Abstract
The use of non-ejaculated sperm coupled with intracytoplasmic sperm injection has become a globally established procedure for couples with azoospermic male partners who wish to have biological offspring. Surgical methods have been developed to retrieve spermatozoa from the epididymides and the testes of such patients. This article reviews the methods currently available for sperm acquisition in azoospermia, with a particular focus on the perioperative, anesthetic and technical aspects of these procedures. A critical analysis of the advantages and disadvantages of these sperm retrieval methods is provided, including the authors' methods of choice and anesthesia preferences.
Keywords: Sperm Retrieval, Assisted Reproductive Techniques, Male Infertility, Azoospermia, Anesthesia, Review
INTRODUCTION
Sperm retrieval techniques (SRTs) are surgical methods that have been developed to obtain spermatozoa from the epididymides and testicles of azoospermic men seeking fertility treatment (1). After sperm acquisition, intracytoplasmic sperm injection (ICSI) is used instead of standard in vitro fertilization (IVF) because ICSI has been shown to result in a significantly higher fertilization rate (2). Alternatively, the retrieved sperm can be cryopreserved for use in future sperm injection attempts (3,4). The use of non-ejaculated sperm and ICSI has become an established procedure for couples whose male partner has azoospermia to obtain biological offspring (5-7).
The method of choice for sperm retrieval (SR) is based on the type of azoospermia, which can be obstructive or non-obstructive, and the attending surgeon's preferences and experience. Obstructive azoospermia (OA) is associated with the inability to detect spermatozoa in the ejaculate and post-ejaculate urine after centrifugation due to the bilateral obstruction of the seminal ducts (8,9). Obstruction of the male reproductive system can be congenital or acquired. Microsurgical ductal reconstruction is generally considered to be a cost-effective treatment that allows for natural conception in selected cases of OA, such as post-vasectomy (10). Despite being highly successful, ductal recanalization may not be an option for some infertile couple or may be impossible in certain cases of congenital obstructions and post-infectious obstruction or failed vasectomy reversals. Spermatozoa can be retrieved from the epididymides or testicles in almost all cases of OA, irrespective of the technique used for sperm collection and the cause of obstruction. Non-obstructive azoospermia (NOA), on the other hand, is a consequence of spermatogenic failure and is the cause of most cases of azoospermia (8). NOA has congenital and acquired etiologies other than hypothalamic-pituitary disease and obstruction of the male genital tract. Unlike men with OA, men with NOA have no treatment options other than attempting testicular sperm retrieval. In such cases, spermatogenesis may be focal, which means that spermatozoa can be found and used for ICSI in approximately 30-60% of men with NOA (1). Testicular sperm extraction (TESE) is the technique of choice for NOA (1,11), and the use of microsurgery for TESE seems to increase retrieval rates (1,12).
Three main goals should be accomplished during sperm retrieval: (i) the acquisition of an adequate number of sperm for both immediate use and cryopreservation, (ii) the retrieval of the highest quality of sperm, and (iii) minimizing the damage to the reproductive tract, thus preserving the option of future retrieval attempts and testicular function (13). A list of the candidates eligible for sperm retrieval is provided in Table 1).
Table 1.
Candidates for sperm retrieval, grouped according to the type and etiology of azoospermia.
| Obstructive Azoospermia | Non-obstructive Azoospermia (Testicular Failure) |
| Congenital Ductal Obstructions: | Congenital Testicular Failure: |
| Congenital bilateral absence of the vas deferens | Testicular dysgenesis/cryptorchidism |
| Young's syndrome (clinical triad of chronic sinusitis, bronchiectasis, and obstructive azoospermia) | Genetic abnormalities (Klinefelter syndrome, Y chromosome microdeletions*) |
| Stenosis or atresia of the ejaculatory ducts | Germ cell aplasia (Sertoli cell-only syndrome) |
| Midline prostatic cysts (utricular and Müllerian cysts) | Spermatogenic (maturation) arrest |
| Ejaculatory duct cysts | |
| Seminal vesicle cysts | |
| Acquired Ductal Obstructions: | Acquired Testicular Failure: |
| Post-infection (epididymitis, prostatitis, seminal vesiculitis) | Testicular trauma |
| Testicular torsion | |
| Post-vasectomy | Post-inflammatory (e.g., mumps orchitis) |
| Post-surgical (epididymal cysts, hernia repair, scrotal surgery, bladder neck surgery, prostatectomy) | Exogenous factors (steroid medications, cytotoxic drugs, irradiation, heat) |
| Iatrogenic (urologic endoscopic instrumentation) | Systemic diseases (liver cirrhosis, renal failure) |
| Testicular tumor | |
| Varicocele | |
| Post-surgical (surgeries that may compromise testicular vascularization, resulting in testicular atrophy) | |
| Idiopathic: | Idiopathic (unknown etiology) |
| Idiopathic epididymal obstruction |
The likelihood of obtaining sperm at sperm retrieval is virtually zero when complete AZFa and/or AZFb Yq microdeletions are found.
The aim of this review is to update readers on the methods currently available for sperm acquisition in azoospermia, focusing in particular on the operative and technical aspects of these procedures. Moreover, a critical expert analysis of the advantages and disadvantages of the sperm acquisition methods is provided, including the authors' methods of choice and anesthesia preferences.
SPERM RETRIEVAL: AVAILABLE METHODS AND TECHNICAL ASPECTS
The two general SR methods are open surgery and percutaneous acquisition. Open surgery can be performed to retrieve spermatozoa from the epididymis or the testicle with or without microsurgery. Percutaneous retrievals, on the other hand, require a needle to be percutaneously inserted into the sperm source, i.e., the epididymis or the testicle. Irrespective of the method used, the goal of SR is to obtain the epididymal fluid or the seminiferous tubules and their contents. Table 2) lists the SR options available and their indications. Table 3) compares the advantages and disadvantages of the different SR methods.
Table 2.
Sperm retrieval techniques, acronyms and indications.
| Technique | Acronym | Indications |
| Percutaneous epididymal sperm aspiration | PESA | Obstructive azoospermia |
| Microsurgical epididymal sperm aspiration | MESA | Obstructive azoospermia |
| Open epididymal fine-needle aspiration | ND | Obstructive azoospermia |
| Percutaneous testicular sperm aspiration; percutaneous testicular fine-needle aspiration | TESA; TEFNA | Obstructive azoospermia; |
| Failed epididymal retrieval in OA cases; | ||
| Epididymal agenesis in CAVD cases; | ||
| Favorable testicular histopathology1 in NOA cases; | ||
| Previous successful TESA/TEFNA attempt in NOA cases | ||
| Testicular sperm extraction (single or multiple biopsies) | TESE | Obstructive azoospermia; |
| Failed epididymal retrieval in OA cases; | ||
| Failed TESA/TEFNA in OA cases; | ||
| Non-obstructive azoospermia | ||
| Single seminiferous tubule biopsy | ND | Obstructive azoospermia; |
| Failed epididymal retrieval in OA cases; | ||
| Failed TESA/TEFNA in OA cases; | ||
| Non-obstructive azoospermia | ||
| Microsurgical testicular sperm extraction | Micro-TESE | Non-obstructive azoospermia |
OA: obstructive azoospermia; NOA: non-obstructive azoospermia. CAVD: congenital absence of the vas deferens. ND: not defined. 1Hypospermatogenesis.
Table 3.
Advantages and disadvantages of sperm retrieval techniques.
| Advantages | Disadvantages | |
| PESA | Fast and low cost; | Few sperm retrieved; |
| Minimal morbidity, repeatable; | Limited number of sperm for cryopreservation; | |
| No microsurgical expertise required; | Fibrosis and obstruction at the aspiration site; | |
| Few instruments and materials; | Risk of hematoma/spermatocele | |
| No open surgical exploration | ||
| Open epididymal fine-needle aspiration | Repeatable; | Open surgical exploration required; |
| No microsurgical expertise required; | Increased cost and time-demanding; | |
| Relatively large number of sperm for cryopreservation; | Fibrosis and obstruction at the aspiration site; | |
| Few instruments and materials | Postoperative discomfort; | |
| Not validated in a large series of patients | ||
| MESA | Large number of sperm retrieved; | Open surgical exploration required; |
| High number of sperm for cryopreservation; | Increased cost and time-demanding; | |
| Reduced risk of hematoma; | Operating microscope required; | |
| Reconstruction possible1 | Microsurgical instruments and expertise required; | |
| Postoperative discomfort | ||
| TESA | Fast and low cost; | Relatively low success rate in NOA cases; |
| Repeatable; | Few sperm retrieved in NOA cases; | |
| No open surgical exploration; | Limited number of sperm for cryopreservation; | |
| No microsurgical expertise required; | Risk of hematoma/testicular atrophy | |
| Few instruments and materials; | ||
| Minimal/mild postoperative discomfort | ||
| TEFNA | Fast and low cost; | Few sperm retrieved in NOA cases; |
| Repeatable; | Limited number of sperm for cryopreservation; | |
| No open surgical exploration; | Risk of hematoma/testicular atrophy; | |
| No microsurgical expertise required; | Not validated in a large series of patients | |
| Few instruments and materials required; | ||
| Minimal/mild postoperative discomfort | ||
| TESE | No microsurgical expertise required; Repeatable | Increased cost and time-demanding; |
| Open surgical exploration required;Relatively few sperm retrieved in NOA cases; | ||
| Risk of testicular atrophy3; | ||
| Risk of testicular androgen production impairment3; | ||
| Postoperative discomfort | ||
| Single seminiferous tubule biopsy | No microsurgical expertise required; | Increased cost and time-demanding; |
| Repeatable | Open surgical exploration required; | |
| Relatively few sperm retrieved in NOA; | ||
| Postoperative discomfort; | ||
| Not validated in a large series of patients | ||
| Micro-TESE | Higher success rates in NOA cases2; | Surgical exploration required; |
| Larger number of sperm retrieved2; | Increased cost and time-demanding; | |
| Relatively higher chance of sperm cryopreservation2; | Operating microscope required; | |
| Low risk of complications | Microsurgical instruments and expertise required; | |
| Postoperative discomfort |
PESA: percutaneous epididymal sperm aspiration; MESA: microsurgical epididymal sperm. aspiration; TESA: percutaneous testicular sperm aspiration; TESE: conventional testicular sperm extraction; micro-TESE: microsurgical testicular sperm extraction. 1in cases of post-vasectomy obstructions. 2compared with TESA and TESE in NOA cases. 3multiple biopsy-TESE.
