Abstract
Enteropathogenic Escherichia coli (EPEC) organisms are an important cause of diarrheal disease in young children. The virulence of EPEC is a multifactorial process and involves a number of distinct stages. Initial adherence to intestinal mucosa is mediated by fimbriae which bring about a distinct form of adhesion, localized adhesion. Intimate adhesion of the bacterium to the eukaryotic membrane occurs, resulting in the activation of signal transduction pathways. Microvilli are disrupted and effaced from the apical membrane which then cups around the organism to form pedestal structures, the attaching and effacing lesion. Diarrhea may be produced by alteration of the permeability of the apical membrane and also through a malabsorption mechanism. The pathways involved in the production of the attaching and effacing lesion are described. EPEC organisms were originally thought to belong to a number of distinct serogroups; it is now apparent that many isolates belonging to these serogroups are not pathogenic or belong to other pathogenic groups of E. coli. In addition, isolates falling outside of these serogroups are considered to be true EPEC. The definition of EPEC based on serotyping is inaccurate and should be replaced by methods that specifically detect the virulence properties of EPEC.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Alam K., Ansaruzzaman M., Montanaro J., Islam M., Faruque S. M., Haider K., Bettelheim K., Tzipori S. Localized adherence and attaching-effacing properties of nonenteropathogenic serotypes of Escherichia coli. Infect Immun. 1991 May;59(5):1864–1868. doi: 10.1128/iai.59.5.1864-1868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Alam K., Islam M., Montanaro J., Rahaman A. S., Haider K., Hossain M. A., Kibriya A. K., Tzipori S. Hafnia alvei, a probable cause of diarrhea in humans. Infect Immun. 1991 Apr;59(4):1507–1513. doi: 10.1128/iai.59.4.1507-1513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Ansaruzzaman M., Faruque S. M., Neogi P. K., Haider K., Tzipori S. An ELISA for the detection of localized adherent classic enteropathogenic Escherichia coli serogroups. J Infect Dis. 1991 Nov;164(5):986–989. doi: 10.1093/infdis/164.5.986. [DOI] [PubMed] [Google Scholar]
- Albert M. J., Faruque S. M., Ansaruzzaman M., Islam M. M., Haider K., Alam K., Kabir I., Robins-Browne R. Sharing of virulence-associated properties at the phenotypic and genetic levels between enteropathogenic Escherichia coli and Hafnia alvei. J Med Microbiol. 1992 Nov;37(5):310–314. doi: 10.1099/00222615-37-5-310. [DOI] [PubMed] [Google Scholar]
- Ananthan S., Subramanian S., Thyagarajan S. P., Jayakumar V., Udayashankar K., Elanchezhiyan M., Shanmugasundaram N. Studies on the pathogenicity of classical enteropathogenic Escherichia coli strains isolated from acute diarrhoea among children 0-5 years of age. Indian J Pathol Microbiol. 1989 Apr;32(2):92–100. [PubMed] [Google Scholar]
- Andrade J. R., Da Veiga V. F., De Santa Rosa M. R., Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989 Jan;28(1):49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Nataro J. P., Kaper J. B. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986 Apr;52(1):334–336. doi: 10.1128/iai.52.1.334-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Brooks S. F., Knutton S., Manjarrez Hernandez H. A., Aitken A., Williams P. H. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1990 Mar;58(3):761–765. doi: 10.1128/iai.58.3.761-765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Knutton S., Sellers L., Hernandez H. A., Aitken A., Williams P. H. Enteroaggregative Escherichia coli strains secrete a heat-labile toxin antigenically related to E. coli hemolysin. Infect Immun. 1992 May;60(5):2092–2095. doi: 10.1128/iai.60.5.2092-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Lee-Delaunay M. B., Knutton S., Williams P. H. Calcium-calmodulin dependence of actin accretion and lethality in cultured HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1993 Feb;61(2):760–763. doi: 10.1128/iai.61.2.760-763.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Ward W., Aitken A., Knutton S., Williams P. H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991 May;59(5):1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqui A. H., Sack R. B., Black R. E., Haider K., Hossain A., Alim A. R., Yunus M., Chowdhury H. R., Siddique A. K. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992 Oct;166(4):792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Hart C. A., McLean L., Saunders J. R. Organ culture of rabbit ileum as a model for the investigation of the mechanism of intestinal damage by enteropathogenic Escherichia coli. Gut. 1987 Oct;28(10):1283–1290. doi: 10.1136/gut.28.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry B., Savarino S. J., Vial P., Kaper J. B., Levine M. M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990 Jun;161(6):1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- Beebakhee G., Louie M., De Azavedo J., Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992 Feb 1;70(1):63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- Benz I., Schmidt M. A. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect Immun. 1992 Jan;60(1):13–18. doi: 10.1128/iai.60.1.13-18.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Orskov I., Orskov F., Zimmermann S., Prada J., Gelderblom H., Stephan R., Whittam T. S. Clonal diversity and virulence factors in strains of Escherichia coli of the classic enteropathogenic serogroup O114. J Infect Dis. 1990 Dec;162(6):1329–1334. doi: 10.1093/infdis/162.6.1329. [DOI] [PubMed] [Google Scholar]
