to emerge in the presence of Fgf3- and Fgf10-blocking antibodies), our studies suggest that Fgf3 and Fgf10 from the forming NH play direct and indispensible roles in attracting H-NH axons towards the midline.
Our in vivo work in the zebrafish substantiates these results and suggests a conserved role for FGFs in guiding H-NH axons. Zebrafish fgf3 is expressed in the ventral hypothalamus, its profile similar to that of its close relative Fgf10 in the chick and mouse. Vp+ neurons differentiate in fgf3 zebrafish mutants, but Vp+ axons fail to project to the NH. Similar results were obtained for Oxytocin+ magnocellular neurons (Gutnick et al., 2011) (H.-M.P. and M.H., unpublished data) and for TH+ parvocellular cells. The innervation defects in fgf3 mutants are apparent at a time when the NH itself appears unaltered, making it unlikely that these are a secondary consequence of compromised NH development. Rather, the NH defects seen in later-stage fgf3 mutants might be secondary consequences of a late requirement for FGF signalling in NH maintenance (Pearson et al., 2011) and/or a consequence of the failed innervation and/or neurohormone release, consistent with data reporting effects of Vp release on pituicyte morphology (George et al., 1987; Rosso et al., 2004). Using heatshock-inducible temporally controlled transgenic expression of a truncated, dominant-negative version of Fgfr1, which cross-reacts with all FGF receptor subtypes (Lee et al., 2005), we further show that the innervation and vascularisation defects of the NH are independent of the earlier role of Fgf3 in AH specification (Herzog et al., 2004). Finally, lack of NH innervation specifically was also seen in mosaic transgenic embryos for hypothalamic neurons expressing the dominant-negative FGF receptor under the control of a Vp-specific promoter. In sum, these in vitro and in vivo data strongly suggest that the chemoattractive effect of Fgf3 and Fgf10 on magno- and parvocellular axons is direct.
A dose-dependent dual function for FGFs in NH innervation
Our studies in chick suggest a dual role for FGFs in regulating H-NH axonal projections, attracting and facilitating growth at lower concentrations, but stalling/repelling growth at high concentrations. Chick Fgf10 and zebrafish fgf3 are expressed in a graded fashion, with highest levels in the posterior-ventral NH, anticipating the anterior-to-posterior innervation of the NH. Strikingly, axons initially avoid regions that display the highest and most persistent fgf3 and Fgf10 expression levels, in line with a dual role. We speculate that this provides a mechanism to ensure that H-NH axons project to, but do not cross, the ventral midline; uniquely in this region of the CNS, axons are non-commissural (see Chédotal, 2011). Our studies raise the possibility that H-NH growth cones integrate FGF signalling over space or time (Dessaud et al., 2008), with low levels of FGF signalling attracting them towards the NH, and higher levels stalling their growth.
FGFs regulate NH vascularisation in partial functional redundancy with other factors
Early in development, both the chick and the zebrafish prosp-NH are surrounded by isolated endothelial cells that subsequently assemble to capillary-like structures. This suggests that rather than, or in addition to, angiogenesis (vessel formation via sprouting of pre-existing blood vessels), NH vascularisation is driven by vasculogenesis (the de novo formation of vessels via the assembly of endothelial cells, which subsequently connect to the existing vascular system).
Our studies reveal both a direct and an indirect contribution of FGFs to NH vascularisation. Chick [CAMv] explants respond to the NH with enhanced outgrowth of endothelial cell projections. This effect is abrogated by pre-exposure of the prosp-NH to Fgf3- and Fgf10-blocking antibodies; conversely, Fgf3 and Fgf10 mimic the ability of the prosp-NH to promote endothelial outgrowth. However, blockade of Fgf3 and Fgf10 only fully eliminates E4 prosp-NH-stimulated endothelial outgrowth. By contrast, blockade is incomplete for E6 NH explants, when the NH is already innervated, indicating a transient FGF requirement and the presence of other vascularisation-promoting factors in the innervated NH. In zebrafish, fgf3 mutants lack both hypothalamic axonal projections and the hypophyseal artery and capillaries. However, transgenic endothelial cells expressing the dominant-negative FGF receptor driven by the cell type-specific fli1a promoter do contribute to pituitary vessel formation, albeit with lower frequencies than do cells carrying a control transgene. This indicates that direct reception of FGF signalling by endothelial cells has an impact on their recruitment to the pituitary vessel system, but is not essential. FGF signal reception by endothelial cells contributing to the hypophyseal capillaries seems to be necessary for proper wiring of the hypophyseal capillaries to the hypohyseal vein, possibly pointing to a crucial role of FGF signalling during the remodelling of the initial capillary plexus to a properly connected and closed vessel system.