Preoperative Considerations
The procedure, results, and potential complications should be reviewed and discussed with the patient and his spouse by experienced staff. The patient should sign an informed consent form prior to surgery and be instructed that someone should accompany him if SR is to be performed on an outpatient basis. In addition, aspirin and/or nonsteroidal anti-inflammatory drugs should be avoided for one week before surgery. Those patients taking anti-coagulating agents should discontinue the medication during the preoperative period. Scrotal hair shaving is required for open retrievals, and patients should be instructed to void the bladder prior to admission to the operating room.
Operating Room and Patient Preparation
All of the instruments and materials used during the sperm retrieval procedure should be assessed for availability and/or operational conditions. For open procedures, a grounding pad should be available to allow the safe use of electrocautery. Ideally, an operating table with motorized control should be available for open procedures. The patient should be positioned on the operating table in a supine position. For microsurgical techniques, the operating microscope should be positioned and adjusted. The skin should be cleansed from mid-abdomen to mid-thigh using a povidone-iodine or similar solution. The surgical staff should scrub and gown properly. Sterile drapes should be positioned in a manner such that only the scrotum is exposed. A list of instruments and materials that are commonly used in sperm retrievals is provided in Table 4).
Table 4.
Materials and instruments commonly used in sperm retrieval techniques.
| Sperm retrieval method | Equipment and Supplies |
| All | Basic instruments and materials: |
| •Unipolar coagulating generator (open retrievals) | |
| •Bipolar coagulating generator (MESA and micro-TESE) | |
| •Antiseptic solution for skin cleaning | |
| •30-cc 1% xylocaine solution (spermatic cord anesthesia) | |
| •19- (40×12) and 22- (25×7) gauge hypodermic needles (spermatic cord anesthesia) | |
| •Sterile towels | |
| •Gauze sponges | |
| •Sterile gowns | |
| •Surgical gloves | |
| •Surgical drapes | |
| •Surgery instrument table (optional) | |
| •Mayo table | |
| •Sterile drapes for tables | |
| •20-cc syringes (spermatic cord anesthesia) | |
| •Saline solution for irrigation (MESA and micro-TESE) | |
| •Unipolar cautery pen (MESA and micro-TESE; optional) | |
| PESA, TESA and TEFNA | •Sharp-beveled fine needle (19-, 22-, 23- or 26-gauge, depending on the surgeon's preference and technique) attached to a 1-mL tuberculin syringe (PESA) or to a 10- or 20-mL syringe coupled to a Cameco (or similar) syringe holder |
| •Tissue-cutting biopsy needle (e.g., Tru-cut™ needle or Biopty™ gun; optional) | |
| TESE, micro-TESE, MESA, Open epididymal fine-needle aspiration, Single seminiferous tubule biopsy | Non-microsurgical set:•Basic set of surgical instruments for delicate surgeries (including small needle holder, small smooth and toothed forceps (Addison forceps), small suture scissors, small curved dissection scissors, a pair of small farabeuf retractors, scalpels, curved kelly clamps, straight mosquito clamps, backhaus clamps)•Sutures (e.g., 4-0 vicryl with tapered needle, 4-0 catgut with tapered needle, 5-0 black monofilament nylon with tapered cut needle (micro-TESE), 9-0 black monofilament nylon with tapered needle (MESA) |
| Micro-TESE and MESA | Microsurgical Set |
| •Straight non-toothed fine-tip forceps (13.5-cm long) | |
| •Curved non-toothed fine-tip forceps (13.5 cm long) | |
| •Non-locking needle holder with a rounded, finely curved tip | |
| •Pair of straight or curved blunt dissecting scissors | |
| •Bipolar cautery with fine-tipped forceps | |
| •Small retractor | |
| •Blunt, long and rounded irrigating needle | |
| •Microsurgical scalpel | |
| •Autoclavable case | |
| •Silicone tubing for protecting instrument tips | |
| Micro-TESE and MESA | Operating Microscope: |
| •Operating microscope equipped with 200-, 300- and 350-mm objective lenses and motorized operated zoom system•Note: The optical, mechanical and electrical microscope components should be checked before surgery to ensure that the operational conditions are adequate. A spare lamp should be readily available. A sterile microscope cover and/or handles should be available to allow for microscope adjustments during surgery. | |
| All | Reagents and Laboratory Supplies: |
| •Sperm culture media (kept at 37 °C) | |
| •6-mL sterile centrifuge polystyrene tubes with caps | |
| •60×15-mm center-well Petri dishes (micro-TESE) |
PESA: percutaneous epididymal sperm aspiration; TESA: testicular sperm aspiration; MESA: microsurgical epididymal sperm aspiration; TESE: testicular sperm extraction; micro-TESE: microdissection testicular sperm extraction.
Anesthesia
Sperm retrievals are relatively simple surgeries that can be safely performed with general anesthesia or spinal blocks. However, because sperm retrievals are typically outpatient procedures, the latest trend is to employ local or locoregional anesthesia with or without intravenous sedation. A review of the anesthesia techniques used for SR is provided in a separate section below.
Conventional Open Sperm Retrieval Methods
Open surgical SR can be used for both epididymal and testicular sperm collection. In both cases, a scrotal incision is made to approach the epididymis or the testis. Testicular delivery to facilitate the exposure of the epididymis or testis is optional, as the procedures can be carried out without testis delivery using the “window” technique (14). In the open epididymal sperm aspiration, the goal is to puncture an epididymal tubule and aspirate the epididymal fluid using a needle. In the open testicular sperm extraction (TESE) procedure, either a large single biopsy or multiple biopsies are performed to obtain seminiferous tubules and their contents. In both cases, the retrieved spermatozoa can be used for fresh sperm injection or cryopreserved for a single or multiple subsequent ICSI attempts. Open epididymal sperm aspirations are only indicated in OA cases, whereas open testicular extractions can be used in both OA and selected NOA cases (Table 2).
Open Epididymal Fine-Needle Aspiration
The epididymis is exposed and a tubule is directly punctured through the tunica without any dissection (15). The epididymal fluid is aspirated using a 26-gauge needle; the epididymal fluid that continues to flow out of the punctured tubule upon needle withdrawal is also aspirated. The tubular opening is not closed. Epididymal fluid can be aspirated from different locations to maximize the number and quality of sperm retrieved. The procedure does not require special equipment or training, but it has not been validated in a large series of patients.
