Abstract
Objective:
To test the efficacy of a hyaluronan derivative (HYADD®4-G) in a model of osteoarthritis (anterior cruciate ligament [ACLT]) and to compare its efficacy with the injection of growth factors.
Design:
In a first experimental set-up, specially selected for treatment scheme with published studies on hyaluronan or growth factor efficacy in osteoarthritis, saline, HYADD®4-G, rh-BMP-7, and the treatments of rh-BMP-7 or rh-BMP-2 with HYADD®4-G were injected after ACLT, for five times starting 3 weeks after ACLT. Euthanasia was at day 70. The knees were evaluated by gross morphological observation, x-ray, and histology (Study A). In a second experimental set-up selected to evaluate the efficacy of three viscosupplement injections, starting 4 weeks after ACTL, HYADD®4-G was compared to saline (Study B).
Results:
(A) X-ray analysis showed more damage in the saline group than all other treatment groups (2.67 ± 0.61 for saline, 0.83 ± 0.26 for HYADD®4-G, 1.67 ± 0.82 for HYADD®4-G with rh-BMP-2, 0.75 ± 0.76 for HYADD®4-G with rh-BMP-7, and 1.58 ± 0.49 for rh-BMP-7), P < 0.05. In the femoral condyle, the Mankin’s score for HYADD®4-G with rh-BMP-2, HYADD®4-G with rh-BMP-7, and rh-BMP7 alone was statistically lower compared to saline in the medial part; in the lateral part a significant lower value was observed in the HYADD®4-G with the rh-BMP-2 group. (B) The Kellgren and Lawrence score and Mankin’s score was lower in the HYADD®4-G group than in the saline group (P < 0.002 and P = 0.0031).
Conclusions:
These two studies suggest that HYADD®4-G delayed the cartilage degeneration and that the association of HYADD®4-G with growth factors is synergistic.
Keywords: osteoarthritis, hyaluronan derivative, rabbit ACLT, cartilage degradation, growth factors
Introduction
Osteoarthritis is the most common joint disorder in the aging population. Although surgical treatment of osteoarthritis can reduce pain and improve joint mobility and function, the operative management of osteoarthritis is associated with significant cost and morbidity.
Hyaluronic acid (HA) is a high-molecular-weight glycosaminoglycan. The average molecular weight of HA in synovial fluid is 4 to 5 × 106 Da.1 HA has both viscous and elastic properties, and the degree to which each of them predominates depends on distinct loading conditions. In arthritic conditions, elastoviscosity of the synovial fluid is abnormally low,2 and thus elastoviscous solutions of high-molecular-weight HA have been developed to be injected intra-articularly.3-7 In 1974, the first clinical study showed that with this treatment the elastoviscosity of the synovial fluid returned to normal levels and the pain associated with the movement of the joint decreased.3 The beneficial effect of the therapeutic intra-articular injection of HA has been confirmed in the most recent published systematic review and meta-analysis.8,9
Currently, there are five injectable HA formulations approved for use in the United States. These preparations differ with respect to HA origin, method of production, treatment schedule, molecular weight, half-life within the joint, rheological properties, pharmacodynamics, and cost.10-13
HYADD®4-G (Fidia Farmaceutici, Abano Terme, Italy) is a novel amide derivative of 500 to 730 kDa HA. In this HA derivative, an aliphatic amine (hexadecylamine) is bound to HA at the carboxylic group of the glucuronic acid (2% substitution). HYADD®4-G is a hydrogel with rheological properties superior to Hyalgan® (Fidia Farmaceutici) and to human synovial fluid.14
HYADD®4-G has shown a beneficial effect on synovial pathological changes in osteoarthritis. The induced increase in high-molecular-weight HA (HMWHA) synthesis and a decreased synovial intimal hyperplasia with fewer injections than Hyalgan treatment suggests potential advantages of this preparation.15,16
BMP-7 has been shown to up-regulate the expression of CD44, the hyaluronan receptor, as well as the expression of hyaluronic acid synthase-2 (HAS2)17,18 and aggrecan19 by chondrocytes, resulting in enhanced extracellular matrix (ECM) deposition and retention.
The objectives of these preclinical studies were the following:
To examine the long-term effect of one course of intra-articular injections of HYADD®4-G on the development of osteoarthritis induced by transection of the anterior cruciate ligament (ACLT) using the application (five time injections) tested previously with Hyalgan in the same animal model20 and to see if the combination of growth factors and HYADD®4-G have a synergic effect with the beginning of treatment in an early phase of the disease.
To evaluate the efficacy of this new hyaluronan derivative with a treatment cycle of only three HYADD®4-G injections and the beginning of treatment in a more advanced stage (injection starting 4 weeks post-ACLT).
Methods
Animals
Sixty-four SPF adult female white New Zealand rabbits (Charles River Laboratory, L’Arbresle Cedex, France) were included in the study. The animals were older than 24 weeks.
Surgery and Treatment
The surgical protocol was approved by the Bernese Cantonal Authorities (BE 2006/38). The surgery was performed under general anesthesia (induction, intramuscular: medetomidine 200 µg/kg, climasolan 0.5 mg/kg, and fentanyl 10 µg/kg; maintenance isoflurane). The rabbit was placed in a left lateral position and the right operated leg was shaved and disinfected with Betadine solution. The knee was approached via a medial parapatellar incision (between the medial collateral ligament and patellar ligament). The patella was retracted medially by extension of the knee to expose the entire trochlea and the upper parts of the condyles, and the anterior cruciate ligament was transected. The patella was replaced gently in the trochlea groove and the incision was sutured in layers. Postoperatively the animals were permitted cage activity without immobilization. The animals were closely monitored for infections and other complications.
Experimental Design
Study A
Group 1 (5Sal + ACTL) underwent ACTL followed by a regimen of five injections of saline. Group 2 (5HYADD + ACLT) underwent ACLT, followed by a course of five injections of HYADD®4-G at concentration 8 mg/ml (500 µl per injection). Group 3 (5BMP7 + ACLT) underwent ACLT, followed by a course of five injections of 1 µg rh-BMP-7 (from R&D Systems, Abingdon, UK) diluted with 500 µl phosphate-buffered saline (PBS). Group 4 (5HYADD/BMP-7 + ACLT) underwent ACLT, followed by a course of five injections of a combination of 1 µg rh-BMP-7 and 500 µl HYADD®4-G. Group 5 (5HYADD/BMP-2 + ACLT) underwent ACLT, followed by a course of five injections of a combination of 3 µg rh-BMP-2 (Reliatech, Braunschweig, Germany) and 500 µl HYADD®4-G. This group was added to verify also the effect of HYADD®4-G associated with a different rh-BMP. Rabbits were injected with those treatments 3 weeks after ACLT five times at 10-day intervals. Six animals per group were included in Study A. This design was selected to compare the present study with similar preclinical studies using similar products.20
Study B
Group 1 (3Sal + ACTL) underwent ACLT followed by a regimen of three injections of saline (500 µl per injection). Group 2 (3HYADD + ACLT) underwent ACLT, followed by a course of three injections of HYADD®4-G at a concentration of 8 mg/ml (500 µl per injection). Rabbits were injected with those treatments 4 weeks after ACLT three times at 10-day intervals. Seventeen animals per group were included in Study B. This design was selected to see if a regimen of fewer injections could still be used in a more advanced disease stage.