- Bhan M. K., Raj P., Levine M. M., Kaper J. B., Bhandari N., Srivastava R., Kumar R., Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989 Jun;159(6):1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzan M., Karch H., Maas M. G., Meyer T., Rüssmann H., Aleksić S., Bockemühl J. Clinical and genetic aspects of Shiga-like toxin production in traditional enteropathogenic Escherichia coli. Zentralbl Bakteriol. 1991 Jan;274(4):496–506. doi: 10.1016/s0934-8840(11)80087-3. [DOI] [PubMed] [Google Scholar]
- Boedeker E. C. Enterocyte adherence of Escherichia coli: its relation to diarrheal disease. Gastroenterology. 1982 Aug;83(2):489–492. [PubMed] [Google Scholar]
- Bower J. R., Congeni B. L., Cleary T. G., Stone R. T., Wanger A., Murray B. E., Mathewson J. J., Pickering L. K. Escherichia coli O114:nonmotile as a pathogen in an outbreak of severe diarrhea associated with a day care center. J Infect Dis. 1989 Aug;160(2):243–247. doi: 10.1093/infdis/160.2.243. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Anderson A. N., Perry M. B. Differences between the LPS cores in adherent and non-adherent strains of enteropathogenic Escherichia coli 0119. FEMS Microbiol Lett. 1991 May 1;64(1):13–17. doi: 10.1016/0378-1097(91)90201-k. [DOI] [PubMed] [Google Scholar]
- Canil C., Rosenshine I., Ruschkowski S., Donnenberg M. S., Kaper J. B., Finlay B. B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993 Jul;61(7):2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Moseley S. L. HeLa cell adherence, actin aggregation, and invasion by nonenteropathogenic Escherichia coli possessing the eae gene. Infect Immun. 1991 Nov;59(11):3924–3929. doi: 10.1128/iai.59.11.3924-3929.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Rowe B. The outer membrane protein of enteropathogenic Escherichia coli, described as the 'localised adherence factor', is OmpF and probably not involved in adhesion to HEp-2 cells. FEMS Microbiol Lett. 1989 Oct 15;52(3):291–295. doi: 10.1016/0378-1097(89)90213-9. [DOI] [PubMed] [Google Scholar]
- Chart H., Said B., Rowe B. Linoleic acid inhibition of adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Lancet. 1991 Jul 13;338(8759):126–127. doi: 10.1016/0140-6736(91)90126-a. [DOI] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Willshaw G. A., Rowe B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to enteropathogenic serogroups. J Gen Microbiol. 1988 May;134(5):1315–1321. doi: 10.1099/00221287-134-5-1315. [DOI] [PubMed] [Google Scholar]
- Chatkaeomorakot A., Echeverria P., Taylor D. N., Bettelheim K. A., Blacklow N. R., Sethabutr O., Seriwatana J., Kaper J. HeLa cell-adherent Escherichia coli in children with diarrhea in Thailand. J Infect Dis. 1987 Oct;156(4):669–672. doi: 10.1093/infdis/156.4.669. [DOI] [PubMed] [Google Scholar]
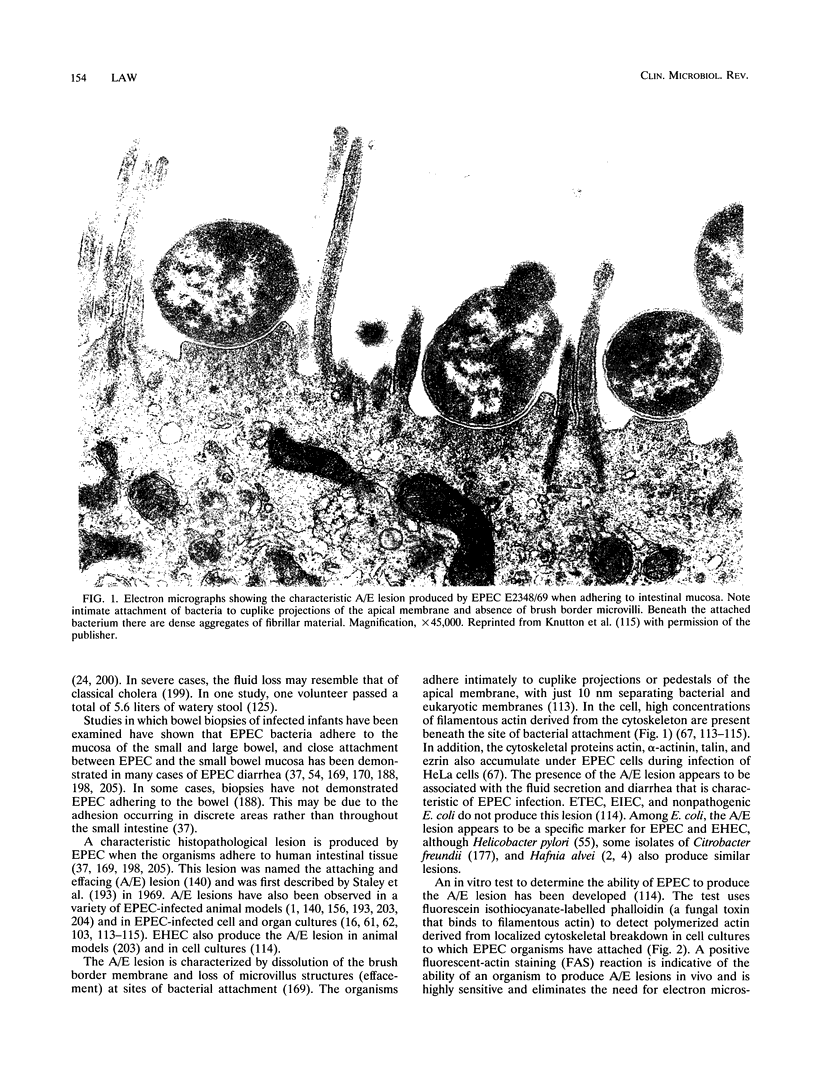
- Clausen C. R., Christie D. L. Chronic diarrhea in infants caused by adherent enteropathogenic Escherichia coli. J Pediatr. 1982 Mar;100(3):358–361. doi: 10.1016/s0022-3476(82)80429-0. [DOI] [PubMed] [Google Scholar]
- Cleary T. G., Mathewson J. J., Faris E., Pickering L. K. Shiga-like cytotoxin production by enteropathogenic Escherichia coli serogroups. Infect Immun. 1985 Jan;47(1):335–337. doi: 10.1128/iai.47.1.335-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. B., Hawkins J. A., Weckbach L. S., Staneck J. L., Levine M. M., Heck J. E. Colonization by enteroaggregative Escherichia coli in travelers with and without diarrhea. J Clin Microbiol. 1993 Feb;31(2):351–353. doi: 10.1128/jcm.31.2.351-353.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravioto A., Reyes R. E., Ortega R., Fernández G., Hernández R., López D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988 Aug;101(1):123–134. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravioto A., Tello A., Navarro A., Ruiz J., Villafán H., Uribe F., Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991 Feb 2;337(8736):262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Tello A., Villafán H., Ruiz J., del Vedovo S., Neeser J. R. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991 Jun;163(6):1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989 Sep;160(3):452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Girón J. A., Nataro J. P., Kaper J. B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992 Nov;6(22):3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991 Dec;59(12):4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker M. M., Polliack A., Yeivin R., Sacks T. G. Immunofluorescent demonstration of enteropathogenic Escherichia coli in tissues of infants dying with enteritis. Pediatrics. 1970 Dec;46(6):855–864. [PubMed] [Google Scholar]
- Dytoc M., Gold B., Louie M., Huesca M., Fedorko L., Crowe S., Lingwood C., Brunton J., Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1993 Feb;61(2):448–456. doi: 10.1128/iai.61.2.448-456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