Testicular Sperm Extraction (TESE)
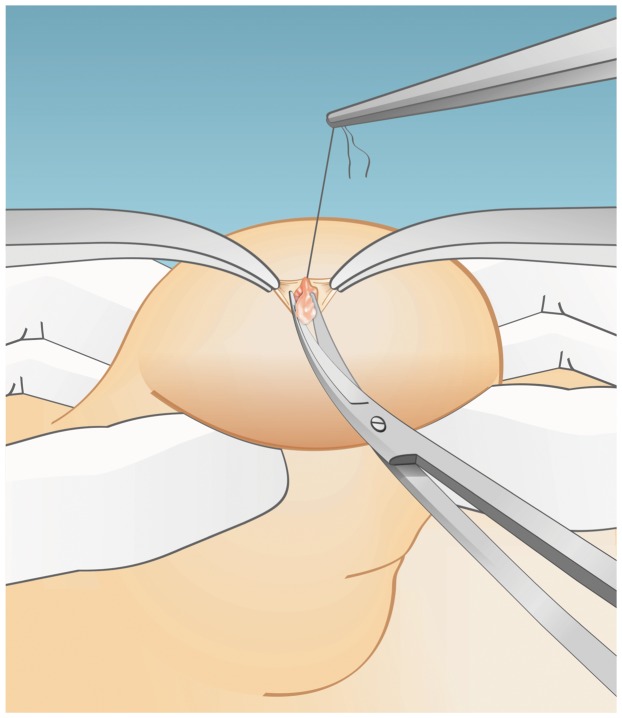
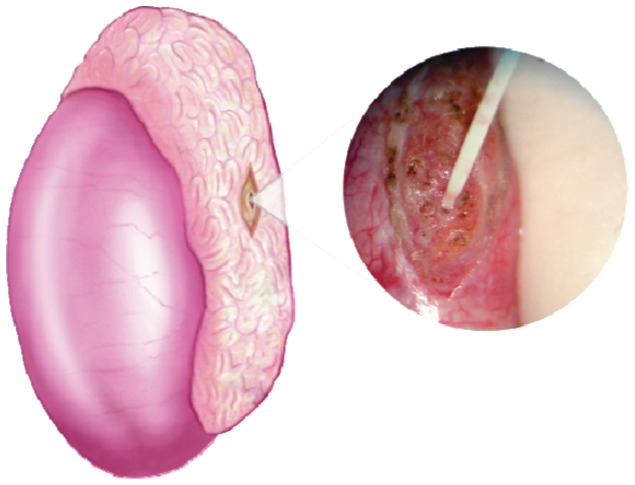
The extraction of the testicular parenchyma for sperm search and isolation was first described in 1995 (16). For conventional TESE, a standard open surgical biopsy technique is used to remove the testicular parenchyma without the aid of optical magnification. This procedure is usually carried out without delivering the testis (14). Briefly, a 2-cm transverse incision is made through the anterior scrotal skin, dartos and tunica vaginalis. A small self-retaining retractor can be used to ensure proper exposure of the tunica albuginea. A 1-cm incision is made in the albuginea, and gentle pressure is applied to the testis to aid the extrusion of the testicular parenchyma. A fragment of approximately 5×5 mm is excised with sharp scissors and placed in sperm culture media (Figure 1). Single or multiple specimens can be extracted from the same incision. Alternatively, individual albuginea incisions can be made in the upper, middle and lower testicular poles in an organized manner for the sampling of different areas. The testicular specimens are sent to the laboratory for processing and immediate microscopic examination. The tunica albuginea is closed with a running, non-absorbable suture.
Figure 1.
Conventional testicular sperm extraction (TESE). The illustration depicts TESE using a single open biopsy (see the text for a detailed description). Adapted from: Esteves SC, Agarwal A. Sperm retrieval techniques. In: Gardner DK, Rizk BRMB, Falcone T, Eds. Human assisted reproductive technology: future trends in laboratory and clinical practice. 1st. edition. Cambridge: Cambridge University Press 2011; pp. 41-53.
Single Seminiferous Tubule Biopsy
This technique is a variation of TESE. The scrotum is opened, and the testis is exposed. An avascular area of the tunica is punctured with a 26-gauge needle. A microforceps tip is used to dilate the puncture site, thus allowing a loop of seminiferous tubule to emerge (15). The seminiferous tubule is pulled out using the microforceps and sent for microscopic examination. If sperm are seen, additional tubule is pulled out from the same site. If no sperm are found or the tubule appears fibrous, the procedure is repeated in a different area. Multiple sites can be sampled until sperm are found or the entire testicular surface has been explored. Albuginea openings are not sutured because these openings are very small. Like the open epididymal FNA, single seminiferous tubule biopsy does not require special equipment or training but has not been validated in a large patient series.
Percutaneous Sperm Retrieval Methods
Since their description in 1994, the use of percutaneous approaches to retrieve sperm from the epididymis has gained popularity (1,5,17,18). Both the epididymal and testicular techniques share similar traits, as they require that a needle be percutaneously inserted into the sperm source (14). The goal of percutaneous epididymal sperm aspiration (PESA) is to obtain the epididymal fluid, which should contain sperm. In testicular sperm aspiration (TESA), the seminiferous tubules and their contents are removed. Percutaneous sperm retrieval may have either a diagnostic or a therapeutic role (19). Regarding the former role, the procedure confirms the presence of viable spermatozoa that can be cryopreserved for future use prior to ICSI. Regarding the latter role, the procedure is performed in conjunction with oocyte retrieval and permits the use of fresh sperm for sperm injections. In addition to offering a less invasive alternative for retrieving sperm, percutaneous techniques can generally be performed under local anesthesia on an outpatient basis. Percutaneous testicular retrievals are indicated in OA cases, as well as selected cases of NOA. In contrast, percutaneous epididymal retrievals are only recommended in OA cases (Table 2).
Percutaneous Epididymal Sperm Aspiration (PESA)
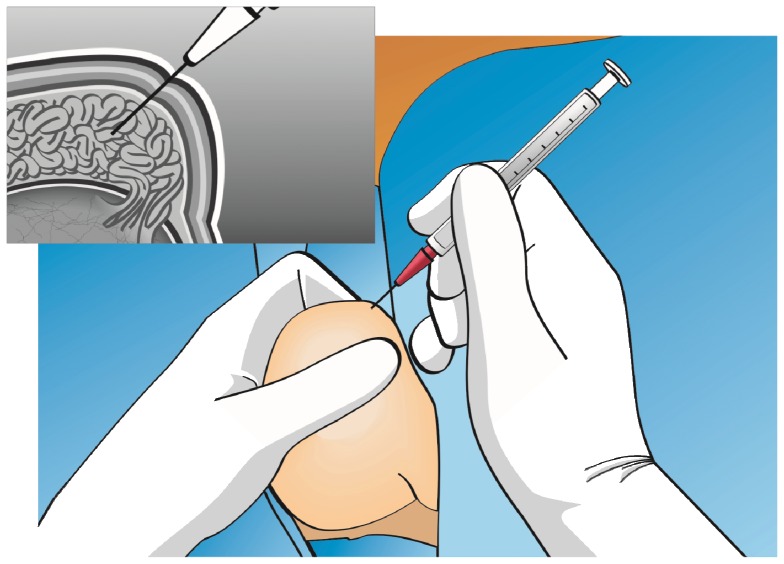
The technical procedure for percutaneous epididymal sperm aspiration involves the insertion of a needle attached to a syringe through the scrotal skin into the epididymis (Figure 2). Originally, the use of a larger butterfly needle was described (17). Currently, most experts use a fine needle (26 gauge) attached to a tuberculin syringe containing sperm washing medium (1,5,14). After creating negative pressure by pulling the syringe plunger, the tip of the needle is gently and slowly moved in and out inside the epididymis until fluid is aspirated. If motile sperm are not obtained, PESA may be repeated at a different site (from the cauda to caput epididymis) until an adequate number of motile sperm is retrieved. These aspirations are usually performed in the corpus epididymis and then in the caput epididymis if needed, as aspirates from the cauda are often rich in poor-quality senescent spermatozoa, debris and macrophages (13). Because PESA is a blind procedure, multiple attempts may be needed before high-quality sperm are found. If PESA fails to enable the retrieval of motile sperm, testicular sperm retrieval can be attempted during the same operation.
Figure 2.
Percutaneous epididymal sperm retrieval. The epididymis is stabilized between the index finger, thumb and forefinger. A needle attached to a tuberculin syringe is inserted into the epididymis through the scrotal skin, and fluid is aspirated (see the text for a detailed description).
Testicular Sperm Aspiration (TESA) and Testicular Fine-Needle Aspiration (TEFNA)
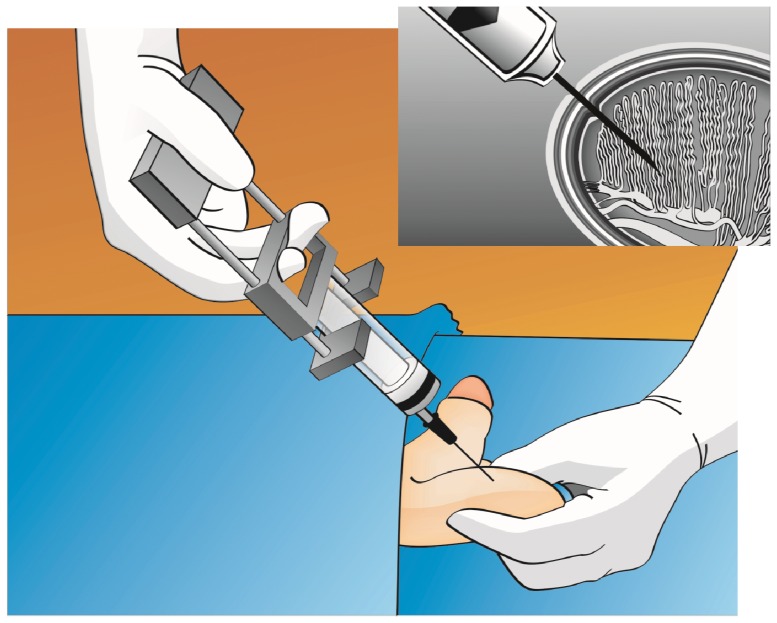
In TESA, a needle is inserted through the scrotal skin into the testis (Figure 3). The needle is usually inserted into the anteromedial or anterolateral portion of the superior testicular pole at an oblique angle toward the medium and lower poles. These areas are the least likely to contain major branches of the testicular artery running superficially underneath the tunica albuginea. These aspirations are usually carried out using either fine (testicular fine-needle aspiration; TEFNA) or large-diameter needles attached to a syringe. The testicular parenchyma is aspirated by creating negative pressure, and the specimen is sent to the laboratory for microscopic examination (Figure 4). TESA can be carried out in the contralateral testis if an insufficient number of or no sperm are obtained during the first attempt. Alternatively, testicular parenchyma can be obtained percutaneously using a tissue-cutting biopsy needle (e.g., a Tru-cut™ needle or Biopty™ gun). For this procedure, the needle is placed against the testis and, upon release of the springer, the needle enters the parenchyma, cuts a piece of tissue and withdraws it into a sheath (21).