All animals were euthanized 70 days after disease induction by injecting an excess dose of pentobarbital. At the time of sacrifice, the cartilage surfaces were photographed, and then the operated site was excised. The joint was subjectively evaluated for gross appearance of the articular cartilage (tibia and femur) and synovial membranes. The explants were processed by fixation.
Assessments
All gross morphological, histological, and radiological analyses were performed by treatment-blind personnel.
X-Rays
X-rays (Philips, Bucky Diagnost Trauma, Eindhoven, Netherlands) were taken after surgery and postmortem in two different planes. For Study B, x-ray evaluation was performed also before the beginning of the intra-articular treatment. Two plane radiographs with straight knees on the operated legs were taken after sacrifice. They were read by an expert radiologist blindly and classified according to the Kellgren and Lawrence scale21,22:
Grade 0, no osteophytes
Grade 1, doubtful osteophytes
Grade 2, minimal osteophytes, possibly with narrowing, cysts, and sclerosis
Grade 3, moderate or definite osteophytes with moderate joint space narrowing
Grade 4, severe with large osteophytes and definite joint space narrowing
Macroscopic Analysis
Macroscopic cartilage assessment was performed on the knee joint. The compartments medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibial plateau (MTP), and lateral tibial plateau (LTP) were graded following India ink application. The knee specimens were painted with an Indian ink solution (Higgins, Waterproof India Ink, Standford Corp, Bellwood, IL) and then the excess ink was washed away with PBS. The condyles were photographed and two independent graders assessed each condyle.
The samples were graded according to the Outerbridge classification23:
Grade 1 = No uptake of India ink, which indicates an intact surface
Grade 2 = Minimal focal uptake of India ink, which indicates a minimal fibrillation
Grade 3 = Evident large focal dark patches of ink uptake, which indicates overt fibrillation
Grade 4 = Large general uptake of India ink, which indicates erosion of cartilage
Histological Processing and Histopathological Observations
Samples were decalcified in 10% ethylenediaminetetraacetic acid in PBS solution and dehydrated in ethanol/xylene prior to paraffin embedding. Two cuts were performed per compartments (MFC, LFC, MTP, and LTP) and stained with Safranin O-fast Green and hematoxylin and eosin (HE). Synovial membranes were embedded in paraffin, cut, and stained with HE. The cartilage surfaces were morphologically described while observing the cartilage and the bone compartment. Two independent pathologists evaluated the histological sections from each of the four compartments using a modified Mankin’s score (Table 1).24 All the samples were scored blindly. An average of the individual observer scores was calculated for each knee. In addition, the synovial membranes were graded based on the following criteria (Table 2)25: intimal hyperplasia, lymphocytic/plasmocytic infiltration, subintimal fibrosis, and vascularity.
Table 1.
Modified Mankin’s Histological Score for Cartilage Evaluation
| Cartilage structure | |
| Normal | 0 |
| Surface irregularities | 1 |
| Pannus and surface irregularities | 2 |
| Clefts to transitional zone | 3 |
| Clefts to radial zone | 4 |
| Clefts to calcified zone | 5 |
| Complete disorganization | 6 |
| Cartilage cells | |
| Normal | 0 |
| Pyknosis, lipid degeneration hypercellularity | 1 |
| Clusters | 2 |
| Hypocellularity | 3 |
| Safranin-O | |
| Normal | 0 |
| Slight reduction | 1 |
| Moderate reduction | 2 |
| Severe reduction | 3 |
| No staining | 4 |
| Tidemark integrity | |
| Intact | 0 |
| Destroyed | 1 |
Table 2.
Synovial Membrane Scoring System
| Intimal hyperplasia | |
| 1 to 2 layers only | 0 |
| 3 to 4 layers, focal | 1 |
| 5 or more layers, focal | 2 |
| 5 or more layers, diffuse | 3 |
| Lymphocytic/plasmocytic infiltration | |
| None | 0 |
| 1 focus of infiltration | 1 |
| 2 to 5 foci of infiltration | 2 |
| Diffuse infiltration or >5 loci | 3 |
| Subintimal fibrosis | |
| None | 0 |
| Light focal collagenous staining | 1 |
| Heavy focal collagenous staining | 2 |
| Heavy diffuse collagenous staining | 3 |
| Vascularity | |
| 0 to 2 vascular elements | 0 |
| 3 to 4 vascular elements | 1 |
| 5 to 8 vascular elements | 2 |
| More than 8 vascular elements | 3 |
Immunohistochemistry Analysis
Immunohistochemistry (IHC) for collagen type I and collagen type II was performed as previously described.26 The sections were incubated overnight with a monoclonal primary antibody against collagen type I (Quartett, Berlin, Germany), collagen type II (II-II6B3, Hybridoma Bank, University of Iowa, Iowa City, IA). Bound antibodies were detected according to the manufacturer’s protocol, using biotinylated secondary antibody and streptavidin alkaline phosphatase conjugated. Visualization was performed with the incubation in DAKO Real detection system.