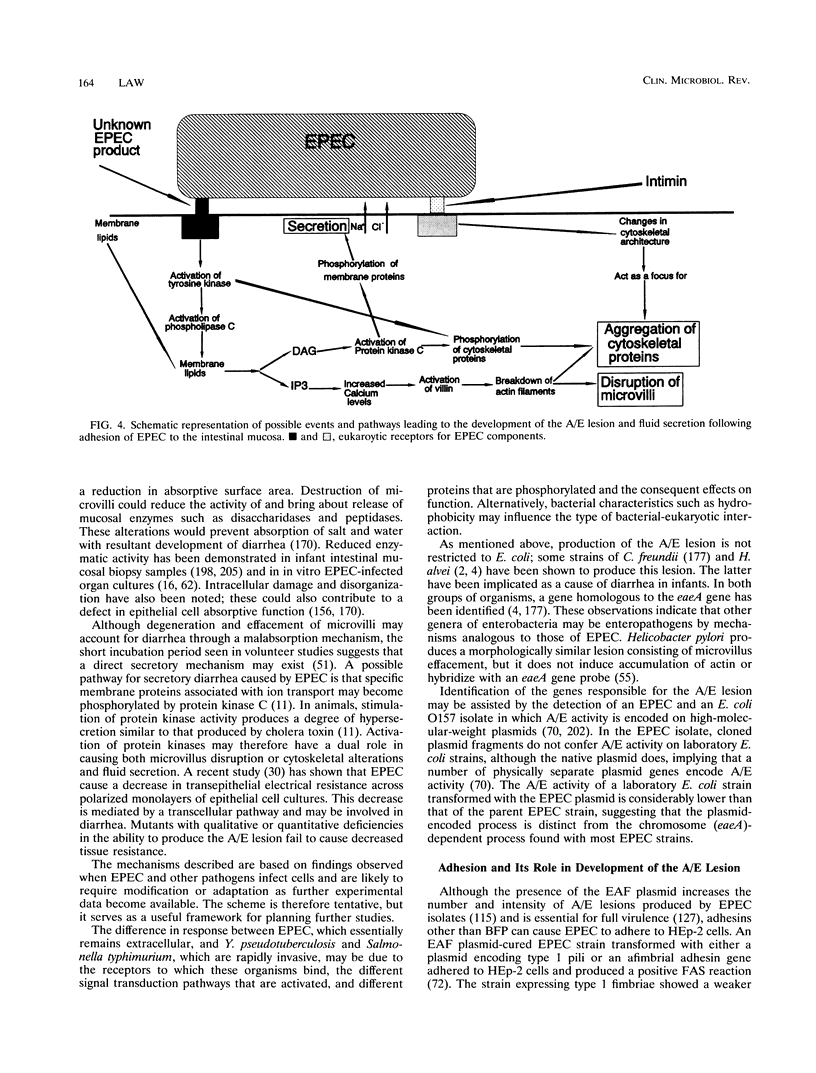
- Echeverria P., Orskov F., Orskov I., Knutton S., Scheutz F., Brown J. E., Lexomboon U. Attaching and effacing enteropathogenic Escherichia coli as a cause of infantile diarrhea in Bangkok. J Infect Dis. 1991 Sep;164(3):550–554. doi: 10.1093/infdis/164.3.550. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Serichantalerg O., Changchawalit S., Baudry B., Levine M. M., Orskov F., Orskov I. Tissue culture-adherent Escherichia coli in infantile diarrhea. J Infect Dis. 1992 Jan;165(1):141–143. doi: 10.1093/infdis/165.1.141. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Taylor D. N., Bettelheim K. A., Chatkaeomorakot A., Changchawalit S., Thongcharoen A., Leksomboon U. HeLa cell-adherent enteropathogenic Escherichia coli in children under 1 year of age in Thailand. J Clin Microbiol. 1987 Aug;25(8):1472–1475. doi: 10.1128/jcm.25.8.1472-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria P., Taylor D. N., Donohue-Rolfe A., Supawat K., Ratchtrachenchai O., Kaper J., Keusch G. T. HeLa cell adherence and cytotoxin production by enteropathogenic Escherichia coli isolated from infants with diarrhea in Thailand. J Clin Microbiol. 1987 Aug;25(8):1519–1523. doi: 10.1128/jcm.25.8.1519-1523.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embaye H., Batt R. M., Saunders J. R., Getty B., Hart C. A. Interaction of enteropathogenic Escherichia coli 0111 with rabbit intestinal mucosa in vitro. Gastroenterology. 1989 Apr;96(4):1079–1086. doi: 10.1016/0016-5085(89)91626-0. [DOI] [PubMed] [Google Scholar]
- Embaye H., Hart C. A., Getty B., Fletcher J. N., Saunders J. R., Batt R. M. Effects of enteropathogenic Escherichia coli on microvillar membrane proteins during organ culture of rabbit intestinal mucosa. Gut. 1992 Sep;33(9):1184–1189. doi: 10.1136/gut.33.9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON W. W., JUNE R. C. Experiments on feeding adult volunteers with Escherichia coli 111, B4, a coliform organism associated with infant diarrhea. Am J Hyg. 1952 Mar;55(2):155–169. doi: 10.1093/oxfordjournals.aje.a119510. [DOI] [PubMed] [Google Scholar]
- Fagundes Neto U., Ferreira V. de C., Patricio F. R., Mostaço V. L., Trabulsi L. R. Protracted diarrhea: the importance of the enteropathogenic E. coli (EPEC) strains and Salmonella in its genesis. J Pediatr Gastroenterol Nutr. 1989 Feb;8(2):207–211. [PubMed] [Google Scholar]
- Faruque S. M., Haider K., Albert M. J., Ahmad Q. S., Alam A. N., Nahar S., Tzipori S. A comparative study of specific gene probes and standard bioassays to identify diarrhoeagenic Escherichia coli in paediatric patients with diarrhoea in Bangladesh. J Med Microbiol. 1992 Jan;36(1):37–40. doi: 10.1099/00222615-36-1-37. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Starnbach M. N., Francis C. L., Stocker B. A., Chatfield S., Dougan G., Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988 Nov;2(6):757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Fletcher J. N., Embaye H. E., Getty B., Batt R. M., Hart C. A., Saunders J. R. Novel invasion determinant of enteropathogenic Escherichia coli plasmid pLV501 encodes the ability to invade intestinal epithelial cells and HEp-2 cells. Infect Immun. 1992 Jun;60(6):2229–2236. doi: 10.1128/iai.60.6.2229-2236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. N., Saunders J. R., Batt R. M., Embaye H., Getty B., Hart C. A. Attaching effacement of the rabbit enterocyte brush border is encoded on a single 96.5-kilobase-pair plasmid in an enteropathogenic Escherichia coli O111 strain. Infect Immun. 1990 May;58(5):1316–1322. doi: 10.1128/iai.58.5.1316-1322.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Darfeuille-Michaud A., Wasch E., Rich C., Petat E., Denis F., Joly B. Adhesive properties of enteropathogenic Escherichia coli isolated from infants with acute diarrhea in Africa. Eur J Clin Microbiol Infect Dis. 1989 Nov;8(11):979–983. doi: 10.1007/BF01967569. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Jerse A. E., Kaper J. B., Falkow S. Characterization of interactions of enteropathogenic Escherichia coli O127:H6 with mammalian cells in vitro. J Infect Dis. 1991 Oct;164(4):693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Gangarosa E. J., Merson M. H. Epidemiologic assessment of the relevance of the so-called enteropathogenic serogroups of Escherichia coli in diarrhea. N Engl J Med. 1977 May 26;296(21):1210–1213. doi: 10.1056/NEJM197705262962106. [DOI] [PubMed] [Google Scholar]
- Ghosh A. R., Nair G. B., Dutta P., Pal S. C., Sen D. Acute diarrhoeal diseases in infants aged below six months in hospital in Calcutta, India: an aetiological study. Trans R Soc Trop Med Hyg. 1991 Nov-Dec;85(6):796–798. doi: 10.1016/0035-9203(91)90459-c. [DOI] [PubMed] [Google Scholar]
- Girón J. A., Ho A. S., Schoolnik G. K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991 Nov 1;254(5032):710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Girón J. A., Jones T., Millán-Velasco F., Castro-Muñoz E., Zárate L., Fry J., Frankel G., Moseley S. L., Baudry B., Kaper J. B. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991 Mar;163(3):507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., DuPont H. L. Enteropathogenic Escherichia coli: lack of correlation of serotype with pathogenicity. J Infect Dis. 1976 Feb;133(2):153–156. doi: 10.1093/infdis/133.2.153. [DOI] [PubMed] [Google Scholar]