Figure 3.
Percutaneous testicular sperm aspiration. A 20-mL needle syringe connected to a Cameco holder is percutaneously inserted into the testis. Negative pressure is created, and the tip of the needle is moved within the testis to disrupt the seminiferous tubules and sample different areas. The testicular parenchyma is aspirated (see the text for a detailed description).
Figure 4.
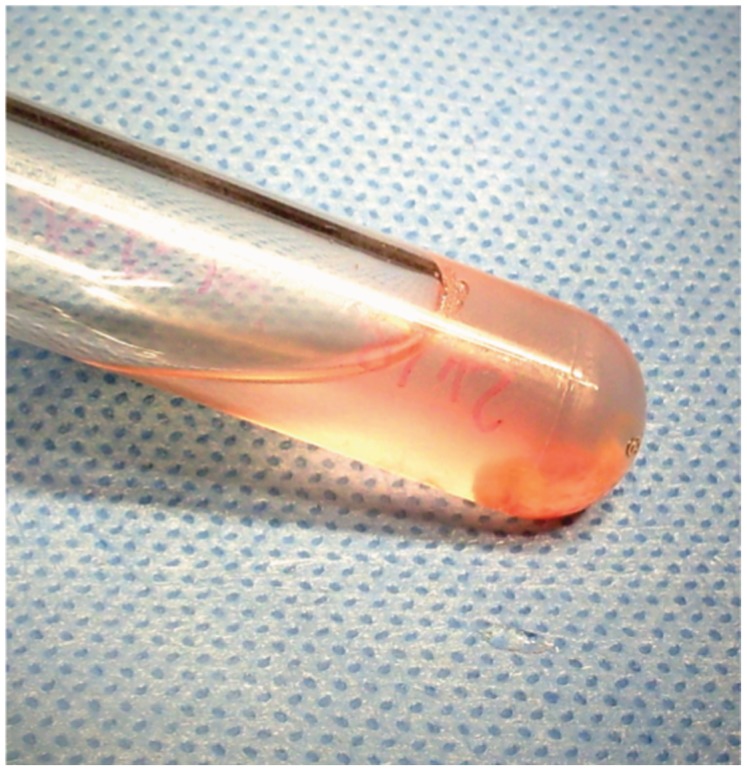
Photograph showing a tube containing one fragment of testicular tissue obtained by percutaneous testicular sperm aspiration (TESA). The fragment is immersed in sperm culture medium.
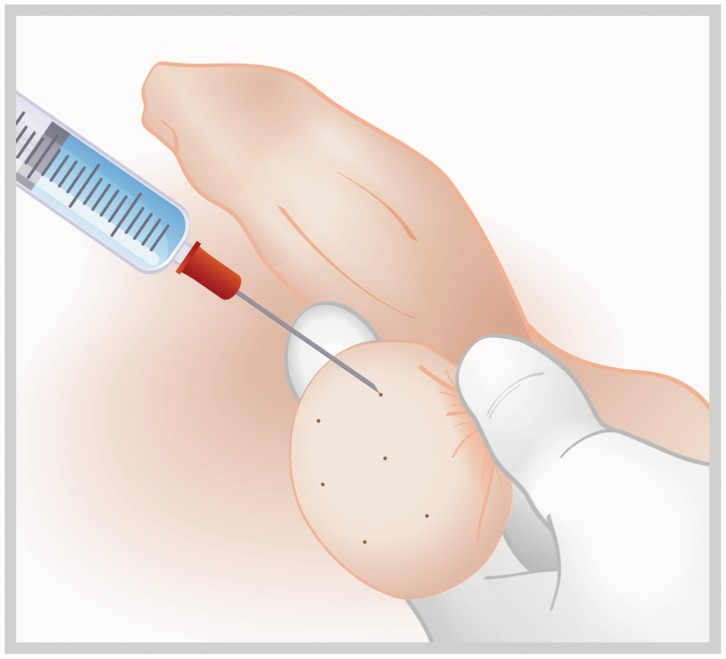
Turek et al. proposed the use of systematic fine-needle aspiration of the testis (FNA mapping) as a diagnostic tool in cases of non-obstructive azoospermia (22). However, testicular fine-needle aspiration (TEFNA) can also be applied for therapeutic sperm retrieval in cases of obstructive and non-obstructive azoospermia (Table 2). The concept behind FNA is to map the testicle to direct biopsies to pre-identified areas of sperm production, thus facilitating sperm retrieval in cases of non-obstructive azoospermia (NOA). Depending on the size of the testis, four to nine systematically placed aspiration sites are mapped (Figure 5). FNA mapping is performed with a sharp-beveled 23-gauge fine needle attached to a 10-mL syringe coupled with a Cameco syringe holder. Suction is applied, and the syringe holder is held steady as the needle is moved in and out within the testis with no change in direction. Twenty to 30 incursions are performed at a depth range of 8 to 12 mm. Suction is released before the needle is withdrawn from the testis. Tissue fragments are expelled from the needle onto a slide after air aspiration and fixed by immersion in 95% ethyl alcohol in the cases in which TEFNA is used for diagnostic purposes. In therapeutic SR, tissue fragments are expelled into pre-identified tubes containing sperm media.
Figure 5.
Testicular fine-needle aspiration (TEFNA). A 23-gauge fine needle attached to a 10-mL syringe coupled to a Cameco syringe holder is percutaneously inserted into the testicle to map different areas. Negative pressure is applied, and the needle is moved in and out within the testis with no change in direction. A tissue fragment from each mapped area is expelled into a pre-identified tube containing sperm culture medium.
Microsurgical Sperm Retrieval Methods
Microsurgical-guided sperm acquisition has been applied in both epididymal and testicular retrievals. The goal of microsurgical epididymal sperm aspiration (MESA) is to identify and open a single epididymal tubule to aspirate a sperm-rich, red blood cell-free fluid that can be used for fresh sperm injection or cryopreserved for a single or multiple later ICSI attempts. In microsurgical testicular sperm extraction (microdissection TESE; micro-TESE), the testicular parenchyma is dissected under magnification to search for enlarged seminiferous tubules, which are more likely to contain germ cells and foci of sperm production compared to non-enlarged or collapsed tubules. Such seminiferous tubules are removed rather than proceeding with the large single or multiple biopsies performed in conventional TESE. Microsurgical techniques and instruments, including an operating microscope, are used throughout both the MESA and micro-TESE procedures. MESA is indicated for cases of OA, whereas micro-TESE is recommended for the most severe cases of NOA (Table 2).
Microsurgical Epididymal Sperm Aspiration (MESA)
MESA was first described in 1985 (23). This surgical technique requires testis delivery through a 2-3-cm transverse scrotal incision. The epididymal tunica is incised, and an enlarged tubule is selected. Then, the epididymal tubule is dissected and opened with sharp microsurgical scissors. The fluid that flows out of the tubule is aspirated with the aid of a silicone tube or a needle attached to a tuberculin syringe (Figure 6). The aspirate is flushed into a tube containing warm sperm medium and is transferred to the laboratory for examination. MESA can be repeated at a different site on the same epididymis (from the cauda to caput regions) and/or the contralateral epididymis until an adequate number of motile sperm is retrieved (1,14). If MESA fails to retrieve motile sperm, TESA or TESE can be performed as part of the same procedure. However, MESA often provides enough sperm for cryopreservation. A single MESA procedure usually enables the retrieval of a large number of high-quality sperm that can be used for ICSI or intentionally cryopreserved for subsequent ICSI attempts (4,24).
Figure 6.
Microsurgical epididymal sperm aspiration (MESA). After exposure of the testis and epididymis, a dilated epididymal tubule is dissected and opened. The fluid is aspirated, diluted with sperm medium and sent to the laboratory for examination.