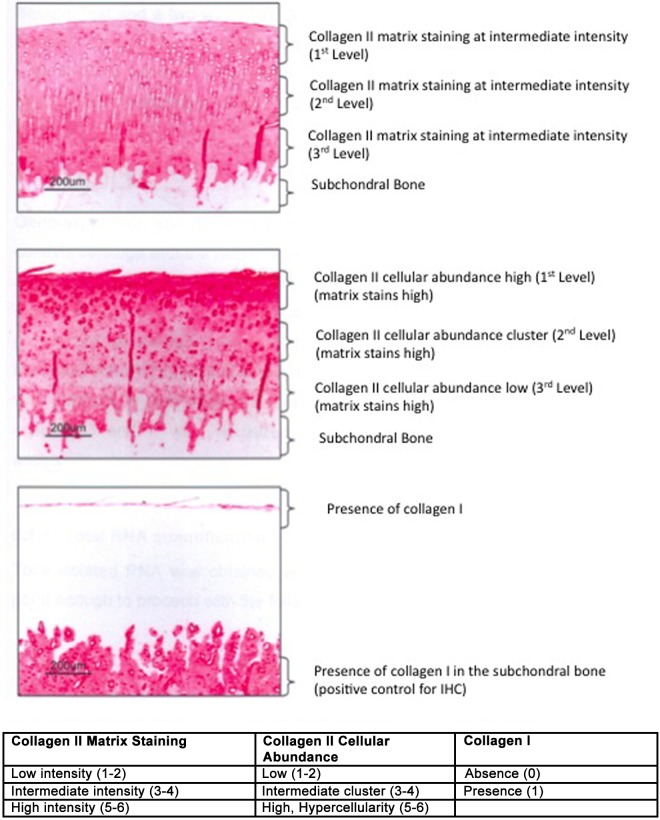
The matrix intensity of the IHC samples was analyzed and graded according to a score that was developed for the purpose of the study. The principles are described in Figure 1. The slides were blindly scored according to their staining intensity by two pathologists. Score ranged from 1 to 6. IHC for anticollagen type II was scored for matrix intensity and cellular abundance. Collagen I was graded for presence or absence within the articular cartilage. This analysis was only conducted in Study A. Reference samples were also stained (healthy rabbit, nonoperated, same strain sex, and age).
Figure 1.
Description of the grading system used to evaluate collagen immunohistochemistry (IHC) cartilage samples. The collagen type II staining is quantified at the matrix level or at the cellular/pericellular level. The matrix is graded based on the staining intensity: grade 1 to 2 for low staining, grade 3 to 4 for reduced staining, and grade 5 to 6 for marked staining. The intensity of the pericellular cell staining for collagen II is graded according to the following score: from 1 to 2 if the stained cells are rare, 3 to 4 if clusters of stained cells are evident, 5 to 6 if the stained cells are abundant. Each zone superficial (I), intermediate (II), or deep (III) are graded individually. No tissue is left because of the degeneration process. The score 0 is not assigned.
Statistical Analysis
All data are reported as the mean ± standard error of the mean. The data from x-ray and microscopic analysis were analyzed using nonparametric test, the Kruskal–Wallis test in Study A, and the Mann–Whitney test in Study B. The Bonferroni test was used when a significance difference was found. The immunohistochemistry data were analyzed using the two-way ANOVA comparison and chi-square test. P values were corrected for multiplicity using the Bonferroni test.
Results
Macroscopic Analysis
Study A
The macroscopic observation of the explanted joints revealed that a moderate to severe synovitis was present in all animals belonging to the saline group. In the rh-BMP-7 group, a moderate synovitis was present in all animals. In all the other groups, the synovitis was not present or was evident in moderate amounts in only one or two animals per group. Osteophytes were present in all groups; however, in the saline group osteophytes were observed in larger amounts. The India ink grading of the four knee compartments (LFC, MFC, MTP, and LTP) was 9.67 ± 1.37 for the saline group, 8.5 ± 0.83 for HYADD®4-G, 8.17 ± 2.13 for HYADD®4-G in combination with rh-BMP-2, 8.66 ± 1.21 for HYADD®4-G in combination with rh-BMP-7, and 8.5 ± 1.76 for rh-BMP-7. These differences were not statistically significant (multiplicity corrected P value < 0.05) (Fig. 2).
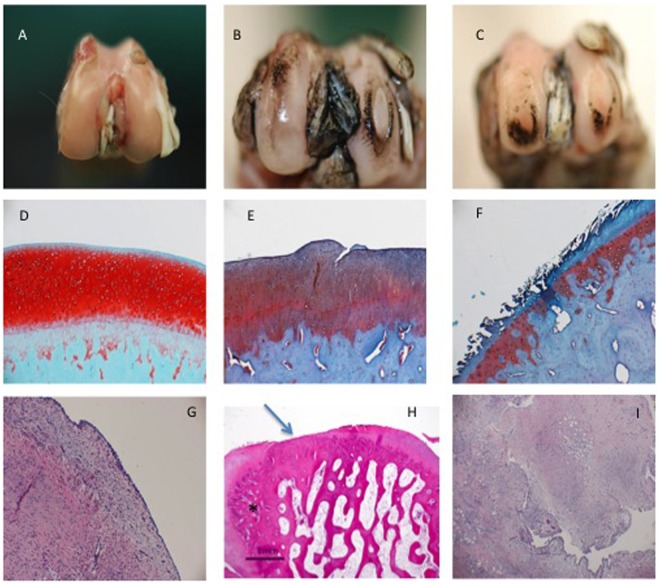
Figure 2.
(A) Macroscopic view of a maintained cartilage surface, no fibrillation of the surface after India ink. Few osteophytes are visible in the trochlea. Animal Allocation: HYADD4-G + BMP-7. (B) Macroscopic view of a deep fibrillation on the medial condyle (Grade IV) and superficial fibrillation on the lateral condyle. Animal allocation HYADD4-G. (C) Macroscopic view of bilateral erosion till the subchondral bone (Grade IV). Animal allocation: Saline. (D) Safranin O staining. Magnification 40×. Maintained Safranin O staining. No fibrillation of the surface. Light hypercellularity. No tide mark duplication. Animal allocation: HYADD4-G + BMP-7. (E) Safranin O staining, magnification 40×. Moderate lost of Safranin O, clefts to the transitional zone, duplication moderate of the tide mark. Animal allocation: HYADD4-G. (F) Safranin O staining, magnification 40×. Severe lost of Safranin O, clefts to the calcified zone and loss of most of the cartilage surface. Animal allocation: Saline. (G) Hematoxylin–eosin (HE) staining, magnification 10×. Absence of intimal hyperplasia, light subtimal hyperplasia. Animal allocation: HYADD4-G. (H) HE staining, magnification 2×. Osteophyte (*) complete ablation of the cartilage layer. Animal allocation: Saline. (I) HE staining, magnification 10×. Severe intimal hyperplasia, heavy focal subtimal hyperplasia, diffuse lymphocytic infiltration, and presence of vascular elements. Animal allocation: Saline.
Study B
The macroscopic observation of the explanted joints revealed that a moderate synovitis was present in all rabbits. Osteophytes were present in all groups. They were more pronounced in the saline group. The India ink grading of the four knee compartments analyzed (LFC, MFC, MTP, and LTP) was 15.3 ± 4.2 for the saline group and 15.5 ± 3.3 for the HYADD®4-G treated group. These differences were not statistically significant; however, a more advanced degeneration was evident when compared to Study A.