- Gomes T. A., Blake P. A., Trabulsi L. R. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J Clin Microbiol. 1989 Feb;27(2):266–269. doi: 10.1128/jcm.27.2.266-269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T. A., Rassi V., MacDonald K. L., Ramos S. R., Trabulsi L. R., Vieira M. A., Guth B. E., Candeias J. A., Ivey C., Toledo M. R. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J Infect Dis. 1991 Aug;164(2):331–337. doi: 10.1093/infdis/164.2.331. [DOI] [PubMed] [Google Scholar]
- Gomes T. A., Vieira M. A., Wachsmuth I. K., Blake P. A., Trabulsi L. R. Serotype-specific prevalence of Escherichia coli strains with EPEC adherence factor genes in infants with and without diarrhea in São Paulo, Brazil. J Infect Dis. 1989 Jul;160(1):131–135. doi: 10.1093/infdis/160.1.131. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L. Bacterial diarrhoea and its treatment. Lancet. 1987 Dec 12;2(8572):1378–1382. doi: 10.1016/s0140-6736(87)91267-0. [DOI] [PubMed] [Google Scholar]
- Griffin P. M., Tauxe R. V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- Gross R. J., Rowe B., Threlfall E. J. Escherichia coli 0142.H6; a drug-resistant enteropathogenic clone? J Hyg (Lond) 1985 Apr;94(2):181–191. doi: 10.1017/s0022172400061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. J., Scotland S. M., Rowe B. Enterotoxin testing of Escherichia coli causing epidemic infantile enteritis in the U.K. Lancet. 1976 Mar 20;1(7960):629–631. doi: 10.1016/s0140-6736(76)90429-3. [DOI] [PubMed] [Google Scholar]
- Guggenbichler J. P. Adherence of enterobacteria in infantile diarrhea and its prevention. Infection. 1983 Jul-Aug;11(4):239–242. doi: 10.1007/BF01641209. [DOI] [PubMed] [Google Scholar]
- Gunzburg S. T., Chang B. J., Burke V., Gracey M. Virulence factors of enteric Escherichia coli in young Aboriginal children in north-west Australia. Epidemiol Infect. 1992 Oct;109(2):283–289. doi: 10.1017/s0950268800050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M., Hinde D., Gross R., Rowe B. A prospective study of enteropathogenic Escherichia coli in endemic diarrheal disease. J Infect Dis. 1978 Mar;137(3):292–297. doi: 10.1093/infdis/137.3.292. [DOI] [PubMed] [Google Scholar]
- Haider K., Faruque S. M., Albert M. J., Nahar S., Neogi P. K., Hossain A. Comparison of a modified adherence assay with existing assay methods for identification of enteroaggregative Escherichia coli. J Clin Microbiol. 1992 Jun;30(6):1614–1616. doi: 10.1128/jcm.30.6.1614-1616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991 Jun;55(2):206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. A., Batt R. M., Saunders J. R., Getty B. Lectin-induced damage to the enterocyte brush border. An electron-microscopic study in rabbits. Scand J Gastroenterol. 1988 Dec;23(10):1153–1159. doi: 10.3109/00365528809090184. [DOI] [PubMed] [Google Scholar]
- Hill S. M., Phillips A. D., Walker-Smith J. A. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991 Feb;32(2):154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. M., Phillips A. D., Walker-Smith J. A., Sanderson I. R., Milla P. J. Antibiotics for Escherichia coli gastroenteritis. Lancet. 1988 Apr 2;1(8588):771–772. doi: 10.1016/s0140-6736(88)91584-x. [DOI] [PubMed] [Google Scholar]
- Househam K. C., Mann M. D., Bowie M. D. Enteropathogens associated with acute infantile diarrhoea in Cape Town. S Afr Med J. 1988 Jan 23;73(2):83–87. [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R., Formal S. B. Colonization, virulence, and mucosal interaction of an enteropathogenic Escherichia coli (strain RDEC-1) expressing shigella somatic antigen in the rabbit intestine. J Infect Dis. 1986 Nov;154(5):742–751. doi: 10.1093/infdis/154.5.742. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990 Feb;7(1):73–82. [PubMed] [Google Scholar]
- JUNE R. C., FERGUSON W. W., WORFEL M. T. Experiments in feeding adult volunteers with Escherichia coli 55, B5, a coliform organism associated with infant diarrhea. Am J Hyg. 1953 Mar;57(2):222–236. doi: 10.1093/oxfordjournals.aje.a119570. [DOI] [PubMed] [Google Scholar]
- Jagannatha H. M., Sharma U. K., Ramaseshan T., Surolia A., Balganesh T. S. Identification of carbohydrate structures as receptors for localised adherent enteropathogenic Escherichia coli. Microb Pathog. 1991 Oct;11(4):259–268. doi: 10.1016/0882-4010(91)90030-e. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Gicquelais K. G., Kaper J. B. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect Immun. 1991 Nov;59(11):3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Kaper J. B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991 Dec;59(12):4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain K. C., Barteluk R. L., Kelly M. T., He X., de Hua G., Ge Y. A., Proctor E. M., Byrne S., Stiver H. G. Etiology of childhood diarrhea in Beijing, China. J Clin Microbiol. 1991 Jan;29(1):90–95. doi: 10.1128/jcm.29.1.90-95.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R. Phage-associated cytotoxin production by and enteroadhesiveness of enteropathogenic Escherichia coli isolated from infants with diarrhea in West Germany. J Infect Dis. 1987 Apr;155(4):707–715. doi: 10.1093/infdis/155.4.707. [DOI] [PubMed] [Google Scholar]