Microsurgical Testicular Sperm Extraction (micro-TESE)
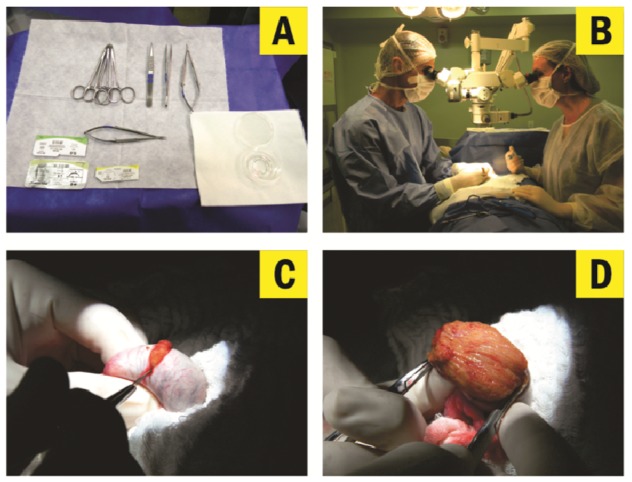
Microdissection testicular sperm extraction was originally described in 1999 in a successful combination of testicular sperm extraction with the assistance of an operating microscope (24). For micro-TESE, the scrotal skin is stretched over the anterior surface of the testis, after which a 2-3-cm transverse incision is made. Alternatively, a single midline scrotal incision can be used (25). The incision extends through the dartos muscle and the tunica vaginalis. The tunica is opened, and identifiable bleeders are cauterized. The testis is delivered extravaginally, and the tunica albuginea is examined. Then, a single, large, mid-portion incision is made in an avascular area of the tunica albuginea under 6-8× magnification, and the testicular parenchyma is widely exposed in its equatorial plane (Figure 7). The testicular parenchyma is dissected at 16-25× magnification to enable the search and isolation of seminiferous tubules that exhibit larger diameters (which are more likely to contain germ cells and eventually normal sperm production) in comparison to non-enlarged or collapsed counterparts (Figure 8). If needed, the superficial and deep testicular regions can be examined, and microsurgical-guided testicular biopsies are performed by carefully removing enlarged tubules using microsurgical forceps. If enlarged tubules are not observed, any tubule that differs from the remaining tubules in size is excised. The excised testicular tissue specimens are placed into the inner well of a Petri dish containing sperm media, and are sent to the laboratory for processing and sperm search (1,13,14) (Figure 9). The tunicas albuginea and vaginalis are then closed in a running fashion using non-absorbable and absorbable sutures. The dartos muscle is closed with interrupted absorbable sutures, respectively. Immediately prior to complete closure, 3 cc of 1% xylocaine solution may be injected into the subcuticular layers. The skin is closed using a continuous subcuticular 4-0 vicryl suture. A fluffy-type scrotal dressing and scrotal supporter are placed.
Figure 7.
Microdissection testicular sperm extraction (micro-TESE). Microsurgical techniques and instruments (A), including an operating microscope (B), are used throughout the procedure. After testis exteriorization, a single large incision is made in an avascular area of the albuginea (C), and the testicular parenchyma is widely exposed (D).
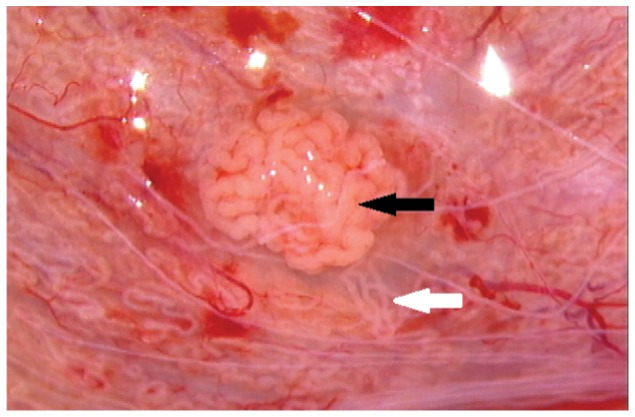
Figure 8.
Photograph showing the micro-TESE intraoperative aspect (25× magnification). The seminiferous tubules with enlarged diameters (black arrow) are likely to contain active spermatogenesis, while the thin tubules usually contain Sertoli cells only (white arrow).
Figure 9.
Photograph showing a petri dish (left) containing seminiferous tubules obtained by microdissection testicular sperm extraction (micro-TESE) immersed in sperm culture medium. The specimen is mechanically minced under stereomicroscopy to release the content of the seminiferous tubules (right).
ANESTHESIA FOR SPERM RETRIEVAL PROCEDURES
There are very few studies describing anesthesia techniques for SR. From the anatomy viewpoint, it is possible to provide efficient anesthesia simply by using local or locoregional anesthesia. However, most patients express great concern about the procedures, most likely because sperm retrievals are carried out in a very delicate part of the male body. For this and other reasons related to the high expectations associated with the procedure, patients undergoing SR have historically been very anxious on the day of surgery.
In a study of 34 patients undergoing PESA and/or TESA, spermatic cord block was performed with 10 mL of 1% lidocaine without epinephrine (27). The authors reported block failure in 6% of the cases, which required the combination of intravenous sedation, and two cases of vasovagal reflex, which required the use of atropine for reversal. They also stated that 16% of the patients reported moderate but tolerable pain. The results of the aforementioned study highlight the fact that the chosen anesthesia technique enabled SR to be performed; however, this method cannot be considered a good-quality anesthesia technique because it did not offer enough comfort to a large proportion of the group studied. Furthermore, 35% of the patients complained about being very anxious preoperatively, which shows that over a third of the patients could have benefited from the coadministration of locoregional anesthesia and sedation.
In another study of 26 patients undergoing MESA, only 38% of the patients tolerated the procedure solely under spermatic cord block through the infiltration of 5-8 mL of 1% lidocaine; the remaining 62% required intravenous sedation (28). The percentage of patients who underwent a bilateral procedure and required intravenous sedation was as high as 75%.
General anesthesia may offer comfort and the efficient management of anxiety. However, when performed with inhalational agents such as N2O and halogenated agents, this approach is associated with a high incidence of postoperative nausea and vomiting (29). These two complaints are among the most frequent causes of hospitalization and the inability to discharge patients scheduled for ambulatory procedures. Additionally, these symptoms are among the most feared by patients undergoing minor surgery, surpassing even postoperative pain (30).
The opposite effect occurs when employing propofol (2,6-diisopropylphenol), as this drug offers antiemetic effects (31). Propofol is a hypnotic intravenous drug that can be used both to induce and maintain general anesthesia and sedation. Moreover, propofol causes a gentle awakening compared with halogenated agents, as patients wake up with a feeling of well-being and a clear mental state. In addition, patients usually experience less postoperative confusion, recognizing the environment and the people around them more easily. Patients also tend to be more cooperative and show less agitation (32). Thus, patients undergoing general anesthesia combined with propofol have a lower incidence of postoperative complaints but still have a slower recovery compared with those receiving propofol alone for intravenous sedation.
The combination of intravenous sedation and local anesthesia offers patients the analgesic effectiveness of local anesthetics combined with the comfort and effective control of anxiety provided by intravenous sedation. When this combination includes propofol, patients experience great satisfaction, recovering quickly and with minimal adverse effects. These patients may also benefit from the advantages of outpatient procedures.
POSTOPERATIVE CARE AND COMPLICATIONS OF SPERM RETRIEVAL
The vast majority of SR procedures are performed on an outpatient basis, with patients usually being discharged 2-3 hours after surgery. Patients should be examined for a scrotal hematoma prior to discharge. A companion should be available, and under no circumstances should the patient be allowed to leave the heath care facility alone or drive if general anesthesia or intravenous sedation has been used. After percutaneous retrievals, patients often resume their normal activities on the following day. Bed rest and the application of an ice pack to the scrotum is recommended for the first 48 hours, especially following open retrievals. For these procedures, patients are instructed to remove the scrotal dressing after 24 hours and are encouraged to take warm showers and wash the incision area with soap and water after 24 hours. Oral analgesics and non-steroidal anti-inflammatory medications are routinely used for 3-5 days. Postoperative antibiotics are not routinely prescribed. Patients are instructed to resume a normal diet and increase their daily activities to a normal level over a 3- to 4-day period. The use of a scrotal supporter is strongly recommended for approximately one week after the procedure. The patient should abstain from sports activities, heavy lifting and sexual intercourse for approximately 10 days. Moreover, patients should be informed of the likelihood of scrotal swelling and ecchymosis at the wound site, as well as mild discomfort that should subside in approximately one week (14,19).
After SR, patients are advised to report any adverse signs and symptoms, including fever, persistent pain or swelling, bleeding or excessive fluid leakage from the wound. A scrotal ultrasound may be indicated in cases with complications. The determination of hormone levels, including total and free testosterone, FSH, LH, and estradiol, is recommended six months after open testicular retrievals.