Radiological Analysis
Study A
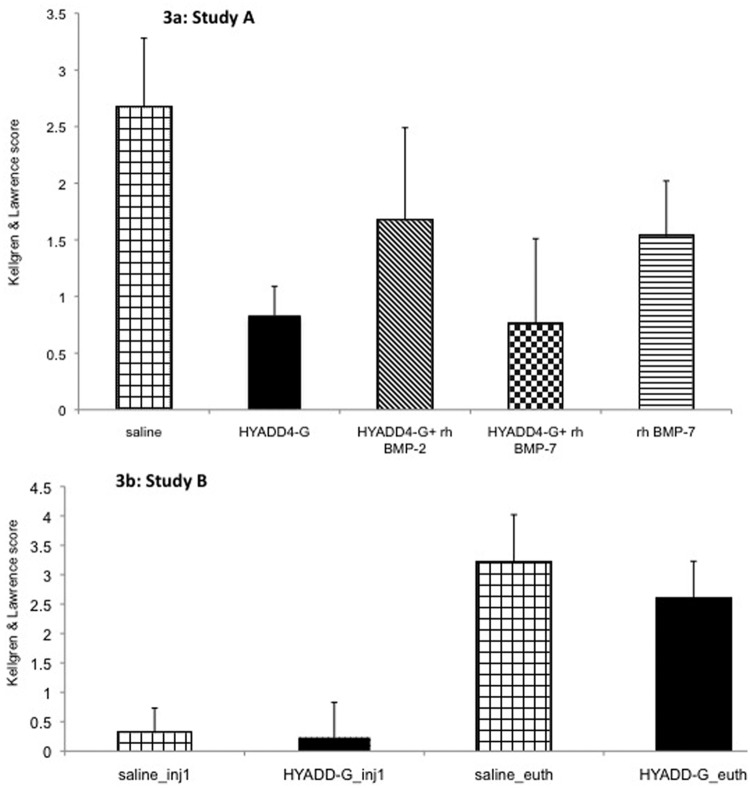
The radiological grading of the knees was 2.67 ± 0.61 for the saline group, 0.83 ± 0.26 for the HYADD®4-G group, and 1.67 ± 0.82 for HYADD®4-G in combination with rh-BMP-2, 0.75 ± 0.76 for HYADD®4-G in combination with rh-BMP-7, and 1.58 ± 0.49 for the rh-BMP-7 group. There were significant differences between the saline group and all other groups (multiplicity corrected P value < 0.05). Moreover the difference between HYADD®4-G in combination with rh-BMP-7 was also significant compared to the rh-BMP-7 group (multiplicity corrected P value < 0.05). The average radiological score between these two groups suggested a synergic effect of HYADD®4-G with rh-BMP-7 (Fig. 3a).
Figure 3.
X-ray analysis of the study. (a) Graphic representation of the Kellgren and Laurence Score of the various treatment groups at euthanasia. (b) Graphic representation of the Kellgren and Laurence score of the various treatment groups prior the first injection and at euthanasia.
Study B
Before the first injection, the radiological grading of the rabbit knees were, respectively, 0.31 ± 0.42 for the saline group and 0.23 ± 0.60 for the HYADD®4-G treated group. At euthanasia, the radiological grading scores were 3.19 ± 0.83 for the saline treated group and 2.59 ± 0.64 for the HYADD®4-G treated group. These results are depicted in Figure 3b. The average Kellgren and Laurence score of the HYADD®4-G group was statistically significantly lower than the control group at euthanasia (multiplicity corrected P value < 0.002).
Histological Analysis
The mean Mankin’s score for normal healthy nonoperated rabbits was 1.1 ± 0.20.
Study A
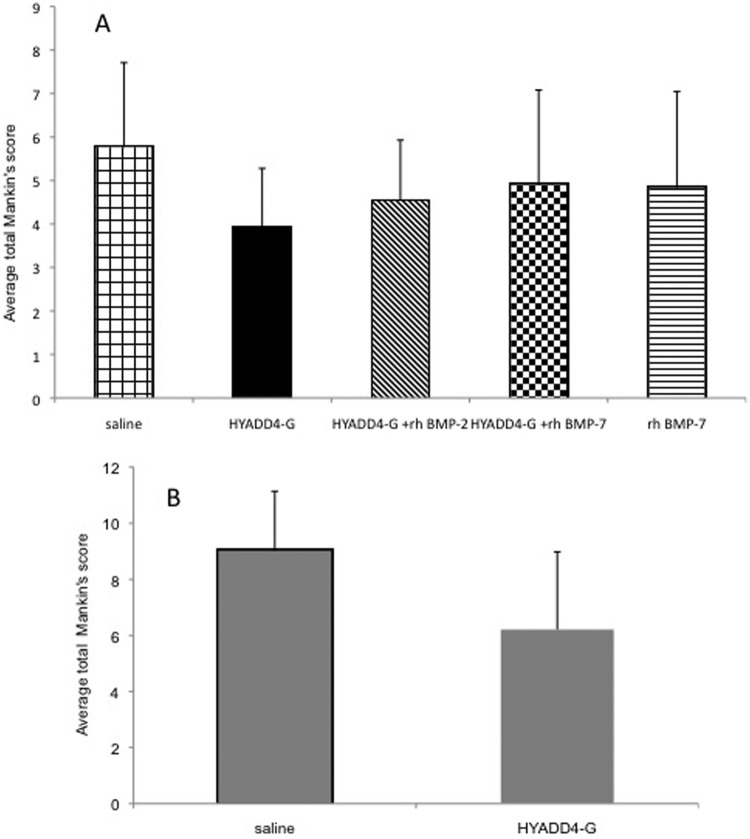
The lower the modified Mankin’s score of an individual animal is the better outcome with respect to potential delay of further progression of an already present osteoarthritis. The mean modified Mankin’s score obtained by the evaluation of all the four compartments was 5.77 ± 1.94 in the saline group and 3.9 ± 1.38 in the HYADD®4-G group. The mean scores for the HYADD®4-G in combination with rh-BMP-2, HYADD®4-G in combination with rh-BMP-7, and rh-BMP-7 alone groups were, respectively, 4.53 ± 1.35, 4.9 ± 2.1, and 4.83 ± 2.05. There was no statistical difference between the different groups (Fig. 4).
Figure 4.
(A) Study A: Histological grading according to the modified Mankin’s score. Overall score, all knee compartments. (B) Study B: Histological grading according to the modified Mankin’s score. Overall score, all knee compartments.