- Katouli M., Jaafari A., Farhoudi-Moghaddam A. A., Ketabi G. R. Aetiological studies of diarrhoeal diseases in infants and young children in Iran. J Trop Med Hyg. 1990 Feb;93(1):22–27. [PubMed] [Google Scholar]
- Katouli M., Pachenary A., Ketabi G. R. Vero cytotoxin production and HeLa cell adherence of enteropathogenic Escherichia coli of infantile diarrhoea in Iran. FEMS Microbiol Lett. 1989 May;50(1-2):177–180. doi: 10.1016/0378-1097(89)90481-3. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Iqbal J., Ghafoor A., Burney M. I. Aetiologic agents of diarrhoeal diseases in hospitalised children in Rawalpindi, Pakistan. J Diarrhoeal Dis Res. 1988 Sep-Dec;6(3-4):228–231. [PubMed] [Google Scholar]
- Klipstein F. A., Rowe B., Engert R. F., Short H. B., Gross R. J. Enterotoxigenicity of enteropathogenic serotypes of Escherichia coli isolated from infants with epidemic diarrhea. Infect Immun. 1978 Jul;21(1):171–178. doi: 10.1128/iai.21.1.171-178.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Phillips A. D., Smith H. R., Gross R. J., Shaw R., Watson P., Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991 Jan;59(1):365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Shaw R. K., Bhan M. K., Smith H. R., McConnell M. M., Cheasty T., Williams P. H., Baldwin T. J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992 May;60(5):2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977 Dec;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J., Delage G., Gosselin F., Chicoine L. Severe protracted diarrhea due to multiresistant adherent Escherichia coli. Am J Dis Child. 1984 Jul;138(7):693–696. doi: 10.1001/archpedi.1984.02140450075023. [DOI] [PubMed] [Google Scholar]
- Laporta M. Z., Silva M. L., Scaletsky I. C., Trabulsi L. R. Plasmids coding for drug resistance and localized adherence to HeLa cells in enteropathogenic Escherichia coli O55:H- and O55:H6. Infect Immun. 1986 Feb;51(2):715–717. doi: 10.1128/iai.51.2.715-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. Detection of verotoxin-producing strains of Escherichia coli. J Infect. 1993 Mar;26(2):228–229. doi: 10.1016/0163-4453(93)93301-j. [DOI] [PubMed] [Google Scholar]
- Law D. Virulence factors of enteropathogenic Escherichia coli. J Med Microbiol. 1988 May;26(1):1–10. doi: 10.1099/00222615-26-1-1. [DOI] [PubMed] [Google Scholar]
- Law D., Wilkie K. M., Freeman R. Examination of enteropathogenic Escherichia coli strains for an adenylcyclase stimulating factor. J Med Microbiol. 1987 Jun;23(4):335–338. doi: 10.1099/00222615-23-4-335. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Bergquist E. J., Nalin D. R., Waterman D. H., Hornick R. B., Young C. R., Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978 May 27;1(8074):1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
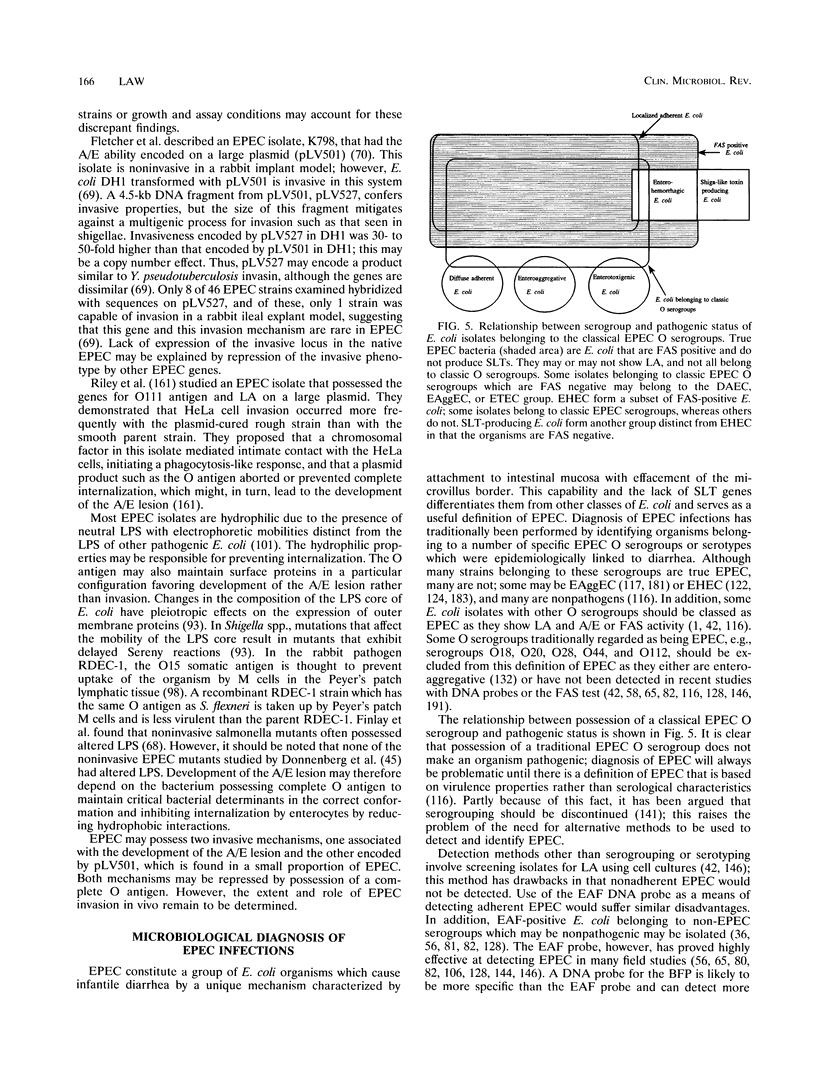
- Levine M. M., Prado V., Robins-Browne R., Lior H., Kaper J. B., Moseley S. L., Gicquelais K., Nataro J. P., Vial P., Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988 Jul;158(1):224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- Long-Krug S. A., Weikel C. S., Tiemens K. T., Hewlett E. L., Levine M. M., Guerrant R. L. Does enteropathogenic Escherichia coli produce heat-labile enterotoxin, heat-stable enterotoxins a or b, or cholera toxin A subunits? Infect Immun. 1984 Nov;46(2):612–614. doi: 10.1128/iai.46.2.612-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarrez-Hernandez H. A., Amess B., Sellers L., Baldwin T. J., Knutton S., Williams P. H., Aitken A. Purification of a 20 kDa phosphoprotein from epithelial cells and identification as a myosin light chain. Phosphorylation induced by enteropathogenic Escherichia coli and phorbol ester. FEBS Lett. 1991 Nov 4;292(1-2):121–127. doi: 10.1016/0014-5793(91)80848-w. [DOI] [PubMed] [Google Scholar]
- Manjarrez-Hernandez H. A., Baldwin T. J., Aitken A., Knutton S., Williams P. H. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoea. Lancet. 1992 Feb 29;339(8792):521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- Marques L. R., Moore M. A., Wells J. G., Wachsmuth I. K., O'Brien A. D. Production of Shiga-like toxin by Escherichia coli. J Infect Dis. 1986 Aug;154(2):338–341. doi: 10.1093/infdis/154.2.338. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Cravioto A. HEp-2 cell adherence as an assay for virulence among diarrheagenic Escherichia coli. J Infect Dis. 1989 Jun;159(6):1057–1060. doi: 10.1093/infdis/159.6.1057. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Johnson P. C., DuPont H. L., Morgan D. R., Thornton S. A., Wood L. V., Ericsson C. D. A newly recognized cause of travelers' diarrhea: enteroadherent Escherichia coli. J Infect Dis. 1985 Mar;151(3):471–475. doi: 10.1093/infdis/151.3.471. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Johnson P. C., DuPont H. L., Satterwhite T. K., Winsor D. K. Pathogenicity of enteroadherent Escherichia coli in adult volunteers. J Infect Dis. 1986 Sep;154(3):524–527. doi: 10.1093/infdis/154.3.524. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Oberhelman R. A., Dupont H. L., Javier de la Cabada F., Garibay E. V. Enteroadherent Escherichia coli as a cause of diarrhea among children in Mexico. J Clin Microbiol. 1987 Oct;25(10):1917–1919. doi: 10.1128/jcm.25.10.1917-1919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M. M., Chart H., Scotland S. M., Smith H. R., Willshaw G. A., Rowe B. Properties of adherence factor plasmids of enteropathogenic Escherichia coli and the effect of host strain on expression of adherence to HEp-2 cells. J Gen Microbiol. 1989 May;135(5):1123–1134. doi: 10.1099/00221287-135-5-1123. [DOI] [PubMed] [Google Scholar]