The incidence of post-SR complications, including persistent pain, swelling, infection, hydrocele, and hematoma, ranges from 0-70% (33-41). The complication rates vary depending on the sperm retrieval technique. Percutaneous retrievals have an increased risk of hematoma compared with open techniques (5,37). Nevertheless, except for minor pain and local swelling, there have been no reports of clinically significant intra- or postoperative complications leading to medical treatment or hospital care when percutaneous techniques are used (1,38). Intratesticular hematomas have been observed on ultrasounds performed three months after surgery in most patients (up to 80%) who undergo TESE with single or multiple biopsies, but they often resolve spontaneously without compromising testicular function (35). Large-volume conventional TESE has been associated with a higher risk of a transient or even permanent decrease in serum testosterone levels due to testicular devascularization and excessive tissue removal (34,39). On the other hand, the incidence of complications is lower following micro-TESE compared to conventional TESE (11,25,34,36). In micro-TESE, the testicular vessels under the tunica albuginea are identified prior to the placement of an incision in the testis. In addition, the use of optical magnification and microsurgical techniques allows the preservation of the intratesticular blood supply (34). However, a significant decrease in serum testosterone has been documented following micro-TESE in men who already have diminished androgen production, such as Klinefelter syndrome patients (33). Nonetheless, testosterone levels return to pre-surgical values in most individuals within 12 months following surgery (39). In fact, Ramasamy et al. reported a return to 95% of the pre micro-TESE testosterone levels after 18 months (34). Given the potential serious postoperative complications of SR, it is recommended that these procedures be performed by surgeons who have specific training in the above-mentioned techniques (39).
EXPERT COMMENTARY
The literature is rich in studies focusing on different sperm retrieval methods. Both percutaneous and microsurgical methods have high success rates, in the range of 90-100%, for OA (37,38),. A series of studies on NOA has reported overall successful retrieval rates (SRRs) ranging from 30-60% (11-14,22,24,25,38),, which means that 30-60% of men with NOA have focal areas of sperm production within the testes.
In a recent study, we reported a cumulative success rate of 97.3% for percutaneous retrievals in OA cases (38). Epididymal sperm retrievals were successful in 78.0% of the cases, and subsequent attempts at testicular retrieval were successful in the vast majority of failed epididymal retrievals. We concluded that percutaneous SR was a reliable method for obtaining sperm for ICSI in OA. Our overall complication rate following percutaneous retrievals was 5.5%, and we noted that complications, albeit of minimal morbidity, occurred more often in the patients undergoing TESA compared with those undergoing PESA. For this reason, we use percutaneous methods for sperm acquisition in OA and preferentially use PESA over TESA. In our recent study, sperm cryopreservation was possible in one-third of the cases. Although increased cryopreservation rates have been reported for open SR, the associated costs of this procedure are significantly higher (5). Percutaneous approaches, on the other hand, can be performed under local anesthesia on an outpatient basis, and, if needed, repeat percutaneous procedures may result in successful SR (37). It is still a matter of debate whether percutaneous retrievals are more cost-effective than MESA. No study has yet compared the cumulative pregnancy rates after repeated cycles of percutaneous retrievals and ICSI with a single MESA attempt for intentional sperm cryopreservation coupled with multiple subsequent ICSI cycles. Nonetheless, the ICSI outcomes using frozen-thawed or fresh sperm retrieved from men with OA are comparable (51).
The testicular SRRs associated with the different etiological categories of non-obstructive azoospermia—namely, cryptorchidism, varicocele, orchitis, genetic, radio-/chemotherapy and idiopathic—are comparable (14,33),. The efficiency of sperm retrieval in NOA males varies depending on the method of sperm collection. The TESA retrieval rates range from 10-30% (11,35,39,46,47,56,57) except in the favorable cases of a previous successful TESA or a testicular histopathology showing hypospermatogenesis. In such cases, the TESA SRRs are greater than 60% (14,53). A recent meta-analysis reported a mean TESE SRR of 49.5% (11). TESE with multiple biopsies has a higher SRR than fine-needle aspiration (TEFNA), especially in cases of Sertoli cell-only (SCO) syndrome and maturation arrest (11). The reported micro-TESE retrieval rates range from 35-77% (5,12,14,25,33,36,41,47,49,51,54,55). Moreover, controlled studies demonstrated that micro-TESE performs better than conventional TESE or TESA (12),. Micro-TESE has been shown to minimize the damage to testicular tissue and maximize sperm recovery because the seminiferous tubules containing active foci of spermatogenesis can be better identified (34). Micro-TESE was shown to be particularly more effective than conventional TESE in recovering sperm from men with a testicular volume of less than 10 mL (42% vs. 27%) (58). It seems that the best chance of sperm recovery during micro-TESE is within the first 2 hours of the operation. However, more than four hours were required to achieve success in up to 37% of men (59).
In a recent controlled study, we compared micro-TESE with conventional single-biopsy TESE in a group of 60 men with NOA (12). Overall, the SRRs were significantly higher when micro-TESE was used (45% vs. 25%). Furthermore, the results were in favor of micro-TESE after patient stratification by the histopathology categories of hypospermatogenesis (93% vs. 64%), maturation arrest (64% vs. 9%) and Sertoli cell-only syndrome (2% vs. 6%). In cases of NOA, our preference is to use micro-TESE over the other SRT. However, our patients exhibiting hypospermatogenesis on previous testicular histopathology or those with a history of a successful SR attempt are eligible for TESA as the first-choice method if their testicular volume is larger than 10 cc. Our SRRs with TESA and micro-TESE have proven comparable (51%) with this treatment algorithm (14,53).
Based on our ten-year experience in the management of azoospermic men seeking fertility treatment, the likelihood of a successful sperm retrieval is 43-fold higher (odds ratio [OR] = 43.0; 95% confidence interval [CI]: 10.3-179.5) in men with OA compared with men with NOA (60).
Sperm retrieval procedures are always carried out in a restricted region. As such, it is often not difficult to implement local or locoregional anesthesia even for more extensive interventions, such as micro-TESE. Despite this fact, general anesthesia and spinal blocks are often used for open procedures because such modalities are safe and effective and, as a rule, most patients are too anxious on the day of the procedure to support the use of local anesthesia. However, simpler, less expensive, and less invasive techniques that offer higher patient satisfaction and quicker recovery are more suitable for outpatient procedures. Due to the brevity and small to moderate intensity of the pain stimulus in PESA, local anesthesia is not required when employing intravenous sedation. The use of propofol as a single agent under spontaneous and/or assisted ventilation with a face mask offers excellent results not only in terms of the surgeon's working conditions during manual testis mobilization but also in terms of patient satisfaction by providing anxiolysis and comfort. The dosage should be tailored to each patient by the anesthetist to obtain the necessary sedation level and is usually in the range of 3 to 4 mg.kg-1. Small amounts of an opioid, such as fentanyl (1 to 3 μg.kg-1) or alfentanil (10 to 20 μg.kg-1), can be added if necessary. In TESA, it is appropriate to add local anesthetic infiltration to the intravenous propofol sedation, as TESA requires further manipulation of the testis. Our preference is to couple sedation with a percutaneous block of the spermatic cord by injecting 6 to 8 mL of 1% lidocaine without a vasoconstrictor at the external inguinal ring. In contrast, open procedures require an incision to be made. Moreover, the testis is usually delivered, thus causing some degree of tension on the spermatic cord. As a result, a more intense nociceptive stimulus is expected, which requires an anesthesia technique capable of providing increased analgesia in addition to autonomic response blockade. Deep sedation with assisted ventilation or even general anesthesia with controlled ventilation using drugs with a short or ultrashort duration is the preferred technique. However, it is also possible to obtain sufficient anesthesia using spermatic cord block associated with mild to moderate sedation (32). For this purpose, we use a 4-mg.kg-1 induction dose of propofol followed by a 60- to 100-μg.kg-1.min-1 infusion under spontaneous or assisted ventilation using a face mask with 100% oxygen, according to the needs and characteristics of the patient. Small amounts of an opioid, such as fentanyl (1 to 2 μg.kg-1) or alfentanil (7 to 15 μg.kg-1), are added before the surgeon injects 1% lidocaine at the incision site. The cord block is achieved with 4 to 6 mL of the same local anesthetic, which is injected by the surgeon when the pampiniform plexus is exposed.
KEY ISSUES
Sperm retrieval techniques (SRTs) are surgical methods that have been developed to retrieve spermatozoa from the epididymides and the testicles of azoospermic men seeking fertility treatment.
After sperm acquisition, intracytoplasmic sperm injection (ICSI) is used in place of standard in vitro fertilization (IVF) because ICSI has been shown to result in a significantly higher fertilization rate.
From the clinical standpoint, the goals of sperm retrieval are two-fold: i) to obtain an adequate number of the highest quality sperm possible, which can be immediately used for ICSI or alternatively cryopreserved for future ICSI attempts, and ii) to minimize damage to the reproductive tract, thus preserving the option of repeated retrieval attempts and testicular function.
The two general SR methods are open surgery and percutaneous acquisition. Open surgery can be carried out to retrieve spermatozoa from the epididymis or the testicle with or without microsurgery. Percutaneous retrievals require a needle to be percutaneously inserted into the sperm source, i.e., the epididymis or the testicle. Irrespective of the method used, the goal is to obtain the epididymal fluid or the seminiferous tubules and their contents.