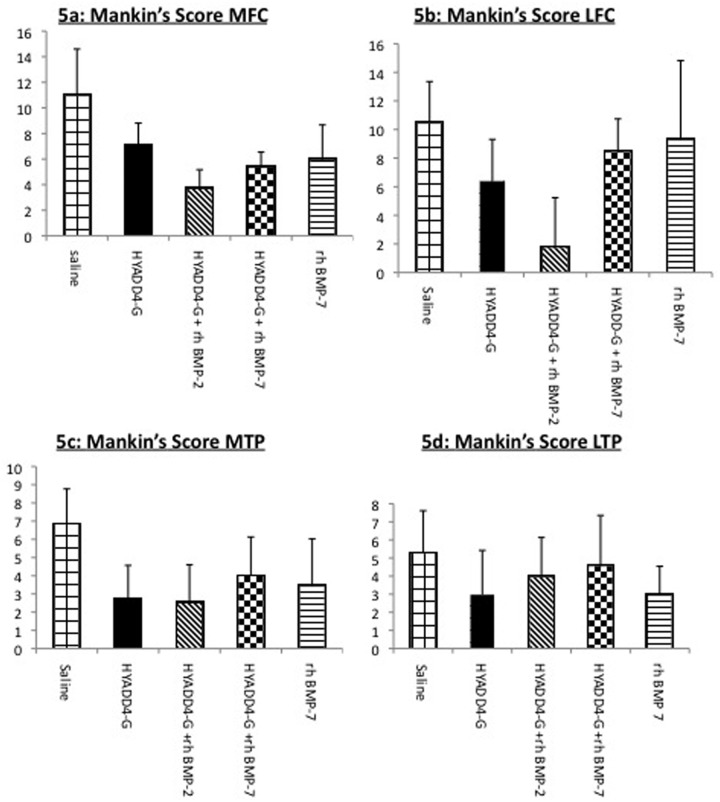
However with the analysis for each different individual compartment a significant difference in the Mankin’s grading could be observed among the groups (Fig. 5). This difference was significant (multiplicity corrected P value < 0.001) in the LFC for HYADD®4-G in combination with rh-BMP-2 in comparison to the others groups. In the MFC, the Mankin’s score values for HYADD®4-G in combination with rh-BMP-2, HYADD®4-G in combination with rh-BMP-7, and rh-BMP-7 alone showed statistically significant differences compared to the other groups (HYADD®4-G and saline) (Fig. 5a). No difference in histological analysis could be seen, however, between HYADD®4-G in combination with rh-BMP-7 and rh-BMP-7 alone.
Figure 5.
Study A: Histological grading according to the modified Mankin’s score. Overall score, individual knee compartments.
In the MTP compartment, a trend in which all the treatment groups had a lower (i.e., better) score compared with saline treatment was seen (Fig. 5c). This difference was only significant for HYADD®4-G alone and HYADD®4-G in combination with rh-BMP-2 compared to saline group (multiplicity corrected P value < 0.05). No significant difference was seen in the LTP (Fig. 5d).
Each single parameter of the modified Mankin’s score in each treatment group is described in Table 3. The parameters that mostly contributed in the decrease of the Mankin’s score were the cartilage structure and the morphological appearance of the cartilage cells.
Table 3.
Individual Scores of Each Category of the Modified Mankin’s Score Is Shown (Mean ± Standard Deviation)
| Cartilage Structure | Cartilage Cells | Safranin-O Staining | Tidemark Integrity | Total Average | |
|---|---|---|---|---|---|
| Nonoperated animal | 0.50 ± 0.25 | 0 ± 0 | 0.5 ± 0.25 | 0 ± 0 | 1.0 ± 0.25 |
| Study A | |||||
| Saline | 3.57 ± 1.30 | 1.80 ± 0.80 | 2.00 ± 0.77 | 0.17 ± 0.23 | 5.77 ± 1.94 |
| HYADD®4-G | 1.87 ± 1.13 | 0.90 ± 0.84 | 1.13 ± 0.72 | 0.00 ± 0.00 | 3.90 ± 1.38 |
| HYADD®4-G + BMP-2 | 2.13 ± 0.90 | 1.07 ± 0.73 | 1.33 ± 0.84 | 0.00 ± 0.00 | 4.53 ± 1.35 |
| HYADD®4-G + BMP-7 | 2.47 ± 1.30 | 0.80 ± 0.46 | 1.60 ± 0.57 | 0.03 ± 0.07 | 4.90 ± 2.10 |
| BMP-7 | 2.33 ± 1.26 | 1.07 ± 0.78 | 1.36 ± 0.97 | 0.07 ± 0.14 | 4.83 ± 2.05 |
| Study B | |||||
| Saline | 4.02 ± 1.11 | 2.11 ± 0.59 | 2.51 ± 0.86 | 0.27 ± 0.16 | 9.03 ± 2.12 |
| HYADD®4-G | 3.04 ± 1.14 | 1.58 ± 0.63 | 1.90 ± 0.88 | 0.14 ± 0.12 | 6.21 ± 2.77 |
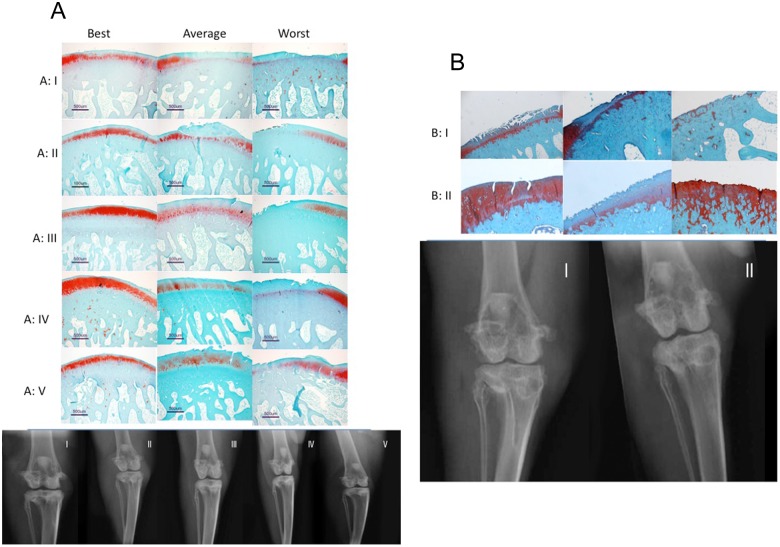
In synovial membranes, in the saline group the average inflammatory score was 6.60 ± 2.88, whereas in the HYADD®4-G group it was 2.01 ± 1.09, in the HYADD®4-G + rh-BMP-2 group it was 3.40 ± 1.67, in the HYADD®4-G + rh-BMP-7 group it was 1.67 ± 1.36, and in the rh-BMP-7 alone group it was 4.33 ± 1.86. The differences were significant (multiplicity corrected P value < 0.05) in the HYADD®4-G, HYADD®4-G + rh-BMP-2, and HYADD®4-G + rh-BMP-7 groups compared to saline. The combination of rh-BMP-7 with HYADD®4-G gave a significantly better result than the growth factor alone in term of inflammatory response. Representative histological pictures are shown in Figures 2 and 7.