- Miliotis M. D., Koornhof H. J., Phillips J. I. Invasive potential of noncytotoxic enteropathogenic Escherichia coli in an in vitro Henle 407 cell model. Infect Immun. 1989 Jul;57(7):1928–1935. doi: 10.1128/iai.57.7.1928-1935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. J., Rao G. G. Conventional screening for enteropathogenic Escherichia coli in the UK. Is it appropriate or necessary? J Hosp Infect. 1992 Jul;21(3):163–167. doi: 10.1016/0195-6701(92)90072-t. [DOI] [PubMed] [Google Scholar]
- Moyenuddin M., Wachsmuth I. K., Moseley S. L., Bopp C. A., Blake P. A. Serotype, antimicrobial resistance, and adherence properties of Escherichia coli strains associated with outbreaks of diarrheal illness in children in the United States. J Clin Microbiol. 1989 Oct;27(10):2234–2239. doi: 10.1128/jcm.27.10.2234-2239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubashir M., Khan A., Baqai R., Iqbal J., Ghafoor A., Zuberi S., Burney M. I. Causative agents of acute diarrhoea in the first 3 years of life: hospital-based study. J Gastroenterol Hepatol. 1990 May-Jun;5(3):264–270. doi: 10.1111/j.1440-1746.1990.tb01627.x. [DOI] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GINO R. M., GORZYNSKI E. A. Demonstration of antibodies against enteropathogenic Escherichia coli in sera of children of various ages. Pediatrics. 1955 Dec;16(6):801–808. [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Deng Y., Maneval D. R., German A. L., Martin W. C., Levine M. M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992 Jun;60(6):2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Maher K. O., Mackie P., Kaper J. B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987 Oct;55(10):2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Scaletsky I. C., Kaper J. B., Levine M. M., Trabulsi L. R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- Olukoya D. K., Daini O., Alabi S. A., Coker A. O., Odugbemi T., Akinrimisi E. O. Antimicrobial resistance patterns and plasmids of enteropathogenic Escherichia coli isolated in Nigeria. Eur J Epidemiol. 1988 Sep;4(3):306–309. doi: 10.1007/BF00148914. [DOI] [PubMed] [Google Scholar]
- Orskov F., Whittam T. S., Cravioto A., Orskov I. Clonal relationships among classic enteropathogenic Escherichia coli (EPEC) belong to different O groups. J Infect Dis. 1990 Jul;162(1):76–81. doi: 10.1093/infdis/162.1.76. [DOI] [PubMed] [Google Scholar]
- PAYNE A. M. M., COOK G. T. A specific serological type of Bact. coli found in infants' home in absence of epidemic diarrhoea. Br Med J. 1950 Jul 22;2(4672):192–195. doi: 10.1136/bmj.2.4672.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Ghose A. C. Identification of plasmid-encoded mannose-resistant hemagglutinin and HEp-2 and HeLa cell adherence factors of two diarrheagenic Escherichia coli strains belonging to an enteropathogenic serogroup. Infect Immun. 1990 Apr;58(4):1106–1113. doi: 10.1128/iai.58.4.1106-1113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky Y. E., Dragunskaya E. M., Seliverstova V. G., Avdeeva T. A., Chakhutinskaya M. G., Kétyi I., Vertényl A., Ralovich B., Emödy L., Málovics I. Pathogenic effect of enterotoxigenic Escherichia coli and Escherichia coli causing infantile diarrhoea. Acta Microbiol Acad Sci Hung. 1977;24(3):221–236. [PubMed] [Google Scholar]
- Rafiee P., Leffler H., Byrd J. C., Cassels F. J., Boedeker E. C., Kim Y. S. A sialoglycoprotein complex linked to the microvillus cytoskeleton acts as a receptor for pilus (AF/R1) mediated adhesion of enteropathogenic Escherichia coli (RDEC-1) in rabbit small intestine. J Cell Biol. 1991 Nov;115(4):1021–1029. doi: 10.1083/jcb.115.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regua A. H., Bravo V. L., Leal M. C., Lobo Leite M. E. Epidemiological survey of the enteropathogenic Escherichia coli isolated from children with diarrhoea. J Trop Pediatr. 1990 Aug;36(4):176–179. doi: 10.1093/tropej/36.4.176. [DOI] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Schoolnik G. K. HeLa cell invasion by a strain of enteropathogenic Escherichia coli that lacks the O-antigenic polysaccharide. Mol Microbiol. 1990 Oct;4(10):1661–1666. doi: 10.1111/j.1365-2958.1990.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Bennett-Wood V. Quantitative assessment of the ability of Escherichia coli to invade cultured animal cells. Microb Pathog. 1992 Feb;12(2):159–164. doi: 10.1016/0882-4010(92)90119-9. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Levine M. M., Rowe B., Gabriel E. M. Failure to detect conventional enterotoxins in classical enteropathogenic (serotyped) Escherichia coli strains of proven pathogenicity. Infect Immun. 1982 Nov;38(2):798–801. doi: 10.1128/iai.38.2.798-801.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Yam W. C., O'Gorman L. E., Bettelheim K. A. Examination of archetypal strains of enteropathogenic Escherichia coli for properties associated with bacterial virulence. J Med Microbiol. 1993 Mar;38(3):222–226. doi: 10.1099/00222615-38-3-222. [DOI] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Duronio V., Finlay B. B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992 Jun;60(6):2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum R. J., Partin J. C., Saalfield K., McAdams A. J. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983 Jun;4(4):291–304. doi: 10.3109/01913128309140582. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Rowe B., Gross R. J., Scotland S. M. Letter: Serotyping of enteropathogenic Escherichia coli. Lancet. 1975 Nov 8;2(7941):925–926. doi: 10.1016/s0140-6736(75)92160-1. [DOI] [PubMed] [Google Scholar]