Epididymal retrievals are only indicated in cases of obstructive azoospermia, whereas testicular extractions can be used in both obstructive and non-obstructive azoospermia cases.
Sperm production is normal and gametes can be easily retrieved from the epididymis or testis in virtually all cases of obstructive azoospermia. In obstructive azoospermia, the choice of sperm retrieval technique should be based on the surgeon's preferences and expertise, as there is no evidence that one particular method is superior to another. Although increased cryopreservation rates have been reported for open surgical retrieval methods, the costs of these methods are significantly higher. Percutaneous approaches, on the other hand, can be performed under local anesthesia on an outpatient basis and, if needed, be repeated to achieve a successful SR. In general, sperm retrieval in obstructive azoospermia is associated with low complication rates and minimal morbidity.
Sperm production is markedly impaired or absent in men with non-obstructive azoospermia. In this clinical scenario, testicular sperm extraction is the method of choice for sperm retrieval. Overall, successful retrieval rates range from 30-60%, which means that 30-60% of the men with NOA have focal areas of sperm production within the testes.
The efficiency of retrieval in non-obstructive azoospermia is related to the method of sperm acquisition. Percutaneous testicular aspiration retrieval rates range from 10-30% and are markedly lower than the 50% success rate reported for testicular sperm extractions.
Microsurgical-guided sperm acquisition has been applied in both epididymal and testicular retrievals. The goal of microsurgical epididymal sperm aspiration (MESA) is to identify and open a single epididymal tubule to enable the aspiration of a sperm-rich, red blood cell-free fluid that can be used for fresh sperm injection or cryopreserved for a single or multiple later ICSI attempts. In microsurgical testicular sperm extraction (micro-TESE), the testicular parenchyma is dissected under magnification to search for enlarged seminiferous tubules, which are more likely to contain germ cells and foci of sperm production. MESA is indicated for OA cases, whereas micro-TESE is recommended for the most severe NOA cases.
Microsurgical retrievals require microsurgical training, microsurgical instruments and an operating microscope. These techniques are associated with increased operative time and costs.
Micro-TESE has superior sperm retrieval rates and requires the removal of much less tissue than conventional open testicular retrievals. Micro-TESE has been successfully used in different populations of men with testicular failure.
Complications after testicular retrievals include intratesticular hematoma, pain, swelling, infection, and hydrocele. Most complications resolve spontaneously without compromising testicular function. The extraction of a large volume of testicular parenchyma may lead to a transient or permanent decrease in serum testosterone levels due to testicular devascularization and excessive tissue removal. The incidence of complications is lower following micro-TESE than conventional TESE because the former procedure enables the excision of a minimal amount of tissue and preserves the vasculature.
Sperm retrieval techniques are relatively simple surgeries that can be safely completed with general anesthesia or spinal blocks. However, because these surgeries are typically outpatient procedures, the latest trend is to employ local or locoregional anesthesia with or without intravenous sedation. The combination of local anesthesia and intravenous sedation offers the patient the analgesic effectiveness of local anesthetics combined with the comfort and effective control of anxiety provided by intravenous sedation. When this combination includes propofol, patients experience greater satisfaction, recovering quickly and with minimal adverse effects. These patients may also benefit from the advantages of outpatient procedures.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37(5):570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 2.Silber SJ, Nagy ZP, Liu J, Godoy H, Devroey P, Vansteirteghem AC. Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Reprod. 1994;9(9):1705–9. doi: 10.1093/oxfordjournals.humrep.a138778. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Salom M, Romero J, Rubio C, Ruiz A, Remohi J, Pellicer A. Intracytoplasmic sperm injection with cryopreserved testicular spermatozoa. Mol Cell Endocrinol. 2000;169(1-2):15–9. doi: 10.1016/s0303-7207(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder-Printzen I, Zumbe J, Bispink L, Palm S, Schneider U, Engelmann U, et al. Microsurgical epididymal sperm aspiration: aspirate analysis and straws available after cryopreservation in patients with non-reconstructable obstructive azoospermia. Hum Reprod. 2000;15(12):2531–5. doi: 10.1093/humrep/15.12.2531. [DOI] [PubMed] [Google Scholar]
- 5.Collaboration ASRM. The management of infertility due to obstructive azoospermia. Fertil Steril. 2008;90:S121–S4. doi: 10.1016/j.fertnstert.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 6.Vernaeve V, Bonduelle M, Tournaye H, Camus M, Van Steirteghem A, Devroey P. Pregnancy outcome and neonatal data of children born after ICSI using testicular sperm in obstructive and non-obstructive azoospermia. Hum Reprod. 2003;18(10):2093–7. doi: 10.1093/humrep/deg403. [DOI] [PubMed] [Google Scholar]
- 7.Belva F, De Schrijver F, Tournaye H, Liebaers I, Devroey P, Haentjens P, et al. Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26(7):1752–8. doi: 10.1093/humrep/der121. [DOI] [PubMed] [Google Scholar]
- 8.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics. 2011;2012;66(4)67(2):691–700. 203. doi: 10.1590/S1807-59322011000400026. Correction in: Clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Krausz C, Tournaye H. Guidelines on male infertility. EAU Guidelines 2012 edition. Uroweb. 2012 doi: 10.1016/j.eururo.2012.04.048. Available from: http://www.uroweb.org/gls/pdf/15_Male_Infertility_LR%20II.pdf. [DOI] [PubMed] [Google Scholar]
- 10.Kolettis PN, Thomas AJ. Vasoepididymostomy for vasectomy reversal: A critical assessment in the era of intracytoplasmic sperm injection. J Urology. 1997;158(2):467–70. doi: 10.1016/s0022-5347(01)64504-x. [DOI] [PubMed] [Google Scholar]
- 11.Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Human reproduction update. 2007;13(6):539–49. doi: 10.1093/humupd/dmm029. [DOI] [PubMed] [Google Scholar]
- 12.Verza S, Esteves SC. Microsurgical versus conventional single-biopsy testicular sperm extraction in nonobstructive azoospermia: a prospective controlled study. Fertil Steril. 2011;96(3):S53. [Google Scholar]
- 13. Esteves SC, Verza S., Jr Nagy Z P, Varghese A C, Agarwal A, PESA/TESA/TESE sperm processing Practical manual of in vitro fertilization 2012New York: Springer; p.207–20.1st edition [Google Scholar]
- 14. Esteves SC, Agarwal A. Gardner D K, Rizk B R M B, Falcone T, Sperm retrieval techniques Human assisted reproductive technology: future trends in laboratory and clinical practice 2011Cambridge: Cambridge University Press; p.41–53.1st edition [Google Scholar]
- 15.Shah R. Surgical sperm retrieval: Techniques and their indications. Indian J Urol. 2011;27(1):102–9. doi: 10.4103/0970-1591.78439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devroey P, Liu J, Nagy Z, Goossens A, Tournaye H, Camus M, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod. 1995;10(6):1457–60. doi: 10.1093/humrep/10.6.1457. [DOI] [PubMed] [Google Scholar]
- 17.Craft I, Shrivastav P. Treatment of male infertility. Lancet. 1994;344(8916):191–2. doi: 10.1016/s0140-6736(94)92792-8. [DOI] [PubMed] [Google Scholar]
- 18.Lewin A, Weiss DB, Friedler S, Ben-Shachar I, Porat-Katz A, Meirow D, et al. Delivery following intracytoplasmic injection of mature sperm cells recovered by testicular fine needle aspiration in a case of hypergonadotropic azoospermia due to maturation arrest. Hum Reprod. 1996;11(4):769–71. doi: 10.1093/oxfordjournals.humrep.a019252. [DOI] [PubMed] [Google Scholar]
- 19.Esteves SC, Miyaoka R, Agarwal A. Surgical treatment of male infertility in the era of intracytoplasmic sperm injection - new insights. Clinics. 2011;66(8):1463–78. doi: 10.1590/S1807-59322011000800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarow JP. Intratesticular arterial anatomy. J Androl. 1990;11(3):255–9. [PubMed] [Google Scholar]
- 21.Morey AF, Deshon GE, Jr, Rozanski TA, Dresner ML. Technique of biopty gun testis needle biopsy. Urology. 1993;42(3):325–6. doi: 10.1016/0090-4295(93)90625-k. [DOI] [PubMed] [Google Scholar]
- 22.Turek PJ, Cha I, Ljung BM. Systematic fine-needle aspiration of the testis: correlation to biopsy and results of organ "mapping" for mature sperm in azoospermic men. Urology. 1997;49(5):743–8. doi: 10.1016/S0090-4295(97)00154-4. [DOI] [PubMed] [Google Scholar]