Figure 7.
(A) Representative histological pictures (best, average, worst) of the study groups in Study A. Typical x-ray representation of Study A. (B) Representative histological pictures (best, average, worst) of the study groups in Study B. Typical x-ray representation of study B.
Study B
The modified Mankin’s score was 9.02 ± 2.12 for the saline treated group and 6.21 ± 2.77 for the HYADD®4-G treated group. The difference was statistically significant (multiplicity corrected P value = 0.0031). These data are summarized in Figures 4b and 6. Representative histological pictures are shown in Figure 7.
Figure 6.
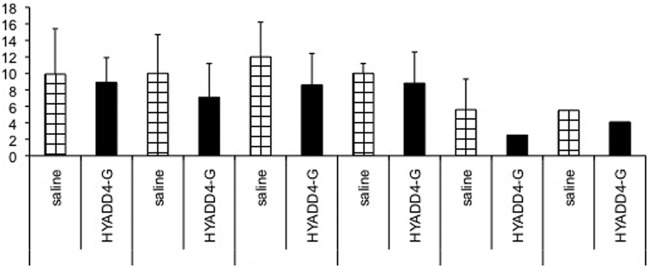
Study B: Histological grading according to the modified Mankin’s score. Overall score, individual knee compartments.
The individual scores of each category of the modified Mankin’s score for the treatment groups are presented in Table 3.
The individual score component of the Mankin’s grading system between saline and HYADD®4-G groups were not statistically significant, although the saline treated group had a tendency toward higher values. Furthermore, individual anatomical compartment was analyzed separately. Among the analyzed compartments (LFC anterior, MFC anterior, LFC posterior, MFC posterior, LTP posterior, and MTP posterior), only LTP posterior showed a significant difference between saline and HYADD®4-G groups (multiplicity corrected P value = 0.0098) (Fig. 7).
In the synovial membranes, the mean synovial membrane score was 4.6 ± 0.94 for the saline group and 3.9 ± 1.5 for the HYADD®4-G group. The difference was not statistically significant. Representative histological pictures are shown in Figure 2.
Immunohistological Analysis
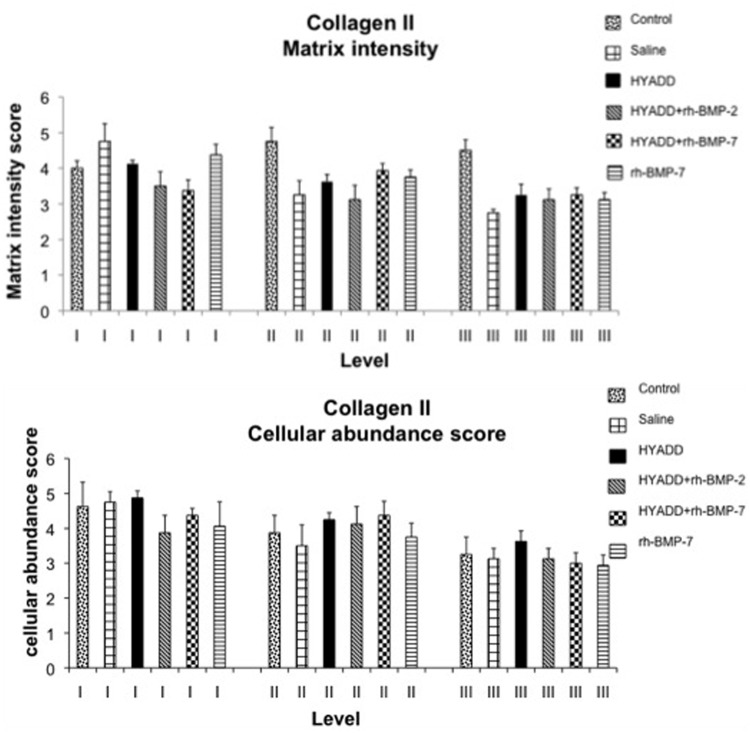
In normal healthy nonoperated rabbits, the mean intensity for matrix staining was 4.0 ± 0.2 at the superficial level, 4.8 ± 0.4 in the intermediate zone, and 4.6 ± 0.3 in the deep zone. The score for pericellular collagen II staining was 4.5 ± 0.6 at the superficial level, 3.8 ± 0.4 at the intermediate level, and 3.3 ± 0.4 in the deep level. Results of the immunohistochemistry are presented in Figure 8.
Figure 8.
Immunohistological grading anticollagen II (Study A). The graph represents the matrix staining intensity and the cellular abundance score according to a grading system (see Fig. 1).
IHC of collagen II: Matrix intensity
At level III, just above the tidemark, there were significant differences (decrease of the collagen staining intensity) between the control and saline (P < 0.01), control versus HYADD®4-G (P < 0.05), control versus HYADD®4-G + rh-BMP-2 (P < 0.05), control versus HYADD4-G–® + rh-BMP-7 (P < 0.05), and control versus rh-BMP-7 (P < 0.05). All the other comparisons were not statistically significant (P > 0.05). All treatments had a positive effect at level II, although statistically not significant in comparison to the saline-treated group.
IHC of collagen II: Cellular abundance
No statistical significant difference could be observed among treatment and control groups (Fig. 8).
IHC of collagen I
In the rh-BMP-7 group, there was a tendency to detect more frequently collagen type I. Chi-square test however revealed no statistically significant differences among the treatment groups (data not shown).