- Rowe B. The role of Escherichia coli in gastroenteritis. Clin Gastroenterol. 1979 Sep;8(3):625–644. [PubMed] [Google Scholar]
- Sack R. B. Letter: Serotyping of E. coli. Lancet. 1976 May 22;1(7969):1132–1132. [PubMed] [Google Scholar]
- Savarino S. J., Fasano A., Robertson D. C., Levine M. M. Enteroaggregative Escherichia coli elaborate a heat-stable enterotoxin demonstrable in an in vitro rabbit intestinal model. J Clin Invest. 1991 Apr;87(4):1450–1455. doi: 10.1172/JCI115151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Milani S. R., Trabulsi L. R., Travassos L. R. Isolation and characterization of the localized adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1988 Nov;56(11):2979–2983. doi: 10.1128/iai.56.11.2979-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer D. B., Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993 Jun;61(6):2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Rowe B. Escherichia coli O128 strains from infants with diarrhea commonly show localized adhesion and positivity in the fluorescent-actin staining test but do not hybridize with an enteropathogenic E. coli adherence factor probe. Infect Immun. 1991 Apr;59(4):1569–1571. doi: 10.1128/iai.59.4.1569-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Said B., Willshaw G. A., Cheasty T., Rowe B. Identification of enteropathogenic Escherichia coli isolated in Britain as enteroaggregative or as members of a subclass of attaching-and-effacing E. coli not hybridising with the EPEC adherence-factor probe. J Med Microbiol. 1991 Nov;35(5):278–283. doi: 10.1099/00222615-35-5-278. [DOI] [PubMed] [Google Scholar]
- Scotland S. M., Willshaw G. A., Smith H. R., Gross R. J., Rowe B. Adhesion to cells in culture and plasmid profiles of enteropathogenic Escherichia coli isolated from outbreaks and sporadic cases of infant diarrhoea. J Infect. 1989 Nov;19(3):237–249. doi: 10.1016/s0163-4453(89)90729-9. [DOI] [PubMed] [Google Scholar]
- Scotland S. M., Willshaw G. A., Smith H. R., Rowe B. Properties of strains of Escherichia coli O26:H11 in relation to their enteropathogenic or enterohemorrhagic classification. J Infect Dis. 1990 Nov;162(5):1069–1074. doi: 10.1093/infdis/162.5.1069. [DOI] [PubMed] [Google Scholar]
- Senerwa D., Mutanda L. N., Gathuma J. M., Olsvik O. Antimicrobial resistance of enteropathogenic Escherichia coli strains from a nosocomial outbreak in Kenya. APMIS. 1991 Aug;99(8):728–734. [PubMed] [Google Scholar]
- Senerwa D., Olsvik O., Mutanda L. N., Lindqvist K. J., Gathuma J. M., Fossum K., Wachsmuth K. Enteropathogenic Escherichia coli serotype O111:HNT isolated from preterm neonates in Nairobi, Kenya. J Clin Microbiol. 1989 Jun;27(6):1307–1311. doi: 10.1128/jcm.27.6.1307-1311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff M., Bhan M. K., Knutton S., Das B. K., Saini S., Kumar R. Evaluation of the fluorescence actin staining test for detection of enteropathogenic Escherichia coli. J Clin Microbiol. 1993 Feb;31(2):386–389. doi: 10.1128/jcm.31.2.386-389.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Cockerill F., 3rd, Soni R., Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991 Mar;59(3):890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Drumm B., Karmali M., Cutz E. Adherence of bacteria to the intestine in sporadic cases of enteropathogenic Escherichia coli-associated diarrhea in infants and young children: a prospective study. Gastroenterology. 1989 Jan;96(1):86–94. doi: 10.1016/0016-5085(89)90768-3. [DOI] [PubMed] [Google Scholar]
- Sjogren R. W., Sherman P. M., Boedeker E. C. Altered intestinal motility precedes diarrhea during Escherichia coli enteric infection. Am J Physiol. 1989 Nov;257(5 Pt 1):G725–G731. doi: 10.1152/ajpgi.1989.257.5.G725. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Scotland S. M., Stokes N., Rowe B. Examination of strains belonging to enteropathogenic Escherichia coli serogroups for genes encoding EPEC adherence factor and Vero cytotoxins. J Med Microbiol. 1990 Apr;31(4):235–240. doi: 10.1099/00222615-31-4-235. [DOI] [PubMed] [Google Scholar]
- Sohel I., Puente J. L., Murray W. J., Vuopio-Varkila J., Schoolnik G. K. Cloning and characterization of the bundle-forming pilin gene of enteropathogenic Escherichia coli and its distribution in Salmonella serotypes. Mol Microbiol. 1993 Feb;7(4):563–575. doi: 10.1111/j.1365-2958.1993.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Jones E. W., Corley L. D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969 Sep;56(3):371–392. [PMC free article] [PubMed] [Google Scholar]