Together, our in vitro and in vivo data indicate that although direct FGF signalling does stimulate NH vascularisation, this function is partly redundant with other vascularisation-stimulating factors, possibly neuropeptides released from H-NH axonal termini, as recently revealed for Oxtl in the zebrafish (Gutnick et al., 2011) (see above).
Supplementary Material
Acknowledgments
We thank P. Ellis and I. Riedl-Quinkertz for technical help; Marc Tessier-Lavigne for helpful discussions; Markus Affolter, Wiebke Herzog, Shlomo Melmed, Joshua Bonkowsky and Kenneth Poss for transgenic zebrafish lines; Chi-Bin Chien for the Tol2kit plasmid vectors; R. Köster for the pCSKalTA4GI plasmid; and K. Kawakami for the iTol2-Amp construct.
Footnotes
Funding
This work was supported by the UK Medical Research Council [GO401310, G0501618 to M.P.]; the Wellcome Trust [077544 to M.P.]; and the German Research Foundation (DFG) [SFB 572; to M.H.]. Deposited in PMC for immediate release.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.080226/-/DC1
References
- Adams R. H., Eichmann A. (2010). Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2, a001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla P. K., Bora A., Monahan P., Sweedler J. V., Raetzman L. T. (2011). The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary. Dev. Biol. 353, 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann W. (1949). About the link between neurosecretory hypothalamus and pituitary. J. Mol. Med. 27, 617–622 [Google Scholar]
- Beenken A., Mohammadi M. (2009). The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J., Schulte-Merker S. (2011). Rapid BAC selection of tol2-mediated transgenesis in zebrafish. Development 138, 4327–4332 [DOI] [PubMed] [Google Scholar]
- Cajal S. R. (1894). Histologie du Systeme Neurveux de L’Homme et de Vertebres (ed. A. Maloine). Ann. Soc. Esp. Hist. Nat. 2a Series. [Google Scholar]
- Carmeliet P., Tessier-Lavigne M. (2005). Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 [DOI] [PubMed] [Google Scholar]
- Charron F., Tessier-Lavigne M. (2007). The Hedgehog, TGF-beta/BMP and Wnt families of morphogens in axon guidance. Adv. Exp. Med. Biol. 621, 116–133 [DOI] [PubMed] [Google Scholar]
- Charron F., Stein E., Jeong J., McMahon A. P., Tessier-Lavigne M. (2003). The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113, 11–23 [DOI] [PubMed] [Google Scholar]
- Chédotal A. (2011). Further tales of the midline. Curr. Opin. Neurobiol. 21, 68–75 [DOI] [PubMed] [Google Scholar]
- Chédotal A., Richards L. J. (2010). Wiring the brain: the biology of neuronal guidance. Cold Spring Harb. Perspect. Biol. 2, a001917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N. C., Shaw R. M., De Val S., Kang G., Jan L. Y., Black B. L., Stainier D. Y. (2008). Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H., Ramakrishnan L. (2005). Multiplex fluorescent in situ hybridization in zebrafish embryos using tyramide signal amplification. Zebrafish 2, 105–111 [DOI] [PubMed] [Google Scholar]
- Cross M. J., Claesson-Welsh L. (2001). FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22, 201–207 [DOI] [PubMed] [Google Scholar]
- Dessaud E., McMahon A. P., Briscoe J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–2503 [DOI] [PubMed] [Google Scholar]
- Distel M., Wullimann M.F., Köster R.W. (2009). Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. USA 106, 13365–13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. C., John T. M., Frombach S. K. (1987). Canada goose posterior lobe pituicytes: seasonal ultrastructural changes in relation to axonal release of neurosecretion. Cytobios 51, 93–101 [PubMed] [Google Scholar]
- Gibson M. J., Ingraham L., Dobrjansky A. (2000). Soluble factors guide gonadotropin-releasing hormone axonal targeting to the median eminence. Endocrinology 141, 3065–3071 [DOI] [PubMed] [Google Scholar]