Discussion
It has been hypothesized that intra-articular HA application causes analgesia by increasing the elastoviscosity of the intercellular matrix of soft tissues around the joint, thus providing a rheological buffer that reduces the force transmitted during joint movements to the transducing membrane elements at sensory nerve terminals innervating the synovial lining and capsule.27-30
The exact mechanism of action in osteoarthritis is however still to be entirely defined, but it is proven that intra-articular HA reduces pain through its effect on peripheral pain receptors, increasing the viscoelastic properties of synovial fluid.31
Several R&D programs are devoted to the chemical modification of HA through coupling or cross-linking to enhance both the molecular weight and the half-life of the injected HA. These reactions increase the HA molecular weight and, consequently, its viscoelastic features, while preserving its biocompatibility.32
Hyalgan® is constituted by 500 to 730 kDa average-molecular-weight HA and has a very low elastic modulus.14 HYADD®4-G is a hydrogel of HA of the same molecular weight but chemically modified with hexadecylamine bound to the glucuronic acid, at a low degree of substitution (2% mol/mol), thus rendering this HA derivative more viscous and elastic.14 In a previous study, it was shown that HYADD®4-G is effective in reducing the excitatory effects of moderate and strong mechanical forces on the impulse activity in fine medial articular nerve afferents of the guinea pig knee joint.33
The HA treatment efficacy is long lasting with improvement of symptoms up to 6 months also with a single intra-articular treatment.34
In vitro and in vivo studies have provided evidence that this natural polymer prevents cartilage degradation and the relevance of the HA molecular weight.11 In osteoarthritic chondrocyte culture the IL-1β induced synthesis of matrix metalloproteinase 13 (MMP-13) is inhibited by the preincubation with HMWHA,35 on the contrary the presence of HA oligosaccharides induces in chondrocyte culture MMP-13 synthesis.36 Growing preclinical and clinical evidences support the notion that, in addition to relieving the symptoms of osteoarthritis, HA modifies the structure of diseased joint, at least early in the evolution of the disease process.37
Intra-articular injections of growth factors in osteoarthritis have been considered a therapeutic option as a disease modifying drug in osteoarthritis since the early 1990s.38
One of the most promising growth factor for the intra-articular treatment of osteoarthritis seems to be rh-BMP-7, already in the clinical phase in osteoarthritic patients.39 Recent preclinical studies have already demonstrated the efficacy of rh-BMP-7 injections in osteoarthritis,40-42 but because of the quick release of the proteins from the articular joints the preclinical studies evaluating the efficacy in osteoarthritis treatment were performed with intra-articular injections every week from the induction of osteoarthritis or at concluded osteoarthritis with the continuous delivery of the growth factor via a minipump applied in the joint or with weekly injections.
To the best of our knowledge, these are the first preclinical results of a combination therapy of a highly morphogenic stimulus for the cartilage, that is, rh-BMP-7 and a new hydrogel HA derivative in an osteoarthritis animal model.
The histological analysis in the individual compartments revealed a significant progression of osteoarthritis in the various compartments 10 weeks after ACLT with the more severe changes in the medial condyle. A statistically lower Mankin’s score was seen for rh-BMP-7 alone, rh-BMP-7 in combination with HYADD®4-G, or rh-BMP-2 in combination with HYADD®4-G. A statistically significant decreased cartilage damage for the joint treated with HYADD®4-G alone was seen, however, in the medial tibial compartment. The results indicate that both HYADD®4-G and the growth factor rh-BMP-7 alone reduce the cartilage damage in some of the analyzed cartilage compartments, but the association of HYADD®4-G and growth factor (rh-BMP-7 or even rhBMP-2) determined a significant reduction in cartilage damage in three (i.e., LFC, MFC, MTP) of the four analyzed cartilage compartments. Radiographically but not histologically a reduction of the osteoarthritis development could be seen with the combination of HYADD®4-G and rh-BMP-7 compared with rh-BMP-7 alone.
Similarly, the synovial membrane analysis revealed a significant decrease in inflammation in all treatment groups employing HYADD®4-G alone or in combination therapy. The collagen II content in the saline group was more severely affected in the basal layer than the other treatment groups.
A diminished anabolic response of osteoarthritis chondrocytes to growth factors has been shown for IGF-1.43 Rogachefsky et al.38 demonstrated the efficacy of IGF-1 intra-articular injection only when combined with intramuscular injection of sodium pentosan polysulfate, a semisynthetic drug inhibiting aggrecanase activity.
A modulation of chondrocyte’s response to growth factors could be demonstrated in an experiment in which the response of chondrocytes to BMP-7 could be restored when those chondrocytes were submitted to hyaluronan.44 Osteoarthritis chondrocytes cannot maintain the balance between ECM synthesis and ECM degradation. The composition of the ECM, chondrocytes surface receptor density, and cytosolic signaling cascade influence the way chondrocytes respond to those growth factor’s stimuli. If this is true that is not solely the concentration of the growth factor that modulate the cell response.44
Hyaluronic acid therefore plays an important role in the cell response to BMP-7 but also other growth factors, which can explain why a combination of HA and growth factors can be beneficial for intra-articular injection.
The efficacy of the viscosupplementation treatment with this novel HA hydrogel treatment was further confirmed in our second study with only three intra-articular injections. The better results demonstrated in this second study with a significant improvement in the overall Mankin’s score, obtained by the analysis of all the analyzed cartilage compartments, is probably related to the higher number of animals and higher cartilage damage.
In conclusion, from our pilot study, it appears that viscoelastic HA hydrogel associated to growth factors have better beneficial effects on the progression of osteoarthritis than the viscosupplement agent alone in this specific animal model of rabbit ACLT. The synergistic effect of viscosupplementation and growth factors need however to be further evaluated in a larger animal study with analysis at longer time points and evaluation of treatment effect also on bone changes. The use of growth factors for osteoarthritis treatment is only at the initial phase of clinical development and the beneficial long-term effects to be proven, with regard to the risk/benefit ratio, although viscosupplementation therapy has long been use in clinical practice and has provided evidence of long-term benefit and safety.13
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Fidia Farmaceutici S.p.A Abano Terme, Italy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The surgical protocol and its execution were approved by the Bernese Cantonal Authorities (BE 2006/38) (Animal safety committee).