- Steele A. D., Geyer A., Alexander J. J., Crewe-Brown H. H., Fripp P. J. Enteropathogens isolated from children with gastro-enteritis at Ga-Rankuwa Hospital, South Africa. Ann Trop Paediatr. 1988 Dec;8(4):262–267. doi: 10.1080/02724936.1988.11748584. [DOI] [PubMed] [Google Scholar]
- Stenderup J., Orskov F. The clonal nature of enteropathogenic Escherichia coli strains. J Infect Dis. 1983 Dec;148(6):1019–1024. doi: 10.1093/infdis/148.6.1019. [DOI] [PubMed] [Google Scholar]
- THOMSON S. The role of certain varieties of Bacterium coli in gastro-enteritis of babies. J Hyg (Lond) 1955 Sep;53(3):357–367. doi: 10.1017/s002217240000084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Moseley S. L., Kay B., Losonsky G., Levine M. M. Challenge studies in volunteers using Escherichia coli strains with diffuse adherence to HEp-2 cells. J Infect Dis. 1990 Aug;162(2):550–552. doi: 10.1093/infdis/162.2.550. [DOI] [PubMed] [Google Scholar]
- Tai Y. H., Gage T. P., McQueen C., Formal S. B., Boedeker E. C. Electrolyte transport in rabbit cecum. I. Effect of RDEC-1 infection. Am J Physiol. 1989 Apr;256(4 Pt 1):G721–G726. doi: 10.1152/ajpgi.1989.256.4.G721. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Hart A., Batt R. M., McDougall C., McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (0111) infection. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- Thorén A. The role of enteropathogenic E. coli in infantile diarrhoea. Aspects on bacteriology, epidemiology and therapy. Scand J Infect Dis Suppl. 1983;37:1–51. doi: 10.3109/inf.1982.14.suppl-37.01. [DOI] [PubMed] [Google Scholar]
- Toledo M. R., Alvariza M. do C., Murahovschi J., Ramos S. R., Trabulsi L. R. Enteropathogenic Escherichia coli serotypes and endemic diarrhea in infants. Infect Immun. 1983 Feb;39(2):586–589. doi: 10.1128/iai.39.2.586-589.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I., Cohen M. L., Rumschlag H. S., Riley L. W., White E. H., Carr J. H., Bond W. W., Wachsmuth I. K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990 May;58(5):1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Gibson R., Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989 Apr;57(4):1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Robins-Browne R. M., Gonis G., Hayes J., Withers M., McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985 Jun;26(6):570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Valentini S. R., Gomes T. A., Falcão D. P. Lack of virulence factors in Escherichia coli strains of enteropathogenic serogroups isolated from water. Appl Environ Microbiol. 1992 Jan;58(1):412–414. doi: 10.1128/aem.58.1.412-414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial P. A., Mathewson J. J., DuPont H. L., Guers L., Levine M. M. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J Clin Microbiol. 1990 May;28(5):882–885. doi: 10.1128/jcm.28.5.882-885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial P. A., Robins-Browne R., Lior H., Prado V., Kaper J. B., Nataro J. P., Maneval D., Elsayed A., Levine M. M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988 Jul;158(1):70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- Vuopio-Varkila J., Schoolnik G. K. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991 Nov 1;174(5):1167–1177. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENTWORTH F. H., BROCK D. W., STULBERG C. S., PAGE R. H. Clinical, bacteriological, and serological observations of two human volunteers following ingestion of Escherichia coli O127:B8. Proc Soc Exp Biol Med. 1956 Apr;91(4):586–588. doi: 10.3181/00379727-91-22338. [DOI] [PubMed] [Google Scholar]
- Wade W. G., Thom B. T., Evans N. Cytotoxic enteropathogenic Escherichia coli. Lancet. 1979 Dec 8;2(8154):1235–1236. doi: 10.1016/s0140-6736(79)92349-3. [DOI] [PubMed] [Google Scholar]
- Wilkins E. G., Roberts C. Screening for enteropathogenic Escherichia coli. J Hosp Infect. 1988 Oct;12(3):177–182. doi: 10.1016/0195-6701(88)90004-7. [DOI] [PubMed] [Google Scholar]
- Willshaw G. A., Scotland S. M., Smith H. R., Rowe B. Properties of Vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J Infect Dis. 1992 Oct;166(4):797–802. doi: 10.1093/infdis/166.4.797. [DOI] [PubMed] [Google Scholar]
- Yakubu D. E., Old D. C., Tavendale A. Production of a mannose-resistant fibrillar haemagglutinin by strains of Escherichia coli of EPEC serotype O111:H12. FEMS Microbiol Lett. 1991 Jun 15;65(2):233–237. doi: 10.1016/0378-1097(91)90308-w. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Koyama Y., Matsumoto M., Sonoda E., Nakayama S., Uchimura M., Paveenkittiporn W., Tamura K., Yokota T., Echeverria P. Localized, aggregative, and diffuse adherence to HeLa cells, plastic, and human small intestines by Escherichia coli isolated from patients with diarrhea. J Infect Dis. 1992 Dec;166(6):1295–1310. doi: 10.1093/infdis/166.6.1295. [DOI] [PubMed] [Google Scholar]
- Yu J., Kaper J. B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992 Feb;6(3):411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]
- da Silva M. L., Mortara R. A., Barros H. C., de Souza W., Trabulsi L. R. Aggregation of membrane-associated actin filaments following localized adherence of enteropathogenic Escherichia coli to HeLa cells. J Cell Sci. 1989 Jul;93(Pt 3):439–446. doi: 10.1242/jcs.93.3.439. [DOI] [PubMed] [Google Scholar]