- Gill J. C., Tsai P. S. (2006). Expression of a dominant negative FGF receptor in developing GNRH1 neurons disrupts axon outgrowth and targeting to the median eminence. Biol. Reprod. 74, 463–472 [DOI] [PubMed] [Google Scholar]
- Gill J. C., Moenter S. M., Tsai P. S. (2004). Developmental regulation of gonadotropin-releasing hormone neurons by fibroblast growth factor signaling. Endocrinology 145, 3830–3839 [DOI] [PubMed] [Google Scholar]
- Gimeno L., Martinez S. (2007). Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev. Dyn. 236, 2285–2297 [DOI] [PubMed] [Google Scholar]
- Gorbman A., Dickhoff W. W., Vigna S. R., Clark N. B., Ralph C. L. (1983). Comparative Endocrinology. New York, NY: Wiley; [Google Scholar]
- Green J. D., Harris G. W. (1946). The neurovascular link between the neurohypophysis and adenohypophysis. J. Endocrinol. 5, 136–146 [DOI] [PubMed] [Google Scholar]
- Gutnick A., Blechman J., Kaslin J., Herwig L., Belting H.-G., Affolter M., Bonkowsky J. L., Levkowitz G. (2011). The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev. Cell 21, 642–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. W. (1955). Neural Control of the Pituitary Gland, Vol. 3 London: Edward Arnold & Co. [Google Scholar]
- Herzog W., Zeng X., Lele Z., Sonntag C., Ting J. W., Chang C. Y., Hammerschmidt M. (2003). Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev. Biol. 254, 36–49 [DOI] [PubMed] [Google Scholar]
- Herzog W., Sonntag C., von der Hardt S., Roehl H. H., Varga Z. M., Hammerschmidt M. (2004). Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development 131, 3681–3692 [DOI] [PubMed] [Google Scholar]
- Hokfelt T., Fuxe K. (1972). Brain Endocrine Interaction. 1, Brain-Endocrine Interaction, Median Eminence, Structures and Function (ed. Knigge K. M., et al.), pp. 228–265 Basel: Karger; [Google Scholar]
- Irving C., Malhas A., Guthrie S., Mason I. (2002). Establishing the trochlear motor axon trajectory: role of the isthmic organiser and Fgf8. Development 129, 5389–5398 [DOI] [PubMed] [Google Scholar]
- Isogai S., Horiguchi M., Weinstein B. M. (2001). The vascular anatomy of the developing zebrafish: an atlas of embronic and early larval development. Dev. Biol. 230, 278–301 [DOI] [PubMed] [Google Scholar]
- Kawauchi H., Abe K., Takahashi A., Hirano T., Hasegawa S., Naito N., Nakai Y. (1983). Isolation and properties of chum salmon prolactin. Gen. Comp. Endocrinol. 49, 446–458 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Larson E. T., O’Malley D. M., Melloni R. H., Jr (2006). Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav. Brain Res. 167, 94–102 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D. (2005). Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183 [DOI] [PubMed] [Google Scholar]
- Liu N. A., Huang H., Yang Z., Herzog W., Hammerschmidt M., Lin S., Melmed S. (2003). Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol. Endocrinol. 17, 959–966 [DOI] [PubMed] [Google Scholar]
- Liu R. Z., Sharma M. K., Sun Q., Thisse C., Thisse B., Denovan-Wright E. M., Wright J. M. (2005). Retention of the duplicated cellular retinoic acid-binding protein 1 genes (crabp1a and crabp1b) in the zebrafish genome by subfunctionalization of tissue-specific expression. FEBS J. 272, 3561–3571 [DOI] [PubMed] [Google Scholar]
- Liu N. A., Liu Q., Wawrowsky K., Yang Z., Lin S., Melmed S. (2006). Prolactin receptor signaling mediates the osmotic response of embryonic zebrafish lactotrophs. Mol. Endocrinol. 20, 871–880 [DOI] [PubMed] [Google Scholar]
- Manning L., Ohyama K., Saeger B., Hatano O., Wilson S. A., Logan M., Placzek M. (2006). Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell 11, 873–885 [DOI] [PubMed] [Google Scholar]
- Markakis E. A. (2002). Development of the neuroendocrine hypothalamus. Front. Neuroendocrinol. 23, 257–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S., McNeill L., Holt C. E. (1995). FGF signaling and target recognition in the developing Xenopus visual system. Neuron 15, 1017–1028 [DOI] [PubMed] [Google Scholar]