- 23.Temple-Smith PD, Southwick GJ, Yates CA, Trounson AO, de Kretser DM. Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J In Vitro Fert Embryo Transf. 1985;2(3):119–22. doi: 10.1007/BF01131497. [DOI] [PubMed] [Google Scholar]
- 24.Van Peperstraten A, Proctor ML, Johnson NP, Philipson G. Techniques for surgical retrieval of sperm prior to ICSI for azoospermia. Cochrane Database Syst Rev. 2006;((3)):CD002807. doi: 10.1002/14651858.CD002807.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14(1):131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14(1):109–15. doi: 10.1038/aja.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgy A, Meniru GI, Naumann N, Beski S, Bates S, Craft IL. The efficacy of local anaesthesia for percutaneous epididymal sperm aspiration and testicular sperm aspiration. Hum Reprod. 1998;13(3):646–50. doi: 10.1093/humrep/13.3.646. [DOI] [PubMed] [Google Scholar]
- 28.Nudell DM, Conaghan J, Pedersen RA, Givens CR, Schriock ED, Turek PJ. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod. 1998;13(5):1260–5. doi: 10.1093/humrep/13.5.1260. [DOI] [PubMed] [Google Scholar]
- 29.Sneyd JR, Carr A, Byrom WD, Bilski AJ. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur J Anaesthesiol. 1998;15(4):433–45. doi: 10.1046/j.1365-2346.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 30.Alghanem SM, Massad IM, Rashed EM, Abu-Ali HM, Daradkeh SS. Optimization of anesthesia antiemetic measures versus combination therapy using dexamethasone or ondansetron for the prevention of postoperative nausea and vomiting. Surg Endosc. 2010;24(2):353–8. doi: 10.1007/s00464-009-0567-3. [DOI] [PubMed] [Google Scholar]
- 31.Borgeat A, Wildersmith OHG, Saiah M, Rifat K. Subhypnotic Doses of Propofol Possess Direct Antiemetic Properties. Anesth Analg. 1992;74(4):539–41. doi: 10.1213/00000539-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10 811 patients. Brit J Anaesth. 2000;84(1):6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 33.Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, Schlegel PN. Success of testicular sperm injection and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocr Metab. 2005;90(11):6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 34.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65(6):1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 35.Carpi A, Fabris FGM, Palego P, Di Coscio G, Romani R, Nardini V, et al. Fine-needle and large-needle percutaneous aspiration biopsy of testicles in men with nonobstructive azoospermia: safety and diagnostic performance. Fertil Steril. 2005;83(4):1029–33. doi: 10.1016/j.fertnstert.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 36.Turunc T, Gul U, Haydardedeoglu B, Bal N, Kuzgunbay B, Peskircioglu L, et al. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: a prospective comparative study. Fertil Steril. 2010;94(6):2157–60. doi: 10.1016/j.fertnstert.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Rosenlund B, Westlander G, Wood M, Lundin K, Reismer E, Hillensjo T. Sperm retrieval and fertilization in repeated percutaneous epididymal sperm aspiration. Hum Reprod. 1998;13(10):2805–7. doi: 10.1093/humrep/13.10.2805. [DOI] [PubMed] [Google Scholar]
- 38.Esteves SC, Lee W, Benjamin DJ, Seol B, Verza A, Jr, Agarwal A. Reproductive potential including neonatal outcomes of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmci sperm injection according to the cause of obstruction. J Urol. 2013;189(1):232–7. doi: 10.1016/j.juro.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 39.Carpi A, Sabanegh E, Mechanick J. Controversies in the management of nonobstructive Azoospermia. Fertil Steril. 2009;91(4):963–70. doi: 10.1016/j.fertnstert.2009.01.083. [DOI] [PubMed] [Google Scholar]
- 40.Harrington TG, Schauer D, Gilbert BR. Percutaneous testis biopsy: an alternative to open testicular biopsy in the evaluation of the subfertile man. J Urol. 1996;156(5):1647–51. doi: 10.1016/s0022-5347(01)65473-9. [DOI] [PubMed] [Google Scholar]
- 41.Silber SJ. Microsurgical TESE and the distribution of spermatogenesis in non-obstructive azoospermia. Hum Reprod. 2000;15(11):2278–84. doi: 10.1093/humrep/15.11.2278. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Hibi H, Asada Y, Suganuma N, Tomoda Y. Epididymal sperm retrieval by epididymal micropuncture combined with intracytoplasmic sperm injection: difference between acquired and congenital irreparable obstructive azoospermia. Urol Int. 1997;58(3):177–80. doi: 10.1159/000282977. [DOI] [PubMed] [Google Scholar]
- 43.Friedler S, Raziel A, Strassburger D, Schachter M, Soffer Y, Ron-El R. Factors influencing the outcome of ICSI in patients with obstructive and non-obstructive azoospermia: a comparative study. Hum Reprod. 2002;17(12):3114–21. doi: 10.1093/humrep/17.12.3114. [DOI] [PubMed] [Google Scholar]
- 44.Glina S, Fragoso JB, Martins FG, Soares JB, Galuppo AG, Wonchockier R. Percutaneous epididymal sperm aspiration (PESA) in men with obstructive azoospermia. Int Braz J Urol. 2003;29(2):141–5. doi: 10.1590/s1677-55382003000200008. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 45.Tournaye H. Surgical sperm recovery for intracytoplasmic sperm injection: which method is to be preferred. Hum Reprod. 1999;14:71–81. doi: 10.1093/humrep/14.suppl_1.71. [DOI] [PubMed] [Google Scholar]
- 46.Ezeh UIO, Moore HDM, Cooke ID. A prospective study of multiple needle biopsies versus a single open biopsy for testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 1998;13(11):3075–80. doi: 10.1093/humrep/13.11.3075. [DOI] [PubMed] [Google Scholar]
- 47.Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, Arakawa S, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urology. 2002;168(3):1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 48.Amer M, Ateyah A, Hany R, Zohdy W. Prospective comparative study between microsurgical and conventional testicular sperm extraction in nonobstructive azoospermia: follow-up by serial ultrasound examinations. Hum Reprod. 2000;15(3):653–6. doi: 10.1093/humrep/15.3.653. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimura A. Microdissection testicular sperm extraction: Prediction, outcome, and complications. Int J Urol. 2007;14(10):883–9. doi: 10.1111/j.1442-2042.2007.01828.x. [DOI] [PubMed] [Google Scholar]
- 50.El-Haggar S, Mostafa T, Abdel Nasser T, Hany R, Abdel Hadi A. Fine needle aspiration vs. mTESE in non-obstructive azoospermia. Int J Androl. 2008;31(6):595–601. doi: 10.1111/j.1365-2605.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 51.Janzen N, Goldstein M, Schlegel PN, Palermo GD, Rosenwaks Z. Use of electively cryopreserved microsurgically aspirated epididymal sperm with IVF and intracytoplasmic sperm injection for obstructive azoospermia. Fertil Steril. 2000;74(4):696–701. doi: 10.1016/s0015-0282(00)01496-5. [DOI] [PubMed] [Google Scholar]
- 52.Schlegel PN. Causes of azoospermia and their management. Reprod Fert Develop. 2004;16(5):561–72. doi: 10.10371/RD03087. [DOI] [PubMed] [Google Scholar]
- 53.Esteves SC, Verza S, Prudencio C, Seol B. Sperm retrieval rates (SRR) in nonobstructive azoospermia (NOA) are related to testicular histopathology results but not to the etiology of azoospermia. Fertil Steril. 2010;94(4):S132. [Google Scholar]
- 54.Chan PTK, Palermo GD, Veeck LL, Rosenwaks Z, Schlegel PN. Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy. Cancer. 2001;92(6):1632–7. doi: 10.1002/1097-0142(20010915)92:6<1632::aid-cncr1489>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 55.Raman JD, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J Urology. 2003;170(4):1287–90. doi: 10.1097/01.ju.0000080707.75753.ec. [DOI] [PubMed] [Google Scholar]
- 56.Hauser R, Yogev L, Paz G, Yavetz H, Azem F, Lessing JB, et al. Comparison of efficacy of two techniques for testicular sperm retrieval in nonobstructive azoospermia: Multifocal testicular sperm extraction versus multifocal testicular sperm aspiration. J Androl. 2006;27(1):28–33. doi: 10.2164/jandrol.05055. [DOI] [PubMed] [Google Scholar]
- 57.Friedler S, Raziel A, Strassburger D, Soffer Y, Komarovsky D, RonEl R. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Hum Reprod. 1997;12(7):1488–93. doi: 10.1093/humrep/12.7.1488. [DOI] [PubMed] [Google Scholar]
- 58.Mulhall JP, Ghaly SW, Aviv N, Ahmed A. The utility of optical loupe magnification for testis sperm extraction in men with nonobstructive azoospermia. J Androl. 2005;26(2):178–81. doi: 10.1002/j.1939-4640.2005.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 59.Ramasamy R, Fisher ES, Ricci JA, Leung RA, Schlegel PN. Duration of microdissection testicular sperm extraction procedures: relationship to sperm retrieval success. J Urology. 2011;185(4):1395–8. doi: 10.1016/j.juro.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 60.Prudencio C, Seol B, Esteves SC. Reproductive potential of azoospermic men undergoing intracytoplasmic sperm injection is dependent on the type of azoospermia. Fertil Steril. 2010;94(4):S232–S3. [Google Scholar]