References
- 1. Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3-9. [PubMed] [Google Scholar]
- 2. Balazs EA. The physical properties of synovial fluid and the special role of hyaluronan acid. In: Helfet A, editor. Disorders of the knee. Philadelphia: J. B. Lippincott; 1982. p. 61-74. [Google Scholar]
- 3. Balazs EA, Denlinger JL. Clinical uses of hyaluronan. Ciba Found Symp 1989;143:265-75. [DOI] [PubMed] [Google Scholar]
- 4. Altman RD. Intra-articular sodium hyaluronate in osteoarthritis of the knee. Semin Arthritis Rheum. 2000;2(Suppl 1):11-8. [DOI] [PubMed] [Google Scholar]
- 5. Adams ME. Viscosupplementation as articular therapy. Perspective. In: Laurent T, editor. The chemistry, biology and medical applications of hyaluronan and its derivatives. London: Portland Press; 1996. p. 243-53. [Google Scholar]
- 6. Weiss C. Hyaluronan and hylan in the treatment of osteoarthritis. In: Kennedy JF, Phillips GO, Williams PA, Hascall VC, editors. Hyaluronan. Cambridge: Woodhead; 2002. p. 467-82. [Google Scholar]
- 7. Balazs EA. Analgesic effect of elastoviscous hyaluronan solutions and the treatment of arthritic pain. Cells Tissues Organs. 2003;174:49-62. [DOI] [PubMed] [Google Scholar]
- 8. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol (Paris). 1974;22:731-6. [PubMed] [Google Scholar]
- 9. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage. 2011;19(6):611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanism of action. Arthritis Res Ther. 2003;5(2):54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10-37. [DOI] [PubMed] [Google Scholar]
- 12. Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25(11):2203-12. [PubMed] [Google Scholar]
- 13. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borzacchiello A, Mayol L, Schiavinato A, Ambrosio L. Effect of hyaluronic acid amide derivative on equine synovial fluid viscoelasticity. J Biomed Mater Res A. 2010;92(3):1162-70. [DOI] [PubMed] [Google Scholar]
- 15. Smith MM, Cake MA, Ghosh P, Schiavinato A, Read RA, Little CB. Significant synovial pathology in a meniscectomy model of osteoarthritis: modifications by intra-articular hyaluronan therapy. Rheumatology (Oxford). 2008;47(8):1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cake MA, Read R, Edwards S, Smith MM, Burkardt D, Little C, et al. Changes in gait after bilateral meniscectomy in sheep: effect of two hyaluronan preparations. J Orthop Sci. 2008;13(6):514-23. [DOI] [PubMed] [Google Scholar]
- 17. Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis Cartilage. 2000;8:127-36. [DOI] [PubMed] [Google Scholar]
- 18. Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W. Osteogenic protein-1 stimulates cell-associated matrix assembly by normal human articular chondrocytes. Arthritis Rheum. 2000;43:206-14. [DOI] [PubMed] [Google Scholar]
- 19. Flechtenmacher J, Huch K, Thonar EJMA, Mollenhauer J, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:478-88. [DOI] [PubMed] [Google Scholar]
- 20. Amiel D, Toyoguchi T, Kobayashi K, Bowden K, Amiel ME, Healey RM. Long-term effect of sodium hyaluronate (Hyalgan) on osteoarthritis progression in a rabbit model. Osteoarthritis Cartilage. 2003;11(9):636-43. [DOI] [PubMed] [Google Scholar]
- 21. Ozlem Nisbet H, Ozak A, Yardimci C, Nisbet C, Yarim M, Bayrak IK, et al. Evaluation of bee venom and hyaluronic acid in the intra-articular treatment of osteoarthritis in an experimental rabbit model. Res Vet Sci. 2012;93(1):488-93. [DOI] [PubMed] [Google Scholar]
- 22. Rijk PC, de Rooy TP, Coerkamp EG, Bernoski FP, van Noorden CJ. Radiographic evaluation of the knee joint after meniscal allograft transplantation. An experimental study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2002;10:241-6. [DOI] [PubMed] [Google Scholar]
- 23. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1962;43:752-7. [DOI] [PubMed] [Google Scholar]
- 24. Mankin HJ, Dorfman H, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523-37. [PubMed] [Google Scholar]
- 25. Cake MA, Smith MM, Young AA, Ghosh P, Read RA. Synovial pathology in an ovine model of osteoarthristis: effect of intraarticular hyaluronan (Hyalgan). Clin Exp Rheumatol. 2008;26(4):561-7. [PubMed] [Google Scholar]
- 26. Giovannini S, Diaz-Romero J, Aigner T, Mainil-Varlet P, Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol. 2010;222(2):411-20. [DOI] [PubMed] [Google Scholar]
- 27. Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3-9. [DOI] [PubMed] [Google Scholar]
- 28. Pawlak M. Mechanoprotective actions of elastoviscous hylans on articular pain receptors. In: Kennedy JF, Phillips GO, Williams PA, Hascall VC, editors. Hyaluronan. Cambridge: Woodhead; 2002. p. 341-52. [Google Scholar]
- 29. Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50(1):314-26. [DOI] [PubMed] [Google Scholar]
- 30. Gomis A, Miralles A, Schmidt RF, Belmonte C. Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the guinea pig: effect of intra-articular hyaluronan application. Pain. 2007;130:126-36. [DOI] [PubMed] [Google Scholar]
- 31. Belmonte C, Pozo MA, Balazs EA. Modulation by hyaluronan and its derivatives (hylans) of sensory nerve activity signalling articular pain. In: Laurent TC, editor. The chemistry, biology and medical applications of hyaluronan and its derivatives. London: Portland Press; 1998. p. 205-17. [Google Scholar]
- 32. Prestwich GD, Kuo JW. Chemically modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol. 2008;9(4):242-5. [DOI] [PubMed] [Google Scholar]
- 33. Gomis A, Miralles A, Schmidt RF, Belmonte C. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage. 2009;17(6):798-804. [DOI] [PubMed] [Google Scholar]
- 34. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomized, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakanura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011;29(2):258-64. [DOI] [PubMed] [Google Scholar]
- 36. Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38MAP kinase in articular chondrocytes. J Biol Chem. 2006;281(26):17952-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216-24. [DOI] [PubMed] [Google Scholar]
- 38. Rogachefsky RA, Dean DD, Howell DS, Altman RD. Treatment of canine osteoarthritis with insulin-like growth factor-1 (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993;1(2):105-14. [DOI] [PubMed] [Google Scholar]
- 39. Hunter DJ, Picke MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):600-6. [DOI] [PubMed] [Google Scholar]
- 41. Badlani N, Oshima Y, Healey R, Coutts R, Amiel D. Use of morphogenic protein-7 as a treatment for osteoarthritis. Clin Orthop Relat Res. 2009;467(12):3221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayashi M, Muneta T, Takahashi T, Ju YI, Tsuji K, Sekiya I. Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration. Orthop Res. 2010;28(11):1502-6. [DOI] [PubMed] [Google Scholar]
- 43. Loeser RF, Shanker RG, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non human primate model of naturally occurring disease. Arthritis Rheum. 2000;43(9):1916-26. [DOI] [PubMed] [Google Scholar]
- 44. Andhare RA, Takahashi N, Knudson W, Knudson CB. Hyaluronan promotes the chondrocyte response to BMP-7. Osteoarthritis Cartilage. 2009;17(7):906-16. [DOI] [PMC free article] [PubMed] [Google Scholar]