- McGrew M. J., Sherman A., Ellard F. M., Lillico S. G., Gilhooley H. J., Kingsman A. J., Mitrophanous K. A., Sang H. (2004). Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 5, 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Raible D. W. (2008). FGF-dependent mechanosensory organ patterning in zebrafish. Science 320, 1774–1777 [DOI] [PubMed] [Google Scholar]
- Nica G., Herzog W., Sonntag C., Hammerschmidt M. (2004). Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol. Endocrinol. 18, 1196–1209 [DOI] [PubMed] [Google Scholar]
- Nishioka R. S., Bern H. A. (1966). Fine structure of the neurohemal areas associated with the hypophysis in the hagfish, Polistotrema stoutii. Gen. Comp. Endocrinol. 7, 457–462 [DOI] [PubMed] [Google Scholar]
- Nowak M., Hammerschmidt M. (2006). Ubc9 regulates mitosis and cell survival during zebrafish development. Mol. Biol. Cell 17, 5324–5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Itoh N. (2001). Fibroblast growth factors. Genome Biol. 2, reviews3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C., Ohyama K., Manning L., Aghamohammadzadeh S., Sang H., Placzek M. (2011). FGF10-dependent midline-derived progenitors in hypothalamo-neuroendocrine axis formation. Development 138, 2613–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Higgins S. E., Porter T. E. (2011). Changes in gene expression during pituitary morphogenesis and organogenesis in the chick embryo. Endocrinology 152, 989–1000 [DOI] [PubMed] [Google Scholar]
- Rogers M. C., Silverman A. J., Gibson M. J. (1997). Gonadotropin-releasing hormone axons target the median eminence: in vitro evidence for diffusible chemoattractive signals from the mediobasal hypothalamus. Endocrinology 138, 3956–3966 [DOI] [PubMed] [Google Scholar]
- Rosso L., Peteri-Brunbäck B., Mienville J. M. (2004). Putative physiological significance of vasopressin V1a receptor activation in rat pituicytes. J. Neuroendocrinol. 16, 313–318 [DOI] [PubMed] [Google Scholar]
- Sánchez-Camacho C., Bovolenta P. (2009). Emerging mechanisms in morphogen-mediated axon guidance. BioEssays 31, 1013–1025 [DOI] [PubMed] [Google Scholar]
- Shin J., Park H. C., Topczewska J. M., Mawdsley D. J., Appel B. (2003). Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 25, 7–14 [DOI] [PubMed] [Google Scholar]
- Shirasaki R., Lewcock J. W., Lettieri K., Pfaff S. L. (2006). FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron 50, 841–853 [DOI] [PubMed] [Google Scholar]
- Suster M. L., Sumiyama K., Kawakami K. (2009). Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genomics 10, 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G., Dent E. W., Callaway J. L., Seys C., Lueth H., Kalil K. (2001). Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J. Neurosci. 21, 3932–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Thisse C. (2005). Functions and regulations of fibroblast growth factor signalling during embryonic development. Dev. Biol. 287, 390–402 [DOI] [PubMed] [Google Scholar]
- Toro S., Wegner J., Muller M., Westerfield M., Varga Z. M. (2009). Identification of differentially expressed genes in the zebrafish hypothalamic-pituitary axis. Gene Expr. Patterns 9, 200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. S., Brooks L. R., Rochester J. R., Kavanaugh S. I., Chung W. C. (2011). Fibroblast growth factor signalling in the developing neuroendocrine hypothalamus. Front. Neuroendocrinol. 32, 95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber C. A., Chen Y. Y., Hehr C. L., Johnston J., McFarlane S. (2005). Multiple signaling pathways regulate FGF-2-induced retinal ganglion cell neurite extension and growth cone guidance. Mol. Cell. Neurosci. 30, 37–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.